Silver Nanowire-Amorphous Indium Zinc Oxide Composite Electrodes for Transparent Film Heaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Films
2.3. Characterization
3. Results and Discussion
3.1. Structural Properties and Surface Morphology
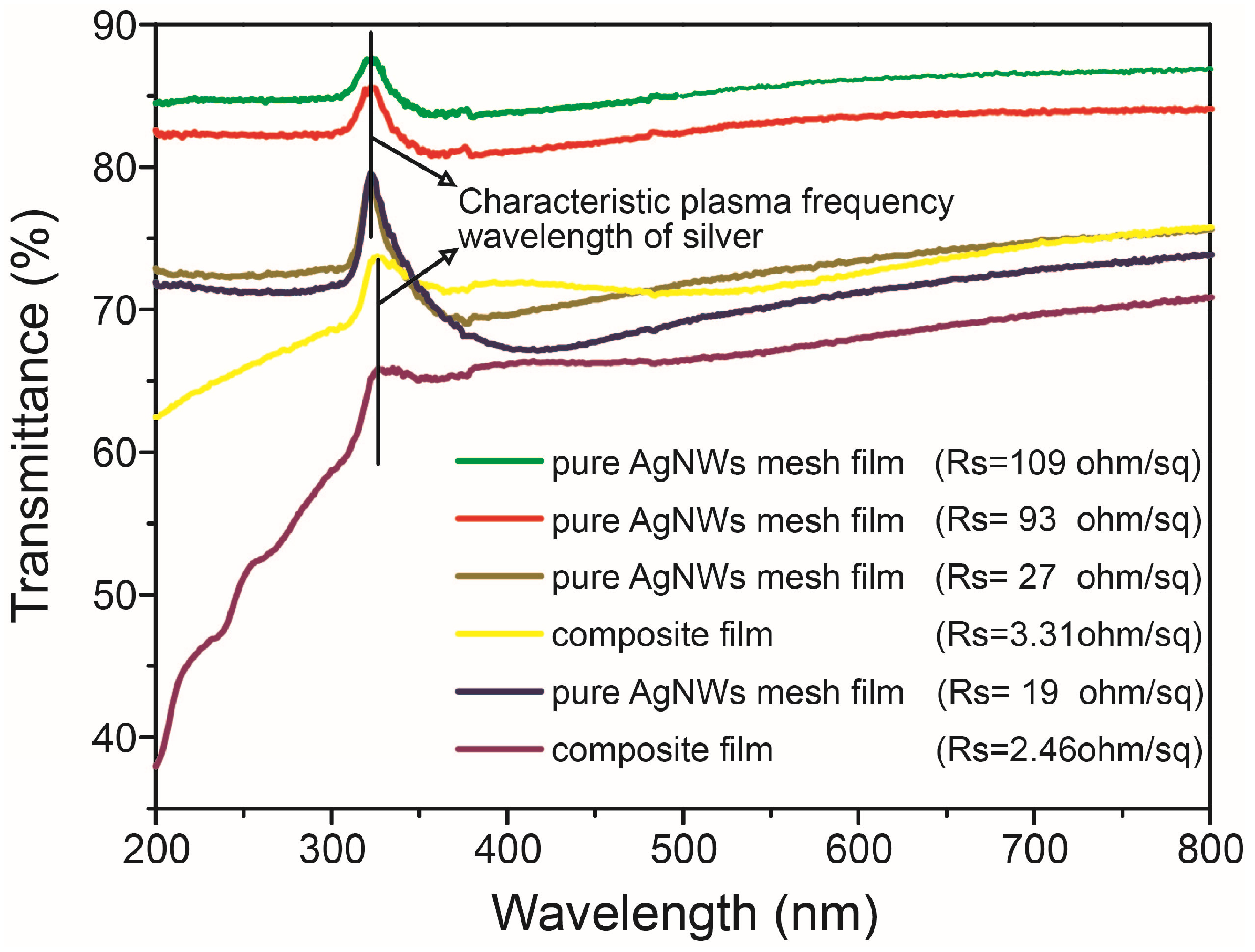
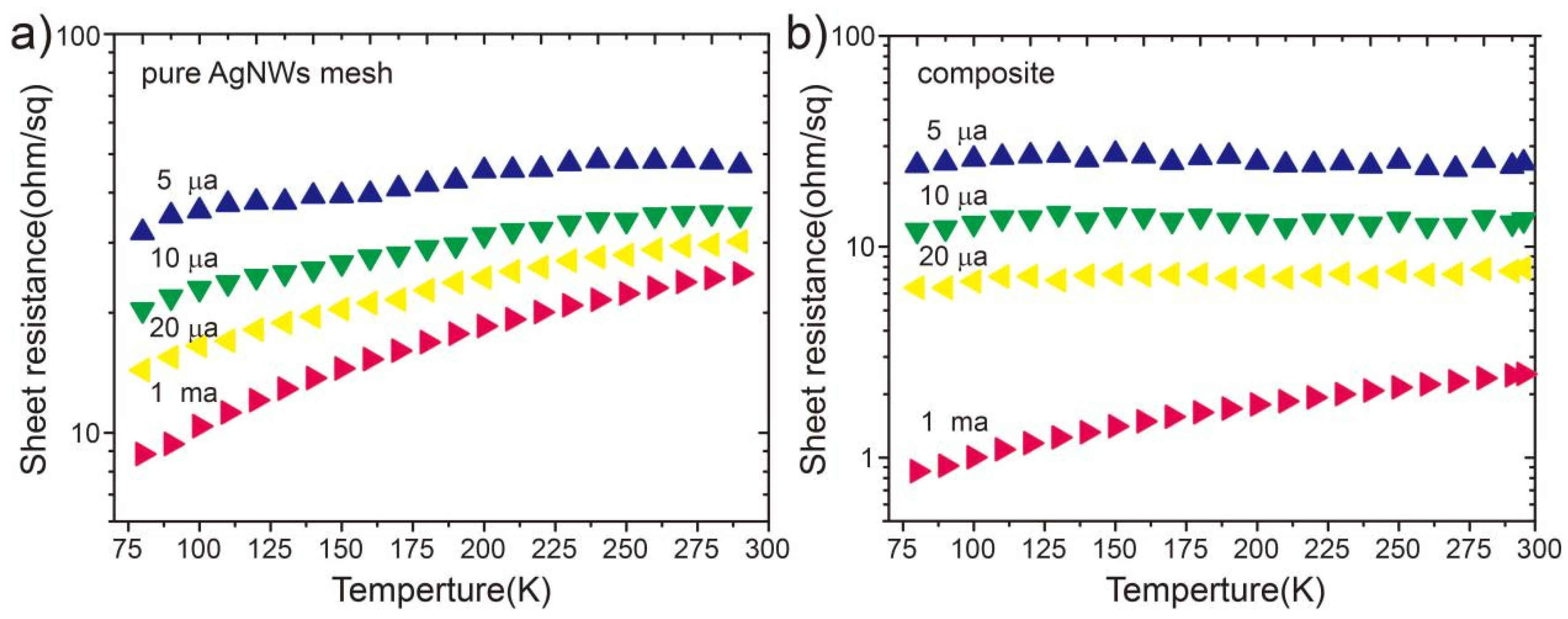
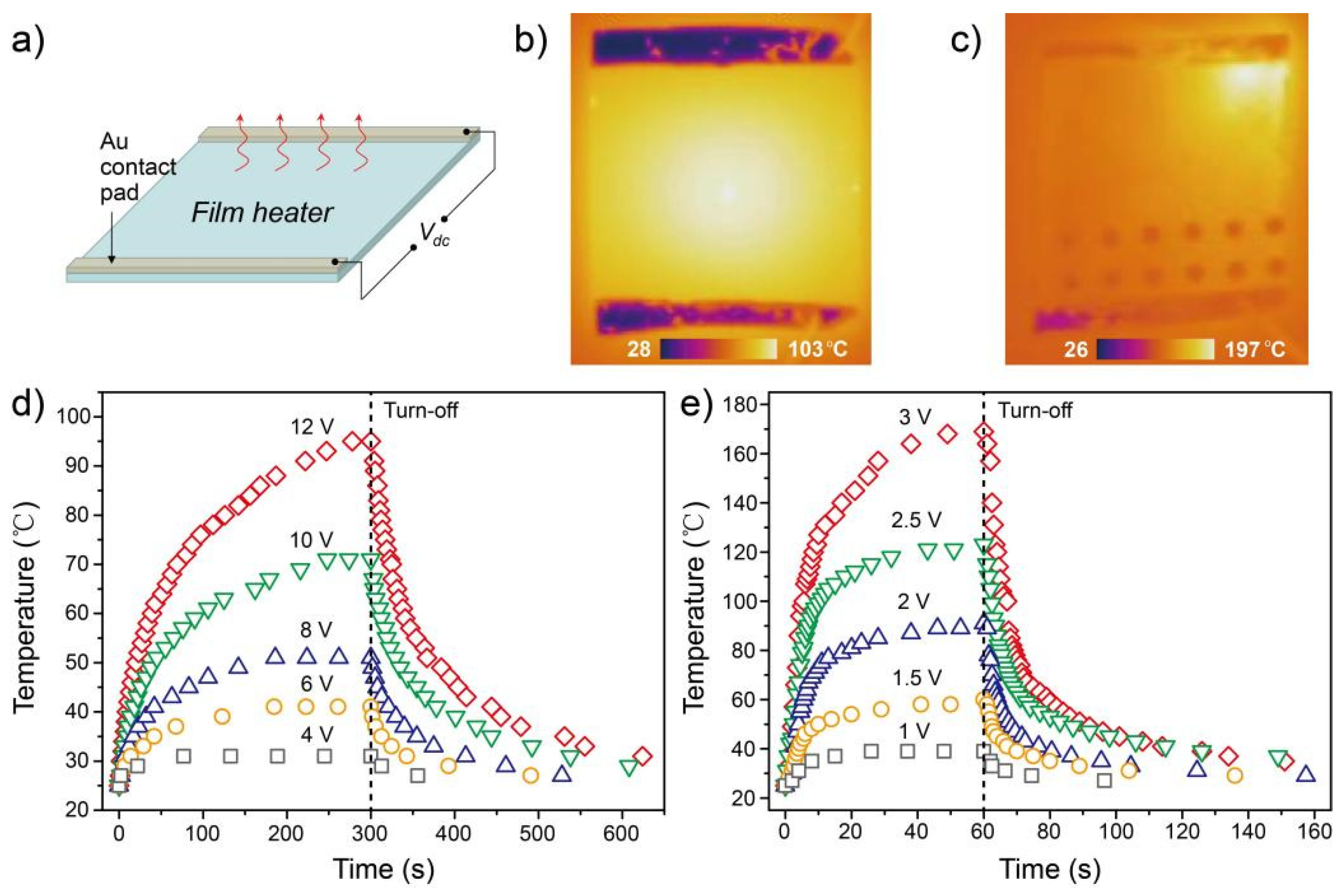
3.2. Electrical and Optical Properties
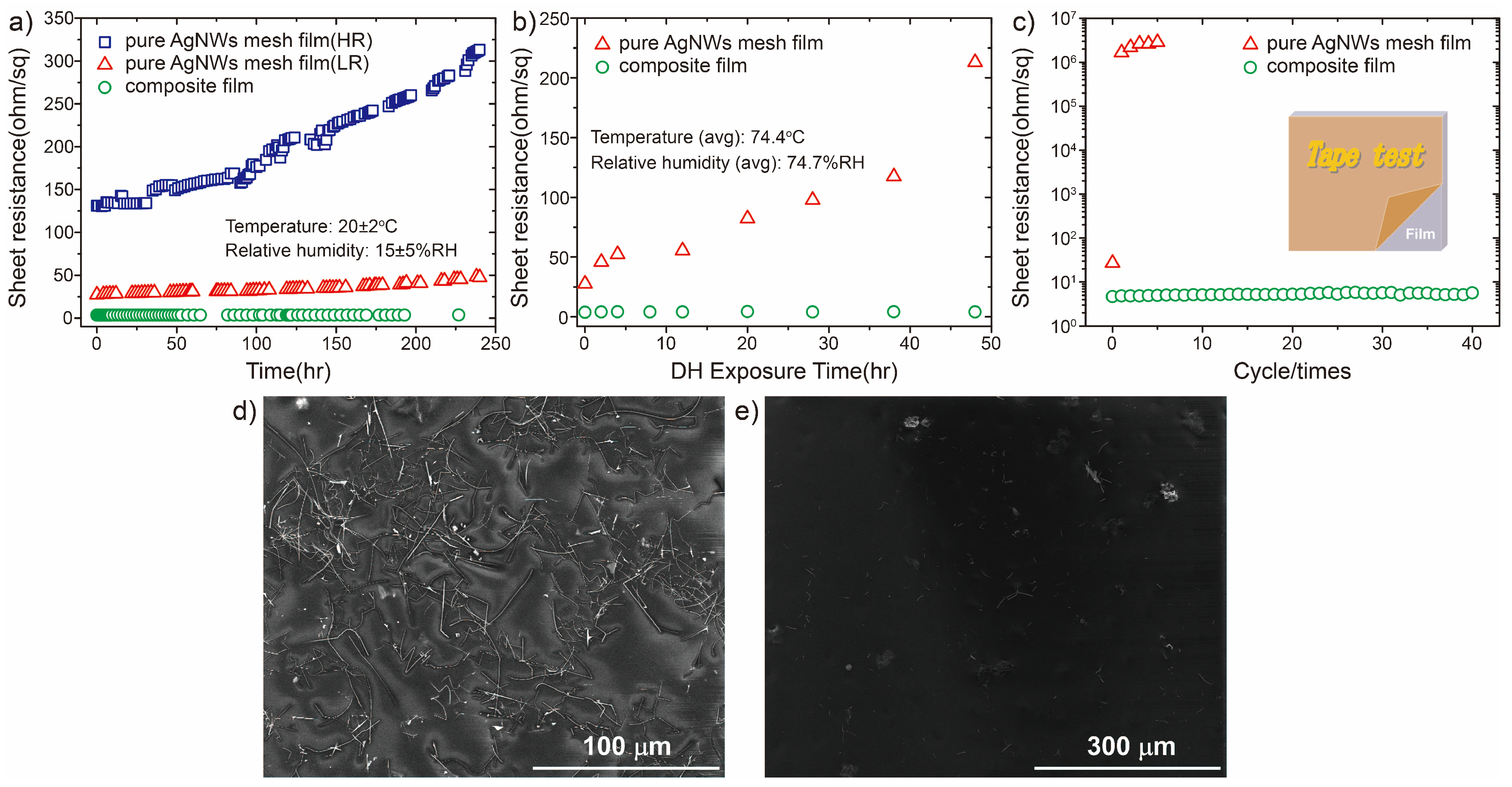
3.3. Material Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Shi, X.L.; Shang, H.J.; Gu, H.W.; Chen, W.Y.; Li, M.; Huang, D.X.; Dong, H.; Wang, X.L.; Ding, F.Z.; et al. High-performance Ag2Se-based thermoelectrics for wearable electronics. Nat. Commun. 2025, 16, 5002. [Google Scholar] [CrossRef]
- Zhang, C.; Ouyang, W.Y.; Zhang, L.; Li, D.C. A dual-mode fiber-shaped flexible capacitive strain sensor fabricated by direct ink writing technology for wearable and implantable health monitoring application. Microsyst. Nanoeng. 2023, 9, 158. [Google Scholar] [CrossRef]
- Wei, R.L.; Li, H.T.; Chen, Z.M.; Hua, Q.L.; Shen, G.Z.; Jiang, K. Revolutionizing wearable technology: Advanced fabrication techniques for body-conformable electronics. npj Flex. Electron. 2024, 8, 83. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Y.Y.; Guo, W.H.; Zhao, M.; Wang, H.; Wei, B.; Miao, Y.Q.; Guo, K.P. MXene/AgNWs/MXene Sandwich-Structured Transparent Electrode for High-Performance Flexible OLEDs. Small 2025, 21, 2409621. [Google Scholar] [CrossRef] [PubMed]
