Strategies for Improving Contact-Electro-Catalytic Efficiency: A Review
Abstract
1. Introduction
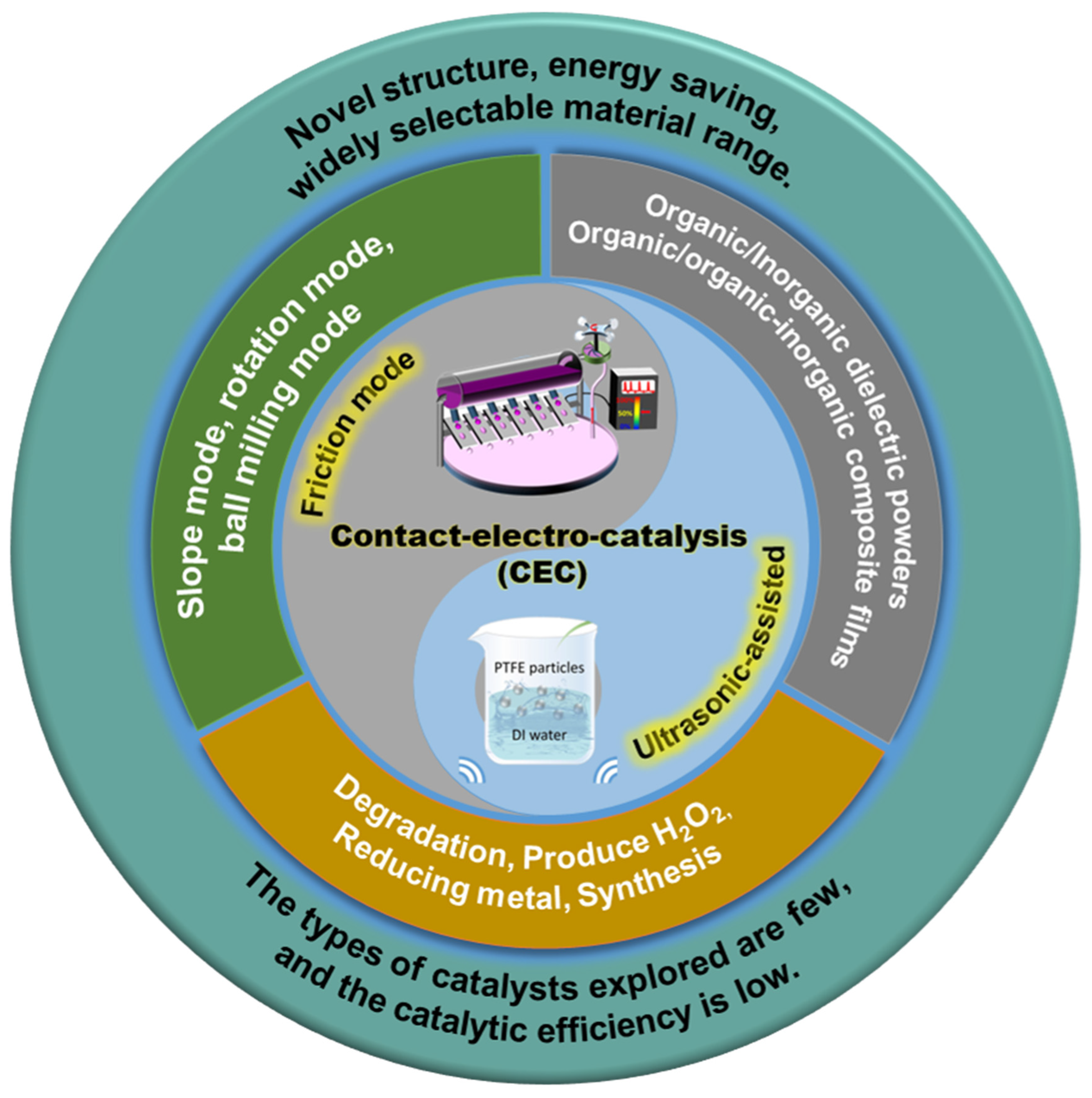
2. Proposal and Fundamental Principles of CEC
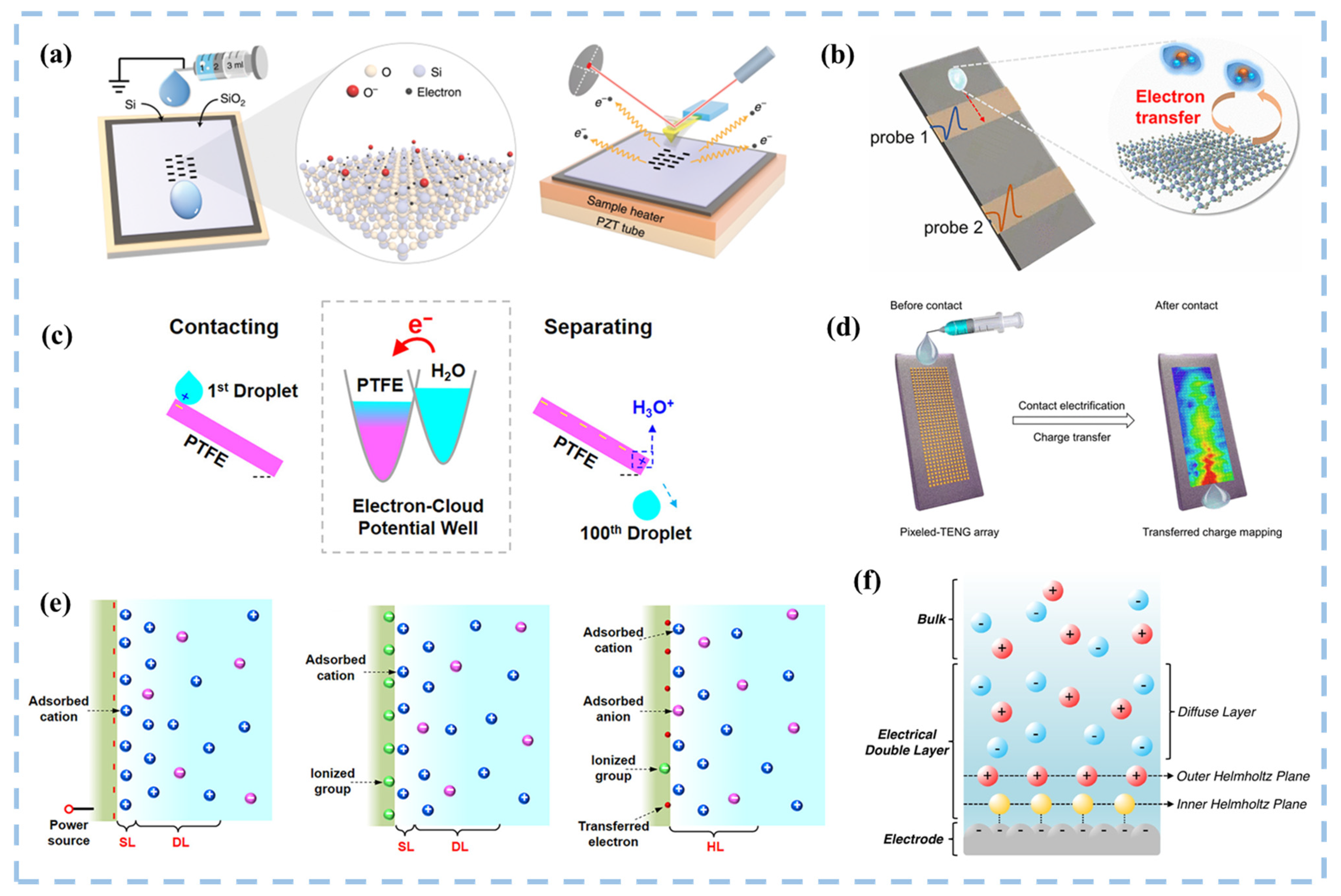
2.1. TENGs and Catalysis
2.2. Mechanochemical Reactions
2.3. Different Catalytic Methods
3. Progress in Applied Research of CEC
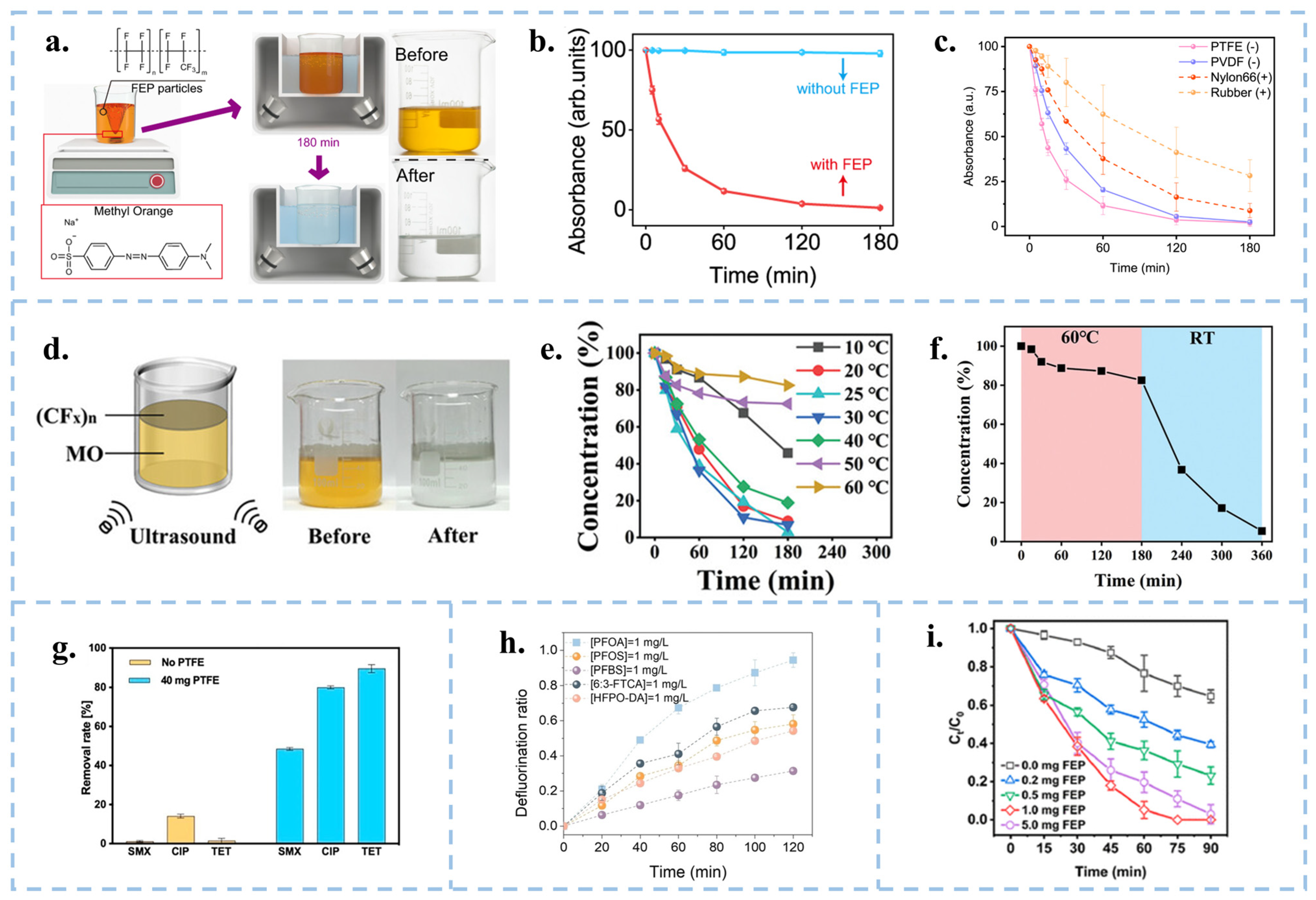
3.1. CEC for the Degradation
3.2. CEC for the Production of H2O2
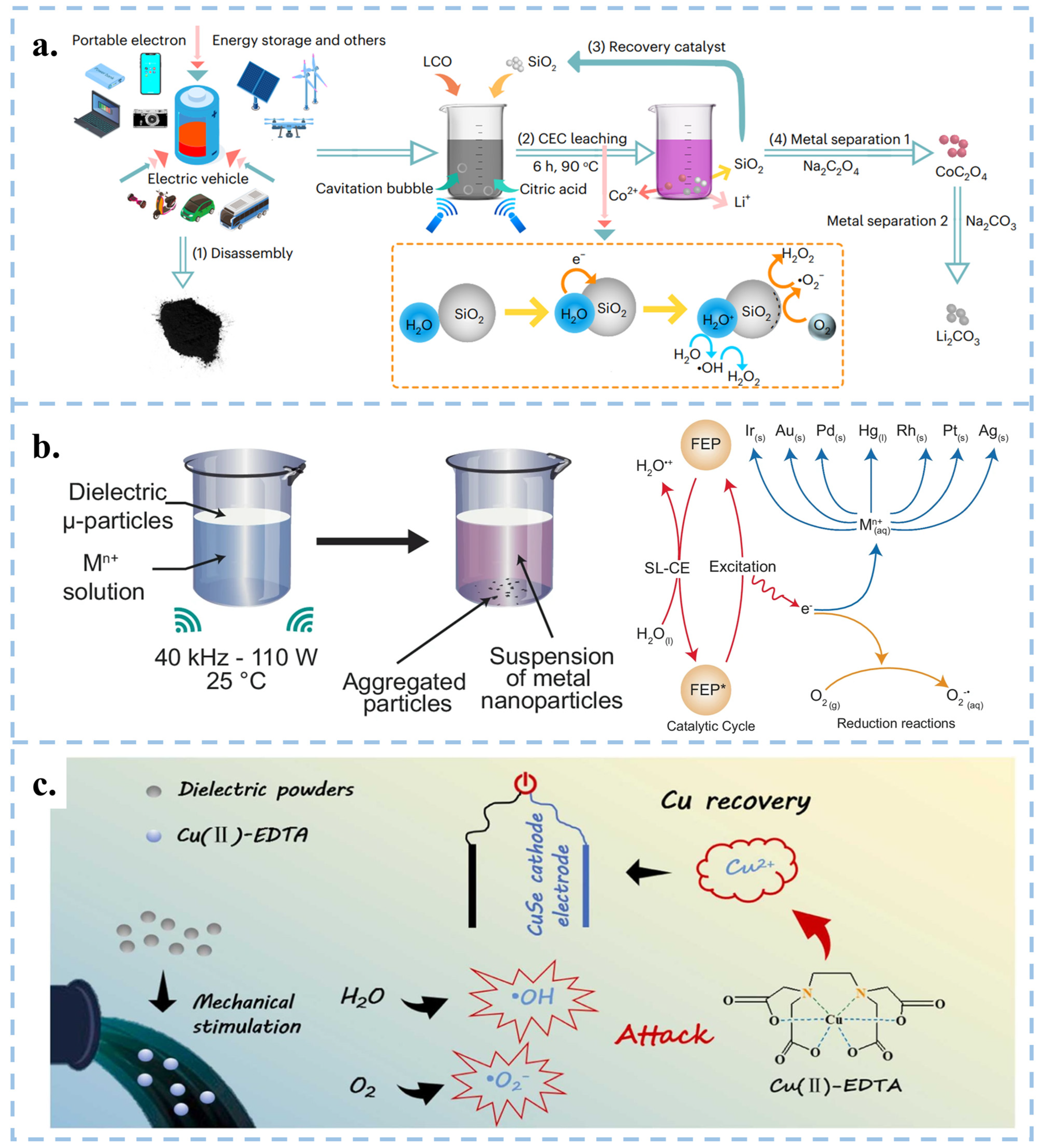
3.3. CEC for the Recovery of Metals
3.4. CEC for Synthesizing Chemical Substances
4. Design and Optimization of Catalytic Methods and Materials
4.1. Change of Catalytic Conditions
4.2. Coupling of CEC with Other Catalytic Methods
4.3. Surface Modification and Functionalization of Catalysts
4.4. Changing the Way of CEC Startup
5. Summaries and Perspectives
- (1)
- Urgent need to explore novel materials for CEC: The predominant catalysts used in CEC are fluorinated polymer powders, with a minimal portion being inorganic materials modified with fluorine or other functional groups. This limited variety in catalyst types raises concerns about potential environmental pollution from fluorides. There is a critical need to explore and develop new materials to expand the catalyst repertoire. Advanced computational techniques such as artificial intelligence and machine learning can play a crucial role in effectively screening;
- (2)
- Exploring the mechanism of CEC: There is an urgent need to delve deeply into the intricate electrocatalytic mechanisms within CEC systems. Currently, there is a notable absence of standardized methodologies for accurately assessing the electronic states associated with frictional charges in insulating materials like polymers. Given the unique degradation barriers of each organic dye, it is imperative to undertake comprehensive theoretical investigations into the charge transfer kinetics, and catalytic reaction kinetics under varying catalytic environments of contact electrocatalysis. Additionally, the development of sophisticated in-situ characterization techniques is paramount, including advanced surface spectroscopic methods and in-situ characterization techniques to study CEC mechanism, which have yet to be comprehensively addressed in the existing literature;
- (3)
- Exploring the structure of CEC materials: Optimizing surface morphology and band structure is expected to improve catalytic performance. Introducing doping and defects can alter the work function of catalysts, thereby increasing the amount of electron transfer. Designing construct hydrophobic surfaces can reduce lateral friction and charge dissipation. Introducing micro/nanostructures to increase contact surface area can improve catalytic efficiency. Theoretically, all materials capable of electron exchange with liquids hold promise for use in CEC, offering an exciting prospect for exploring the structure of the materials;
- (4)
- Designing novel operational modes for CEC: Implement innovative operating models, combine different catalytic methods, integrate wind energy, solar energy, potential energy, thermal energy, and magnetic energy storage, and improve catalytic efficiency;
- (5)
- Developing standards and novel applications of CEC: CEC is currently at a preliminary stage of development within laboratory environments. The lack of standardized experimental protocols and comprehensive data reporting protocols poses challenges to the reproducibility and comparability of experimental results. Moreover, potential issues in scalability, energy conversion efficiency or material degradation over time need to be addressed. Therefore, it is imperative to establish rigorous standards and guidelines in laboratory practices, facilitating accurate and equitable assessments of catalytic performance across various research facilities. Furthermore, the progression of CEC hinges on collaborative efforts spanning multiple disciplines, including materials science, electrochemistry, fluid dynamics, physics, and environmental engineering. At present, CEC has demonstrated multifaceted capabilities including organic molecules degradation, H2O2 production, metal recovery, depending on specific solution compositions and gas environments. This versatility provides the possibility for its future expansion into diverse fields such as eco-friendly chemical synthesis, biomedical applications, and environmental remediation. Potential applications include but are not limited to water splitting, tooth whitening, pharmaceutical loading, heavy metal reduction, bio-inspired and enzyme-mimetic catalysts, etc.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Wang, Y.; Chen, Y.; Shi, J. Nanocatalytic Tumor Therapy by Single-Atom Catalysts. ACS Nano 2019, 13, 2643–2653. [Google Scholar] [CrossRef]
