A Focus on Thermal Durability and Oxidation Resistance and Morphology of Polymer Capped Copper Particles Through a Synthesis-Driven, Precursor-Influenced Approach
Abstract
1. Introduction
2. Materials and Methods
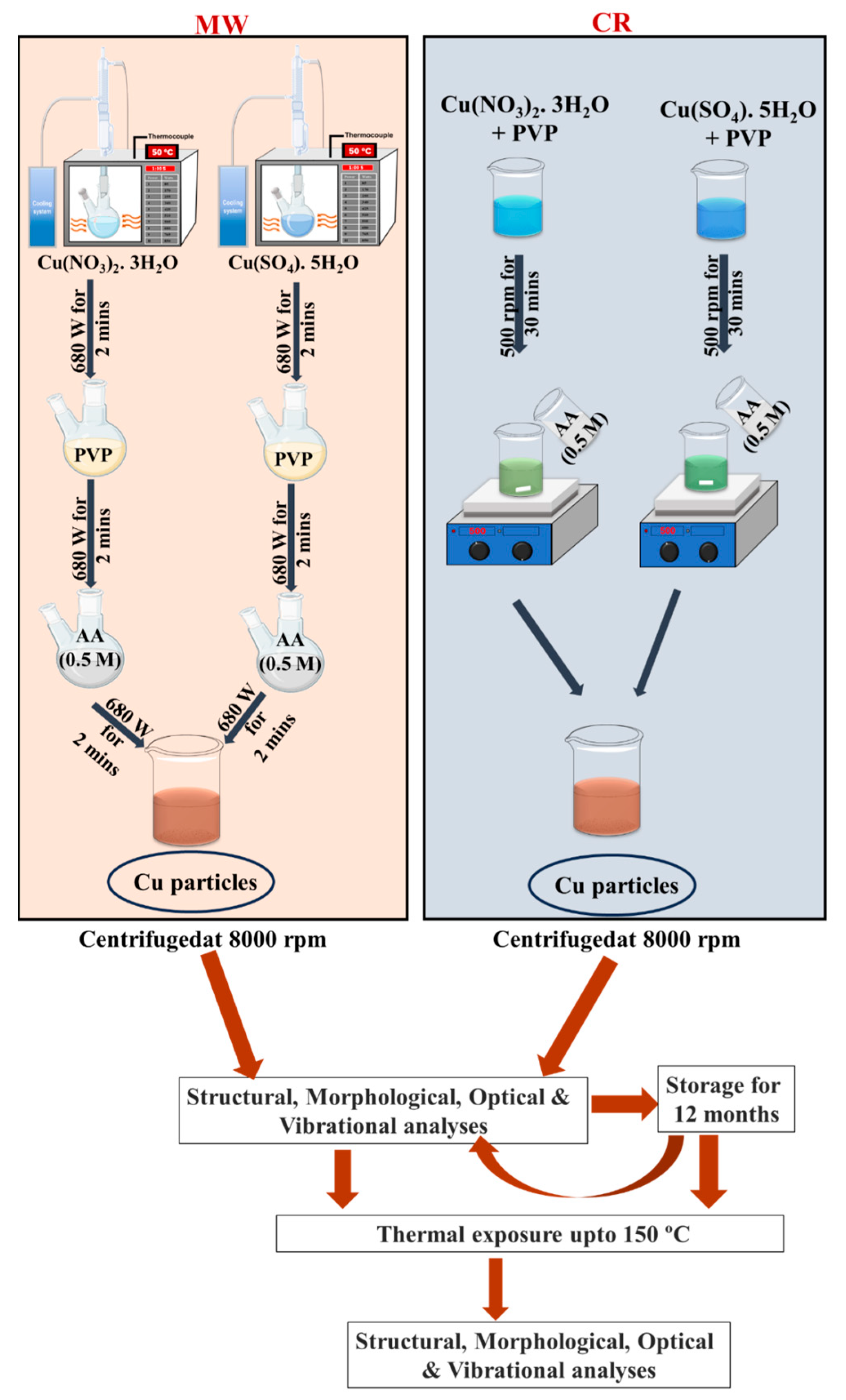
2.1. Preparation of Cu Structures
2.1.1. Synthesis Protocol A—Microwave Synthesis
2.1.2. Synthesis Protocol B—Chemical Reduction
2.2. Protocols Followed for Stability Assessment
- Group 1: Post-synthesized (PS) samples were tested immediately after synthesis. Each sample (100 μL) was drop-cast onto a 1 cm × 1 cm glass substrate and characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM). These samples were subjected to thermal exposure at 150 °C for 5 min in a custom high-temperature furnace, with a ramp rate of 10 °C per minute under ambient conditions.
- Group 2: Samples stored in deionized water for twelve months were selected from our previous report [57] specifically, NC2 and SC2. These aged samples were drop-cast and characterized before thermal treatment, followed by identical exposure at 150 °C for 5 min.
