Room-Temperature Synthesis of Carbon Nanochains via the Wurtz Reaction
Abstract
1. Introduction
2. Experimental Section
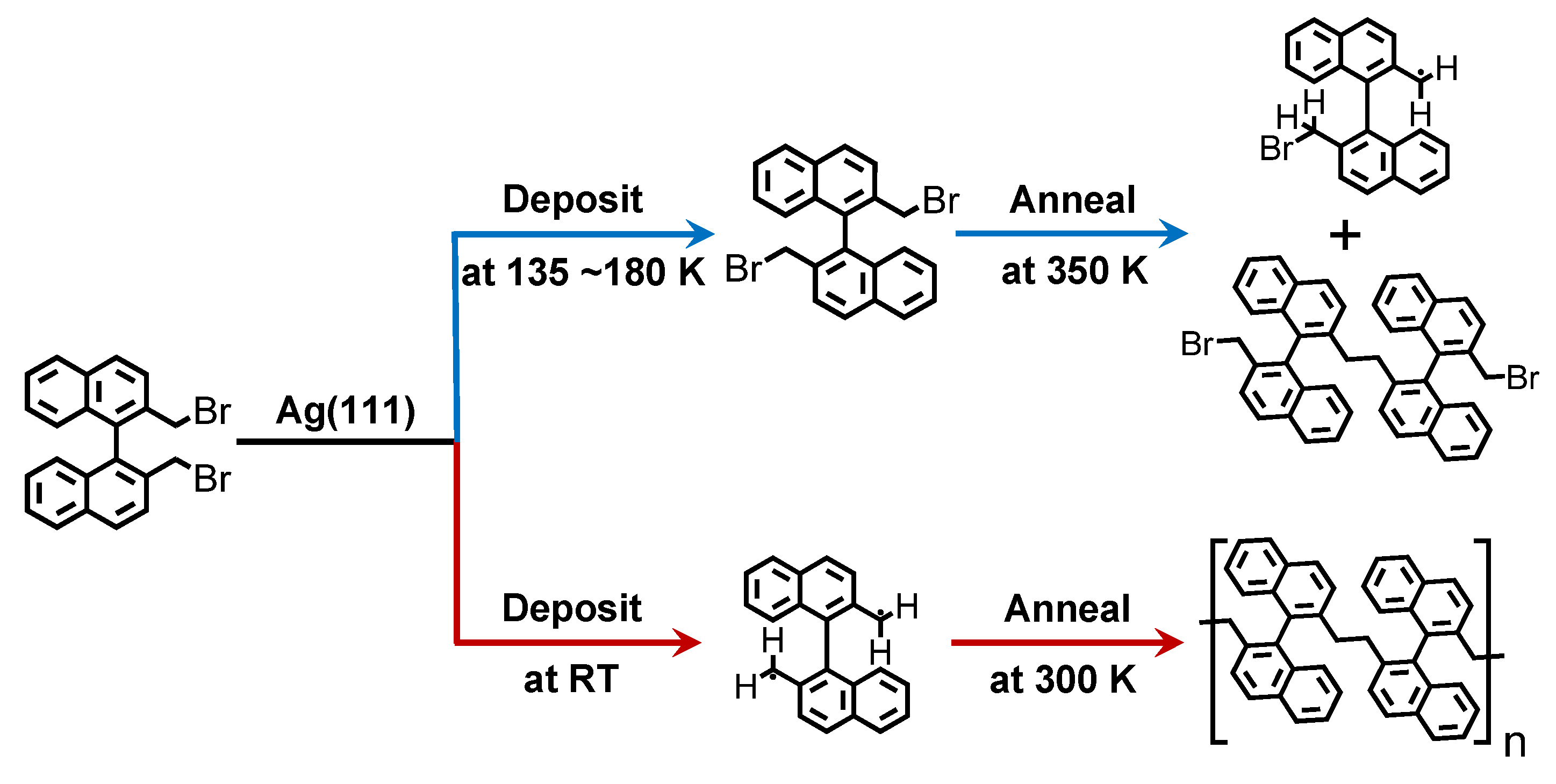
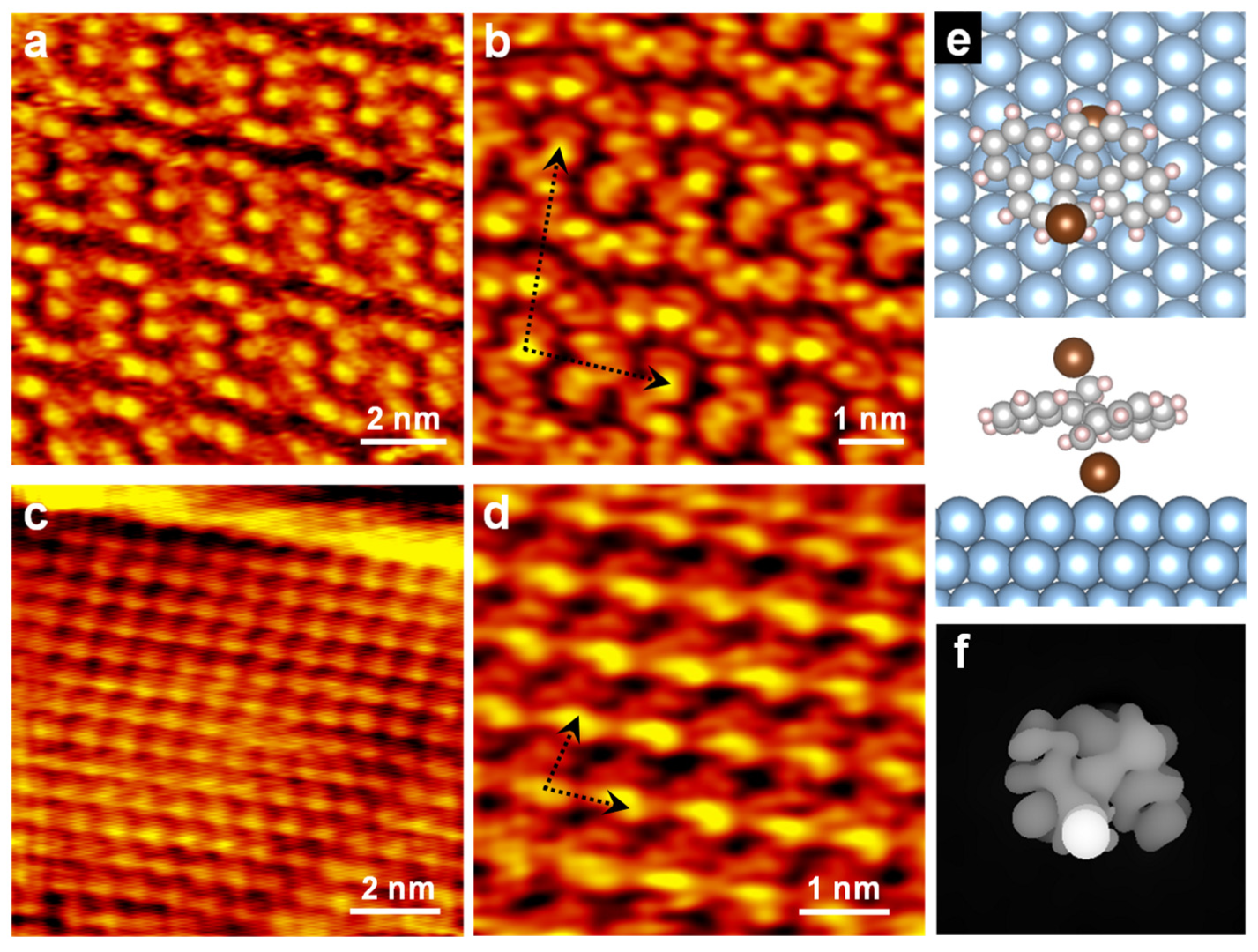
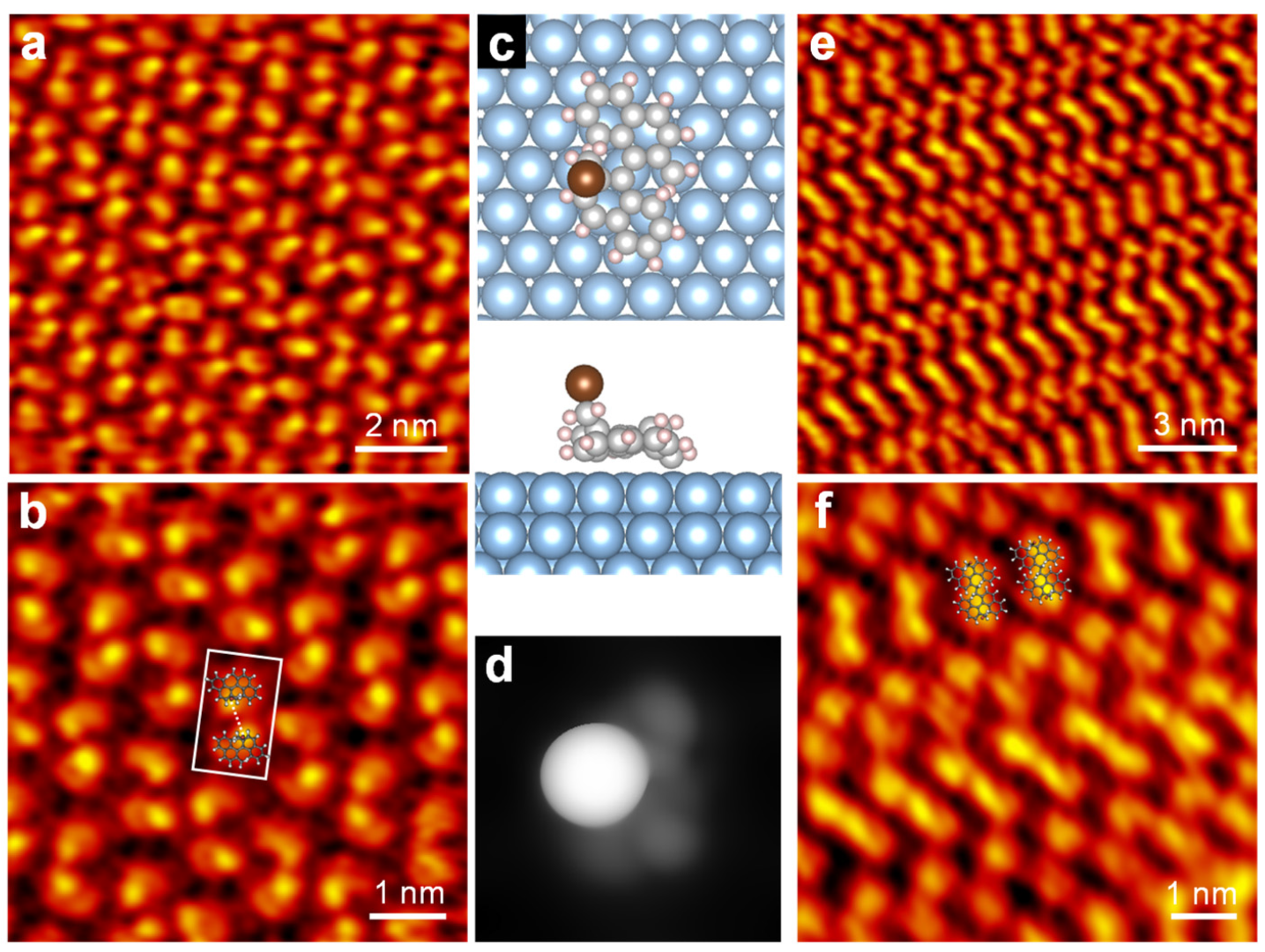
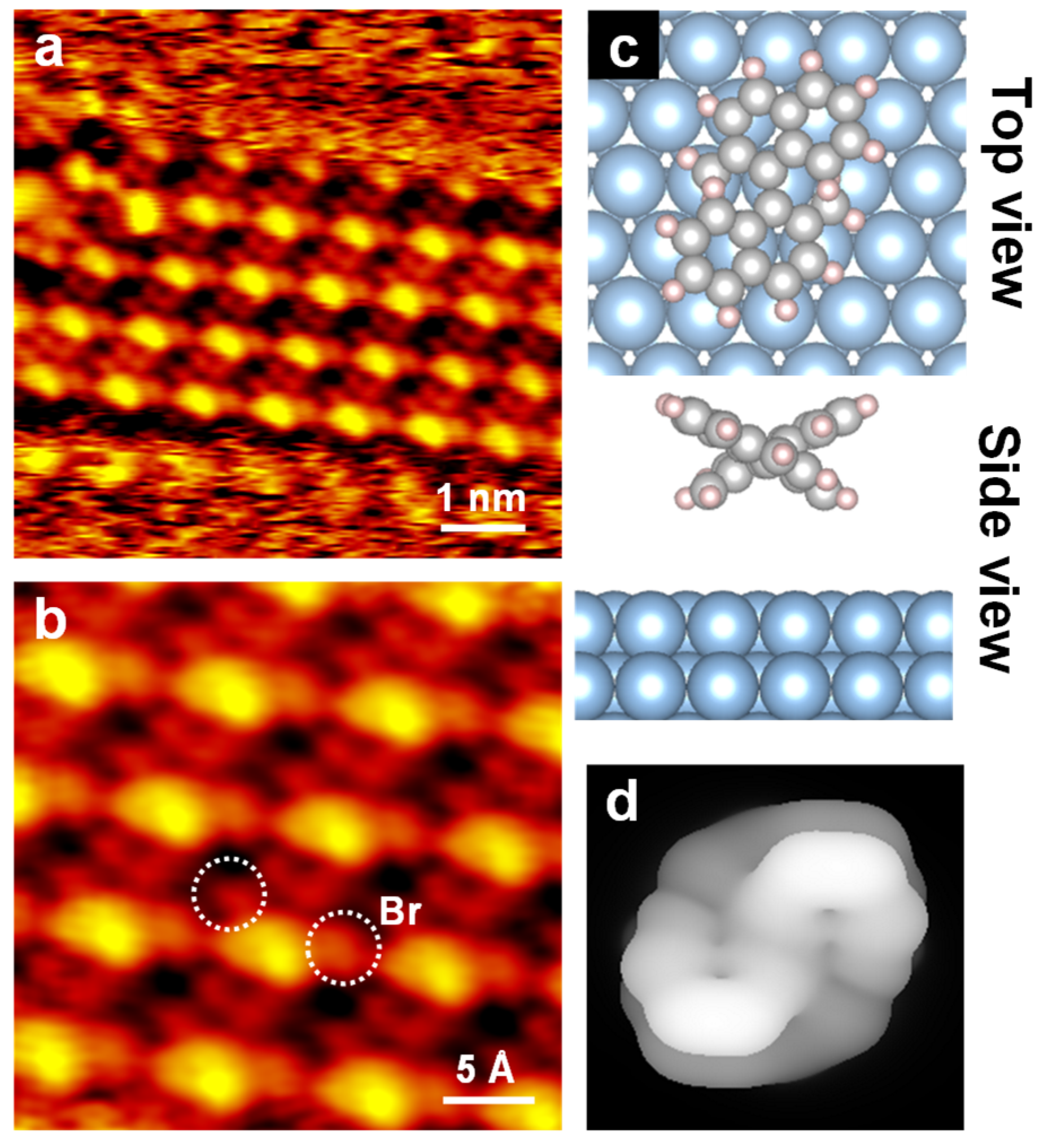
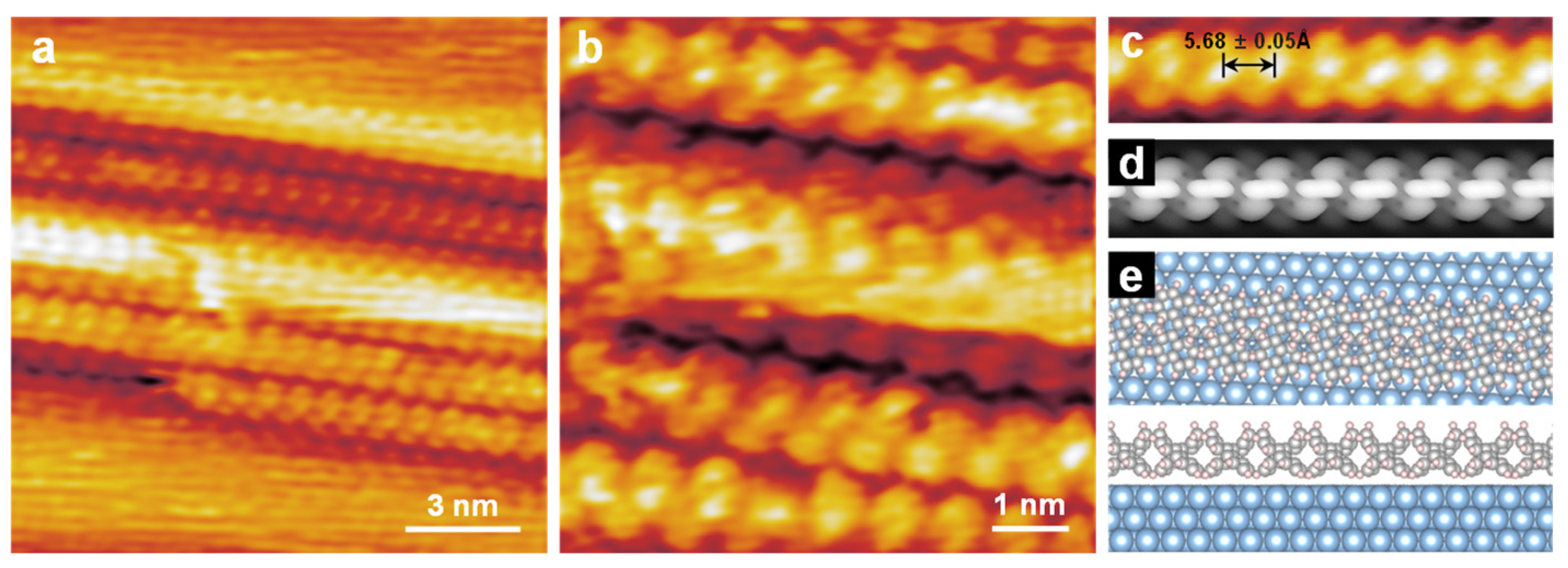
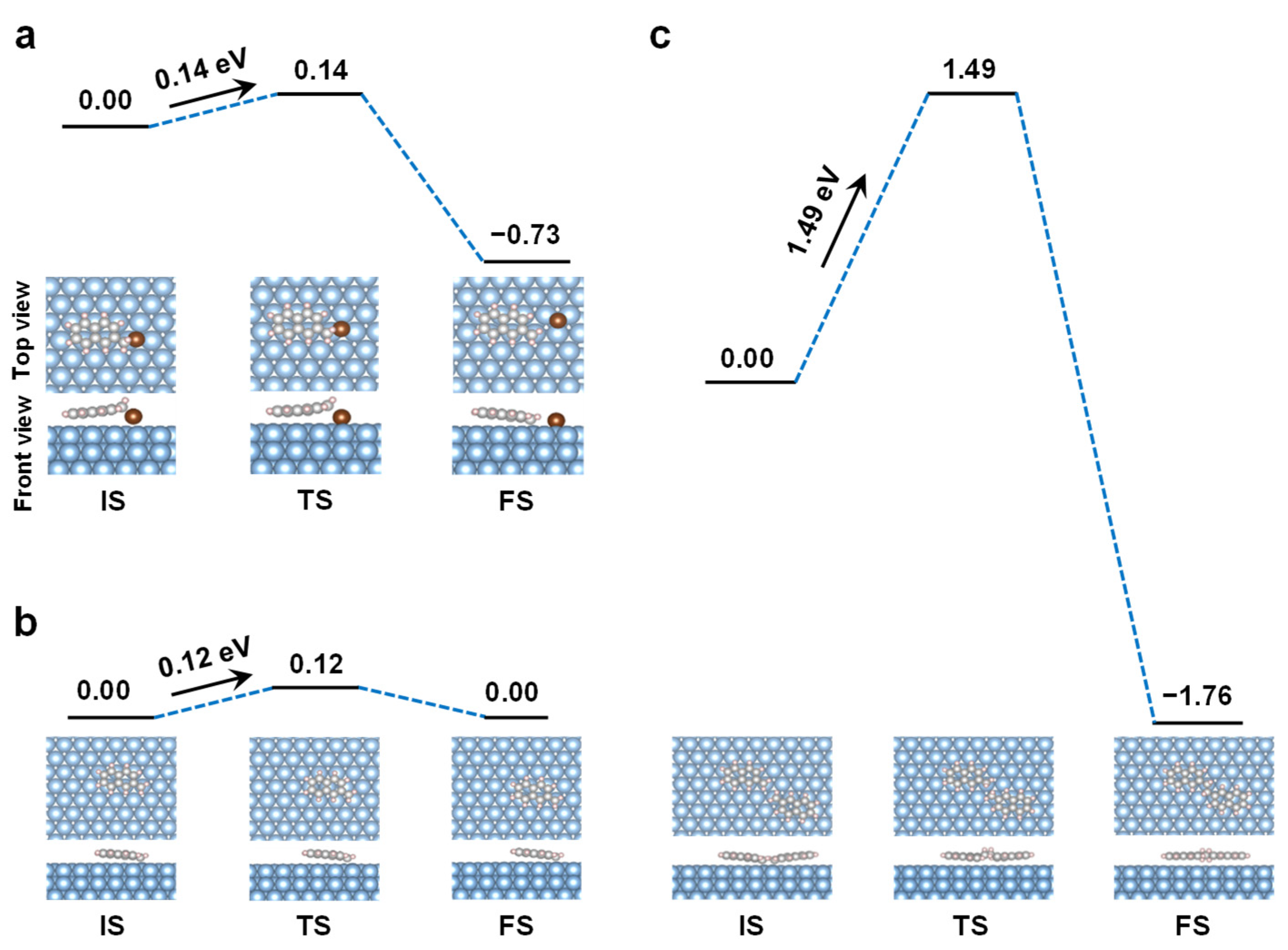
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UHV | ultrahigh vacuum |
| STM | scanning tunneling microscopy |
| DFT | Density functional theory |
| VASP | Vienna Ab Initio Simulation Package |
| PBE | Perdew–Burke–Ernzerhof |
| vdWs | van der Waals |
| LDOS | local density of states |
| BBMBN | (R)-2,2-bis(bromomethyl)-1,1-binaphthalene |
| RT | room temperature |
| LT | low temperature |
References
- Gao, W.; Cai, L.; Kang, F.; Shang, L.; Zhao, M.; Zhang, C.; Xu, W. Bottom-Up Synthesis of Metalated Carbyne Ribbons via Elimination Reactions. J. Am. Chem. Soc. 2023, 145, 6203–6209. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, X.; Lin, H.; Liu, M.; Cai, L.; Qiu, X.; Yang, D.; Fan, X.; Qiu, X.; Xu, W. Bond-Scission-Induced Structural Transformation from Cumulene to Diyne Moiety and Formation of Semiconducting Organometallic Polyyne. J. Am. Chem. Soc. 2020, 142, 8085–8089. [Google Scholar] [CrossRef] [PubMed]
- Rashed, E.A.; Palacio, C. Calculations of Constant-Height STM Images of Fullerene C60 Adsorbed onto a Surface. J. Spectrosc. 2023, 2023, 8841630. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y. Direct etching of nano/microscale patterns with both few-layer graphene and high-depth graphite structures by the raster STM electric lithography in the ambient conditions. J. Microsc. 2023, 292, 37–46. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef]
- Lafferentz, L.; Eberhardt, V.; Dri, C.; Africh, C.; Comelli, G.; Esch, F.; Hecht, S.; Grill, L. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 2012, 4, 215–220. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, R.; Qiu, J.; Liu, R.; Xu, W. On-Surface Synthesis of Carbon Nanostructures. Adv. Mater. 2018, 30, 1705630. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Z.; Xu, W. Scanning probe microscopy in probing low-dimensional carbon-based nanostructures and nanomaterials. Mater. Futures 2022, 1, 032301. [Google Scholar] [CrossRef]
- Liu, G.; Xu, S.G.; Ma, Y.; Shao, X.; Xiong, W.; Wu, X.; Zhang, S.; Liao, C.; Chen, C.; Wang, X.; et al. Arsenic Monolayers Formed by Zero-Dimensional Tetrahedral Clusters and One-Dimensional Armchair Nanochains. ACS Nano 2022, 16, 17087–17096. [Google Scholar] [CrossRef]
- Kunitake, M.; Uemura, S. Construction and Scanning Probe Microscopy Imaging of Two-dimensional Nanomaterials. Chem. Lett. 2020, 49, 565–573. [Google Scholar] [CrossRef]
- Deshpande, A.; Sham, C.H.; Alaboson, J.M.; Mullin, J.M.; Schatz, G.C.; Hersam, M.C. Self-assembly and photopolymerization of sub-2 nm one-dimensional organic nanostructures on graphene. J. Am. Chem. Soc. 2012, 134, 16759–16764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, Y.; Yi, Z.; Zhang, C.; Xu, W. Separation of Halogen Atoms by Sodium from Dehalogenative Reactions on a Au(111) Surface. ACS Nano 2024, 18, 9082–9091. [Google Scholar] [CrossRef] [PubMed]
