Nanotechnology Driven Innovations in Modern Pharmaceutics: Therapeutics, Imaging, and Regeneration
Abstract
1. Introduction
1.1. Overview of Nanomaterials in Pharmaceutics
1.2. Emergence of Smart Nanomaterials
1.3. Objectives and Scope of This Review
- To provide a detailed overview of the design and functionality of smart nanomaterials in pharmaceutics.
- To critically evaluate their applications across drug delivery, diagnostics, and regenerative medicine.
- To discuss translational challenges and propose future perspectives for clinical adoption.
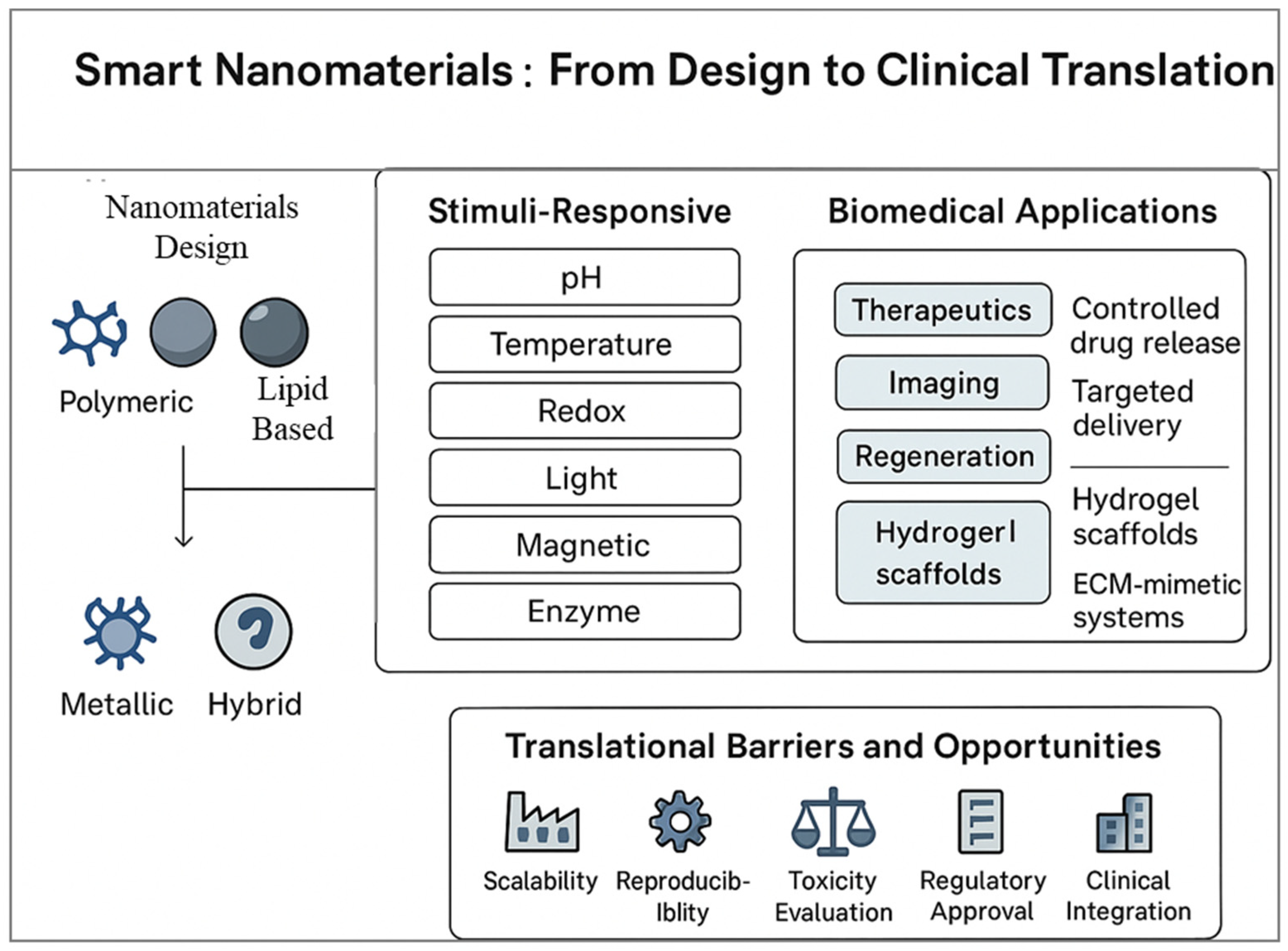
2. Design and Properties of Smart Nanomaterials
2.1. Stimuli-Responsive Behavior
2.1.1. pH Responsive Nanomaterials
2.1.2. Temperature Responsive Systems
2.1.3. Redox Responsive Nanoplatforms
2.1.4. Light and Magnetic Field Responsive Materials
2.2. Biocompatibility and Biodegradability Considerations
2.3. Surface Modification and Functionalization Strategies
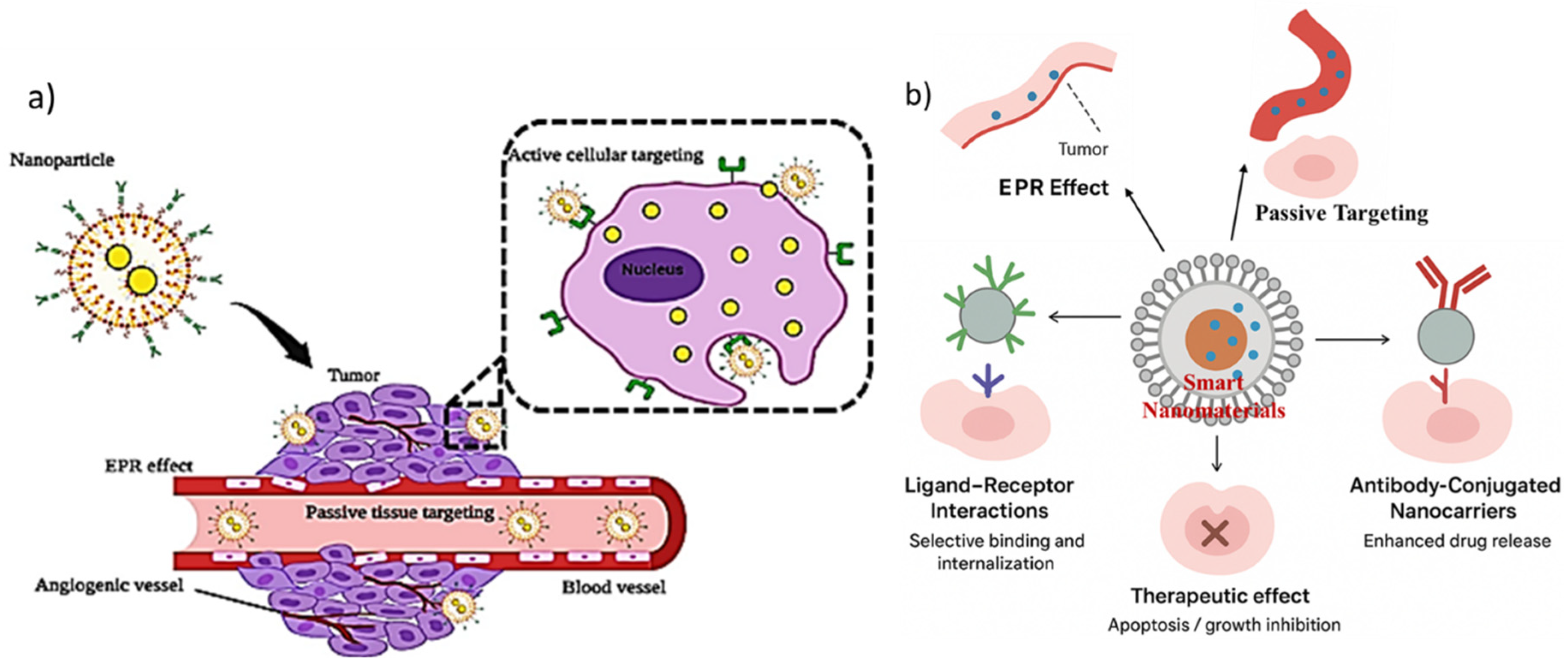
3. Smart Nanomaterials for Targeted Drug Delivery
3.1. Active Targeting Approaches
3.1.1. Ligand Receptor Interactions
3.1.2. Antibody Conjugated Nanocarriers
3.2. Passive Targeting Mechanisms (EPR Effect)
3.3. Controlled and Stimuli-Triggered Drug Release
3.4. Case Studies: Recent Advances in Targeted Delivery Systems
4. Smart Nanomaterials in Diagnostic Applications
4.1. Nanomaterials for Imaging and Biosensing
4.1.1. Fluorescence Imaging Nanoprobes
4.1.2. Magnetic Resonance Imaging (MRI) Nanocontrast Agents
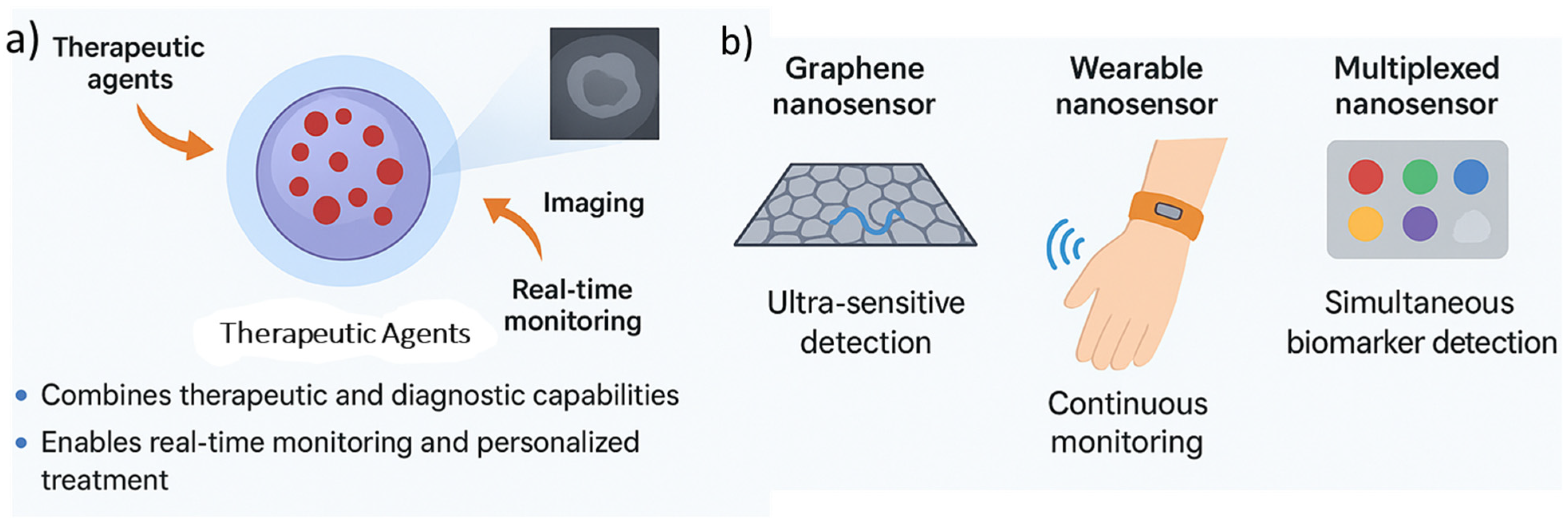
4.2. Theranostic Nanoplatforms: Combined Therapy and Diagnosis
4.3. Emerging Trends in Nanosensors for Disease Monitoring
5. Nanomaterials in Regenerative Medicine
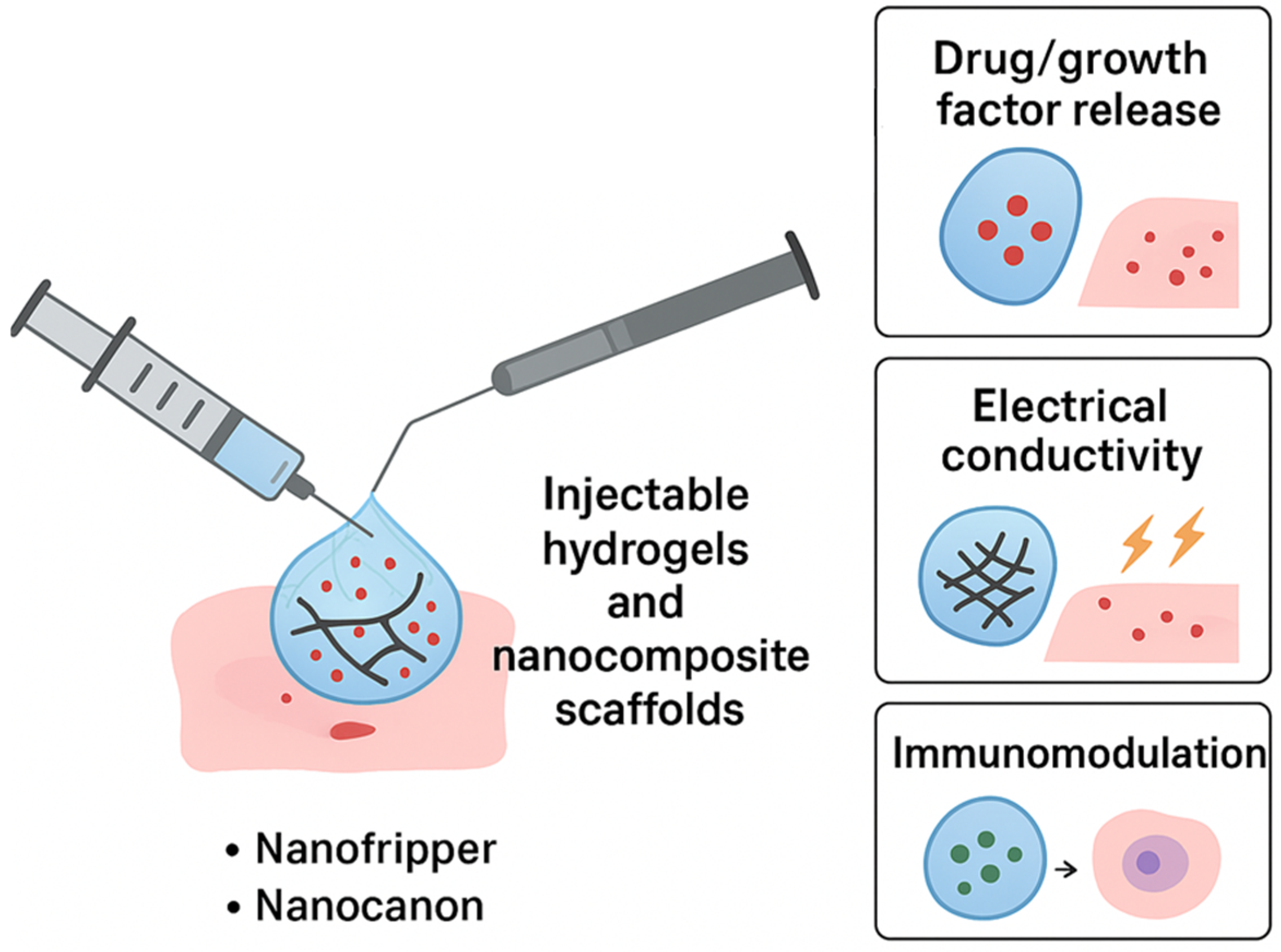
5.1. Injectable Hydrogels and Nanocomposite Scaffolds
5.2. Nanoparticles for Stem Cell Therapy
5.3. Applications in Musculoskeletal and Neural Regeneration
5.4. Biomimetic Nanomaterials for Tissue Engineering
6. Clinical Translation and Challenges
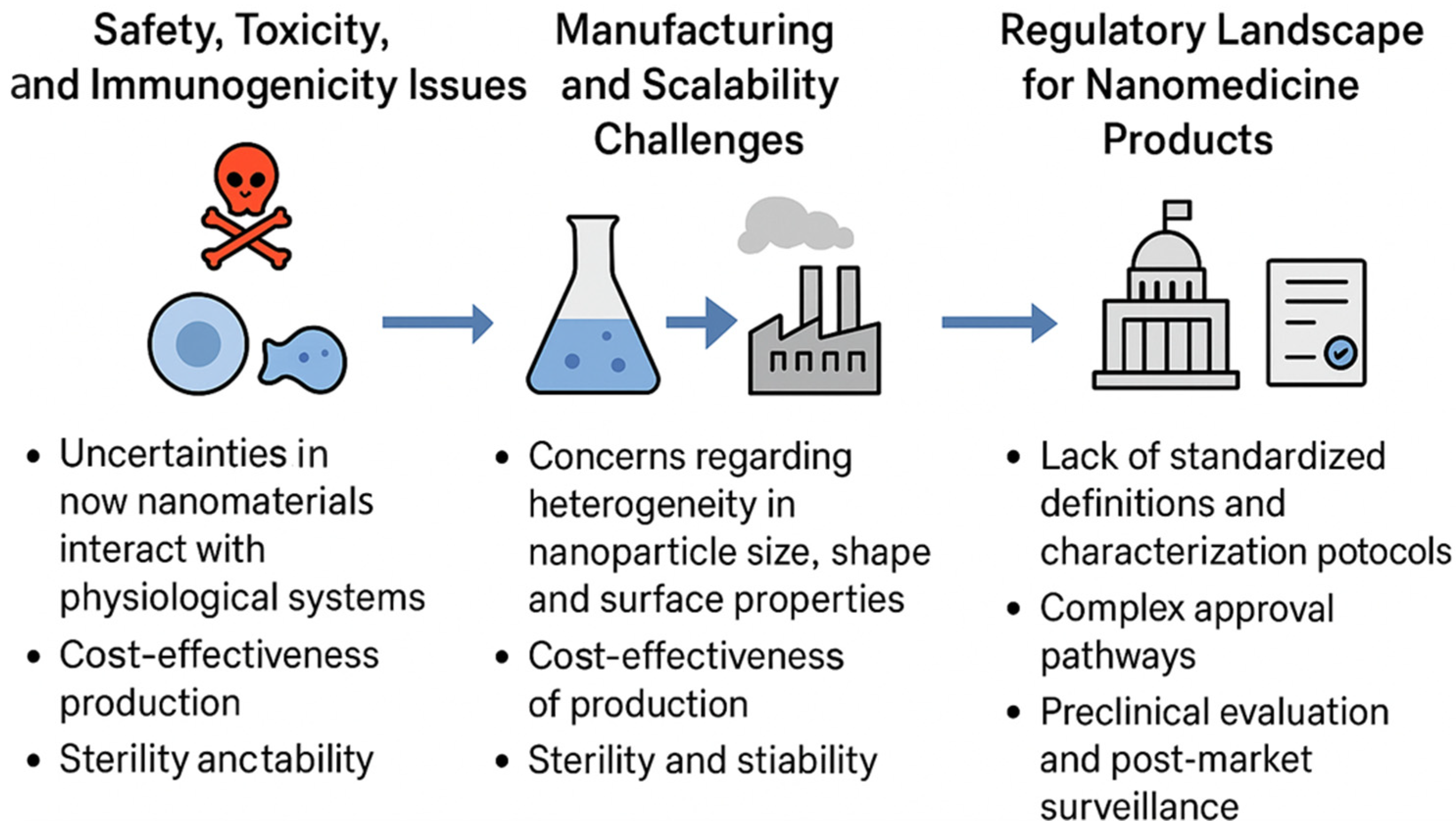
6.1. Safety, Toxicity, and Immunogenicity Issues
6.2. Manufacturing and Scalability Challenges
6.3. Regulatory Landscape for Nanomedicine Products
6.4. Case Studies of Clinically Approved Nanomaterial-Based Therapeutics
7. Future Perspectives and Opportunities
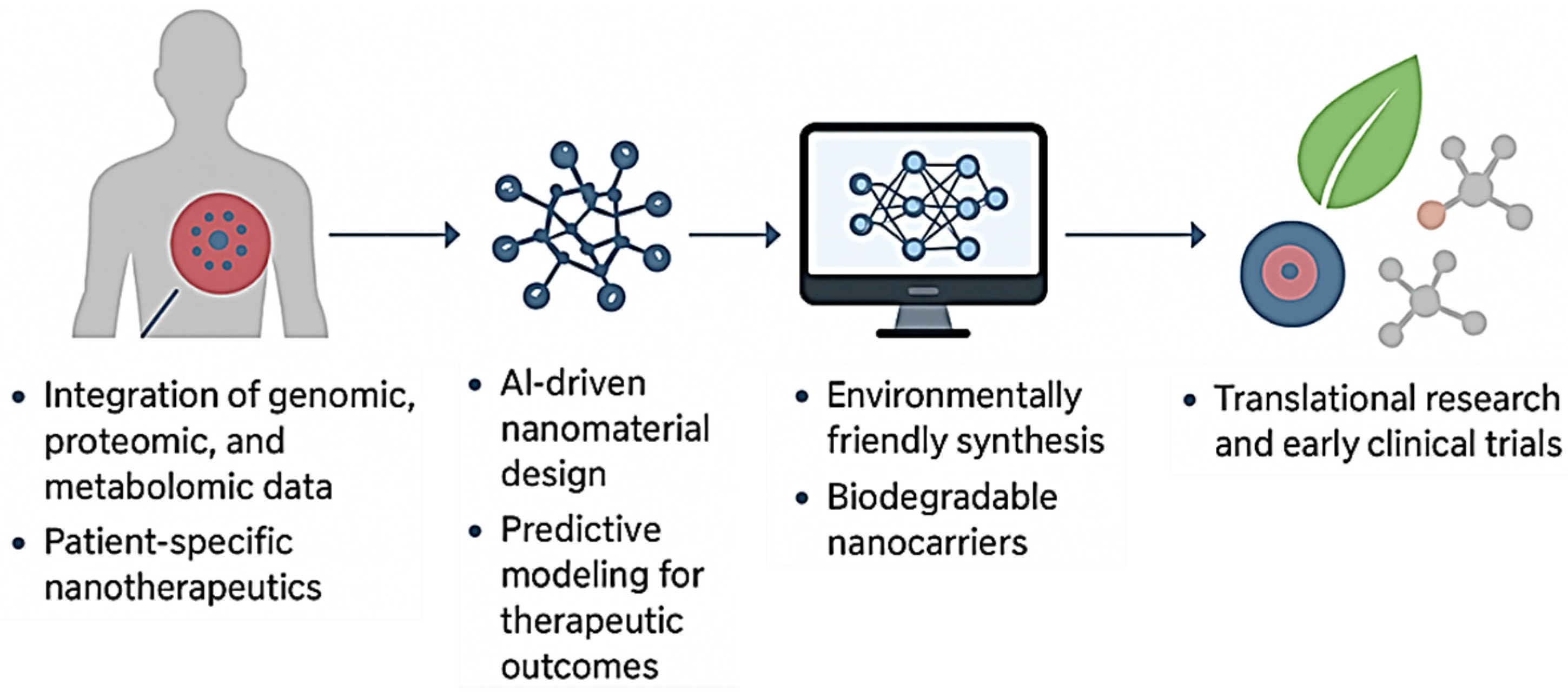
7.1. Personalized Nanomedicine
7.2. Artificial Intelligence and Smart Nanomaterials
7.3. Green and Sustainable Synthesis of Smart Nanomaterials
7.4. Bridging the Gap Between Research and Clinical Application
7.5. Clinical Translation and Regulatory Perspectives
8. Conclusions
8.1. Summary of Key Insights
8.2. Outlook on Smart Nanomaterials in Modern Pharmaceutics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raut, S.S.; Singh, R.; Lekhak, U.M. Naturally Occurring Nanoparticles (NONPs): A Review. Next Sustain. 2024, 3, 100037. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N. Rapid Detection of Bacteria by Carbon Quantum Dots. J. Biomed. Nanotechnol. 2011, 7, 846–848. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Dually Emissive P,N-Co-Doped Carbon Dots for Fluorescent and Photoacoustic Tissue Imaging in Living Mice. Microchim. Acta 2017, 184, 1117–1125. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-Based Strategies to Combat Antibiotic Resistance: Mechanisms and Applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare. Nanomaterials 2024, 14, 1085. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in Nanoparticles in Targeted Drug Delivery–A Review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Epameinondas Georgakopoulou, V.; Papalexis, P.; Trakas, N. Nanotechnology-Based Approaches for Targeted Drug Delivery for the Treatment of Respiratory Tract Infections. J. Biol. Methods 2024, 11, e99010032. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, D.; Wang, H.; Zhang, P. Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases. Pharmaceutics 2023, 15, 1899. [Google Scholar] [CrossRef]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How Nanomaterials Are Transforming Drug Delivery, Bio-Imaging, and Diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Naghib, S.M.; Amiri, S.; Mozafari, M.R. Stimuli-Responsive Chitosan-Based Nanocarriers for Drug Delivery in Wound Dressing Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100497. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Salahshoori, I.; Yazdanbakhsh, A.; Namayandeh Jorabchi, M.; Kazemabadi, F.Z.; Khonakdar, H.A.; Mohammadi, A.H. Recent Advances and Applications of Stimuli-Responsive Nanomaterials for Water Treatment: A Comprehensive Review. Adv. Colloid Interface Sci. 2024, 333, 103304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.; Li, Y.; Han, X.; Yang, Y.; Chen, Z.; Zhao, X.; Qian, Y.; Liu, X.; Zhou, F.; et al. Magnetic Nanomaterials for Hyperthermia-Based Therapy and Controlled Drug Delivery. Bioact. Mater. 2025, 53, 591–629. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 1901714. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases. J. Funct. Biomater. 2025, 16, 24. [Google Scholar] [CrossRef]
- Kim, C.-D.; Koo, K.-M.; Kim, H.-J.; Kim, T.-H. Recent Advances in Nanomaterials for Modulation of Stem Cell Differentiation and Its Therapeutic Applications. Biosensors 2024, 14, 407. [Google Scholar] [CrossRef]
- Laib, I.; Gheraissa, N.; Benaissa, A.; Benkhira, L.; Azzi, M.; Benaissa, Y.; Abdelaziz, A.G.; Tian, F.; Walsh, M.; Bechelany, M.; et al. Tailoring Innovative Silver Nanoparticles for Modern Medicine: The Importance of Size and Shape Control and Functional Modifications. Mater. Today Bio 2025, 33, 102071. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Roy, P. Polyelectrolyte Carbon Quantum-Dots: New Player as a Noninvasive Imaging Probe in Drosophila. J. Nanosci. Nanotechnol. 2013, 13, 6499–6505. [Google Scholar] [CrossRef]
- Harishbhai Tilala, M.; Kumar Chenchala, P.; Choppadandi, A.; Kaur, J.; Naguri, S.; Saoji, R.; Devaguptapu, B. Ethical Considerations in the Use of Artificial Intelligence and Machine Learning in Health Care: A Comprehensive Review. Cureus 2024, 16, e62443. [Google Scholar] [CrossRef]
- Mu, Y.; Gong, L.; Peng, T.; Yao, J.; Lin, Z. Advances in PH-Responsive Drug Delivery Systems. OpenNano 2021, 5, 100031. [Google Scholar] [CrossRef]
- Guo, Y.; Li, N.; Zhang, D.; Gu, J.; Liao, Z.; Teng, Z.; Du, X.; Timashev, P.S.; Chen, S.; Huo, S. Advancing Design Strategies in Smart Stimulus-responsive Liposomes for Drug Release and Nanomedicine. BMEMat 2025, e70045. [Google Scholar] [CrossRef]
- Torres, J.; Valenzuela Oses, J.K.; Rabasco-Álvarez, A.M.; González-Rodríguez, M.L.; García, M.C. Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers. Pharmaceutics 2025, 17, 245. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; Irshad Khan, M.Z.; Rácz, Á.; Béni, S. Acid-Sensitive Prodrugs; a Promising Approach for Site-Specific and Targeted Drug Release. Eur. J. Med. Chem. 2024, 276, 116699. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Rubab, M.; Vijayalakshmi, S.; Karuvelan, M.; Barathikannan, K.; Oh, D.-H. Liposomes for Drug Delivery: Classification, Therapeutic Applications, and Limitations. Next Nanotechnol. 2025, 8, 100209. [Google Scholar] [CrossRef]