- Du, M.Y.; Yang, Z.; Miao, Y.Q.; Wang, C.; Dong, P.; Wang, H.; Guo, K.P. Facile Nanowelding Process for Silver Nanowire Electrodes Toward High-Performance Large-Area Flexible Organic Light-Emitting Diodes. Adv. Funct. Mater. 2024, 34, 2404567. [Google Scholar] [CrossRef]
- Yu, Z.B.; Zhang, Q.W.; Li, L.; Chen, Q.; Niu, X.F.; Liu, J.; Pei, Q.B. Highly Flexible Silver Nanowire Electrodes for Shape-Memory Polymer Light-Emitting Diodes. Adv. Mater. 2010, 23, 664–668. [Google Scholar] [CrossRef]
- Yan, X.Z.; Ma, J.G.; Xu, H.Y.; Wang, C.L.; Liu, Y.C. Fabrication of silver nanowires and metal oxide composite transparent electrodes and their application in UV light-emitting diodes. J. Phys. D Appl. Phys. 2016, 49, 325103. [Google Scholar] [CrossRef]
- Yan, Y.D.; Duan, B.W.; Ru, M.; Gu, Q.Y.; Li, S.S.; Zhao, W.C. Toward Flexible and Stretchable Organic Solar Cells: A Comprehensive Review of Transparent Conductive Electrodes, Photoactive Materials, and Device Performance. Adv. Energy Mater. 2024, 15, 2404233. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, K.R.; Liu, F.; Ran, G.L.; Wang, M.N.; Zhang, T.; Xu, R.J.; Liu, H.; Zhang, W.K.; Wei, Z.X.; et al. 20.6% Efficiency Organic Solar Cells Enabled by Incorporating a Lower Bandgap Guest Nonfullerene Acceptor Without Open-Circuit Voltage Loss. Adv. Mater. 2025, 37, 2500282. [Google Scholar] [CrossRef]