2.3. Characterizations
3. Results
3.1. Choice of Reagents and Concentrations

3.2. Discussions on Synthesis Method-Based Variations
3.2.1. Morphological Analyses
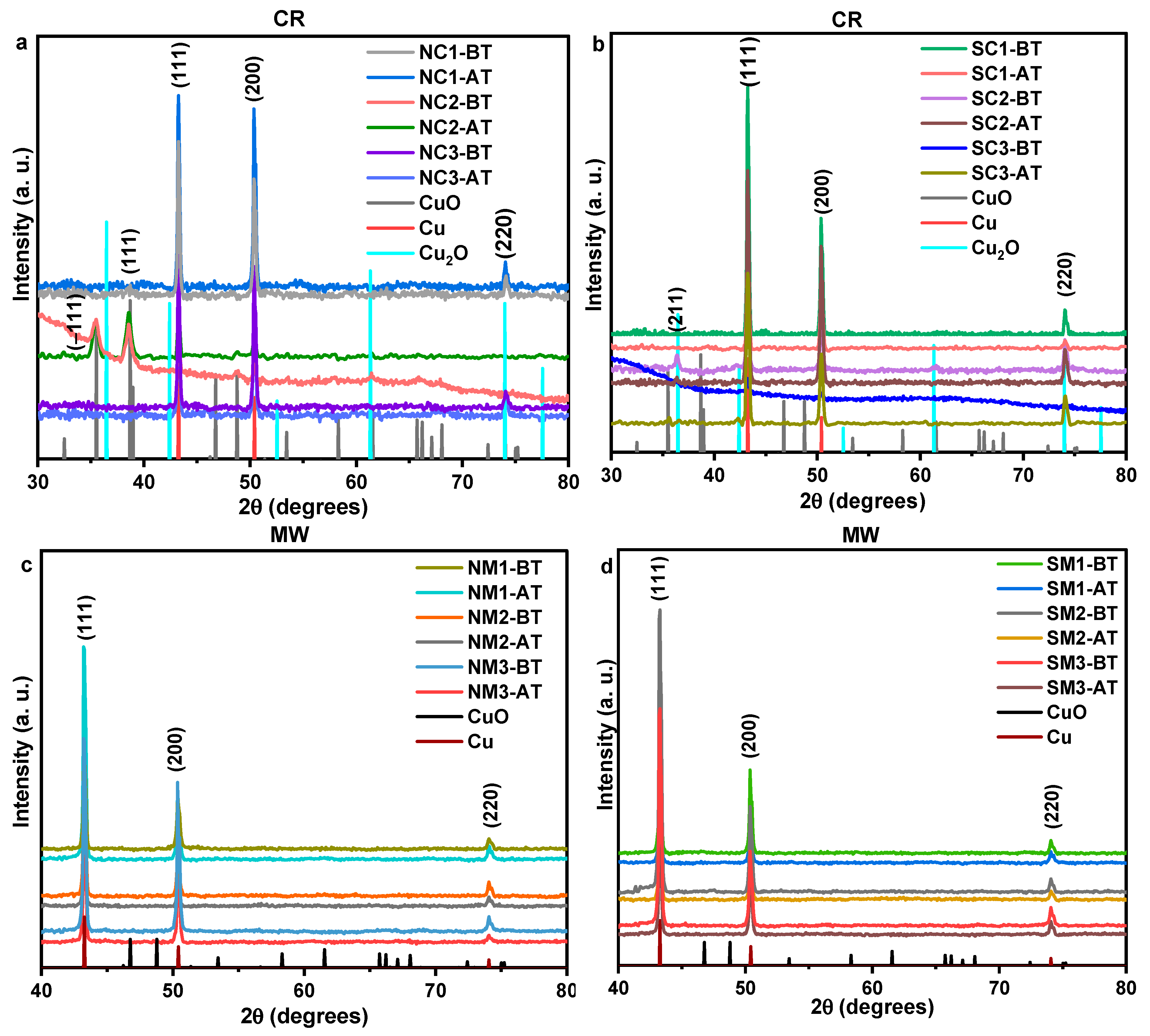
3.2.2. Lattice-Level Visualizations
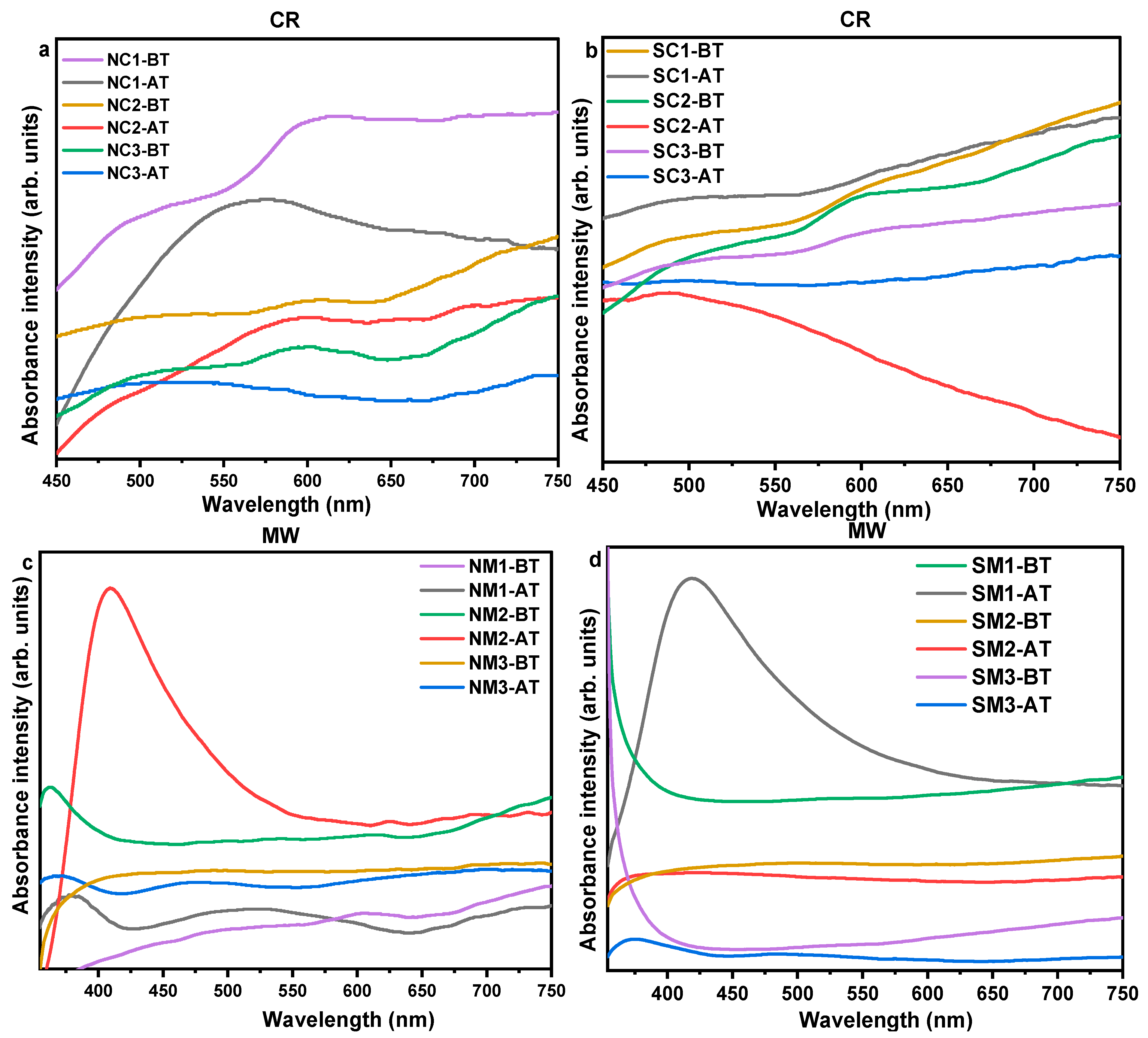
3.2.3. Optical Analyses
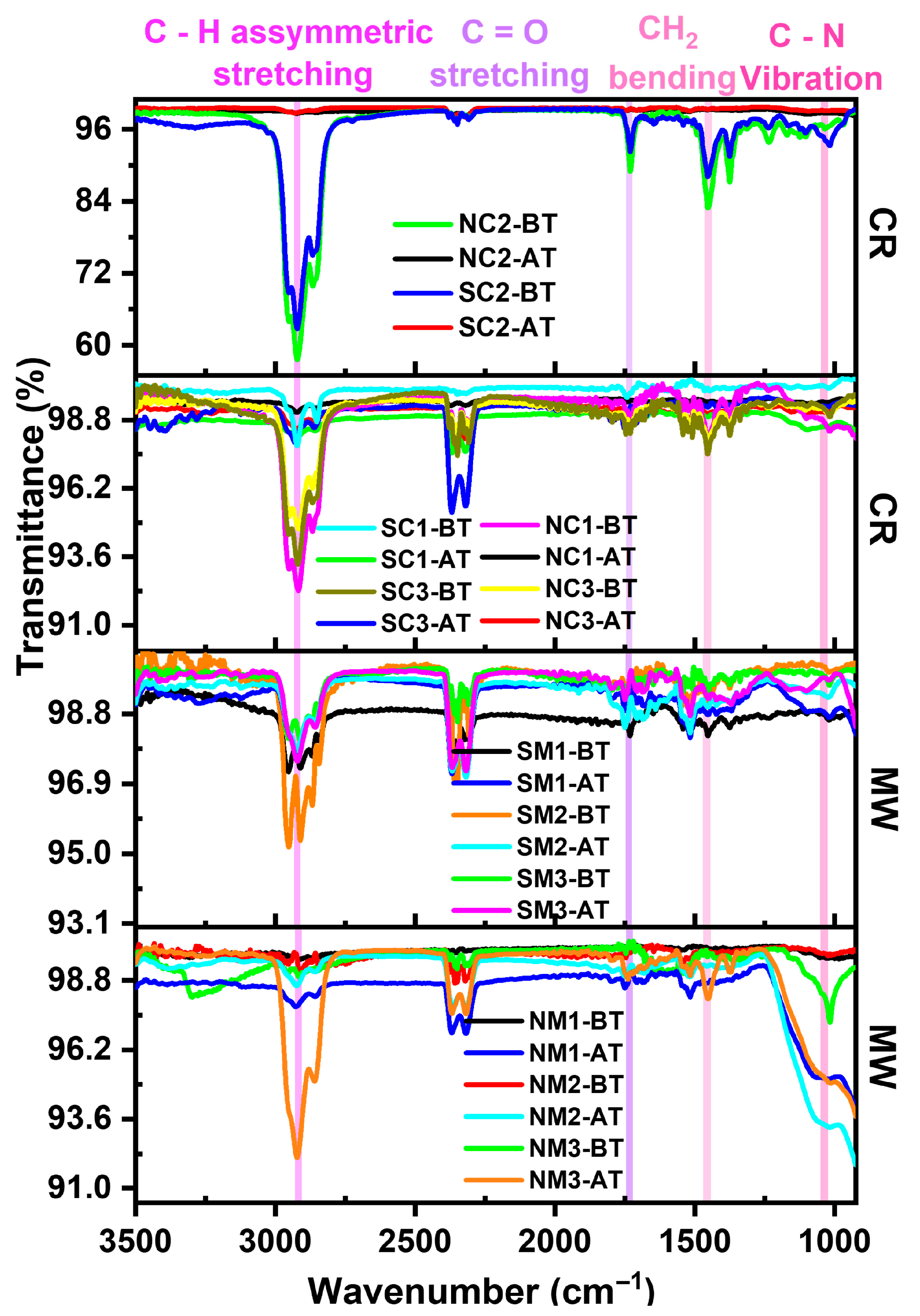
3.2.4. Vibrational Analyses
3.2.5. Electrokinetic Analysis
3.3. Investigations on Sample Variations Post Time/Thermal Exposures
3.3.1. Influence of Ambient Storage
3.3.2. Influences of Thermal Exposures
3.4. An Integrated Physicochemical and Stability Analysis
3.5. Comparative Studies on Pristine Cu and Integrated Cu Phases with Published Research
4. Observational Summary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Narayanan, K.; Gnanaprakash, D. Branched Gold Nanostructures Through a Facile Fructose Mediated Microwave Route. J. Clust. Sci. 2022, 33, 227–240. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Simulation of the Optical Absorption Spectra of Gold Nanorods as a Function of Their Aspect Ratio and the Effect of the Medium Dielectric Constant. J. Phys. Chem. B 1999, 103, 3073–3077, Erratum in J. Phys. Chem. B 2005, 109, 10531–10532. https://doi.org/10.1021/jp058091f. [Google Scholar] [CrossRef]
- Indhu, A.R.; Keerthana, L.; Dharmalingam, G. Plasmonic nanotechnology for photothermal applications—An evaluation. Beilstein J. Nanotechnol. 2023, 14, 380–419. [Google Scholar] [CrossRef]
- Indhu, A.R.; Dharanya, C.; Dharmalingam, G. Plasmonic Copper: Ways and Means of Achieving, Directing, and Utilizing Surface Plasmons. Plasmonics 2023, 19, 1303–1357. [Google Scholar] [CrossRef]
- Dabera, G.D.M.; Walker, M.; Sanchez, A.M.; Pereira, H.J.; Beanland, R.; Hatton, R.A. Retarding oxidation of copper nanoparticles without electrical isolation and the size dependence of work function. Nat. Commun. 2017, 8, 1894. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ward, M.R.; Lloyd, D.C.; Gai, P.L.; Boyes, E.D. Visualizing the Cu/Cu2O Interface Transition in Nanoparticles with Environmental Scanning Transmission Electron Microscopy. J. Am. Chem. Soc. 2017, 139, 179–185. [Google Scholar] [CrossRef]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic Properties of Copper Nanoparticles Fabricated by Nanosphere Lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Kim, J.H.; Ehrman, S.H.; Germer, T.A. Influence of particle oxide coating on light scattering by submicron metal particles on silicon wafers. Appl. Phys. Lett. 2004, 84, 1278–1280. [Google Scholar] [CrossRef]
- Pedersen, D.B.; Wang, S.; Liang, S.H. Charge-Transfer-Driven Diffusion Processes in Cu@Cu-Oxide Core−Shell Nanoparticles: Oxidation of 3.0 ± 0.3 nm Diameter Copper Nanoparticles. J. Phys. Chem. C 2008, 112, 8819–8826. [Google Scholar] [CrossRef]
- Ramsey, J.A.; Garlick, G.F.J.; Roberts, J.K. Theory of the oxidation of metals Some interactions of gases with metals and crystalline solids. Rep. Prog. Phys. 1949, 12, 163. [Google Scholar]
- Yang, J.C.; Kolasa, B.; Gibson, J.M.; Yeadon, M. Self-limiting oxidation of copper. Appl. Phys. Lett. 1998, 73, 2841–2843. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yamaguchi, T.; Sugawara, K.-I.; Koga, K. Role of Stress in the Self-Limiting Oxidation of Copper Nanoparticles. J. Phys. Chem. B 2005, 109, 20669–20672. [Google Scholar] [CrossRef] [PubMed]
- Cudennec, Y.; Lecerf, A.; Gérault, Y. Synthesis of Cu(OH)2 and CuO by soft chemistry. Eur. J. Solid State Inorg. Chem. 1995, 32, 1013–1022. [Google Scholar]
