On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells
Abstract
:1. Introduction
2. Experimental
2.1. Characterization
2.2. Synthesis of RuCu-ANPs/IL-GA
2.3. Preparation of Three-Electrode System
2.4. Fabrication of Microfluidic Electrochemical Sensing Chip
2.5. Cell Culture in Sensing Chip for Real-Time Monitoring
3. Results and Discussion
3.1. Morphological and Structural Characterization
3.2. Electrochemical Catalytic and Sensing Performances
3.3. The Anti-Interference Ability
3.4. Reproducibility and Stability
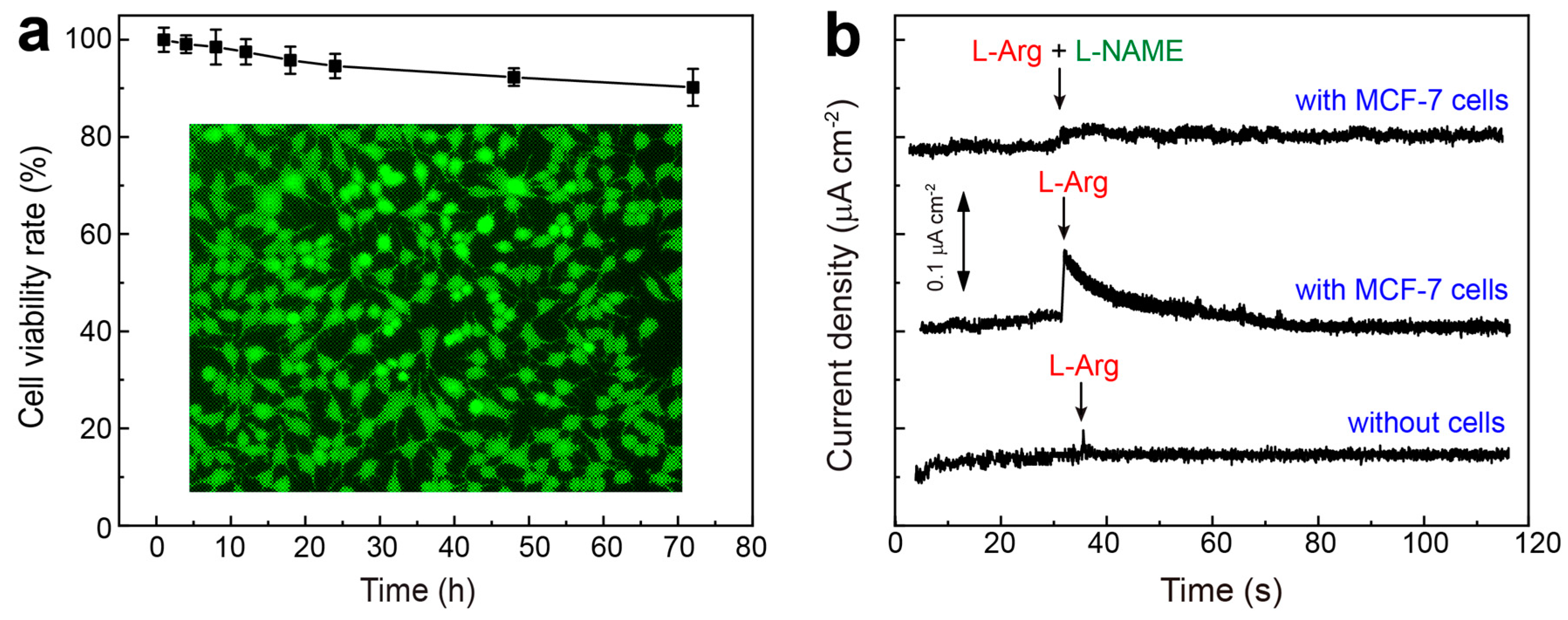
3.5. On-Chip Detection of NO Released from Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Ridnour, L.A.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Nitric oxide in wound-healing. Microsurgery 2005, 25, 442–451. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef]
- Nakamura, T.; Oh, C.K.; Liao, L.; Zhang, X.; Lopez, K.M.; Gibbs, D.; Deal, A.K.; Scott, H.R.; Spencer, B.; Masliah, E.; et al. Noncanonical transnitrosylation network contributes to synapse loss in Alzheimer’s disease. Science 2021, 371, eaaw0843. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 19–48. [Google Scholar] [CrossRef]
- Beal, M.F. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann. Neurol. 1998, 44, S110–S114. [Google Scholar] [CrossRef]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Webb, A.; Bond, R.; McLean, P.; Uppal, R.; Benjamin, N.; Ahluwalia, A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA 2004, 101, 13683–13688. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R., Jr. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef]
- Hall, C.N.; Garthwaite, J. What is the real physiological NO concentration in vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef]
- Almeida, B.; Rogers, K.E.; Nag, O.K.; Delehanty, J.B. Sensing nitric oxide in cells: Historical technologies and future outlook. ACS Sens. 2021, 6, 1695–1703. [Google Scholar] [CrossRef]
- Hu, F.X.; Hu, G.; Wang, D.P.; Duan, X.; Feng, L.; Chen, B.; Liu, Y.; Ding, J.; Guo, C.; Yang, H.B. Integrated biochip−electronic system with single-atom nanozyme for in vivo analysis of nitric oxide. ACS Nano 2023, 17, 8575–8585. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electroanalysis and biosensors. Anal. Chem. 1999, 71, 328R–332R. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, R.; Duan, Y.; Deng, D.; Chen, H.; Xia, T.; Duan, Y.; Lei, H.; Luo, L. Ultrasensitive lab-on-paper electrochemical device via heterostructure copper/cuprous sulfide@N-doped C@Au hollow nanoboxes as signal amplifier for alpha-fetoprotein detection. Biosens. Bioelectron. 2025, 267, 116827. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Hu, Y.; Zhang, Q.; Wang, S.; Guo, Z.; Qing, Z. Portable detection of lysine acetyltransferase activity in lung cancer cells based on a miniature electrochemical sensor. Anal. Chem. 2024, 96, 5546–5553. [Google Scholar] [CrossRef]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef]
