Structural, Adsorptive, and Antibacterial Properties of a Novel Silver (Diethyldithiocarbamate)-Decorated Reduced Graphene Oxide Nanocomposite for Sustainable Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Green Synthesis of the Ag(DDTC)@rGO Nanocomposite
2.4. Batch Sorption Study
2.5. Effect of Temperature and Sonication on the Antibacterial Activity of the Nanocomposite
2.6. MTT Assay
2.7. Statistical Analysis
3. Results and Discussion
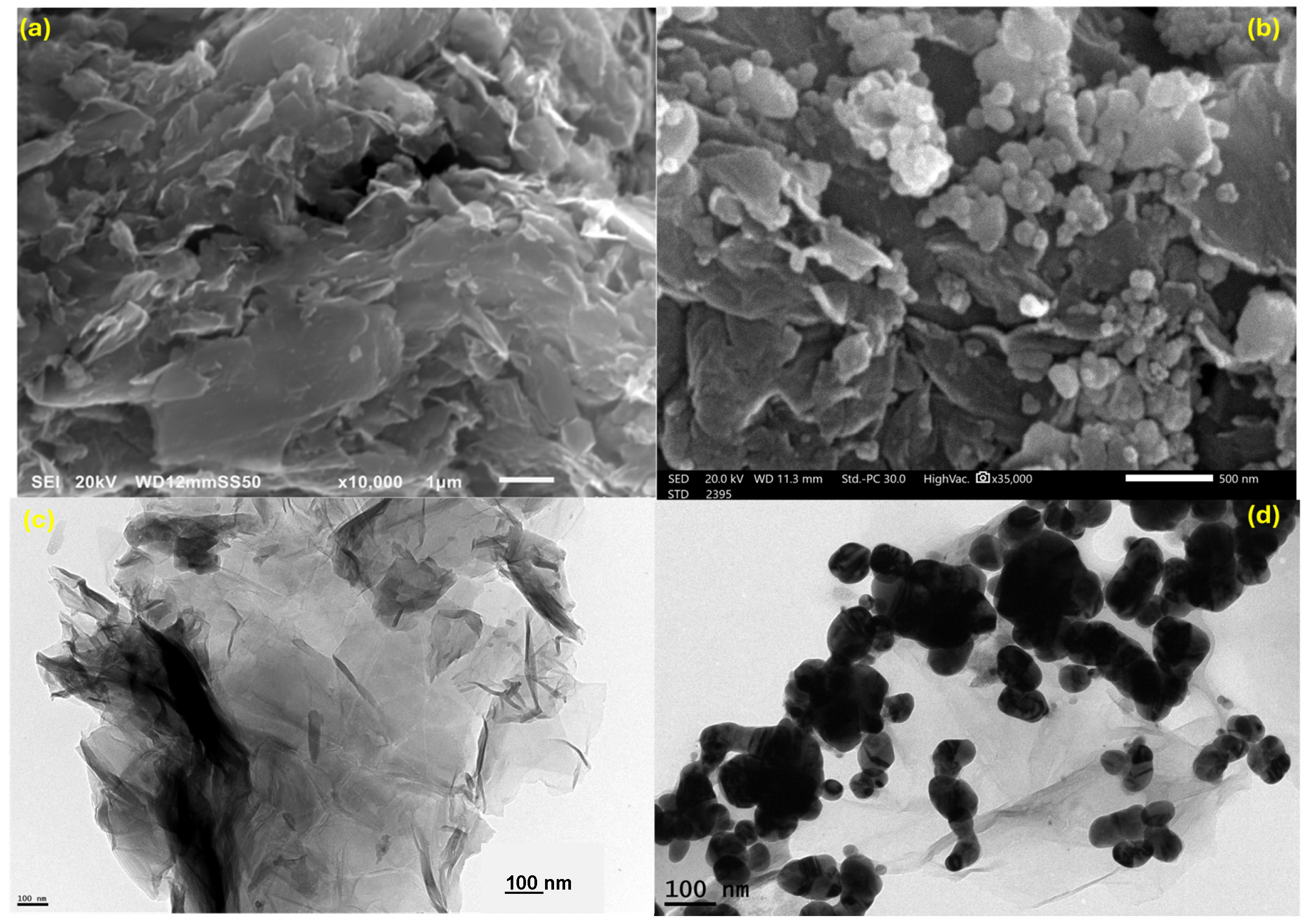
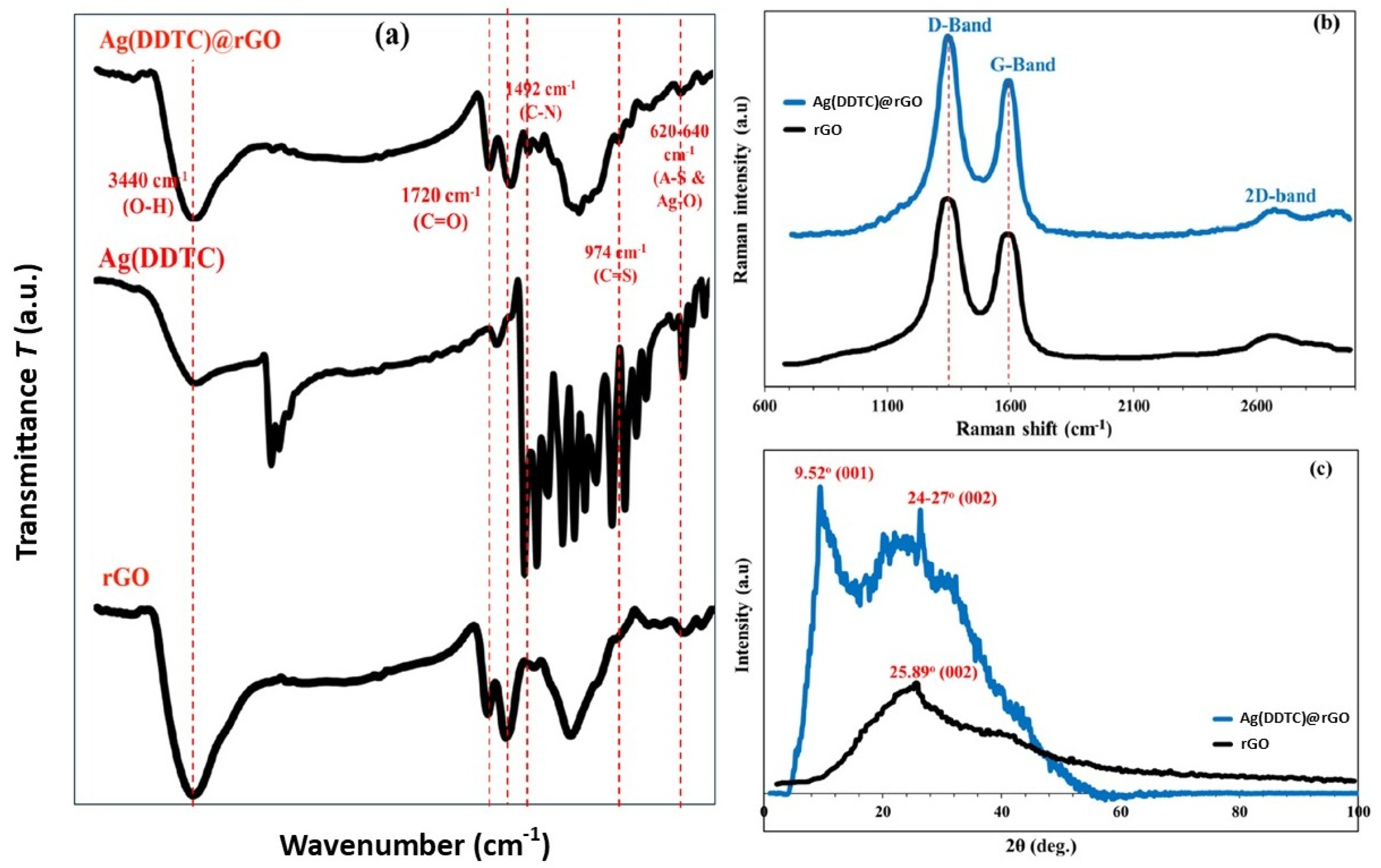
3.1. Characterization and Morphology of the Ag(DDTC)@rGO Nanocomposite
3.2. Batch Sorption Parameters
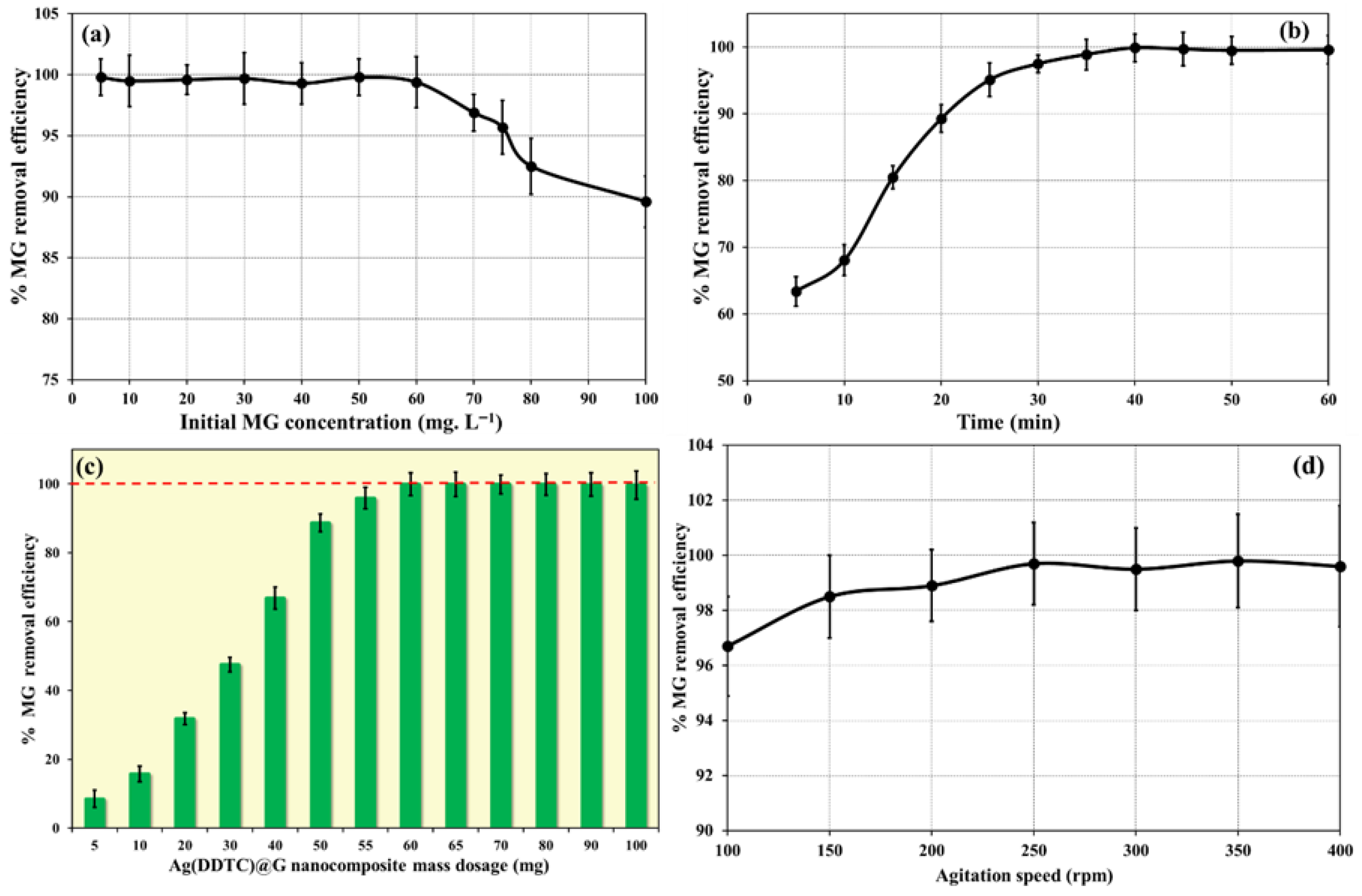
3.2.1. Effect of Initial Dye Concentration
