Synthesis of Silicon Dioxide (SiO2) Nanowires via a Polyethylene Glycol-Based Emulsion Template Method in Isopropanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SiO2NWs
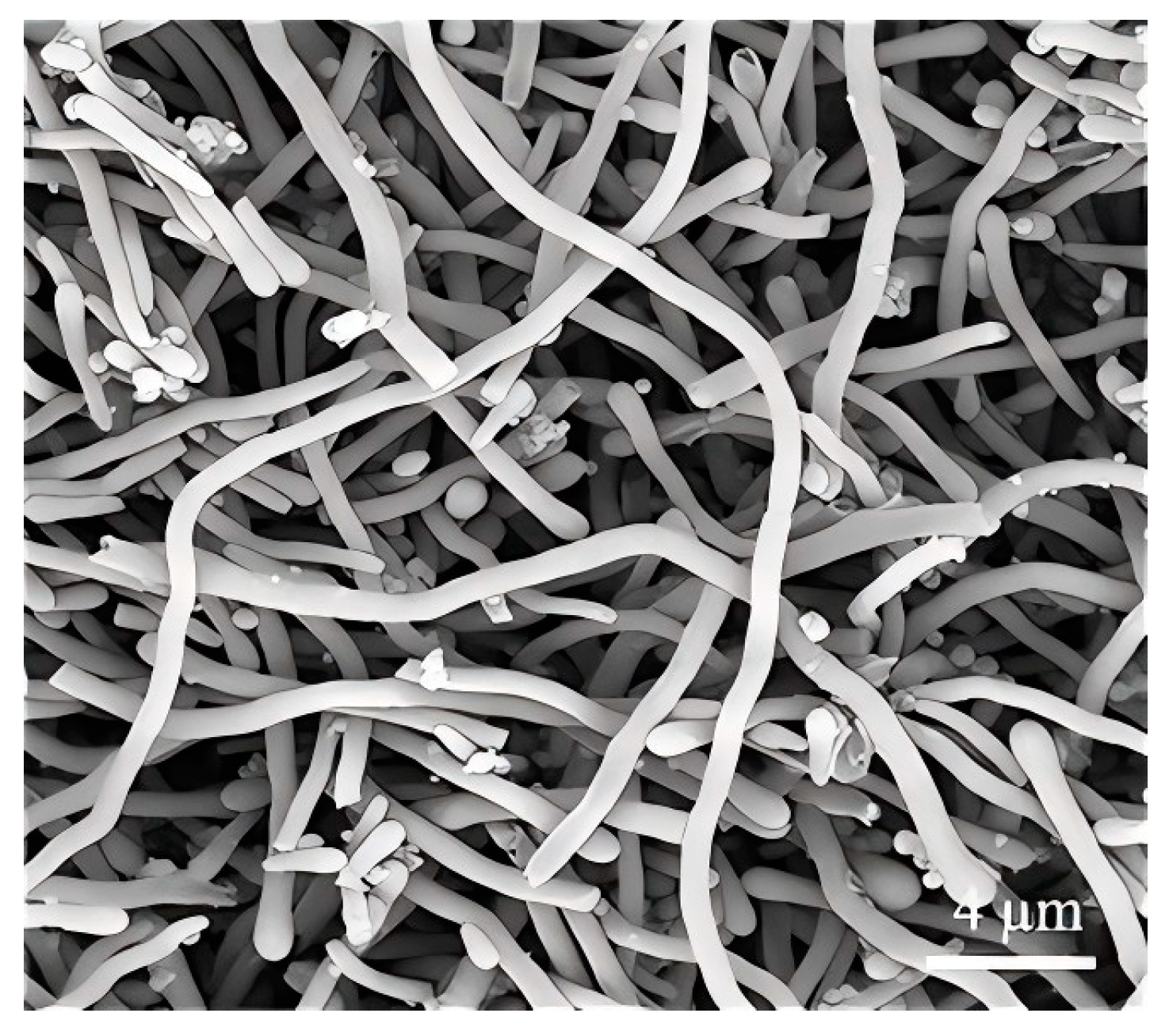
2.3. Scale-Up Experiment
2.4. Characterization
3. Results and Discussion
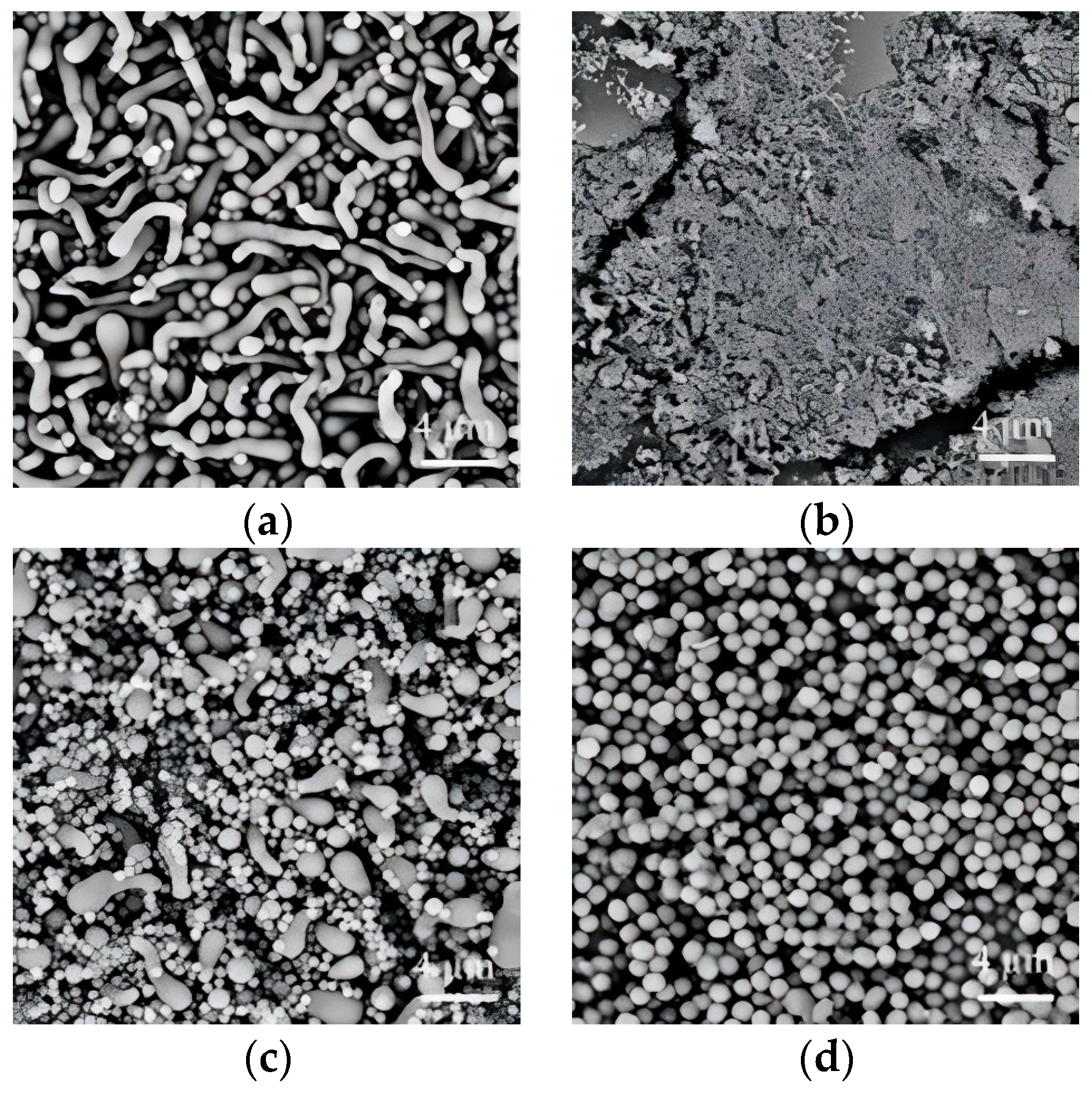
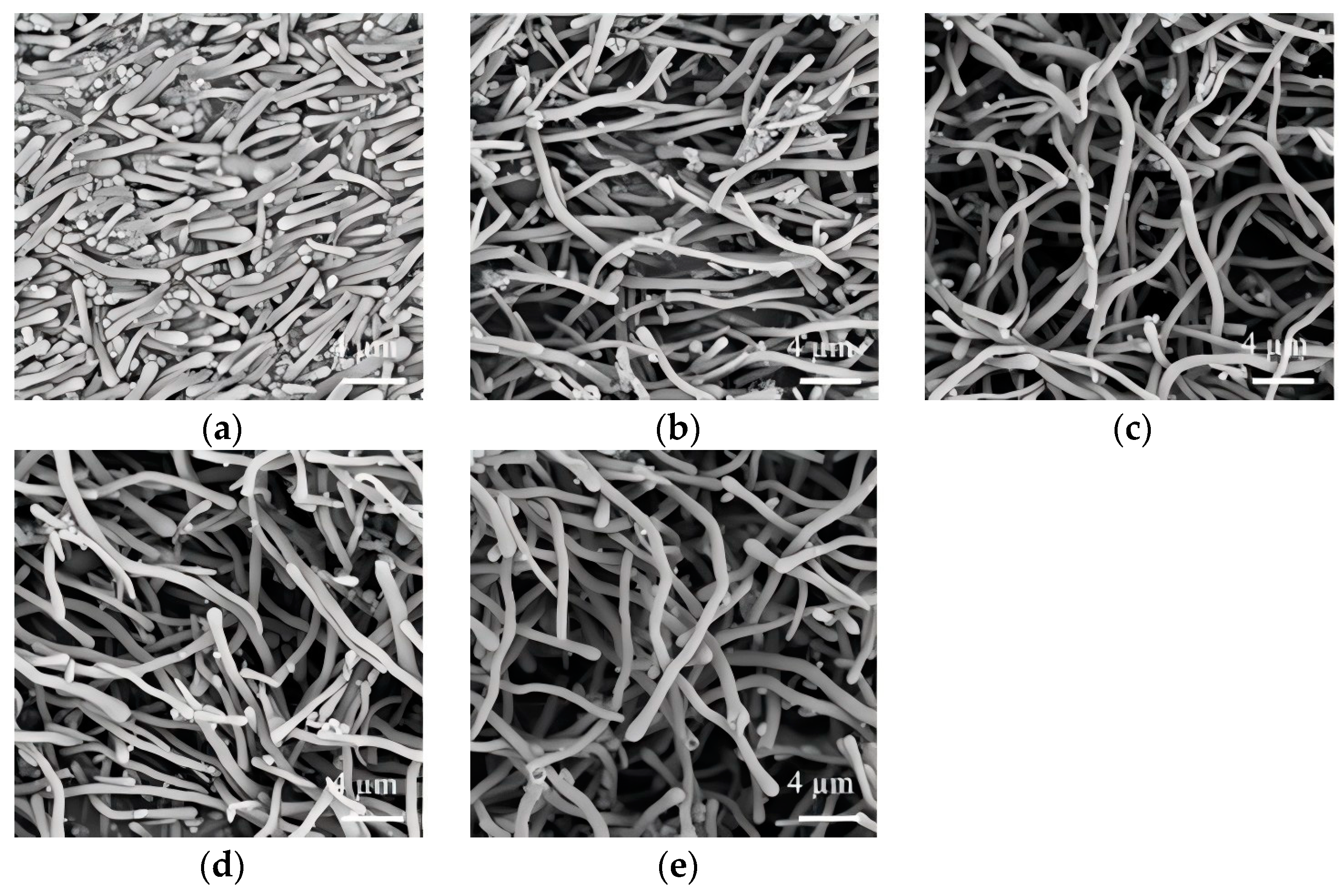
3.1. Effect of Solvent
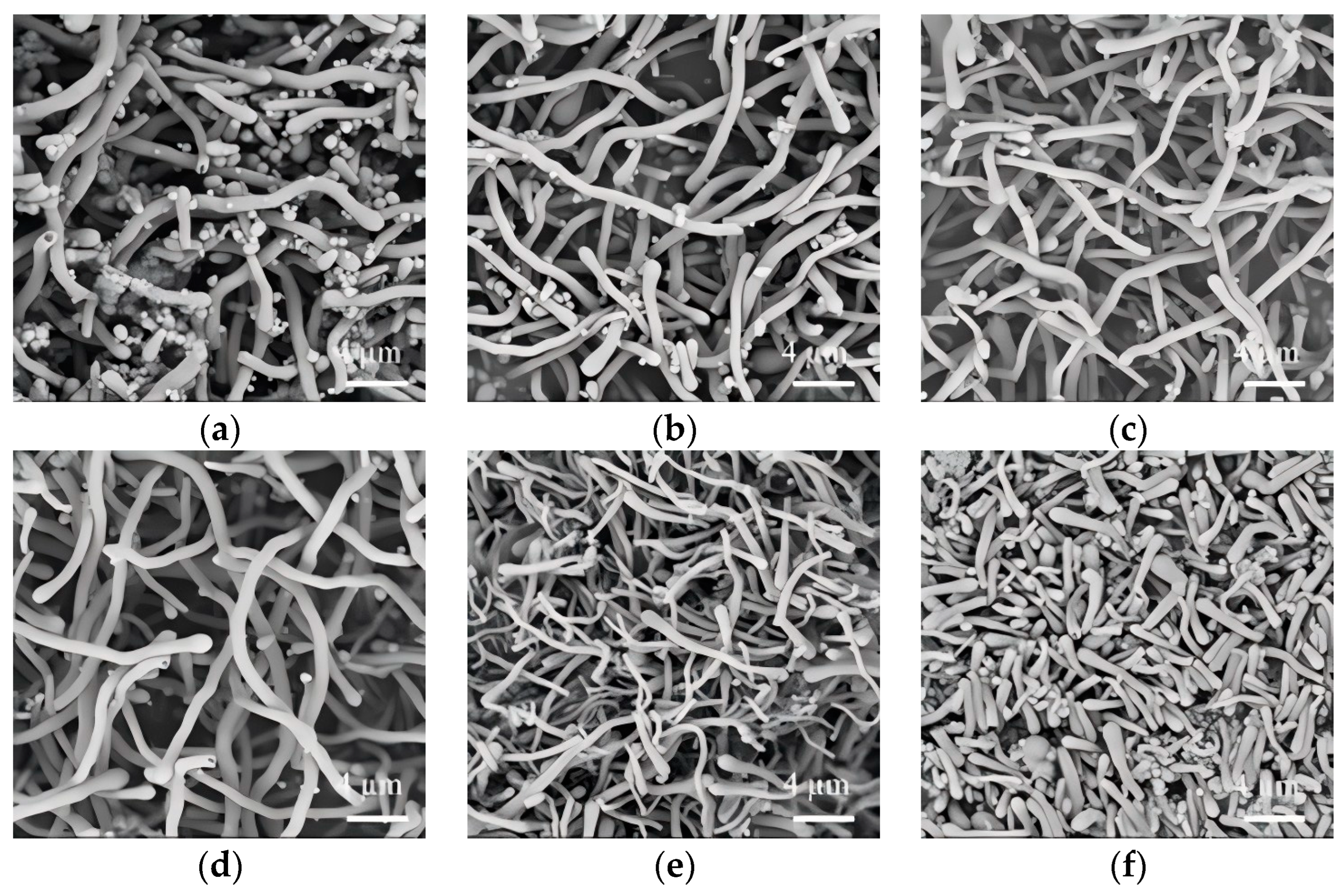
3.2. Effect of Molecular Weight and Dosage of PEG
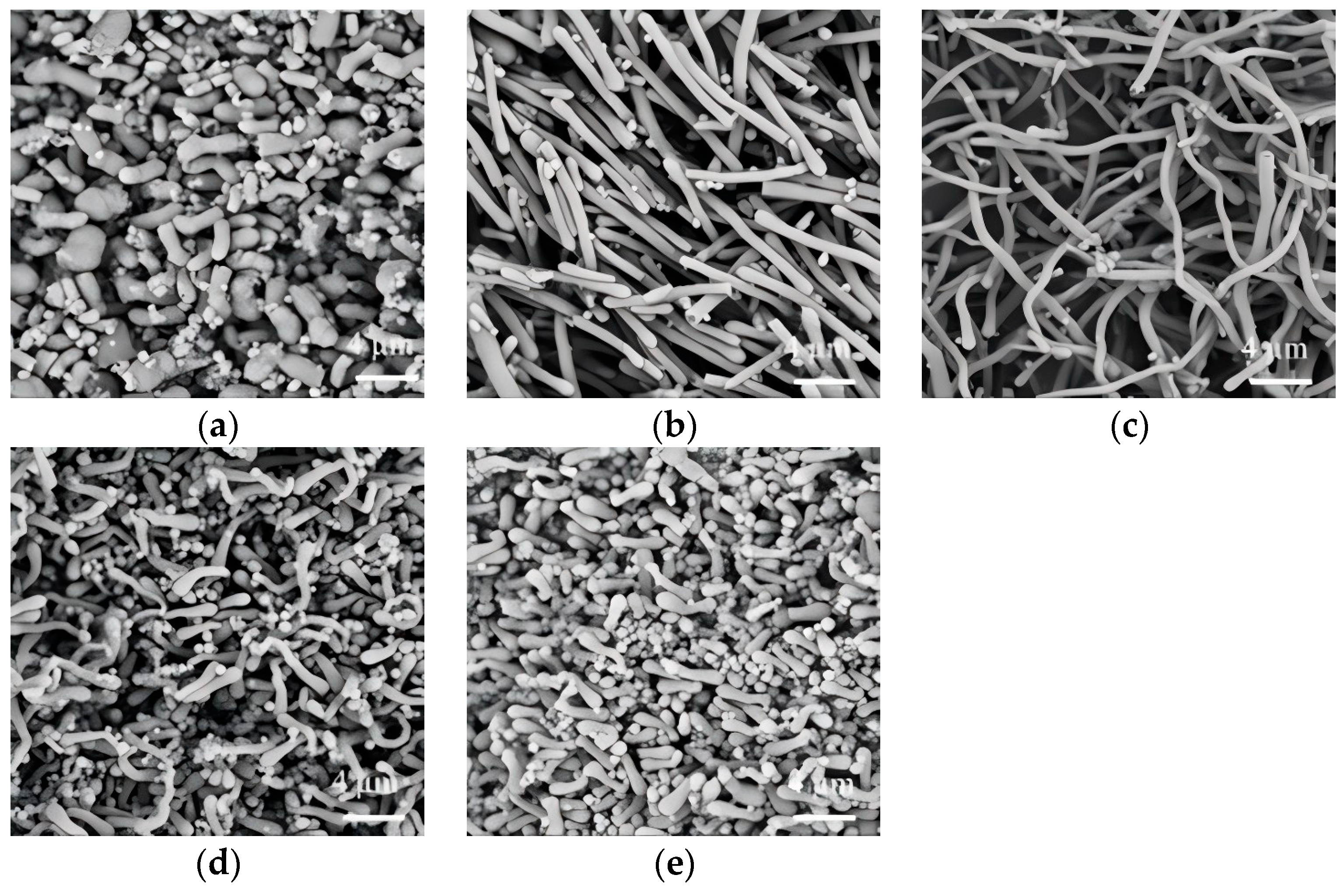
3.3. Effect of Sodium Citrate Dosage
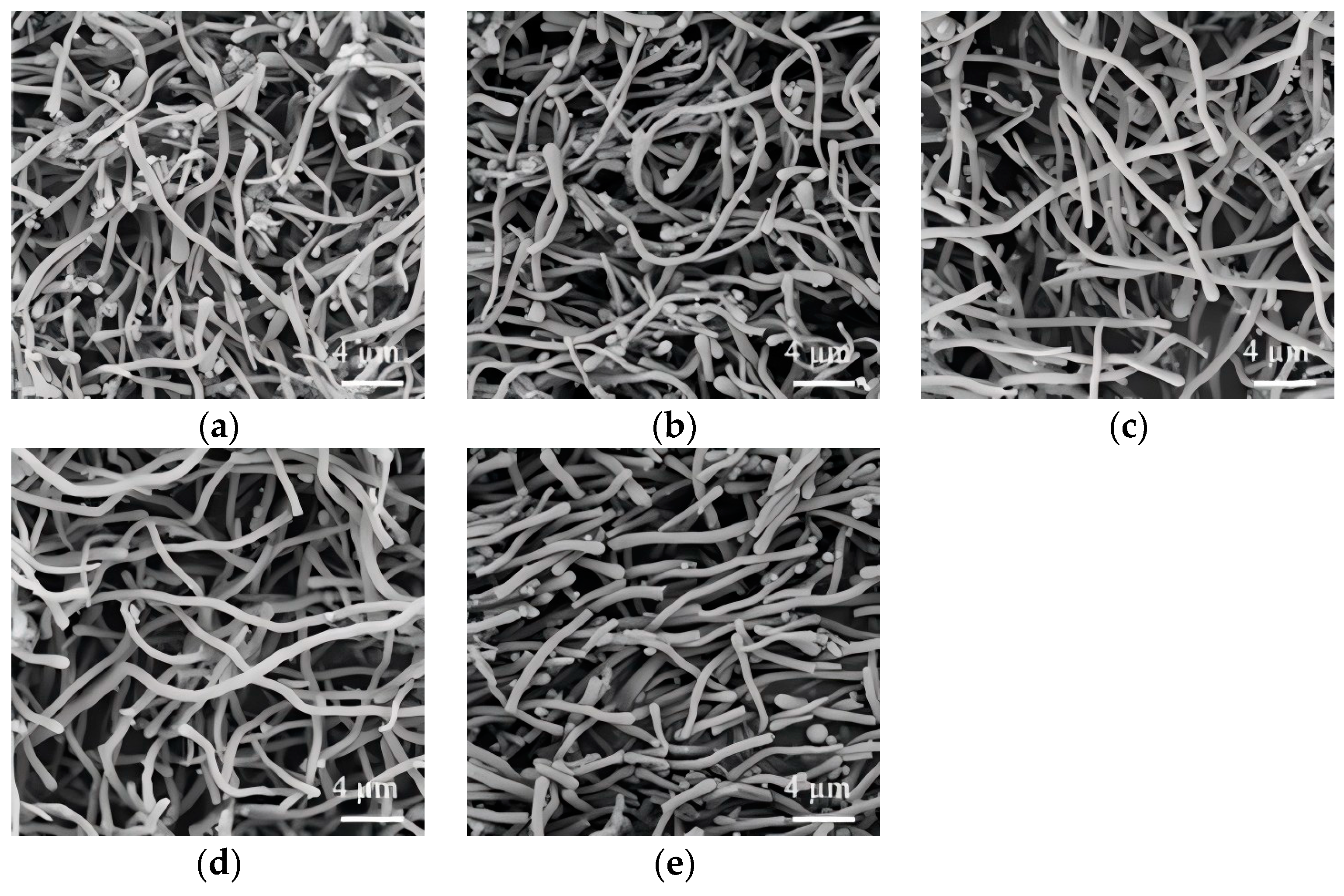
3.4. Effect of Ammonia Dosage
3.5. Effect of TEOS Dosage
3.6. Effect of Incubation Temperature and Time
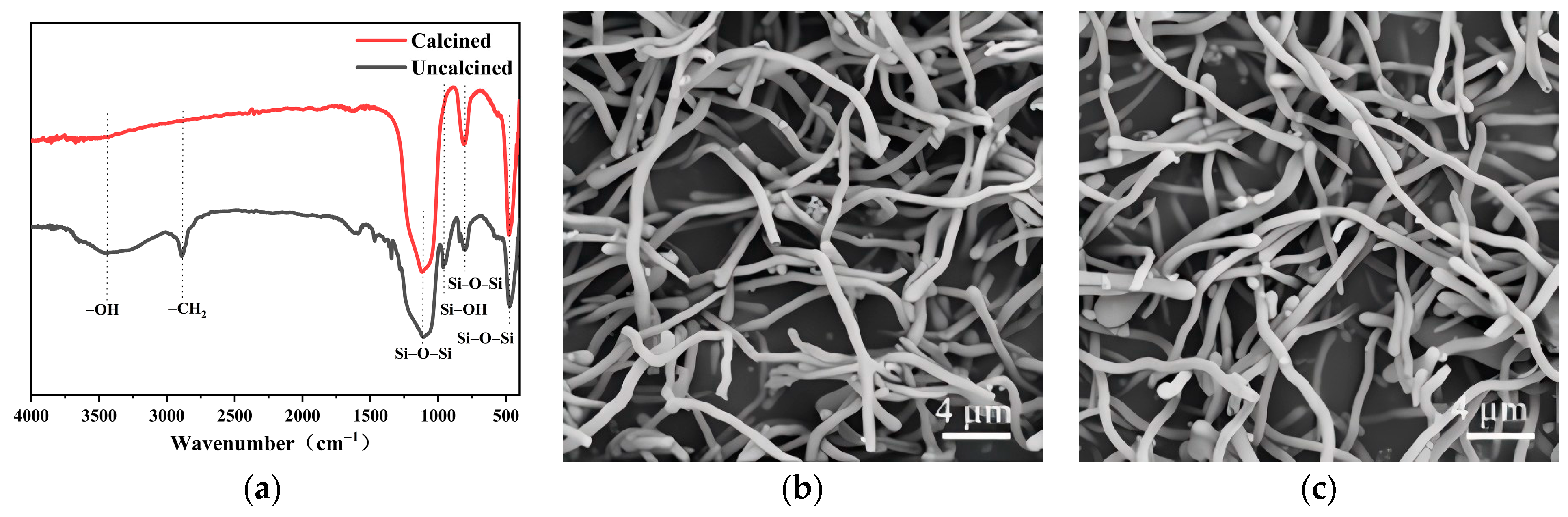
3.7. Calcination of Nanowires and Product from Scale-Up Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, B.; Deng, J.; Lu, B.; Xu, C.; Wang, Y.; Wan, J.; Chen, Y. Nanofabrication of silicon nanowires with high aspect ratio for photo-electron sensing. Microelectron. Eng. 2018, 195, 139–144. [Google Scholar] [CrossRef]
- Sreejith, S.; Ajayan, J.; Reddy, N.V.U.; Manikandan, M. Recent Advances and Prospects in Silicon Nanowire Sensors: A Critical Review. Silicon 2024, 16, 485–511. [Google Scholar] [CrossRef]