- Brown, M.D.; Schoenfisch, M.H. Electrochemical nitric oxide sensors: Principles of design and characterization. Chem. Rev. 2019, 119, 11551–11575. [Google Scholar] [CrossRef]
- Li, J.X.; Fan, W.T.; Sun, M.Y.; Zhao, Y.; Lu, Y.F.; Yang, Y.B.; Huang, W.H.; Liu, Y.L. Flexible fiber sensors for real-time monitoring of redox signaling molecules in exercise-mimicking engineered skeletal muscle. Angew. Chem. Int. Ed. 2024, e202421684. [Google Scholar] [CrossRef]
- Guo, J.; Li, M.; Long, S.; Zhu, J.; Miao, P.; Wei, T.; Gao, T. Bio-inspired electrochemical detection of nitric oxide promoted by coordinating the histamine-iron phthalocyanine catalytic center on microelectrode. Anal. Chem. 2023, 95, 8842–8849. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, J.; Zhang, P.; Zhang, Y.; Miao, Y.; Gao, S.; Deng, Y.; Geng, L. Recent advances in microfluidic chip integrated electronic biosensors for multiplexed detection. Biosens. Bioelectron. 2018, 121, 272–280. [Google Scholar] [CrossRef]
- Sameenoi, Y.; Koehler, K.; Shapiro, J.; Boonsong, K.; Henry, C.S. Microfluidic electrochemical sensor for on-line monitoring of aerosol oxidative activity. J. Am. Chem. Soc. 2012, 134, 10562–10568. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dong, X.; He, H.; Zhang, Y.; Chi, K.; Xu, Y.; Asif, M.; Yang, X.; He, W.; Liao, K.; et al. 2D carbon network arranged into high-order 3D nanotube arrays on a flexible microelectrode: Integration into electrochemical microbiosensor devices for cancer detection. NPG Asia Mater. 2023, 15, 6. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, W.; Zhang, Y.; Duan, H.; Xiao, F. Electrochemical microfluidic multiplexed bioanalysis by a highly active bottlebrush-like nanocarbon microelectrode. Anal. Chem. 2022, 94, 4463–4473. [Google Scholar] [CrossRef]
- Zhao, A.; Lin, T.; Xu, Y.; Zhang, W.; Asif, M.; Sun, Y.; Xiao, F. Integrated electrochemical microfluidic sensor with hierarchically porous nanoarrays modified graphene fiber microelectrode for bioassay. Biosens. Bioelectron. 2022, 205, 114095. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; She, J.; Zhao, A.; Yang, S.; Yang, X.; Xiao, F.; Sun, Y. On-chip electrochemical sensing of neurotransmitter in nerve cells by functionalized graphene fiber microelectrode. Sens. Actuators B-Chem. 2022, 365, 131874. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, W.; Duan, H.; Xiao, F. Bimetal–organic framework-integrated electrochemical sensor for on-chip detection of H2S and H2O2 in cancer tissues. Biosens. Bioelectron. 2024, 260, 116463. [Google Scholar] [CrossRef]
- Asif, M.; Ashraf, G.; Aziz, A.; Iftikhar Ta Wang, Z.; Xiao, F.; Sun, Y. Tuning the redox chemistry of copper oxide nanoarchitectures integrated with rGOP via facet engineering: Sensing H2S toward SRB detection. ACS Appl. Mater. Interfaces 2022, 14, 19480–19490. [Google Scholar] [CrossRef]
- Hafeez, A. Two-dimensional nanomaterials. In Engineering Materials: Fundamentals, Processing and Properties; Springer: Cham, Switzerland, 2024; pp. 181–204. [Google Scholar] [CrossRef]