- Cudennec, Y.; Lecerf, A.; Riou, A.; Gérault, Y.; Cudennec, Y.; Lecerf, A.; Riou, A.; Synthesis, Y.G. Synthesis and study of sodium hydroxi-cuprate: Na2Cu(OH)4 and copper hydroxide: Cu(OH)2. Eur. J. Solid State Inorg. Chem. 1988, 25, 351–358. [Google Scholar]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Azarian, A.; Zad, A.I.; Dolati, A.; Ghorbani, M. Time dependence of the surface plasmon resonance of copper nanorods. J. Phys. Condens. Matter 2007, 19, 446007. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.-C.; Wang, X.; Wang, Z.; Liu, G.; Zhou, W.; Wen, L.; Li, Q.; Wang, X.; Chen, X.; et al. Facile synthesis of pentacle gold–copper alloy nanocrystals and their plasmonic and catalytic properties. Nat. Commun. 2014, 5, 4327. [Google Scholar] [CrossRef]
- Plascencia, G.; Utigard, T.; Marín, T. The oxidation resistance of copper-aluminum alloys at temperatures up to 1000 °C. JOM 2005, 57, 80–84. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kazuma, E.; Sakai, N.; Tatsuma, T. Photoelectrochemical Responses from Polymer-coated Plasmonic Copper Nanoparticles on TiO2. Chem. Lett. 2012, 41, 1340–1342. [Google Scholar] [CrossRef]
- Kanninen, P.; Johans, C.; Merta, J.; Kontturi, K. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, Y.; Zhuo, Y.; Dong, L.; Li, L.; Li, M. Controlled Synthesis of Various Hollow Cu Nano/MicroStructures via a Novel Reduction Route. Adv. Funct. Mater. 2007, 17, 933–938. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Valdez-Salas, B.; Valdez-Salas, E.; Pérez-Cortéz, G.; Nedev, N. Synthesis, Characterization, and In Situ Antifungal and Cytotoxicity Evaluation of Ascorbic Acid-Capped Copper Nanoparticles. J. Nanomater. 2019, 2019, 5287632. [Google Scholar] [CrossRef]
- Biçer, M.; Şişman, I. Controlled synthesis of copper nano/microstructures using ascorbic acid in aqueous CTAB solution. Powder Technol. 2010, 198, 279–284. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.; Jain, T.K. Ascorbic acid-mediated synthesis and characterisation of iron oxide/gold core–shell nanoparticles. J. Exp. Nanosci. 2016, 11, 370–382. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI. Appl. Nanosci. 2015, 5, 435–441. [Google Scholar] [CrossRef]
- Indhu, A.R.; Dharmalingam, G. Microwave Prepared Oxidation Resistant Cu Microstructures with Tailored Morphologies. Chem. Afr. 2024, 7, 2715–2724. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.; Li, G.; Zhao, T.; Fu, X.; Sun, R.; Zhou, F.; Wong, C.-P. Facile Preparation of Monodisperse, Impurity-Free, and Antioxidation Copper Nanoparticles on a Large Scale for Application in Conductive Ink. ACS Appl. Mater. Interfaces 2014, 6, 560–567. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Yu, A.-N.; Wang, K. Effects of reaction parameters on self-degradation of L-ascorbic acid and self-degradation kinetics. Food Sci. Biotechnol. 2016, 25, 97–104. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Sadeghi, B.; Sadjadi, M.A.S.; Pourahmad, A. Effects of protective agents (PVA & PVP) on the formation of silver nanoparticles. Int. J. Nanosci. Nanotechnol. 2008, 4, 3–12. [Google Scholar]
- del Caño, R.; Gisbert-González, J.M.; González-Rodríguez, J.; Sánchez-Obrero, G.; Madueño, R.; Blázquez, M.; Pineda, T. Effective replacement of cetyltrimethylammonium bromide (CTAB) by mercaptoalkanoic acids on gold nanorod (AuNR) surfaces in aqueous solutions. Nanoscale 2020, 12, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Aljadaani, A.H.A.; Al-Thabaiti, S.A.; Khan, Z. SDS capped Cu nanorods: Photosynthesis, stability, and their catalytic activity for trypan blue oxidative degradation. J. Mater. Res. Technol. 2021, 15, 6841–6854. [Google Scholar] [CrossRef]
