Ion Substitution-Induced Distorted MOF Lattice with Deviated Energy and Dielectric Properties for Quasi-Solid-State Ion Conductor
Abstract
1. Introduction
2. Experimental
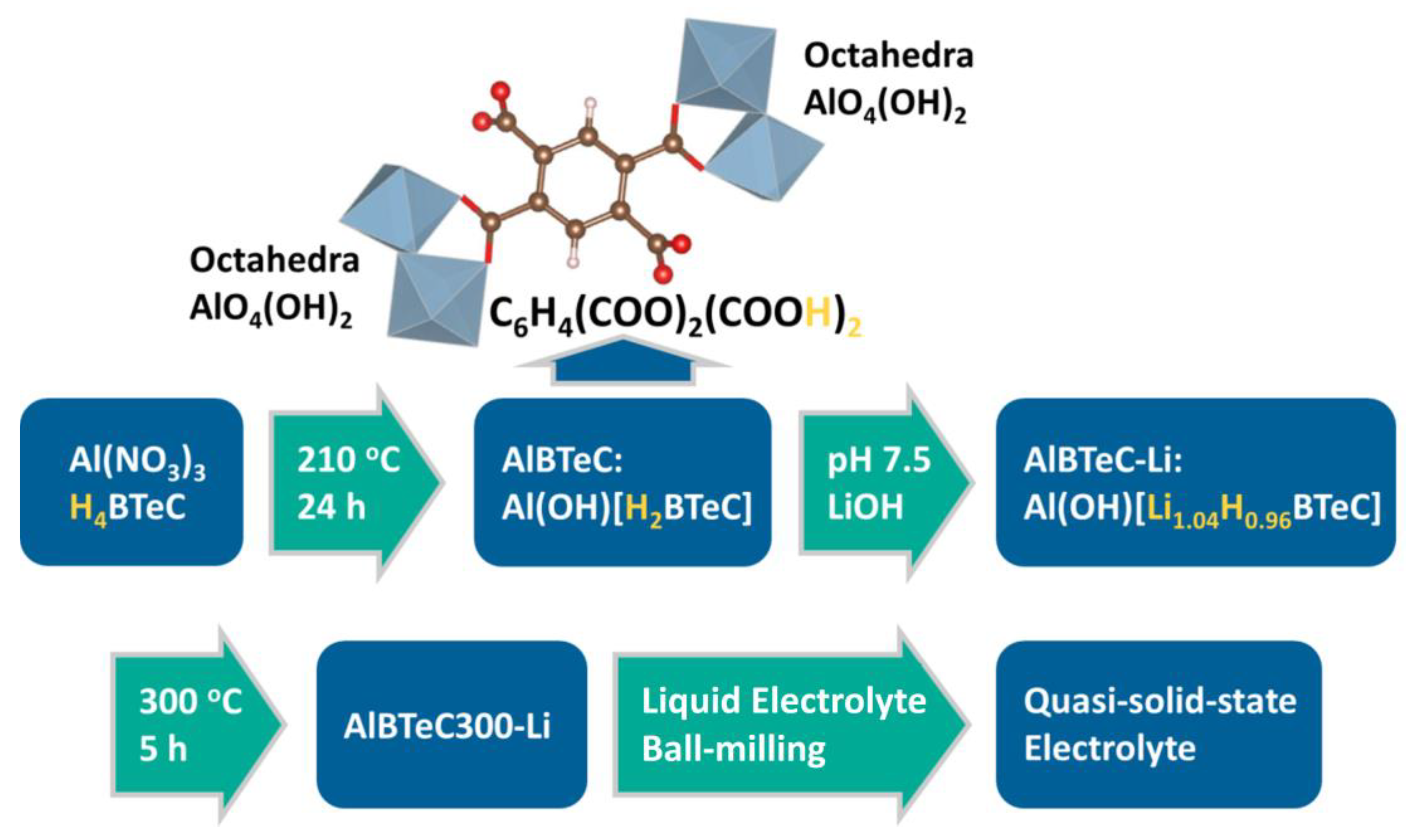
2.1. Synthesis
2.2. Electrochemical Measurements
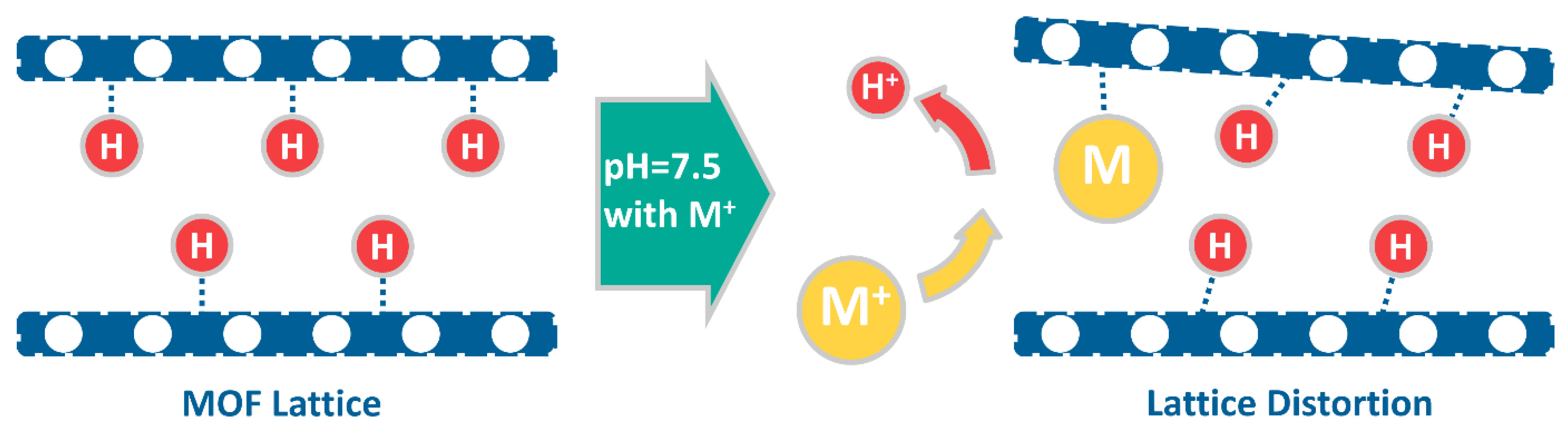
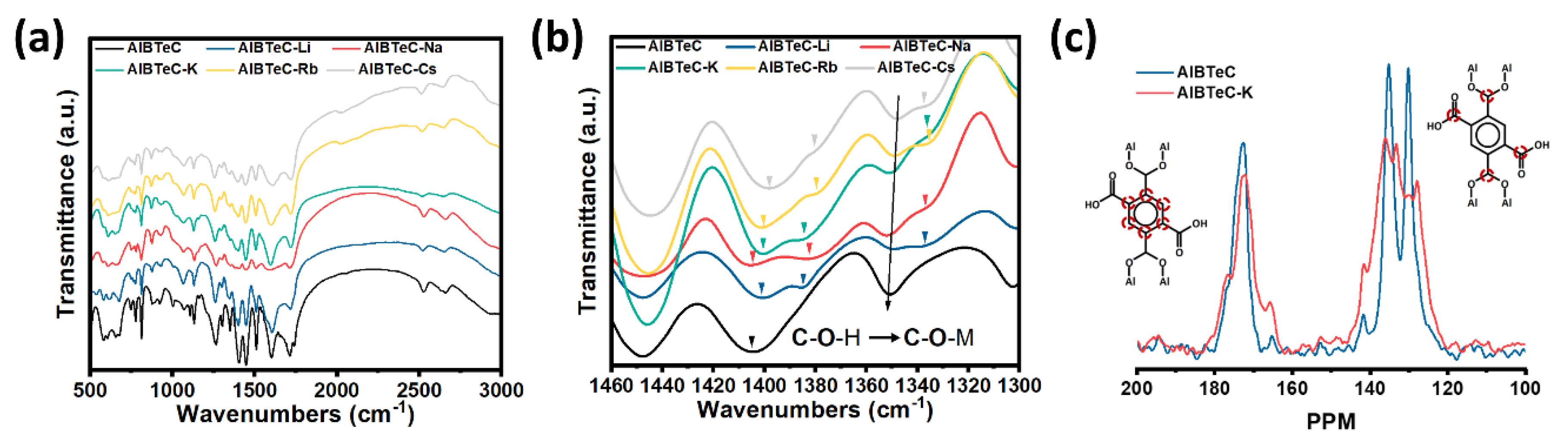
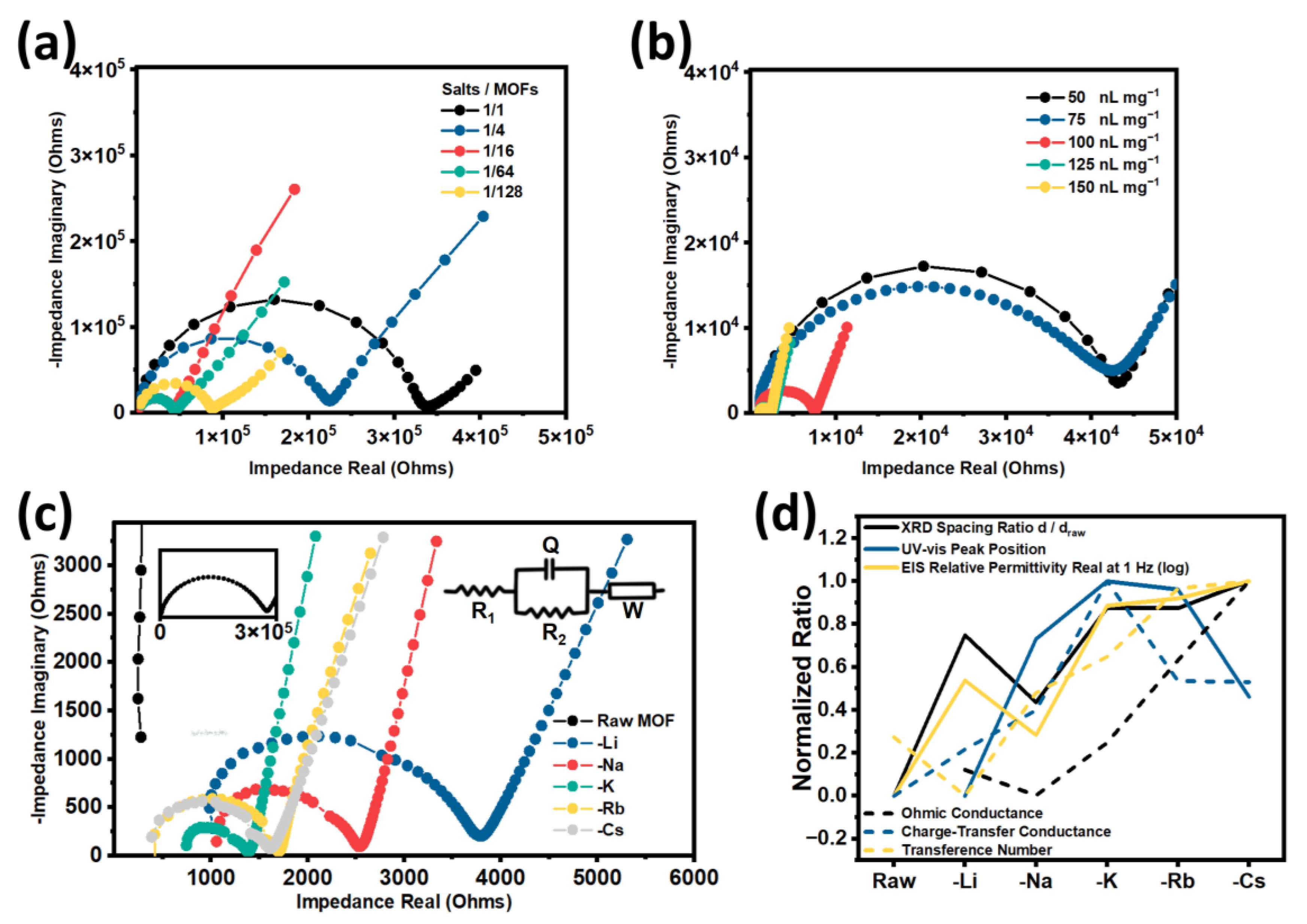
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Zhao, C.L.; Liu, L.L.; Qi, X.G.; Lu, Y.X.; Wu, F.X.; Zhao, J.M.; Yu, Y.; Hu, Y.S.; Chen, L.Q. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 201703012. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Liu, H.; Zhao, C.Z.; Lu, Y.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. Unlocking the Failure Mechanism of Solid State Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2100748. [Google Scholar] [CrossRef]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-state batteries: The critical role of mechanics. Science 2023, 381, 1300. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, F.; Martinez-Ibañez, M.; Feng, W.; Forsyth, M.; Zhou, Z.; Armand, M.; Zhang, H. A reflection on polymer electrolytes for solid-state lithium metal batteries. Nat. Commun. 2023, 14, 4884. [Google Scholar] [CrossRef]
- Ji, X.; Hou, S.Y.; Wang, P.F.; He, X.Z.; Piao, N.; Chen, J.; Fan, X.L.; Wang, C.S. Solid-State Electrolyte Design for Lithium Dendrite Suppression. Adv. Mater. 2020, 32, e202002741. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.T.; Chen, S.M.; Li, N.; Liu, Z.X.; Tang, Z.J.; Zapien, J.A.; Chen, S.M.; Fan, J.; Zhi, C.Y. Hydrogen-Free and Dendrite-Free All-Solid-State Zn-Ion Batteries. Adv. Mater. 2020, 32, e1908121. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Roddatis, V.; Chandran, C.V.; Ma, Q.L.; Uhlenbruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Guan, S.D.; Ma, C.; Li, L.L.; Nan, C.W. Role of Interfaces in Solid-State Batteries. Adv. Mater. 2023, 35, e2206402. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Shi, Y.; Yang, Y.; Hou, Y.; Wu, Y. A Composite Gel Polymer Electrolyte with High Performance Based on Poly(Vinylidene Fluoride) and Polyborate for Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300647. [Google Scholar] [CrossRef]
- Kang, J.B.; Deng, N.P.; Cheng, B.W.; Kang, W.M. Progress in the application of polymer fibers in solid electrolytes for lithium metal batteries. J. Energy Chem. 2024, 92, 26–42. [Google Scholar] [CrossRef]
- Martinez-Juarez, A.; Pecharroman, C.; Iglesias, J.E.; Rojo, J.M. Relationship between activation energy and bottleneck size for Li+ ion conduction in NASICON materials of composition LiMM′(PO4)3; M, M′ = Ge, Ti, Sn, Hf. J. Phys. Chem. B 1998, 102, 372–375. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 856. [Google Scholar] [CrossRef]
- Wang, Q.D.; Zhou, Y.N.; Wang, X.L.; Guo, H.; Gong, S.P.; Yao, Z.P.; Wu, F.T.; Wang, J.L.; Ganapathy, S.; Bai, X.D.; et al. Designing lithium halide solid electrolytes. Nat. Commun. 2024, 15, 1–10. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Li6ALa2Ta2O12 (A = Sr, Ba): Novel Garnet-Like Oxides for Fast Lithium Ion Conduction. Adv. Funct. Mater. 2005, 15, 107–112. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Archer, L.A.; Koch, D.L. Stabilizing electrodeposition in elastic solid electrolytes containing immobilized anions. Sci. Adv. 2016, 2, e1600320. [Google Scholar] [CrossRef]
