Fabrication of an Optical Sensor Based on Eosin-Y-Doped Electrospun Fibers for Ammonia Detection via Wavelength Shifts
Abstract
1. Introduction
| NH3 Probe | Method/Type | Range | Sensitivity/Linearity | Sensing Analysis | Reference |
|---|---|---|---|---|---|
| TMOF 6 (Cl) | Deposition/drop quartz plate | 0–400 ppm | 5/Linear | Intensity | [29] |
| CsPbBr3–SiO2 | Drop coating | 2160–3600 ppm | 0.8/Linear | Intensity | [30] |
| ZnO: Eu2+ | Spin coating | 0–80 ppm | 0.05/Linear | Intensity | [31] |
| MAPbBr3-TBA | Spin coating | 0–100 ppm | 2.5/Linear | Intensity | [32] |
| Mp-TiO2-based MAPbBr3 | Spin coating | 0–100 ppm | 9/Linear | Intensity | [33] |
| CsPbBr3 QDs | Electrospinning | 0–30 mg/L | 0.8/Linear | Intensity | [34] |
| CsPbBr3 QDs | Electrospinning | 0–350 ppm | 0.2/Linear | Intensity | [35] |
| MMPyP and TMPyP | Dip coating | 0–600 ppm | None/None | Intensity | [36] |
| CdSe/SiO2 | Dip coating | 0–400 ppm | 42.4/Linear | Intensity | [37] |
| Eosin-Y | Spin coating | 0–1000 ppm | 4.8/Non-linear | Intensity | [38] |
| Eosin-Y | Spin coating | 0–1000 ppm | 20/Non-linear | Intensity | [39] |
| Eosin-Y | Liquid fluxion coating | 0–500 ppm | None/None | Intensity | [40] |
| Eosin-Y | Electrospinning | 0–500 ppm | 8.11/Linear | Intensity/Wavelength Shift | This work |
2. Experiments
2.1. Materials
2.2. Experimental of Ammonia Sensing
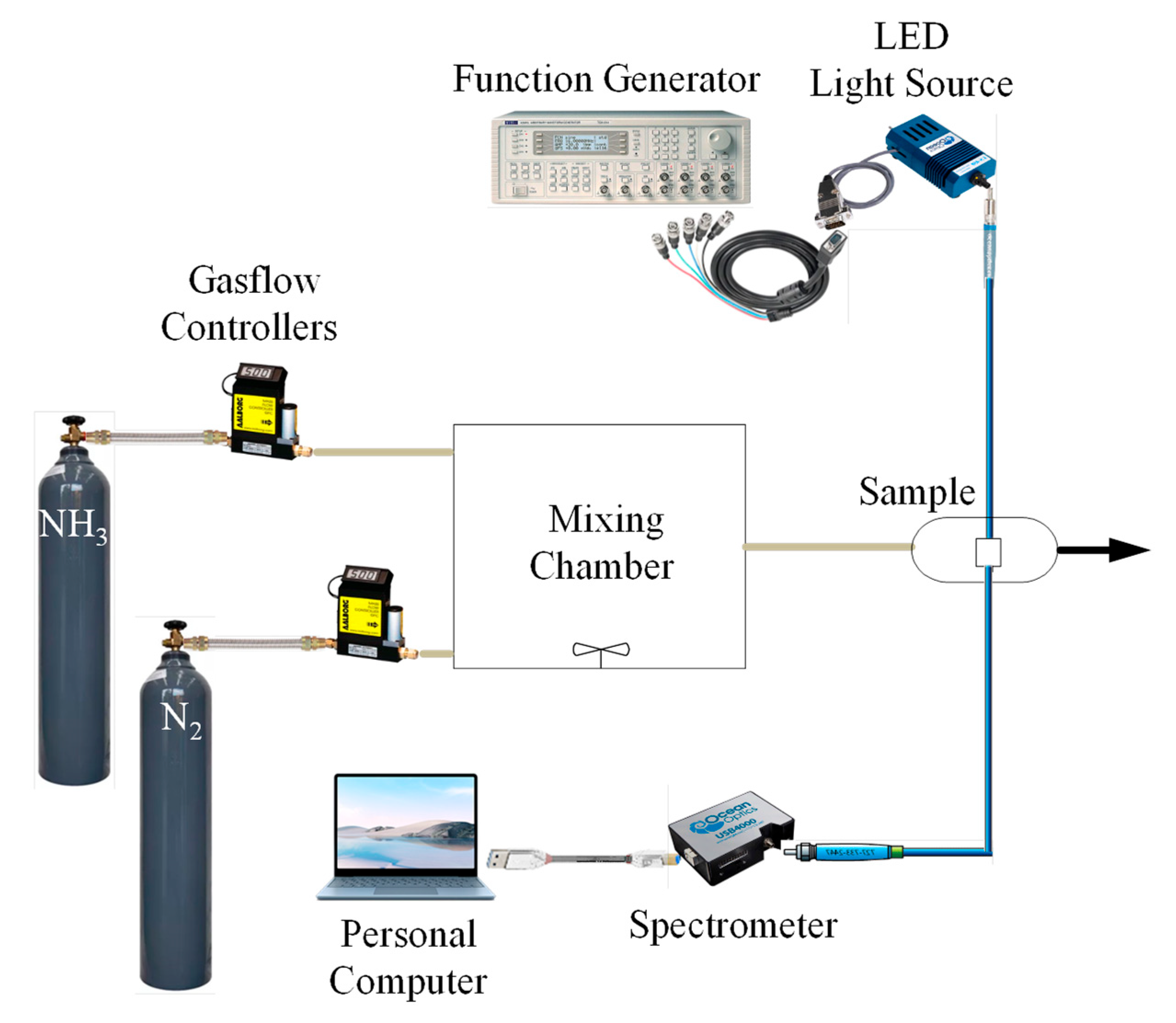
2.3. Optical Sensing Instruments
3. Results and Discussion
3.1. Optical Characterization of the Material Used
3.2. Ammonia Sensing Properties of Single Fiber
3.3. Wavelength Shift of Ammonia-Sensitive Dye
3.4. Photostability of Optical Ammonia Sensor
3.5. Dynamic Response Time of Optical Ammonia Sensors
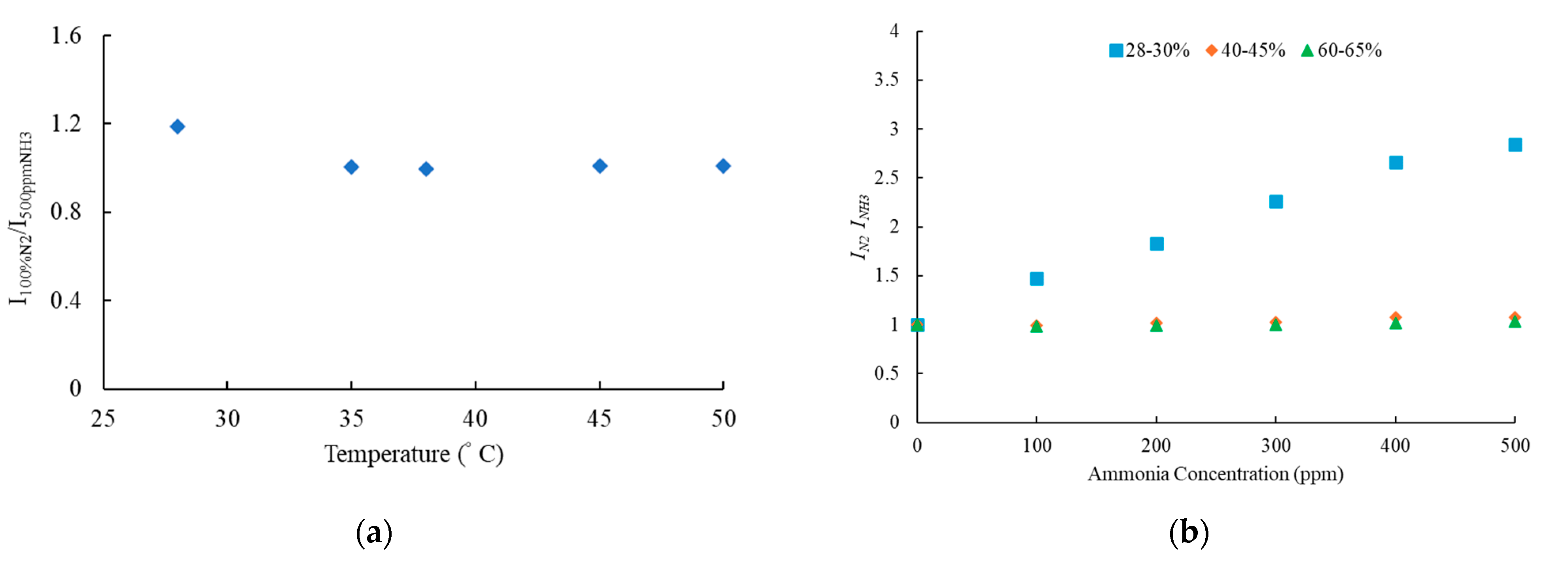
3.6. Effect of Temperature and Humidity of Optical Ammonia Sensor
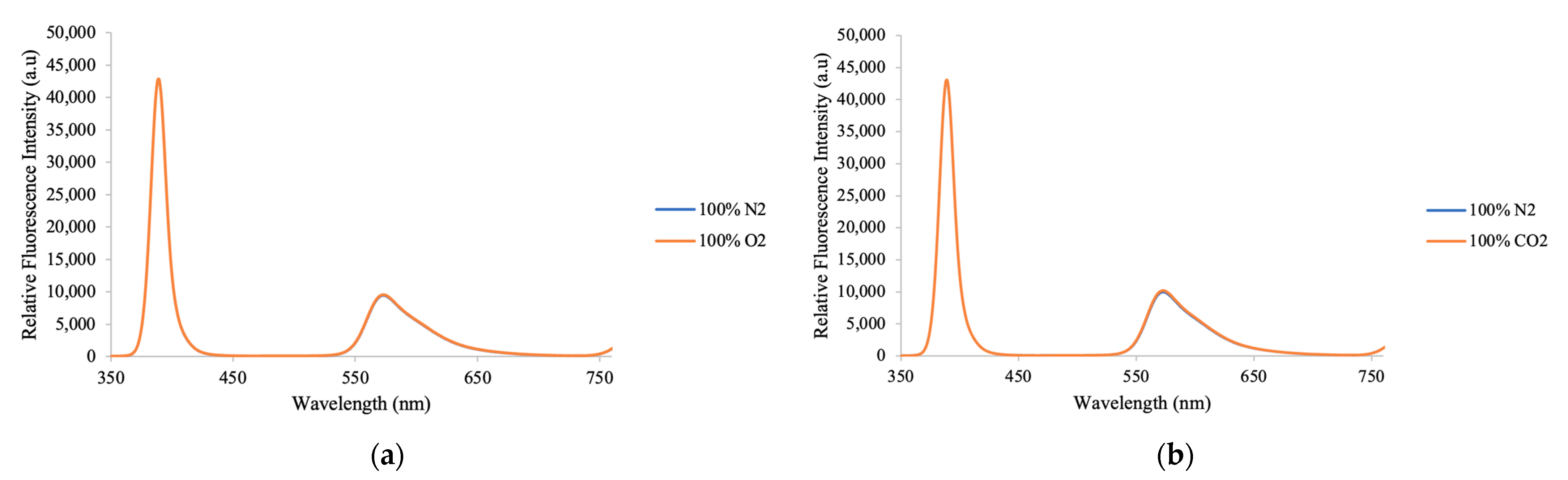
3.7. Selectivity of Optical Ammonia Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bagchi, S.; Achla, R.; Mondal, S.K. Electrospun polypyrrole-polyethylene oxide coated optical fiber sensor probe for detection of volatile compounds. Sens. Actuators B Chem. 2017, 250, 52–60. [Google Scholar] [CrossRef]