- Song, W.; Ye, Q.R.; Chen, Z.Y.; Ge, J.F.; Xie, L.; Ge, Z.Y. Advances in Stretchable Organic Photovoltaics: Flexible Transparent Electrodes and Deformable Active Layer Design. Adv. Mater. 2024, 36, 2311170. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.W.C.; Bolding, J.; Roldan-Carmona, C.; Ventosinos, F.; Paliwal, A.; Gil-Escrig, L.; Palazon, F.; Sessolo, M.; Zanoni, K.P.S.; Bolink, H.J. Room Temperature Pulsed Laser Deposition of Aluminum Zinc Oxide (AZO): Enabling Scalable Indium-Free Transparent Conductive Oxides. Adv. Funct. Mater. 2024, 35, 2418069. [Google Scholar] [CrossRef]
- Kim, G.W.; Back, S.A.; Park, J.E.; Arthanari, S.; Lee, H.S.; Hwang, J.S.; Yang, M.Y. Selective metallic reinforcement of silver nanowire junctions for improving the electrical conductivity of transparent electrodes. J. Micromech. Microeng. 2025, 35, 115002. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-Processed Metal Nanowire Mesh Transparent Electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef]
- Hussein, R.N.; Gomes, T.C.; Ng, E.; Rondeau-Gagne, S.; Carmichael, T.B. Composites of Shellac and Silver Nanowires as Flexible, Biobased, and Corrosion-Resistant Transparent Conductive Electrodes. Adv. Funct. Mater. 2025; early view. [Google Scholar] [CrossRef]
- Wang, J.T.; Fan, J.J.; Wan, T.; Hu, L.; Li, Z.; Chu, D.W. Progress in Silver Nanowire-Based Transparent Conductive Electrodes. Adv. Energy Sustain. Res. 2025, 6, 2500033. [Google Scholar] [CrossRef]
