Precision Fabrication and Optimization of Nanostructures for Exosome Detection via Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates Preparation
2.2. Cell Culture and Exosome Isolation
2.3. Raman Spectroscopy Detection
2.4. Characterization
3. Results and Discussion
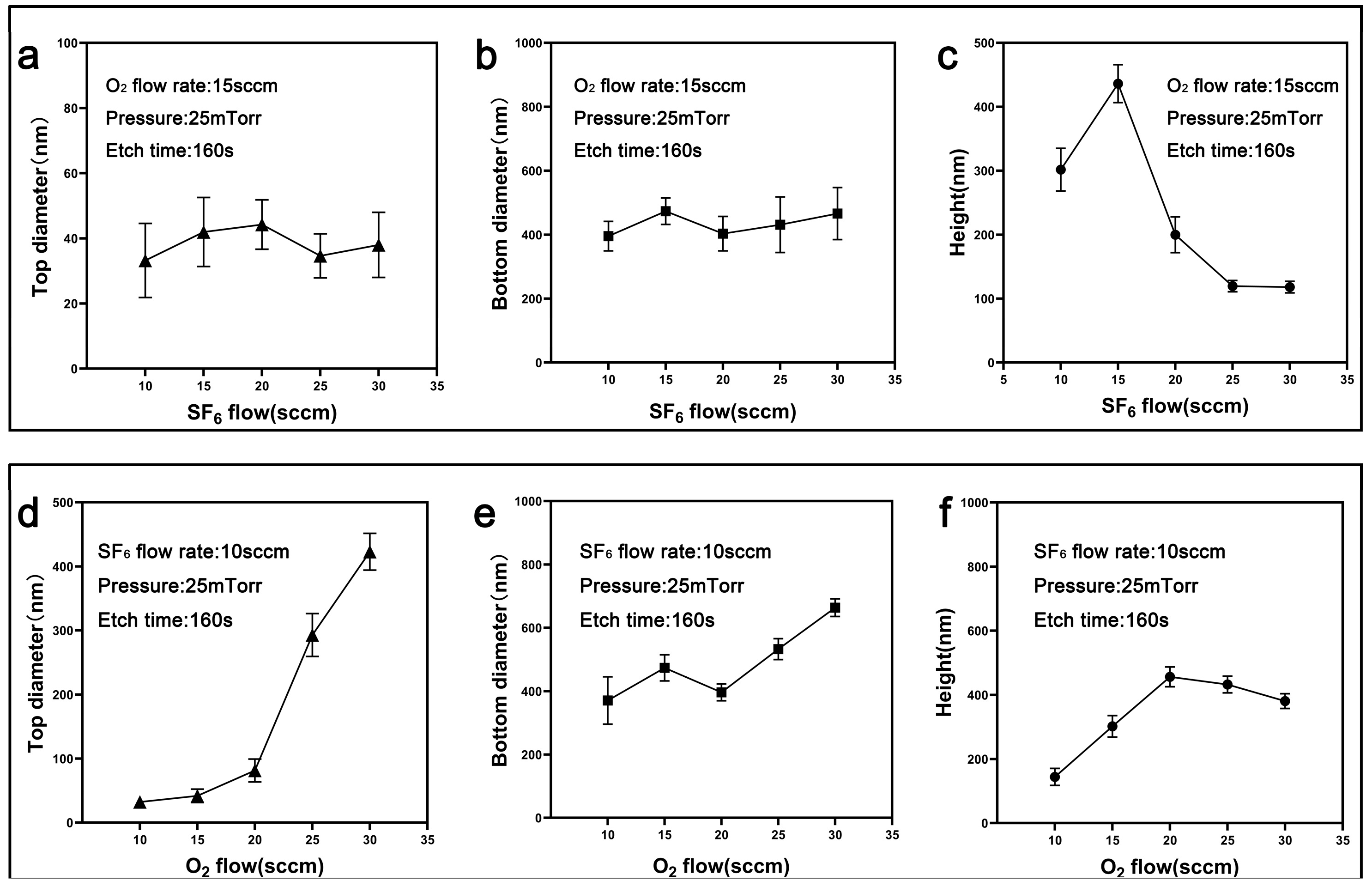
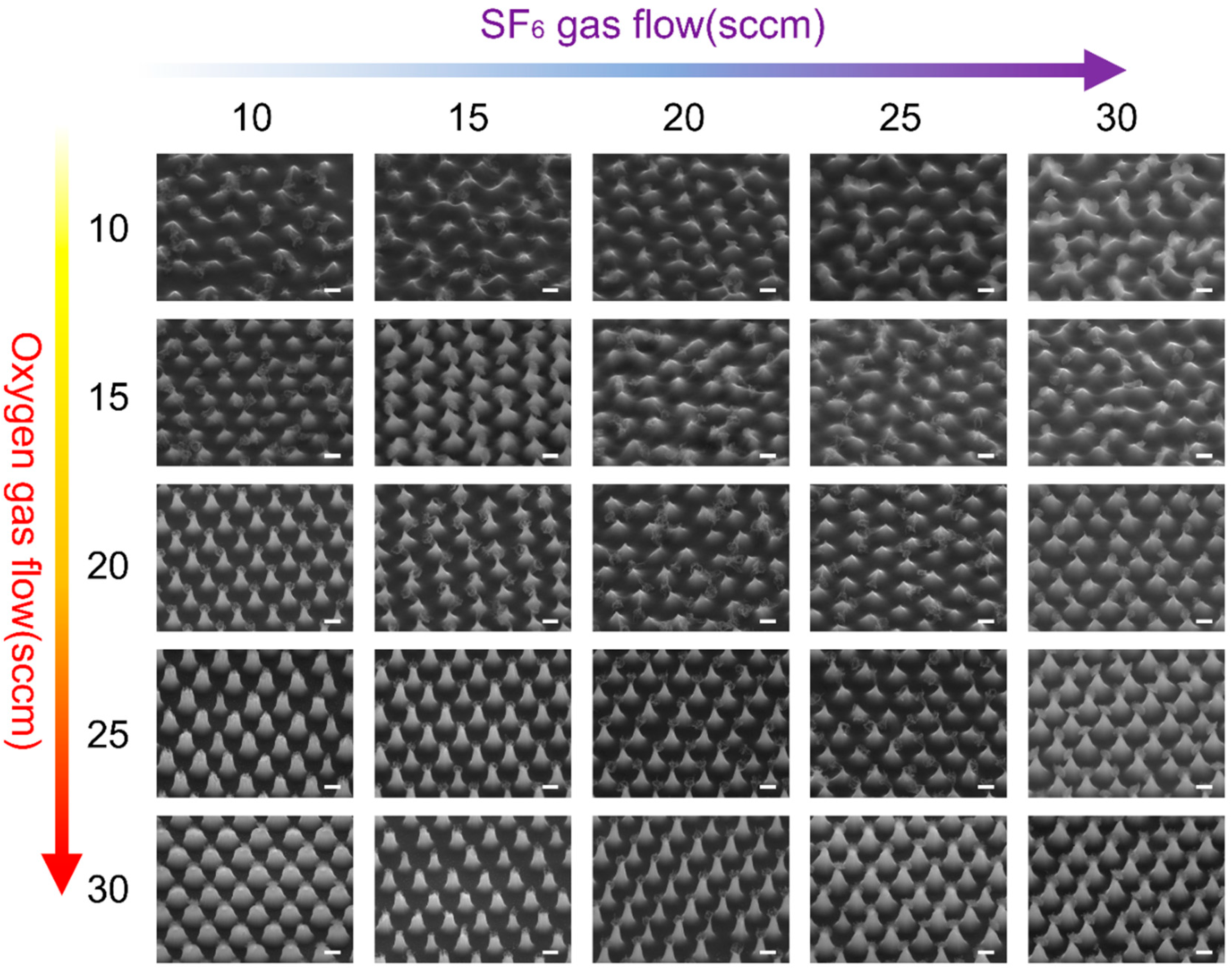
3.1. Fabrication and Optimization Analysis of Nanocone Substrate
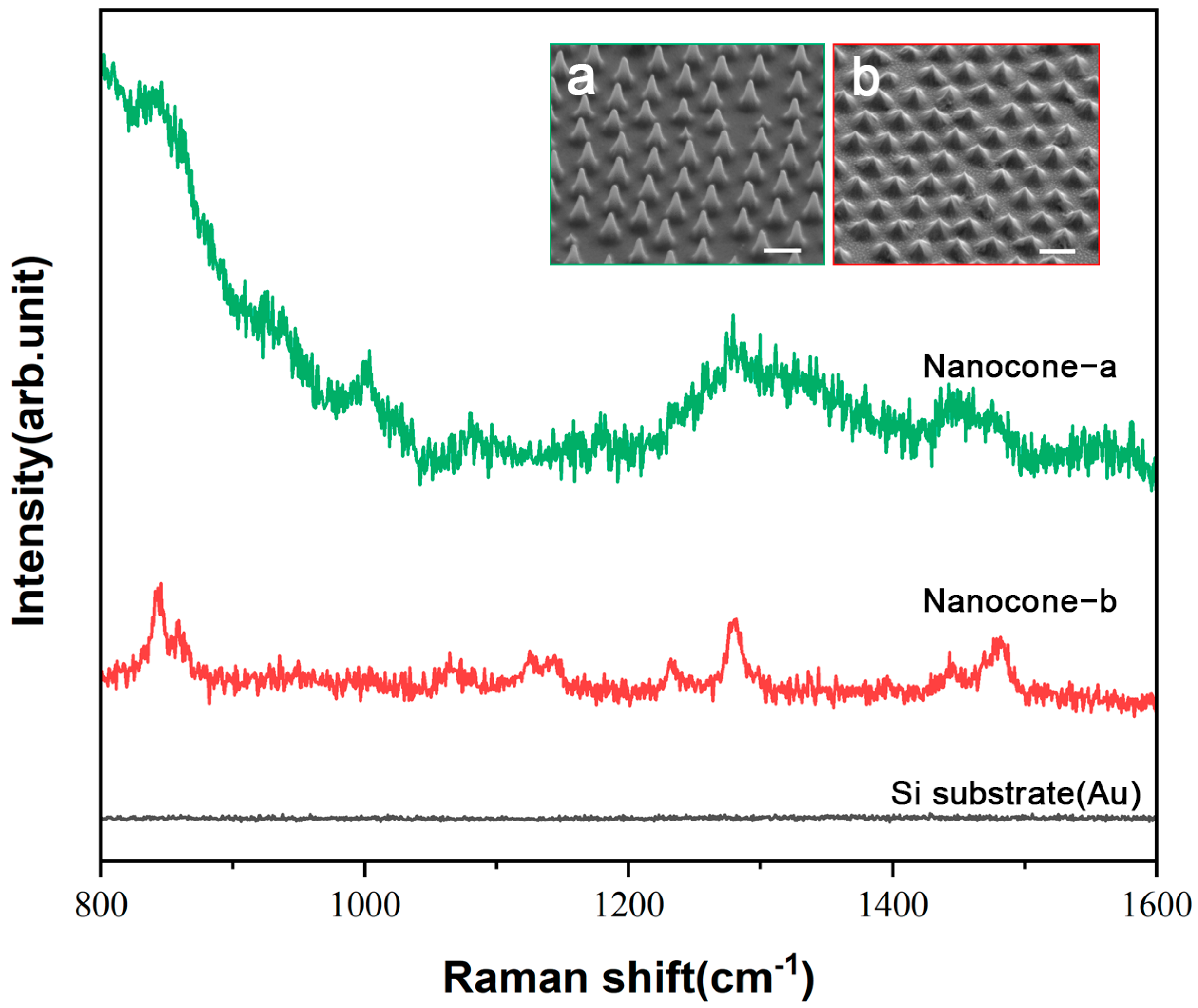
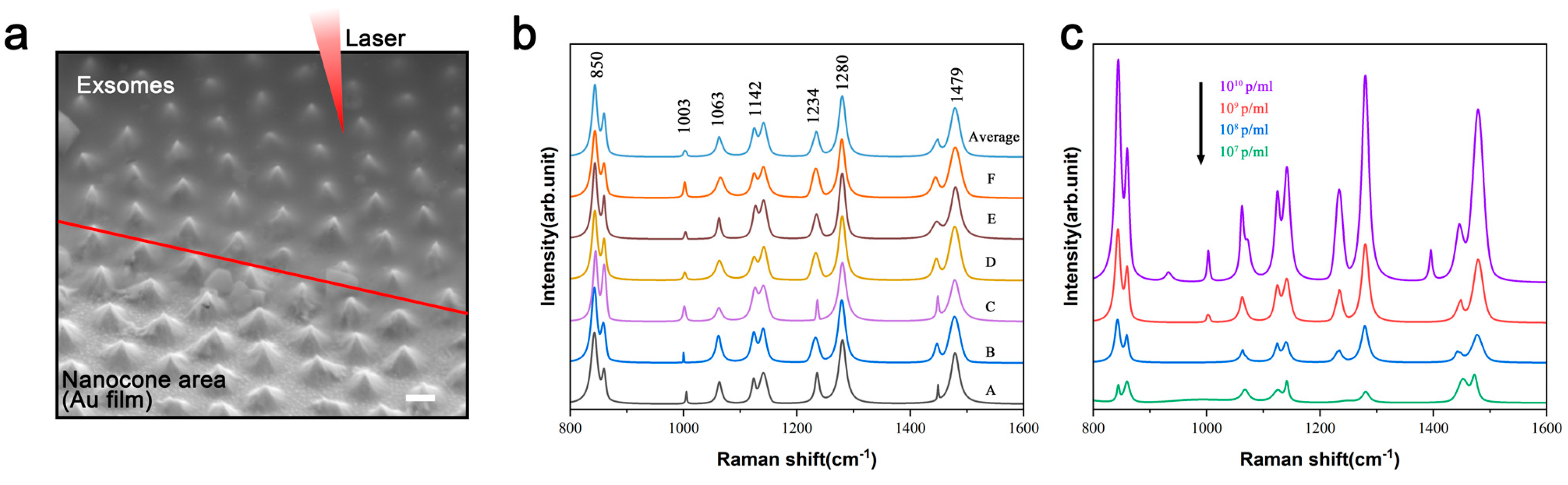
3.2. Raman Performance Characterization of the Fabricated Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simons, M.; Raposo, G. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gu, Y.; Du, Y.; Liu, J. Exosomes: Diagnostic biomarkers and therapeutic delivery vehicles for cancer. Mol. Pharm. 2019, 16, 3333–3349. [Google Scholar] [CrossRef] [PubMed]
- Boriachek, K.; Islam, M.N.; Möller, A.; Salomon, C.; Nguyen, N.-T.; Hossain, M.S.A.; Yamauchi, Y.; Shiddiky, M.J.A. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small 2018, 14, 1702153. [Google Scholar] [CrossRef]
- Umeche, I.E.; Olaniyan, M.F. Exosomes: Emerging biomarkers unveiling cellular mysteries—A narrative review. J. Bio-X Res. 2023, 6, 104–115. [Google Scholar] [CrossRef]
- Bhat, E.A.; Sajjad, N.; Thokar, F.M. Current advancement of exosomes as biomarkers for cancer diagnosis and forecasting. Cancer Treat. Res. Commun. 2021, 28, 100417. [Google Scholar] [CrossRef]
- Tan, H.-S.; Wang, T.; Sun, H.-N.; Liu, A.; Li, S.-S. Advances of surface-enhanced Raman spectroscopy in exosomal biomarkers analysis. TrAC Trends Anal. Chem. 2023, 167, 117253. [Google Scholar] [CrossRef]
- Kurian, T.K.; Banik, S.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Mol. Biotechnol. 2021, 63, 249–266. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation–efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Lima Moura, S.; Martì, M.; Pividori, M.I. Matrix Effect in the Isolation of Breast Cancer-Derived Nanovesicles by Immunomagnetic Separation and Electrochemical Immunosensing—A Comparative Study. Sensors 2020, 20, 965. [Google Scholar] [CrossRef]