- Engel, L.; Benito-Altamirano, I.; Tarantik, K.R.; Pannek, C.; Dold, M.; Prades, J.D.; Wöllenstein, J. Printed sensor labels for colorimetric detection of ammonia, formaldehyde and hydrogen sulfide from the ambient air. Sens. Actuators B Chem. 2021, 330, 129281. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas sensors based on electrospun nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef]
- Imran, M.; Motta, N.; Shafiei, M. Electrospun one-dimensional nanostructures: A new horizon for gas sensing materials. Beilstein J. Nanotechnol. 2018, 9, 2128–2170. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, X.; Liu, Y.; Wang, J.; Evans, S.; Yang, M. Unpacking Additive Manufacturing Challenges and Opportunities in Moving towards Sustainability: An Exploratory Study. Sustainability 2023, 15, 3827. [Google Scholar] [CrossRef]
- Grand View Research. Gas Detection Equipment Market Size, Share & Trends Analysis Report by Product (Fixed Gas Detector, Portable Gas Detector), by Technology, by End-Use, by Region, and Segment Forecasts, 2025–2030; Grand View Research: San Francisco, CA, USA, 2024; p. 130. [Google Scholar]
- DataIntelo. Fixed Ammonia Gas Detector Market. In DataIntelo Market Research Report; DataIntelo: Maharashtra, India, 2023. [Google Scholar]
- Fuerte, A.; Valenzuela, R.X.; Escudero, M.J.; Daza, L. Ammonia as efficient fuel for SOFC. J. Power Sources 2009, 192, 170–174. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W. Direct ammonia solid oxide fuel cell based on thin proton-conducting electrolyte. J. Power Sources 2008, 179, 92–95. [Google Scholar] [CrossRef]
- Chellappa, A.S.; Fischer, C.M.; Thomson, W.J. Ammonia Decomposition Kinetics over Ni-Pt/Al2O3 for PEM Fuel Cell Applications. Appl. Catal. A Gen. 2022, 227, 231–240. [Google Scholar] [CrossRef]
- Hejze, T.; Besenhard, J.O.; Kordesch, K.; Cifrain, M.; Aronsson, R.R. Current status of combined systems using alkaline fuel cells and ammonia as a hydrogen carrier. J. Power Sources 2008, 176, 490–493. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia-hydrogen fuelled internal combustion engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Int. J. Hydrogen Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B. Selective detection of ammonia using spray pyrolysis deposited pure and nickel doped ZnO thin films. Appl. Surf. Sci. 2014, 311, 405–412. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelmoez, S.; Klapçtke, T.M. Electrospun nanofibers from a tricyanofuran-based molecular switch for colorimetric recognition of ammonia gas. Chem. A.European. J. 2016, 22, 4157–4163. [Google Scholar] [CrossRef]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells—A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Mesin, R.; Chu, C.S.; Tseng, Z.L. Ratiometric optical oxygen sensor based on perovskite quantum dots and Rh110 embedded in an ethyl cellulose matrix. Opt. Mater. Express 2023, 13, 945–955. [Google Scholar] [CrossRef]
- Rogacheva, S.M.; Shipovskaya, A.B.; Ivanova, I.A.; Gegel, N.O. Film polysaccharide matrices for immobilization of hydrophilic fluorescence probes. Sens. Int. 2020, 1, 100022. [Google Scholar] [CrossRef]
- Rispandi; Chu, C.S.; Simanjuntak, M.S. Ehanced sensing properties of optical ammonia sensor based on electrospun fibers containing eosin-Y and silver nanoparticles. Mater. Today Commun. 2024, 41, 110524. [Google Scholar] [CrossRef]
- Mesin, R.; Chu, C.S. Optical dual gas sensor for simultaneous detection of nitric oxide and oxygen. Chemosensors 2023, 11, 454. [Google Scholar] [CrossRef]
- Chu, C.S.; Hsieh, M.W.; Su, Z.R. Optical sensing of H2O2 based on red-shift of emission wavelength of carbon quantum dots. Opt. Mater. Express 2016, 6, 759–766. [Google Scholar] [CrossRef]
- Kumar, D.; Mesin, R.; Chu, C.S. Optical fluorescent sensor based on perovskite QDs for nitric oxide gas detection. Appl. Opt. 2023, 62, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Rispandi; Chu, C.S.; Nugroho, S.; Ammarullah, M.I. Optical dual gas sensor for biomedical monitoring of NO and O2 based on electrospun fibers containing CsPbBr3 QDs and PtTFPP. Results Opt. 2025, 18, 100781. [Google Scholar] [CrossRef]
- Sundaramurthy, J.; Li, N.; Kumar, P.S.; Ramakrishna, S. Perspective of Electrospun Nanofibers in Energy and Environment. Biofuel Res. J. 2014, 1, 44–54. [Google Scholar] [CrossRef]
