Multi-Batch Consecutive Foliar Spraying Zinc–Carbon Dot Nano-Fertilizer Improving Soil Health for Bok Choy Cultivation Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experiment Design
2.2. Bok Choy Cultivation Sample Testing
2.3. Soil Sample Collection and Determination of Physicochemical Parameters
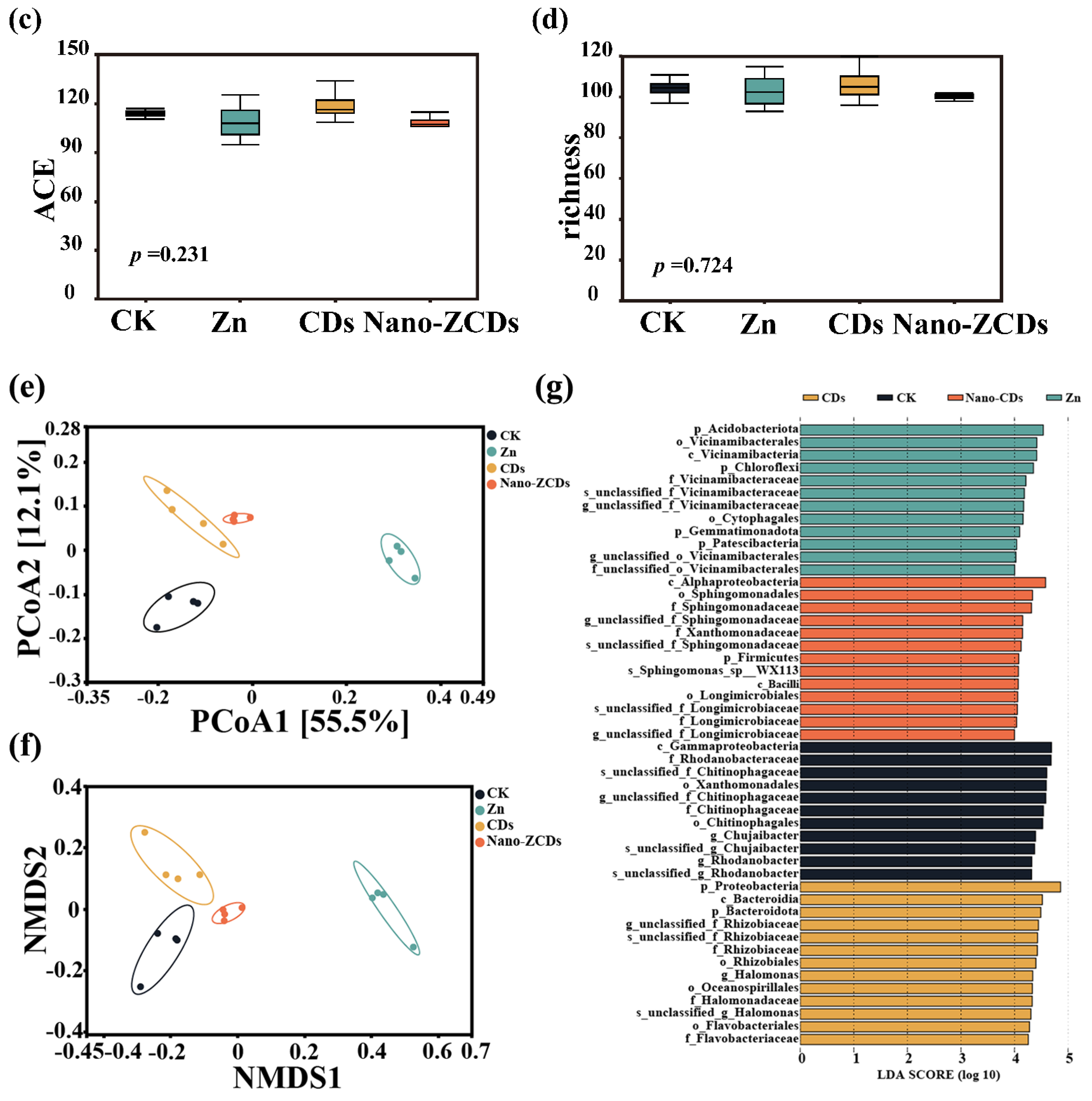
2.4. Soil Microbial Community Analysis
2.5. Calculation of Soil Health Index
2.6. Data Analysis
3. Result and Discussion
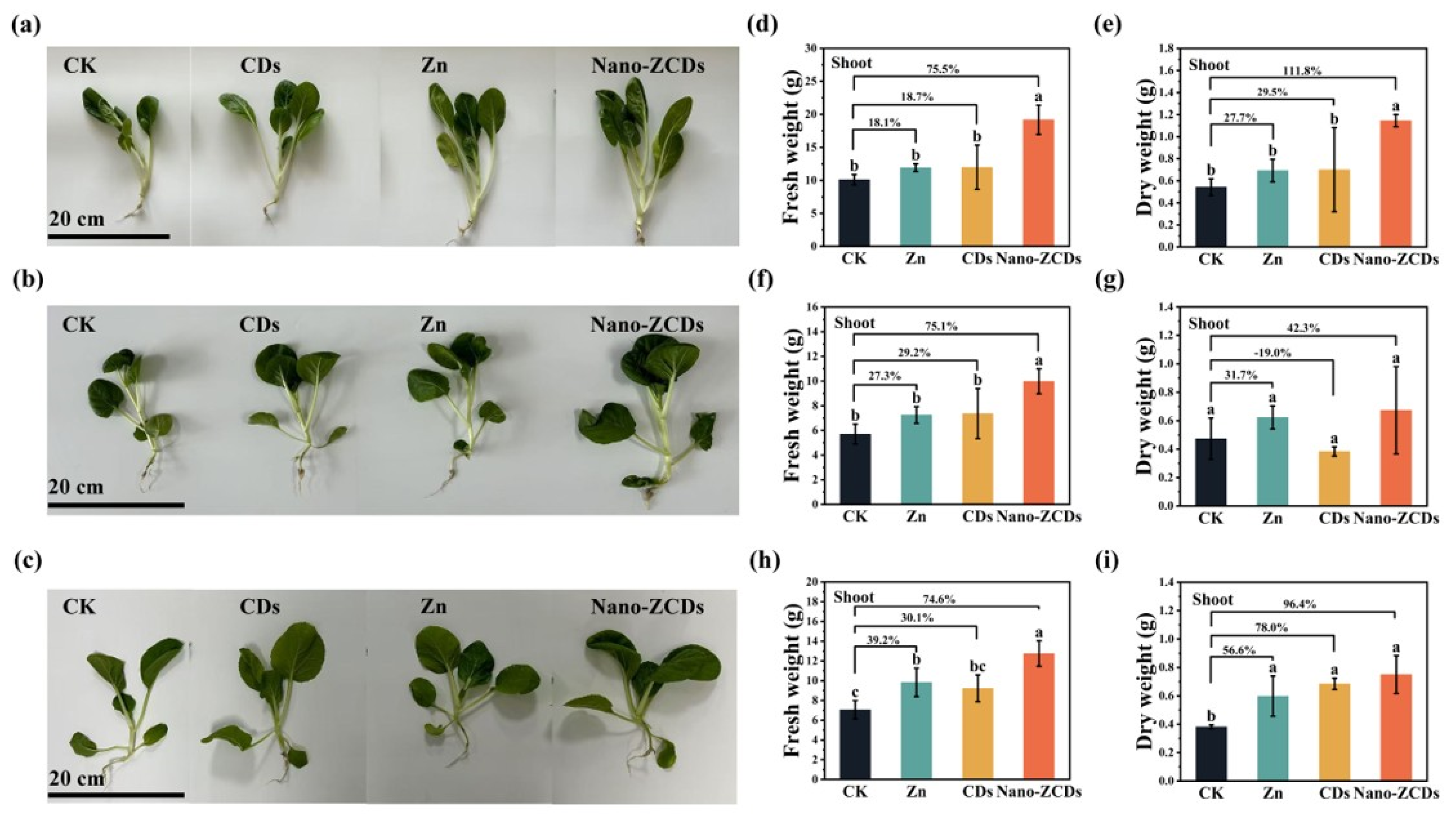
3.1. Effects of Multi-Batch Consecutive Foliar Application Nano-ZCDs on Bok Choy Growth
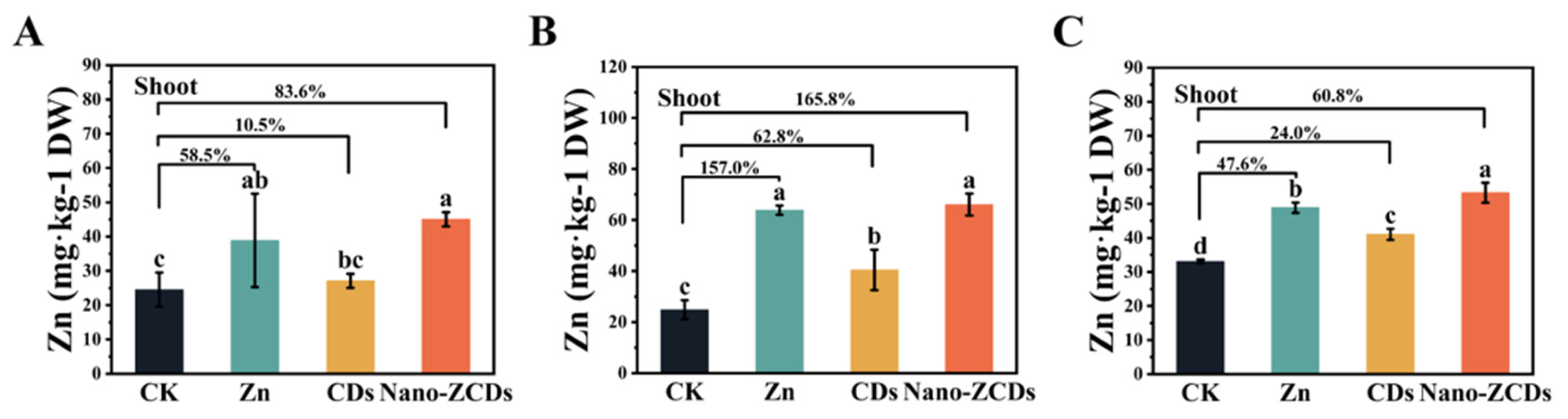
3.2. Effects of Multi-Batch Consecutive Foliar Application Nano-ZCDs on the Quality and Zn Content in Bok Choy
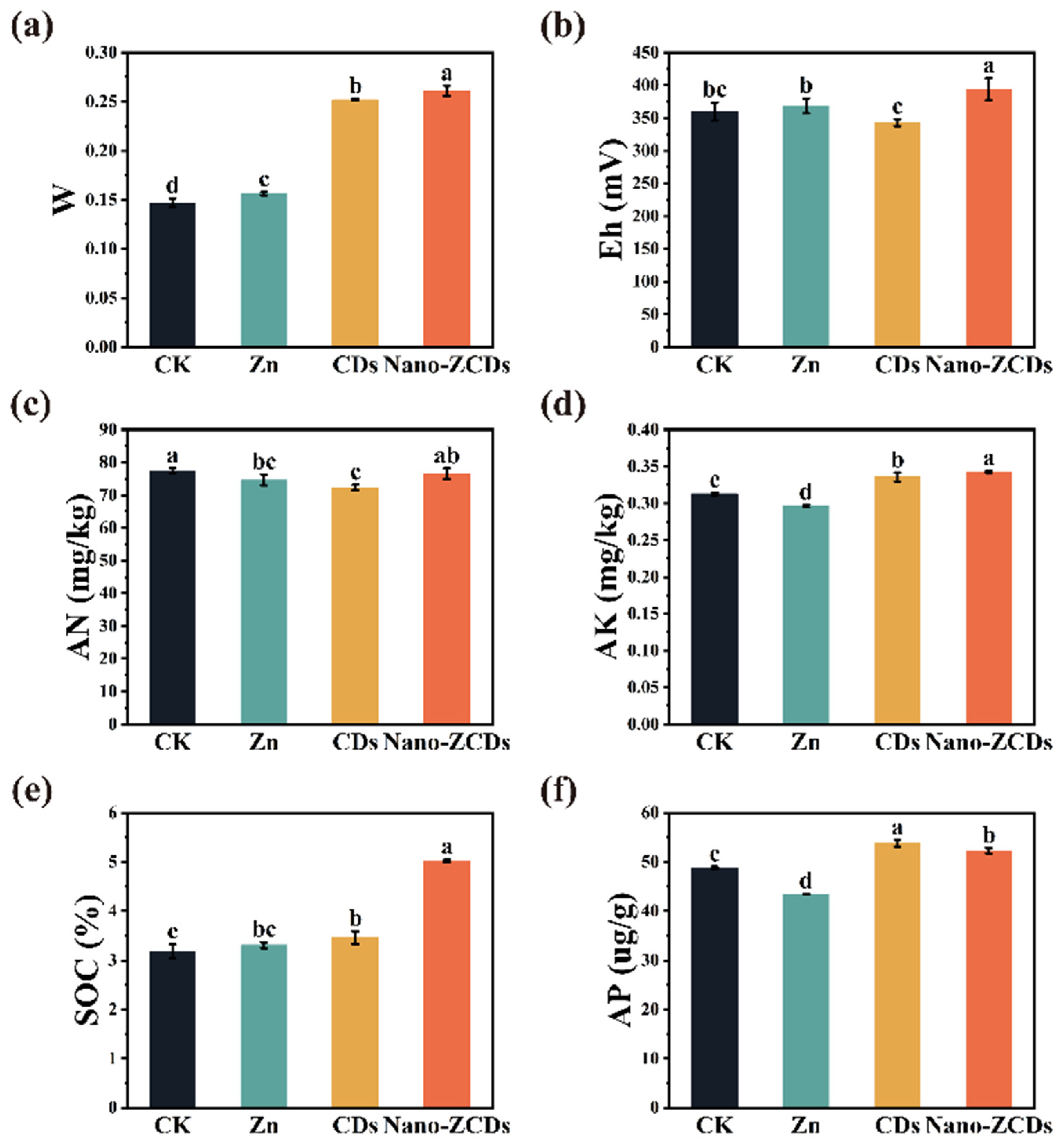
3.3. Effects of Multi-Batch Consecutive Foliar Application of Nano-ZCDs on Soil Physico-Chemical Properties
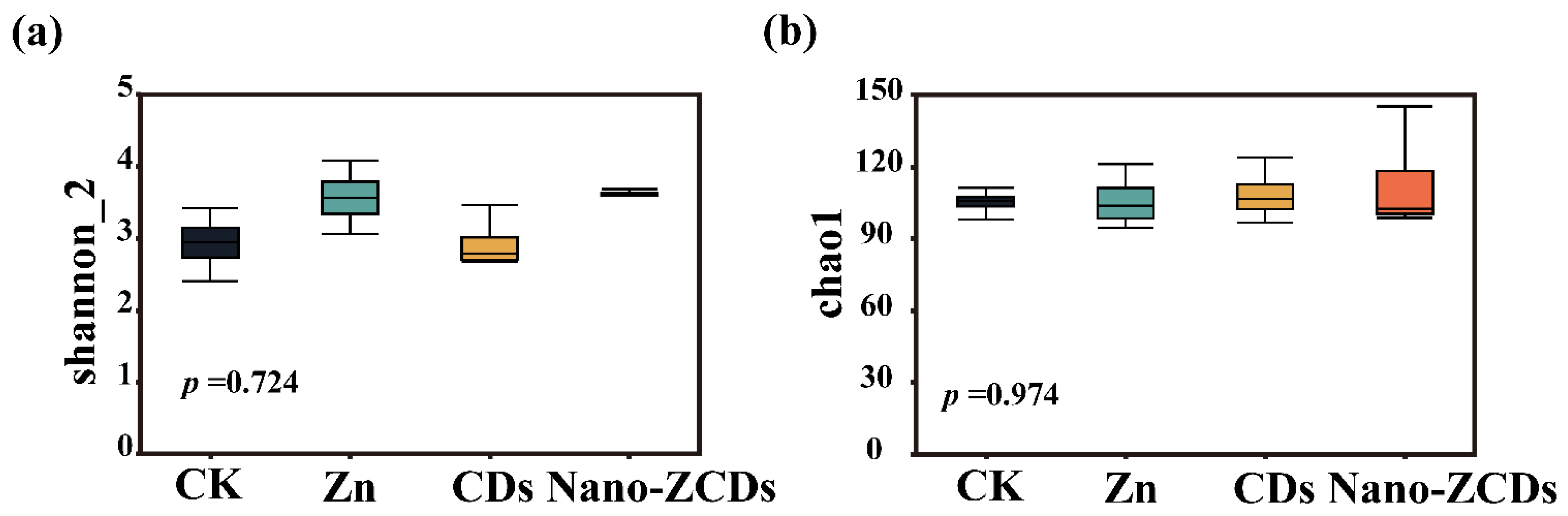
3.4. Effects of Multi-Batch Consecutive Foliar Application of Nano-ZCDs on Soil Microbial Community
3.5. Soil Health Assessment Under Multi-Batch Consecutive Foliar Application of Nano-ZCDs
3.6. Limitations, Challenges, and Prospects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Huang, J.; Zhang, M.L.; Wang, Y.; Wang, H.B.; Ma, Y.R.; Zhao, X.D.; Wang, X.; Liu, C.H.; Huang, H.; et al. Carbon Dots Enable Efficient Delivery of Functional DNA in Plants. ACS Appl. Bio Mater. 2020, 3, 8857–8864. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Huang, R.R.; Huang, H.; Tao, S.T.; Luo, Z.Y.; Zhao, Y.J.; She, L.W.; Yang, J.J.; Lei, Y.J.; Li, Q.F.; Liu, Y.; et al. Carbon dots—Driven epigenome reprogramming reshapes DNA methylation landscapes in rice. Carbon 2025, 242, 120464. [Google Scholar] [CrossRef]