- Tayebi, M.; Zhou, Y.; Tripathi, P.; Chandramohanadas, R.; Ai, Y. Exosome Purification and Analysis Using a Facile Microfluidic Hydrodynamic Trapping Device. Anal. Chem. 2020, 92, 10733–10742. [Google Scholar] [CrossRef]
- Amrollahi, P.; Rodrigues, M.; Lyon, C.J.; Goel, A.; Han, H.; Hu, T.Y. Ultra-Sensitive Automated Profiling of EpCAM Expression on Tumor-Derived Extracellular Vesicles. Front. Genet. 2019, 10, 1273. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, P.; Zhang, Y.; Du, L.; Wang, Y.; Zhang, C.; Wang, C. Exosome detection via surface-enhanced Raman spectroscopy for cancer diagnosis. Acta Biomater. 2022, 144, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-Activated Platforms for Immunoassay: Probes, Encoding Methods, and Applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef]
- Nishino, T. Surface-enhanced Raman Spectroscopy. Anal. Sci. 2018, 34, 1061–1062. [Google Scholar] [CrossRef]
- Zhu, W.; Hutchison, J.A.; Dong, M.; Li, M. Frequency Shift Surface-Enhanced Raman Spectroscopy Sensing: An Ultrasensitive Multiplex Assay for Biomarkers in Human Health. ACS Sens. 2021, 6, 1704–1716. [Google Scholar] [CrossRef]
- Ouyang, L.; Ren, W.; Zhu, L.; Irudayaraj, J. Prosperity to challenges: Recent approaches in SERS substrate fabrication. Rev. Anal. Chem. 2017, 36, 20160027. [Google Scholar] [CrossRef]
- Li, Q.; Zhuo, X.; Li, S.; Ruan, Q.; Xu, Q.; Wang, J. Production of Monodisperse Gold Nanobipyramids with Number Percentages Approaching 100% and Evaluation of Their Plasmonic Properties. Adv. Opt. Mater. 2015, 3, 801–812. [Google Scholar] [CrossRef]
- Sun, A.Y.; Lee, Y.-C.; Chang, S.-W.; Chen, S.-L.; Wang, H.-C.; Wan, D.; Chen, H.-L. Diverse Substrate-Mediated Local Electric Field Enhancement of Metal Nanoparticles for Nanogap-Enhanced Raman Scattering. Anal. Chem. 2021, 93, 4299–4307. [Google Scholar] [CrossRef]