- Wu, Z.C.; Chen, Z.H.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, Conductive Carbon Nanotube Films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.J.; Rossi, J.E.; Broderick, D.L.; Puchades, I.; Landi, B.J. Free-Standing, Transparent Carbon Nanotube Thin Films with High Specific Shielding Effectiveness. Adv. Mater. Technol. 2024, 10, 2401392. [Google Scholar] [CrossRef]
- Mastrippolito, D.; Colle, A.; Gureghian, C.; Gemo, T.; Khalili, A.; Cavallo, M.; Bossavit, E.; Zhang, H.C.; Ma, Y.J.; Prado, Y.; et al. Graphene as Infrared and Electron Transparent Electrode Applied to the Design of Narrow Bandgap Nanocrystal-Based Photodiode. Adv. Opt. Mater. 2025, 13, 2500708. [Google Scholar] [CrossRef]
- Spechler, J.A.; Koh, T.W.; Herb, J.T.; Rand, B.P.; Arnold, C.B. Flexible Electronics: A Transparent, Smooth, Thermally Robust, Conductive Polyimide for Flexible Electronics. Adv. Funct. Mater. 2015, 25, 7547. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Coleman, E.; Kelly, A.; Gabbett, C.; Doolan, L.; Liu, S.X.; Yadav, N.; Vij, J.K.; Coleman, J.N. Extracting the Temperature Dependence of Both Nanowire Resistivity and Junction Resistance from Electrical Measurements on Printed Silver Nanowire Networks. ACS Appl. Electron. Mater. 2025, 7, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Yang, X.J.; Chen, J.B.; Yang, N.J.; Zhu, Y.W. Stability of silver nanowire transparent conductive film and strategies for improvement. Crit. Rev. Solid State Mater. Sci. 2025, 50, 1–53. [Google Scholar] [CrossRef]
- Meza, L.; Shukla, D.; Sadeghifar, H.; Hsiao, L.; Zhu, Y.; Venditti, R.A. Sustainable Soft Electronics with Biodegradable Regenerated Cellulose Films and Printed Recyclable Silver Nanowires. Adv. Sustain. Syst. 2025, 9, 2400713. [Google Scholar] [CrossRef]