- Judd, C.J.; Junqueira, F.L.Q.; Haddow, S.L.; Champness, N.R.; Duncan, D.A.; Jones, R.G.; Saywell, A. Structural characterisation of molecular conformation and the incorporation of adatoms in an on-surface Ullmann-type reaction. Comm. Chem. 2020, 3, 166. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Song, F.; Nguyen, M.T.; Li, Z.; Studener, F.; Stohr, M. Comparing Ullmann Coupling on Noble Metal Surfaces: On-Surface Polymerization of 1,3,6,8-Tetrabromopyrene on Cu(111) and Au(111). Chem. Eur. J. 2016, 22, 5937–5944. [Google Scholar] [CrossRef]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M.V.; Hecht, S. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2007, 2, 687–691. [Google Scholar] [CrossRef]
- Zint, S.; Ebeling, D.; Schloder, T.; Ahles, S.; Mollenhauer, D.; Wegner, H.A.; Schirmeisen, A. Imaging Successive Intermediate States of the On-Surface Ullmann Reaction on Cu(111): Role of the Metal Coordination. ACS Nano 2017, 11, 4183–4190. [Google Scholar] [CrossRef]
- Kohlmann, T.; Kerzig, C.; Goez, M. Laser-Induced Wurtz-Type Syntheses with a Metal-Free Photoredox Catalytic Source of Hydrated Electrons. Chem. Eur. J 2019, 25, 9991–9996. [Google Scholar] [CrossRef]
- McLeish, T. Physics meets polymerisation chemistry: Modelling the Wurtz reaction. Polym. Int. 2009, 58, 239–241. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, L.; Ding, Y.; Ma, H.; Yuan, C.; Xu, W. Single-molecule insight into Wurtz reactions on metal surfaces. Phys. Chem. Chem. Phys. 2016, 18, 2730–2735. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, L.; Ding, Y.; Xie, L.; Zhang, C.; Tan, Q.; Xu, W. Dehydrogenative Homocoupling of Terminal Alkenes on Copper Surfaces: A Route to Dienes. Angew. Chem.-Int. Ed. 2015, 54, 4549–4552. [Google Scholar] [CrossRef]
- Cai, L.; Kang, F.; Sun, Q.; Gao, W.; Yu, X.; Ma, H.; Yuan, C.; Xu, W. The Stereoselective Formation of trans-Cumulene through Dehalogenative Homocoupling of Alkenyl gem-Dibromides on Cu(110). ChemCatChem 2019, 11, 5417–5420. [Google Scholar] [CrossRef]