- Dzhagan, V.; Mazur, N.; Kapush, O.; Skoryk, M.; Pirko, Y.; Yemets, A.; Dzhahan, V.; Shepeliavyi, P.; Valakh, M.; Yukhymchuk, V. Self-Organized SERS Substrates with Efficient Analyte Enrichment in the Hot Spots. ACS Omega 2024, 9, 4819–4830. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, C.; Lee, Y.; Lee, J.; Park, S.J.; Park, S.; Nam, J.M. Synthesis, Assembly, Optical Properties, and Sensing Applications of Plasmonic Gap Nanostructures. Adv. Mater. 2021, 33, 2006966. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, H. Hot spots in different metal nanostructures for plasmon-enhanced Raman spectroscopy. Nanoscale 2013, 5, 10794–10805. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.Y.; Kim, D.J.; Park, S.-G.; Kim, S.-H. Nanostructured plasmonic substrates for use as SERS sensors. Nano Converg. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Kawasaki, D.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Gold Nanocone Array with Extensive Electromagnetic Fields for Highly Reproducible Surface-Enhanced Raman Scattering Measurements. Micromachines 2022, 13, 1182. [Google Scholar] [CrossRef]
- Yan, S.; Sun, J.; Chen, B.; Wang, L.; Bian, S.; Sawan, M.; Tang, H.; Wen, L.; Meng, G. Manipulating Coupled Field Enhancement in Slot-under-Groove Nanoarrays for Universal Surface-Enhanced Raman Scattering. ACS Nano 2023, 17, 22766–22777. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Chen, L.; Guan, P.; Chen, B.; Fujita, T.; Yamaguchi, Y.; Iwasaki, H.; Xue, Q.-K.; Chen, M. Large-scale growth of sharp gold nano-cones for single-molecule SERS detection. RSC Adv. 2016, 6, 2882–2887. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Tian, D.; Ke, P.; Yang, X.; Wu, Q.; Chen, J.; Hu, C.; Ji, H. Assembly of long silver nanowires into highly aligned structure to achieve uniform “Hot Spots” for Surface-enhanced Raman scattering detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 273, 121030. [Google Scholar] [CrossRef]