- Qu, K.; Yuan, Z.; Wang, Y.; Song, Z.; Gong, X.; Zhao, Y.; Mu, Q.; Zhan, Q.; Xu, W.; Wang, L. Structures, Properties, and Applications of Zwitterionic Polymers. ChemPhysMater 2022, 1, 294–309. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. PH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Farhoudi, L.; Hosseinikhah, S.M.; Kazemi-Beydokhti, A.; Arabi, L.; Alavizadeh, S.H.; Moosavian, S.A.; Jaafari, M.R. PH-Sensitive Polymeric Micelles Enhance the Co-Delivery of Doxorubicin and Docetaxel: An Emerging Modality for Treating Breast Cancer. Cancer Nanotechnol. 2024, 15, 37. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST Polymers: Thermoresponsive Nanostructured Assemblies towards Bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Dethe, M.R.; Prabakaran, A.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG Copolymer Based Injectable Thermosensitive Hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; ten Hagen, T.L.M. Hyperthermia and Smart Drug Delivery Systems for Solid Tumor Therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Xiong, X.Y. Research Progress on Nano Delivery Systems of Doxorubicin. Part. Part. Syst. Charact. 2025, e00049. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-Manipulating Nanocarriers for Anticancer Drug Delivery: A Systematic Review. J. Nanobiotechnol. 2024, 22, 587. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Pandey, V.; Pandey, T. A Mechanistic Understanding of Reactive Oxygen Species (ROS)-Responsive Bio-Polymeric Nanoparticles: Current State, Challenges and Future Toward Precision Therapeutics. Biopolymers 2025, 116, e70027. [Google Scholar] [CrossRef]
- Guo, J.; Fathi Kazerooni, A.; Toorens, E.; Akbari, H.; Yu, F.; Sako, C.; Mamourian, E.; Shinohara, R.T.; Koumenis, C.; Bagley, S.J.; et al. Integrating Imaging and Genomic Data for the Discovery of Distinct Glioblastoma Subtypes: A Joint Learning Approach. Sci. Rep. 2024, 14, 4922. [Google Scholar] [CrossRef]
- Kaplan, Ö.; Bal, K.; Şentürk, S.; Demir, K.; Gök, M.K. Redox-Responsive Lipoic Acid-Modified Poly (β-Amino Ester) Nanoparticles for Enhanced Gene Delivery. React. Funct. Polym. 2025, 214, 106318. [Google Scholar] [CrossRef]
- Fu, S.; Puche, V.; Zhao, B.; Zhang, X.; McKenzie, V.A.A.; Garcia, S.; Zhang, F. Redox-Sensitive Camptothecin Prodrug: A Promising Drug Delivery Strategy with Ultrahigh Drug Loading and Tunable Drug Release. Nano TransMed 2025, 4, 100088. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef]
- Badir, A.; Refki, S.; Sekkat, Z. Utilizing Gold Nanoparticles in Plasmonic Photothermal Therapy for Cancer Treatment. Heliyon 2025, 11, e42738. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Ghavidast, A.; Pashabadi, A. Phototriggered Structures: Latest Advances in Biomedical Applications. Acta Pharm. Sin. B 2023, 13, 2844–2876. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Balusamy, S.R.; Madhusudanan, M.; Singh, H.; Amsath Haseef, H.M.; Mijakovic, I. Advanced Nanomaterials for Cancer Therapy: Gold, Silver, and Iron Oxide Nanoparticles in Oncological Applications. Adv. Healthc. Mater. 2025, 14, e2403059. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, K.; Singh, S.; Chaturvadi, V.K.; Singh, A.K.; Verma, A. Recent Advances in NIR-II Emitting Nanomaterials: Design and Biomedical Applications of Lanthanide Complexes and Functionalized Mesoporous Silica Nanoparticles (MSNs). J. Mater. Chem. B 2025, 13, 9720–9744. [Google Scholar] [CrossRef]
- Basaran, S.; Dey, S.; Bhusari, S.; Sankaran, S.; Kraus, T. Plasmonic Stimulation of Gold Nanorods for the Photothermal Control of Engineered Living Materials. Biomater. Adv. 2023, 147, 213332. [Google Scholar] [CrossRef]
- Bianchi, L.; Mooney, R.; Cornejo, Y.R.; Schena, E.; Berlin, J.M.; Aboody, K.S.; Saccomandi, P. Thermal Analysis of Laser Irradiation-Gold Nanorod Combinations at 808 Nm, 940 Nm, 975 Nm and 1064 Nm Wavelengths in Breast Cancer Model. Int. J. Hyperth. 2021, 38, 1099–1110. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological Interactions of Carbon-Based Nanomaterials: From Coronation to Degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Guo, S.; Shi, Y.; Liang, Y.; Liu, L.; Sun, K.; Li, Y. Relationship and Improvement Strategies between Drug Nanocarrier Characteristics and Hemocompatibility: What Can We Learn from the Literature. Asian J. Pharm. Sci. 2021, 16, 551–576. [Google Scholar] [CrossRef]
- van den Berg, A.I.S.; Yun, C.-O.; Schiffelers, R.M.; Hennink, W.E. Polymeric Delivery Systems for Nucleic Acid Therapeutics: Approaching the Clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef]
- Gidwani, B.; Sahu, V.; Shukla, S.S.; Pandey, R.; Joshi, V.; Jain, V.K.; Vyas, A. Quantum Dots: Prospectives, Toxicity, Advances and Applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102308. [Google Scholar] [CrossRef]
- Ly, P.-D.; Ly, K.-N.; Phan, H.-L.; Nguyen, H.H.T.; Duong, V.-A.; Nguyen, H.V. Recent Advances in Surface Decoration of Nanoparticles in Drug Delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Graván, P.; Peña-Martín, J.; de Andrés, J.L.; Pedrosa, M.; Villegas-Montoya, M.; Galisteo-González, F.; Marchal, J.A.; Sánchez-Moreno, P. Exploring the Impact of Nanoparticle Stealth Coatings in Cancer Models: From PEGylation to Cell Membrane-Coating Nanotechnology. ACS Appl. Mater. Interfaces 2024, 16, 2058–2074. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical Applications and Prospects of Polylactic Acid Materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef]
- Patra, P.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; Kesari, K.K. Alginate-Chitosan Biodegradable and Biocompatible Based Hydrogel for Breast Cancer Immunotherapy and Diagnosis: A Comprehensive Review. ACS Appl. Bio Mater. 2024, 7, 3515–3534. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, S.; Wu, X.; Tan, S.; He, Y. Biodegradability of Mesoporous Silica Nanoparticles. Ceram. Int. 2021, 47, 31031–31041. [Google Scholar] [CrossRef]
- Tripathi, D.; Pandey, P.; Sharma, S.; Rai, A.K.; Prabhu BH, M. Advances in Nanomaterials for Precision Drug Delivery: Insights into Pharmacokinetics and Toxicity. BioImpacts 2024, 15, 30573. [Google Scholar] [CrossRef]
- Ewii, U.E.; Attama, A.A.; Olorunsola, E.O.; Onugwu, A.L.; Nwakpa, F.U.; Anyiam, C.; Chijioke, C.; Ogbulie, T. Nanoparticles for Drug Delivery: Insight into in Vitro and in Vivo Drug Release from Nanomedicines. Nano TransMed 2025, 4, 100083. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Bhardwaj, S.; Sikarwar, M.; Sriwastaw, S.; Sharma, G.; Gupta, M. Nanotoxicity Unveiled: Evaluating Exposure Risks and Assessing the Impact of Nanoparticles on Human Health. J. Trace Elem. Miner. 2025, 13, 100252. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, Y.; Shen, Y.; Zhou, Z. Surface Engineering of Nanoparticles for Precision Medicine. Precis. Med. Eng. 2025, 2, 100037. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Taher, M.; Susanti, D.; Haris, M.S.; Rushdan, A.A.; Widodo, R.T.; Syukri, Y.; Khotib, J. PEGylated Liposomes Enhance the Effect of Cytotoxic Drug: A Review. Heliyon 2023, 9, e13823. [Google Scholar] [CrossRef]
- Ibrahim, M.A.I.; Othman, R.; Chee, C.F.; Ahmad Fisol, F. Evaluation of Folate-Functionalized Nanoparticle Drug Delivery Systems—Effectiveness and Concerns. Biomedicines 2023, 11, 2080. [Google Scholar] [CrossRef]
- Vilaça-Faria, H.; Noro, J.; Reis, R.L.; Pirraco, R.P. Extracellular Matrix-Derived Materials for Tissue Engineering and Regenerative Medicine: A Journey from Isolation to Characterization and Application. Bioact. Mater. 2024, 34, 494–519. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, W.; Liu, R.; Li, X.; Li, H.; Liu, L.; Chen, Y.; Lv, C.; Liu, Y. PH- and Enzyme-Triggered Drug Release as an Important Process in the Design of Anti-Tumor Drug Delivery Systems. Biomed. Pharmacother. 2019, 118, 109340. [Google Scholar] [CrossRef] [PubMed]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Marin, E.; Tapeinos, C.; Sarasua, J.R.; Larrañaga, A. Exploiting the Layer-by-Layer Nanoarchitectonics for the Fabrication of Polymer Capsules: A Toolbox to Provide Multifunctional Properties to Target Complex Pathologies. Adv. Colloid Interface Sci. 2022, 304, 102680. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Tambe, V.; Pofali, P.; Vora, L.K. Cell Membrane-Coated Nanoparticles: A New Frontier in Immunomodulation. Adv. NanoBiomed Res. 2024, 4, 2400012. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, D.; Li, L.; Sun, K. Charge Reversal Nano-Systems for Tumor Therapy. J. Nanobiotechnol. 2022, 20, 31. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Chao, Y.-C.; Xu, S.-Q.; Peng, Y.-R.; Syu, J.-J.; Yang, X.-H.; Pan, Y.-K.; Lin, P.-C.; Weng, L.-L.; Chen, I.-C.; et al. Surface Functionalization of Gold Nanoparticles Using Alkyne Derivatives: Applications in Chemical Sensing. ACS Appl. Mater. Interfaces 2024, 16, 58262–58273. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The Protein Corona from Nanomedicine to Environmental Science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimnejad, P.; Sodagar Taleghani, A.; Asare-Addo, K.; Nokhodchi, A. An Updated Review of Folate-Functionalized Nanocarriers: A Promising Ligand in Cancer. Drug Discov. Today 2022, 27, 471–489. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD Peptide in Cancer Targeting: Benefits, Challenges, Solutions, and Possible integrin–RGD Interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef]
- Pereira Gomes, C.; Leiro, V.; Ferreira Lopes, C.D.; Spencer, A.P.; Pêgo, A.P. Fine Tuning Neuronal Targeting of Nanoparticles by Adjusting the Ligand Grafting Density and Combining PEG Spacers of Different Length. Acta Biomater. 2018, 78, 247–259. [Google Scholar] [CrossRef]
- Selepe, C.T.; Dhlamini, K.S.; Tshweu, L.; Moralo, M.; Kwezi, L.; Ray, S.S.; Ramalapa, B. Trastuzumab-based Nanomedicines for Breast Cancer Therapy: Recent Advances and Future Opportunities. Nano Sel. 2024, 5, 2300191. [Google Scholar] [CrossRef]
- Jiao, J.; Qian, Y.; Lv, Y.; Wei, W.; Long, Y.; Guo, X.; Buerliesi, A.; Ye, J.; Han, H.; Li, J.; et al. Overcoming Limitations and Advancing the Therapeutic Potential of Antibody-Oligonucleotide Conjugates (AOCs): Current Status and Future Perspectives. Pharmacol. Res. 2024, 209, 107469. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-Based Blood-Brain-Barrier (BBB) Crossing Strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Lucchi, R.; Bentanachs, J.; Oller-Salvia, B. The Masking Game: Design of Activatable Antibodies and Mimetics for Selective Therapeutics and Cell Control. ACS Cent. Sci. 2021, 7, 724–738. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Augmentation of the EPR Effect by Mild Hyperthermia to Improve Nanoparticle Delivery to the Tumor. Biochim. Biophys. Acta–Rev. Cancer 2024, 1879, 189109. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.; Golzaryan, A.; Soltani, M. Charge-Switchable Nanoparticles to Enhance Tumor Penetration and Accumulation. Eur. J. Pharm. Biopharm. 2024, 199, 114310. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Moradi Kashkooli, F.; Kiani Shahvandi, M.; Chiani, M.; Shariati, F.S.; Mehrabi, M.R.; Munn, L.L. Towards Principled Design of Cancer Nanomedicine to Accelerate Clinical Translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Batool, S.; Sohail, S.; ud Din, F.; Alamri, A.H.; Alqahtani, A.S.; Alshahrani, M.A.; Alshehri, M.A.; Choi, H.G. A Detailed Insight of the Tumor Targeting Using Nanocarrier Drug Delivery System. Drug Deliv. 2023, 30, 2183815. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, J.; Yang, W. Stimuli-Responsive Nanocarriers for Therapeutic Applications in Cancer. Cancer Biol. Med. 2021, 18, 319–335. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Joo, S.W. PH-Responsive Biocompatible Fluorescent Core-Shell Nanogel for Intracellular Imaging and Control Drug Release. Part. Part. Syst. Charact. 2021, 38, 2100110. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Nagajyothi, P.C.; Reddy, P.M.; Reddy, N.R.; Joo, S.W. Highly Fluorescent Doped Fe3O4@C Nanoparticles Cross the Blood–Brain Barrier: Help in Brain Imaging and Blocking the Life Cycle of Mosquitoes. J. Clust. Sci. 2021, 32, 1761–1767. [Google Scholar] [CrossRef]
- Fu, S.; Rempson, C.M.; Puche, V.; Zhao, B.; Zhang, F. Construction of Disulfide Containing Redox-Responsive Polymeric Nanomedicine. Methods 2022, 199, 67–79. [Google Scholar] [CrossRef]
- Hajebi, S.; Chamanara, M.; Nasiri, S.S.; Ghasri, M.; Mouraki, A.; Heidari, R.; Nourmohammadi, A. Advances in Stimuli-Responsive Gold Nanorods for Drug-Delivery and Targeted Therapy Systems. Biomed. Pharmacother. 2024, 180, 117493. [Google Scholar] [CrossRef]
- Day, N.B.; Wixson, W.C.; Shields, C.W. Magnetic Systems for Cancer Immunotherapy. Acta Pharm. Sin. B 2021, 11, 2172–2196. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Mandal, T.K.; Joo, S.W. Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics. J. Funct. Biomater. 2024, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K.; Woo Joo, S. Synthesis of Heteroatom-Doped Polymer-Coated Nanomaterials for Slow and Controlled Drug Release in the Physiological Microenvironment. IEEE Trans. Nanobiosci. 2025, 24, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-Responsive Liposomal Nanoformulations in Cancer Therapy: Pre-Clinical & Clinical Approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Innovative Micro- and Nano-Architectures in Biomedical Engineering for Therapeutic and Diagnostic Applications. Micromachines 2025, 16, 419. [Google Scholar] [CrossRef]
- Naik, H.; Sonju, J.J.; Singh, S.; Chatzistamou, I.; Shrestha, L.; Gauthier, T.; Jois, S. Lipidated Peptidomimetic Ligand-Functionalized HER2 Targeted Liposome as Nano-Carrier Designed for Doxorubicin Delivery in Cancer Therapy. Pharmaceuticals 2021, 14, 221. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Joo, S.-W. The Impact of COVID-19 on RNA Therapeutics: A Surge in Lipid Nanoparticles and Alternative Delivery Systems. Pharmaceutics 2024, 16, 1366. [Google Scholar] [CrossRef]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational Design of Polymeric Micelles for Targeted Therapeutic Delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics. Gels 2025, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Hernandez, Y.; Miyasaki, K.F.; Kwon, E.J. Engineered Nanomaterials That Exploit Blood-Brain Barrier Dysfunction for Delivery to the Brain. Adv. Drug Deliv. Rev. 2023, 197, 114820. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Magnetic Resonance Imaging and Iron-Oxide Nanoparticles in the Era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Enhancing Vaccine Efficacy and Stability: A Review of the Utilization of Nanoparticles in MRNA Vaccines. Biomolecules 2024, 14, 1036. [Google Scholar] [CrossRef]
- Paurević, M.; Šrajer Gajdošik, M.; Ribić, R. Mannose Ligands for Mannose Receptor Targeting. Int. J. Mol. Sci. 2024, 25, 1370. [Google Scholar] [CrossRef]
- Mandal, T.K.; Lee, Y.R.; Parvin, N. Red Phosphorus Decorated Graphene Oxide Nanosheets: Label-Free DNA Detection. Biomater. Sci. 2020, 8, 125–131. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M. The Use of RNA-Based Treatments in the Field of Cancer Immunotherapy. Mol. Cancer 2023, 22, 106. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Christopher, A.T.; Lekan, O.K.; Aworinde, O.R.; Faderin, E.; Obembe, O.; Abdulsalam_Akanji, T.F.; Igboanugo, J.C.; Udogu, U.; Ogidi, G.O.; et al. Advancements in Tantalum Based Nanoparticles for Integrated Imaging and Photothermal Therapy in Cancer Management. RSC Adv. 2024, 14, 33681–33740. [Google Scholar] [CrossRef]
- Sun, X.; Xiang, T.; Xie, L.; Ren, Q.; Chang, J.; Jiang, W.; Jin, Z.; Yang, X.; Ren, W.; Yu, Y. Recent Advances in Fluorescent Nanomaterials Designed for Biomarker Detection and Imaging. Mater. Today Bio 2025, 32, 101763. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Cutting-Edge Hydrogel Technologies in Tissue Engineering and Biosensing: An Updated Review. Materials 2024, 17, 4792. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Parvin, N.; Mishra, K.; Mohandoss, S.; Lee, Y.R. Sensitive and Selective Fluorometric Determination of DNA by Using Layered Hexagonal Nanosheets of a Covalent Organic Framework Prepared from P-Phenylenediamine and Benzene-1,3,5-Tricarboxaldehyde. Microchim. Acta 2019, 186, 833. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; He, S.; Cheng, Z.; Hu, J. Enhancing the Fluorescence Emission of the NIR-II Fluorophores: Strategies, Mechanisms, Challenges, and Opportunities. Coord. Chem. Rev. 2025, 532, 216511. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a Highly Fluorescence Nitrogen-Doped Carbon Quantum Dots Bioimaging Probe and Its in Vivo Clearance and Printing Applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Salvi, A.; Kharbanda, S.; Thakur, P.; Shandilya, M.; Thakur, A. Biomedical Application of Carbon Quantum Dots: A Review. Carbon Trends 2024, 17, 100407. [Google Scholar] [CrossRef]
- Soman, S.; Kulkarni, S.; Sherin, F.; Roy, A.A.; Mukharya, A.; Pokale, R.; Mutalik, S. Bioinspired Quantum Dots: Advancing Diagnostic and Therapeutic Strategies in Breast Cancer. RSC Adv. 2025, 15, 27738–27771. [Google Scholar] [CrossRef]
- Grover, V.P.B.; Tognarelli, J.M.; Crossey, M.M.E.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J.W. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 246–255. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Electroactive Polymers for Self-Powered Actuators and Biosensors: Advancing Biomedical Diagnostics Through Energy Harvesting Mechanisms. Actuators 2025, 14, 257. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Ellis, C.M.; Pellico, J.; Davis, J.J. Magnetic Nanoparticles Supporting Bio-Responsive T1/T2 Magnetic Resonance Imaging. Materials 2019, 12, 4096. [Google Scholar] [CrossRef]
- Rizwan, A.; Sridharan, B.; Park, J.H.; Kim, D.; Vial, J.-C.; Kyhm, K.; Lim, H.G. Nanophotonic-Enhanced Photoacoustic Imaging for Brain Tumor Detection. J. Nanobiotechnol. 2025, 23, 170. [Google Scholar] [CrossRef]
- Chohan, D.P.; Dey, B.; Tarkunde, A.; Vyas, V.; De Sarkar, S.; Sundara, B.K. Advancing Autonomous Nanomedicine: Bridging the Gap from Concept to Potential Clinical Studies. J. Clust. Sci. 2024, 35, 2607–2635. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mohammadnejad, J.; Salamat, S.; Beiram Zadeh, Z.; Tanhaei, M.; Ramakrishna, S. Theranostic Polymeric Nanoparticles as a New Approach in Cancer Therapy and Diagnosis: A Review. Mater. Today Chem. 2023, 29, 101400. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Pouya Hadipour Moghaddam, S.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A Review on the Latest Developments of Mesoporous Silica Nanoparticles as a Promising Platform for Diagnosis and Treatment of Cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef] [PubMed]
- Fahim, Y.A.; Hasani, I.W.; Mahmoud Ragab, W. Promising Biomedical Applications Using Superparamagnetic Nanoparticles. Eur. J. Med. Res. 2025, 30, 441. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Ferdous, U.T.; Nizela, A.; Hasan, A.; Shakoor, A.; Zia, A.W.; Uddin, S. Mechanistic Study of Cancer Drug Delivery: Current Techniques, Limitations, and Future Prospects. Eur. J. Med. Chem. 2025, 290, 117535. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Khan, T.; Islam, R.U.; Naseem, S. Nanomedicine Breakthroughs in Cancer and Neurodegenerative Disease Therapy. Next Nanotechnol. 2025, 8, 100233. [Google Scholar] [CrossRef]
- Francisco, T.N.; Malafaia, D.; Melo, L.; Silva, A.M.S.; Albuquerque, H.M.T. Recent Advances in Fluorescent Theranostics for Alzheimer’s Disease: A Comprehensive Survey on Design, Synthesis, and Properties. ACS Omega 2024, 9, 13556–13591. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials 2025, 15, 895. [Google Scholar] [CrossRef]
- Teng, L.; Bi, Y.; Xing, X.; Yao, G. Nano-Oncology Revisited: Insights on Precise Therapeutic Advances and Challenges in Tumor. Fundam. Res. 2025, 5, 1845–1859. [Google Scholar] [CrossRef]
- Upadhaya, A.; Pegu, J.; Singh, Y.D.; Ngomle, S. Nanoparticle-Enabled Portable Biosensors for Early Detection and Monitoring of Non-Communicable Diseases: A Focus on Diabetes, Cardiovascular, and Cancer Diagnostics. Biosens. Bioelectron. X 2025, 26, 100675. [Google Scholar] [CrossRef]
- Song, J.; Luo, Y.; Hao, Z.; Qu, M.; Huang, C.; Wang, Z.; Yang, J.; Liang, Q.; Jia, Y.; Song, Q.; et al. Graphene-Based Wearable Biosensors for Point-of-Care Diagnostics: From Surface Functionalization to Biomarker Detection. Mater. Today Bio 2025, 32, 101667. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.-S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Park, S.; Mandal, T.K.; Joo, S.W. Innovative Wearable Electronics: Next-Generation Nitrogen-Doped Lutetium-Carbon Microspheres Composites for Robust Energy Harvesting. Small 2025, 21, e2407386. [Google Scholar] [CrossRef]
- Lutomia, D.; Poria, R.; Kala, D.; Garg, P.; Nagraik, R.; Kaushal, A.; Gupta, S.; Kumar, D. 2D Nanomaterials in Biosensing: Synthesis, Characterization, Integration in Biosensors and Their Applications. Biosens. Bioelectron. X 2025, 24, 100615. [Google Scholar] [CrossRef]
- Safarkhani, M.; Aldhaher, A.; Heidari, G.; Zare, E.N.; Warkiani, M.E.; Akhavan, O.; Huh, Y.; Rabiee, N. Nanomaterial-Assisted Wearable Glucose Biosensors for Noninvasive Real-Time Monitoring: Pioneering Point-of-Care and Beyond. Nano Mater. Sci. 2024, 6, 263–283. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Unlocking the Future: Carbon Nanotubes as Pioneers in Sensing Technologies. Chemosensors 2025, 13, 225. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Ravi, P.; Reddy, M.C.; Thummala, C.; Mandal, T.K. Zinc Oxide Nanoparticles in Modern Science and Technology: Multifunctional Roles in Healthcare, Environmental Remediation, and Industry. Nanomaterials 2025, 15, 754. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and Their Applications in Early Diagnosis of Cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Ghorbian, M.; Ghobaei-Arani, M.; Babaei, M.R.; Ghorbian, S. Nanotechnology and Nanosensors in Personalized Healthcare: A Comprehensive Review. Sens. Bio-Sens. Res. 2025, 47, 100740. [Google Scholar] [CrossRef]
- Dhahi, T.S.; Yousif Dafhalla, A.K.; Al-Mufti, A.W.; Elobaid, M.E.; Adam, T.; Gopinath, S.C.B. Application of Nanobiosensor Engineering in the Diagnosis of Neurodegenerative Disorders. Results Eng. 2024, 24, 102790. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Liao, C. AI-Driven Wearable Bioelectronics in Digital Healthcare. Biosensors 2025, 15, 410. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical Applications of Functional Hydrogels: Innovative Developments, Relevant Clinical Trials and Advanced Products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lv, Z.; Wang, Z.; Wang, Y.; Xu, J.; Yang, X.; Wang, H.; Bian, Y.; Zhu, Y.; Feng, B.; et al. Two-Dimensional Nanomaterials for Bone Disease Therapy: Multifunctional Platforms for Regeneration, Anti-Infection and Tumor Ablation. J. Nanobiotechnol. 2025, 23, 566. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wei, H.; Qi, Y.; Ding, S.; Li, H.; Si, S. Advances in Hybrid Hydrogel Design for Biomedical Applications: Innovations in Drug Delivery and Tissue Engineering for Gynecological Cancers. Cell Biol. Toxicol. 2025, 41, 115. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.; Joo, S. Application of Bimetallic Heterojunction Nanoparticle-Based Multishelled Porous Hollow Microspheres as a Two-in-One Inorganic UV Filter. ACS Sustain. Chem. Eng. 2023, 11, 16133–16143. [Google Scholar] [CrossRef]
- Merino, J.J.; Cabaña-Muñoz, M.E. Nanoparticles and Mesenchymal Stem Cell (MSC) Therapy for Cancer Treatment: Focus on Nanocarriers and a Si-RNA CXCR4 Chemokine Blocker as Strategies for Tumor Eradication In Vitro and In Vivo. Micromachines 2023, 14, 2068. [Google Scholar] [CrossRef]
- Abu-El-Rub, E.; Khasawneh, R.R.; Almahasneh, F. Prodigious Therapeutic Effects of Combining Mesenchymal Stem Cells with Magnetic Nanoparticles. World J. Stem Cells 2022, 14, 513–526. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2013, 2, 244–260. [Google Scholar] [CrossRef]
- Kumar, V.; Mandal, T.K.; Parvin, N.; Joo, S.W.; Park, S.-S. The Multifunctional Composites Based on New Generation Carbon Microsphere and Their Hybrids with Robust Interfacial Mechanical Strength. Surf. Interfaces 2023, 42, 103378. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef]
- Szustak, M.; Gendaszewska-Darmach, E. Nanocellulose-Based Scaffolds for Chondrogenic Differentiation and Expansion. Front. Bioeng. Biotechnol. 2021, 9, 736213. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ou, X.; Cheng, D. How Advancing Is Peripheral Nerve Regeneration Using Nanofiber Scaffolds? A Comprehensive Review of the Literature. Int. J. Nanomed. 2023, 18, 6763–6779. [Google Scholar] [CrossRef] [PubMed]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication Methods for Reconstructing Extracellular Matrix Mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef]
- Parvin, N.; Aslam, M.; Joo, S.W.; Mandal, T.K. Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules 2025, 30, 3177. [Google Scholar] [CrossRef]
- Amani, H.; Alipour, M.; Shahriari, E.; Taboas, J.M. Immunomodulatory Biomaterials: Tailoring Surface Properties to Mitigate Foreign Body Reaction and Enhance Tissue Regeneration. Adv. Healthc. Mater. 2024, 13, e2401253. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing Factors and Strategies of Enhancing Nanoparticles into Tumors in Vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Batir-Marin, D.; Boev, M.; Cioanca, O.; Lungu, I.-I.; Marin, G.-A.; Burlec, A.F.; Mitran, A.-M.; Mircea, C.; Hancianu, M. Exploring Oxidative Stress Mechanisms of Nanoparticles Using Zebrafish (Danio Rerio): Toxicological and Pharmaceutical Insights. Antioxidants 2025, 14, 489. [Google Scholar] [CrossRef]
- Moayedi, S.; Xia, W.; Lundergan, L.; Yuan, H.; Xu, J. Zwitterionic Polymers for Biomedical Applications: Antimicrobial and Antifouling Strategies toward Implantable Medical Devices and Drug Delivery. Langmuir 2024, 40, 23125–23145. [Google Scholar] [CrossRef]
- Halamoda-Kenzaoui, B.; Bremer-Hoffmann, S. Main Trends of Immune Effects Triggered by Nanomedicines in Preclinical Studies. Int. J. Nanomed. 2018, 13, 5419–5431. [Google Scholar] [CrossRef]
- Hofer, S.; Hofstätter, N.; Punz, B.; Hasenkopf, I.; Johnson, L.; Himly, M. Immunotoxicity of Nanomaterials in Health and Disease: Current Challenges and Emerging Approaches for Identifying Immune Modifiers in Susceptible Populations. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1804. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.B.; Pradhan, S. Nanoparticles Induced Health Hazards through Environmental Exposure and Direct Contact. Proc. Inst. Mech. Eng. Part N J. Nanomater. Nanoeng. Nanosyst. 2025, 23977914241312438. [Google Scholar] [CrossRef]
- Pogribna, M.; Hammons, G. Epigenetic Effects of Nanomaterials and Nanoparticles. J. Nanobiotechnol. 2021, 19, 2. [Google Scholar] [CrossRef]
- Dormont, F.; Rouquette, M.; Mahatsekake, C.; Gobeaux, F.; Peramo, A.; Brusini, R.; Calet, S.; Testard, F.; Lepetre-Mouelhi, S.; Desmaële, D.; et al. Translation of Nanomedicines from Lab to Industrial Scale Synthesis: The Case of Squalene-Adenosine Nanoparticles. J. Control. Release 2019, 307, 302–314. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive Stimulus-responsive Nanocarriers for Targeted Delivery of Therapeutic Agents. WIREs Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef]
- Kafetzis, K.N.; Papalamprou, N.; McNulty, E.; Thong, K.X.; Sato, Y.; Mironov, A.; Purohit, A.; Welsby, P.J.; Harashima, H.; Yu-Wai-Man, C.; et al. The Effect of Cryoprotectants and Storage Conditions on the Transfection Efficiency, Stability, and Safety of Lipid-Based Nanoparticles for MRNA and DNA Delivery. Adv. Healthc. Mater. 2023, 12, e2203022. [Google Scholar] [CrossRef]
- Rose, K.A.; Molaei, M.; Boyle, M.J.; Lee, D.; Crocker, J.C.; Composto, R.J. Particle Tracking of Nanoparticles in Soft Matter. J. Appl. Phys. 2020, 127, 191101. [Google Scholar] [CrossRef]
- Saeed, M.M.; Carthy, E.; Dunne, N.; Kinahan, D. Advances in Nanoparticle Synthesis Assisted by Microfluidics. Lab Chip 2025, 25, 3060–3093. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Obeid, M.A.; Gammoh, O.; El-Tanani, M.; Mishra, V.; Mishra, Y.; Kapre, S.; Srivatsa Palakurthi, S.; Hassan, S.S.; Nawn, D.; et al. Nanomaterial-Driven Precision Immunomodulation: A New Paradigm in Therapeutic Interventions. Cancers 2024, 16, 2030. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Png Yi Jie, J.; Malinovskaya, J.; Kovshova, T.; Jain, P.; Naik, S.; Khopade, A.; Bhowmick, S.; Shahi, P.; Chakra, A.; et al. A Design-Conversed Strategy Establishes the Performance Safe Space for Doxorubicin Nanosimilars. ACS Nano 2024, 18, 6162–6175. [Google Scholar] [CrossRef] [PubMed]
- Vandghanooni, S.; Jaymand, M.; Eskandani, M. The Use of Nanomedicines in the Healthcare Systems: A Policy Brief. Drug Dev. Ind. Pharm. 2025, 51, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Mustafa, G.; Abdel-Wahab, B.A.; Pathak, K.; Das, A.; Sahariah, J.J.; Kalita, P.; Alam, A.; Borthakur, P.P. From Bench to Bedside: Advancing Liposomal Doxorubicin for Targeted Cancer Therapy. Results Surf. Interfaces 2025, 19, 100473. [Google Scholar] [CrossRef]
- Saxena, A.; Schneider, B.J.; Christos, P.J.; Audibert, L.F.; Cagney, J.M.; Scheff, R.J. Treatment of Recurrent and Platinum-Refractory Stage IV Non-Small Cell Lung Cancer with Nanoparticle Albumin-Bound Paclitaxel (Nab-Paclitaxel) as a Single Agent. Med. Oncol. 2016, 33, 13. [Google Scholar] [CrossRef]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing Ferumoxytol: Diagnostic and Therapeutic Applications of an FDA-Approved Nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, W.; Balthasar, J.P. Camptothein-Based Anti-Cancer Therapies and Strategies to Improve Their Therapeutic Index. Cancers 2025, 17, 1032. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The Promise of Nanotechnology in Personalized Medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-Based Drug Delivery Systems Targeting Cancer Cell Surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lin, S.; Yang, F.; Situ, J.; Lin, S.; Luo, Y. Aptamer-Conjugated Multifunctional Polymeric Nanoparticles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems for Treatment of Castration-Resistant Prostate Cancer. Biomed Res. Int. 2020, 2020, 9186583. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A Review on Microfluidic-Assisted Nanoparticle Synthesis, and Their Applications Using Multiscale Simulation Methods. Discov. Nano 2023, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhuo, S.; Zhang, Y.; Wu, L.; Gao, X.; He, S.; Bo, X.; Zhou, W. Machine Learning Reshapes the Paradigm of Nanomedicine Research. Acta Pharm. Sin. B, 2025; in press. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Li, C.; Xu, B.; Xu, S.; Liu, B. Machine Learning-Enhanced Nanoparticle Design for Precision Cancer Drug Delivery. Adv. Sci. 2025, 12, e03138. [Google Scholar] [CrossRef]
- Bhange, M.; Telange, D. Convergence of Nanotechnology and Artificial Intelligence in the Fight against Liver Cancer: A Comprehensive Review. Discov. Oncol. 2025, 16, 77. [Google Scholar] [CrossRef]
- Chou, W.; Canchola, A.; Zhang, F.; Lin, Z. Machine Learning and Artificial Intelligence in Nanomedicine. WIREs Nanomed. Nanobiotechnol. 2025, 17, e70027. [Google Scholar] [CrossRef]
- Savarkar, S.; Gibson, J.; Balasubramanian, V.; Moudgil, B.; Hennig, R. Data Curation to Develop Machine Learning Models for Assessing the Toxicity of Nanoparticles. KONA Powder Part. J. 2025, 2026019. [Google Scholar] [CrossRef]
- Patra, A.R.; Pattnaik, A.; Ghosh, P. The Latest Breakthroughs in Green and Hybrid Nanoparticle Synthesis for Multifaceted Environmental Applications. J. Taiwan Inst. Chem. Eng. 2025, 106157. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- Yin, Z.; Li, S.; Li, X.; Shi, W.; Liu, W.; Gao, Z.; Tao, M.; Ma, C.; Liu, Y. A Review on the Synthesis of Metal Oxide Nanomaterials by Microwave Induced Solution Combustion. RSC Adv. 2023, 13, 3265–3277. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Jain, A.; Bhise, K. Nano-Pharmacokinetics and Pharmacodynamics of Green-Synthesized ZnO Nanoparticles: A Pathway to Safer Therapeutic Applications. Xenobiotica 2025, 55, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.; Lenzi, G.G. Advances in the Use of Green and Sustainable Synthesis to Obtain Nanomaterials. In Green Chemistry for Environmental Sustainability—Prevention-Assurance-Sustainability (P-A-S) Approach; IntechOpen: London, UK, 2023. [Google Scholar]
- Loh, J.S.; Low, C.Y.; Low, W.P.; Ng, Y.L.; Mak, W.Q.; Stella Tan, L.K.; Chan, H.H.; Ong, Y.S.; Cheah, S.K.A.; Albert, E.L.; et al. Traversing the Valley of Death for Nanotechnology-Based Natural Products: Strategies and Insights from Pharmaceutical Stakeholders. Drug Deliv. Transl. Res. 2025, 15, 4126–4140. [Google Scholar] [CrossRef]
- Bae, H.; Ji, H.; Konstantinov, K.; Sluyter, R.; Ariga, K.; Kim, Y.H.; Kim, J.H. Artificial Intelligence-Driven Nanoarchitectonics for Smart Targeted Drug Delivery. Adv. Mater. 2025, 37, e10239. [Google Scholar] [CrossRef]
- Sampaio, A.R.; Maia, R.F.; Ciardulli, M.C.; Santos, H.A.; Sarmento, B. Organ-on-Chip Platforms for Nanoparticle Toxicity and Efficacy Assessment: Advancing beyond Traditional in Vitro and in Vivo Models. Mater. Today Bio 2025, 33, 102053. [Google Scholar] [CrossRef]
- Souto, E.B.; Blanco-Llamero, C.; Krambeck, K.; Kiran, N.S.; Yashaswini, C.; Postwala, H.; Severino, P.; Priefer, R.; Prajapati, B.G.; Maheshwari, R. Regulatory Insights into Nanomedicine and Gene Vaccine Innovation: Safety Assessment, Challenges, and Regulatory Perspectives. Acta Biomater. 2024, 180, 1–17. [Google Scholar] [CrossRef]
- Patne, A.Y.; Mohapatra, S.; Mohapatra, S.S. Role of Nanomedicine in Transforming Pharmacotherapy for Substance Use Disorder (SUD). WIREs Nanomed. Nanobiotechnol. 2025, 17, e70008. [Google Scholar] [CrossRef]
- Haripriyaa, M.; Suthindhiran, K. Pharmacokinetics of Nanoparticles: Current Knowledge, Future Directions and Its Implications in Drug Delivery. Futur. J. Pharm. Sci. 2023, 9, 113. [Google Scholar] [CrossRef]

| Stimuli Type | Design/Mechanism | Applications | Key Limitations/Challenges | References |
|---|---|---|---|---|
| pH Responsive | Acid labile bonds (hydrazone, acetal, imine, cis aconityl) cleave in acidic conditions; charge switch polymers gain positive charge in low pH | Tumor targeted drug release, improved intracellular uptake, theranostics using gold or iron oxide nanoparticles | Small pH differences between tissues; risk of premature release in systemic acidosis | [23,24,25,26,27,28,29] |
| Temperature Responsive | LCST type polymers (e.g., PNIPAM) collapse above 32 °C; hydrogels form gel depots at body temperature; thermosensitive liposomes destabilize under mild heat (40–42 °C) | Controlled drug release, injectable depots, cancer therapy (ThermoDox® trials) | Achieving uniform heating in deep tissues; risk of off-target hyperthermia | [16,30,31,32] |
| Redox Responsive | Disulfide or thioketal linkages cleave in high intracellular GSH or ROS environments | Targeted release in tumor cytoplasm; gene and anti-inflammatory therapies | Variations in GSH/ROS levels among patients; potential premature degradation | [34,35,36,37,38,39] |
| Light Responsive | Gold nanostructures convert NIR light to heat (photothermal); light cleavable bonds enable photolysis and gel–sol transitions | On demand release, photothermal therapy, implantable light triggered systems | Limited tissue penetration; possible phototoxicity in prolonged exposure | [40,41] |
| Magnetic Responsive | Iron oxide nanoparticles (IONPs) generate local heat under alternating magnetic fields and act as MRI contrast agents | Theranostic use in magnetic hyperthermia, guided drug delivery, MRI imaging | Requires precise tuning of magnetic field; variable heating efficiency | [16,42,43] |
| Enzyme Responsive | Peptide or polymer linkages cleaved by enzymes such as MMPs, cathepsins, or esterases overexpressed in diseased tissues | Targeted release in tumor and inflammatory microenvironments; tissue specific therapy | Enzyme expression varies across diseases; risk of off-target activation | [45,46,47] |
| Nanomaterial Class | Primary Composition/Examples | Main Biodegradation Mechanism | Primary Clearance Route | Key Translational Considerations | References |
|---|---|---|---|---|---|
| Polymeric Nanomaterials | PLGA, PLA, PCL, PEG based copolymers | Hydrolysis of ester bonds into lactic and glycolic acids; enzymatic degradation by esterases | Renal or hepatic clearance of soluble degradation products | Widely used in approved drug delivery systems; degradation rate tunable by copolymer ratio and molecular weight | [53,54,55,56] |
| Lipid-Based Nanocarriers | Liposomes, solid lipid nanoparticles, nanoemulsions | Enzymatic hydrolysis by lipases and phospholipases into fatty acids and glycerol | Metabolized via hepatic lipid pathways; minimal long-term accumulation | Excellent biocompatibility and clearance; stability and storage conditions remain key challenges | [57,58] |
| Metallic Nanoparticles | Gold, iron oxide, silver, zinc oxide | Oxidation, dissolution, or surface ligand exchange; partial biodegradation depending on particle size and coating | Phagocytic uptake and sequestration in liver, spleen, and lymph nodes; limited renal clearance for larger (>10 nm) particles | Long-term retention and potential chronic toxicity; requires surface modification for improved excretion | [56,59] |
| Hybrid and Composite Nanomaterials | Metal–polymer or silica–lipid composites | Combined hydrolytic, oxidative, and enzymatic degradation depending on components | Mixed clearance through hepatobiliary and renal pathways | Complexity offers multifunctionality but complicates predictability of degradation and regulatory approval | [60,61] |
| Targeting/Release Strategy | Nanocarrier Type | Examples/Applications | Key Advantages | Limitations/Challenges | References |
|---|---|---|---|---|---|
| Active Targeting (Ligand–Receptor Interactions) | Liposomes, polymeric micelles, polymer drug conjugates | Folic acid functionalized nanoparticles for tumors; transferrin modified nanocarriers for proliferating cancer cells; RGD peptide systems for angiogenesis imaging/therapy | High selectivity, improved intracellular uptake, reduced systemic toxicity, adaptable across multiple receptors | Receptor heterogeneity across patients/tumors; ligand density optimization; risk of immune recognition | [11,72,75] |
| Active Targeting (Aptamer Functionalization) | Polymeric nanoparticles, liposomes, gold nanostructures | Aptamer conjugated nanocarriers for PSMA targeted prostate cancer therapy | High binding specificity, non-immunogenic, tunable for different targets | Limited stability in vivo, potential degradation by nucleases | [4,74] |
| Antibody Conjugated Nanocarriers | Liposomes, polymeric micelles, dendrimers, nanoparticles | Trastuzumab conjugated liposomes for HER2+ breast cancer; antibody–nanocarriers for ICAM 1 targeting in inflammation; brain targeting antibodies for CNS disorders | Dual functionality (therapeutic + targeting); strong specificity to well characterized biomarkers | Immunogenicity, high cost, large size reduces deep tissue penetration; scale-up difficulties | [76,77,78,79] |
| Passive Targeting (EPR Effect) | Liposomes, polymeric micelles, albumin bound nanoparticles | Doxil® (liposomal doxorubicin), Abraxane® (albumin bound paclitaxel) | Exploits tumor vasculature leakiness, improved accumulation in tumor tissue, FDA-approved nanomedicines | Heterogeneity of EPR across patients/tumors, limited predictability, poor penetration in dense tumors | [80,83,86,87] |
| Stimuli-Responsive Release (Internal Stimuli) | pH sensitive liposomes, redox sensitive polymeric carriers, enzyme responsive nanoparticles | pH sensitive liposomes releasing drugs in acidic tumor microenvironment; disulfide bond carriers for GSH triggered release; MMP responsive carriers for cancer therapy | On demand drug release at pathological site, higher therapeutic index, minimized systemic toxicity | Complex synthesis, variable pathological conditions across patients, premature degradation risk | [18,88,92] |
| Stimuli-Responsive Release (External Stimuli) | Gold nanorods, SPIONs, carbon nanomaterials, ultrasound responsive carriers | Photothermal therapy with gold nanorods (NIR triggered release); SPIONs for magnetic field triggered targeting; ultrasound triggered carriers | Spatiotemporal control, clinician directed release, useful for precision oncology | Limited tissue penetration of external triggers (e.g., NIR, ultrasound); scalability issues | [21,94,95,96] |
| Hybrid Stimuli-Responsive Platforms | Dual pH–redox responsive polymeric micelles, multi responsive nanoplatforms | Sequential drug release: pH triggered extracellular release + redox triggered intracellular release | Enhanced efficiency, multi-level control, ensures delivery to intracellular targets | Complex synthesis, clinical translation barriers, reproducibility concerns | [97] |
| Case Study—Antibody Targeted Liposomes | Liposomal doxorubicin (trastuzumab modified) | HER2+ breast cancer therapy | Improved tumor selectivity, reduced cardiotoxicity, enhanced therapeutic index | High production costs, immune clearance, variable patient response | [99,100] |
| Case Study—Ligand Functionalized Micelles | Polymeric micelles (folic acid modified) | Paclitaxel delivery in folate receptor–positive cancers | High tumor accumulation, low toxicity to healthy tissues | Dependence on receptor expression, limited scalability | [101,102] |
| Case Study—Brain Targeting Nanoparticles | Transferrin modified polymeric nanoparticles | Alzheimer’s therapy, glioblastoma treatment | Crosses BBB, improves CNS drug delivery | BBB heterogeneity, limited translation in humans | [91,103] |
| Theranostic Nanoplatforms (Imaging + Therapy) | Iron oxide nanoparticles, gold nanorods, polymer drug conjugates | Iron oxide nanoparticles for MRI + drug delivery; gold nanorods for photothermal + chemotherapy | Real time monitoring, multimodal therapy, personalized medicine | High cost, regulatory challenges, potential long-term toxicity | [104,105] |
| Case Study—Infectious Disease Targeting | Mannose functionalized nanoparticles | Anti-tuberculosis therapy (targeting macrophages) | Enhances antibiotic efficacy, reduces systemic toxicity | Variability in pathogen host interactions, immune recognition risk |
| Strategy/Application | Examples of Nanomaterials | Advantages | Limitations/Challenges | References |
|---|---|---|---|---|
| Fluorescence Imaging Nanoprobes | Quantum dots (QDs), carbon dots (CDs), upconversion nanoparticles (UCNPs), dye doped silica nanoparticles | High sensitivity, photostability, multiplexed biomarker detection, deep tissue penetration (UCNPs) | Possible toxicity (QDs), limited penetration for visible range probes, stability issues in vivo | [6,94,112] |
| Biosensing Platforms | Gold nanostructures, graphene oxide, magnetic nanoparticles | Signal amplification, detection of low abundance biomarkers (ctDNA, miRNA, proteins), real-time monitoring | Risk of nonspecific binding, reproducibility challenges, potential interference from complex biological fluids | [110,111] |
| MRI Nanocontrast Agents | Superparamagnetic iron oxide nanoparticles (SPIONs), manganese oxide nanoparticles, hybrid core–shell nanostructures | High resolution imaging, targeted delivery via ligands, multimodal imaging integration (MRI + fluorescence/photoacoustic) | Safety concerns for long-term accumulation, potential immunogenicity, complex regulatory approval | [116,119] |
| Theranostic Nanoplatforms (Oncology) | Gold nanostructures, mesoporous silica nanoparticles (MSNs), drug loaded SPIONs | Simultaneous diagnosis and therapy, spatiotemporal drug release, reduced systemic toxicity | Stability in circulation, immune clearance, challenges in large scale reproducibility | [21,121,128] |
| Stimuli-Responsive Theranostics | pH sensitive polymer coated QDs, enzyme activated fluorophores, redox responsive nanoparticles | Controlled drug release at diseased sites, dual function imaging + therapy, real-time monitoring | Risk of premature activation, variability in microenvironment stimuli, safety of degradation products | [123,124] |
| Applications Beyond Cancer | Theranostic liposomes for cardiovascular imaging/therapy, PET nanoplatforms for Alzheimer’s | Disease specific imaging, early detection + therapy integration, versatility across multiple disorders | Limited clinical validation, off-target accumulation, translational hurdles for chronic diseases | [126,127] |
| Strategy/Application | Examples of Nanomaterials | Biomedical Advantages | Limitations/Challenges | References |
|---|---|---|---|---|
| Injectable Hydrogels and Nanocomposite Scaffolds | Natural polymers (alginate, chitosan, collagen, gelatin), Synthetic polymers (PEG), Nanofillers (graphene oxide, silica NPs, carbon nanotubes, nanoclays, mesoporous silica, polypyrrole, cerium oxide NPs) | Minimally invasive delivery; conformability to defect sites; mechanical reinforcement; controlled release of growth factors (BMPs, VEGF); electroconductivity for neural/cardiac repair; immunomodulation via macrophage polarization; antioxidant protection | Risk of nanoparticle aggregation; potential immune responses; ensuring long-term biocompatibility and biodegradability | [5,102,143,146] |
| Nanoparticles for Stem Cell Therapy | Gold NPs, Mesoporous silica NPs, Polymeric NPs, Magnetic NPs (MNPs, SPIONs), Hydroxyapatite NPs, Bioactive glass NPs, Graphene, Carbon nanotubes, Cerium oxide NPs | Targeted gene/drug delivery; controlled release; stem cell differentiation (osteogenic, neuronal, cardiac); magnetic guidance of stem cells; MRI tracking; topographical/electrical cues; antioxidant and anti-inflammatory protection | Off-target uptake; cytotoxicity concerns; long-term safety and clearance issues; reproducibility in clinical settings | [6,19,95,147] |
| Applications in Musculoskeletal and Neural Regeneration | Nanohydroxyapatite, Carbon based nanomaterials, Metallic NPs, Nanocellulose, Graphene oxide, Electrospun nanofibers, Conductive polymers, Neurotrophic factor loaded NPs | Bone/cartilage regeneration; osteoinductive signaling; antimicrobial activity; chondrocyte proliferation; ECM deposition; neural guidance; sustained neurotrophic release; enhanced electrical activity for axonal regeneration | Mechanical wear in cartilage scaffolds; chronic inflammation risk; limited spontaneous repair in avascular tissues; challenges in precise neural integration | [102,111,151] |
| Biomimetic Nanomaterials for Tissue Engineering | Self-assembling peptide nanofibers, Nanostructured calcium phosphate, Growth factor loaded nanocarriers, ECM mimicking coatings, RGD modified nanofibers | Mimic ECM structure; guide cell adhesion, proliferation, differentiation; osteointegration of implants; adaptive responses to stimuli (pH, mechanical stress); controlled release of signaling molecules; immune modulation (M2 macrophage polarization); improved graft survival | Need for scalable fabrication; immune compatibility in diverse patient populations; maintaining dynamic responsive | [152] |
| Aspect | Key Issues/Challenges | Proposed Strategies/Solutions | Examples/Case Studies | References |
|---|---|---|---|---|
| Safety, Toxicity, & Immunogenicity | Uncertain biodistribution and clearance (small < 10 nm → renal elimination; large > 200 nm → liver/spleen accumulation). Long-term organ retention (e.g., AuNPs, carbon nanomaterials). Oxidative stress and ROS generation (e.g., ZnO, TiO2). Cationic surface charge causing membrane disruption. Complement activation related pseudoallergy (CARPA) and cytokine storms. | Surface modifications (PEGylation, zwitterionic coatings, biomimetic cloaking). Personalized immunotoxicity profiling. Standardized long-term toxicity studies. Development of biodegradable and stimuli-responsive nanomaterials. Use of organ on chip and in silico predictive toxicology. | Doxil® associated with CARPA hypersensitivity. Persistent gold nanoparticles linked to chronic toxicity. | [11,157,159] |
| Manufacturing & Scalability | Reproducibility issues in nanoparticle size, shape, and surface chemistry during scale-up. High costs of materials (e.g., AuNPs, graphene oxide). Instability of lipid/polymer nanocarriers (aggregation, oxidation, hydrolysis). GMP compliance and batch to batch quality control. | Microfluidic synthesis and high-pressure homogenization for reproducibility. Lyophilization with cryoprotectants for storage stability. GMP compliant facilities with advanced nanoparticle characterization. AI-driven process optimization and continuous manufacturing. | Scale-up difficulties in liposomes, polymeric nanoparticles, metallic nanostructures. | [165,168] |
| Regulatory Landscape | Lack of standardized nanomaterial definitions and global harmonization. Complex classification (drug, biologic, device, or combination). Conventional assays fail to predict in vivo behavior. Long approval timelines due to regulatory uncertainty. | Case by case evaluation of physicochemical properties, biodistribution, long-term safety. Adaptive and risk-based frameworks. Early industry–regulator dialogue. Integration of post marketing surveillance and real-world evidence. | Doxil® approved under drug framework. Nanoparticle coated stents classified as combination products. | [171,173] |
| Clinically Approved Nanomedicines (Case Studies) | Successes show clear benefits in pharmacokinetics, safety, or delivery. Failures highlight unpredictable toxicity or lack of efficacy advantage. | Liposomal encapsulation to reduce toxicity. Albumin bound nanocarriers for solubility. Theranostic nanomaterials (dual therapy & imaging). | Doxil®: PEGylated liposomal doxorubicin, reduces cardiotoxicity. Abraxane®: albumin bound paclitaxel, solvent free with improved tumor penetration. Feraheme®: iron oxide nanoparticles, used in anemia and MRI imaging. mRNA COVID 19 vaccines: lipid nanoparticle carriers. Failures: polymeric NPs of camptothecin discontinued due to safety. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Aslam, M.; Alam, M.N.; Mandal, T.K. Nanotechnology Driven Innovations in Modern Pharmaceutics: Therapeutics, Imaging, and Regeneration. Nanomaterials 2025, 15, 1733. https://doi.org/10.3390/nano15221733
Parvin N, Aslam M, Alam MN, Mandal TK. Nanotechnology Driven Innovations in Modern Pharmaceutics: Therapeutics, Imaging, and Regeneration. Nanomaterials. 2025; 15(22):1733. https://doi.org/10.3390/nano15221733
Chicago/Turabian StyleParvin, Nargish, Mohammad Aslam, Md Najib Alam, and Tapas K. Mandal. 2025. "Nanotechnology Driven Innovations in Modern Pharmaceutics: Therapeutics, Imaging, and Regeneration" Nanomaterials 15, no. 22: 1733. https://doi.org/10.3390/nano15221733
APA StyleParvin, N., Aslam, M., Alam, M. N., & Mandal, T. K. (2025). Nanotechnology Driven Innovations in Modern Pharmaceutics: Therapeutics, Imaging, and Regeneration. Nanomaterials, 15(22), 1733. https://doi.org/10.3390/nano15221733