- Rispandi; Chu, C.-S.; Nugroho, S.; Ammarullah, M.I. Ratiometric optical dual gas sensor for simultaneous detection of nitric oxide and oxygen intended for healthcare, biological, and biomedical applications. RSC Adv. 2024, 14, 38135–38145. [Google Scholar] [CrossRef] [PubMed]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, I.; et al. Supramolecular chemistry in solution and solid–gas interfaces: Synthesis and photophysical properties of monocolor and bicolor fluorescent sensors for barium tagging in neutrinoless double beta decay. RSC Appl. Interfaces 2025, 2, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.; Yang, C.; Yin, J.; Song, X.; Li, J.; Fei, H. Fabrication of robust and porous lead chloride-based metal-organic frameworks toward a selective and sensitive smart NH3 sensor. ACS Appl. Mater. Interfaces 2021, 13, 52765–52774. [Google Scholar] [CrossRef]
- Huangfu, C.; Wang, Y.; Wang, Z.; Hu, Q.; Feng, L. A stable and humidity resistant NH3 sensor based on luminous CsPbBr3 perovskite nanocrystals. Talanta 2023, 253, 124070. [Google Scholar] [CrossRef]
- Yang, W.; Feng, W.; Yang, X.; Chen, H.; He, Y.; Deng, D.; Peng, Z. Optical ammonia sensor based on ZnO:E2+ fluorescence quenching nanoparticles. Z. Naturforsch. 2018, 73, 781–784. [Google Scholar] [CrossRef]
- Li, G.; Zhang, W.; She, C.; Jia, S.; Liu, S.; Yue, F.; Jing, C.; Cheng, Y.; Chu, J. Stable fluorescent NH3 sensor based on MAPbBr3 encapsulated by tetrabutylammonium cations. J. Alloys Compd. 2020, 835, 155386. [Google Scholar] [CrossRef]
- Li, G.; She, C.; Zhang, Y.; Li, H.; Liu, S.; Yue, F.; Jing, C.; Cheng, Y.; Chu, J. A “turn-on” fluorescence perovskite sensor based on MAPbBr3/mesoporous TiO2 for NH3 and amine vapor detections. Sens. Actuators B Chem. 2021, 327, 128918. [Google Scholar] [CrossRef]
- Park, B.; Kim, S.; Kwak, C.H.; Shanmugam, K.R.; Han, Y.K.; Cho, Y.; Huh, Y.S. Visual colorimetric detection of ammonia under gaseous and aqueous state: Approach on cesium lead bromide perovskite-loaded porous electrospun nanofibers. J. Ind. Eng. Chem. 2021, 97, 515–522. [Google Scholar] [CrossRef]
- Huang, H.; Hao, M.; Song, Y.; Dang, S.; Liu, X.; Dong, Q. Dynamic passivation in perovskite quantum dots for specific ammonia detection at room temperature. Nano Mic. Small 2020, 16, e1904462. [Google Scholar] [CrossRef] [PubMed]