- Lu, Q.; He, Y.-B.; Yu, Q.; Li, B.; Kaneti, Y.V.; Yao, Y.; Kang, F.; Yang, Q.-H. Dendrite-Free, High-Rate, Long-Life Lithium Metal Batteries with a 3D Cross-Linked Network Polymer Electrolyte. Adv. Mater. 2017, 29, 1604460. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Guo, J.P.; Ning, D.; Huang, Y.K.; Lei, W.; Li, J.; Li, J.D.; Schuck, G.; Shen, J.J.; Guo, Y.; et al. Design of metal-organic frameworks for improving pseudo-solid-state magnesium-ion electrolytes: Open metal sites, isoreticular expansion, and framework topology. J. Mater. Sci. Technol. 2023, 144, 15–27. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Xia, Q.; Li, X.; Liu, B.; Hu, T.; Tebyetekerwa, M.; Hu, S.; Knibbe, R.; Chou, S. Confining Polymer Electrolyte in MOF for Safe and High-Performance All-Solid-State Sodium Metal Batteries. Angew. Chem. Int. Ed. 2024, 63, e202318822. [Google Scholar] [CrossRef]
- Zettl, R.; Lunghammer, S.; Gadermaier, B.; Boulaoued, A.; Johansson, P.; Wilkening, H.M.R.; Hanzu, I. High Li+ and Na+ Conductivity in New Hybrid Solid Electrolytes based on the Porous MIL-121 Metal Organic Framework. Adv. Energy Mater. 2021, 11, 2003542. [Google Scholar] [CrossRef]
- Jiang, S.; Lv, T.T.; Peng, Y.; Pang, H. MOFs Containing Solid-State Electrolytes for Batteries. Adv. Sci. 2023, 10, 202206887. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Xie, X.X.; Zhang, P.; Li, X.H.; Wang, Z.X.; Qin, X.; Shao, M.H.; Zhang, L.Q.; Zhou, W.D. Rational Design of F-Modified Polyester Electrolytes for Sustainable All-Solid-State Lithium Metal Batteries. J. Am. Chem. Soc. 2024, 146, 5940–5951. [Google Scholar] [CrossRef]