3.2.2. Effect of Contact Time
3.2.3. Effect of Ag(DDTC)@rGO Mass Dosage
3.2.4. Effect of Stirring Speed
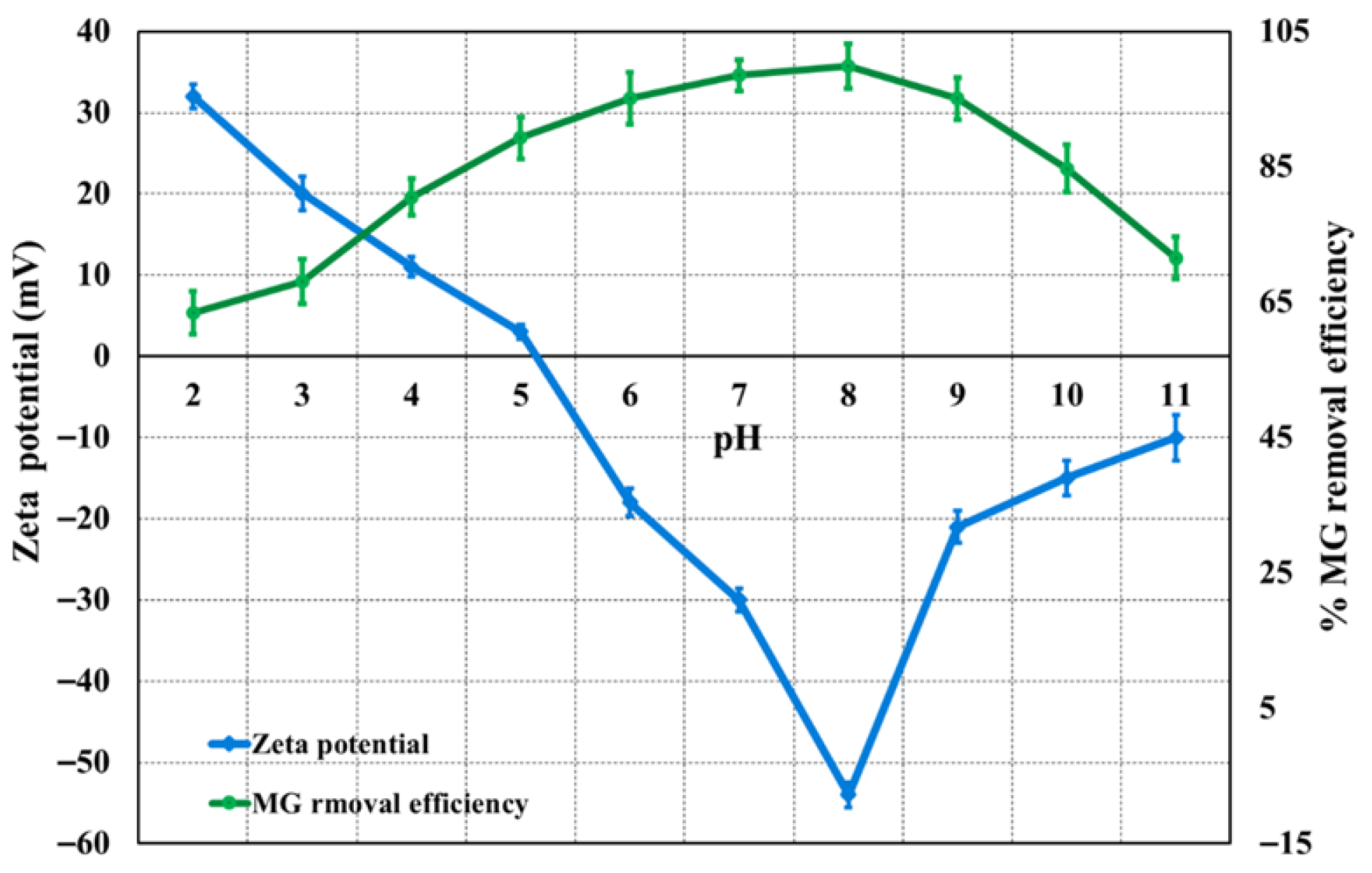
3.2.5. Effect of pH
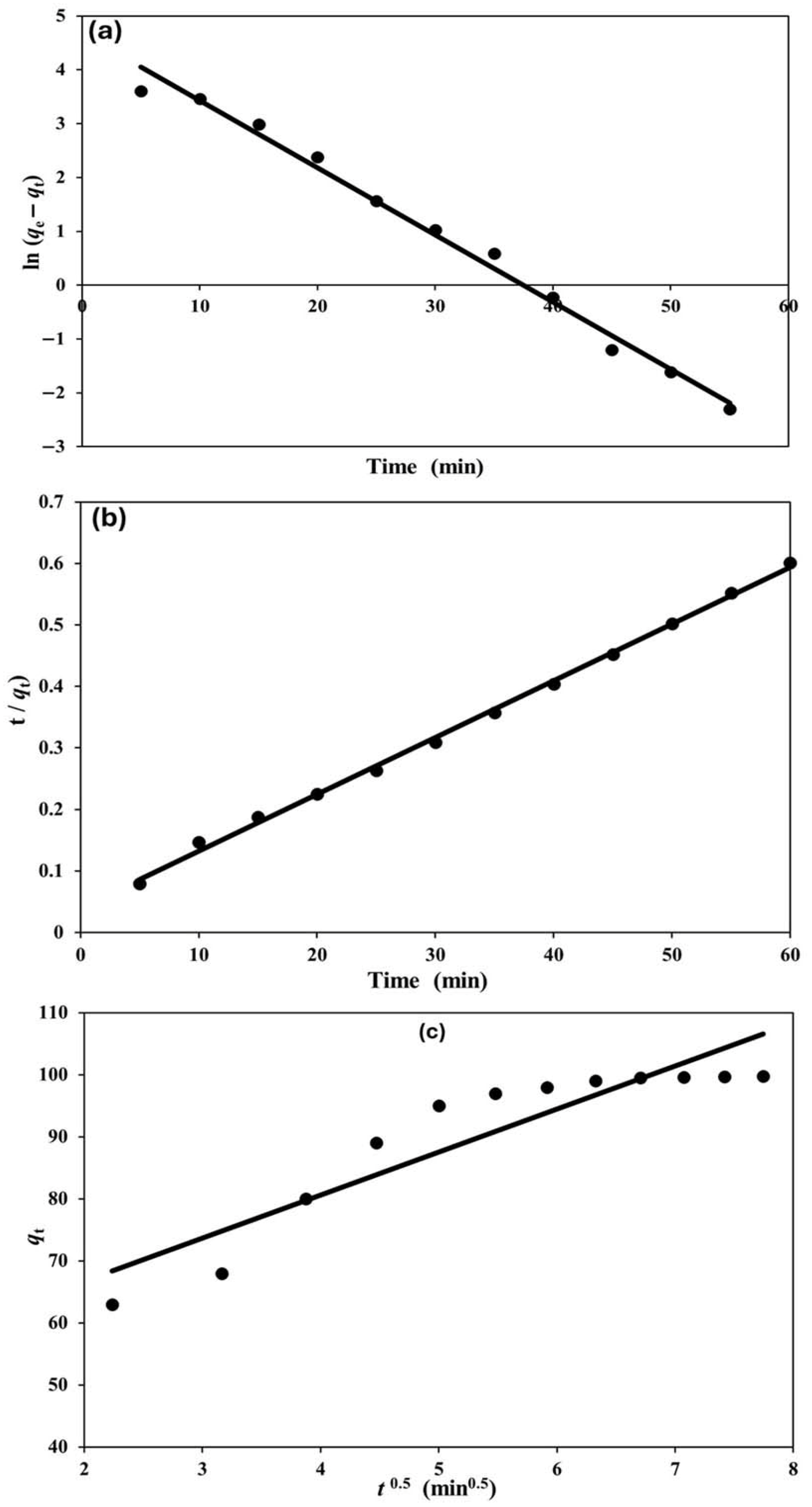
3.3. Kinetic Study of Adsorption
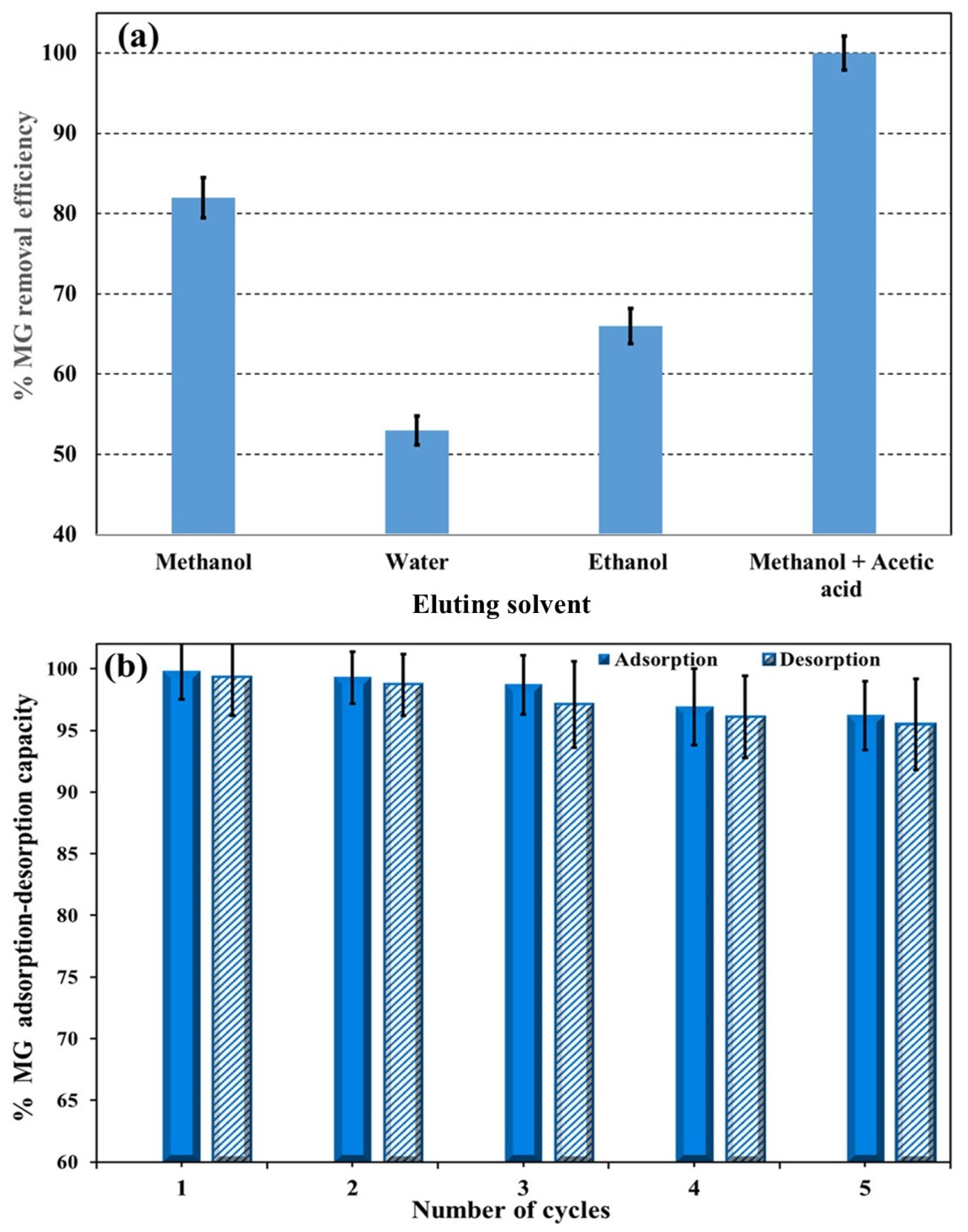
3.4. Regeneration and Reusability of Ag(DDTC)@rGO
3.5. Mechanism of MG Dye Adsorption on the Ag(DDTC)@rGO Nanocomposite