- Tan, M.; Balela, M.D. Oleylamine Assisted Synthesis of Ultralong Copper Nanowires. In MATEC Web of Conferences, Proceedings of the 2015 4th International Conference on Engineering and Innovative Materials (ICEIM 2015), Penang, Malaysia, 3–4 September 2015; EDP Sciences: Les Ulis, France, 2015; Volume 27, p. 03003. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, N.; Kim, J.H.; Park, Y.I.; Piao, Y. Shape-Controlled Synthesis of Au Nanostructures Using EDTA Tetrasodium Salt and Their Photothermal Therapy Applications. Nanomaterials 2018, 8, 252. [Google Scholar] [CrossRef]
- Meng, F.; Jin, S. The Solution Growth of Copper Nanowires and Nanotubes is Driven by Screw Dislocations. Nano Lett. 2012, 12, 234–239. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Ye, J.-Y.; Attard, G.A.; Brew, A.; Zhou, Z.-Y.; Sun, S.-G.; Morgan, D.J.; Willock, D.J. Explicit Detection of the Mechanism of Platinum Nanoparticle Shape Control by Polyvinylpyrrolidone. J. Phys. Chem. C 2016, 120, 7532–7542. [Google Scholar] [CrossRef]
- Rónavári, A.; Bélteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Kónya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles—The Colloidal, Chemical, and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef]
- Centa, U.G.; Mihelčič, M.; Sterniša, M.; Perše, L.S. Tackling microbial adhesion to surfaces by adding mesoporous SiO2 nanoparticles to nanocomposite based on PVDF-HFP and PVP polymers. Surfaces Interfaces 2025, 56, 105713. [Google Scholar] [CrossRef]
- Falqi, F.H.; Bin-Dahman, O.A.; Hussain, M.; Al-Harthi, M.A. Preparation of Miscible PVA/PEG Blends and Effect of Graphene Concentration on Thermal, Crystallization, Morphological, and Mechanical Properties of PVA/PEG (10 wt%) Blend. Int. J. Polym. Sci. 2018, 2018, 8527693. [Google Scholar] [CrossRef]
- Marques, A.C.; Pinheiro, T.; Morais, M.; Martins, C.; Andrade, A.F.; Martins, R.; Sales, M.G.F.; Fortunato, E. Bottom-up microwave-assisted seed-mediated synthesis of gold nanoparticles onto nanocellulose to boost stability and high performance for SERS applications. Appl. Surf. Sci. 2021, 561, 150060. [Google Scholar] [CrossRef]
- Wu, S. Preparation of fine copper powder using ascorbic acid as reducing agent and its application in MLCC. Mater. Lett. 2007, 61, 1125–1129. [Google Scholar] [CrossRef]
- Rehan, M.; Mowafi, S.; Aly, S.A.; Elshemy, N.S.; Haggag, K. Microwave-heating for in-situ Ag NPs preparation into viscose fibers. Eur. Polym. J. 2017, 86, 68–84. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Jahan, I.; Erci, F.; Isildak, I. Facile microwave-mediated green synthesis of non-toxic copper nanoparticles using Citrus sinensis aqueous fruit extract and their antibacterial potentials. J. Drug Deliv. Sci. Technol. 2021, 61, 102172. [Google Scholar] [CrossRef]
- Sreeju, N.; Rufus, A.; Philip, D. Microwave-assisted rapid synthesis of copper nanoparticles with exceptional stability and their multifaceted applications. J. Mol. Liq. 2016, 221, 1008–1021. [Google Scholar] [CrossRef]
- Tanghatari, M.; Sarband, Z.N.; Rezaee, S.; Larijani, K. Microwave assisted green synthesis of copper nanoparticles. Bulg. Chem. Commun. Spec. Issue J. 2017, 49, 347–352. [Google Scholar]