- Shi, L.; Liu, M.; Zhang, L.; Tian, Y. A Liquid Interfacial SERS Platform on a Nanoparticle Array Stabilized by Rigid Probes for the Quantification of Norepinephrine in Rat Brain Microdialysates. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117125. [Google Scholar] [CrossRef]
- Zhou, B.; Qu, C.; Du, S.; Gao, W.; Zhang, Y.; Ding, Y.; Wang, H.; Hou, R.; Su, M.; Liu, H. Multi-analyte High-Throughput Microplate-SERS Reader with Controllable Liquid Interfacial Arrays. Anal. Chem. 2022, 94, 7528–7535. [Google Scholar] [CrossRef]
- Paramanik, D.; Motayed, A.; King, M.; Ha, J.Y.; Kryluk, S.; Davydov, A.V.; Talin, A. Fabrication of high quality GaN nanopillar arrays by dry and wet. arXiv 2013, arXiv:1311.0321. [Google Scholar]
- Magdi, S.; El-Rifai, J.; Swillam, M.A. One step fabrication of Silicon nanocones with wide-angle enhanced light absorption. Sci. Rep. 2018, 8, 4001. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Yang, S.Y. Development of Nanoparticle Fluid Imprinting Technology in Microstructure Manufacture. Polym.-Plast. Technol. Eng. 2009, 48, 209–215. [Google Scholar] [CrossRef]
- Suresh, V.; Ding, L.; Chew, A.B.; Yap, F.L. Fabrication of Large-Area Flexible SERS Substrates by Nanoimprint Lithography. ACS Appl. Nano Mater. 2018, 1, 886–893. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Faro, M.J.L.; Irrera, A. Silicon Nanowires Synthesis by Metal-Assisted Chemical Etching: A Review. Nanomaterials 2021, 11, 383. [Google Scholar] [CrossRef]
- Teng, F.; Li, N.; Xu, D.; Xiao, D.; Yang, X.; Lu, N. Precise regulation of tilt angle of Si nanostructures via metal-assisted chemical etching. Nanoscale 2017, 9, 449–453. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X. Functional semiconductor nanowires via vapor deposition. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2011, 29, 060801. [Google Scholar] [CrossRef][Green Version]