- Maholiya, A.; Ranjan, P.; Khan, R.; Murali, S.; Nainwal, R.C.; Chauhan, P.S.; Sathish, N.; Chaurasia, J.P.; Srivastava, A.K. An insight into the role of carbon dots in the agriculture system: A review. Environ. SCI-Nano 2023, 10, 959–995. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, W.Y.; Yao, X.; Chen, L.L.; Li, G.J.; Gu, J.J.; Chen, L.; Li, Z.H.; Wu, H.H. Cell Wall Pectin Content Refers to Favored Delivery of Negatively Charged Carbon Dots in Leaf Cells. ACS Nano 2023, 17, 23442–23454. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Aslan, H.; Zhang, P.; Zhu, S.J.; Xiao, Y.; Chen, L.X.; Khan, N.; Boesen, T.; Wang, Y.L.; Liu, Y.; et al. Carbon dots-fed Shewanella oneidensis MR-1 for bioelectricity enhancement. Nat. Commun. 2020, 11, 1379. [Google Scholar] [CrossRef]
- Wang, B.Y.; Wei, Z.H.; Sui, L.Z.; Yu, J.K.; Zhang, B.W.; Wang, X.Y.; Feng, S.N.; Song, H.Q.; Yong, X.; Tian, Y.X.; et al. Electron-phonon coupling-assisted universal red luminescence of o-phenylenediamine-based carbon dots. Light Sci. App. 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Kou, E.F.; Yao, Y.Y.; Yang, X.; Song, S.W.; Li, W.; Kang, Y.Y.; Qu, S.N.; Dong, R.Y.; Pan, X.Q.; Li, D.N.; et al. Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato. ACS Sustain. Chem. Eng. 2021, 9, 944–953. [Google Scholar] [CrossRef]
- Cheng, B.X.; Yang, Z.L.; Chen, F.R.; Yue, L.; Cao, X.S.; Li, J.; Qian, H.L.; Yan, X.P.; Wang, C.X.; Wang, Z.Y. Biomass-derived carbon dots with light conversion and nutrient provisioning capabilities facilitate plant photosynthesis. Sci. Total Environ. 2023, 901, 165973. [Google Scholar] [CrossRef]
- Ji, Y.H.; Yue, L.; Cao, X.S.; Chen, F.R.; Li, J.; Zhang, J.S.; Wang, C.X.; Wang, Z.Y.; Xing, B.S. Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: Reactive oxygen species scavenging and nitrogen metabolism. Sci. Total Environ. 2023, 856, 159125. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wu, J.Q.; Wang, Y.M.; Qin, H.; Fan, R.F.; Yang, C.X.; Liu, X.B.; Tan, L.; Yang, C.H.; Chen, Z.J.; et al. The Foliar Application of Carbon Dots Promoted the Photosynthesis Performance, Growth, and Pharmaceutical Ingredient Enrichment in Isatis tinctoria L. ACS Appl. Mater. Interfaces 2025, 17, 36334–36344. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.F.; Kang, Y.Y.; Wang, H.; Huang, R.M.; Lei, B.F.; Zhong, M.; Yang, X. The roles of Salvia miltiorrhiza-derived carbon dots involving in maintaining quality by delaying senescence of postharvest flowering Brassica chinensis L. cultivation. Food Chem. 2023, 404, 134704. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atf, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Adeel, M.; Shakoor, N.; Ali, I.; Ishfaq, M.; Haider, F.U.; Deng, X. Unraveling the roles of modified nanomaterials in nano enabled agriculture. Plant Physiol. Biochem. 2023, 202, 107944. [Google Scholar] [CrossRef]
- Wang, S.J.; Fang, R.T.; Yuan, X.J.; Chen, J.; Mi, K.L.; Wang, R.; Zhang, H.P.; Zhang, H.C. Foliar Spraying of ZnO Nanoparticles Enhanced the Yield, Quality, and Zinc Enrichment of Rice Grains. Foods 2023, 12, 3677. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liang, W.; Shang, W.; Xie, Z.; Cheng, J.; Yu, B.; Fang, Y.; Sun, L.; Zhao, J. Bidirectional Uptake, Transfer, and Transport of Dextran-Based Nanoparticles in Plants for Multidimensional Enhancement of Pesticide Utilization. Small 2023, 20, 2305693. [Google Scholar] [CrossRef]
- Lei, C.L.; Ding, Z.C.; Tao, M.N.; Lu, Y.B.; Xu, L.Q.; Cheng, B.X.; Wang, C.X.; Wang, Z.Y. Unraveling the Distribution, Metabolization, and Catabolism of Foliar Sprayed Carbon Dots in Maize and Effect on Soil Environment. J. Agric. Food Chem. 2024, 72, 19710–19720. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Arancibia-Miranda, N.; Mlih, R.; Cáceres-Jensen, L.; Bolan, N.; Mora, M.d.l.L. Impact on Some Soil Physical and Chemical Properties Caused by Metal and Metallic Oxide Engineered Nanoparticles: A Review. Nanomaterials 2023, 13, 572. [Google Scholar] [CrossRef]