- Osman, A.I.; Elgarahy, A.M.; Eltaweil, A.S.; El-Monaem, E.M.A.; El-Aqapa, H.G.; Park, Y.; Hwang, Y.; Ayati, A.; Farghali, M.; Ihara, I.; et al. Biofuel production, hydrogen production and water remediation by photocatalysis, biocatalysis and electrocatalysis. Environ. Chem. Lett. 2023, 21, 1315–1379. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Das, T.K.; Jesionek, M.; Çelik, Y.; Poater, A. Catalytic polymer nanocomposites for environmental remediation of wastewater. Sci. Total Environ. 2023, 901, 165772. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, L.; Lu, Z.; Han, Q.; Wu, L.; Wang, L.; Xiong, Y.; Ye, J.; Zou, Z.; Zhou, Y. Comprehensive Insight into Indium Oxide-Based Catalysts for CO2 Hydrogenation: Thermal, Photo, and Photothermal Catalysis. Adv. Funct. Mater. 2024, 51, 2409904. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Y.; Lin, Q.; Zhang, L.; Zhang, X.; Wang, H. Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis. Energy Environ. Sci. 2021, 14, 2954–3009. [Google Scholar] [CrossRef]
- Wang, N.; Yang, W.-H.; Wang, R.-X.; Li, Z.-J.; Xu, X.-F.; Long, Y.-Z.; Zhang, H.-D. Oxygen Vacancy-Enhanced Centrosymmetric Breaking of SrFeO3−x for Piezoelectric-Catalyzed Synthesis of H2O2. Small 2024, 20, 2307291. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Han, C.; Zhang, M.; Vongsvivut, J.; Dong, T.; Liu, L.; Wang, S.; Liu, S. Kesterite-Type Narrow Bandgap Piezoelectric Catalysts for Highly Efficient Piezocatalytic Fenton System. Adv. Funct. Mater. 2024, 35, 2412258. [Google Scholar] [CrossRef]
- Wu, M.; Lei, H.; Chen, J.; Dong, X. Friction energy harvesting on bismuth tungstate catalyst for tribocatalytic degradation of organic pollutants. J. Colloid Interface Sci. 2021, 587, 883–890. [Google Scholar] [CrossRef]
- Guo, W.; Guo, T.; Zhang, Y.; Yin, L.; Dai, Y. Progress on simultaneous photocatalytic degradation of pollutants and production of clean energy: A review. Chemosphere 2023, 339, 139486. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Ahuja, T.; Brighu, U.; Saxena, K. Recent advances in photocatalytic materials and their applications for treatment of wastewater: A review. J. Water Process Eng. 2023, 53, 103759. [Google Scholar] [CrossRef]
- Li, T.; Li, R.; Yang, L.; Wang, R.; Liu, R.; Chen, Y.; Yan, S.; Ramakrishna, S.; Long, Y.Z. Flexible PTh/GQDs/TiO2 composite with superior visible-light photocatalytic properties for rapid degradation pollutants. RSC Adv. 2023, 13, 1765–1778. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, L.; Wang, R.X.; Zhang, W.J.; Liu, R.; Yun, Y.Z.; Wang, N.; Ramakrishna, S.; Jiao, L.; Long, Y.Z. Constructing CoO/Mo2C Heterostructures with Interfacial Electron Redistribution Induced by Work Functions for Boosting Overall Water Splitting. Small 2023, 19, 2304086. [Google Scholar] [CrossRef]
- Wang, Z.; Berbille, A.; Feng, Y.; Li, S.; Zhu, L.; Tang, W.; Wang, Z.L. Contact-electro-catalysis for the degradation of organic pollutants using pristine dielectric powders. Nat. Commun. 2022, 13, 130. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, X.; Tang, W.; Wang, Z.L. Contact-electro-catalysis (CEC). Chem. Soc. Rev. 2024, 53, 4349–4373. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Xu, J.; Tang, W.; Wang, Z.L.; Fan, F.R. Contact-electro-catalysis for Direct Synthesis of H2O2 under Ambient Conditions. Angew. Chem. 2023, 135, e202300604. [Google Scholar] [CrossRef]