- Nguyen, A.G.; Park, C.J. Insights into tailoring composite solid polymer electrolytes for solid-state lithium batteries. J. Membr. Sci. 2023, 675, 121552. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Gu, T.; Rui, G.; Shi, P.; Fu, W.; Chen, L.; Liu, X.; Zeng, J.; Kang, B.; Yan, Z.; et al. A relaxor ferroelectric polymer with an ultrahigh dielectric constant largely promotes the dissociation of lithium salts to achieve high ionic conductivity. Energy Environ. Sci. 2021, 14, 6021–6029. [Google Scholar] [CrossRef]
- Wang, D.; Xie, H.; Liu, Q.; Mu, K.; Song, Z.; Xu, W.; Tian, L.; Zhu, C.; Xu, J. Low-Cost, High-Strength Cellulose-based Quasi-Solid Polymer Electrolyte for Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302767. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, H.; Tang, Q.; Peng, H.; Huang, J.; He, H.; Zhang, H.; Yang, W. Coaxially MXene-confined solid-state electrolyte for flexible high-rate lithium metal battery. Nano Energy 2024, 122, 109312. [Google Scholar] [CrossRef]
- Volkringer, C.; Loiseau, T.; Guillou, N.; Férey, G.; Haouas, M.; Taulelle, F.; Elkaim, E.; Stock, N. High-Throughput Aided Synthesis of the Porous Metal−Organic Framework-Type Aluminum Pyromellitate, MIL-121, with Extra Carboxylic Acid Functionalization. Inorg. Chem. 2010, 49, 9852–9862. [Google Scholar] [CrossRef]
- Siddique, A.B.; Shaheen, M.A.; Abbas, A.; Zaman, Y.; Bratty, M.A.; Najmi, A.; Hanbashi, A.; Mustaqeem, M.; Alhazmi, H.A.; Rehman, Z.u.; et al. Thermodynamic and kinetic insights into azo dyes photocatalytic degradation on biogenically synthesized ZnO nanoparticles and their antibacterial potential. Heliyon 2024, 10, e40679. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Zeng, J.-P.; Li, S.-F.; Dai, C.; Liu, J.-F.; Liu, C.; He, Y.-B. Conformational Regulation of Dielectric Poly(Vinylidene Fluoride)-Based Solid-State Electrolytes for Efficient Lithium Salt Dissociation and Lithium-Ion Transportation. Adv. Energy Mater. 2023, 13, 2203888. [Google Scholar] [CrossRef]