- Yallappa, S.; Manjanna, J.; Sindhe, M.A.; Satyanarayan, N.D.; Pramod, S.N.; Nagaraja, K. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 108–115. [Google Scholar] [CrossRef]
- Zhu, H.-T.; Zhang, C.-Y.; Yin, Y.-S. Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradiation. J. Cryst. Growth 2004, 270, 722–728. [Google Scholar] [CrossRef]
- Blosi, M.; Albonetti, S.; Dondi, M.; Martelli, C.; Baldi, G. Microwave-assisted polyol synthesis of Cu nanoparticles. J. Nanoparticle Res. 2011, 13, 127–138. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Marracci, M.; Piccinelli, F.; Tellini, B. Novel microwave-synthesis of Cu nanoparticles in the absence of any stabilizing agent and their antibacterial and antistatic applications. Appl. Surf. Sci. 2013, 280, 610–618. [Google Scholar] [CrossRef]
- El-Berry, M.F.; Sadeek, S.A.; Abdalla, A.M.; Nassar, M.Y. Microwave-assisted fabrication of copper nanoparticles utilizing different counter ions: An efficient photocatalyst for photocatalytic degradation of safranin dye from aqueous media. Mater. Res. Bull. 2021, 133, 111048. [Google Scholar] [CrossRef]
- Naik, R.; Shivashankar, S.A.; Bindu, P.J. Microwave-assisted synthesis of copper nanoparticles: Influence of copper nanoparticles morphology on the antimicrobial activity. J. Mat. NanoSci. 2020, 7, 62–67. [Google Scholar]
- Zhang, X.; Cheng, X.; Yin, H.; Yuan, J.; Xu, C. Preparation of needle shaped nano-copper by microwave-assisted water system and study on its application of enhanced epoxy resin coating electrical conductivity. Appl. Surf. Sci. 2008, 254, 5757–5759. [Google Scholar] [CrossRef]
- Indhu, A.R.; Dharmalingam, G. Oxidative and Colloidal Kinetics of Size-Controlled Copper Structures through Surface Plasmon-Regulated Examinations for Broadband Absorbance. ChemistrySelect 2024, 9, e202305087. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, D.; Chen, J.; Zhou, H.; Wan, H. The use of CTAB to control the size of copper nanoparticles and the concentration of alkylthiols on their surfaces. Mater. Sci. Eng. A 2006, 415, 156–161. [Google Scholar] [CrossRef]
- Aromaa, J.; Kekkonen, M.; Mousapour, M.; Jokilaakso, A.; Lundström, M. The Oxidation of Copper in Air at Temperatures up to 100 °C. Corros. Mater. Degrad. 2021, 2, 625–640. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.; Fang, L.; Meng, M.; Liu, Z.; Di, Y.; Cai, W.; Huang, S.; Gan, Z. Copper nanoparticles with near-unity, omnidirectional, and broadband optical absorption for highly efficient solar steam generation. Nanotechnology 2018, 30, 015402. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Lu, X.; Nejati, S.; Jaramillo, H.; Elimelech, M. In situ surface functionalization of reverse osmosis membranes with biocidal copper nanoparticles. Desalination 2016, 388, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Ni, X.; Zheng, H. One-step preparation of copper nanorods with rectangular cross sections. Solid State Commun. 2006, 139, 412–414. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wang, Z. Surfactant-assisted preparation of single-crystalline Fe3O4 nanowires under low magnetic field. Mater. Lett. 2007, 61, 1629–1632. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, F.; Wang, Y.-X.J.; Yu, J.C.; Leung, K.C.-F. Facile synthesis of size-controllable monodispersed ferrite nanospheres. J. Mater. Chem. 2010, 20, 5086–5094. [Google Scholar] [CrossRef]
