A Facile Two-Step High-Throughput Screening Strategy of Advanced MOFs for Separating Argon from Air
Abstract
1. Introduction
2. Methods
2.1. MOF Database and Descriptors
2.2. Grand Canonical Monte Carlo (GCMC) Simulation
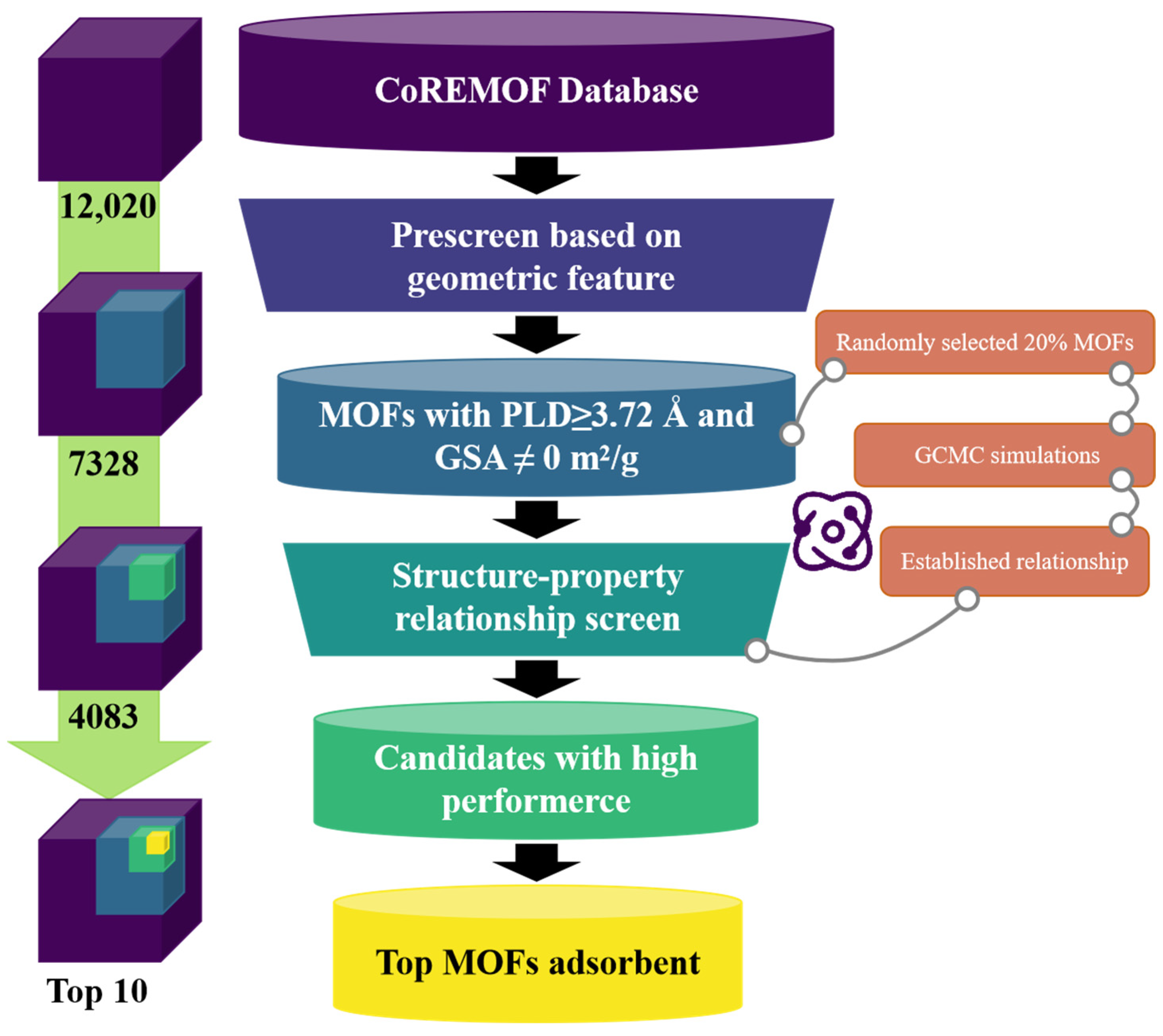
2.3. Two-Step Screening Strategy
2.3.1. Pre-Screening
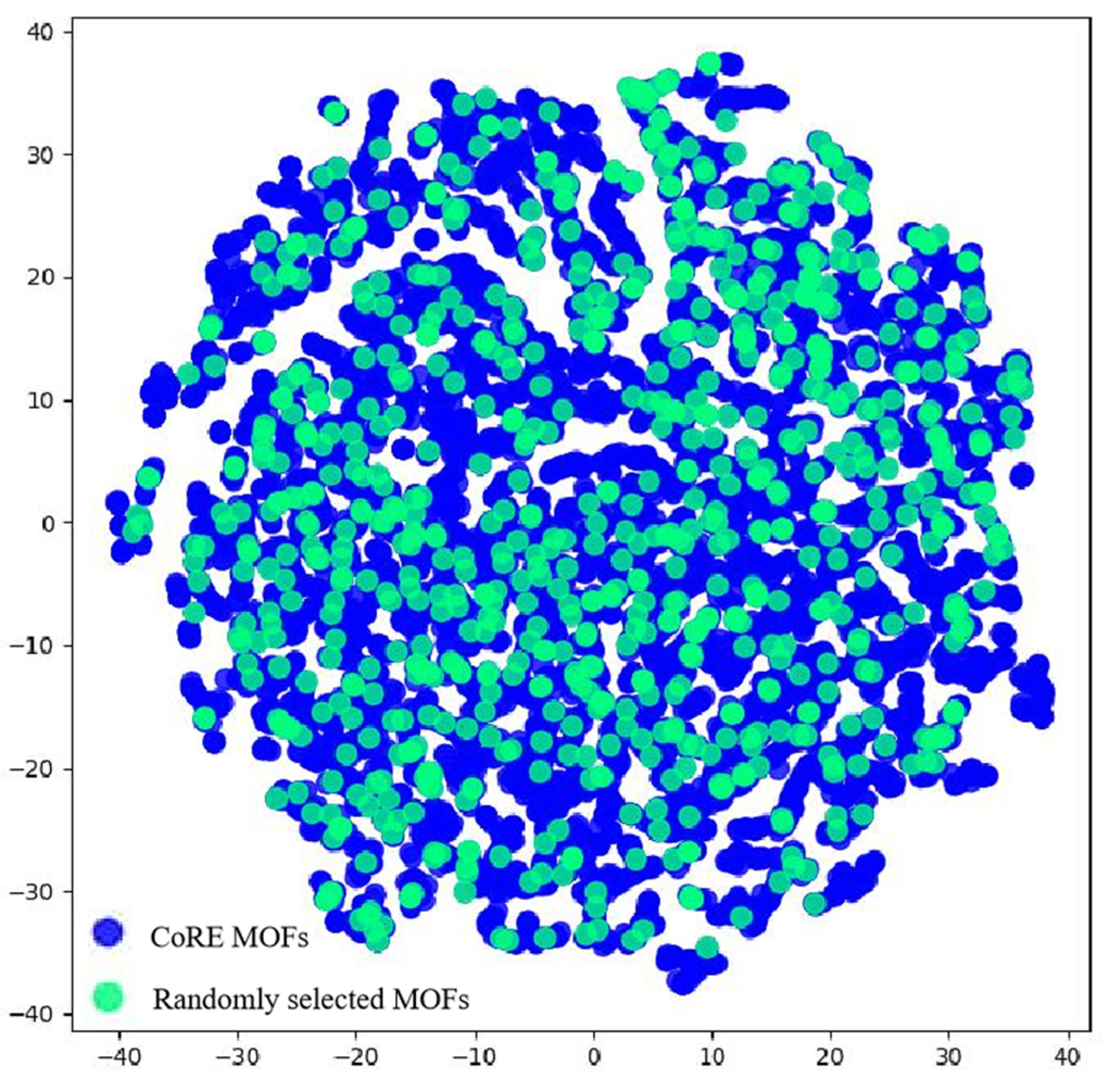
2.3.2. Screening Based on Structure–Property Relationship
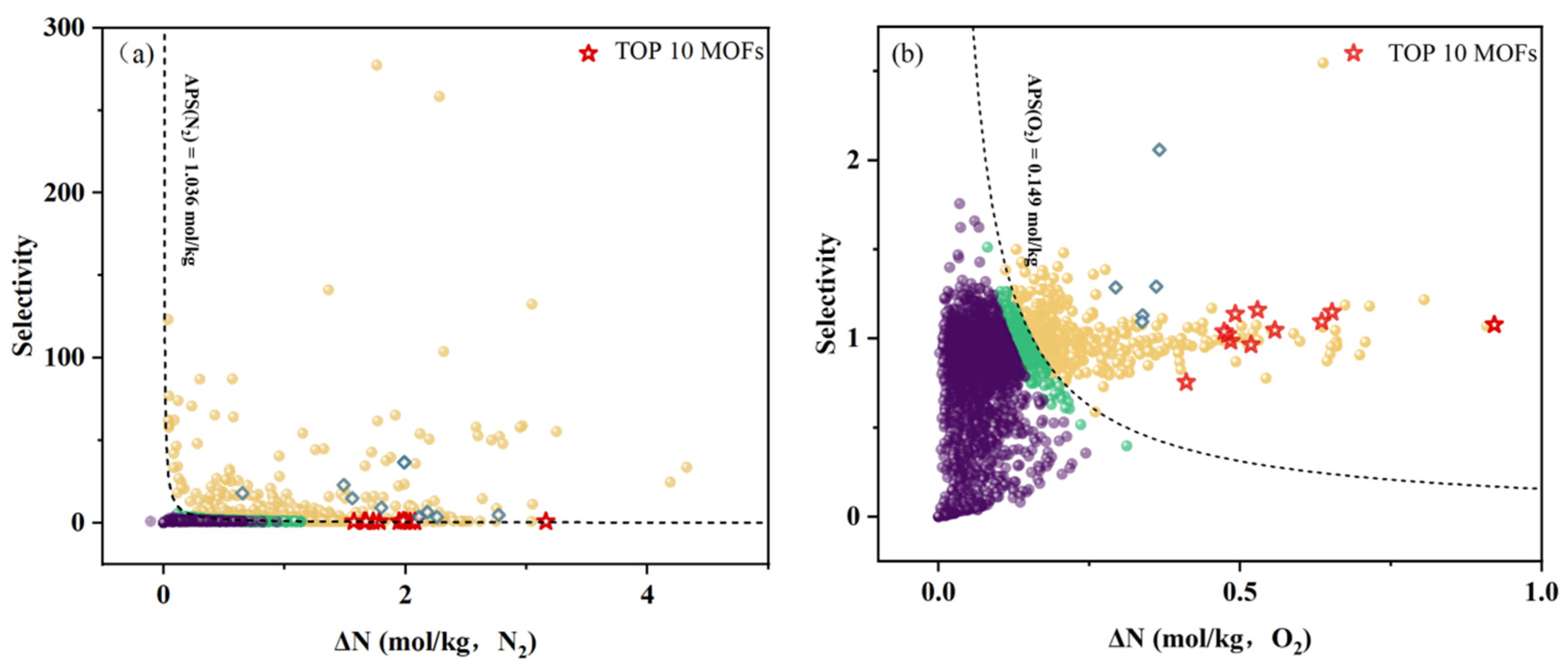
2.3.3. Top MOFs Selection
3. Results and Discussion
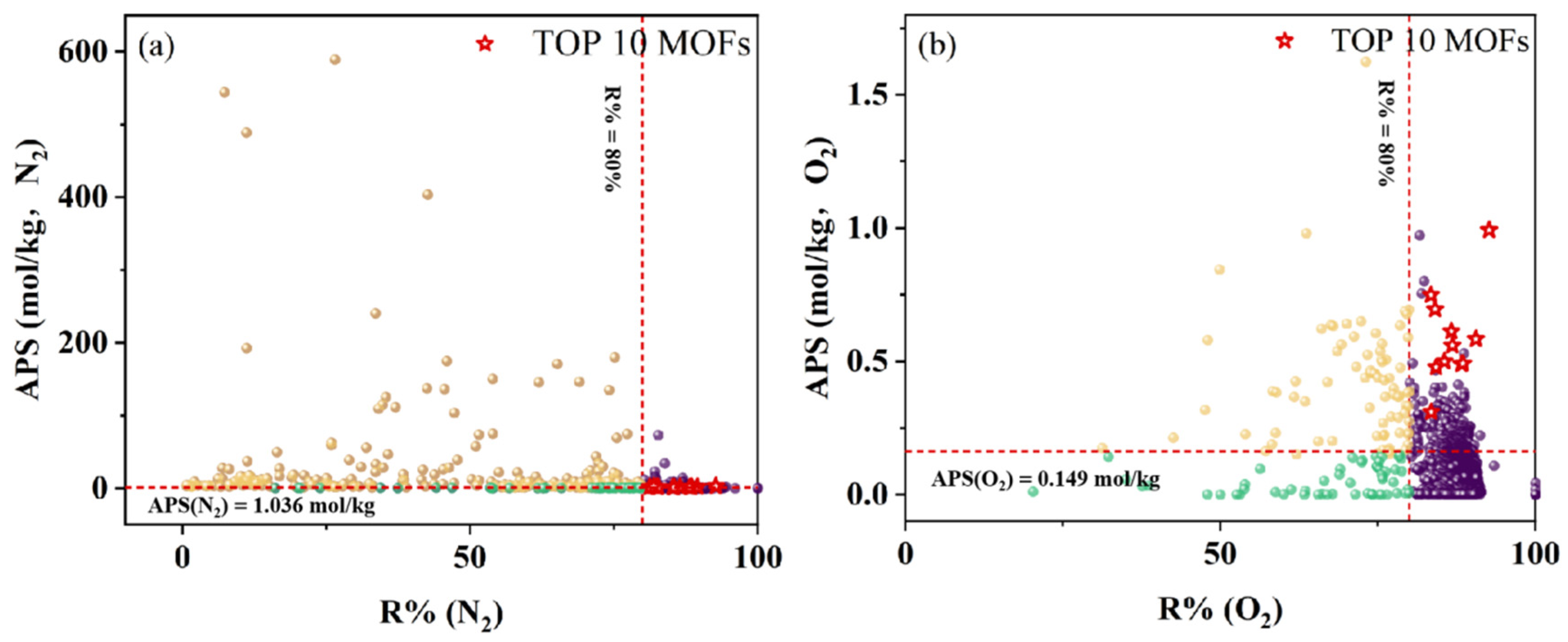
3.1. Separation Performances of MOFs
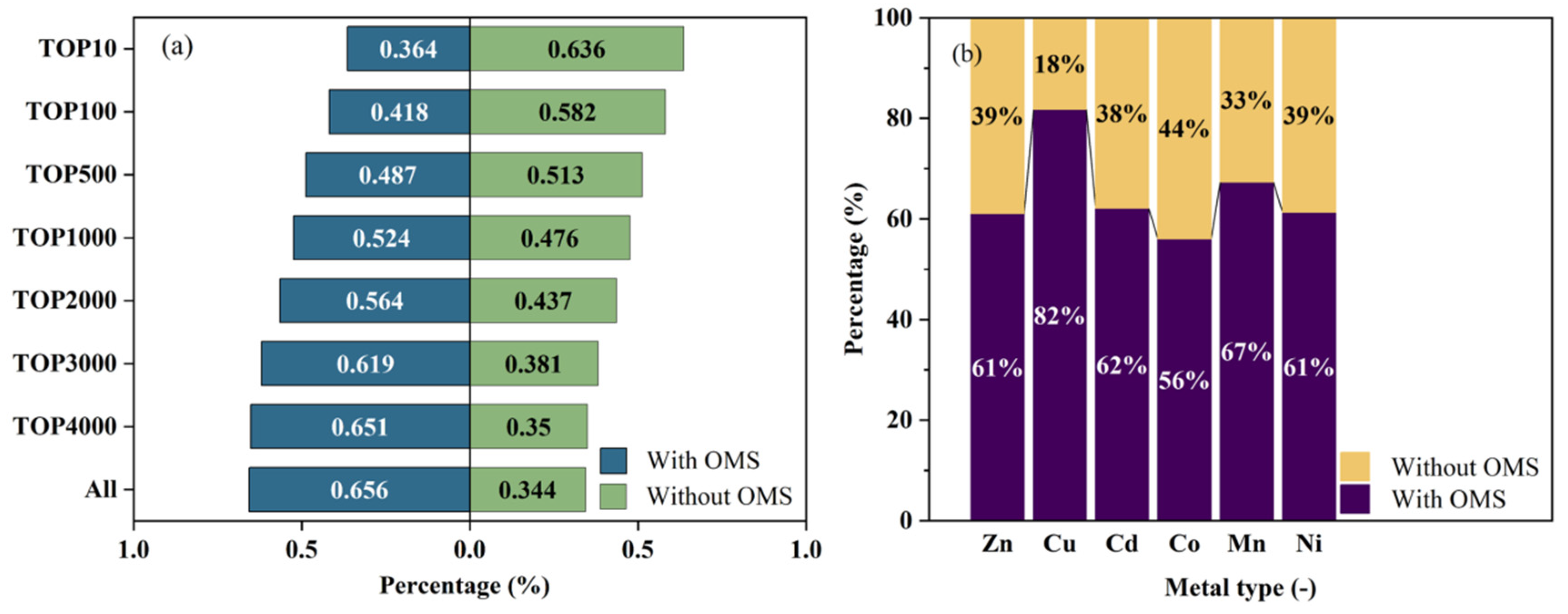
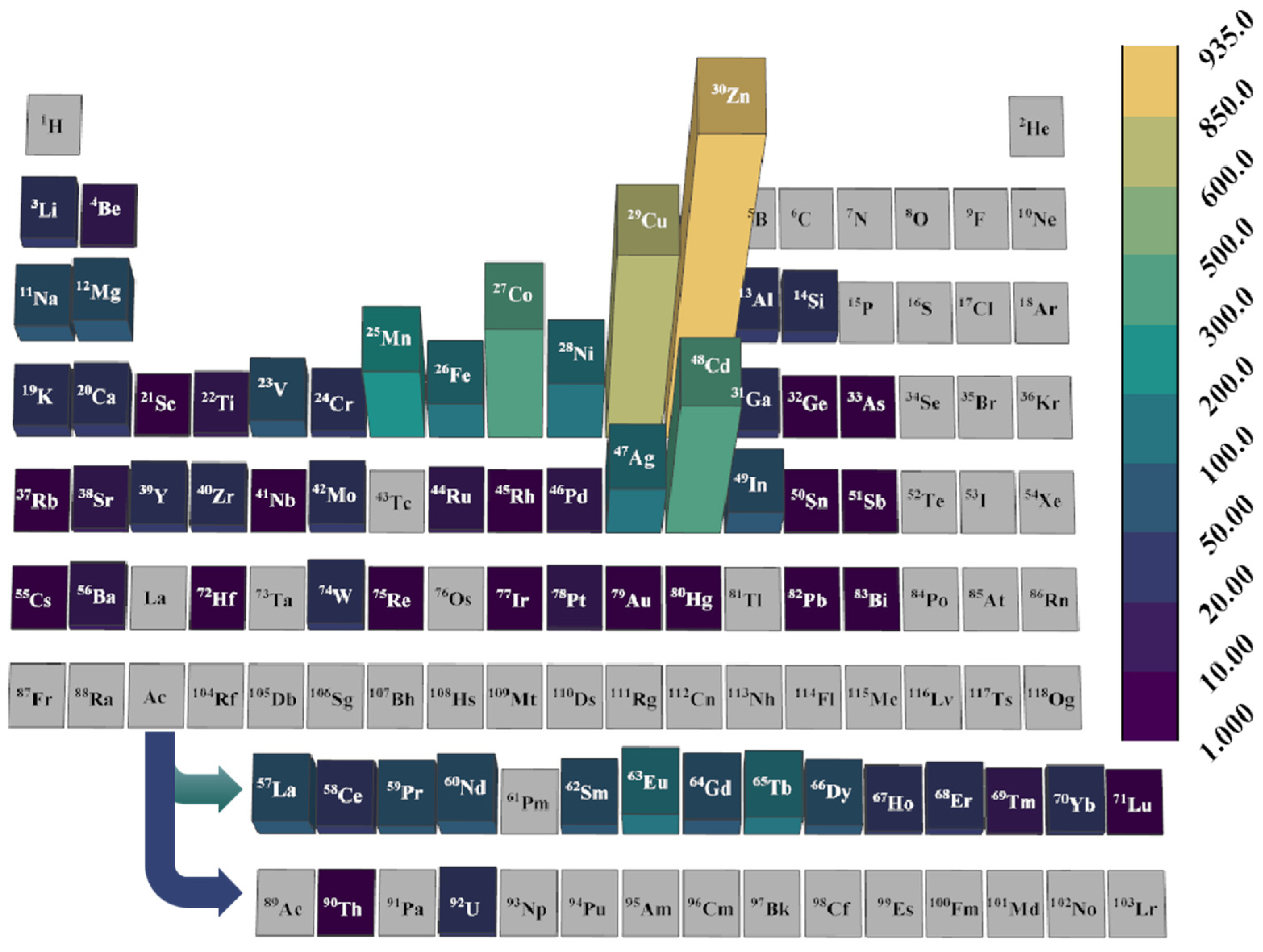
3.2. Metals and OMSs
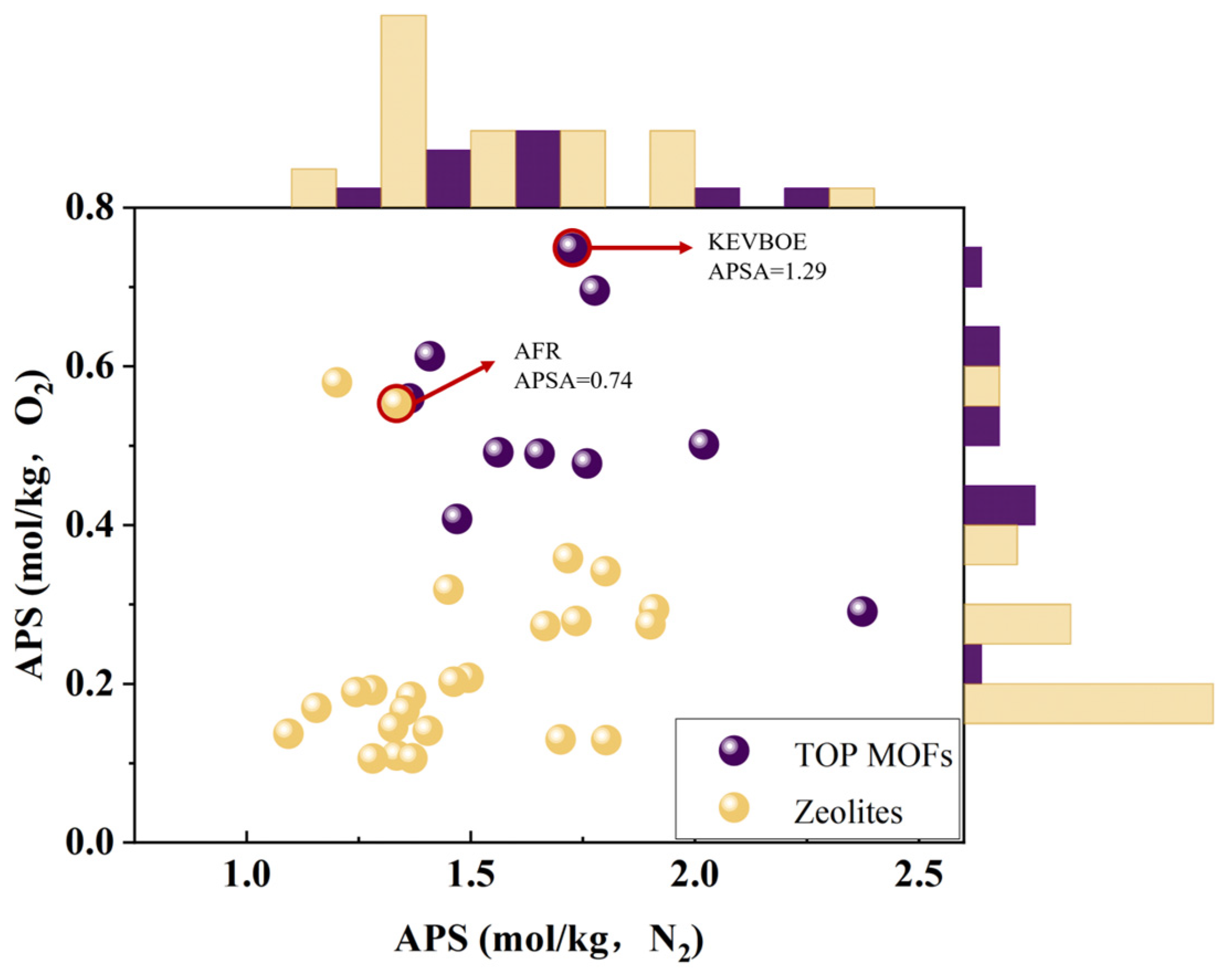
3.3. Comparison with Molecular Sieve Separation Data
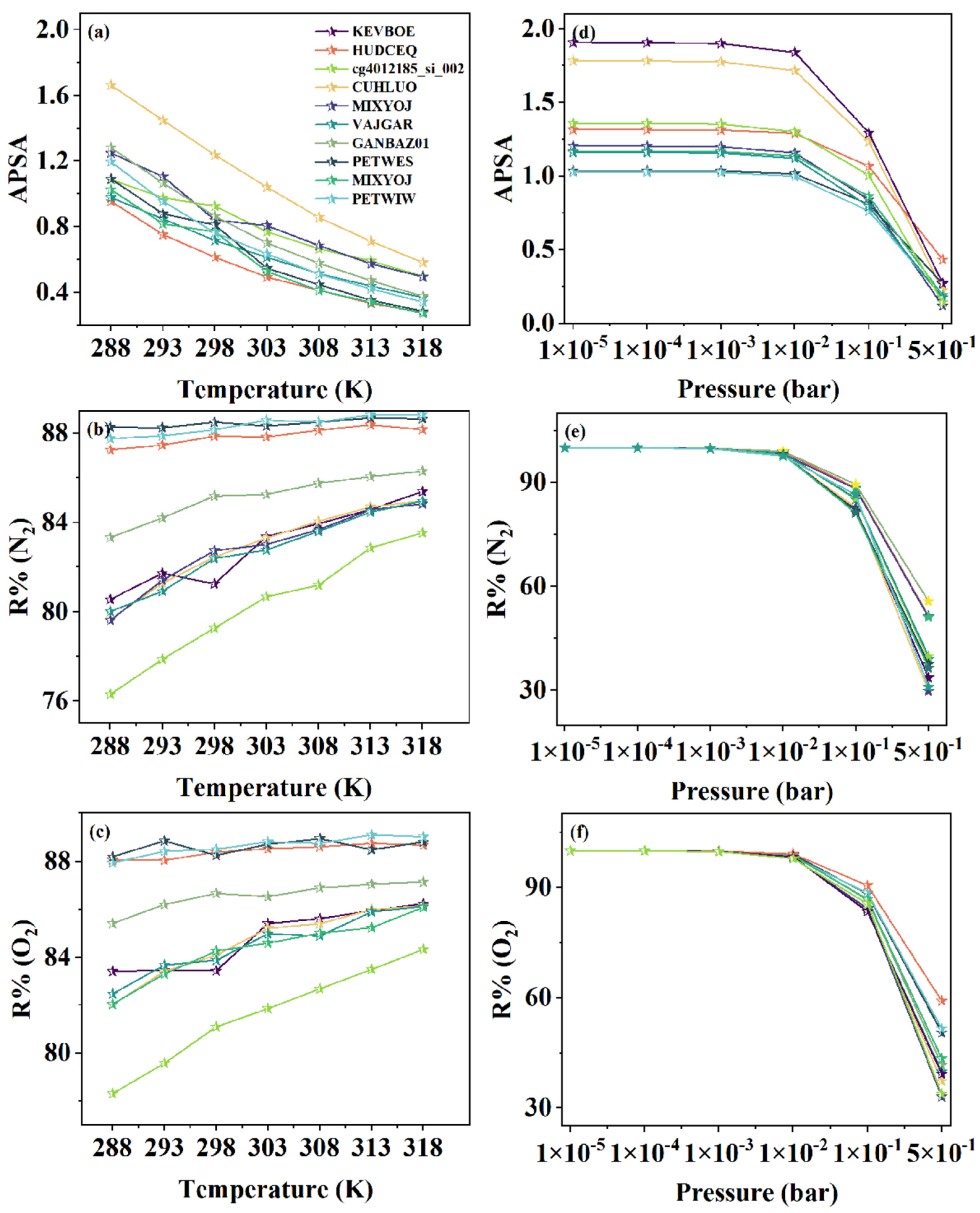
3.4. Analysis of Temperature, Pressure, and Compatibility
3.5. The Performance of the Adsorbent Under Real Gas Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APS | adsorbent performance score |
| APSA | adsorbent performance score for separating argon from air |
| CIF | Crystallographic Information File |
| CoRE | computationally ready experimental |
| GCMC | Grand Canonical Monte Carlo |
| GSA | geometric surface area |
| HTCS | high-throughput computational screening |
| LCD | largest cavity diameter |
| MOF | metal–organic framework |
| OMS | existence of open metal site |
| PLD | pore-limiting diameter |
| PSA | pressure swing adsorption |
| UFF | Universal Force Field |
| VSA | volume surface area |
References
- Sutour, C.; Stumpf, C.; Kosinski, J.-P.; Surget, A.; Hervouët, G.; Yardin, C.; Madec, T.; Gosset, A. Determination of the Argon Concentration in Ambient Dry Air for the Calculation of Air Density. Metrologia 2007, 44, 448. [Google Scholar] [CrossRef]