- Kim, D.S.; Hwang, N.-M. Synthesis of nanostructures using charged nanoparticles spontaneously generated in the gas phase during chemical vapor deposition. J. Phys. D Appl. Phys. 2018, 51, 463002. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Ma, Y.; Weng, D.; Li, Z.; Song, L.; Zhang, X.; Yu, G.; Wang, J. Ultrafast Fabrication of Large-Area Colloidal Crystal Micropatterns via Self-Assembly and Transfer Printing. Adv. Funct. Mater. 2022, 32, 2205462. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Weng, D.; Chen, C.; Li, Z.; Wang, J. Tension gradient-driven rapid self-assembly method of large-area colloidal crystal film and its application in multifunctional structural color displays. Chem. Eng. J. 2022, 427, 130658. [Google Scholar] [CrossRef]
- Yu, G.; Ma, Y.; Li, X.; Yu, B.; Zhang, X.; Zhang, X.; Chen, Y.; Liang, Z.; Pang, Z.; Weng, D.; et al. Analysis of the Pattern Shapes Obtained By Micro/Nanospherical Lens Photolithography. Langmuir 2023, 39, 14328–14335. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ma, Y.; Wang, Y.; Song, L.; Yu, G.; Zhang, X.; Wang, Q.; Pang, Z.; Zhang, Y.; Wang, Q.; et al. Self-Assembly Hybrid Manufacture of Nanoarrays for Metasurfaces. Small Methods 2024, e2401288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Yu, G.; Song, L.; Weng, D.; Ma, Y.; Wang, J. Rapid Fabrication of High-Resolution Flexible Electronics via Nanoparticle Self-Assembly and Transfer Printing. Nano Lett. 2024, 24, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Wu, X.Z.; Di, D.; Dong, P.T.; Xiao, R.; Wang, S.Q. Orientation-dependent nanostructure arrays based on anisotropic silicon wet-etching for repeatable surface-enhanced Raman scattering. Nanoscale 2016, 8, 4672–4680. [Google Scholar] [CrossRef]
- Wakawaiachi, S.M.; Tezani, L.L.; Pessoa, R.S.; Medeiros, H.S.; Maciel, H.S.; Petraconi, G. Morphological and Chemical Analysis of Silicon Etched by SF6+O2 and CF4+O2 Low Pressure Constricted Plasma Jet. ECS Trans. 2011, 39, 409. [Google Scholar] [CrossRef]