- Jung, Y.; Pyun, K.R.; Yu, S.; Ahn, J.; Kim, J.; Park, J.J.; Lee, M.J.; Lee, B.; Won, D.; Bang, J.; et al. Laser-Induced Nanowire Percolation Interlocking for Ultrarobust Soft Electronics. Nano-Micro Lett. 2025, 17, 127. [Google Scholar] [CrossRef]
- Yan, X.Z.; Zhou, L.; Chu, X.F.; Wang, H.; Yang, F.; Wang, C.; Chi, Y.D.; Yang, X.T. Effect of Silver Nanowire Plasmons on Graphene Oxide Coatings Reduction for Highly Transparent Electrodes. Adv. Condens. Matter Phys. 2018, 2018, 5045427. [Google Scholar] [CrossRef]
- Kim, A.; Won, Y.; Woo, K.; Jeong, S.; Moon, J. All-Solution-Processed Indium-Free Transparent Composite Electrodes based on Ag Nanowire and Metal Oxide for Thin-Film Solar Cells. Adv. Funct. Mater. 2014, 24, 2462–2471. [Google Scholar] [CrossRef]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating Simulation and Experiments To Predict Sheet Resistance and Optical Transmittance in Nanowire Films for Transparent Conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, K.; Gasse, F.; Pagui, R.; Polywka, A.; Behrendt, A.; Trost, S.; Heiderhoff, R.; Gorrn, P.; Riedl, T. Highly Robust Indium-Free Transparent Conductive Electrodes Based on Composites of Silver Nanowires and Conductive Metal Oxides. Adv. Funct. Mater. 2014, 24, 1671–1678. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Russo, U.; Ielmini, D.; Cagli, C.; Lacaita, A.L.; Spiga, S.; Wiemer, C.; Perego, M.; Fanciulli, M. Conductive-filament switching analysis and self-accelerated thermal dissolution model for reset in NiO-based RRAM. In Proceedings of the 2007 IEEE International Electron Devices Meeting 2007, Washington, DC, USA, 10–12 December 2007; Volume 1, p. 2775. [Google Scholar] [CrossRef]



| Sample | Sheet Resistance (ohm/sq) | Transmittance (%) at 550 nm | Figure of Merit (10−3 ohm−1) |
|---|---|---|---|
| AgNWs mesh film Sample 3 | 27 | 72.7 | 1.529 |
| AgNWs-IZO composite film C1 | 3.3 | 71.5 | 10.645 |
| Pure AgNWs mesh film Sample 4 | 19 | 70.2 | 1.532 |
| AgNWs-IZO composite film C1 | 2.46 | 67.1 | 7.490 |
| Sputtered ITO | 40 | 90.5 | 9.210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Lyu, M.; Niu, Z. Silver Nanowire-Amorphous Indium Zinc Oxide Composite Electrodes for Transparent Film Heaters. Nanomaterials 2025, 15, 1883. https://doi.org/10.3390/nano15241883
Yan X, Lyu M, Niu Z. Silver Nanowire-Amorphous Indium Zinc Oxide Composite Electrodes for Transparent Film Heaters. Nanomaterials. 2025; 15(24):1883. https://doi.org/10.3390/nano15241883
Chicago/Turabian StyleYan, Xingzhen, Mengying Lyu, and Ziyao Niu. 2025. "Silver Nanowire-Amorphous Indium Zinc Oxide Composite Electrodes for Transparent Film Heaters" Nanomaterials 15, no. 24: 1883. https://doi.org/10.3390/nano15241883
APA StyleYan, X., Lyu, M., & Niu, Z. (2025). Silver Nanowire-Amorphous Indium Zinc Oxide Composite Electrodes for Transparent Film Heaters. Nanomaterials, 15(24), 1883. https://doi.org/10.3390/nano15241883