3.6. Antibacterial Activity and Cytotoxicity
3.6.1. Impact of Temperature and Sonication on Ag(DDTC)@rGO’ Antibacterial Activity
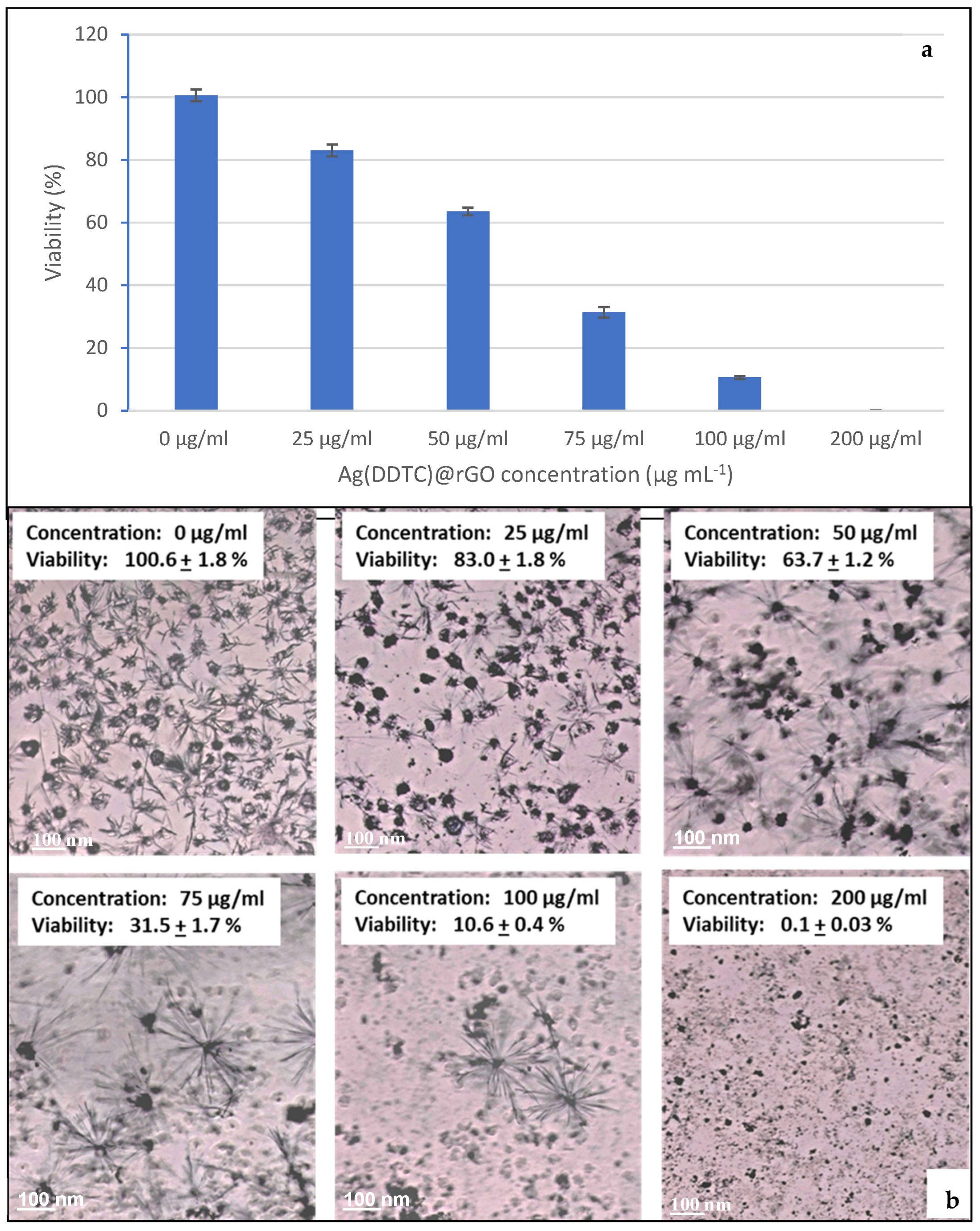
3.6.2. Cell Viability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Day, N.P.; Peacock, S.J.; Limmathurotsakul, D. Epidemiology and Burden of Multidrug-Resistant Bacterial Infection in a Developing Country. eLife 2016, 5, e18082. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The Threat of Antimicrobial Resistance in Developing Countries: Causes and Control Strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- World Health Organization. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 November 2023).