- Alam, A.B.M.K.; Kuittinen, M.; Laukkanen, J. In Etching Process Development of SiO2 Etching Using Inductively Coupled Plasma. Master’s Thesis, Itä-Suomen yliopisto, Kuopio, Finland, 2015. [Google Scholar]
- Tian, F.; Li, M.; Wu, S.; Li, L.; Hu, H. A hybrid and scalable nanofabrication approach for bio-inspired bactericidal silicon nanospike surfaces. Colloids Surf. B Biointerfaces 2023, 222, 113092. [Google Scholar] [CrossRef]
- Arana, L.R.; de Mas, N.; Schmidt, R.T.; Franz, A.J.; Schmidt, M.A.; Jensen, K.F. Isotropic etching of silicon in fluorine gas for MEMS micromachining. J. Micromech. Microeng. 2007, 17, 384–392. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Maciel, H.S.; Petraconi, G.; Massi, M.; da Silva Sobrinho, A.S. Effect of gas residence time on the morphology of silicon surface etched in SF6 plasmas. Appl. Surf. Sci. 2008, 255, 749–751. [Google Scholar] [CrossRef]
- Wongwanitwattana, C.; Shah, V.A.; Myronov, M.; Parker, E.H.C.; Whall, T.; Leadley, D.R. Precision plasma etching of Si, Ge, and Ge:P by SF6 with added O2. J. Vac. Sci. Technol. A 2014, 32, 031302. [Google Scholar] [CrossRef]
- Aydinoglu, F.; Pan, A.; Zhu, C.; Cui, B. Effect of oxygen plasma cleaning on nonswitching pseudo-Bosch etching of high aspect ratio silicon pillars. J. Vac. Sci. Technol. B 2020, 38, 012804. [Google Scholar] [CrossRef]
- Legtenberg, R.; Jansen, H.V.; Boer, M.D.; Elwenspoek, M. Anisotrapic Reactive Ion Etching of Silicon Using SF6/O2/CHF3 Gas Mixtures. J. Electrochem. Soc. 2005, 142, 2020. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Gartia, M.R.; Whitlock, D.; Lian, Y.; Liu, G.L. Ultrahigh Throughput Silicon Nanomanufacturing by Simultaneous Reactive Ion Synthesis and Etching. ACS Nano 2011, 5, 8002–8012. [Google Scholar] [CrossRef] [PubMed]