- Castillero, P.; Roales, J.; Lopes-Costa, T.; Sanchez-Valenciia, J.R.; Barranco, A.; Gonalez-Elipe, A.R.; Pedrosa, J.M. Optical gas sensing of ammonia and amines based on protonated porphyrin/TiO2 composite thin films. Sensors 2017, 17, 24. [Google Scholar] [CrossRef]
- Sung, T.W.; Lo, Y.L. Ammonia vapor sensor based on CdSe/SiO2 core-shell nanoparticles embedded in sol-gel matrix. Sens. Actuators B Chem. 2013, 188, 702–708. [Google Scholar] [CrossRef]
- Liu, C.Y.; Deb, M.; Sadhu, A.S.; Karmakar, R.; Huang, P.T.; Lin, Y.N.; Chu, C.S.; Pal, B.N.; Chang, S.H.; Biring, S. Resolving cross-sensitivity effect in fluorescence quenching for simultaneously sensing oxygen and ammonia concentrations by an optical dual gas sensor. Sensors 2021, 21, 6940. [Google Scholar] [CrossRef]
- Biring, S.; Sadhu, A.S.; Deb, M. An effective optical dual gas sensor for simultaneous detection of oxygen and ammonia. Sensors 2019, 19, 5124. [Google Scholar] [CrossRef]
- Peng, L.; Yang, X.; Yuan, L.; Wang, L.; Zhao, E.; Tian, F.; Liu, Y. Gaseous ammonia fluorescence probe based on cellulose acetate modified microstructured optical fiber. Opt. Commun. 2011, 284, 4810–4814. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rispandi; Simanjuntak, M.S.; Chu, C.-S. Fabrication of an Optical Sensor Based on Eosin-Y-Doped Electrospun Fibers for Ammonia Detection via Wavelength Shifts. Nanomaterials 2025, 15, 273. https://doi.org/10.3390/nano15040273
Rispandi, Simanjuntak MS, Chu C-S. Fabrication of an Optical Sensor Based on Eosin-Y-Doped Electrospun Fibers for Ammonia Detection via Wavelength Shifts. Nanomaterials. 2025; 15(4):273. https://doi.org/10.3390/nano15040273
Chicago/Turabian StyleRispandi, Manna Septriani Simanjuntak, and Cheng-Shane Chu. 2025. "Fabrication of an Optical Sensor Based on Eosin-Y-Doped Electrospun Fibers for Ammonia Detection via Wavelength Shifts" Nanomaterials 15, no. 4: 273. https://doi.org/10.3390/nano15040273
APA StyleRispandi, Simanjuntak, M. S., & Chu, C.-S. (2025). Fabrication of an Optical Sensor Based on Eosin-Y-Doped Electrospun Fibers for Ammonia Detection via Wavelength Shifts. Nanomaterials, 15(4), 273. https://doi.org/10.3390/nano15040273