- Shi, P.R.; Ma, J.B.; Liu, M.; Guo, S.K.; Huang, Y.F.; Wang, S.W.; Zhang, L.H.; Chen, L.K.; Yang, K.; Liu, X.T.; et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries. Nat. Nanotechnol. 2023, 18, 602. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Zhu, X.; Lin, D.; Li, Q.; Liu, W.; Xu, Z. Colossal dielectric permittivity in hydrogen-reduced rutile TiO2 crystals. J. Alloy. Compd. 2017, 692, 375–380. [Google Scholar] [CrossRef]
- Boutoumit, A.; Elhawary, M.; Bellaouchou, A.; Boudalia, M.; Hammani, O.; José Garcia, A.; Amin, H.M.A. Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl. Coatings 2024, 14, 783. [Google Scholar] [CrossRef]
- Najem, A.; Campos, O.S.; Girst, G.; Raji, M.; Hunyadi, A.; García-Antón, J.; Bellaouchou, A.; Amin, H.M.A.; Boudalia, M. Experimental and DFT Atomistic Insights into the Mechanism of Corrosion Protection of Low-Carbon Steel in an Acidic Medium by Polymethoxyflavones from Citrus Peel Waste. J. Electrochem. Soc. 2023, 170, 093512. [Google Scholar] [CrossRef]
- Watanabe, M.; Nagano, S.; Sanui, K.; Ogata, N. Estimation of Li+ transport number in polymer electrolytes by the combination of complex impedance and potentiostatic polarization measurements. Solid State Ionics 1988, 28–30, 911–917. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]



| In Mole Ratio | Al | Li | Na | K | Rb | Cs |
|---|---|---|---|---|---|---|
| AlBTeC300 | 1.000 | 0.000 | 0.019 | 0.000 | 0.000 | 0.000 |