- Zhiting, G.; Zhuang, M.; Lihong, G.; Qiang, L.; Yuxiang, W.; Yanbo, L.; Lidong, W.; Yuyang, H.; Yuanhan, D. Etching mechanism of high-aspect-ratio array structure. Microelectron. Eng. 2023, 279, 112060. [Google Scholar] [CrossRef]
- Chang, L.; Liu, X.; Luo, J.; Lee, C.-Y.; Zhang, J.; Fan, X.; Zhang, W. Physiochemical Coupled Dynamic Nanosphere Lithography Enabling Multiple Metastructures from Single Mask. Adv. Mater. 2024, 36, 2310469. [Google Scholar] [CrossRef]
- Manciu, F.S.; Ciubuc, J.D.; Parra, K.; Manciu, M.; Bennet, K.E.; Valenzuela, P.; Sundin, E.M.; Durrer, W.G.; Reza, L.; Francia, G. Label-Free Raman Imaging to Monitor Breast Tumor Signatures. Technol. Cancer Res. Treat. 2017, 16, 461–469. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Fang, Y.; Zheng, D.; Lin, T.; Wang, H. Label-Free Exosomal Detection and Classification in Rapid Discriminating Different Cancer Types Based on Specific Raman Phenotypes and Multivariate Statistical Analysis. Molecules 2019, 24, 2947. [Google Scholar] [CrossRef]
- Kallaway, C.; Almond, L.M.; Barr, H.; Wood, J.; Hutchings, J.; Kendall, C.; Stone, N. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagnosis Photodyn. Ther. 2013, 10, 207–219. [Google Scholar] [CrossRef]
- Galler, K.; Requardt, R.P.; Glaser, U.; Markwart, R.; Bocklitz, T.; Bauer, M.; Popp, J.; Neugebauer, U. Single cell analysis in native tissue: Quantification of the retinoid content of hepatic stellate cells. Sci. Rep. 2016, 6, 24155. [Google Scholar] [CrossRef]
- Willets, K.A. Surface-enhanced Raman scattering (SERS) for probing internal cellular structure and dynamics. Anal. Bioanal. Chem. 2009, 394, 85–94. [Google Scholar] [CrossRef]
- Shetty, G.; Kendall, C.; Shepherd, N.; Stone, N.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef]
- Tirinato, L.; Gentile, F.; Di Mascolo, D.; Coluccio, M.L.; Das, G.; Liberale, C.; Pullano, S.A.; Perozziello, G.; Francardi, M.; Accardo, A.; et al. SERS analysis on exosomes using super-hydrophobic surfaces. Microelectron. Eng. 2012, 97, 337–340. [Google Scholar] [CrossRef]
