Nanostructured Thin Films: Properties, Fabrication and Applications—A Short Review
Abstract
1. Introduction
2. Properties of Nanostructured Thin Films
2.1. Physical Properties
2.2. Chemical Properties
2.3. Optical Properties
| Property | Phenomenon | Physical Mechanism | Theoretical Model/Approach | References |
|---|---|---|---|---|
| Physical Properties | High reactivity, phase transitions at lower T | High surface-to-volume ratio → high surface energy → atomic unsaturation | Surface thermodynamics; Gibbs free energy minimization; surface diffusion models | [34,35,36,37,38,39] |
| Grain growth, densification, coarsening | Enhanced surface diffusion during/after deposition | Diffusion models (Arrhenius-type activation), sintering models | [34,35,36,37,38] | |
| Structural reconstruction | Reduced coordination → lower activation energy for rearrangement | Surface reconstruction theory; thermodynamic stability models | [39] | |
| Chemical Properties | High catalytic activity | Abundant surface active sites (edges, corners, vacancies) | Langmuir–Hinshelwood adsorption kinetics; surface reaction models | [40,41,42,43,44] |
| Quantum-size-modified chemical reactivity | Bandgap shifting via quantum confinement | Particle-in-a-box/confinement models | [42,43] | |
| Ion diffusion, redox behavior | Grain boundary-driven high ion diffusivity | Defect chemistry; diffusion/ion-transport models | [45,46] | |
| Photochemical activity | Surface defects, charge trapping | Charge-transfer models, defect-state theories | [47,48,49] | |
| Optical Properties | LSPR in metal nanostructures | Collective oscillation of conduction electrons | Mie theory; plasmonics models | [50,51] |
| Quantum confinement and bandgap widening | Reduced dimensions < de Broglie wavelength | Effective mass approximation; confinement models | [52,53] | |
| Interference effects | Thin-film interference (multi-layer reflections) | Fresnel equations; thin-film optical models | [54] | |
| Scattering, refractive index modification | Surface roughness and porosity | Effective medium theory; scattering theory | [55] | |
| SHG enhancement | Field localization, exciton–BIC coupling | Nonlinear optics (χ(2) theory); interface-mode modeling | [62,63,64,65] | |
| SERS enhancement | Electromagnetic field amplification in hot-spots | Plasmonic enhancement models (EM enhancement), charge-transfer theory | [66,67,68,69] | |
| Electrical Properties | Tunable conductivity, transparency | Nanostructure size → percolation pathways | Percolation theory; effective-medium approximations | [62,63,64,65,66,67,68,69,70,71,72] |
| Carrier scattering/transport | Grain boundaries as potential barriers | Seto’s grain boundary model; transport scattering models | [72] | |
| Defect-modulated conductivity | Vacancies, interstitials trap carriers | Polaron models; trap-state theory | [73] | |
| Thin-film device optimization | Charge transport determined by film thickness/crystallinity | Semiconductor device physics; drift–diffusion models | [74,75,76,77,78,79,80,81,82] | |
| Magnetic Properties | Perpendicular magnetic anisotropy (PMA) | Interface-driven spin–orbit coupling | Magnetic anisotropy models; micromagnetics | [83,84] |
| Superparamagnetism | KV ≈ kBT → thermally induced magnet reversal | Néel–Arrhenius model | [85] | |
| Tunable AFM–FM transition | Doping (Co, Pd, Ir) modifies magnetic phase stability | Phase transition theories; alloy energetics | [86,87,88,89,90] | |
| Exchange bias | Interfacial uncompensated spins | Meiklejohn–Bean model | [91] | |
| GMR/TMR | Spin-dependent electron scattering/tunneling | Spin-transport models; Jullière model | [92,93,94,95,96,97] | |
| Defect Engineering in 2D Materials | Defect-induced magnetism | Vacancies/dopants create localized magnetic moments | DFT (first-principles); exchange interaction calculations | [98,99] |
| Strain-controlled defect energetics | External strain interacts with local defect fields | Strain–defect coupling models; DFT mechanical simulations | [100,101] | |
| Gas sensing enhancement | Charge transfer at defect sites | Adsorption energy and charge-transfer models | [100] |
| Property | Nanostructured Thin Films | Epitaxial Thin Films | Implications |
|---|---|---|---|
| Surface energy | High | Low | Reactivity and phase transitions |
| Grain boundaries | Numerous | Few | Catalytic activity vs. electronic uniformity |
| Morphology | Tunable via deposition | Smooth, uniform | Morphology control vs. electronic precision |
| Phase transitions | Lower temperature | Bulk-like | Processing flexibility vs. stability |
| Optical Effect | Nanostructured Films | Epitaxial Films | Applications |
|---|---|---|---|
| LSPR | Strong, tunable | Weak | Sensing applications |
| SHG | Enhanced via hot spots | Moderate | Nonlinear photonics |
| SERS | High enhancement | Limited | Substrate uniformity vs. signal |
| Quantum confinement | Tunable bandgap | Bulk-like | LEDs, photovoltaics |
2.4. Electrical Properties
2.5. Magnetic Properties
2.6. Defect Engineering in Two-Dimensional (2D) Materials
3. Fabrication and Characterization Methods of Nanostructured Thin Films
4. Advancements and Applications
4.1. Energy Conversion and Storage
4.2. Smart Coatings and Self-Cleaning Surfaces
4.3. Sensing and Biomedical Applications
4.4. Future Outlook and Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benelmekki, M.; Erbe, A. Nanostructured thin films–background, preparation and relation to the technological revolution of the 21st century. In Frontiers of Nanoscience; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Du, X.; Yang, T.; Li, Q.; Jin, L. Preparation and optical properties of nanostructure thin films. Appl. Nanosci. 2021, 11, 1967–1976. [Google Scholar] [CrossRef]