- Cozzoli, P.D.; Fanizza, E.; Comparelli, R.; Curri, M.L.; Agostiano, A.; Laub, D. Role of Metal Nanoparticles in TiO2 /Ag Nanocomposite-Based Microheterogeneous Photocatalysis. J. Phys. Chem. B 2004, 108, 9623–9630. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Silver Nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Parmar, S.; Kaur, H.; Singh, J.; Matharu, A.S.; Ramakrishna, S.; Bechelany, M. Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance. Nanomaterials 2022, 12, 1115. [Google Scholar] [CrossRef]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of Toxicity of Ag Nanoparticles in Comparison to Bulk and Ionic Ag on Mussel Hemocytes and Gill Cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef]
- Xiong, Y.; Brunson, M.; Huh, J.; Huang, A.; Coster, A.; Wendt, K.; Fay, J.; Qin, D. The Role of Surface Chemistry on the Toxicity of Ag Nanoparticles. Small 2013, 9, 2628–2638. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Andjelković, L.; Šuljagić, M.; Pavlović, V.; Mirković, M.; Vrbica, B.; Novaković, I.; Stanković, D.; Kremenović, A.; Uskoković, V. Mechanical Activation and Silver Supplementation as Determinants of the Antibacterial Activity of Titanium Dioxide Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133890. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.; Chen, W.; Wang, X.; Ji, H.; Fu, Y.; Lü, C. Synthesis, Antibacterial Activity and Action Mechanism of Silver-Based Nanomaterials with Thermosensitive Polymer-Decorated Graphene Oxide as a Stable Support. Mater. Today Commun. 2023, 36, 106598. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Chen, S.; Jiao, X.; Li, X.; Chen, W. Synthesis, in Vitro Biocompatibility and Antibacterial Property of Novel Silk fibroin@Ag Spheres. Mater. Lett. 2024, 357, 135681. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.d.S.; Hasan, M.d.N. Enhanced Toxic Dye Removal from Wastewater Using Biodegradable Polymeric Natural Adsorbent. J. Mol. Liq. 2021, 328, 115468. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Y.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Liu, Q. Optimized Preparation of Activated Carbon from Coconut Shell and Municipal Sludge. Mater. Chem. Phys. 2020, 241, 122327. [Google Scholar] [CrossRef]
- Teimouri, Z.; Salem, A.; Salem, S. Regeneration of Wastewater Contaminated by Cationic Dye by Nanoporous Activated Carbon Produced from Agriculture Waste Shells. Environ. Sci. Pollut. Res. 2019, 26, 7718–7729. [Google Scholar] [CrossRef]
- Arora, C.; Soni, S.; Sahu, S.; Mittal, J.; Kumar, P.; Bajpai, P.K. Iron Based Metal Organic Framework for Efficient Removal of Methylene Blue Dye from Industrial Waste. J. Mol. Liq. 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Essien, E.R.; Atasie, V.N.; Oyebanji, T.O.; Nwude, D.O. Biomimetic Synthesis of Magnesium Oxide Nanoparticles Using Chromolaena odorata (L.) Leaf Extract. Chem. Pap. 2020, 74, 2101–2109. [Google Scholar] [CrossRef]
- Heidari, Z.; Pelalak, R.; Malekshah, R.E.; Pishnamazi, M.; Marjani, A.; Sarkar, S.M.; Shirazian, S. Molecular Modeling Investigation on Mechanism of Cationic Dyes Removal from Aqueous Solutions by Mesoporous Materials. J. Mol. Liq. 2021, 329, 115485. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-Pot Green Synthesis of Magnetic Fullerene Nanocomposite for Adsorption Characteristics. J. Water Process Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured Metal Oxides and Its Hybrids for Photocatalytic and Biomedical Applications. Adv. Colloid Interface Sci. 2020, 281, 102178. [Google Scholar] [CrossRef] [PubMed]
- Shabaan, O.A.; Jahin, H.S.; Mohamed, G.G. Removal of Anionic and Cationic Dyes from Wastewater by Adsorption Using Multiwall Carbon Nanotubes. Arab. J. Chem. 2020, 13, 4797–4810. [Google Scholar] [CrossRef]
- Yin, G.; Sun, Z.; Gao, Y.; Xu, S. Preparation of Expanded Graphite for Malachite Green Dye Removal from Aqueous Solution. Microchem. J. 2021, 166, 106190. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, B.; Senapati, P.; Murmu, R.; Sahu, D. Mechanical, Thermal, and Morphological Properties of Graphene Nanoplatelet-Reinforced Polypropylene Nanocomposites: Effects of Nanofiller Thickness. J. Compos. Sci. 2021, 5, 24. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Gas and Solution Uptake Properties of Graphene Oxide-Based Composite Materials: Organic vs. Inorganic Cross-Linkers. J. Compos. Sci. 2019, 3, 80. [Google Scholar] [CrossRef]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Thermodynamic and Kinetic Studies of Crystal Violet Dye Adsorption with Poly(Methyl Methacrylate)–Graphene Oxide and Poly(Methyl Methacrylate)–Graphene Oxide–Zinc Oxide Nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47495. [Google Scholar] [CrossRef]