- Kast, R.E.; Serhatkulu, G.K.; Cao, A.; Pandya, A.K.; Dai, H.; Thakur, J.S.; Naik, V.M.; Naik, R.; Klein, M.D.; Auner, G.W.; et al. Raman spectroscopy can differentiate malignant tumors from normal breast tissue and detect early neoplastic changes in a mouse model. Biopolymers 2008, 89, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Li, X.; Hou, S.; Li, H.; Qi, G.; Jin, Y. Machine Learning-Based Label-Free SERS Profiling of Exosomes for Accurate Fuzzy Diagnosis of Cancer and Dynamic Monitoring of Drug Therapeutic Processes. Anal. Chem. 2023, 95, 7552–7559. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, X.; Wen, Y.; Zheng, C.; Li, M. Artificial Intelligent Label-Free SERS Profiling of Serum Exosomes for Breast Cancer Diagnosis and Postoperative Assessment. Nano Lett. 2022, 22, 7910–7918. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yu, B.; Yang, B.; Zhang, X.; Yu, G.; Wang, Z.; Qin, H.; Ma, Y. Precision Fabrication and Optimization of Nanostructures for Exosome Detection via Surface-Enhanced Raman Spectroscopy. Nanomaterials 2025, 15, 266. https://doi.org/10.3390/nano15040266
Wang Q, Yu B, Yang B, Zhang X, Yu G, Wang Z, Qin H, Ma Y. Precision Fabrication and Optimization of Nanostructures for Exosome Detection via Surface-Enhanced Raman Spectroscopy. Nanomaterials. 2025; 15(4):266. https://doi.org/10.3390/nano15040266
Chicago/Turabian StyleWang, Qingyi, Bowen Yu, Bingbing Yang, Xuanhe Zhang, Guoxu Yu, Zeyu Wang, Hua Qin, and Yuan Ma. 2025. "Precision Fabrication and Optimization of Nanostructures for Exosome Detection via Surface-Enhanced Raman Spectroscopy" Nanomaterials 15, no. 4: 266. https://doi.org/10.3390/nano15040266
APA StyleWang, Q., Yu, B., Yang, B., Zhang, X., Yu, G., Wang, Z., Qin, H., & Ma, Y. (2025). Precision Fabrication and Optimization of Nanostructures for Exosome Detection via Surface-Enhanced Raman Spectroscopy. Nanomaterials, 15(4), 266. https://doi.org/10.3390/nano15040266