- Wiley, B.J.; Wang, Z.; Wei, J.; Yin, Y.; Cobden, D.H.; Xia, Y. Synthesis and Electrical Characterization of Silver Nanobeams. Nano Lett. 2006, 6, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-Y.; Huang, P.-J. Magnetic nanoprobes for rapid detection of copper ion in aqueous environment by surface-enhanced Raman spectroscopy. RSC Adv. 2022, 12, 921–928. [Google Scholar] [CrossRef]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef]
- Filankembo, A.; Giorgio, S.; Lisiecki, I.; Pileni, M.P. Is the Anion the Major Parameter in the Shape Control of Nanocrystals? J. Phys. Chem. B 2003, 107, 7492–7500. [Google Scholar] [CrossRef]
- Whitehead, C.B.; Özkar, S.; Finke, R.G. LaMer’s 1950 model of particle formation: A review and critical analysis of its classical nucleation and fluctuation theory basis, of competing models and mechanisms for phase-changes and particle formation, and then of its application to silver halide, semiconductor, metal, and metal-oxide nanoparticles. Mater. Adv. 2021, 2, 186–235. [Google Scholar] [CrossRef]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
- Herring, N.P.; AbouZeid, K.; Mohamed, M.B.; Pinsk, J.; El-Shall, M.S. Formation Mechanisms of Gold–Zinc Oxide Hexagonal Nanopyramids by Heterogeneous Nucleation using Microwave Synthesis. Langmuir 2011, 27, 15146–15154. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, L.; Li, Y.; Zhang, C. Synthesis and Characterization of Monodispersed Copper Colloids in Polar Solvents. Nanoscale Res. Lett. 2009, 4, 465–470. [Google Scholar] [CrossRef]
- Borodko, Y.; Lee, H.S.; Joo, S.H.; Zhang, Y.; Somorjai, G. Spectroscopic Study of the Thermal Degradation of PVP-Capped Rh and Pt Nanoparticles in H2 and O2 Environments. J. Phys. Chem. C 2010, 114, 1117–1126. [Google Scholar] [CrossRef]
- Averitt, R.D.; Westcott, S.L.; Halas, N.J. Linear optical properties of gold nanoshells. J. Opt. Soc. Am. B 1999, 16, 1824–1832. [Google Scholar] [CrossRef]
- White, A.H.; Germer, L.H. The Rate of Oxidation of Copper at Room Temperature. Trans. Electrochem. Soc. 1942, 81, 305–319. [Google Scholar] [CrossRef]
- Sekhon, J.S.; Verma, S.S. Cu, CuO, and Cu2O Nanoparticle Plasmons for Enhanced Scattering in Solar Cells. In Proceedings of the Optical Instrumentation for Energy and Environmental Applications 2011, Austin, TX, USA, 2–3 November 2011; pp. 10–13. [Google Scholar] [CrossRef]
- Harris, N.; Blaber, M.G.; Schatz, G.C. Optical properties of metal nanoparticles. In Encyclopedia of Nanotechnology; Springer: Dordrecht, The Netherlands, 2016; pp. 3027–3048. [Google Scholar] [CrossRef]
- Jin, H.; Kahk, J.M.; Papaconstantopoulos, D.A.; Ferreira, A.; Lischner, J. Plasmon-Induced Hot Carriers from Interband and Intraband Transitions in Large Noble Metal Nanoparticles. PRX Energy 2022, 1, 013006. [Google Scholar] [CrossRef]
- Shaheen, N.; Nazar, R.; Mehmood, U.; Raza, S.A.; Iftikhar, F.; Naz, R.; Habib, M.S.; Rafiq, M.A.; Sharif, S.; Farooq, M. Development of Polyaniline/Polyvinylpyrrolidone (PANI/PVP) Composite Films for Piezoresistive Strain-Sensing Applications. Arab. J. Sci. Eng. 2023, 48, 16419–16429. [Google Scholar] [CrossRef]