| AlBTeC300-Li | 1.000 | 1.042 | 0.030 | 0.000 | 0.000 | 0.000 |
| AlBTeC300-Na | 1.000 | 0.000 | 0.971 | 0.000 | 0.000 | 0.000 |
| AlBTeC300-K | 1.000 | 0.000 | 0.045 | 0.823 | 0.000 | 0.000 |
| AlBTeC300-Rb | 1.000 | 0.000 | 0.000 | 0.000 | 0.700 | 0.000 |
| AlBTeC300-Cs | 1.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Zheng, Y.; Guo, Y.; Zhang, Q.; Shen, Y.; Zhang, H.; Liu, Y.; Zheng, Y.; Jia, P.; Chen, R.; et al. Ion Substitution-Induced Distorted MOF Lattice with Deviated Energy and Dielectric Properties for Quasi-Solid-State Ion Conductor. Nanomaterials 2025, 15, 274. https://doi.org/10.3390/nano15040274
Huang Y, Zheng Y, Guo Y, Zhang Q, Shen Y, Zhang H, Liu Y, Zheng Y, Jia P, Chen R, et al. Ion Substitution-Induced Distorted MOF Lattice with Deviated Energy and Dielectric Properties for Quasi-Solid-State Ion Conductor. Nanomaterials. 2025; 15(4):274. https://doi.org/10.3390/nano15040274
Chicago/Turabian StyleHuang, Yike, Yun Zheng, Yan Guo, Qi Zhang, Yingying Shen, Hebin Zhang, Yinan Liu, Yihao Zheng, Pingshan Jia, Rong Chen, and et al. 2025. "Ion Substitution-Induced Distorted MOF Lattice with Deviated Energy and Dielectric Properties for Quasi-Solid-State Ion Conductor" Nanomaterials 15, no. 4: 274. https://doi.org/10.3390/nano15040274
APA StyleHuang, Y., Zheng, Y., Guo, Y., Zhang, Q., Shen, Y., Zhang, H., Liu, Y., Zheng, Y., Jia, P., Chen, R., Long, L., Zhang, Z., Zhang, C., Hou, Y., Yan, K., Huang, Z., Zhang, M., Jiang, J., Dong, S., ... Shao, H. (2025). Ion Substitution-Induced Distorted MOF Lattice with Deviated Energy and Dielectric Properties for Quasi-Solid-State Ion Conductor. Nanomaterials, 15(4), 274. https://doi.org/10.3390/nano15040274