- Salhi, B.; Hossain, M.K.; Mukhaimer, A.W.; Al-Sulaiman, F.A. Nanowires: A New Pathway to Nanotechnology-Based Applications. J. Electroceram. 2016, 37, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.L.; Cui, H.; Wang, C.X. Synthesis and photoluminescence of ultralong amorphous SiO2 nanowires catalyzed by germanium. CrystEngComm 2011, 13, 4082. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Liu, D.; Nie, E.; Jiao, Z.; Jin, Y.; He, Y.; Gong, M.; Sun, X. Selective synthesis of SiO2 NWs on Si substrate and their adjustable photoluminescence. J. Non-Cryst. Solids 2010, 356, 2207–2210. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Rheima, A.M.; Abbas, Z.S. Nanowires Properties and Applications: A Review Study. S. Afr. J. Chem. Eng. 2023, 46, 286–311. [Google Scholar] [CrossRef]
- Liao, H.; Chen, H.; Zhou, F.; Zhang, Z. A novel SiO2 nanofiber-supported organic–inorganic gel polymer electrolyte for dendrite-free lithium metal batteries. J. Mater. Sci. 2020, 55, 9504–9515. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Ma, M.; Zhou, J.; Liu, Y.; Chen, Y. Preparation of SiO2 nanowire arrays as anode material with enhanced lithium storage performance. RSC Adv. 2018, 8, 33652–33658. [Google Scholar] [CrossRef] [PubMed]