- Berbille, A.; Li, X.F.; Su, Y.; Li, S.; Zhao, X.; Zhu, L.; Wang, Z.L. Mechanism for Generating H2O2 at Water-Solid Interface by Contact-Electrification. Adv. Mater. 2023, 35, 2304387. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Berbille, A.; Wang, Z.L.; Tang, W. Degradation of methyl orange by dielectric films based on contact-electro-catalysis. Nanoscale 2023, 15, 6243–6251. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, X.; Li, X.-F.; Feng, Y.; Li, S.; Tang, W.; Wang, Z.L. A contact-electro-catalysis process for producing reactive oxygen species by ball milling of triboelectric materials. Nat. Commun. 2024, 15, 757. [Google Scholar] [CrossRef]
- Kang, Z.; Qin, N.; Lin, E.; Wu, J.; Yuan, B.; Bao, D. Effect of Bi2WO6 nanosheets on the ultrasonic degradation of organic dyes: Roles of adsorption and piezocatalysis. J. Clean. Prod. 2020, 261, 121125. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Vaish, R. Transparent ferroelectric glass–ceramics for wastewater treatment by piezocatalysis. Commun. Mater. 2020, 1, 100. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Liu, W.; Ji, H.; Li, F.; Shen, C.; Fang, X.; Li, X.; Duan, X. A novel electrocatalytic filtration system with carbon nanotube supported nanoscale zerovalent copper toward ultrafast oxidation of organic pollutants. Water Res. 2021, 194, 116961. [Google Scholar] [CrossRef]
- Nugroho, D.; Wannakan, K.; Nanan, S.; Benchawattananon, R. The Synthesis of carbon dots//zincoxide (CDs/ZnO-H400) by using hydrothermal methods for degradation of ofloxacin antibiotics and reactive red azo dye (RR141). Sci. Rep. 2024, 14, 2455. [Google Scholar] [CrossRef]
- Li, X.F.; Berbille, A.; Wang, T.Y.; Zhao, X.; Li, S.; Su, Y.; Li, H.; Zhang, G.; Wang, Z.; Zhu, L.; et al. Defect Passivation Toward Designing High-Performance Fluorinated Polymers for Liquid–Solid Contact-Electrification and Contact-Electro-Catalysis. Adv. Funct. Mater. 2024, 34, 2315817. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Zhang, X.; Chen, Y.; Liu, X.; Zhang, Y. Contact-Electro-Catalysis Through Electret Behavior to Facilitate Electron Transfer. ACS Appl. Mater. Interfaces 2024, 16, 42293–42304. [Google Scholar] [CrossRef]
- Song, W.Z.; Zhang, M.; Qiu, H.J.; Li, C.L.; Chen, T.; Jiang, L.L.; Yu, M.; Ramakrishna, S.; Wang, Z.L.; Long, Y.Z. Insulator polymers achieve efficient catalysis under visible light due to contact electrification. Water Res. 2022, 226, 119242. [Google Scholar] [CrossRef]
- Fan, F.; Tian, Z.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Cheng, T.; Shao, J.; Wang, Z.L. Triboelectric nanogenerators. Nat. Rev. Methods Primers 2023, 3, 38. [Google Scholar] [CrossRef]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2019, 9, 1802906. [Google Scholar] [CrossRef]
- Lin, S.; Chen, X.; Wang, Z.L. Contact Electrification at the Liquid–Solid Interface. Chem. Rev. 2022, 122, 5209–5232. [Google Scholar] [CrossRef]
- Lin, S.; Chen, X.; Wang, Z.L. The tribovoltaic effect and electron transfer at a liquid-semiconductor interface. Nano Energy 2020, 76, 105070. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Y.; Li, R.; Li, D.; Xiang, B.; Li, J.; Liu, Q. Liquid-solid contact electrification through the lens of surface and interface science. Nano Energy 2023, 116, 108834. [Google Scholar] [CrossRef]
- Lin, S.; Xu, L.; Wang, A.C.; Wang, Z.L. Quantifying electron-transfer in liquid-solid contact electrification and the formation of electric double-layer. Nat. Commun. 2020, 11, 399. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Zheng, M.; Wang, Z.L. Triboelectric Nanogenerator as a Probe for Measuring the Charge Transfer between Liquid and Solid Surfaces. ACS Nano 2021, 15, 14830–14837. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Wang, Z.L. Triboelectric Nanogenerator Array as a Probe for In Situ Dynamic Mapping of Interface Charge Transfer at a Liquid–Solid Contacting. ACS Nano 2023, 17, 1646–1652. [Google Scholar] [CrossRef]
- Zhan, F.; Wang, A.C.; Xu, L.; Lin, S.; Shao, J.; Chen, X.; Wang, Z.L. Electron Transfer as a Liquid Droplet Contacting a Polymer Surface. ACS Nano 2020, 14, 17565–17573. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Yan, X.; Wang, L.; Hu, H.; Wang, K. Triboelectric nanogenerators: The beginning of blue dream. Front. Chem. Sci. Eng. 2023, 17, 635–678. [Google Scholar] [CrossRef]