- Nezhad, A.A.; Alimoradi, M.; Ramezani, M. One-Step Preparation of Graphene Oxide/Polypyrrole Magnetic Nanocomposite and Its Application in the Removal of Methylene Blue Dye from Aqueous Solution. Mater. Res. Express 2018, 5, 025508. [Google Scholar] [CrossRef]
- Raghu, M.S.; Yogesh Kumar, K.; Prashanth, M.K.; Prasanna, B.P.; Vinuth, R.; Pradeep Kumar, C.B. Adsorption and Antimicrobial Studies of Chemically Bonded Magnetic Graphene Oxide-Fe3O4 Nanocomposite for Water Purification. J. Water Process Eng. 2017, 17, 22–31. [Google Scholar] [CrossRef]
- Ben Ameur Mehdi, R.; Shaaban, K.A.; Rebai, I.K.; Smaoui, S.; Bejar, S.; Mellouli, L. Five Naturally Bioactive Molecules Including Two Rhamnopyranoside Derivatives Isolated from the Streptomyces sp. Strain TN58. Nat. Prod. Res. 2009, 23, 1095–1107. [Google Scholar] [CrossRef]
- Horiuchi, N.; Nakagawa, K.; Sasaki, Y.; Minato, K.; Fujiwara, Y.; Nezu, K.; Ohe, Y.; Saijo, N. In Vitro Antitumor Activity of Mitomycin C Derivative (RM-49) and New Anticancer Antibiotics (FK973) against Lung Cancer Cell Lines Determined by Tetrazolium Dye (MTT) Assay. Cancer Chemother. Pharmacol. 1988, 22, 246–250. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Soares, E.A.; Téllez, C.; Fortes, S.A.; Coelho, A.; Versiane, O.; Ferreira, G.B.; Mondragón, M.A.; TéllezS, C.A. Fourier Transform Infrared and Raman Spectra of the Complex Cation Diethyldithiocarbamate Cr(III) Di-Hydrate, [Cr(DDTC)2(OH2)2]+. UV-Vis Spectrum, DFT:B3LYP/6-311G(d, p) Structural Determination, Vibrational and Natural Bond Orbital Analysis. J. Mol. Struct. 2022, 1256, 132555. [Google Scholar] [CrossRef]
- Costa Júnior, A.C.; Versiane, O.; Faget Ondar, G.; Ramos, J.M.; Ferreira, G.B.; Martin, A.A.; Téllez Soto, C.A. An Experimental and Theoretical Approach of Spectroscopic and Structural Properties of the Bis(Diethyldithiocarbamate)–Cobalt(II). J. Mol. Struct. 2012, 1029, 119–134. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Ramakrishna, S. Effective Removal of Mercury, Arsenic and Lead from Aqueous Media Using Polyaniline-Fe3O4- Silver Diethyldithiocarbamate Nanostructures. J. Clean. Prod. 2019, 239, 118023. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Biris, A.R.; Lazar, M.D.; Pruneanu, S.; Neamtu, C.; Watanabe, F.; Kannarpady, G.K.; Dervishi, E.; Biris, A.S. Catalytic One-Step Synthesis of Pt-Decorated Few-Layer Graphenes. RSC Adv. 2013, 3, 26391. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption Characteristics of Congo Red onto the Chitosan/Montmorillonite Nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from Water Using Pineapple Peel Derived Biochars: Adsorption Potential and Re-Usability Assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Konar, M.; Rath, J.; Kumar, D.; Sahoo, H. Magnetic Hydroxyapatite Nanocomposite: Impact on Eriochrome Black-T Removal and Antibacterial Activity. J. Mol. Liq. 2019, 294, 111596. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Konar, M.; Rath, J.; Kumar, D.; Sahoo, H. Hexagonal Strontium Ferrite: Cationic Dye Adsorption and Antibacterial Activity. Sep. Sci. Technol. 2020, 55, 415–430. [Google Scholar] [CrossRef]
- He, C.; Yang, Z.; Ding, J.; Chen, Y.; Tong, X.; Li, Y. Effective Removal of Cr(VI) from Aqueous Solution by 3-Aminopropyltriethoxysilane-Functionalized Graphene Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 448–458. [Google Scholar] [CrossRef]
- Yakout, A.A.; Albishri, H.M. Solvothermal Synthesis of EDTA-Functionalized Magnetite-Carboxylated Graphene Oxide Nanocomposite as a Potential Magnetic Solid Phase Extractor of p-Phenylenediamine from Environmental Samples. J. Dispers. Sci. Technol. 2019, 40, 369–377. [Google Scholar] [CrossRef]
- Almalki, S.G.; Alshitari, W.H.; Yakout, A.A. An Insight into the Utilization of High-Sulfur Green Saudi Arabian Petcoke as an Ecofriendly Sorbent for Enhanced Simultaneous Sequestration of Heavy Metal Ions from Industrial Wastewaters. Pet. Sci. Technol. 2025, 43, 2700–2719. [Google Scholar] [CrossRef]
- Al-Harthy, E.A.; Shaker, M.A.; Yakout, A.A. Synergetic Enhancement of Copper and Manganese Dioxide Nanoparticles with the Biobased Chitosan-Crosslinked-Graphene Nanocomposite for Potential Adsorptive Remediation of Tetracycline Antibiotics from Food Samples. Mater. Today Commun. 2025, 47, 112930. [Google Scholar] [CrossRef]
- Shaker, M.A.; Alshitari, W.H.; Basha, M.T.; Aly, N.A.; Asim, M.; Albishri, H.M.; Bhawani, S.A.; Yakout, A.A. Synergetic Impact of Copper Nanoparticles and Polyaniline Reinforced Graphene Oxide Nanocomposite on the Sequestration of Tetracycline Antibiotic from Milk and Wastewaters Samples. Mater. Today Commun. 2024, 38, 107869. [Google Scholar] [CrossRef]
- Yakout, A.A.; Basha, M.T.; Al-Raimi, D.S.; Aljadaani, A.H.; Ali Zainy, F.M. Synergistic Impact of Cu and MnO2 Nanoparticles in Cu/MnO2/Chitosan/Graphene Oxide Hybrid Nanocomposite for Improved Antibacterial Activity and Fast Adsorption of Environmentally Relevant Anionic and Cationic Dyes from Industrial Wastewater. Int. J. Biol. Macromol. 2025, 318, 144839. [Google Scholar] [CrossRef]
- Yakout, A.A.; Alshitari, W.; Akhdhar, A. Synergistic Effect of Cu-Nanoparticles and β-Cyclodextrin Functionalized Reduced Graphene Oxide Nanocomposite on the Adsorptive Remediation of Tetracycline Antibiotics. Carbohydr. Polym. 2021, 273, 118528. [Google Scholar] [CrossRef]
- Yakout, A.A.; Khan, Z.A. High Performance Zr-MnO2@reduced Graphene Oxide Nanocomposite for Efficient and Simultaneous Remediation of Arsenates As(V) from Environmental Water Samples. J. Mol. Liq. 2021, 334, 116427. [Google Scholar] [CrossRef]
- Robati, D.; Rajabi, M.; Moradi, O.; Najafi, F.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Kinetics and Thermodynamics of Malachite Green Dye Adsorption from Aqueous Solutions on Graphene Oxide and Reduced Graphene Oxide. J. Mol. Liq. 2016, 214, 259–263. [Google Scholar] [CrossRef]
- Amri, A.; Lesbani, A.; Mohadi, R. Malachite Green Dye Adsorption from Aqueous Solution Using a Ni/al Layered Double Hydroxide-Graphene Oxide Composite Material. Sci. Technol. Indones. 2023, 8, 280–287. [Google Scholar] [CrossRef]
- Hosseinkhani, O.; Hamzehlouy, A.; Dan, S.; Sanchouli, N.; Tavakkoli, M.; Hashemipour, H. Graphene Oxide/ZnO Nanocomposites for Efficient Removal of Heavy Metal and Organic Contaminants from Water. Arab. J. Chem. 2023, 16, 105176. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Qin, P.; Muneeswaran, T.; Rajivgandhi, G.; Quero, F.; Song, J. Graphene-Zinc Oxide Nanocomposites (G-ZnO NCs): Synthesis, Characterization and Their Photocatalytic Degradation of Dye Molecules. Mater. Sci. Eng. B 2020, 254, 114516. [Google Scholar] [CrossRef]
- Feng, S.; Liu, S.; Zhang, Z.; Feng, S.; Yuan, B.; Cheng, P.; Wang, R. Efficient Removal of Malachite Green Dye from Aqueous Solution Using Functionalized GO/Fe3O4 Nanocomposite: Kinetic, Equilibrium and Thermodynamic Studies. Desalination Water Treat. 2018, 109, 241–252. [Google Scholar] [CrossRef]
- Piras, S.; Zannotti, M.; Appignanesi, D.; Ferraro, S.; Minicucci, M.; Giovannetti, R. Silver Nanoparticles-Anchored Reduced Graphene Oxide Obtained by Orange Peel Extract-Mediated Synthesis for Visible Light Photodegradation of Organic Dye. J. Mol. Liq. 2025, 434, 128059. [Google Scholar] [CrossRef]
- Upoma, B.P.; Yasmin, S.; Ali Shaikh, M.d.A.; Jahan, T.; Haque, M.d.A.; Moniruzzaman, M.; Kabir, M.H. A Fast Adsorption of Azithromycin on Waste-Product-Derived Graphene Oxide Induced by H-Bonding and Electrostatic Interactions. ACS Omega 2022, 7, 29655–29665. [Google Scholar] [CrossRef]
- Han, F.; Ma, L.; Sun, Q.; Lei, C.; Lu, A. Rationally Designed Carbon-Coated Fe3O4 Coaxial Nanotubes with Hierarchical Porosity as High-Rate Anodes for Lithium Ion Batteries. Nano Res. 2014, 7, 1706–1717. [Google Scholar] [CrossRef]
- Tang, M.; Wu, T.; Xu, X.; Zhang, L.; Wu, F. Factors That Affect the Stability, Type and Morphology of Pickering Emulsion Stabilized by Silver Nanoparticles/Graphene Oxide Nanocomposites. Mater. Res. Bull. 2014, 60, 118–129. [Google Scholar] [CrossRef]
- Rajabi, M.; Mahanpoor, K.; Moradi, O. Preparation of PMMA/GO and PMMA/GO-Fe3O4 Nanocomposites for Malachite Green Dye Adsorption: Kinetic and Thermodynamic Studies. Compos. Part B Eng. 2019, 167, 544–555. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Low Cost and Efficient Synthesis of Magnetic Iron Oxide/Activated Sericite Nanocomposites for Rapid Removal of Methylene Blue and Crystal Violet Dyes. Mater. Charact. 2020, 163, 110275. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef] [PubMed]
- Hamidzadeh, S.; Torabbeigi, M.; Shahtaheri, S.J. Removal of Crystal Violet from Water by Magnetically Modified Activated Carbon and Nanomagnetic Iron Oxide. J. Environ. Health Sci. Eng. 2015, 13, 8. [Google Scholar] [CrossRef]
- Hoang, B.N.; Nguyen, T.T.; Bui, Q.P.T.; Bach, L.G.; Vo, D.N.; Trinh, C.D.; Bui, X.; Nguyen, T.D. Enhanced Selective Adsorption of Cation Organic Dyes on Polyvinyl Alcohol/Agar/Maltodextrin Water-resistance Biomembrane. J. Appl. Polym. Sci. 2020, 137, 48904. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mukherjee, A.; Das, S.; Raju Maddela, N.; Iram, S.; Das, P. Study on Isotherm, Kinetics, and Thermodynamics of Adsorption of Crystal Violet Dye by Calcium Oxide Modified Fly Ash. Environ. Eng. Res. 2020, 26, 190372. [Google Scholar] [CrossRef]
- Kaur, I.; Ellis, L.-J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; