On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells
Abstract
1. Introduction
2. Experimental
2.1. Characterization
2.2. Synthesis of RuCu-ANPs/IL-GA
2.3. Preparation of Three-Electrode System
2.4. Fabrication of Microfluidic Electrochemical Sensing Chip
2.5. Cell Culture in Sensing Chip for Real-Time Monitoring
3. Results and Discussion
3.1. Morphological and Structural Characterization
3.2. Electrochemical Catalytic and Sensing Performances
3.3. The Anti-Interference Ability
3.4. Reproducibility and Stability
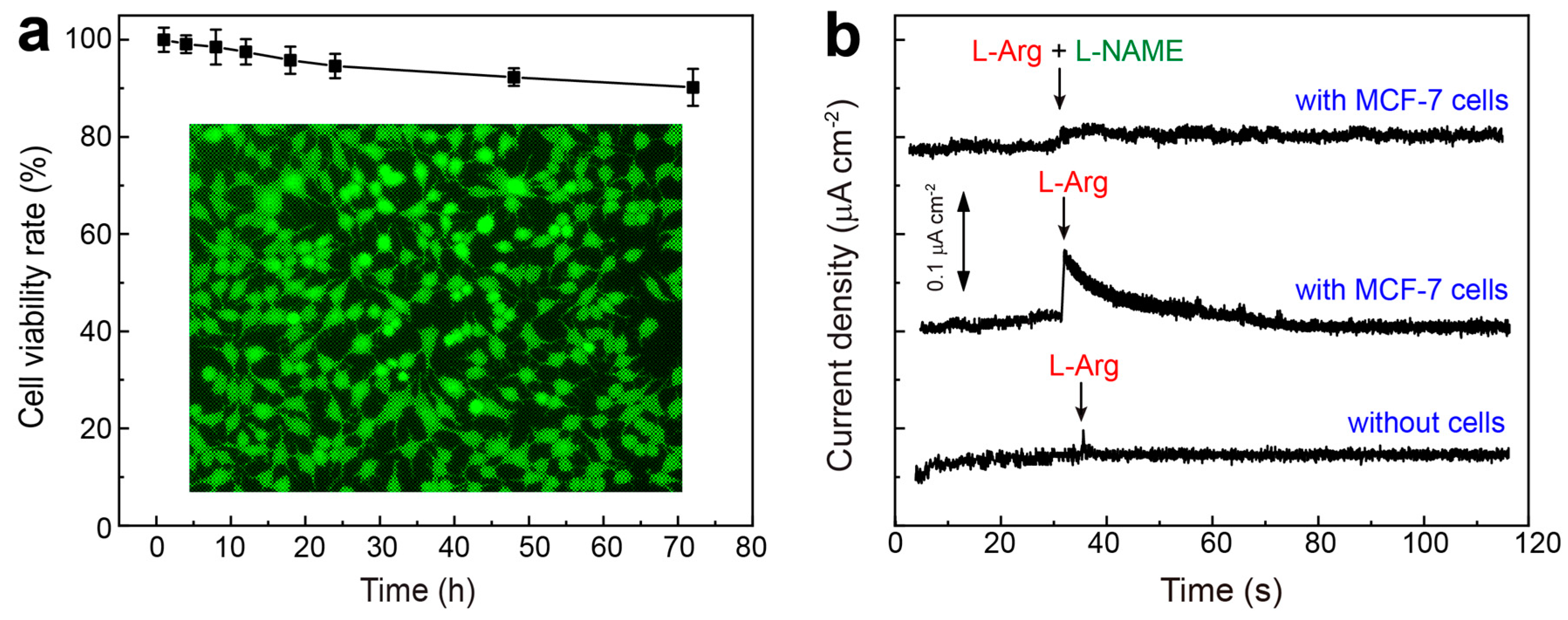
3.5. On-Chip Detection of NO Released from Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Ridnour, L.A.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Nitric oxide in wound-healing. Microsurgery 2005, 25, 442–451. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef]
- Nakamura, T.; Oh, C.K.; Liao, L.; Zhang, X.; Lopez, K.M.; Gibbs, D.; Deal, A.K.; Scott, H.R.; Spencer, B.; Masliah, E.; et al. Noncanonical transnitrosylation network contributes to synapse loss in Alzheimer’s disease. Science 2021, 371, eaaw0843. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 19–48. [Google Scholar] [CrossRef]
- Beal, M.F. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann. Neurol. 1998, 44, S110–S114. [Google Scholar] [CrossRef]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Webb, A.; Bond, R.; McLean, P.; Uppal, R.; Benjamin, N.; Ahluwalia, A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA 2004, 101, 13683–13688. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R., Jr. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef]
- Almeida, B.; Rogers, K.E.; Nag, O.K.; Delehanty, J.B. Sensing nitric oxide in cells: Historical technologies and future outlook. ACS Sens. 2021, 6, 1695–1703. [Google Scholar] [CrossRef]
- Hu, F.X.; Hu, G.; Wang, D.P.; Duan, X.; Feng, L.; Chen, B.; Liu, Y.; Ding, J.; Guo, C.; Yang, H.B. Integrated biochip−electronic system with single-atom nanozyme for in vivo analysis of nitric oxide. ACS Nano 2023, 17, 8575–8585. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electroanalysis and biosensors. Anal. Chem. 1999, 71, 328R–332R. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, R.; Duan, Y.; Deng, D.; Chen, H.; Xia, T.; Duan, Y.; Lei, H.; Luo, L. Ultrasensitive lab-on-paper electrochemical device via heterostructure copper/cuprous sulfide@N-doped C@Au hollow nanoboxes as signal amplifier for alpha-fetoprotein detection. Biosens. Bioelectron. 2025, 267, 116827. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Hu, Y.; Zhang, Q.; Wang, S.; Guo, Z.; Qing, Z. Portable detection of lysine acetyltransferase activity in lung cancer cells based on a miniature electrochemical sensor. Anal. Chem. 2024, 96, 5546–5553. [Google Scholar] [CrossRef]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef]
- Brown, M.D.; Schoenfisch, M.H. Electrochemical nitric oxide sensors: Principles of design and characterization. Chem. Rev. 2019, 119, 11551–11575. [Google Scholar] [CrossRef]
- Li, J.X.; Fan, W.T.; Sun, M.Y.; Zhao, Y.; Lu, Y.F.; Yang, Y.B.; Huang, W.H.; Liu, Y.L. Flexible fiber sensors for real-time monitoring of redox signaling molecules in exercise-mimicking engineered skeletal muscle. Angew. Chem. Int. Ed. 2024, e202421684. [Google Scholar] [CrossRef]
- Guo, J.; Li, M.; Long, S.; Zhu, J.; Miao, P.; Wei, T.; Gao, T. Bio-inspired electrochemical detection of nitric oxide promoted by coordinating the histamine-iron phthalocyanine catalytic center on microelectrode. Anal. Chem. 2023, 95, 8842–8849. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, J.; Zhang, P.; Zhang, Y.; Miao, Y.; Gao, S.; Deng, Y.; Geng, L. Recent advances in microfluidic chip integrated electronic biosensors for multiplexed detection. Biosens. Bioelectron. 2018, 121, 272–280. [Google Scholar] [CrossRef]
- Sameenoi, Y.; Koehler, K.; Shapiro, J.; Boonsong, K.; Henry, C.S. Microfluidic electrochemical sensor for on-line monitoring of aerosol oxidative activity. J. Am. Chem. Soc. 2012, 134, 10562–10568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dong, X.; He, H.; Zhang, Y.; Chi, K.; Xu, Y.; Asif, M.; Yang, X.; He, W.; Liao, K.; et al. 2D carbon network arranged into high-order 3D nanotube arrays on a flexible microelectrode: Integration into electrochemical microbiosensor devices for cancer detection. NPG Asia Mater. 2023, 15, 6. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, W.; Zhang, Y.; Duan, H.; Xiao, F. Electrochemical microfluidic multiplexed bioanalysis by a highly active bottlebrush-like nanocarbon microelectrode. Anal. Chem. 2022, 94, 4463–4473. [Google Scholar] [CrossRef]
- Zhao, A.; Lin, T.; Xu, Y.; Zhang, W.; Asif, M.; Sun, Y.; Xiao, F. Integrated electrochemical microfluidic sensor with hierarchically porous nanoarrays modified graphene fiber microelectrode for bioassay. Biosens. Bioelectron. 2022, 205, 114095. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; She, J.; Zhao, A.; Yang, S.; Yang, X.; Xiao, F.; Sun, Y. On-chip electrochemical sensing of neurotransmitter in nerve cells by functionalized graphene fiber microelectrode. Sens. Actuators B-Chem. 2022, 365, 131874. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, W.; Duan, H.; Xiao, F. Bimetal–organic framework-integrated electrochemical sensor for on-chip detection of H2S and H2O2 in cancer tissues. Biosens. Bioelectron. 2024, 260, 116463. [Google Scholar] [CrossRef]
- Asif, M.; Ashraf, G.; Aziz, A.; Iftikhar Ta Wang, Z.; Xiao, F.; Sun, Y. Tuning the redox chemistry of copper oxide nanoarchitectures integrated with rGOP via facet engineering: Sensing H2S toward SRB detection. ACS Appl. Mater. Interfaces 2022, 14, 19480–19490. [Google Scholar] [CrossRef]
- Hafeez, A. Two-dimensional nanomaterials. In Engineering Materials: Fundamentals, Processing and Properties; Springer: Cham, Switzerland, 2024; pp. 181–204. [Google Scholar] [CrossRef]
- Kizhepat, S.; Rasal, A.S.; Chang, H.F. Development of two-dimensional functional nanomaterials for biosensor applications: Opportunities, challenges, and future prospects. Nanomaterials 2023, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Conquest, O.J.; Verdi, C.; Stampfl, C. Electronic and optical properties of 2D heterostructure bilayers of graphene, borophene and 2D boron carbides from first principles. Nanomaterials 2024, 14, 1659. [Google Scholar] [CrossRef]
- Huang, W.; Xu, Y.; Wang, Z.; Liao, K.; Zhang, Y.; Sun, Y. Dual nanozyme based on ultrathin 2D conductive MOF nanosheets intergraded with gold nanoparticles for electrochemical biosensing of H2O2 in cancer cells. Talanta 2022, 249, 123612. [Google Scholar] [CrossRef]
- Yan, C.; Liu, Y.; Zeng, Q.; Wang, G.; Han, J. 2D nanomaterial supported single-metal atoms for heterogeneous photo/electrocatalysis. Adv. Funct. Mater. 2023, 33, 2210837. [Google Scholar] [CrossRef]
- Sanjayan, C.G.; Gokavi, L.; Ravikumar, C.H.; Balarkishna, R.G. Antibody-modified 2D mxene nanosheet probes for selective, picolevel detection of cancer biomarkers. Biosens. Bioelectron. 2025, 271, 117028. [Google Scholar] [CrossRef]
- Chen, Z.; Asif, M.; Wang, R.; Li, Y.; Zeng, X.; Yao, W.; Sun, Y.; Liao, K. Recent trends in synthesis and applications of porous MXene assemblies: A topical review. Chem. Rec. 2022, 22, e202100261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, J.; Lv, Q.; Wang, L.; Dong, X.; Asif, M.; Ren, J.; He, W.; Sun, Y.; Xiao, F.; et al. In situ electrochemical sensing and real-time monitoring live cells based on freestanding nanohybrid paper electrode assembled from 3D functionalized graphene framework. ACS Appl. Mater. Interfaces 2017, 9, 38201–38210. [Google Scholar] [CrossRef]
- Ding, G.; Yang, B.; Chen, K.; Wang, H.; Chen, J.; Mei, Q. Enhanced self–assembly and spontaneous separation for ultrathin, airfloating graphene macrofilms and their application in ultrasensitive in-site growth sensors. Adv. Mater. 2024, 36, e2408550. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qi, Y.; Cai, G.; Zhao, Y.; Ming, X.; Lin, Z.; Ma, W.; Lin, J.; Li, H.; et al. Bidirectionally promoting assembly order for ultrastiff and highly thermally conductive graphene fibres. Nat. Commun. 2024, 15, 409. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, C.; Yang, L.; Wu, J.; Li, J. Preparation of graphene/polydimethylsiloxane flexible resistive pressure sensors based on direct ink writing 3D printing. Sens. Actuators A-Phys. 2025, 382, 116148. [Google Scholar] [CrossRef]
- Kumar, A.; Ubaidullah, M.; Pham, P.V.; Gupta, R.K. Three-dimensional macro-structures of graphene-based materials for emerging heterogeneous electrocatalysis: Concepts, syntheses, principles, applications, and perspectives. Chem. Eng. J. 2024, 499, 156664. [Google Scholar] [CrossRef]
- Lee, J.; Han, H.; Noh, D.; Lee, J.; Lim, D.D.; Park, J.; Gu, G.X.; Choi, W. Multiscale porous architecture consisting of graphene aerogels and metastructures enabling robust thermal and mechanical functionalities of phase change materials. Adv. Funct. Mater. 2024, 34, 2405625. [Google Scholar] [CrossRef]
- Fan, K.; Zhou, S.; Xie, L.; Jia, S.; Zhao, L.; Liu, X.; Liang, K.; Jiang, L.; Kong, B. Interfacial assembly of 2D graphene-derived ion channels for water-based green energy conversion. Adv. Mater. 2024, 36, 2307849. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.L.; Sharma, R.; Radhakrishnan, J.; Abbaszadeh, S.; Shahbazi, M.; Tafreshi, O.A.; Karamikamkar, S.; Maleki, H. Emerging 2D nanomaterials-integrated hydrogels: Advancements in designing theragenerative materials for bone regeneration and disease therapy. Adv. Sci. 2024, 11, e2403204. [Google Scholar] [CrossRef]
- Zhao, A.; She, J.; Xiao, C.; Xi, J.; Xu, Y.; Manoj, D.; Sun, Y.; Xiao, F. Green and controllable synthesis of multi-heteroatoms co-doped graphene fiber as flexible and biocompatible microelectrode for in situ electrochemical detection of biological samples. Sens. Actuators B-Chem. 2021, 335, 129683. [Google Scholar] [CrossRef]
- Zeng, W.; Devaraj, M.; Sun, H.; Yi, R.; Huang, X.; Sun, Y. One-pot synthesis of high-density Pd nanoflowers decorated 3D carbon nanotube-graphene network modified on printed electrode as portable electrochemical sensing platform for sensitive detection of nitroaromatic explosives. J. Electroanal. Chem. 2019, 833, 527–535. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, G.; Du, Y.; Shi, P.; Li, N.; Li, Y.; Qin, Z.; Jiao, T.; He, X. Highly stretchable, low-hysteresis, and adhesive TA@MXene-composited organohydrogels for durable wearable sensors. Adv. Funct. Mater. 2024, 34, 2315813. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zheng, W.; Yao, J.; Zhao, Y. RuCu cage/alloy nanoparticles with controllable electroactivity for specific electroanalysis applications. Anal. Chem. 2021, 93, 13080–13088. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Liang, J.; Li, S.; Shi, H.; Liu, J.; Wang, T.; Han, J.; Li, Q. Protrusion-rich Cu@NiRu core@shell nanotubes for efficient alkaline hydrogen evolution electrocatalysis. Small 2022, 18, 2202496. [Google Scholar] [CrossRef]
- Li, L.; Qiu, H.; Zhu, Y.; Chen, G.; She, S.; Guo, X.; Li, H.; Liu, T.; Lin, Z.; Zhou, H.; et al. Atomic ruthenium modification of nickel-cobalt alloy for enhanced alkaline hydrogen evolution. Appl. Catal. B 2023, 331, 122710. [Google Scholar] [CrossRef]
- Cui, Z.; Ren, Z.; Ma, C.; Chen, B.; Chen, G.; Lu, R.; Zhu, W.; Gan, T.; Wang, Z.; Zhuang, Z. Dilute RuCo alloy synergizing single Ru and Co atoms as efficient and co-resistant anode catalyst for anion exchange membrane fuel cells. Angew. Chem. Int. Ed. 2024, 63, e202404761. [Google Scholar] [CrossRef]
- Kim, C.; Song, J.Y.; Choi, C.; Ha, J.P.; Lee, W.; Nam, Y.T.; Lee, D.M.; Kim, G.; Gereige, I.; Jung, W.B.; et al. Atomic-scale homogeneous RuCu alloy nanoparticles for highly efficient electrocatalytic nitrogen reduction. Adv. Mater. 2022, 34, e2205270. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Le Boeuf, W.; Maheshwari, V. Au–Pt–Ni nanochains as dopamine catalysts: Role of elements and their spatial distribution. Nanoscale Adv. 2023, 5, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Jiang, Y.; Wang, G.; Wu, W.; Chen, W.; Yu, P.; Lin, Y.; Mao, J.; Mao, L. Single-atom Ni-N4 provides a robust cellular NO sensor. Nat. Commun. 2020, 11, 3188. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au nanoparticles-3D graphene hydrogel nanocomposite to boost synergistically in situ detection sensitivity toward cell-released nitric oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef]
- Paul, V. The effect of N-nitro-L-arginine methyl ester posttreatment on the anticonvulsant effect of phenobarbitone and diazepam on picrotoxin-induced convulsions in rats. Pharmacol. Biochem. Behav. 2003, 74, 789–794. [Google Scholar] [CrossRef]
- Liu, Z.; Nemec-Bakk, A.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide release from cardiac and cancer cells via a hierarchical nanoporous gold microelectrode. Anal. Chem. 2017, 89, 8036–8043. [Google Scholar] [CrossRef]
- Tang, L.; Sun, X.; Gao, X.; Wang, L.; Yang, P.; Ling, P. Ionic liquid functionalized metal-organic framework nanowires for sensitive and real-time electrochemical monitoring of nitric oxide released from living cells. Anal. Methods 2023, 15, 729–737. [Google Scholar] [CrossRef]
- Arul, P.; Huang, S.T.; Nandhini, C.; Huang, C.H.; Gowthaman, N.S.K.; Huang, C.H. Development of a nanozyme-based electrochemical catalyst for real-time biomarker sensing of superoxide and nitric oxide anions released from living cells and exogenous donors. Biosens. Bioelectron. 2024, 261, 116485. [Google Scholar] [CrossRef]
- Zhu, P.; Li, S.; Zhou, S.; Ren, N.; Ge, S.; Zhang, Y.; Wang, Y.; Yu, J. In situ grown COFs on 3D strutted graphene aerogel for electrochemical detection of NO released from living cells. Chem. Eng. J. 2020, 420, 127559. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yao, K.; Hu, M.; Li, S.; Yang, S.; Zhao, A. On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials 2025, 15, 417. https://doi.org/10.3390/nano15060417
Liu H, Yao K, Hu M, Li S, Yang S, Zhao A. On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials. 2025; 15(6):417. https://doi.org/10.3390/nano15060417
Chicago/Turabian StyleLiu, Haibo, Kaiyuan Yao, Min Hu, Shanting Li, Shengxiong Yang, and Anshun Zhao. 2025. "On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells" Nanomaterials 15, no. 6: 417. https://doi.org/10.3390/nano15060417
APA StyleLiu, H., Yao, K., Hu, M., Li, S., Yang, S., & Zhao, A. (2025). On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials, 15(6), 417. https://doi.org/10.3390/nano15060417