- Cai, L.; Yu, X.; Liu, M.; Sun, Q.; Bao, M.; Zha, Z.; Pan, J.; Ma, H.; Ju, H.; Hu, S.; et al. Direct Formation of C–C Double-Bonded Structural Motifs by On-Surface Dehalogenative Homocoupling of gem-Dibromomethyl Molecules. ACS Nano 2018, 12, 7959–7966. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wei, D.; Sun, J.; Wong, S.L.; Feng, Y.P.; Neto, A.H.; Wee, A.T. Spatially resolved electronic structures of atomically precise armchair graphene nanoribbons. Sci. Rep. 2012, 2, 983. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, R.; Shen, Z.; Song, Y.; She, L.; Wang, X.; Jia, Y.; Zhang, Z.; Zhang, W. On-Surface Synthesis of Self-Assembled Covalently Linked Wavy Chains with Site-Selective Conformational Switching. J. Am. Chem. Soc. 2023, 145, 1660–1667. [Google Scholar] [CrossRef]
- Hermann Walch, R.G.; Sirtl, T.; Eder, G.; Lackinger, M. Material- and Orientation-Dependent Reactivity for Heterogeneously Catalyzed Carbon-Bromine Bond Homolysis. J. Phys. Chem. C 2010, 114, 12604–12609. [Google Scholar] [CrossRef]