- Holder, G.D.; Corbin, G.; Papadopoulos, K.D. Thermodynamic and Molecular Properties of Gas Hydrates from Mixtures Containing Methane, Argon, and Krypton. Ind. Eng. Chem. Fund. 1980, 19, 282–286. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, M.; Lv, Q.; Hou, H.; Yang, J.; Liu, G.; Liu, J.; Qiao, G. Effects of the High-Temperature Sensitization in Argon Atmosphere on the Microstructure and Properties of Polycrystalline PbSe Films. Mater. Sci. Semicond. Process. 2023, 162, 107486. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Hamdaoui, O. Methanol Sono-Pyrolysis for Hydrogen Recovery: Effect of Methanol Concentration under an Argon Atmosphere. Chem. Eng. J. 2022, 433, 133272. [Google Scholar] [CrossRef]
- Maitre, P.-A.; Long, J.; Bieniek, M.S.; Bannerman, M.N.; Kechagiopoulos, P.N. Investigating the Effects of Helium, Argon and Hydrogen Co-Feeding on the Non-Oxidative Coupling of Methane in a Dielectric Barrier Discharge Reactor. Chem. Eng. Sci. 2022, 259, 117731. [Google Scholar] [CrossRef]
- Phakpeetinan, P.; Chianpairot, A.; Viyanit, E.; Hartung, F.; Lothongkum, G. Effects of Nitrogen and Hydrogen in Argon Shielding Gas on Bead Profile, Delta-Ferrite and Nitrogen Contents of the Pulsed GTAW Welds of AISI 316L Stainless Steel. Mater. Test. 2016, 58, 489–494. [Google Scholar] [CrossRef]
- Nikolaev, I.V.; Geydt, P.V.; Korobeishchikov, N.G.; Kapishnikov, A.V.; Volodin, V.A.; Azarov, I.A.; Strunin, V.I.; Gerasimov, E.Y. The Influence of Argon Cluster Ion Bombardment on the Characteristics of AlN Films on Glass-Ceramics and Si Substrates. Nanomaterials 2022, 12, 670. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Jang, H.-J.; Jung, E.; Bae, G.; Lee, S.; Park, C.-S.; Shin, B.; Tae, H.-S. Improvement of the Uniformity and Electrical Properties of Polyaniline Nanocomposite Film by Addition of Auxiliary Gases during Atmospheric Pressure Plasma Polymerization. Nanomaterials 2021, 11, 2315. [Google Scholar] [CrossRef]