- Toma, F.T.Z.; Rahman, M.S.; Maria, K.H. A review of recent advances in ZnO nanostructured thin films by various deposition techniques. Discov. Mater. 2025, 5, 60. [Google Scholar] [CrossRef]
- Hai, Z.; Wei, Z.; Xue, C.; Xu, H.; Verpoort, F. Nanostructured tungsten oxide thin film devices: From optoelectronics and ionics to iontronics. J. Mater. Chem. C 2019, 7, 12968–12990. [Google Scholar] [CrossRef]
- Khan, Z.R.; Shkir, M. Third order optical nonlinearities in CdS nanostructured thin films: A comprehensive review. J. Mater. Sci. Mater. Electron. 2021, 32, 24176–24197. [Google Scholar] [CrossRef]
- Patwary, M.A.M.; Hossain, M.A.; Ghos, B.C.; Chakrabarty, J.; Haque, S.R.; Rupa, S.A.; Uddin, J.; Tanaka, T. Copper oxide nanostructured thin films processed by SILAR for optoelectronic applications. RSC Adv. 2022, 12, 32853–32884. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Sang, D.; Zou, L.; Yao, Y.; Zhou, C.; Fu, H.; Xi, H.; Fan, J.; Meng, L.; Wang, C. A review on the progress of optoelectronic devices based on TiO2 thin films and nanomaterials. Nanomaterials 2023, 13, 1141. [Google Scholar] [CrossRef]
- Flory, F.; Escoubas, L.; Berginc, G. Optical properties of nanostructured materials: A review. J. Nanophotonics 2011, 5, 052502. [Google Scholar] [CrossRef]
- Abdul, S.; Judit, T.; Ilona, F.; Nikoletta, M. Functional thin films and nanostructures for sensors. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 485–519. [Google Scholar] [CrossRef]
- Moura, P.C.; Sério, S. Recent applications and future trends of nanostructured Thin Films-Based gas sensors produced by Magnetron Sputtering. Coatings 2024, 14, 1214. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Meenakshi, G.A.; Vinothini, S.; Yu, C.; Chen, C.; Chiu, T.; Vittayakorn, N. A review of Thin-Film growth, properties, applications, and future prospects. Processes 2025, 13, 587. [Google Scholar] [CrossRef]
- Nunes, D.; Fortunato, E.; Martins, R. Flexible nanostructured TiO2-based gas and UV sensors: A review. Discov. Mater. 2022, 2, 2. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Bhowmik, B. Micro/nanostructured gas sensors: The physics behind the nanostructure growth, sensing and selectivity mechanisms. Nanoscale Adv. 2020, 3, 73–93. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Pasuk, I.; Stan, G.E.; Stan, M.S.; Popa, M.; Chifiriuc, M.C.; Hapenciuc, C.; Oktar, F.N.; et al. Fish bone derived Bi-Phasic calcium phosphate coatings fabricated by pulsed laser deposition for biomedical applications. Mar. Drugs 2020, 18, 623. [Google Scholar] [CrossRef]
- Kacica, C.T.; Biswas, P. Improved conductivity and ionic mobility in nanostructured thin films via aliovalent doping for ultra-high rate energy storage. Nanoscale Adv. 2020, 2, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, M.; Solterbeck, C.; Es-Souni, M. Clay nanomaterial thin film electrodes for electrochemical energy storage applications. RSC Adv. 2014, 4, 46976–46979. [Google Scholar] [CrossRef]
- Shen, B.; Li, Y.; Sun, N.; Zhao, Y.; Hao, X. Enhanced energy-storage performance of an all-inorganic flexible bilayer-like antiferroelectric thin film via using electric field engineering. Nanoscale 2020, 12, 8958–8968. [Google Scholar] [CrossRef]
- Litra, D.; Chiriac, M.; Ababii, N.; Lupan, O. Acetone sensors based on Al-Coated and Ni-Doped Copper oxide nanocrystalline thin films. Sensors 2024, 24, 6550. [Google Scholar] [CrossRef]
- Jang, Y.; Park, S.; Char, K. Functionalization of polymer multilayer thin films for novel biomedical applications. Korean J. Chem. Eng. 2011, 28, 1149–1160. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, L.S.; Zhang, K.; Baumgart, H. Advanced nested coaxial Thin-Film ZnO nanostructures synthesized by atomic layer deposition for improved sensing performance. Appl. Sci. 2024, 14, 10959. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Gaballah, A.E.H.; Tihtih, M.; Derkaoui, I.; Zaki, S.E.; Eker, Y.R.; Ateş, Ş. Thermionic emission of atomic layer deposited MoO3/Si UV photodetectors. Materials 2023, 16, 2766. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Mitrofanov, I.; Nazarov, D.; Medvedev, O.; Kim, A.; Ezhov, I.; Rumyantsev, A.; Popovich, A.; Maximov, M.Y. Atomic layer deposition of Ni-Co-O Thin-Film electrodes for Solid-State LIBs and the influence of chemical composition on overcapacity. Nanomaterials 2021, 11, 907. [Google Scholar] [CrossRef]
- Stramarkou, M.; Bardakas, A.; Krokida, M.; Tsamis, C. Fabrication of ZnO Thin Films Doped with Na at Different Percentages for Sensing CO2 in Small Quantities at Room Temperature. Sensors 2025, 25, 2705. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B.; Closset, R. Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of annealing on structural and optical properties. Crystals 2023, 14, 6. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Eftimie, M.; Elisa, M.; Vasiliu, I.C.; Bartha, C.; Enculescu, M.; Filipescu, M.; Aguado, C.E.; Lopez, D.; Sava, B.A.; et al. Nanostructured PbS-Doped inorganic film synthesized by Sol-Gel Route. Nanomaterials 2022, 12, 3006. [Google Scholar] [CrossRef]
- Ssennyimba, K.W.; Nkele, A.C.; Byaruhanga, P.; Waligo, D.; Ezema, F.I. Recent advances in titanium nitride (TiN) nanostructures and thin films for bio-sensing applications. JOM 2025, 77, 4286–4300. [Google Scholar] [CrossRef]
- Martinuzzi, S.M.; Caporali, S.; Taurino, R.; Gabellini, L.; Berretti, E.; Schmeer, E.; Calisi, N. Nanostructured chromium PVD thin films fabricated through Copper–Chromium selective dissolution. Materials 2025, 18, 894. [Google Scholar] [CrossRef]