- Bayat, H.; Kolahchi, Z.; Valaey, S.; Rastgou, M.; Mahdavi, S. Iron and magnesium nano-oxide effects on some physical and mechanical properties of a loamy Hypocalcic Cambisol. Geoderma 2019, 335, 57–68. [Google Scholar] [CrossRef]
- Hu, Z.K.; Delgado-Baquerizo, M.; Fanin, N.; Chen, X.Y.; Zhou, Y.; Du, G.Z.; Hu, F.; Jiang, L.; Hu, S.J.; Liu, M.Q. Nutrient-induced acidification modulates soil biodiversity-function relationships. Nat. Commun. 2024, 15, 2858. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Wang, X.K.; Mustafa, A. Exploring the link between soil health and crop productivity. Ecotoxicol. Environ. Saf. 2025, 289, 117703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Zhao, L.Y.; He, Y.Y.; Hu, J.P.; Hu, G.W.; Zhu, Y.; Khan, A.; Xiong, Y.C.; Zhang, J.L. Potential roles of iron nanomaterials in enhancing growth and nitrogen fixation and modulating rhizomicrobiome in alfalfa (Medicago sativa L.). Bioresour. Technol. 2024, 391, 129987. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, C.X.; Chen, F.R.; Yue, L.; Cao, X.S.; Li, J.; Zhao, X.L.; Wu, F.C.; Wang, Z.Y.; Xing, B.S. Foliar carbon dot amendment modulates carbohydrate metabolism, rhizospheric properties and drought tolerance in maize seedling. Sci. Total Environ. 2022, 809, 151105. [Google Scholar] [CrossRef]
- Xue, R.; Wang, C.; Liu, M.L.; Zhang, D.; Li, K.L.; Li, N. A newmethodfor soil health assessment based on Analytic Hierarchy Process and meta-analysis. Sci. Total Environ. 2019, 650, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liang, Y.; Lakshmanan, P.; Guan, X.L.; Liu, D.Y.; Chen, X.P. Magnesium application reduced heavy metal-associated health risks and improved nutritional quality of field-grown Brassica chinensis L. cultivation. Environ. Pollut. 