- Kizhepat, S.; Rasal, A.S.; Chang, H.F. Development of two-dimensional functional nanomaterials for biosensor applications: Opportunities, challenges, and future prospects. Nanomaterials 2023, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Conquest, O.J.; Verdi, C.; Stampfl, C. Electronic and optical properties of 2D heterostructure bilayers of graphene, borophene and 2D boron carbides from first principles. Nanomaterials 2024, 14, 1659. [Google Scholar] [CrossRef]
- Huang, W.; Xu, Y.; Wang, Z.; Liao, K.; Zhang, Y.; Sun, Y. Dual nanozyme based on ultrathin 2D conductive MOF nanosheets intergraded with gold nanoparticles for electrochemical biosensing of H2O2 in cancer cells. Talanta 2022, 249, 123612. [Google Scholar] [CrossRef]
- Yan, C.; Liu, Y.; Zeng, Q.; Wang, G.; Han, J. 2D nanomaterial supported single-metal atoms for heterogeneous photo/electrocatalysis. Adv. Funct. Mater. 2023, 33, 2210837. [Google Scholar] [CrossRef]
- Sanjayan, C.G.; Gokavi, L.; Ravikumar, C.H.; Balarkishna, R.G. Antibody-modified 2D mxene nanosheet probes for selective, picolevel detection of cancer biomarkers. Biosens. Bioelectron. 2025, 271, 117028. [Google Scholar] [CrossRef]
- Chen, Z.; Asif, M.; Wang, R.; Li, Y.; Zeng, X.; Yao, W.; Sun, Y.; Liao, K. Recent trends in synthesis and applications of porous MXene assemblies: A topical review. Chem. Rec. 2022, 22, e202100261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, J.; Lv, Q.; Wang, L.; Dong, X.; Asif, M.; Ren, J.; He, W.; Sun, Y.; Xiao, F.; et al. In situ electrochemical sensing and real-time monitoring live cells based on freestanding nanohybrid paper electrode assembled from 3D functionalized graphene framework. ACS Appl. Mater. Interfaces 2017, 9, 38201–38210. [Google Scholar] [CrossRef]
- Ding, G.; Yang, B.; Chen, K.; Wang, H.; Chen, J.; Mei, Q. Enhanced self–assembly and spontaneous separation for ultrathin, airfloating graphene macrofilms and their application in ultrasensitive in-site growth sensors. Adv. Mater. 2024, 36, e2408550. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qi, Y.; Cai, G.; Zhao, Y.; Ming, X.; Lin, Z.; Ma, W.; Lin, J.; Li, H.; et al. Bidirectionally promoting assembly order for ultrastiff and highly thermally conductive graphene fibres. Nat. Commun. 2024, 15, 409. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, C.; Yang, L.; Wu, J.; Li, J. Preparation of graphene/polydimethylsiloxane flexible resistive pressure sensors based on direct ink writing 3D printing. Sens. Actuators A-Phys. 2025, 382, 116148. [Google Scholar] [CrossRef]
- Kumar, A.; Ubaidullah, M.; Pham, P.V.; Gupta, R.K. Three-dimensional macro-structures of graphene-based materials for emerging heterogeneous electrocatalysis: Concepts, syntheses, principles, applications, and perspectives. Chem. Eng. J. 2024, 499, 156664. [Google Scholar] [CrossRef]