- Fan, Q.; Gottfried, J.M.; Zhu, J. Surface-catalyzed C-C covalent coupling strategies toward the synthesis of low-dimensional carbon-based nanostructures. Acc. Chem. Res 2015, 48, 2484–2494. [Google Scholar] [CrossRef]
- Besenbacher, F. Scanning tunnelling microscopy studies of metal surfaces. Rep. Prog. Phys 1996, 59, 1737–1802. [Google Scholar] [CrossRef]
- Besenbacher, E.L.L.Ö.P.T.P.B.R.I.S.F. A high-pressure scanning tunneling microscope. Rev. Sci. Instrum. 2001, 72, 3537–3542. [Google Scholar] [CrossRef]
- Kresse, G.a.J.F. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initiomolecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Tersoff, J.; Hamann, D.R. Theory of the scanning tunneling microscope. Phys. Rev. B 1985, 31, 805–813. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Han, Z.; Czap, G.; Chiang, C.L.; Xu, C.; Wagner, P.J.; Wei, X.; Zhang, Y.; Wu, R.; Ho, W. Imaging the halogen bond in self-assembled halogenbenzenes on silver. Science 2017, 358, 206–210. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2006, 13, 291–296. [Google Scholar] [CrossRef]
- Houtsma, R.S.K.; van Zuilen, J.; Stöhr, M. On-Surface Ullmann-Type Coupling: Reaction Intermediates and Organometallic Polymer Growth. Adv. Mater. Interfaces 2023, 11, 2300728. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.; Gong, Y.; Yang, M.; Zhao, M. Room-Temperature Synthesis of Carbon Nanochains via the Wurtz Reaction. Nanomaterials 2025, 15, 407. https://doi.org/10.3390/nano15050407
Pu J, Gong Y, Yang M, Zhao M. Room-Temperature Synthesis of Carbon Nanochains via the Wurtz Reaction. Nanomaterials. 2025; 15(5):407. https://doi.org/10.3390/nano15050407
Chicago/Turabian StylePu, Juxiang, Yongqing Gong, Menghao Yang, and Mali Zhao. 2025. "Room-Temperature Synthesis of Carbon Nanochains via the Wurtz Reaction" Nanomaterials 15, no. 5: 407. https://doi.org/10.3390/nano15050407
APA StylePu, J., Gong, Y., Yang, M., & Zhao, M. (2025). Room-Temperature Synthesis of Carbon Nanochains via the Wurtz Reaction. Nanomaterials, 15(5), 407. https://doi.org/10.3390/nano15050407