- Soller, S.; Kirchberger, C.; Kuhn, M.; Langener, T.; Bouchez, M.; Steelant, J. Experimental Investigation of Cooling Techniques and Materials for Highspeed Flight Propulsion Systems. In Proceedings of the 16th AIAA/DLR/DGLR International Space Planes and Hypersonic Systems and Technologies Conference, Bremen, Germany, 19–22 October 2009; American Institute of Aeronautics and Astronautics: Sunrise Valley Drive, VI, USA, 2009. [Google Scholar]
- Heinrich, V.; Zunabovic, M.; Nehm, L.; Bergmair, J.; Kneifel, W. Influence of Argon Modified Atmosphere Packaging on the Growth Potential of Strains of Listeria monocytogenes and Escherichia coli. Food Control 2016, 59, 513–523. [Google Scholar] [CrossRef]
- Che, C.; Dong, F.; Wu, X.; Wang, W.; Jiang, L. Argon Gas Knife Combined with Cryotherapy for Amyloidosis Leading to Severe Airway Stenosis. Respir. Med. Case Rep. 2019, 28, 100948. [Google Scholar] [CrossRef]
- De-fu, L.I.; Yong-hong, X.I.A.; Ying, M.A. Effect of Argon Knife in Hepatic Resection for Hepatocellular Carcinoma on Postoperative Recurrence and Survival Rate. Chin. Hepatolgy 2020, 25, 819. [Google Scholar]
- Korkmaz, Ş.; Ekici, F.; Sül, S. Argon Laser-Assisted Treatment of Benign Eyelid Lesions. Lasers Med. Sci. 2015, 30, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Barrlett, W.J. Chemistry in Incandescent Lamp Manufacture. Ind. Eng. Chem. 1929, 21, 970–973. [Google Scholar] [CrossRef]
- Agrawal, R.; Woodward, D.W.; Yee, T.F. Argon Production from Air Distillation: Use of a Heat Pump in a Ternary Distillation with a Side Rectifier. Gas Sep. Purif. 1994, 8, 37–43. [Google Scholar] [CrossRef]
- Kingston, D.; Wilhelmsen, Ø.; Kjelstrup, S. Minimum Entropy Production in a Distillation Column for Air Separation Described by a Continuous Non-Equilibrium Model. Chem. Eng. Sci. 2020, 218, 115539. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Zhou, Z. Optimization of Cryogenic Air Separation Distillation Columns. In Proceedings of the 2006 6th World Congress on Intelligent Control and Automation, Dalian, China, 21–23 June 2006; Volume 2, pp. 7702–7705. [Google Scholar]