- Oh, G.-H.; Hwang, H.-J.; Kim, H.-S. Effect of copper oxide shell thickness on flash light sintering of copper nanoparticle ink. RSC Adv. 2017, 7, 17724–17731. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, H.; Peng, R.; He, R.; Li, M.; Feng, W.; Zhao, Y.; Liu, Z. Experimental Study at the Phase Interface of a Single-Crystal Ni-Based Superalloy Using TEM. Materials 2022, 15, 6915. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F. Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Mater. Lett. 2009, 63, 441–443. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; Delfin-Narciso, D.; Juárez-Cortijo, L.; Nazario-Naveda, R. Influence of the volume of ascorbic acid in the synthesis of copper nanoparticles mediated by chemical pathway and its stability over time. IOP Conf. Ser. Earth Environ. Sci. 2021, 897, 012010. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; He, Y.; Huang, H.; Wu, Y.; Hou, L.; Liu, X.; Yang, T.; Zou, J.; Huang, B. Synthesis, growth mechanism and thermal stability of copper nanoparticles encapsulated by multi-layer graphene. Carbon 2012, 50, 2119–2125. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. The influence of solvents and surfactants on the preparation of copper nanoparticles by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 025004. [Google Scholar] [CrossRef]
- Ng, C.H.B.; Fan, W.Y. Shape Evolution of Cu2O Nanostructures via Kinetic and Thermodynamic Controlled Growth. J. Phys. Chem. B 2006, 110, 20801–20807. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, N.; Ferdous, S.; Henneke, D. Colloidal synthesis of copper nanoparticles in a two-phase liquid–liquid system. Mater. Lett. 2010, 64, 45–48. [Google Scholar] [CrossRef]
- Dang, C.M.; Trinh, C.D.; Dang, D.M.T.; Blanc, E.F. Characteristics of colloidal copper particles prepared by using polyvinyl pyrrolidone and polyethylene glycol in chemical reduction method. Int. J. Nanotechnol. 2013, 10, 296. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Sánchez-Iglesias, A.; Rodríguez-González, B.; Liz-Marzán, L.M. Aerobic Synthesis of Cu Nanoplates with Intense Plasmon Resonances. Small 2009, 5, 440–443. [Google Scholar] [CrossRef]
- Jeong, S.; Woo, K.; Kim, D.; Lim, S.; Kim, J.S.; Shin, H.; Xia, Y.; Moon, J. Controlling the Thickness of the Surface Oxide Layer on Cu Nanoparticles for the Fabrication of Conductive Structures by Ink-Jet Printing. Adv. Funct. Mater. 2008, 18, 679–686. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.-R.; Lee, K.J.; Stott, N.E.; Kim, D. Large-scale synthesis of copper nanoparticles by chemically controlled reduction for applications of inkjet-printed electronics. Nanotechnology 2008, 19, 415604. [Google Scholar] [CrossRef]
- Choudhary, S.; Sarma, J.V.N.; Pande, S.; Ababou-Girard, S.; Turban, P.; Lepine, B.; Gangopadhyay, S. Oxidation mechanism of thin Cu films: A gateway towards the formation of single oxide phase. AIP Adv. 2018, 8, 055114. [Google Scholar] [CrossRef]
- Curtis, A.C.; Duff, D.G.; Edwards, P.P.; Jefferson, D.A.; Johnson, B.F.G.; Kirkland, A.I.; Wallace, A.S. Preparation and structural characterization of an unprotected copper sol. J. Phys. Chem. 1988, 92, 2270–2275. [Google Scholar] [CrossRef]
- Nilsson, S.; El Berch, J.N.; Albinsson, D.; Fritzsche, J.; Mpourmpakis, G.; Langhammer, C. The Role of Grain Boundary Sites for the Oxidation of Copper Catalysts during the CO Oxidation Reaction. ACS Nano 2023, 17, 20284–20298. [Google Scholar] [CrossRef]
- Cuya Huaman, J.L.; Urushizaki, I.; Jeyadevan, B. Large-Scale Cu Nanowire Synthesis by PVP-Ethylene Glycol Route. J. Nanomater. 2018, 2018, 1698357. [Google Scholar] [CrossRef]












| PVP Concentrations | Samples from the Nitrate Precursor | Samples from the Sulphate Precursor | ||
|---|---|---|---|---|
| CR * | MW * | CR | MW | |
| 0.03 mM | * NC1 | * NM1 | * SC1 | * SM1 |
| 0.3 mM | NC2 | NM2 | SC2 | SM2 |
| 3 mM | NC3 | NM3 | SC3 | SM3 |