2021, 289, 117881. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhang, D.G.; Cheng, B.X.; Chen, B.; Yue, L.; Cao, X.S.; Wang, C.X.; Wang, Z.Y. Foliar Spraying Zinc–Carbon Dot Nanofertilizer Promotes Yield and Quality of Lettuce (Lactuca sativa L.) through Leaf–Root Regulation. ACS Agric. Sci. Technol. 2025, 5, 371–380. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Liu, L.Y.; Chen, L.X.; Yin, X.Q.; Tan, W.; Li, Y.; Shen, T.H. Synergistic changes in bacterial community composition, function, and soil characteristics of tomato rhizosphere soil under long-term monoculture conditions. Rhizosphere 2024, 31, 100950. [Google Scholar] [CrossRef]
- Teng, J.; Hou, R.; Dungait, J.A.J.; Zhou, G.; Kuzyakov, Y.; Zhang, J.; Tian, J.; Cui, Z.; Zhang, F.; Delgado-Baquerizo, M. Conservation agriculture improves soil health and sustains crop yields after long-term warming. Nat. Commun. 2024, 15, 8785. [Google Scholar] [CrossRef]
- Singh, S.; Jagadamma, S.; Yoder, D.; Yin, X.H.; Walker, F. Cropping system management responses to Cornell and Alabama soil health assessment methods in the southeastern United States. Soil. Sci. Soc. Am. J. 2022, 86, 106–117. [Google Scholar] [CrossRef]
- Yang, X.L.; Xiong, J.R.; Du, T.S.; Ju, X.T.; Gan, Y.T.; Li, S.; Xia, L.L.; Shen, Y.J.; Pacenka, S.; Steenhuis, T.S.; et al. Diversifying crop rotation increases food production, reduces net greenhouse gas emissions and improves soil health. Nat. Commun. 2024, 15, 198. [Google Scholar] [CrossRef]
- Han, S.K.; Liu, H.; Han, Y.; He, Y.H.; Nan, Y.Y.; Qu, W.; Rao, J.P. Effects of calcium treatment on malate metabolism and γ-aminobutyric acid (GABA) pathway in postharvest apple fruit. Food Chem. 2021, 334, 127479. [Google Scholar] [CrossRef]
- Sun, C.; Jin, L.F.; Cai, Y.T.; Huang, Y.N.; Zheng, X.D.; Yu, T. L-Glutamate treatment enhances disease resistance of tomato fruit by inducing the expression of glutamate receptors and the accumulation of amino acids. Food Chem. 2019, 293, 263–270. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.C.; He, M.L.; Chen, P.R.; Li, M.Y.; Liu, J.T.; Li, Y.W.; Lu, W.; Jiang, C.Y.; Liu, D.S.; Quzha, K.; et al. Proanthocyanidins delay the senescence of young asparagus stems by regulating antioxidant capacity and synthesis of phytochemicals. Food Chem. X 2024, 21, 101222. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Howlader, P.; Jia, X.C.; Wang, W.X.; Heng, Y. Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via Abscisic acid signaling in strawberry. Food Chem. 2019, 283, 665–674. [Google Scholar] [CrossRef]
- Khan, M.N.; Fu, C.; Li, J.; Tao, Y.; Li, Y.; Hu, J.; Chen, L.; Khan, Z.; Wu, H.; Li, Z. Seed nanopriming: How do nanomaterials improve seed tolerance to salinity and drought? Chemosphere 2023, 310, 136911. [Google Scholar] [CrossRef]
- Khan, M.N.; Fu, C.C.; Liu, X.H.; Li, Y.H.; Yan, J.S.; Yue, L.; Li, J.Q.; Khan, Z.; Nie, L.X.; Wu, H.H. Nanopriming with selenium doped carbon dots improved rapeseed germination and seedling salt tolerance. Crop J. 2024, 12, 1333–1343. [Google Scholar] [CrossRef]
- Murshed, F. Zinc and boron foliar application, An influential treatment on the quality and seed yield of soybean. DYSONA-Appl. Sci. 2024, 5, 20–24. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Yang, Z.; Cheng, P.; Li, F.; Wang, M.; Liu, T. The Kinetics of Aging and Reducing Processes of Cr(VI) in Two Soils. Bull. Environ. Contam. Toxicol. 2019, 103, 82–89. [Google Scholar] [CrossRef]
- Ratié, G.; Vantelon, D.; Lotfi Kalahroodi, E.; Bihannic, I.; Pierson-Wickmann, A.C.; Davranche, M. Iron speciation at the riverbank surface in wetland and potential impact on the mobility of trace metals. Sci. Total Environ. 2019, 651, 443–455. [Google Scholar] [CrossRef]
- Li, G.Q.; Chen, Y.D.; Li, H.X.; Li, S.Y.; Qiao, Q.Q.; Zhang, X.L.; Wang, D.; Wang, J.M.; Wang, M.C.; Ye, J.M.; et al. Soil pH and Organic Carbon Content Governing the Active Iron in Tea Plantation and Maize Field. Land Degrad. Dev. 2025, 1–14. [Google Scholar] [CrossRef]
- Liu, D.; Iqbal, S.; Gui, H.; Xu, J.C.; An, S.S.; Xing, B.S. Nano-Iron Oxide (Fe3O4) Mitigates the Effects of Microplastics on a Ryegrass Soil−Microbe−Plant System. ACS Nano 2023, 17, 24867–24882. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Li, X.; Qiao, L.; Huang, Y.P.; Li, D.C.; Xu, M.G.; Ge, T.D.; Meersmans, J.; Zhang, W.J. Manuring improves soil health by sustaining multifunction at relatively high levels in subtropical area. Agr. Ecosyst. Environ. 2023, 353, 108539. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.B.; Yin, J.; Huang, S.M. Bacterial Community Structure after Long-term Organic and Inorganic Fertilization Reveals Important Associations between Soil Nutrients and Specific Taxa Involved in Nutrient Transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Mapelli, F.; Zanardini, E.; Terzaghi, E.; Di Guardo, A.; Morosini, C.; Raspa, G.; Borin, S. Phyto-rhizoremediation of polychlorinated biphenyl contaminated soils: An outlook on plant-microbe beneficial interactions. Sci. Total Environ. 2017, 575, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. Mineral and Organic Fertilizers Distinctly Affect Fungal Communities in the Crop Rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Liu, J.J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.K.; Chen, X.L.; Jin, J.; Liu, X.B.; Wang, G.H. Effects of over 30-year of different fertilization regimes on fungal community compositions in the black soils of northeast China. Agr. Ecosyst. Environ. 2017, 248, 113–122. [Google Scholar] [CrossRef]
- Li, Y.N.; Yang, R.Y.; Pan, Y.H.; Geng, Y.J.; Sun, J.N.; Zhang, Z.H. Mechanisms of Biochar Effects on Plant Growth of Seepweed (Suaeda salsa) and Hybrid Sorrel (Rumex patientia × Rumex tianschanicus) in a Coastal Saline Soil over Two Cropping Seasons: Soil–Plant–Microbe Interactions. J. Soil. Sci. Plant Nut. 2023, 23, 569–580. [Google Scholar] [CrossRef]
- Mukherjee, A.; Singh, S.; Gaurav, A.K.; Chouhan, G.K.; Jaiswal, D.K.; Araujo Pereira, A.P.; Passari, A.K.; Abdel-Azeem, A.M.; Verma, J.P. Harnessing of phytomicrobiome for developing potential biostimulant consortium for enhancing the productivity of chickpea and soil health under sustainable agriculture. Sci. Total Environ. 2022, 836, 155550. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, M.; Zhang, J.; Ren, Y.; Zhang, D.; Cheng, B.; Wang, C. Multi-Batch Consecutive Foliar Spraying Zinc–Carbon Dot Nano-Fertilizer Improving Soil Health for Bok Choy Cultivation Production. Nanomaterials 2025, 15, 1714. https://doi.org/10.3390/nano15221714
Tao M, Zhang J, Ren Y, Zhang D, Cheng B, Wang C. Multi-Batch Consecutive Foliar Spraying Zinc–Carbon Dot Nano-Fertilizer Improving Soil Health for Bok Choy Cultivation Production. Nanomaterials. 2025; 15(22):1714. https://doi.org/10.3390/nano15221714
Chicago/Turabian StyleTao, Mengna, Jiangshan Zhang, Yuying Ren, Dingge Zhang, Bingxu Cheng, and Chuanxi Wang. 2025. "Multi-Batch Consecutive Foliar Spraying Zinc–Carbon Dot Nano-Fertilizer Improving Soil Health for Bok Choy Cultivation Production" Nanomaterials 15, no. 22: 1714. https://doi.org/10.3390/nano15221714
APA StyleTao, M., Zhang, J., Ren, Y., Zhang, D., Cheng, B., & Wang, C. (2025). Multi-Batch Consecutive Foliar Spraying Zinc–Carbon Dot Nano-Fertilizer Improving Soil Health for Bok Choy Cultivation Production. Nanomaterials, 15(22), 1714. https://doi.org/10.3390/nano15221714