- Schurink, B.; Van Den Beld, W.T.E.; Tiggelaar, R.M.; Van De Kruijs, R.W.E.; Bijkerk, F. Synthesis and characterization of boron thin films using chemical and physical vapor depositions. Coatings 2022, 12, 685. [Google Scholar] [CrossRef]
- Fernández-González, C.; Ruiz-Gómez, S.; Arché-Núñez, A.; Pérez, L.; De Sousa, C.T. Nano-patterning using ultra-thin alumina membranes. Mater. Today Nano 2024, 29, 100553. [Google Scholar] [CrossRef]
- Ha, C.; Chung, Y.J. Thin films as practical quantum materials: A status quo and beyond. APL Mater. 2024, 12, 120901. [Google Scholar] [CrossRef]
- Şahin, B. Flexible nanostructured CuO thin film: A promising candidate for wearable real-time sweat rate monitoring devices. Sens. Actuators A Phys. 2022, 341, 113604. [Google Scholar] [CrossRef]
- Singh, D.; Padha, N.; Hussain, Z.; Ahmed, Z.; Dolma, P. Growth of nanostructured ZnTe thin films through annealing of the MSELD-prepared Stack of precursors for photonic applications. Chem. Phys. Impact 2025, 10, 100837. [Google Scholar] [CrossRef]
- Karvounis, A.; Timpu, F.; Vogler-Neuling, V.V.; Savo, R.; Grange, R. Barium titanate nanostructures and thin films for photonics. Adv. Opt. Mater. 2020, 8, 2001249. [Google Scholar] [CrossRef]
- Abdallah, B.; Zetoun, W.; Tello, A. Deposition of ZnO thin films with different powers using RF magnetron sputtering method: Structural, electrical and optical study. Heliyon 2024, 10, e27606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, S.K.; Sharma, H.; Kumar, S.; Banerjee, M.; Vij, A. Investigation of structural and optical properties of ZnO thin films of different thickness grown by pulsed laser deposition method. Phys. B Condens. Matter 2018, 552, 221–226. [Google Scholar] [CrossRef]
- Khiari, M.; Gilliot, M.; Lejeune, M.; Lazar, F.; Hadjadj, A. Preparation of very thin zinc oxide films by liquid deposition process: Review of key processing parameters. Coatings 2022, 12, 65. [Google Scholar] [CrossRef]
- Kaźmierczak-Bałata, A.; Grządziel, L.; Guziewicz, M.; Venkatachalapathy, V.; Kuznetsov, A.; Krzywiecki, M. Correlations of thermal properties with grain structure, morphology, and defect balance in nanoscale polycrystalline ZnO films. Appl. Surf. Sci. 2021, 546, 149095. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.; Pedrero, E.; Rodríguez-Lazcano, Y.; Rodríguez, J. Morphology, Microstructure and Optical Properties of Nanostructured Ultrasonically Sprayed ZnO Thin Films with Different Thicknesses. Opt. Mater. 2025, 162, 116899. [Google Scholar] [CrossRef]
- Zarzycki, A.; Perzanowski, M.; Krupinski, M.; Marszalek, M. Phase transformations and magnetism in patterned FePd thin films. Nanoscale 2025, 17, 11739–11751. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Chaib, H.; Mohammedi, L.; Benmebrouk, L.; Boukraa, A.; Daoudi, B.; Achouri, A. Effect of metal atom substitutions in Li based hydrides for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 28920–28929. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Ren, X.; Sangle, A.; Zhang, S.; Yuan, S.; Zhao, Y.; Shi, L.; Hoye, R.L.Z.; Cho, S.; Li, D.; MacManus-Driscoll, J.L. Photoelectrochemical water splitting strongly enhanced in fast-grown ZnO nanotree and nanocluster structures. J. Mater. Chem. A 2016, 4, 10203–10211. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.A.; Raina, A.; Mohan, S.; Singh, R.A.; Jayalakshmi, S.; Haq, M.I.U. Nanostructured Coatings: Review on processing techniques, corrosion behaviour and tribological performance. Nanomaterials 2022, 12, 1323. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K. The Li-Ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Gilani, M.R.H.S.; Maaz, M.; Akhter, P.; Hussain, M.; Jeong, K.; Kwon, E.E.; Bae, S.; Park, Y. Advancements in TiO2-based photocatalysis for environmental remediation: Strategies for enhancing visible-light-driven activity. Chemosphere 2023, 349, 140703. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Krishna, S.B.N.; Kumar, P.; Kumar, V.; Ghosh, K.S.; Swart, H.C.; Bellucci, S.; Cho, J. Recent advances on metal oxide based Nano-Photocatalysts as potential antibacterial and antiviral agents. Catalysts 2022, 12, 1047. [Google Scholar] [CrossRef]
- Patil, M.; Shaikh, S.; Ganesh, I. Recent Advances on TiO2 Thin Film Based Photocatalytic Applications (A Review). Curr. Nanosci. 2015, 11, 271–285. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Klimov, V.I. Spectral and dynamical properties of multiexcitons in semiconductor nanocrystals. Annu. Rev. Phys. Chem. 2006, 58, 635–673. [Google Scholar] [CrossRef] [PubMed]
- Ekimov, A.; Efros, A.; Onushchenko, A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- MacLeod, H.A.; Macleod, H.A. Thin-Film Optical Filters; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2011, 61, 1–7. [Google Scholar] [CrossRef]
- Fujiwara, H. Spectroscopic Ellipsometry; Wiley: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Baida, F.I.; Yepes, J.J.R.; Ndao, A. Giant second harmonic generation in etch-less lithium niobate thin film. J. Appl. Phys. 2023, 133, 124501. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Y.; Dang, Z.; Zeng, C.; Qi, X.; Guo, G.; Ren, X.; Xia, J. Strongly enhanced second harmonic generation in a thin film lithium niobate heterostructure cavity. Phys. Rev. Lett. 2021, 127, 153901. [Google Scholar] [CrossRef]
- Krajczewski, J.; Ambroziak, R.; Kudelski, A. Substrates for Surface-Enhanced Raman Scattering Formed on Nanostructured Non-Metallic Materials: Preparation and Characterization. Nanomaterials 2020, 11, 75. [Google Scholar] [CrossRef]
- Mura, S.; Greppi, G.; Innocenzi, P.; Piccinini, M.; Figus, C.; Marongiu, M.L.; Guo, C.; Irudayaraj, J. Nanostructured thin films as surface-enhanced Raman scattering substrates. J. Raman Spectrosc. 2012, 44, 35–40. [Google Scholar] [CrossRef]
- Tognazzi, A.; Franceschini, P.; Biechteler, J.; Baù, E.; Cino, A.C.; Tittl, A.; De Angelis, C.; Sortino, L. Interface second harmonic generation enhancement in bulk WS2/MoS2 hetero-bilayer van der Waals nanoantennas. Light Sci. Appl. 2025, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, G.; Zhu, P.; Zhang, K.; Xu, P.; Zhu, Z. Efficient Second-Harmonic Generation in Thin-Film Lithium Tantalate Through Modal Phase-Matching. Photonics 2024, 11, 1150. [Google Scholar] [CrossRef]
- He, J.; Liu, L.; Lin, M.; Chen, H.; Ma, F. Efficient Second-Harmonic Generation in Adapted-Width Waveguides Based on Periodically Poled Thin-Film Lithium Niobate. Micromachines 2024, 15, 1145. [Google Scholar] [CrossRef]
- Zhao, J.; Rüsing, M.; Javid, U.A.; Ling, J.; Li, M.; Lin, Q.; Mookherjea, S. Shallow-etched thin-film lithium niobate waveguides for highly-efficient second-harmonic generation. Opt. Express 2020, 28, 19669–19682. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Buonomo, M.; Casalini, S.; Bonacchi, S.; Meneghetti, M.; Litti, L. High performance multi-purpose nanostructured thin films by inkjet printing: Au micro-electrodes and SERS substrates. Nanoscale Adv. 2023, 5, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Li, B.; Song, H.; Tan, C.L.; Shi, Y.; Yan, S. Semiconducting Tungsten Trioxide Thin Films for High-Performance SERS Biosensors. Nanomaterials 2025, 15, 1393. [Google Scholar] [CrossRef]
- Visbal, C.A.; Cervantes, W.R.; Marín, L.; Betancourt, J.; Pérez, A.; Diosa, J.E.; Rodríguez, L.A.; Mosquera-Vargas, E. The Fabrication of Gold Nanostructures as SERS Substrates for the Detection of Contaminants in Water. Nanomaterials 2024, 14, 1525. [Google Scholar] [CrossRef]
- Raj, D.; Scaglione, F.; Rizzi, P. Rapid Fabrication of Fe and Pd Thin Films as SERS-Active Substrates via Dynamic Hydrogen Bubble Template Method. Nanomaterials 2023, 13, 135. [Google Scholar] [CrossRef]
- Wu, F.; Cai, W.; Gao, J.; Loo, Y.; Yao, N. Nanoscale electrical properties of epitaxial Cu3Ge film. Sci. Rep. 2016, 6, 28818. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, T.S.; Wanger, D.D.; Ray, N.; Walker, B.J.; Strasfeld, D.; Bawendi, M.G.; Kastner, M.A. Nanopatterned electrically conductive films of semiconductor nanocrystals. Nano Lett. 2012, 12, 4404–4408. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Feng, Y.P. Semiconductor Nanowires and Nanotubes: Effects of size and Surface-to-Volume ratio. ACS Nano 2008, 2, 2410–2414. [Google Scholar] [CrossRef]
- Zaouche, C. Electrical and Magnetic properties of nanostructured NiO thin films prepared by spray pyrolysis Method. Biomed. J. Sci. Tech. Res. 2023, 52, 43761–43766. [Google Scholar] [CrossRef]
- Liu, T. Evaluating the impact of microstructure modifications on thin film photoelectric properties. J. Meas. Eng. 2024, 13, 120–129. [Google Scholar] [CrossRef]
- Smaha, R.W.; Mangum, J.S.; Leahy, I.A.; Calder, J.; Hautzinger, M.P.; Muzzillo, C.P.; Perkins, C.L.; Talley, K.R.; Eley, S.; Gorai, P.; et al. Structural and optoelectronic properties of thin film LaWN3. Phys. Rev. Mater. 2023, 7, 084411. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, R.; Genc, A.; Raychaudhuri, P.; Fraser, H.L.; Ayyub, P. Size induced metal–insulator transition in nanostructured niobium thin films: Intra-granular and inter-granular contributions. J. Phys. Condens. Matter 2006, 18, 4553–4566. [Google Scholar] [CrossRef]
- Peters, C.; Weber, A.; Butz, B.; Gerthsen, D.; Ivers-Tiffée, E. Grain-Size effects in YSZ Thin-Film electrolytes. J. Am. Ceram. Soc. 2009, 92, 2017–2024. [Google Scholar] [CrossRef]
- Qian, C.; Sun, J.; Gao, Y. Transport of charge carriers and optoelectronic applications of highly ordered metal phthalocyanine heterojunction thin films. Phys. Chem. Chem. Phys. 2021, 23, 9631–9642. [Google Scholar] [CrossRef]
- Benítez-Rico, A.; García-Sánchez, M.F.; Picquart, M.; Monroy-Peláez, B.M.; Santana-Rodríguez, G. Understanding the high ionic conductivity in nanostructured ytterbium stabilized zirconia thin films. J. Nanomater. 2015, 2015, 692648. [Google Scholar] [CrossRef]
- De, S.; King, P.J.; Lyons, P.E.; Khan, U.; Coleman, J.N. Size Effects and the Problem with Percolation in Nanostructured Transparent Conductors. ACS Nano 2010, 4, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, R.; Takano, Y. Preparation of SnS films in chemical solution using microwave irradiation. Thin Solid Film. 2017, 636, 171–176. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- King, B.; Lessard, B.H. Review of recent advances and sensing mechanisms in solid-state organic thin-film transistor (OTFT) sensors. J. Mater. Chem. C 2024, 12, 5654–5683. [Google Scholar] [CrossRef]
- Song, J.; Liu, H.; Zhao, Z.; Lin, P.; Yan, F. Flexible organic transistors for biosensing: Devices and applications. Adv. Mater. 2023, 36, e2300034. [Google Scholar] [CrossRef]
- Hao, Z.; Wu, Z.; Liu, S.; Tang, X.; Chen, J.; Liu, X. High-performance organic thin-film transistors: Principles and strategies. J. Mater. Chem. C 2024, 12, 9427–9454. [Google Scholar] [CrossRef]
- Prasad, K.H.; Vinoth, S.; Juliet, A.V.; Ganesh, V.; Yahia, I.S.; AlAbdulaal, T.H. Terbium-doped SnS thin films: Comprehensive physical and optoelectronic characterizations for the photodetector applications. Ionics 2024, 30, 5079–5091. [Google Scholar] [CrossRef]
- Aydın, R.; Akkaya, A.; Kahveci, O.; Şahin, B. Nanostructured CuO Thin-Film-Based conductometric sensors for Real-Time tracking of sweat loss. ACS Omega 2023, 8, 20009–20019. [Google Scholar] [CrossRef]
- Liang, B.; Chen, X.; Yuan, H.; Wang, X.; Hou, G.; Zhao, Y.; Zhang, X. Enhancing optical and electrical performances via nanocrystalline Si-Based thin films for Si heterojunction solar cells. ACS Omega 2024, 9, 49935–49944. [Google Scholar] [CrossRef]
- Enesio, M.J.; Villegas, C.E.P.; Venezuela, P.; Rocha, A.R. Photovoltaic efficiency of transition metal dichalcogenides thin films by ab initio excited-state methods. arXiv 2023, arXiv:2312.10284. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhou, X.; Yu, J.; Lan, X.; Shi, Y.; Li, J.; Liu, B.; Li, Y.; Chen, G.; Hu, R.; et al. Performance improvement of resistive switching memory achieved by reducing the size of MoS2 embedded in poly(vinyl alcohol) films. J. Mater. Chem. C 2025, 13, 5660–5672. [Google Scholar] [CrossRef]
- Nadalini, G.; Borghi, F.; Košutová, T.; Falqui, A.; Ludwig, N.; Milani, P. Engineering the structural and electrical interplay of nanostructured Au resistive switching networks by controlling the forming process. Sci. Rep. 2023, 13, 19713. [Google Scholar] [CrossRef]
- Chappert, C.; Fert, A.; Van Dau, F.N. The emergence of spin electronics in data storage. Nat. Mater. 2007, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.D.; Worledge, D.C. A new spin on magnetic memories. Nat. Nanotechnol. 2015, 10, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Dormann, J.L.; Fiorani, D.; Tronc, E. Magnetic relaxation in Fine-Particle systems. In Advances in Chemical Physics; Wiley & Sons, Inc.: New York, NY, USA, 1997; pp. 283–494. [Google Scholar] [CrossRef]
- Lee, C.; Anbalagan, A.K.; Chang, T.; Fan, C.; Chung, J.; Chien, S. The exchange bias effect on single layer of Fe-rich FeRh thin film. Mater. Lett. 2019, 254, 309–311. [Google Scholar] [CrossRef]
- Komlev, A.S.; Vaschenkova, A.R.; Yusupov, D.M.; Rusakov, V.S.; Amirov, A.A.; Chirkova, A.M.; Aliev, A.M.; Ilina, T.S.; Kiselev, D.A.; Baranov, N.V.; et al. Cobalt-doped FeRh system: Studies of compositional homogeneity and property stability. J. Alloys Compd. 2025, 1036, 181971. [Google Scholar] [CrossRef]
- Seo, S.; Park, M.; Jung, M. Impact of Co doping on the magnetic and transport properties of FeRh. APL Mater. 2024, 12, 121111. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Zhou, X.; Wang, Y.; Pan, F.; Song, C. Influence of film composition on the transition temperature of FeRh films. J. Cryst. Growth 2016, 438, 19–24. [Google Scholar] [CrossRef]
- Graët, C.L.; Charlton, T.R.; McLaren, M.; Loving, M.; Morley, S.A.; Kinane, C.J.; Brydson, R.M.D.; Lewis, L.H.; Langridge, S.; Marrows, C.H. Temperature controlled motion of an antiferromagnet- ferromagnet interface within a dopant-graded FeRh epilayer. APL Mater. 2015, 3, 041802. [Google Scholar] [CrossRef]
- Nogués, J.; Schuller, I.K. Exchange bias. J. Magn. Magn. Mater. 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Elsafi, B. Tailoring the magnetic properties of giant magnetoresistance multilayers via modification of ultrathin Non-Magnetic spacer thickness. J. Electron. Mater. 2024, 53, 2852–2860. [Google Scholar] [CrossRef]
- Marchal, N.; Da Câmara Santa Clara Gomes, T.; Araujo, F.A.; Piraux, L. Giant Magnetoresistance and Magneto-Thermopower in 3D Interconnected NixFe1−x/Cu Multilayered Nanowire Networks. Nanomaterials 2021, 11, 1133. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, L.; Hellenes, A.B.; González-Hernández, R.; Sinova, J.; Jungwirth, T. Giant and Tunneling Magnetoresistance in Unconventional Collinear Antiferromagnets with Nonrelativistic Spin-Momentum Coupling. Phys. Rev. X 2022, 12, 011028. [Google Scholar] [CrossRef]
- Acharya, J.; Goul, R.; Wu, J. High Tunneling Magnetoresistance in Magnetic Tunnel Junctions with Subnanometer Thick Al2O3 Tunnel Barriers Fabricated Using Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2020, 13, 15738–15745. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Zhao, W. Tunable Tunneling Magnetoresistance in van der Waals Magnetic Tunnel Junctions with 1T-CrTe2 Electrodes. ACS Appl. Mater. Interfaces 2020, 13, 1214–1221. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Gurung, G.; Zhu, M.; Zhang, P.; Zheng, F.; Tsymbal, E.Y.; Zhang, J. Tunneling magnetoresistance in noncollinear antiferromagnetic tunnel junctions. Phys. Rev. Lett. 2022, 128, 197201. [Google Scholar] [CrossRef]
- Afrid, S.M.T. Defect engineered magnetism induction and electronic structure modulation in monolayer MoS2. Heliyon 2023, 10, e23384. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Guo, H.; Yang, T.; Zhang, Z. Strain-induced magnetism in MoS2 monolayer with defects. J. Appl. Phys. 2014, 115, 054305. [Google Scholar] [CrossRef]
- Fu, X.; Qiao, Z.; Zhou, H.; Xie, D. Defect Engineering in Transition Metal Dichalcogenide-Based Gas Sensors. Chemosensors 2024, 12, 85. [Google Scholar] [CrossRef]
- Santra, P.; Ghaderzadeh, S.; Ghorbani-Asl, M.; Komsa, H.-P.; Besley, E.; Krasheninnikov, A.V. Strain-modulated defect engineering of two-dimensional materials. npj 2D Mater. Appl. 2024, 8, 33. [Google Scholar] [CrossRef]
- Nasir, T.; Han, Y.; Blackman, C.; Beanland, R.; Hector, A.L. Zinc Oxide Nanostructure Deposition into Sub-5 nm Vertical Mesopores in Silica Hard Templates by Atomic Layer Deposition. Materials 2024, 17, 2272. [Google Scholar] [CrossRef]
- Antonova, I.V.; Seleznev, V.A.; Nebogatikova, N.A.; Ivanov, A.I.; Voloshin, B.V.; Volodin, V.A.; Kurkina, I.I. Thin V2O5 films synthesized by plasma-enhanced atomic layer deposition for memristive applications. Phys. Chem. Chem. Phys. 2023, 25, 32132–32141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Cheng, H.; Jia, Y.; Liu, T.; Liu, D. Infrared electrochromic devices based on thin metal films. Adv. Mater. Interfaces 2023, 10, 2202505. [Google Scholar] [CrossRef]
- Belosludtsev, A.; Sytchkova, A.; Baltrusaitis, K.; Vaicikauskas, V.; Jasulaitiene, V.; Gric, T. Growth of Magnetron-Sputtered ultrathin chromium films: In situ monitoring and ex situ film properties. Coatings 2023, 13, 347. [Google Scholar] [CrossRef]
- State, S.; Enache, L.; Potorac, P.; Prodana, M.; Enachescu, M. Synthesis of copper nanostructures for Non-Enzymatic glucose sensors via Direct-Current magnetron sputtering. Nanomaterials 2022, 12, 4144. [Google Scholar] [CrossRef]
- Ruiz, E.P.E.; Lago, J.L.; Thirumuruganandham, S.P. Experimental Studies on TiO2 NT with Metal Dopants through Co-Precipitation, Sol–Gel, Hydrothermal Scheme and Corresponding Computational Molecular Evaluations. Materials 2023, 16, 3076. [Google Scholar] [CrossRef]
- Peng, Z.; Yuan, L.; Luo, X.; Yu, J.; Tian, C.; Liu, Z. Mechanical properties and thermal shock resistance performance of spark plasma sintered MgO–Al2O3–SiO2 ceramics. Ceram. Int. 2022, 48, 28548–28556. [Google Scholar] [CrossRef]
- Lee, G.; Sim, J.; Oh, G.; Won, M.; Mantry, S.P.; Kim, D. Electrostatic spray deposition of Al-Doped ZnO thin films for acetone gas detection. Processes 2023, 11, 3390. [Google Scholar] [CrossRef]
- Santana, J.J.; Izquierdo, J.; Souto, R.M. Uses of Scanning Electrochemical Microscopy (SECM) for the Characterization with Spatial and Chemical Resolution of Thin Surface Layers and Coating Systems Applied on Metals: A Review. Coatings 2022, 12, 637. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.; Gu, X.; Zou, Y.; Zhan, D.; Peng, J. Recent advances in scanning electrochemical microscopy for probing the sites in electrocatalysts. J. Mater. Chem. A 2024, 12, 18733–18750. [Google Scholar] [CrossRef]
- Xu, J.; Chen, R.; Song, J.; Liu, S.; Shen, Y.; Zhang, Y. Emerging techniques and scenarios of scanning electrochemical microscopy for the characterization of electrocatalytic reactions. Chem. Sci. 2025, 16, 9564–9576. [Google Scholar] [CrossRef] [PubMed]
- Aich, P.; Meneghini, C.; Tortora, L. Advances in Structural and Morphological Characterization of Thin Magnetic Films: A Review. Materials 2023, 16, 7331. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, C.; Chen, C.; Gao, Y.; Sun, F.; Li, C.; Yin, X.; Luo, C.; Kentsch, U.; Cai, X.; et al. Tripling energy storage density through order-disorder transition induced polar nanoregions in PbZrO3 thin films by ion implantation. arXiv 2022, arXiv:2211.15896. [Google Scholar] [CrossRef]
- Jakubauskas, G.; Paluckiene, E.; Usoviene, E.; Petrasauskiene, N. Influence of different chemical methods used for the deposition of CdSe/ZnO layers. Coatings 2025, 15, 415. [Google Scholar] [CrossRef]
- Daskalova, D.; Flores, G.A.; Plachetka, U.; Möller, M.; Wolters, J.; Wintgens, T.; Lemme, M.C. Combined structural and plasmonic enhancement of Nanometer-Thin film photocatalysis for Solar-Driven wastewater treatment. ACS Appl. Nano Mater. 2023, 6, 15204–15212. [Google Scholar] [CrossRef]
- Li, M.; Yue, Z.; Ye, Z.; Li, H.; Luo, H.; Yang, Q.; Zhou, Y.; Huo, Y.; Cheng, Y. Improving the efficiency and stability of MAPbI3 perovskite solar cells by dipeptide molecules. Small 2024, 20, e2311400. [Google Scholar] [CrossRef]
- Hou, X.; Liu, J.; Wu, Y.; Ma, H.; Zhu, S. Simulation Research of Hole Transport Layer Free CsPbI3/MAPbI3 Heterojunction Perovskite Solar Cells with an Efficiency of 30.33%. J. Phys. Chem. Solids 2024, 192, 112108. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, S.; Cao, J.; Yuan, L.; Liu, W. Controlled crystallization and enhanced performance of γ-CsPbI3 perovskite through methylammonium Iodide-Assisted coevaporation. Small Methods 2024, 9, e2400796. [Google Scholar] [CrossRef]
- Ahmad, K.; Oh, T.H. Recent progress in MXene-Based materials for supercapacitors and electrochemical sensing applications. Biosensors 2025, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Zhang, X.; Wang, K.; Sun, X.; Ma, Y. Research progress on novel MXene materials in supercapacitors. Power Supply Technol. 2017, 41, 665–667. [Google Scholar]
- Hu, Z. Principle of MXene Supercapacitors and their applications in power electronics. Appl. Comput. Eng. 2025, 140, 73–83. [Google Scholar] [CrossRef]
- Huang, M.; Lin, J.; Liang, Z.; Chen, S.; Zhong, Y.; Wang, F.; Chen, B.; Zhang, D. Effect of Sulfurization Temperature on Properties of Cu2ZnSnS4 Thin Films and Diffusion of Ti Substrate Elements. Crystals 2024, 14, 910. [Google Scholar] [CrossRef]
- Zaki, M.Y.; Sava, F.; Simandan, I.D.; Mihai, C.; Velea, A. Structural and Compositional Analysis of CZTSSe Thin Films by Varying S/(S+Se) Ratio. Energies 2024, 17, 3684. [Google Scholar] [CrossRef]
- Mihai, C.; Simandan, I.; Sava, F.; Buruiana, A.; Bocirnea, A.E.; Tite, T.; Zaki, M.Y.; Velea, A. Synthesis of wrinkled MOS2 thin films using a Two-Step method consisting of magnetron sputtering and sulfurization in a confined space. Sustainability 2024, 16, 3819. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, M.; Cai, Y.; Zhuang, Y.; Wang, L. The advanced progress of MOS2 and WS2 for Multi-Catalytic Hydrogen Evolution Reaction Systems. Catalysts 2023, 13, 1148. [Google Scholar] [CrossRef]
- Gardella, M.; Zambito, G.; Ferrando, G.; Bisio, F.; Giordano, M.C.; De Mongeot, F.B. Large area van der Waals MoS2–WS2 heterostructures for visible-light energy conversion. RSC Appl. Interfaces 2024, 1, 1001–1011. [Google Scholar] [CrossRef]
- Liu, Y.; Clark, M.; Zhang, Q.; Yu, D.; Liu, D.; Liu, J.; Cao, G. V2O5 Nano-Electrodes with High Power and Energy Densities for Thin Film Li-Ion Batteries. Adv. Energy Mater. 2011, 1, 194–202. [Google Scholar] [CrossRef]
- Lin, T.; Jheng, B.; Yen, H.; Huang, W. Thermal annealing effects of V2O5 thin film as an ionic storage layer for electrochromic application. Materials 2022, 15, 4598. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Lee, J.K.; Ravi, G. Sputtering deposition of a Binder-Free V2O5/ZnO thin film for transparent supercapacitor applications. ACS Appl. Electron. Mater. 2024, 6, 1504–1513. [Google Scholar] [CrossRef]
- Shaban, M.; Zayed, M.; Hamdy, H. Nanostructured ZnO thin films for self-cleaning applications. RSC Adv. 2017, 7, 617–631. [Google Scholar] [CrossRef]
- He, C.; He, J.; Cui, S.; Fan, X.; Li, S.; Yang, Y.; Tan, X.; Zhang, X.; Mao, J.; Zhang, L.; et al. Novel effective photocatalytic Self-Cleaning coatings: TiO2-Polyfluoroalkoxy coatings prepared by suspension plasma spraying. Nanomaterials 2023, 13, 3123. [Google Scholar] [CrossRef] [PubMed]
- Lukong, V.T.; Ukoba, K.; Jen, T. Fabrication of vanadium dioxide thin films and application of its thermochromic and photochromic nature in self-cleaning: A review. Energy Environ. 2022, 34, 3495–3528. [Google Scholar] [CrossRef]
- Kaufman, M.; Vlcek, J.; Houska, J.; Farrukh, S.; Haviar, S.; Cerstvy, R.; Kozak, T. A low-temperature synthesis of strongly thermochromic W and Sr co-doped VO2 films with a low transition temperature. arXiv 2024, arXiv:2411.03005. [Google Scholar] [CrossRef]
- Petrov, V.V.; Ivanishcheva, A.P.; Volkova, M.G.; Storozhenko, V.Y.; Gulyaeva, I.A.; Pankov, I.V.; Volochaev, V.A.; Khubezhov, S.A.; Bayan, E.M. High gas sensitivity to nitrogen dioxide of nanocomposite ZnO-SnO2 films activated by a surface electric field. Nanomaterials 2022, 12, 2025. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Joshi, M.; Myung, J. Sputtered SnO2/ZnO heterostructures for improved NO2 gas sensing properties. Chemosensors 2020, 8, 67. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Su, S.; He, J.; Ai, Y. Improving surface structures of Al-Doped zinc oxide thin films to apply in CO Gas-Sensing property by designing processes through RF magnetron sputtering. J. Electron. Mater. 2024, 53, 2410–2420. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal oxide thin films prepared by magnetron sputtering technology for volatile organic compound detection in the microwave frequency range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.R.; Chang, C.; Gupta, S.; Anbalagan, A.K.; Lee, C.; Li, S.; Tai, N. Polyethylenimine/Nitrogen-Doped Reduced Graphene Oxide/ZNO nanoRod layered composites for carbon dioxide sensing at room temperature. ACS Appl. Nano Mater. 2022, 5, 6543–6554. [Google Scholar] [CrossRef]
- Haldar, T.; Shiu, J.; Yang, R.; Wang, W.; Wu, H.; Mao, H.; Chen, C.; Yu, C. Exploring MOF-Derived CuO/rGO heterostructures for highly efficient room temperature CO2 sensors. ACS Sens. 2024, 9, 5856–5865. [Google Scholar] [CrossRef]
- Anwar, N.; Jiang, G.; Wen, Y.; Ahmed, M.; Zhong, H.; Ao, S.; Li, Z.; Ling, Y.; Schneider, G.F.; Fu, W.; et al. Evaluating the potential of two-dimensional materials for innovations in multifunctional electrochromic biochemical sensors: A review. Moore More 2024, 1, 12. [Google Scholar] [CrossRef]
- Kruger, D.D.; García, H.; Primo, A. Molten salt derived MXenes: Synthesis and applications. Adv. Sci. 2024, 11, e2307106. [Google Scholar] [CrossRef]
- Jussambayev, M.; Shakenov, K.; Sultakhan, S.; Zhantikeyev, U.; Askaruly, K.; Toshtay, K.; Azat, S. MXENES for Sustainable Energy: A Comprehensive Review on Conservation and Storage Applications. Carbon Trends 2025, 19, 100471. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Sun, Y.; Wang, F.; Yang, J.; Dou, Y.; Zhang, C.; Kong, J. One-Step fabrication for CsPbBr3 perovskite thin film via a facile Ion-Solution spraying approach. Crystals 2024, 14, 604. [Google Scholar] [CrossRef]
- Masawa, S.M.; Zhao, C.; Liu, J.; Xu, J.; Yao, J. Fabrication and Characterization of a Lead-Free Cesium Bismuth Iodide Perovskite through Antisolvent-Assisted Crystallization. Nanomaterials 2024, 14, 626. [Google Scholar] [CrossRef]
- Jabeen, N.; Zaidi, A.; Hussain, A.; Hassan, N.U.; Ali, J.; Ahmed, F.; Khan, M.U.; Iqbal, N.; Elnasr, T.A.S.; Helal, M.H. Single- and Multilayered Perovskite Thin Films for Photovoltaic Applications. Nanomaterials 2022, 12, 3208. [Google Scholar] [CrossRef] [PubMed]
- Gollino, L.; Mercier, N.; Pauporté, T. Exploring Solar Cells Based on Lead- and Iodide-Deficient Halide Perovskite (d-HP) Thin Films. Nanomaterials 2023, 13, 1245. [Google Scholar] [CrossRef]
- Kim, H.J.; Chong, M.; Rhee, T.G.; Khim, Y.G.; Jung, M.-H.; Kim, Y.-M.; Jeong, H.Y.; Choi, B.K.; Chang, Y.J. Machine-learning-assisted analysis of transition metal dichalcogenide thin-film growth. Nano Converg. 2023, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Efficient inverse design of optical multilayer nano-thin films using neural network principles: Backpropagation and gradient descent. Nanoscale 2024, 16, 17165–17175. [Google Scholar] [CrossRef]
- Ignacz, G.; Baig, M.I.; Gopalsamy, K.; Villa, A.; Nunes, S.; Ghanem, B.; Shastry, T.; Kumar, S.K.; Szekely, G. A data-driven approach to interfacial polymerization exploiting machine learning for predicting thin-film composite membrane formation. Mater. Horiz. 2025, 12, 9009–9025. [Google Scholar] [CrossRef]
- Nandishwara, K.M.; Cheng, S.; Liu, P.; Zhu, H.; Guo, X.; Massabuau, F.C.; Hoye, R.L.Z.; Sun, S. Data-driven microstructural optimization of Ag-Bi-I perovskite-inspired materials. npj Comput. Mater. 2025, 11, 210. [Google Scholar] [CrossRef]
- Wang, C.; Kim, Y.; Vriza, A.; Batra, R.; Baskaran, A.; Shan, N.; Li, N.; Darancet, P.; Ward, L.; Liu, Y.; et al. Autonomous platform for solution processing of electronic polymers. Nat. Commun. 2025, 16, 1498. [Google Scholar] [CrossRef]
- Bonner, J.C.; Piper, R.T.; Bhandari, B.; Allen, C.R.; Bower, C.T.; Ostendorf, M.A.; Davis, M.; Valdez, M.; Lee, M.; Hsu, J.W.P. “Green” Fabrication of High-performance Transparent Conducting Electrodes by Blade Coating and Photonic Curing on PET for Perovskite Solar Cells. Mater. Sustain. 2025, 1, 2. [Google Scholar] [CrossRef]
- Das, A.; Chatterjee, R.; Sarkar, S.; Ninave, G.; Bose, D.; Dutta, A.K.; Biswas, S.; Mukherjee, M.; Venkatesan, R.; Majee, R.; et al. Green Chemistry-Assisted synthesis of metal nanoparticles and fabrication of microstructurally engineered conductive and endurable M0@PEO functional films. ACS Omega 2025, 10, 38609–38628. [Google Scholar] [CrossRef] [PubMed]
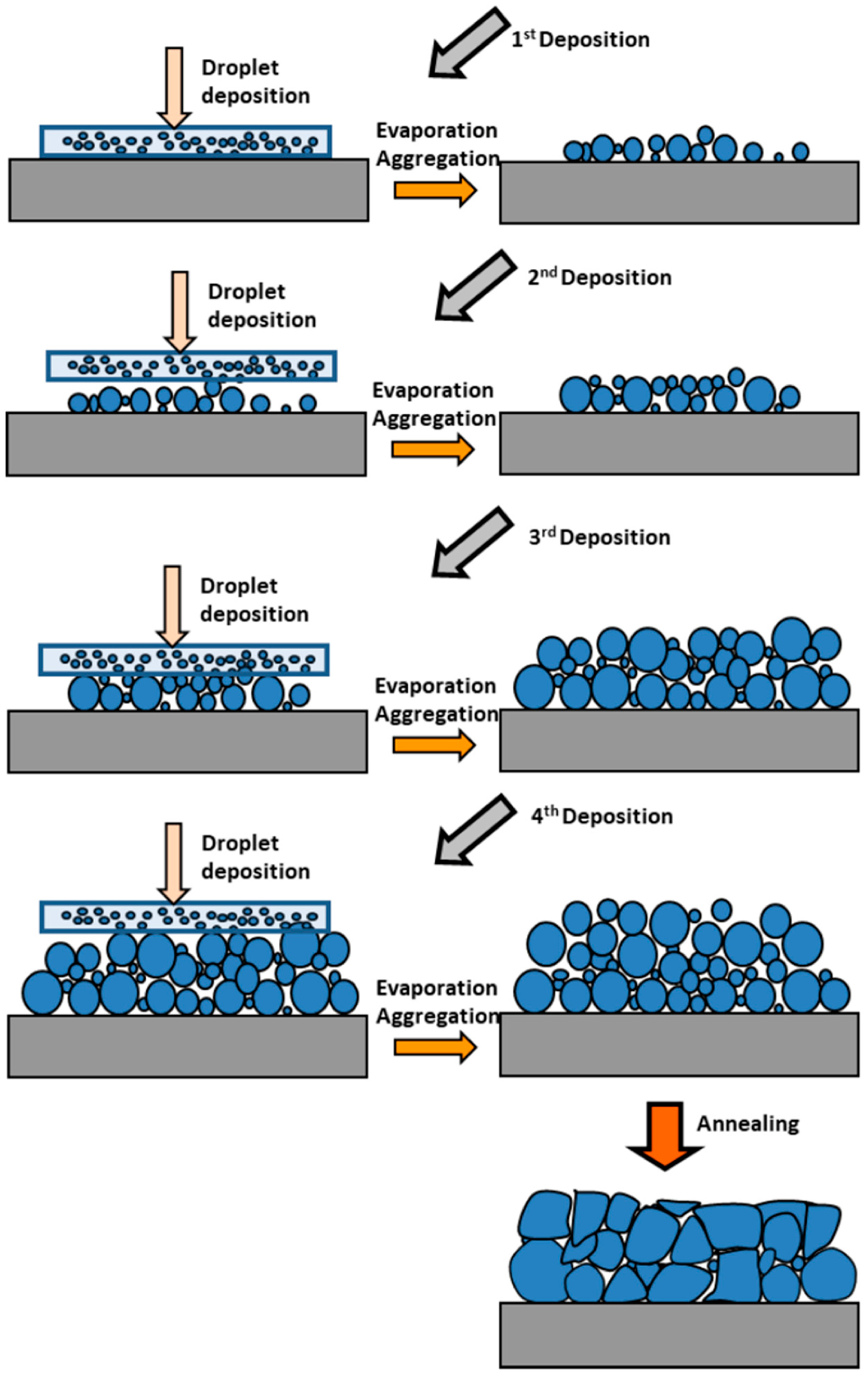
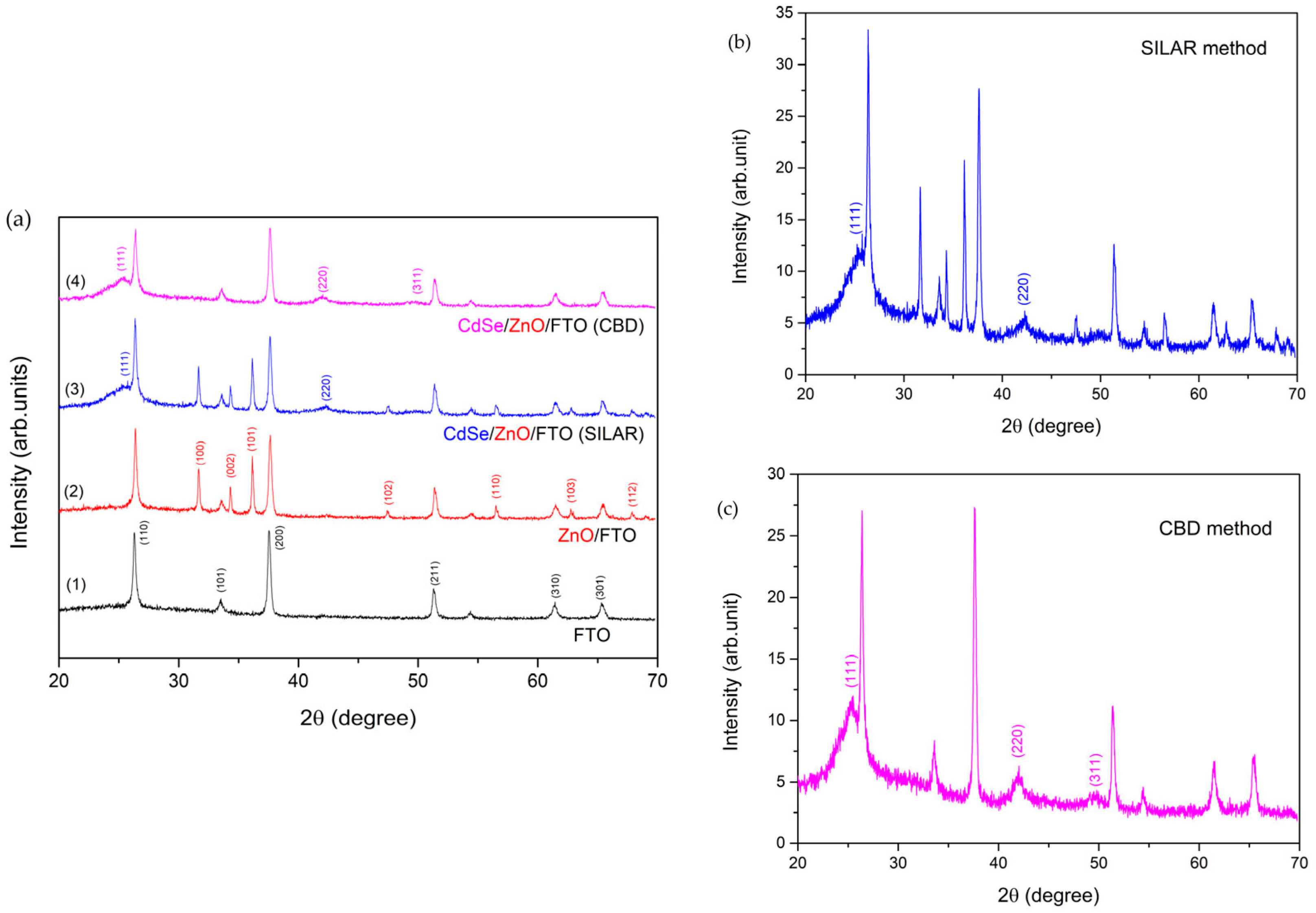
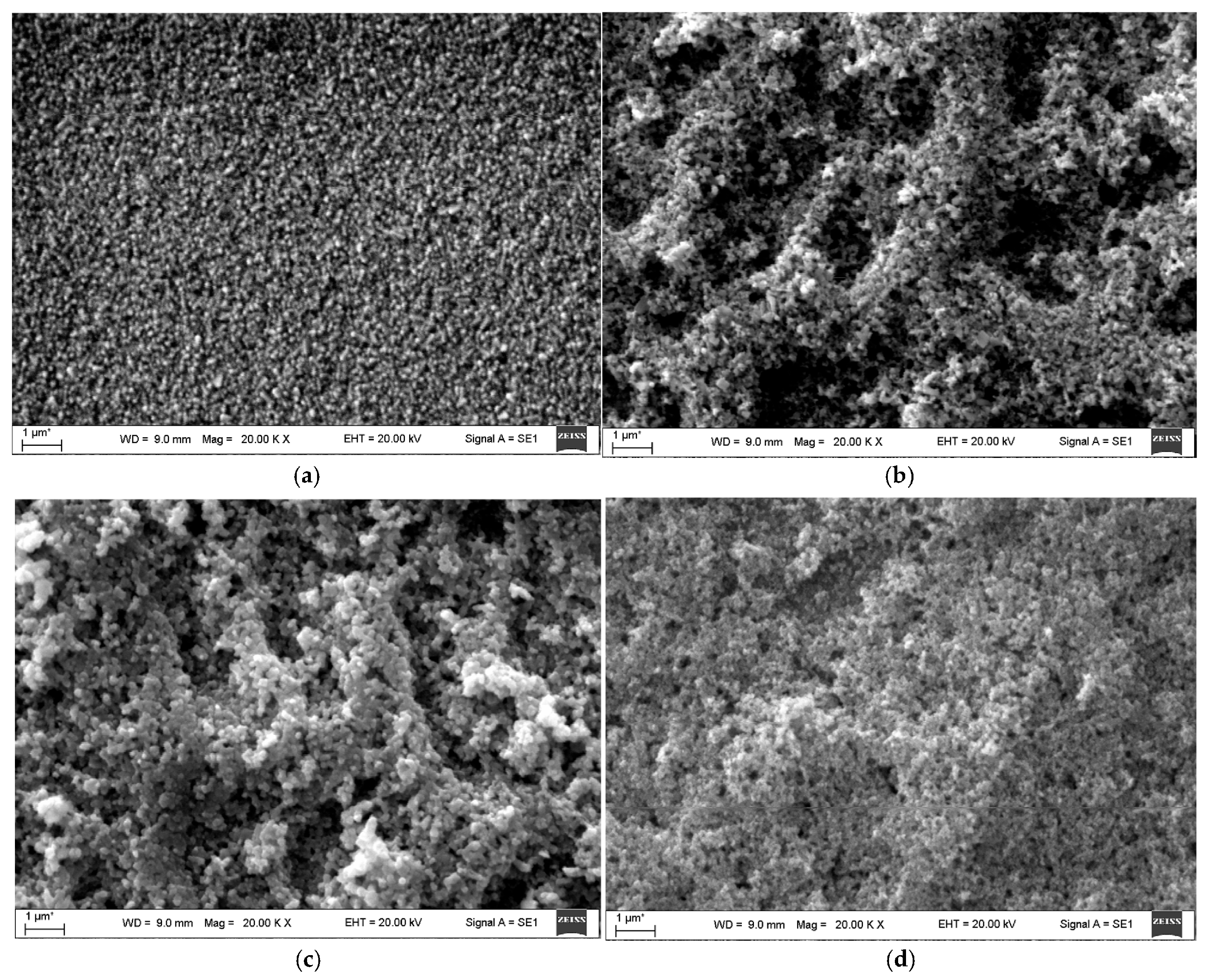

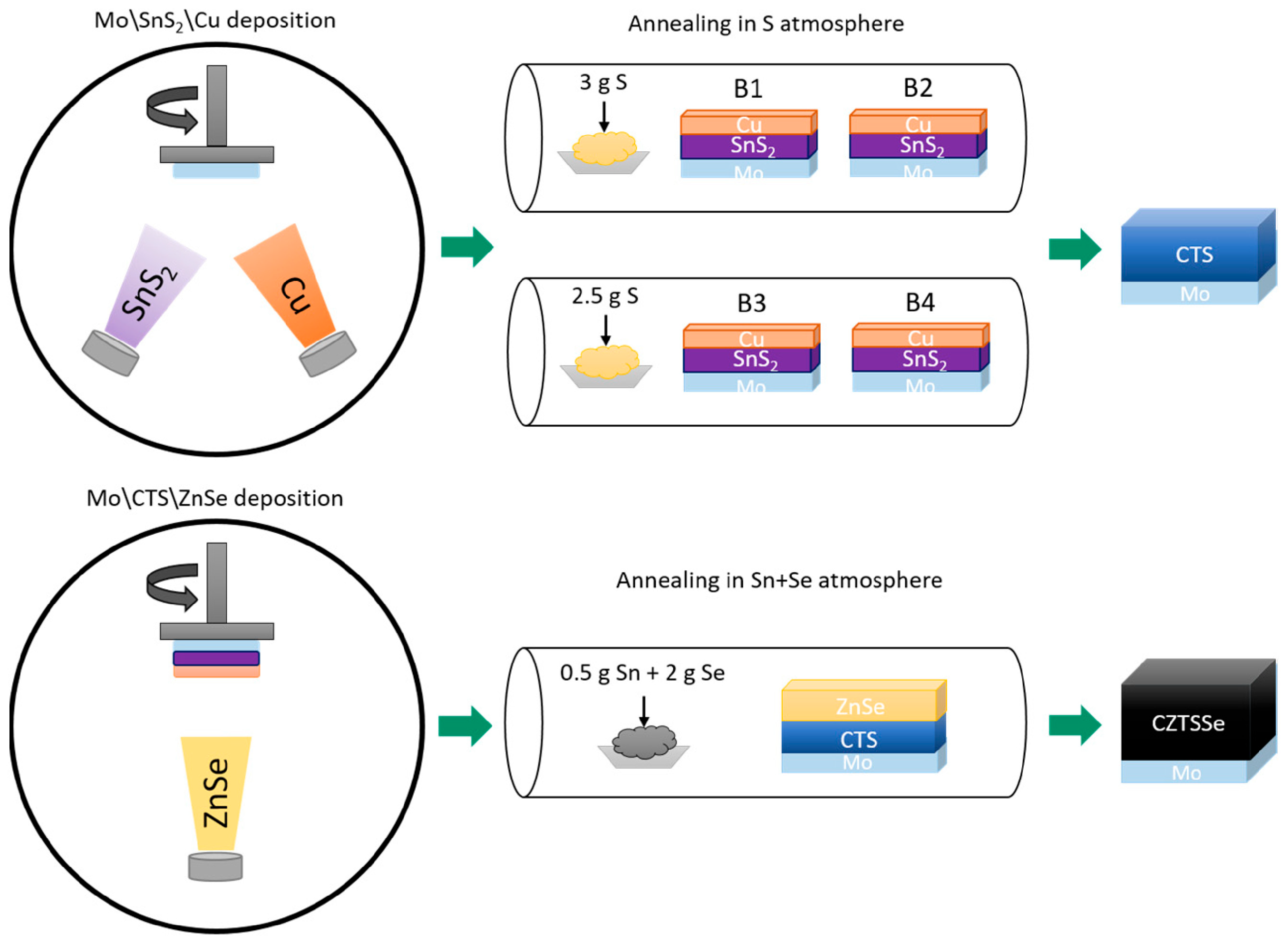
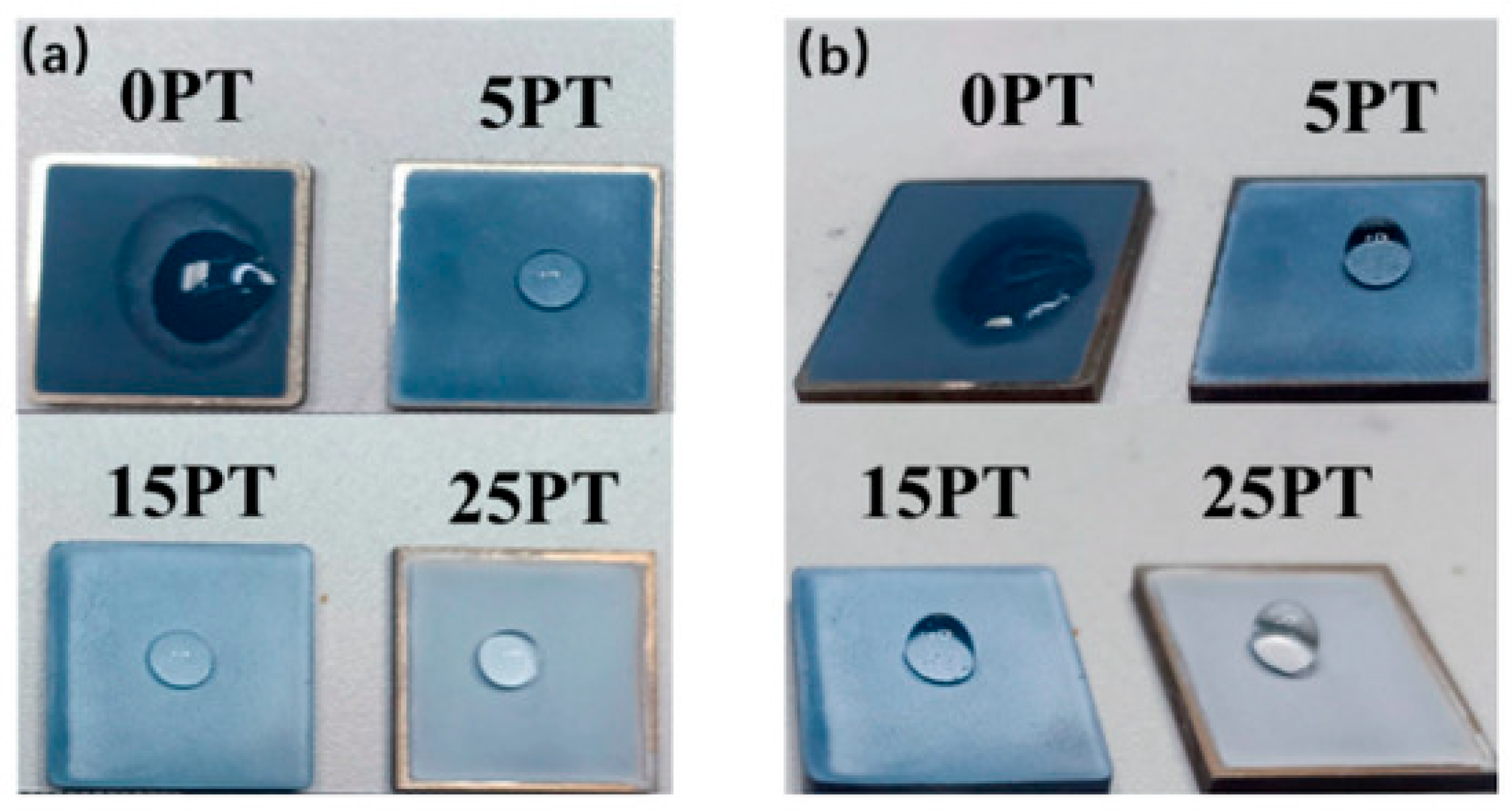
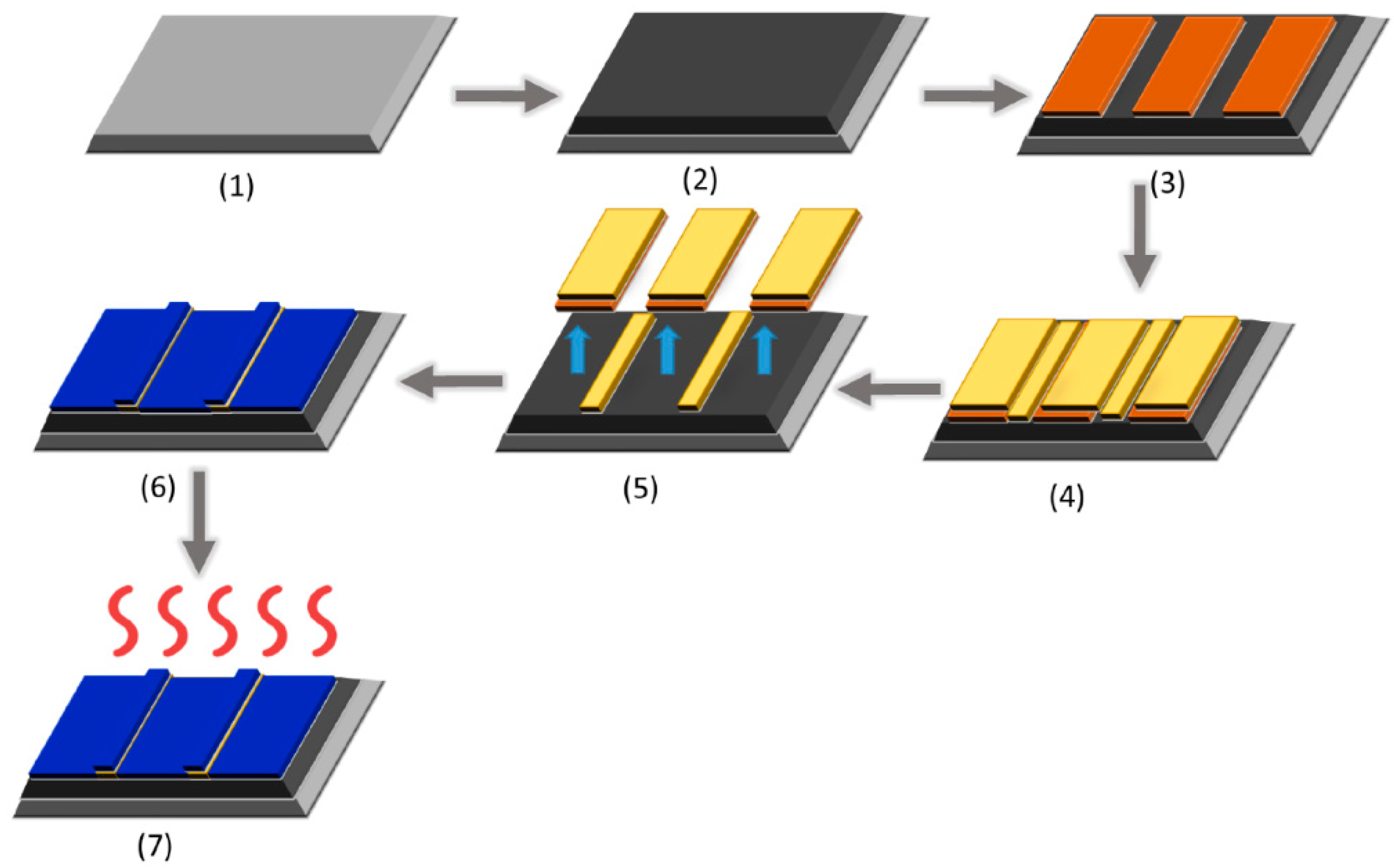
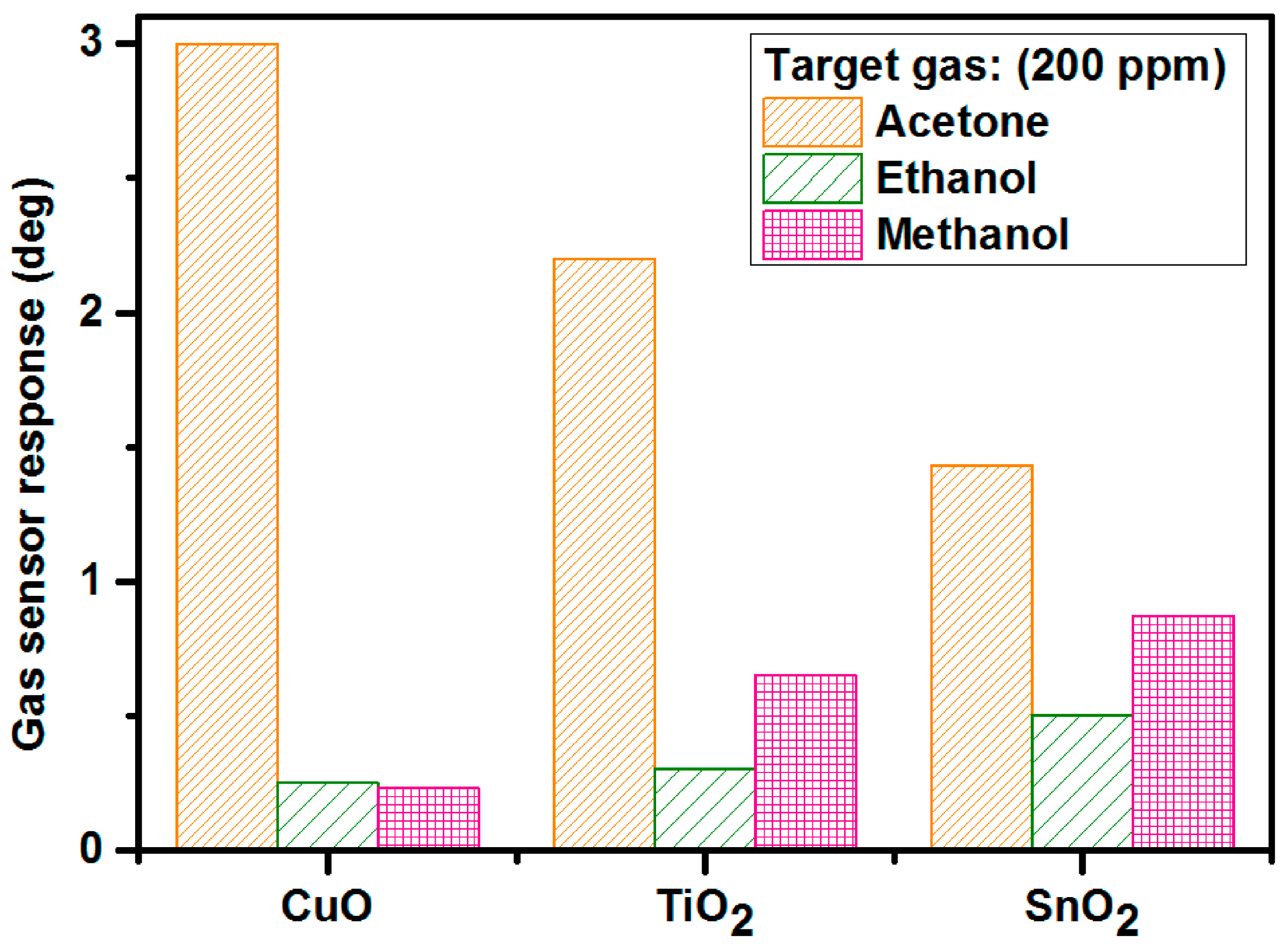
| 2D Material | Nanostructured Films | Epitaxial Films | Applications |
|---|---|---|---|
| MoS2 | Defect-enhanced magnetism | Uniform, low defects | Spintronics, sensors |
| WS2 | Quantum confinement effects | Smooth, ordered | Optoelectronics |
| h-BN | Tunable vacancy sites | High-quality dielectric | Substrates, tunneling barriers |
| Type of Perovskite/System | Article | Novelty Statement |
|---|---|---|
| CsPbBr3 perovskite thin films | 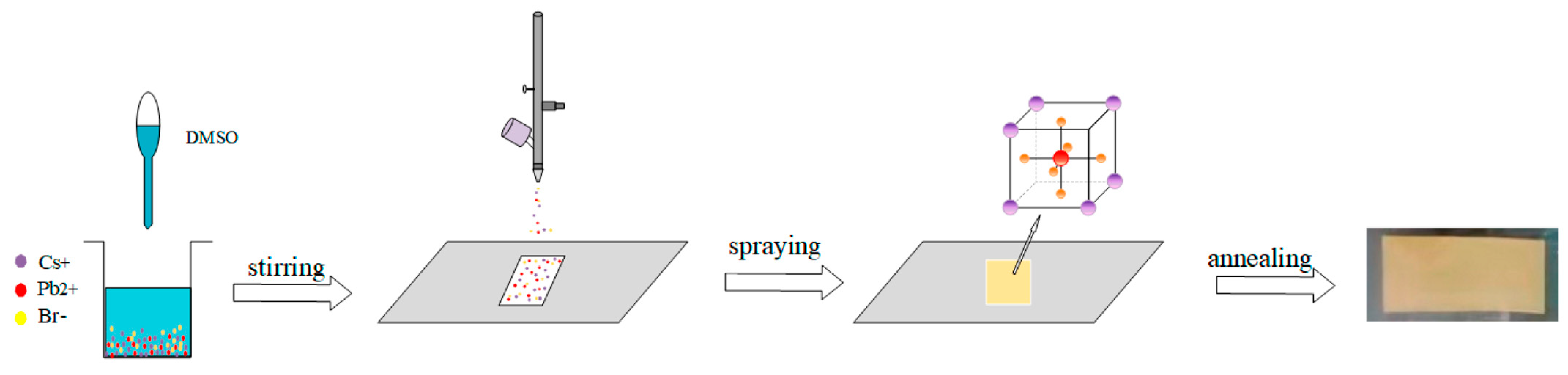 | Introduced an ambient, one-step ion-spray method enabling scalable fabrication of high-quality CsPbBr3 perovskite thin films with tunable microstructure [152]. |
| Lead-free CsBi3I0 perovskite thin films | 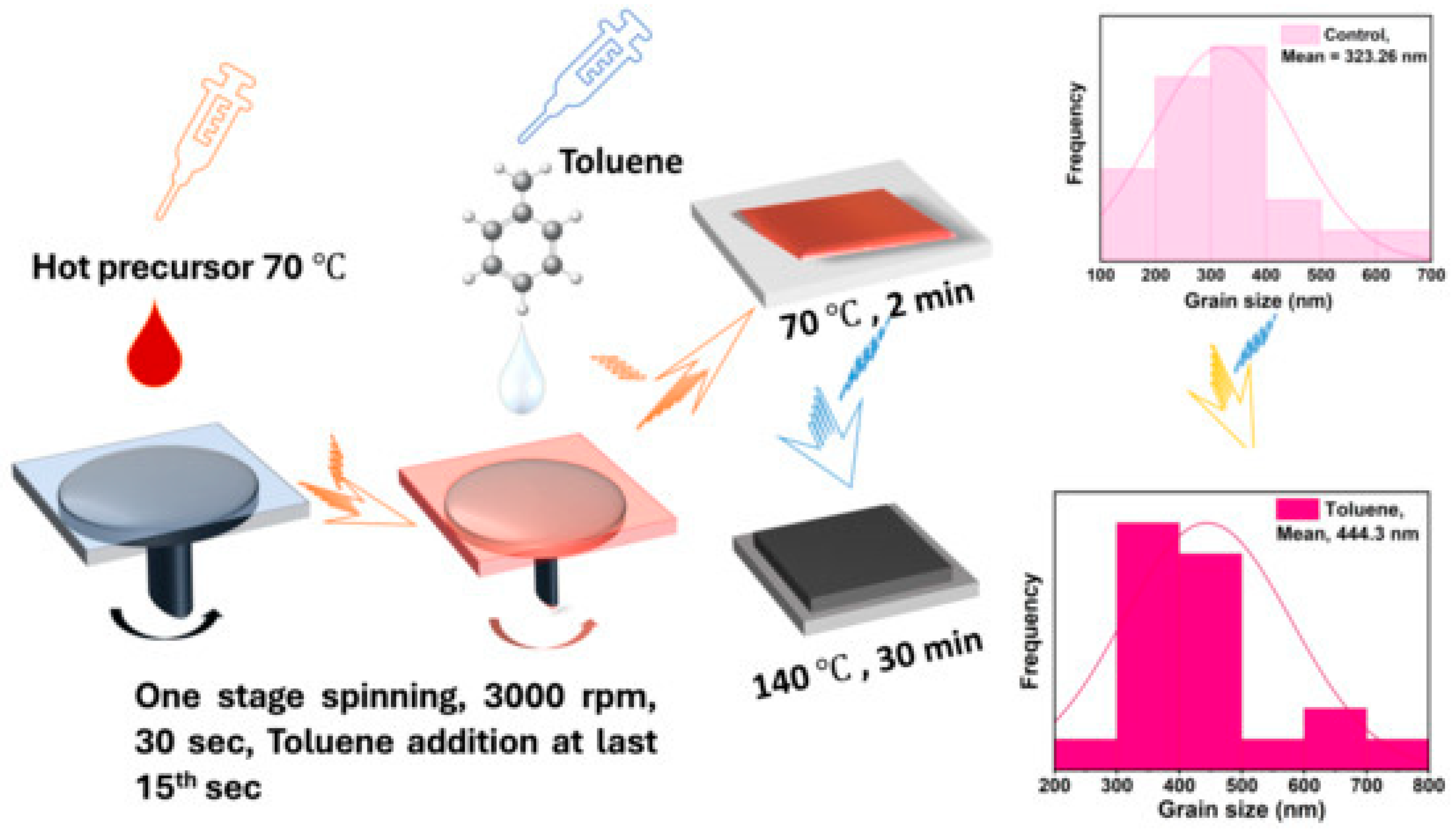 | A novel approach to creating lead-free perovskite thin films, focusing on enhancing the optoelectronic properties of cesium bismuth iodide (CsBi3I10) perovskites by employing antisolvent-assisted crystallization techniques [153]. |
| Multilayered CH3NH3PbIBr2 perovskite thin films | 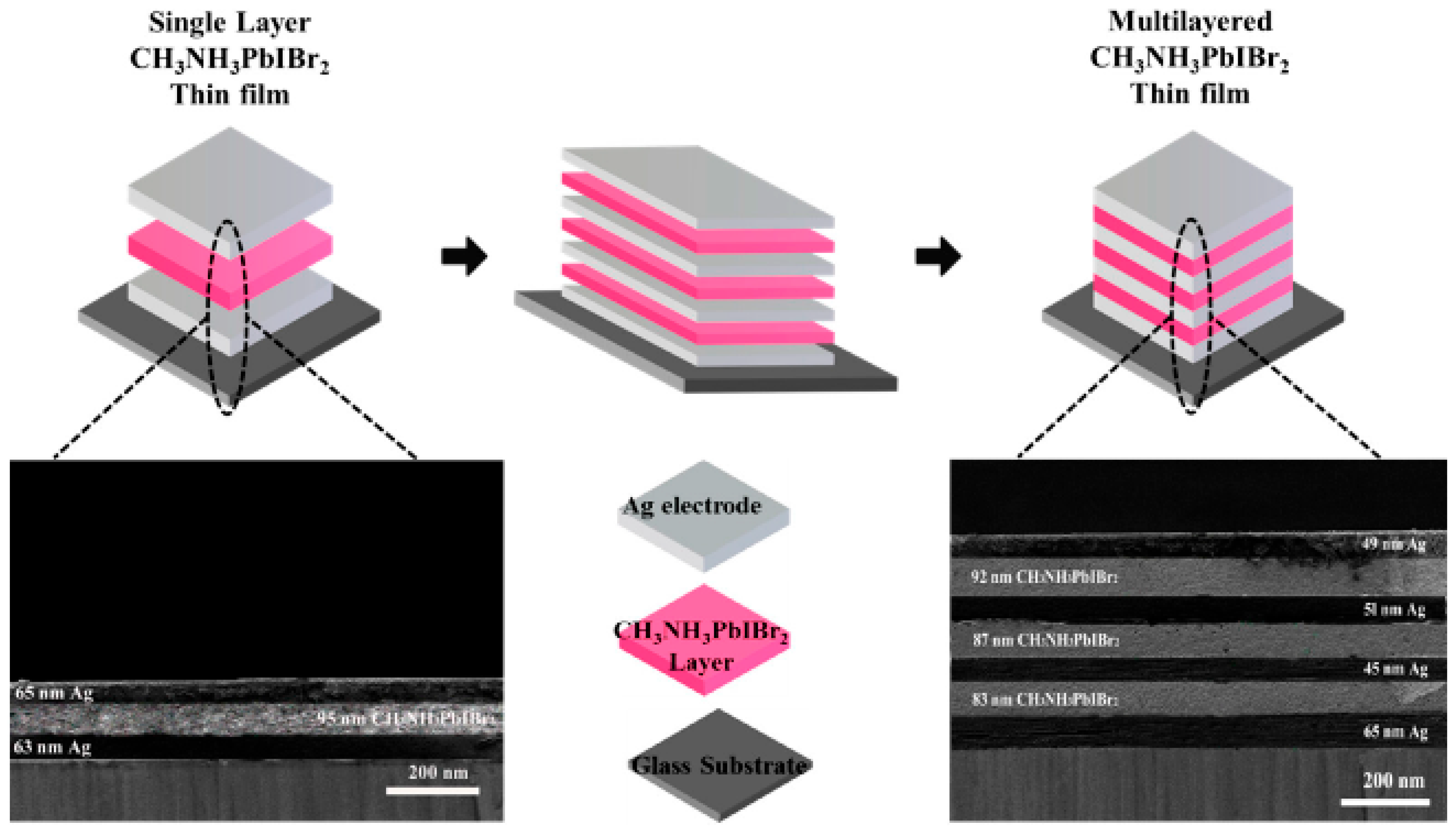 | Multilayered CH3NH3PbIBr2 perovskite thin films achieve enhanced crystallinity, larger grain sizes, reduced defects, and higher photovoltaic efficiency (~13.8%) compared to single-layer films, demonstrating that multilayer architectures directly improve optoelectronic performance and stability [154]. |
| d-MAPbI3-HEA and d-FAPbI3-TEA perovskite thin films | 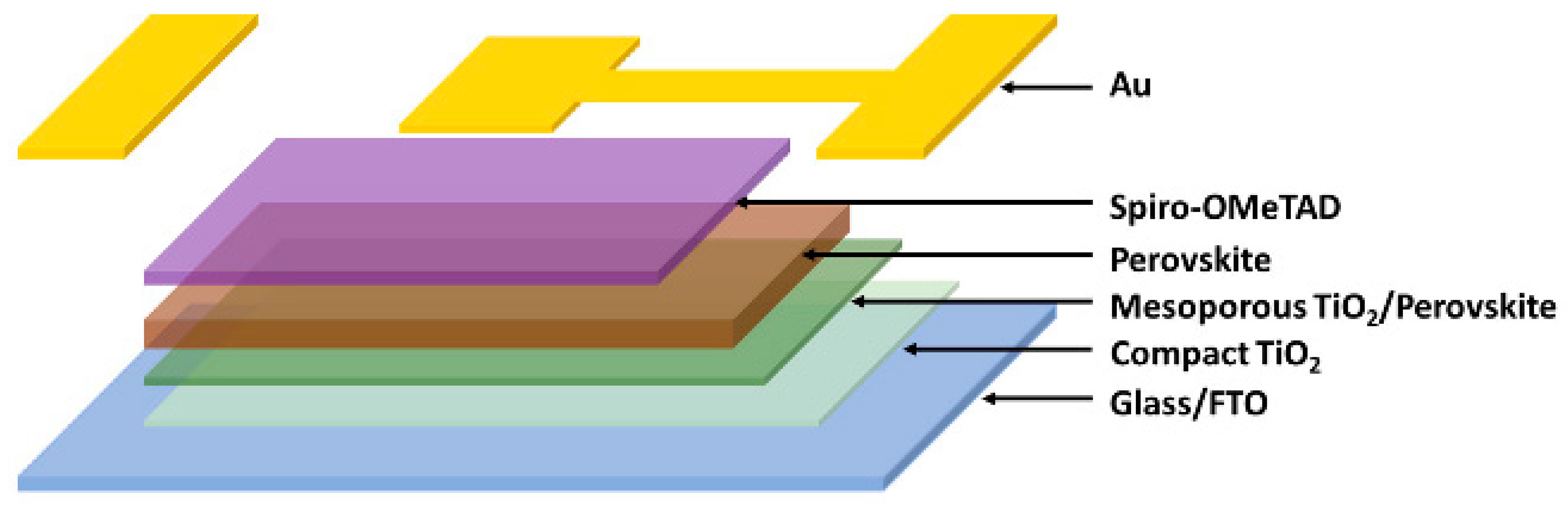 | Introducing lead- and iodide-deficient (d-HP) perovskite thin films that exhibit enhanced stability and tunable optoelectronic properties, enabling a better balance between efficiency and environmental resilience compared to standard stoichiometric perovskite films [155]. |
| Property to Be Tailored | Approach/Method | Observed Effect | Also Affected | References |
|---|---|---|---|---|
| Refractive index | Modify nanoporosity | Higher porosity → smaller refractive index | Air-to-vacuum spectral shifts, mechanical properties, light scattering | [54,55] |
| Catalytic activity | Control grain size, surface morphology | Increased active site density → improved catalytic performance | Adsorption/desorption kinetics, phase stability | [40,41,42,43] |
| Bandgap/optical absorption | Reduce nanostructure size (quantum confinement) | Bandgap widening, altered absorption/emission spectra | Charge carrier mobility, exciton dynamics | [52,53] |
| Electrical conductivity | Annealing/grain growth | Enhanced carrier mobility, lower resistivity | Transparency, defect-state distribution | [62,63,64,65,66,67,68,69,70,71,72] |
| AFM → FM transition temperature | Doping (Co, Pd, Ir) | Tunable magnetic phase transition temperature | Electrical resistivity, magnetic anisotropy | [86,87,88,89,90] |
| Surface-enhanced Raman scattering (SERS) | Nanostructure patterning/porous metal deposition | Strong electromagnetic hot-spots → enhanced Raman signal | Morphology-dependent reproducibility, plasmon resonance shifts | [66,67] |
| Photocatalytic activity | Introduce surface defects/porosity | Enhanced charge separation → higher photocatalytic efficiency | Bandgap modulation, surface stability | [47,48,49] |
| Magnetic anisotropy (PMA) | Control thickness/interface engineering | Transition from in-plane to perpendicular anisotropy | Spin–orbit coupling, domain structure, coercivity | [83,84] |
| Surface energy/reactivity | Reduce particle size/increase surface-to-volume ratio | Higher surface energy → improved reactivity | Phase transitions at lower temperature, densification | [34,35,36,37,38,39] |
| Gas sensing | Introduce surface defects or dopants | Increased adsorption → higher sensitivity | Selectivity, response/recovery kinetics | [40,100] |
| Ion diffusion/conductivity | Increase grain boundary networks/nanoscale porosity | Enhanced ionic transport | Mechanical stability, film density | [45,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, A.-M.; Caramizoiu, S.; Iordache, A.-M.; Iordache, S.-M.; Bita, B. Nanostructured Thin Films: Properties, Fabrication and Applications—A Short Review. Nanomaterials 2025, 15, 1760. https://doi.org/10.3390/nano15231760
Florea A-M, Caramizoiu S, Iordache A-M, Iordache S-M, Bita B. Nanostructured Thin Films: Properties, Fabrication and Applications—A Short Review. Nanomaterials. 2025; 15(23):1760. https://doi.org/10.3390/nano15231760
Chicago/Turabian StyleFlorea (Raduta), Ana-Maria, Stefan Caramizoiu, Ana-Maria Iordache, Stefan-Marian Iordache, and Bogdan Bita. 2025. "Nanostructured Thin Films: Properties, Fabrication and Applications—A Short Review" Nanomaterials 15, no. 23: 1760. https://doi.org/10.3390/nano15231760
APA StyleFlorea, A.-M., Caramizoiu, S., Iordache, A.-M., Iordache, S.-M., & Bita, B. (2025). Nanostructured Thin Films: Properties, Fabrication and Applications—A Short Review. Nanomaterials, 15(23), 1760. https://doi.org/10.3390/nano15231760