- Lin, Z.H.; Cheng, G.; Lin, L.; Lee, S.; Wang, Z.L. Water–Solid Surface Contact Electrification and its Use for Harvesting Liquid-Wave Energy. Angew. Chem. 2013, 125, 12777–12781. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, T.; Wu, J.; Xu, T.; Wang, X.; Han, X.; Cui, H.; Xu, X.; Pan, C.; Li, X. Energy Conversion Analysis of Multilayered Triboelectric Nanogenerators for Synergistic Rain and Solar Energy Harvesting. Adv. Mater. 2022, 34, 2202238. [Google Scholar] [CrossRef]
- Cheng, G.; Lin, Z.H.; Du, Z.L.; Wang, Z.L. Simultaneously Harvesting Electrostatic and Mechanical Energies from Flowing Water by a Hybridized Triboelectric Nanogenerator. ACS Nano 2014, 8, 1932–1939. [Google Scholar] [CrossRef]
- Wang, Z.L.; Jiang, T.; Xu, L. Toward the blue energy dream by triboelectric nanogenerator networks. Nano Energy 2017, 39, 9–23. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, N.; Yang, D.; Wang, J.; Lu, W.; Wang, D. Robust Solid-Liquid Triboelectric Nanogenerators: Mechanisms, Strategies and Applications. Adv. Funct. Mater. 2023, 33, 2300764. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, H.; Cao, Z.; Zhang, E.; Xu, S.; Li, W.; Yao, H.; Wan, L.; Liu, G. 0.5 m Triboelectric Nanogenerator for Efficient Blue Energy Harvesting of All-Sea Areas. Adv. Sci. 2022, 9, 2204407. [Google Scholar] [CrossRef]
- Chen, T.; Song, W.Z.; Zhang, M.; Sun, D.J.; Zhang, D.S.; Li, C.L.; Cui, W.Y.; Fan, T.T.; Ramakrishna, S.; Long, Y.Z. Acid and alkali-resistant fabric-based triboelectric nanogenerator for self-powered intelligent monitoring of protective clothing in highly corrosive environments. RSC Adv. 2023, 13, 11697–11705. [Google Scholar] [CrossRef]
- Zhou, L.N.; Song, W.Z.; Sun, D.J.; Yan, B.Y.; Chen, T.; Li, T.; Zhang, J.; Yu, G.F.; Ramakrishna, S.; Long, Y.Z. Transparent, Stretchable, and Recyclable Triboelectric Nanogenerator Based on an Acid- and Alkali-Resistant Hydrogel. ACS Appl. Electron. Mater. 2023, 5, 216–226. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Z.Q.; Qin, S.Y.; Hu, J.; Yuan, S.M.; Wang, Z.L.; Chen, X.Y. A wearable triboelectric impedance tomography system for noninvasive and dynamic imaging of biological tissues. Sci. Adv. 2024, 10, eadr9139. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Lin, S.Q.; Yang, P.; Qin, S.Y.; Hua, J.; Chen, X.Y. A wiping-type semiconductor-liquid generator utilizing water-bearing solid materials and hydrated biological tissues. Energy Environ. Sci. 2024, 17, 6582. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, S.W. Nanogenerators to Power Implantable Medical Systems. Joule 2020, 4, 1398–1407. [Google Scholar] [CrossRef]
- Li, C.L.; Song, W.Z.; Sun, D.J.; Zhang, M.; Zhang, J.; Chen, Y.Q.; Ramakrishna, S.; Long, Y.Z. A self-priming air filtration system based on triboelectric nanogenerator for active air purification. Chem. Eng. J. 2023, 452, 139428. [Google Scholar] [CrossRef]
- Qin, S.Y.; Yang, P.; Liu, Z.Q.; Hu, J.; Li, N.; Ding, L.M.; Chen, X.Y. Triboelectric sensor with ultra-wide linear range based on water-containing elastomer and ion-rich interface. Nat. Commun. 2024, 15, 10640. [Google Scholar] [CrossRef]
- Sun, D.-J.; Song, W.Z.; Li, C.L.; Chen, T.; Zhang, D.S.; Zhang, J.; Ramakrishna, S.; Long, Y.Z. High-voltage direct current triboelectric nanogenerator based on charge pump and air ionization for electrospinning. Nano Energy 2022, 101, 107599. [Google Scholar] [CrossRef]
- Dunwell, M.; Yan, Y.; Xu, B. Understanding the influence of the electrochemical double-layer on heterogeneous electrochemical reactions. Curr. Opin. Chem. Eng. 2018, 20, 151–158. [Google Scholar] [CrossRef]
- Sharma, M.K.; Khan, I.; Kaswan, K.; Barman, S.R.; Saha, S.; Hsieh, W.C.; Chueh, Y.L.; Wang, Y.L.; Lee, S.; Choi, D.; et al. Emergence of Non-photoresponsive Catalytic Techniques for Environmental Remediation and Energy Generation. Chem. Asian J. 2023, 18, e202300090. [Google Scholar] [CrossRef]
- Xu, C.; Zi, Y.; Wang, A.C.; Zou, H.; Dai, Y.; He, X.; Wang, P.; Wang, Y.C.; Feng, P.; Li, D.; et al. On the Electron-Transfer Mechanism in the Contact-Electrification Effect. Adv. Mater. 2018, 30, 1706790. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis mechanisms: Electron transfer and transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, A.C. On the origin of contact-electrification. Mater. Today 2019, 30, 34–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, Y.; Li, S.; Liu, Y.; Wang, Z.L.; Jiang, P. The process of free radical generation in contact electrification at solid-liquid interface. Nano Energy 2023, 112, 108464. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Lloyd, E.M.; Vakil, J.R.; Yao, Y.; Sottos, N.R.; Craig, S.L. Covalent Mechanochemistry and Contemporary Polymer Network Chemistry: A Marriage in the Making. J. Am. Chem. Soc. 2023, 145, 751–768. [Google Scholar] [CrossRef]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- Gonnet, L.; Chamayou, A.; André-Barrès, C.; Micheau, J.C.; Guidetti, B.; Sato, T.; Baron, M.; Baltas, M.; Calvet, R. Elucidation of the Diels–Alder Reaction Kinetics between Diphenylfulvene and Maleimide by Mechanochemistry and in Solution. ACS Sustain. Chem. Eng. 2021, 9, 4453. [Google Scholar] [CrossRef]
- Pladevall, B.S.; de Aguirre, A.; Maseras, F. Understanding Ball Milling Mechanochemical Processes with DFT Calculations and Microkinetic Modeling. ChemSusChem 2021, 14, 2763–2768. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; Nicolosi, V. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Ganguly, P.; Jha, A.K.; Am, J. Synthesis and Characterization of Tungsten–Bronze Structured Nanocrystalline Ba5SmTi3Nb7O30 Ferroelectric Ceramics by High-Energy Ball Milling. J. Ceram. Soc. 2011, 94, 1725–1730. [Google Scholar] [CrossRef]
- Shi, K.; Chai, B.; Zou, H.; Shen, P.; Sun, B.; Jiang, P.; Shi, Z.; Huang, X. Interface induced performance enhancement in flexible BaTiO3/PVDF-TrFE based piezoelectric nanogenerators. Nano Energy 2021, 80, 105515. [Google Scholar] [CrossRef]
- Ferdowsi, P.; Ochoa-Martinez, E.; Steiner, U.; Saliba, M. One-Step Solvent-Free Mechanochemical Incorporation of Insoluble Cesium Salt into Perovskites for Wide Band-Gap Solar Cells. Chem. Mater. 2021, 33, 3971–3979. [Google Scholar] [CrossRef]
- Wu, W.; Fang, H.; Ma, H.; Wu, L.; Wang, Q.; Wang, H. Self-Powered Rewritable Electrochromic Display based on WO3-x Film with Mechanochemically Synthesized MoO3–y Nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 20326–20335. [Google Scholar] [CrossRef]
- Guo, B.; Ma, R.; Li, Z.; Guo, S.; Luo, J.; Yang, M.; Liu, Q.; Thomas, T.; Wang, J. Hierarchical N-Doped Porous Carbons for Zn–Air Batteries and Supercapacitors. Nano-Micro Lett. 2020, 12, 20. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Yu, J.; Liao, J.; Zhao, B.; Huang, L.; Abdelhafiz, A.; Zhang, H.; Wang, J.-H.; Guo, Z.; et al. A self-healing layered GeP anode for high-performance Li-ion batteries enabled by low formation energy. Nano Energy 2019, 61, 594–603. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327. [Google Scholar] [CrossRef]
- Jin, C.; Liu, D.; Hu, J.; Wang, Y.; Zhang, Q.; Lv, L.; Zhuge, F. The role of microstructure in piezocatalytic degradation of organic dye pollutants in wastewater. Nano Energy 2019, 59, 372–379. [Google Scholar] [CrossRef]
- Amaechi, I.C.; Youssef, A.H.; Dörfler, A.; González, Y.; Katoch, R.; Ruediger, A. Catalytic Applications of Non-Centrosymmetric Oxide Nanomaterials. Angew. Chem. Int. Ed. 2022, 61, e202207975. [Google Scholar] [CrossRef]
- Wang, Q.; Bowen, C.R.; Valev, V.K. Plasmonic-Pyroelectric Materials and Structures. Adv. Funct. Mater. 2024, 34, 2312245. [Google Scholar] [CrossRef]
- Yuan, X.; Shi, J.; Kang, Y.; Dong, J.; Pei, Z.; Ji, X. Piezoelectricity, Pyroelectricity, and Ferroelectricity in Biomaterials and Biomedical Applications. Adv. Mater. 2024, 36, 2308726. [Google Scholar] [CrossRef]
- Meroni, D.; Djellabi, R.; Ashokkumar, M.; Bianchi, C.L.; Boffito, D.C. Sonoprocessing: From Concepts to Large-Scale Reactors. Chem. Rev. 2022, 122, 3219–3258. [Google Scholar] [CrossRef]
- Zeitler, S.M.; Golder, M.R. Shake, shear, and grind!—The evolution of mechanoredox polymerization methodology. Chem. Commun. 2024, 60, 26–35. [Google Scholar] [CrossRef]
- Manickam, S.; Boffito, D.C.; Flores, E.M.M.; Leveque, J.-M.; Pflieger, R.; Pollet, B.G.; Ashokkumar, M. Ultrasonics and sonochemistry: Editors’ perspective. Ultrason. Sonochem. 2023, 99, 106540. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles–An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kozuka, T.; Towata, A.; Iida, Y. Relationship between the bubble temperature and main oxidant created inside an air bubble under ultrasound. J. Chem. Phys. 2007, 127, 154502. [Google Scholar] [CrossRef]
- Rochebrochard d’Auzay, S.D.L.; Blais, J.-F.; Naffrechoux, E. Comparison of characterization methods in high frequency sonochemical reactors of differing configurations. Ultrason. Sonochem. 2010, 17, 547–554. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Gogate, P.R. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason. Sonochemistry 2017, 36, 527–543. [Google Scholar] [CrossRef]
- Yasui, K. Influence of ultrasonic frequency on multibubble sonoluminescence. J. Acoust. Soc. Am. 2002, 112, 1405–1413. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.-H.; Zhang, Y.; Liu, M.-N.; Wang, L.-Y.; Yu, Z.-C.; Yang, W.-H.; Zhang, J.; Long, Y.-Z. Contact-Electro-Catalysis for Organic Pollutants Degradation Based on 2D Fluorinated Graphite. Adv. Funct. Mater. 2024, 34, 2406417. [Google Scholar] [CrossRef]
- Cao, D.-Q.; Fang, R.-K.; Song, Y.-X.; Ma, M.-G.; Li, H.; Hao, X.-D.; Wu, R.; Chen, X. Contact-electro-catalysis for degradation of trace antibiotics in wastewater. Chem. Eng. J. 2024, 487, 150531. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, W.; Yao, J.; Liu, J.; He, H.; Gu, C.; Gao, G.; Jin, X. Electrostatic Field in Contact-Electro-Catalysis Driven C−F Bond Cleavage of Perfluoroalkyl Substances. Angew. Chem. Int. Ed. 2024, 63, e202402440. [Google Scholar] [CrossRef]
- Li, K.; Lai, Y.; Lin, S.; Zhou, L.; He, M.; Lin, H.; Yuan, Y. Contact-Electro-Catalysis for the Degradation of Pentachlorophenol Using Inert Fluorinated Ethylene Propylene Powders. ACS EST Eng. 2024, 4, 2485–2494. [Google Scholar] [CrossRef]
- Chen, B.; Xia, Y.; He, R.; Sang, H.; Zhang, W.; Li, J.; Chen, L.; Wang, P.; Guo, S.; Yin, Y.; et al. Water–solid contact electrification causes hydrogen peroxide production from hydroxyl radical recombination in sprayed microdroplets. Proc. Natl. Acad. Sci. USA 2022, 119, e2209056119. [Google Scholar] [CrossRef]
- Li, H.; Berbille, A.; Zhao, X.; Wang, Z.; Tang, W.; Wang, Z.L. A contact-electro-catalytic cathode recycling method for spent lithium-ion batteries. Nat. Energy 2023, 8, 1137–1144. [Google Scholar] [CrossRef]
- Su, Y.; Berbille, A.; Li, X.-F.; Zhang, J.; PourhosseiniAsl, M.; Li, H.; Liu, Z.; Li, S.; Liu, J.; Zhu, L.; et al. Reduction of precious metal ions in aqueous solutions by contact-electro-catalysis. Nat. Commun. 2024, 15, 4196. [Google Scholar] [CrossRef]
- Shen, X.; Wang, S.; Zhao, L.; Song, H.; Li, W.; Li, C.; Lv, S.; Wang, G. Simultaneous Cu(II)-EDTA decomplexation and Cu(II) recovery using integrated contact-electro-catalysis and capacitive deionization from electroplating wastewater. J. Hazard. Mater. 2024, 472, 134548. [Google Scholar] [CrossRef]
- Chen, X.; Xia, Y.; Zhang, Z.; Hua, L.; Jia, X.; Wang, F.; Zare, R.N. Hydrocarbon Degradation by Contact with Anoxic Water Microdroplets. J. Am. Chem. Soc. 2023, 145, 21538–21545. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Zhang, Y.; Yin, Y.; Chen, B.; Liang, Y.; Jiang, G.; Zare, R.N. Contact between water vapor and silicate surface causes abiotic formation of reactive oxygen species in an anoxic atmosphere. Proc. Natl. Acad. Sci. USA 2023, 120, e2302014120. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, D.; Liang, C.; Zhang, X. Spontaneous Reduction of Transition Metal Ions by One Electron in Water Microdroplets and the Atmospheric Implications. J. Am. Chem. Soc. 2023, 145, 2800–2805. [Google Scholar] [CrossRef]
- Xing, D.; Meng, Y.; Yuan, X.; Jin, S.; Song, X.; Zare, R.N.; Zhang, X. Capture of Hydroxyl Radicals by Hydronium Cations in Water Microdroplets. Angew. Chem. Int. Ed. 2022, 61, e202207587. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, X.; Gong, C.; Zhang, X. High Electric Field on Water Microdroplets Catalyzes Spontaneous and Ultrafast Oxidative C–H/N–H Cross-Coupling. J. Am. Chem. Soc. 2022, 144, 16184–16190. [Google Scholar] [CrossRef]
- Mehrgardi, M.A.; Mofidfar, M.; Zare, R.N. Sprayed Water Microdroplets Are Able to Generate Hydrogen Peroxide Spontaneously. J. Am. Chem. Soc. 2022, 144, 7606–7609. [Google Scholar] [CrossRef]
- Song, X.; Basheer, C.; Zare, R.N. Making ammonia from nitrogen and water microdroplets. Proc. Natl. Acad. Sci. USA 2023, 120, e2301206120. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y.; Song, X.; Chen, B.; Zare, R.N. Continuous ammonia synthesis from water and nitrogen via contact electrification. Proc. Natl. Acad. Sci. USA 2024, 121, e2318408121. [Google Scholar] [CrossRef]
- Li, W.; Sun, J.; Wang, M.; Xu, J.; Wang, Y.; Yang, L.; Yan, R.; He, H.; Wang, S.; Deng, W.-Q.; et al. Contact-Electro-Catalysis for Direct Oxidation of Methane under Ambient Conditions. Angew. Chem. Int. Ed. 2024, 63, e202403114. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, W.; Yang, J.; Feng, H.; Zheng, Y.; Wang, S.; Li, B.; Heng, J.Z.X.; Ong, W.C.; Tan, H.R.; et al. Contact-electro-catalytic CO2 reduction from ambient air. Nat. Commun. 2024, 15, 5913. [Google Scholar] [CrossRef]
- Shinde, R.S.; Khairnar, S.D.; Patil, M.R.; Adole, V.A.; Koli, P.B.; Deshmane, V.V.; Halwar, D.K.; Shinde, R.A.; Pawar, T.B.; Jagdale, B.S.; et al. Synthesis and Characterization of ZnO/CuO Nanocomposites as an Effective Photocatalyst and Gas Sensor for Environmental Remediation. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1045–1066. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Wang, Z.; Wang, G.; Zhang, B.; Zhang, Q.; Yang, D. Rapid fabrication of SnO2 nanoparticle photocatalyst: Computational understanding and photocatalytic degradation of organic dye. Inorg. Chem. Front. 2018, 5, 3005–3014. [Google Scholar] [CrossRef]
- Wu, M.-H.; Lee, J.-T.; Chung, Y.J.; Srinivaas, M.; Wu, J.-M. Ultrahigh efficient degradation activity of single- and few-layered MoSe2 nanoflowers in dark by piezo-catalyst effect. Nano Energy 2017, 40, 369–375. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Guo, X.; Wang, L.; Xu, T.; Bian, J.; Yang, Y.; Liu, Q.; Du, Y.; Lou, X. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Z.; Berbille, A.; Zhao, X.; Tang, W.; Wang, Z.L. Investigations on the contact-electro-catalysis under various ultrasonic conditions and using different electrification particles. Nano Energy 2022, 99, 107346. [Google Scholar] [CrossRef]
- Jiang, B.; Xue, X.; Mu, Z.; Zhang, H.; Li, F.; Liu, K.; Wang, W.; Zhang, Y.; Li, W.; Yang, C.; et al. Contact-Piezoelectric Bi-Catalysis of an Electrospun ZnO@PVDF Composite Membrane for Dye Decomposition. Molecules 2022, 27, 8579. [Google Scholar] [CrossRef]
- Su, Y.; Berbille, A.; Wang, Z.L.; Tang, W. Water-solid contact electrification and catalysis adjusted by surface functional groups. Nano Res. 2024, 17, 3344–3351. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Y.; Liu, X.; Li, J.; Liu, Q. Novel magnetic catalysts for organic pollutant degradation via contact electro-catalysis. Nano Energy 2023, 108, 108198. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Ao, X.; Gu, Y.; Li, C.; Wu, Y.; Wu, C.; Xun, S.; Nikiforov, A.; Xu, C.; Jia, J.; Cai, W.; et al. Sulfurization-functionalized 2D metal-organic frameworks for high-performance urea fuel cell. Appl. Catal. B Environ. 2022, 315, 121586. [Google Scholar] [CrossRef]
- Ahmed, I.; Mondol, M.M.H.; Jung, M.J.; Lee, G.H.; Jhung, S.H. MOFs with bridging or terminal hydroxo ligands: Applications in adsorption, catalysis, and functionalization. Coord. Chem. Rev. 2023, 475, 214912. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, X.; Ma, Y.; Cho, H.S.; Ding, D.; Wang, C.; Wu, J.; Oleynikov, P.; Jia, M.; Cheng, J.; et al. Filling metal–organic framework mesopores with TiO2 for CO2 photoreduction. Nature 2020, 586, 549–554. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, T.; Han, X.; Yang, W.; Gong, W.; Li, K.; Guo, Y. Molecular-functionalized metal-organic frameworks enabling contact-electro-catalytic organic decomposition. Nano Energy 2023, 111, 108433. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Z.; Hou, Y.; Feng, Y.; Berbille, A.; Li, H.; Wang, Z.L.; Tang, W. Regulating Contact-Electro-Catalysis Using Polymer/Metal Janus Composite Catalysts. J. Am. Chem. Soc. 2024, 146, 28110–28118. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.Z.; Chen, T.; Sun, D.J.; Zhang, D.S.; Li, C.L.; Li, R.; Zhang, J.; Ramakrishna, S.; Long, Y.Z. Rotation-mode liquid-solid triboelectric nanogenerator for efficient contact-electro-catalysis and adsorption. Nano Energy 2023, 110, 108329. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Liu, J.-H.; Liu, M.-N.; Yin, F.; Yu, Z.-C.; Li, M.-J.; Zhang, Y.; Zhang, H.-D.; Zhang, J.; Long, Y.-Z. Contact-electro-catalytic degradation of organic dyes based on solid-liquid-solid friction. Nano Energy 2024, 128, 109910. [Google Scholar] [CrossRef]
- Li, W.; Tu, J.; Sun, J.; Zhang, Y.; Fang, J.; Wang, M.; Liu, X.; Tian, Z.-Q.; Fan, F.R. Boosting Reactive Oxygen Species Generation via Contact-Electro-Catalysis with FeIII-Initiated Self-cycled Fenton System. Angew. Chem. Int. Ed. 2024, 64, e202413246. [Google Scholar] [CrossRef]



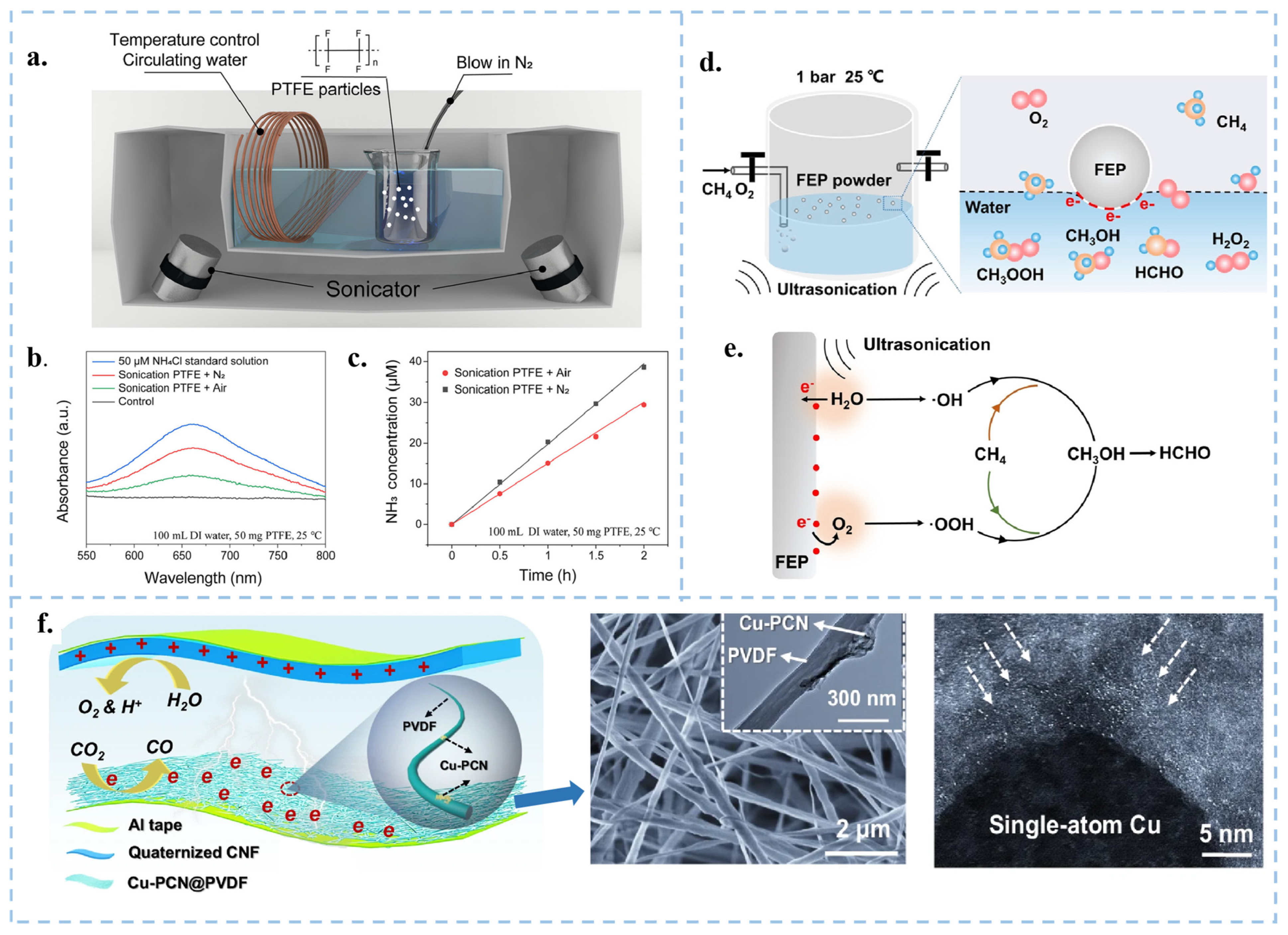
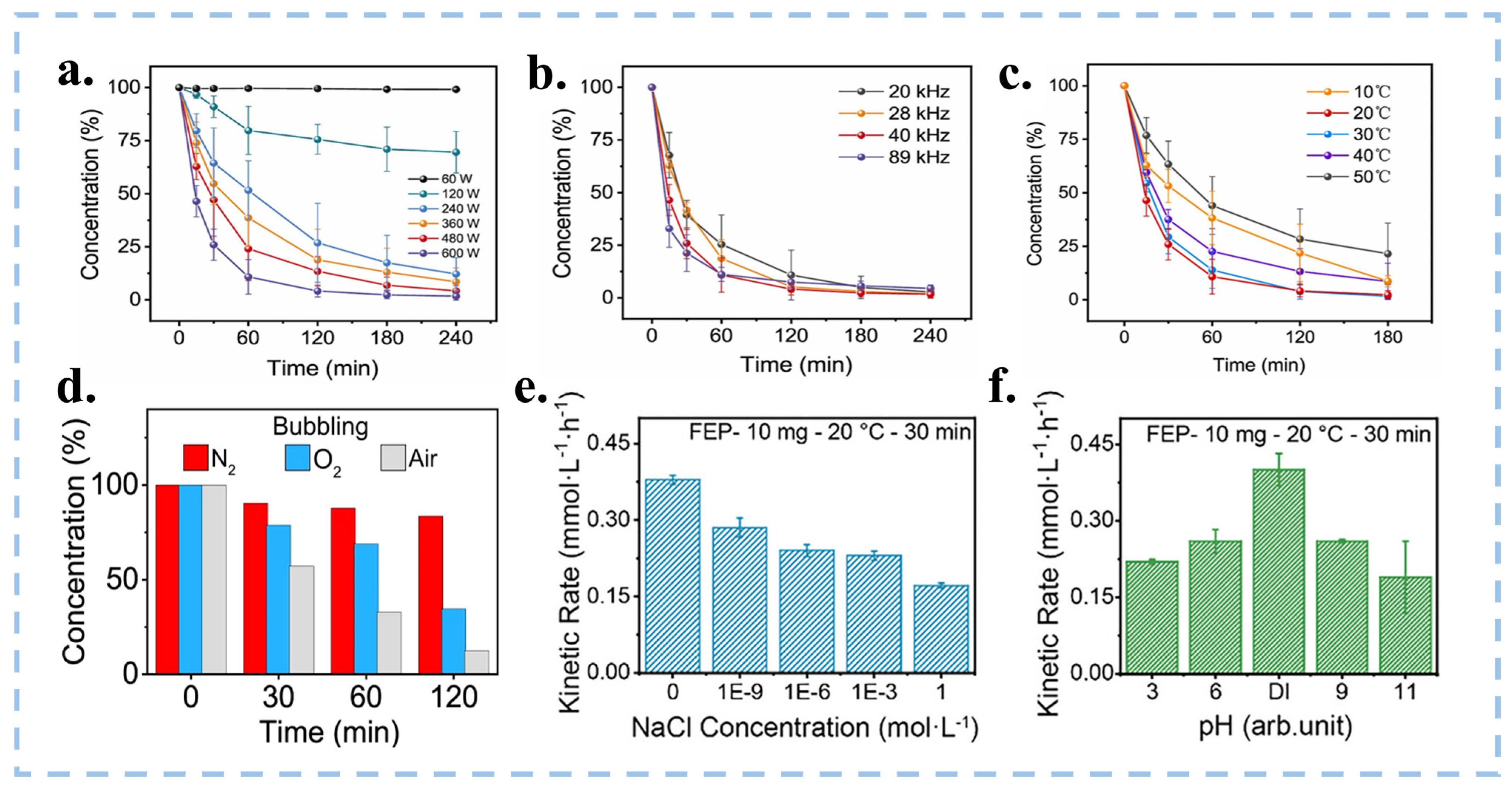
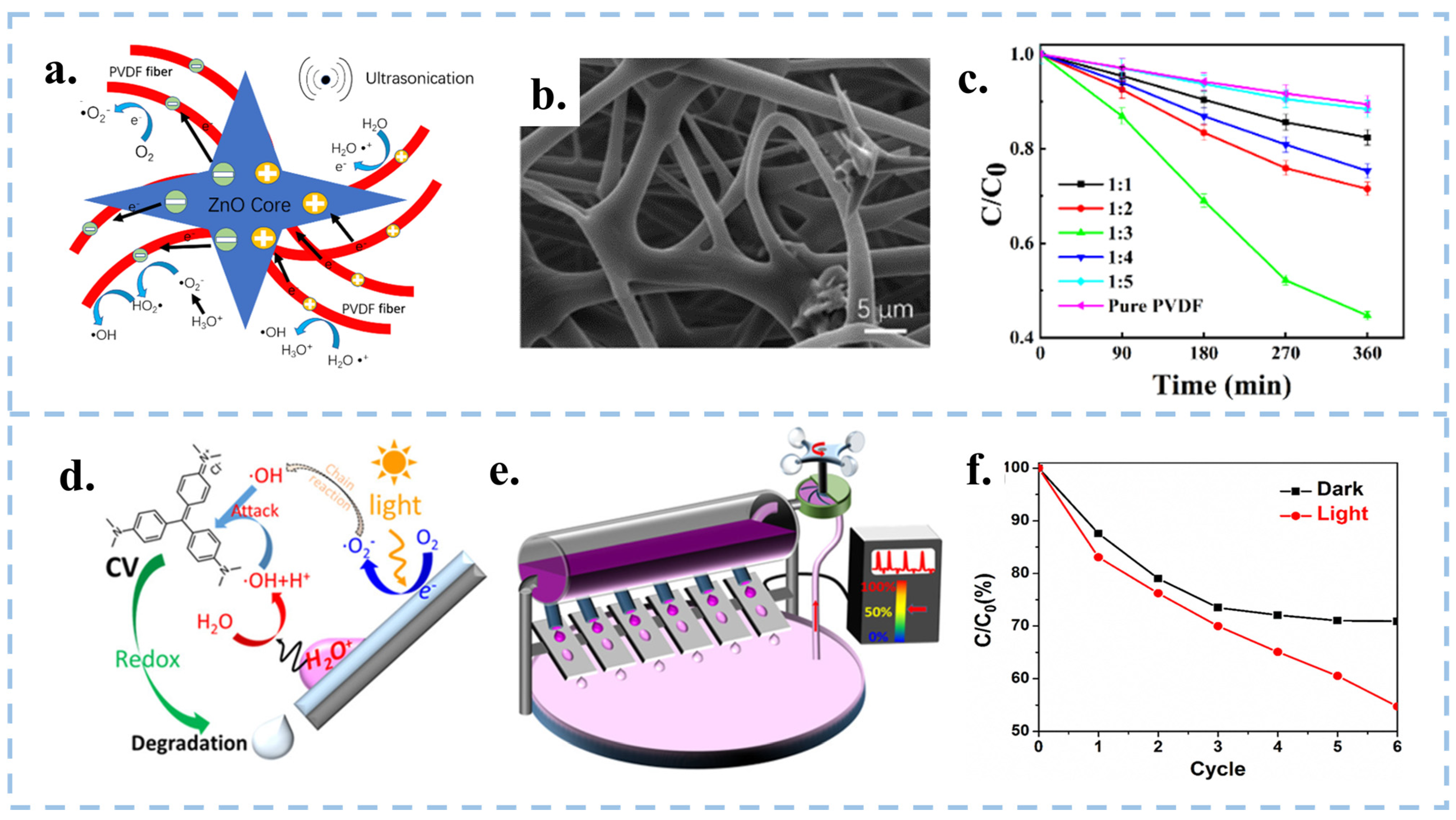
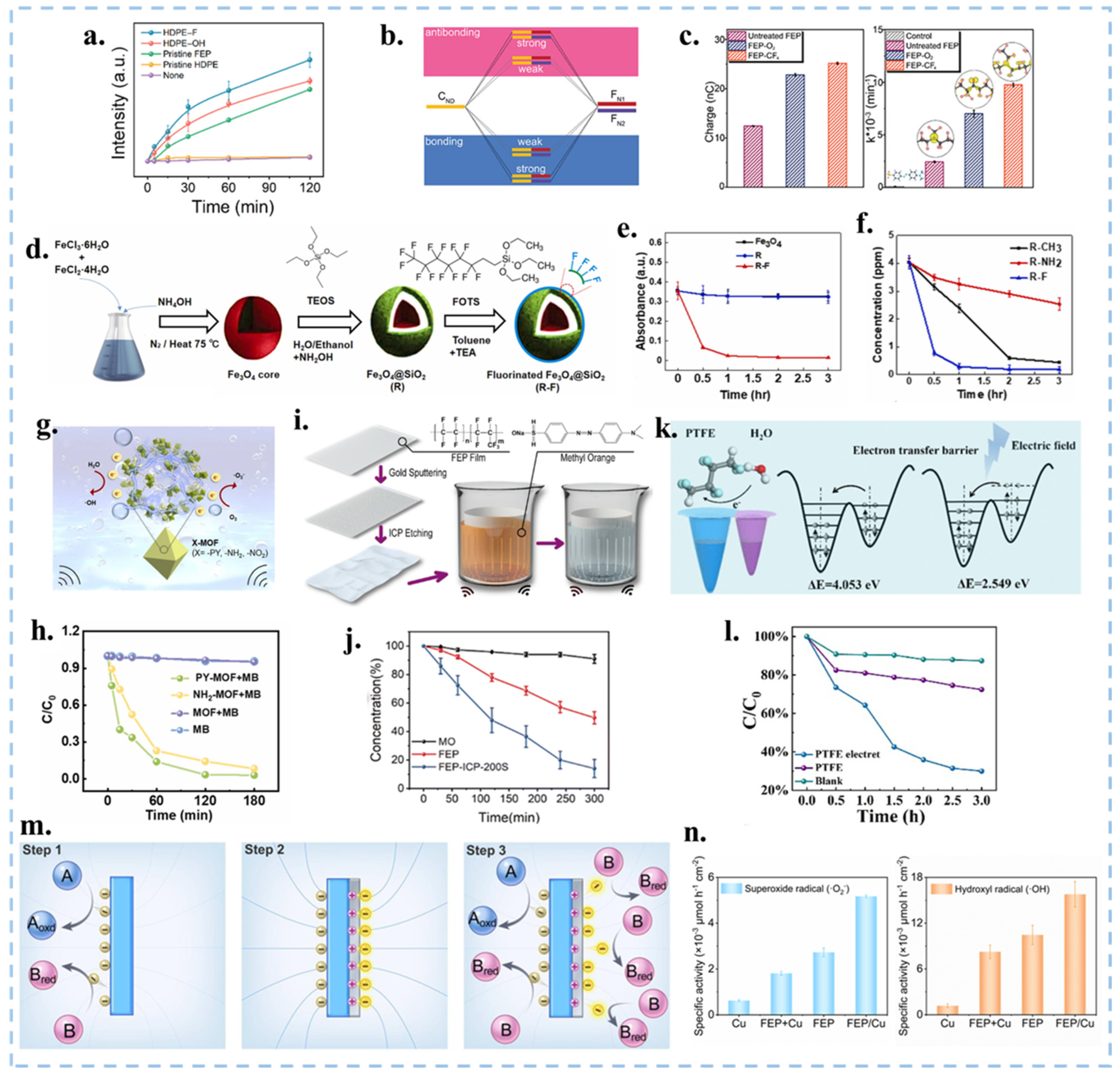

| Catalytic Mode | Characteristics | Advantages | Disadvantages |
|---|---|---|---|
| Electrocatalysis | Precious metal (Pt, Ru, Ir), and metal oxides, etc. [15,16]. | Good stability and catalytic activity | Need external electric field, high energy consumption |
| Photocatalysis | Metal oxide, metal sulfide and other semiconductor materials, etc. [13,14]. | High oxidation efficiency and low cost | Short life of catalyst, limited light-responsive wavelength range and needs light excitation |
| Piezoelectric Catalysis | A metal or semiconductor material having piezoelectric properties [77,78] | Environmental protection, without external excitation and light sources | Limited selection of materials |
| Tribocatalysis | Combination of semiconductor materials and high electronegativity materials [60] | Wide selection of materials | Low catalytic efficiency |
| Contact-electro-catalysis | Dielectric material with high electronegativity [28] | Wide selection of materials and applications | Need to explore types of catalysts |
| Catalysts | Catalyst Characteristics | Dye Species | Dye Concentration (mg/L) | Form Mechanical Action | Catalytic Activity | Ref. |
|---|---|---|---|---|---|---|
| FEP Powders | Contact-electro-catalysis | MO | 5 | Ultrasonication (120 W/40 kHz) | ~98.1%, 180 min | [17] |
| (CFx)n | Contact-electro-catalysis | MO | 5 | Ultrasonication (600 W/45 kHz) | ~95%, 180 min | [89] |
| PTFE | Contact-electro-catalysis | TET | 5 | Ultrasonication | ~90%, 90 min | [90] |
| ZnO/CuO | Photocatalysis | CV | 5 | UV–vis light irradiation | ~90%, 120 min | [107] |
| SnO2 | Photocatalysis | MB | 10 | 175W high-pressure mercury lamp irradiation (365 nm) | ~90%, 50 min | [108] |
| MoSe2 nanoflowers | Piezoelectric catalysis | RhB | 10 | Ultrasonication (250 W/40 kHz) | ~90%, 0.5 min | [109] |
| SrFeO3−x | Piezoelectric catalysis | TC | 30 | Ultrasonication (150 W/40 kHz) | ~96%, 75 min | [8] |
| CdS nanowires | Tribocatalysis | RhB | 5 | Stirring (300 rpm) | ~98%, 420 min | [110] |
| BaSrTiO3 nanoparticle | Tribocatalysis | RhB | 5 | Stirring (300 rpm) | ~99%, 180 min | [111] |
| TiO2 nanoparticles | Tribocatalysis | MB | 20 | Stirring (400 rpm) | ~94.7%, 180 min | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.-N.; Liu, J.-H.; Wang, L.-Y.; Yin, F.; Zheng, G.; Li, R.; Zhang, J.; Long, Y.-Z. Strategies for Improving Contact-Electro-Catalytic Efficiency: A Review. Nanomaterials 2025, 15, 386. https://doi.org/10.3390/nano15050386
Liu M-N, Liu J-H, Wang L-Y, Yin F, Zheng G, Li R, Zhang J, Long Y-Z. Strategies for Improving Contact-Electro-Catalytic Efficiency: A Review. Nanomaterials. 2025; 15(5):386. https://doi.org/10.3390/nano15050386
Chicago/Turabian StyleLiu, Meng-Nan, Jin-Hua Liu, Lu-Yao Wang, Fang Yin, Gang Zheng, Ru Li, Jun Zhang, and Yun-Ze Long. 2025. "Strategies for Improving Contact-Electro-Catalytic Efficiency: A Review" Nanomaterials 15, no. 5: 386. https://doi.org/10.3390/nano15050386
APA StyleLiu, M.-N., Liu, J.-H., Wang, L.-Y., Yin, F., Zheng, G., Li, R., Zhang, J., & Long, Y.-Z. (2025). Strategies for Improving Contact-Electro-Catalytic Efficiency: A Review. Nanomaterials, 15(5), 386. https://doi.org/10.3390/nano15050386