| CR | MW | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | NC1 | SC1 | NC2 | SC2 | NC3 | SC3 | NM1 | SM1 | NM2 | SM2 | NM3 | SM3 |
| Crystallite size (nm) | 39.89 | 36.56 | 37.38 | 39.38 | 36.56 | 36.56 | 38.15 | 39.89 | 38.15 | 36.56 | 38.15 | 36.56 |
| Sample Name | hkl | Crystal Size (nm) | Average Crystal Size (nm) | |
|---|---|---|---|---|
| NC2 (Post synthesis) | Cu | (111) | 37.38 | 36.86 |
| (200) | 33.25 | |||
| (220) | 39.96 | |||
| NC2 (Post 12 months) | CuO | (−111) | 13.79 | 14.48 |
| (111) | 15.15 | |||
| Cu | (111) | 25.04 | 24.35 | |
| (200) | 23.64 | |||
| SC2 (Post synthesis) | Cu | (111) | 39.38 | 35.86 |
| (200) | 32.35 | |||
| SC2 (Post 12 months) | Cu2O | (211) | 26.85 | 26.85 |
| Cu | (111) | 29.22 | 28.04 | |
| (200) | 27.30 | |||
| (220) | 27.61 | |||
| S. NO. | Capping Molecule | Stability for | Oxide Planes Observed | Stored Environment | Temperature (°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Oleylamine | 1 Hour | (111), (220) | Air | 230 | [82] | |
| 2 | AA | 11 days | Not available | Not available | [83] | ||
| 3 | Graphene | 60 days | [84] | ||||
| 4 | PVP and AA | 22 days | Water | 210 | [85] | ||
| 5 | CTAB and alkanethiols | During Drying | (110) | Vacuum | 80 | [58] | |
| 6 | Tetraoctylammonium Bromide | Not available | n-dodecane | 150 | [86] | ||
| 7 | PVP | 30 min | (110), (211), (311), (222) | Water | Not available | [87] | |
| 8 | PEG | 2 days | Not available | Water | Room temperature | [88] | |
| 9 | PVP | 1 day | (220), (110) | Air | Room temperature | [89] | |
| 10 | PVP | Not available | Vacuum | 200 | [90] | ||
| 11 | PVP | 20 days | Not employed | Air | 180 | [91] | |
| 12 | Sodium metaphosphate | Not available | 200 | [44] | |||
| 13 | Not employed | 30 min | (200), (111), (−111) | 150 | [92] | ||
| 14 | 3-mercaptopropionic acid | 7 days | Not available | Room temperature | [6] | ||
| 15 | AA | Not available | 237 | [44] | |||
| 16 | Lauric acid | 2 day | (111), (220) | Room temperature | [21] | ||
| 17 | Not employed | Not available | (111) | [93] | |||
| 18 | SiNx | 30 min | (111), (220) | CO | 400 | [94] | |
| 19 | PVP | 240 min | - | Methanol | 182 | [95] | |
| 20 | PVP and AA | 12 months | (−111), (111), and (211) | Air | 150 | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indhu, A.R.; Minakshi, M.; Sivasubramanian, R.; Dharmalingam, G. A Focus on Thermal Durability and Oxidation Resistance and Morphology of Polymer Capped Copper Particles Through a Synthesis-Driven, Precursor-Influenced Approach. Nanomaterials 2025, 15, 1852. https://doi.org/10.3390/nano15241852
Indhu AR, Minakshi M, Sivasubramanian R, Dharmalingam G. A Focus on Thermal Durability and Oxidation Resistance and Morphology of Polymer Capped Copper Particles Through a Synthesis-Driven, Precursor-Influenced Approach. Nanomaterials. 2025; 15(24):1852. https://doi.org/10.3390/nano15241852
Chicago/Turabian StyleIndhu, A. R., Manickam Minakshi, R. Sivasubramanian, and Gnanaprakash Dharmalingam. 2025. "A Focus on Thermal Durability and Oxidation Resistance and Morphology of Polymer Capped Copper Particles Through a Synthesis-Driven, Precursor-Influenced Approach" Nanomaterials 15, no. 24: 1852. https://doi.org/10.3390/nano15241852
APA StyleIndhu, A. R., Minakshi, M., Sivasubramanian, R., & Dharmalingam, G. (2025). A Focus on Thermal Durability and Oxidation Resistance and Morphology of Polymer Capped Copper Particles Through a Synthesis-Driven, Precursor-Influenced Approach. Nanomaterials, 15(24), 1852. https://doi.org/10.3390/nano15241852