- Meng, Y.; Wang, S.; Zhang, Y.; Chen, S.; Hou, Y.; Chen, L. Experimental Evaluation of the Performance of a Cryogenic Distillation System under Offshore Conditions. Chem. Eng. Sci. 2022, 263, 118084. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Potential for Improving the Energy Efficiency of Cryogenic Air Separation Unit (ASU) Using Binary Heat Recovery Cycles. Appl. Therm. Eng. 2015, 81, 223–231. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Rodrigues, A.E. Activated Carbon for Hydrogen Purification by Pressure Swing Adsorption: Multicomponent Breakthrough Curves and PSA Performance. Chem. Eng. Sci. 2011, 66, 303–317. [Google Scholar] [CrossRef]
- Jin, X.; Malek, A.; Farooq, S. Production of Argon from an Oxygen−Argon Mixture by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2006, 45, 5775–5787. [Google Scholar] [CrossRef]
- Wang, Y. A Study of Kinetic Air Separation at Low Temperatures: Oxygen, Nitrogen, and Argon in Carbon Molecular Sieve. Sep. Purif. Technol. 2023, 312, 123372. [Google Scholar] [CrossRef]
- Hayashi, S.; Tsuchiya, H.; Haruna, K. Process for Obtaining High Concentration Argon by Pressure-Swing-Adsorption. U.S. Patent No. 4,529,412, 16 July 1985. [Google Scholar]
- Hayashi, S.; Kawai, M.; Kaneko, T. Dynamics of High Purity Oxygen PSA. Gas Sep. Purif. 1996, 10, 19–23. [Google Scholar] [CrossRef]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Osazuwa, O.U.; Elimian, E.A.; Eshiemogie, S.O.; Oyefolu, P.K.; Kusuma, H.S. A Comprehensive Review of Recent Advances in the Synthesis and Application of Metal-Organic Frameworks (MOFs) for the Adsorptive Sequestration of Pollutants from Wastewater. Sep. Purif. Technol. 2023, 311, 123246. [Google Scholar] [CrossRef]