- Lee, J.; Han, H.; Noh, D.; Lee, J.; Lim, D.D.; Park, J.; Gu, G.X.; Choi, W. Multiscale porous architecture consisting of graphene aerogels and metastructures enabling robust thermal and mechanical functionalities of phase change materials. Adv. Funct. Mater. 2024, 34, 2405625. [Google Scholar] [CrossRef]
- Fan, K.; Zhou, S.; Xie, L.; Jia, S.; Zhao, L.; Liu, X.; Liang, K.; Jiang, L.; Kong, B. Interfacial assembly of 2D graphene-derived ion channels for water-based green energy conversion. Adv. Mater. 2024, 36, 2307849. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.L.; Sharma, R.; Radhakrishnan, J.; Abbaszadeh, S.; Shahbazi, M.; Tafreshi, O.A.; Karamikamkar, S.; Maleki, H. Emerging 2D nanomaterials-integrated hydrogels: Advancements in designing theragenerative materials for bone regeneration and disease therapy. Adv. Sci. 2024, 11, e2403204. [Google Scholar] [CrossRef]
- Zhao, A.; She, J.; Xiao, C.; Xi, J.; Xu, Y.; Manoj, D.; Sun, Y.; Xiao, F. Green and controllable synthesis of multi-heteroatoms co-doped graphene fiber as flexible and biocompatible microelectrode for in situ electrochemical detection of biological samples. Sens. Actuators B-Chem. 2021, 335, 129683. [Google Scholar] [CrossRef]
- Zeng, W.; Devaraj, M.; Sun, H.; Yi, R.; Huang, X.; Sun, Y. One-pot synthesis of high-density Pd nanoflowers decorated 3D carbon nanotube-graphene network modified on printed electrode as portable electrochemical sensing platform for sensitive detection of nitroaromatic explosives. J. Electroanal. Chem. 2019, 833, 527–535. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, G.; Du, Y.; Shi, P.; Li, N.; Li, Y.; Qin, Z.; Jiao, T.; He, X. Highly stretchable, low-hysteresis, and adhesive TA@MXene-composited organohydrogels for durable wearable sensors. Adv. Funct. Mater. 2024, 34, 2315813. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zheng, W.; Yao, J.; Zhao, Y. RuCu cage/alloy nanoparticles with controllable electroactivity for specific electroanalysis applications. Anal. Chem. 2021, 93, 13080–13088. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Liang, J.; Li, S.; Shi, H.; Liu, J.; Wang, T.; Han, J.; Li, Q. Protrusion-rich Cu@NiRu core@shell nanotubes for efficient alkaline hydrogen evolution electrocatalysis. Small 2022, 18, 2202496. [Google Scholar] [CrossRef]
- Li, L.; Qiu, H.; Zhu, Y.; Chen, G.; She, S.; Guo, X.; Li, H.; Liu, T.; Lin, Z.; Zhou, H.; et al. Atomic ruthenium modification of nickel-cobalt alloy for enhanced alkaline hydrogen evolution. Appl. Catal. B 2023, 331, 122710. [Google Scholar] [CrossRef]
- Cui, Z.; Ren, Z.; Ma, C.; Chen, B.; Chen, G.; Lu, R.; Zhu, W.; Gan, T.; Wang, Z.; Zhuang, Z. Dilute RuCo alloy synergizing single Ru and Co atoms as efficient and co-resistant anode catalyst for anion exchange membrane fuel cells. Angew. Chem. Int. Ed. 2024, 63, e202404761. [Google Scholar] [CrossRef]