- Slimani, Y.; Selmi, A.; Hannachi, E.; Almessiere, M.A.; AlFalah, G.; AlOusi, L.F.; Yasin, G.; Iqbal, M. Study on the addition of SiO2 nanowires to BaTiO3: Structure, morphology, electrical and dielectric properties. J. Phys. Chem. Solids 2021, 156, 110183. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Li, Z.; Ding, S.; Wang, Y.; Qiu, G.; Meng, A.; Li, Q. Ultralong SiC/SiO2 Nanowires: Simple Gram-Scale Production and Their Effective Blue-Violet Photoluminescence and Microwave Absorption Properties. ACS Sustain. Chem. Eng. 2018, 6, 3596–3603. [Google Scholar] [CrossRef]
- Tasleem, S.; Sabah, A.; Tahir, M.; Sabir, A.; Shabbir, A.; Nazir, M. Alkyl Silica Hybrid Nanowire Assembly in Improved Superhydrophobic Membranes for RO Filtration. ACS Omega 2022, 7, 3940–3948. [Google Scholar] [CrossRef]
- He, J.; Ma, C.; Yang, J.; Zou, X.; Sun, B.; Sun, Y.; Wang, C. Transparent ultrathin SiO2 nanowire aerogel displaying novel properties when interacting with water: A promising versatile functional platform. Fundam. Res. 2023, 3, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Fang, M.; Liu, H. Growth, structure, and luminescence properties of novel silica nanowires and interconnected nanorings. Sci. Rep. 2017, 7, 10482. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wu, Y.; Yang, P.; Liu, J. Synthesis of Ultra-Long and Highly Oriented Silicon Oxide Nanowires from Liquid Alloys. Adv. Mater. 2002, 14, 122–124. [Google Scholar] [CrossRef]
- An, G.H.; Jeong, S.Y.; Seong, T.Y.; Ahn, H.J. One-pot fabrication of hollow SiO2 nanowires via an electrospinning technique. Mater. Lett. 2011, 65, 2377–2380. [Google Scholar] [CrossRef]
- Liu, S.; Shan, H.; Xia, S.; Yan, J.; Yu, J.; Ding, B. Polymer template synthesis of flexible SiO2 nanofibers to upgrade composite electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 31439–31447. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Y.; Li, Z.; Zhang, J.; Shen, T.; Chen, Y.; Yang, D.; Sacher, E. Synthesis of amorphous SiO2 nanowires by one-step low temperature hydrothermal process. Mater. Res. Express 2019, 6, 115202. [Google Scholar] [CrossRef]
- Bathla, A.; Narula, C.; Chauhan, R.P. Hydrothermal synthesis and characterization of silica nanowires using rice husk ash: An agricultural waste. J. Mater. Sci. Mater. Electron. 2018, 29, 6225–6231. [Google Scholar] [CrossRef]
- Li, H.; Guan, L.; Xu, Z.; Zhao, Y.; Sun, J.; Wu, J.; Xu, N. Synthesis and characterization of amorphous SiO2 nanowires via pulsed laser deposition accompanied by N2 annealing. Appl. Surf. Sci. 2016, 389, 705–712. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, X.; Huang, F.; Zhang, Z.; Muhammad, J.; Li, X.; Rong, Z.; Guo, X.; Jung, Y.; Dong, X. Instantaneous vapor-liquid-solid growth of amorphous SiO2 nanowires within a local-equilibrium plasma and the optimized lithiation/delithiation activity. Appl. Surf. Sci. 2021, 557, 149848. [Google Scholar] [CrossRef]
- Lv, H.; Yan, T.; Yang, X.; Zhao, J.; Liu, S.; Chen, S.; Wang, Q.; Zhu, G.; Lu, X.; Liu, B.; et al. Preparation and characterization of SiO2 nanowires using a SnO2 catalyst. Phys. Lett. A 2020, 384, 126174. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, H.; Cao, K.; Song, H.; Sheng, W.; Wu, Q. Templated-assisted one-dimensional silica-nanotubes: Synthesis and applications. J. Mater. Chem. 2011, 21, 6122–6135. [Google Scholar] [CrossRef]