- Meng, X.; Dai, Z.; Jia, C.Q.; Yang, L.; Jiang, W.; Yao, L.; Zhou, Q.; Xu, B. Hierarchical Porous MOF-199 and Zeolite Composites with High Adsorption Performance for Both Toluene and Acetone. Ind. Eng. Chem. Res. 2023, 62, 19702–19714. [Google Scholar] [CrossRef]
- Preißler-Kurzhöfer, H.; Lange, M.; Möllmer, J.; Erhart, O.; Kobalz, M.; Krautscheid, H.; Gläser, R. Hydrocarbon Sorption in Flexible MOFs—Part III: Modulation of Gas Separation Mechanisms. Nanomaterials 2024, 14, 241. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Zhou, T.; Sundmacher, K. Computational Screening of Metal-Organic Frameworks for Ethylene Purification from Ethane/Ethylene/Acetylene Mixture. Nanomaterials 2022, 12, 869. [Google Scholar] [CrossRef]
- Chung, Y.G.; Camp, J.; Haranczyk, M.; Sikora, B.J.; Bury, W.; Krungleviciute, V.; Yildirim, T.; Farha, O.K.; Sholl, D.S.; Snurr, R.Q. Computation-Ready, Experimental Metal-Organic Frameworks: A Tool to Enable High-Throughput Screening of Nanoporous Crystals. Chem. Mater. 2014, 26, 6185–6192. [Google Scholar] [CrossRef]
- Altintas, C.; Erucar, I.; Keskin, S. High-Throughput Computational Screening of the Metal Organic Framework Database for CH4/H2 Separations. ACS Appl. Mater. Interfaces 2018, 10, 3668–3679. [Google Scholar] [CrossRef] [PubMed]
- Gulbalkan, H.C.; Haslak, Z.P.; Altintas, C.; Uzun, A.; Keskin, S. Assessing CH4/N2 Separation Potential of MOFs, COFs, IL/MOF, MOF/Polymer, and COF/Polymer Composites. Chem. Eng. J. 2022, 428, 131239. [Google Scholar] [CrossRef]
- Qiao, Z.; Xu, Q.; Jiang, J. Computational Screening of Hydrophobic Metal–Organic Frameworks for the Separation of H2S and CO2 from Natural Gas. J. Mater. Chem. A 2018, 6, 18898–18905. [Google Scholar] [CrossRef]
- Ansón, A.; Kuznicki, S.M.; Kuznicki, T.; Dunn, B.C.; Eyring, E.M.; Hunter, D.B. Separation of Argon and Oxygen by Adsorption on a Titanosilicate Molecular Sieve. Sep. Sci. Technol. 2009, 44, 1604–1620. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. Kinetic Separation of Oxygen and Argon Using Molecular Sieve Carbon. Adsorption 2000, 6, 15–22. [Google Scholar] [CrossRef]
- Shi, M.; Kim, J.; Sawada, J.; Lam, J.; Sarabadan, S.; Kuznicki, T.; Kuznicki, S. Production of Argon Free Oxygen by Adsorptive Air Separation on Ag-ETS-10. AIChE J. 2013, 59, 982–987. [Google Scholar] [CrossRef]
- Willems, T.F.; Rycroft, C.H.; Kazi, M.; Meza, J.C.; Haranczyk, M. Algorithms and Tools for High-Throughput Geometry-Based Analysis of Crystalline Porous Materials. Microporous Mesoporous Mater. 2012, 149, 134–141. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, M.; Luo, L.; Ji, X.; Liu, C.; Bi, K.; Zhou, L. High-Throughput Screening of Metal–Organic Frameworks for Hydrogen Purification. Chem. Eng. J. 2023, 451, 138436. [Google Scholar] [CrossRef]
- Azar, A.N.V.; Velioglu, S.; Keskin, S. Large-Scale Computational Screening of Metal Organic Framework (MOF) Membranes and MOF-Based Polymer Membranes for H2/N2 Separations. ACS Sustain. Chem. Eng. 2019, 7, 9525–9536. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, P.Z.; Islamoglu, T.; Goswami, S.; Exley, J.; Fantham, M.; Kaminski, C.F.; Snurr, R.Q.; Farha, O.K.; Fairen-Jimenez, D. Computer-Aided Discovery of a Metal–Organic Framework with Superior Oxygen Uptake. Nat. Commun. 2018, 9, 1378. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular Simulation Software for Adsorption and Diffusion in Flexible Nanoporous Materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Mehio, N.; Dai, S.; Jiang, D. Quantum Mechanical Basis for Kinetic Diameters of Small Gaseous Molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.R.; O’koy, I.P.; Thomas, K.M. Adsorption of Gases on Carbon Molecular Sieves Used for Air Separation. Spherical Adsorptives as Probes for Kinetic Selectivity. Langmuir 1998, 14, 2415–2425. [Google Scholar] [CrossRef]
- GitHub—Emmhald/Open_Metal_Detector. Available online: https://github.com/emmhald/open_metal_detector (accessed on 22 February 2025).
- Manos, G.; Dunne, L.J. Predicting the Features of Methane Adsorption in Large Pore Metal-Organic Frameworks for Energy Storage. Nanomaterials 2018, 8, 818. [Google Scholar] [CrossRef]
- Pillai, R.S.; Peter, S.A.; Jasra, R.V. Adsorption of Carbon Dioxide, Methane, Nitrogen, Oxygen and Argon in NaETS-4. Microporous Mesoporous Mater. 2008, 113, 268–276. [Google Scholar] [CrossRef]
- Daglar, H.; Keskin, S. Recent Advances, Opportunities, and Challenges in High-Throughput Computational Screening of MOFs for Gas Separations. Coord. Chem. Rev. 2020, 422, 213470. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.I.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Orhan, I.B.; Daglar, H.; Keskin, S.; Le, T.C.; Babarao, R. Prediction of O2/N2 Selectivity in Metal–Organic Frameworks via High-Throughput Computational Screening and Machine Learning. ACS Appl. Mater. Interfaces 2022, 14, 736–749. [Google Scholar] [CrossRef]
- Míguez, J.M.; González-Salgado, D.; Legido, J.L.; Piñeiro, M.M. Calculation of Interfacial Properties Using Molecular Simulation with the Reaction Field Method: Results for Different Water Models. J. Chem. Phys. 2010, 132, 184102. [Google Scholar] [CrossRef]
- Sadeghi, M.; Esmaeilzadeh, F.; Mowla, D.; Zandifar, A. Improving CO2 Capture in UTSA-16(Zn) via Alkali and Alkaline Earth Metal Introduction: GCMC and MD Simulations Study. Sep. Purif. Technol. 2024, 338, 126534. [Google Scholar] [CrossRef]
- Jiang, J.; Sandler, S.I. Monte Carlo Simulation of O2 and N2 Mixture Adsorption in Nanoporous Carbon (C168 Schwarzite). Langmuir 2003, 19, 5936–5941. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Parkes, M.V.; Staiger, C.L.; Iv, J.J.P.; Allendorf, M.D.; Greathouse, J.A. Screening Metal–Organic Frameworks for Selective Noble Gas Adsorption in Air: Effect of Pore Size and Framework Topology. Phys. Chem. Chem. Phys. 2013, 15, 9093–9106. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, Y.; Cong, S.; Liu, X.; Wang, Z. N2-Selective Adsorbents and Membranes for Natural Gas Purification. Sep. Purif. Technol. 2022, 300, 121808. [Google Scholar] [CrossRef]
- van der Maaten, L.; Hinton, G. Visualizing Data Using T-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Kulkarni, S.; Kaware, J.; Mumbai, N. Regeneration and Recovery in Adsorption—A Review. Int. J. Innov. Sci. Eng. Technol. 2014, 1, 61–64. [Google Scholar]
- González-Galán, C.; Madero-Castro, R.M.; Luna-Triguero, A.; Vicent-Luna, J.M.; Calero, S. Understanding the Role of Open Metal Sites in MOFs for the Efficient Separation of Benzene/Cyclohexane Mixtures. Sep. Purif. Technol. 2024, 348, 127606. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: https://www.iza-structure.org/databases/ (accessed on 25 December 2024).