- Kim, C.; Song, J.Y.; Choi, C.; Ha, J.P.; Lee, W.; Nam, Y.T.; Lee, D.M.; Kim, G.; Gereige, I.; Jung, W.B.; et al. Atomic-scale homogeneous RuCu alloy nanoparticles for highly efficient electrocatalytic nitrogen reduction. Adv. Mater. 2022, 34, e2205270. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Le Boeuf, W.; Maheshwari, V. Au–Pt–Ni nanochains as dopamine catalysts: Role of elements and their spatial distribution. Nanoscale Adv. 2023, 5, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Jiang, Y.; Wang, G.; Wu, W.; Chen, W.; Yu, P.; Lin, Y.; Mao, J.; Mao, L. Single-atom Ni-N4 provides a robust cellular NO sensor. Nat. Commun. 2020, 11, 3188. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au nanoparticles-3D graphene hydrogel nanocomposite to boost synergistically in situ detection sensitivity toward cell-released nitric oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef]
- Paul, V. The effect of N-nitro-L-arginine methyl ester posttreatment on the anticonvulsant effect of phenobarbitone and diazepam on picrotoxin-induced convulsions in rats. Pharmacol. Biochem. Behav. 2003, 74, 789–794. [Google Scholar] [CrossRef]
- Liu, Z.; Nemec-Bakk, A.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide release from cardiac and cancer cells via a hierarchical nanoporous gold microelectrode. Anal. Chem. 2017, 89, 8036–8043. [Google Scholar] [CrossRef]
- Tang, L.; Sun, X.; Gao, X.; Wang, L.; Yang, P.; Ling, P. Ionic liquid functionalized metal-organic framework nanowires for sensitive and real-time electrochemical monitoring of nitric oxide released from living cells. Anal. Methods 2023, 15, 729–737. [Google Scholar] [CrossRef]
- Arul, P.; Huang, S.T.; Nandhini, C.; Huang, C.H.; Gowthaman, N.S.K.; Huang, C.H. Development of a nanozyme-based electrochemical catalyst for real-time biomarker sensing of superoxide and nitric oxide anions released from living cells and exogenous donors. Biosens. Bioelectron. 2024, 261, 116485. [Google Scholar] [CrossRef]
- Zhu, P.; Li, S.; Zhou, S.; Ren, N.; Ge, S.; Zhang, Y.; Wang, Y.; Yu, J. In situ grown COFs on 3D strutted graphene aerogel for electrochemical detection of NO released from living cells. Chem. Eng. J. 2020, 420, 127559. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yao, K.; Hu, M.; Li, S.; Yang, S.; Zhao, A. On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials 2025, 15, 417. https://doi.org/10.3390/nano15060417
Liu H, Yao K, Hu M, Li S, Yang S, Zhao A. On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials. 2025; 15(6):417. https://doi.org/10.3390/nano15060417
Chicago/Turabian StyleLiu, Haibo, Kaiyuan Yao, Min Hu, Shanting Li, Shengxiong Yang, and Anshun Zhao. 2025. "On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells" Nanomaterials 15, no. 6: 417. https://doi.org/10.3390/nano15060417
APA StyleLiu, H., Yao, K., Hu, M., Li, S., Yang, S., & Zhao, A. (2025). On-Chip Electrochemical Sensor Based on 3D Graphene Assembly Decorated Ultrafine RuCu Alloy Nanocatalyst for In Situ Detection of NO in Living Cells. Nanomaterials, 15(6), 417. https://doi.org/10.3390/nano15060417