- Ren, X.; Lun, Z. Mesoporous Silica Nanowires Synthesized by Electrodeposition in AAO. Mater. Lett. 2012, 68, 228–229. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, H.; Yang, R.; Li, X.; Qi, L. Morphology-Controlled Synthesis of SnO2 Nanotubes by Using 1D Silica Mesostructures as Sacrificial Templates and Their Applications in Lithium-Ion Batteries. Small 2010, 6, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, L.; Ravichandran, A.; Ballamurugan, A.M. Organic and Inorganic Template-Assisted Synthesis of Silica Nanotubes and Evaluation of Their Properties. Appl. Biochem. Biotechnol. 2022, 194, 167–175. [Google Scholar] [CrossRef] [PubMed]
- AlOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Sharma, J. Finite-Sized One-Dimensional Silica Microstructures (Rods): Synthesis, Assembly, and Applications. ChemNanoMat 2017, 3, 214–222. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Zhao, S. Anisotropic Colloids: From Non-Templated Synthesis to Patchy Templated Synthesis. Chem. Eur. J. 2018, 24, 10562–10570. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Wang, Z.; Ming, N. Au-Induced Polyvinylpyrrolidone Aggregates with Bound Water for the Highly Shape-Selective Synthesis of Silica Nanostructures. Chem. Eur. J. 2008, 14, 4374–4380. [Google Scholar] [CrossRef] [PubMed]
- Kuijk, A.; van Blaaderen, A.; Imhof, A. Synthesis of Monodisperse, Rodlike Silica Colloids with Tunable Aspect Ratio. J. Am. Chem. Soc. 2011, 133, 2346–2349. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, B.; Long, Y.; Zheng, L.; Tung, C.-H.; Song, K. New Pickering Emulsions Stabilized by Silica Nanowires. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 639–646. [Google Scholar] [CrossRef]
- He, J.; Yu, B.; Hourwitz, M.J.; Liu, Y.; Perez, M.T.; Yang, J.; Nie, Z. Wet-Chemical Synthesis of Amphiphilic Rodlike Silica Particles and Their Molecular Mimetic Assembly in Selective Solvents. Angew. Chem. Int. Ed. 2012, 51, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Xu, C.; Tang, R.; Zhang, X.; Caruso, F.; Wang, Y. Synthesis of Discrete Alkyl-Silica Hybrid Nanowires and Their Assembly into Nanostructured Superhydrophobic Membranes. Angew. Chem. Int. Ed. 2016, 55, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Hagemans, F.; Pujala, R.K.; Hotie, D.S.; Thies-Weesie, D.M.E.; de Winter, D.A.M.; Meeldijk, J.D.; van Blaaderen, A.; Imhof, A. Shaping Silica Rods by Tuning Hydrolysis and Condensation of Silica Precursors. Chem. Mater. 2019, 31, 521–531. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, K.; Zhang, J.; Liu, M.; Liu, Y.; Cheng, C. Synthesis of anisotropic silica colloids. RSC Adv. 2017, 7, 37542. [Google Scholar] [CrossRef]
- Nakashima, Y.; Fukushima, M.; Hyuga, H. Fiber Template Approach toward Preparing One-Dimensional Silica Nanostructures with Rough Surface. Adv. Powder Technol. 2021, 32, 1099–1105. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Sun, Y.; Yang, T. Synthesis of Silicon Dioxide (SiO2) Nanowires via a Polyethylene Glycol-Based Emulsion Template Method in Isopropanol. Nanomaterials 2025, 15, 326. https://doi.org/10.3390/nano15050326
Liu J, Sun Y, Yang T. Synthesis of Silicon Dioxide (SiO2) Nanowires via a Polyethylene Glycol-Based Emulsion Template Method in Isopropanol. Nanomaterials. 2025; 15(5):326. https://doi.org/10.3390/nano15050326
Chicago/Turabian StyleLiu, Jian, Yonghua Sun, and Tianfeng Yang. 2025. "Synthesis of Silicon Dioxide (SiO2) Nanowires via a Polyethylene Glycol-Based Emulsion Template Method in Isopropanol" Nanomaterials 15, no. 5: 326. https://doi.org/10.3390/nano15050326
APA StyleLiu, J., Sun, Y., & Yang, T. (2025). Synthesis of Silicon Dioxide (SiO2) Nanowires via a Polyethylene Glycol-Based Emulsion Template Method in Isopropanol. Nanomaterials, 15(5), 326. https://doi.org/10.3390/nano15050326