- Abascal, J.L.F.; Vega, C. A General Purpose Model for the Condensed Phases of Water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, L.; He, Y.; Feng, Y. An Anionic Metal–Organic Framework Constructed from a Triazole-Functionalized Diisophthalate Featuring Hierarchical Cages for Selective Adsorptive C2H2/CH4 and CO2/CH4 Separation. CrystEngComm 2017, 19, 2795–2801. [Google Scholar] [CrossRef]
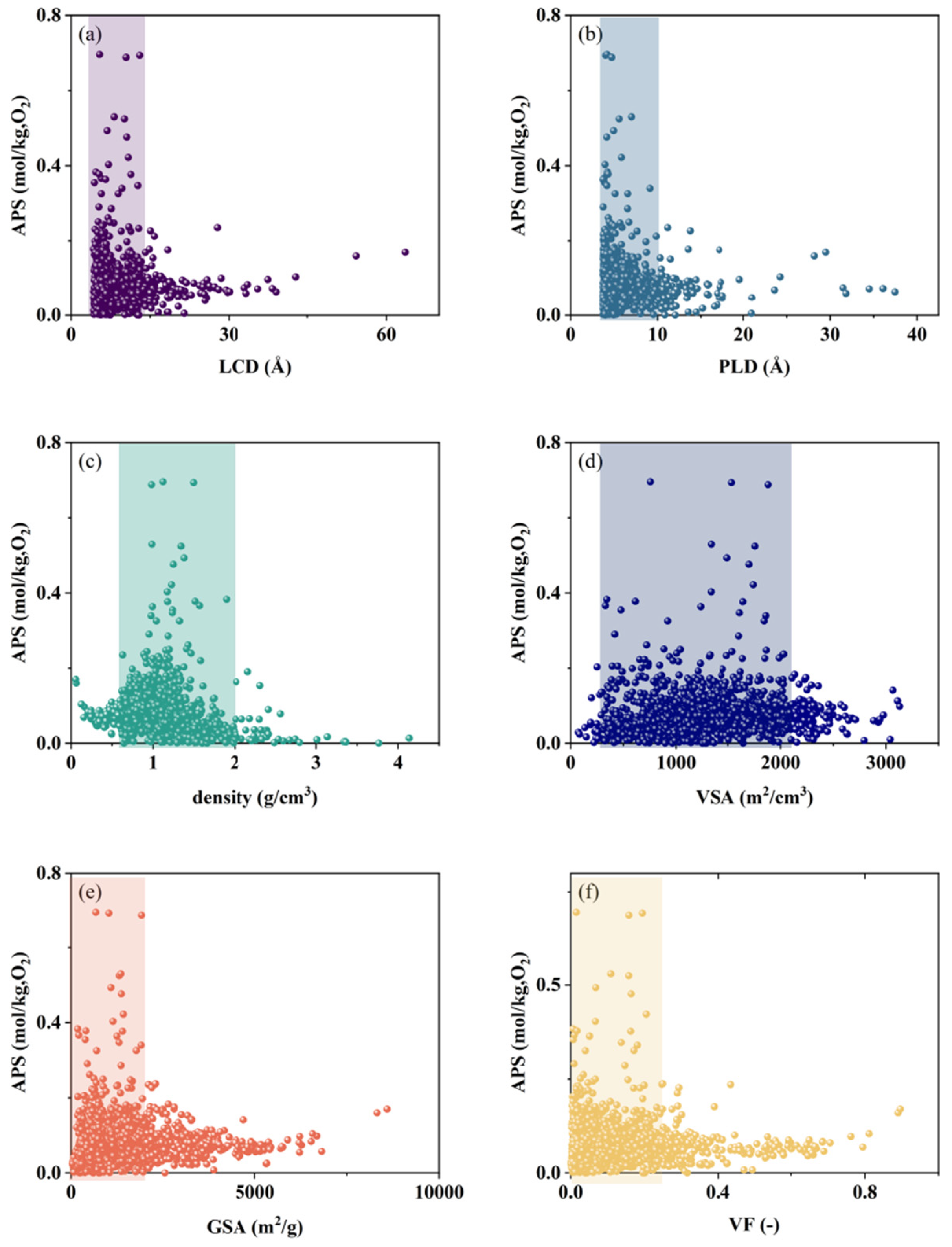
| Descriptor | Unit | Optimal N2 Adsorption Interval | Optimal O2 Adsorption Interval | Target Optimal Structural Interval |
|---|---|---|---|---|
| LCD | Å | 2.5~17 | 3~15 | 3~15 |
| PLD | Å | 3~12.5 | 3~10 | 3~10 |
| density | g/cm3 | 0.5~2 | 0.5~2 | 0.5~2 |
| VSA | m2/cm3 | 250~2250 | 250~2250 | 250~2250 |
| GSA | m2/g | 0~2800 | 0~2250 | 0~2250 |
| VF | — | 0~0.35 | 0~0.25 | 0~0.25 |
| MOF Name | APS (mol/kg, N2) | R% (N2) | APS (mol/kg, O2) | R% (O2) | APSA |
|---|---|---|---|---|---|
| KEVBOE | 1.723 | 81.25 | 0.75 | 83.43 | 1.29 |
| CUHLUO | 1.78 | 82.45 | 0.70 | 84.08 | 1.24 |
| HUDCEQ | 1.83 | 89.50 | 0.58 | 90.56 | 1.07 |
| cg4012185_si_002 | 2.01 | 86.49 | 0.50 | 85.56 | 1.01 |
| GANBAZ01 | 1.41 | 85.189 | 0.61 | 86.66 | 0.86 |
| MIXYOJ | 1.76 | 82.74 | 0.48 | 84.26 | 0.84 |
| PETWES | 1.65 | 88.50 | 0.49 | 88.25 | 0.81 |
| VAJGAR | 2.56 | 81.94 | 0.31 | 83.47 | 0.79 |
| PETWIW | 1.56 | 88.16 | 0.49 | 88.51 | 0.77 |
| GANBAZ | 1.36 | 85.45 | 0.56 | 86.83 | 0.76 |
| MOF Name | S (CO2) | S (H2O) | APSA | APSA’ | Has OMS |
|---|---|---|---|---|---|
| KEVBOE | 88.14 | 0.50 | 1.29 | 1.24 | NO |
| CUHLUO | 55.40 | 0.41 | 1.24 | 1.08 | NO |
| HUDCEQ | 34.25 | 0.50 | 1.07 | 0.67 | NO |
| cg4012185_si_002 | 74.93 | 0.49 | 1.01 | 0.79 | YES |
| GANBAZ01 | 87.33 | 0.44 | 0.86 | 0.81 | NO |
| MIXYOJ | 66.88 | 3.51 | 0.84 | 0.80 | NO |
| PETWES | 24.98 | 0.47 | 0.81 | 0.76 | YES |
| VAJGAR | 225.09 | 2.28 | 0.79 | 0.03 | YES |
| PETWIW | 21.85 | 0.49 | 0.77 | 0.73 | YES |
| GANBAZ | 76.17 | 0.54 | 0.76 | 0.76 | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Xin, B.; Dai, Z.; Liu, C.; Zhou, L.; Ji, X.; Dai, Y. A Facile Two-Step High-Throughput Screening Strategy of Advanced MOFs for Separating Argon from Air. Nanomaterials 2025, 15, 412. https://doi.org/10.3390/nano15060412
Xu X, Xin B, Dai Z, Liu C, Zhou L, Ji X, Dai Y. A Facile Two-Step High-Throughput Screening Strategy of Advanced MOFs for Separating Argon from Air. Nanomaterials. 2025; 15(6):412. https://doi.org/10.3390/nano15060412
Chicago/Turabian StyleXu, Xiaoyi, Bingru Xin, Zhongde Dai, Chong Liu, Li Zhou, Xu Ji, and Yiyang Dai. 2025. "A Facile Two-Step High-Throughput Screening Strategy of Advanced MOFs for Separating Argon from Air" Nanomaterials 15, no. 6: 412. https://doi.org/10.3390/nano15060412
APA StyleXu, X., Xin, B., Dai, Z., Liu, C., Zhou, L., Ji, X., & Dai, Y. (2025). A Facile Two-Step High-Throughput Screening Strategy of Advanced MOFs for Separating Argon from Air. Nanomaterials, 15(6), 412. https://doi.org/10.3390/nano15060412









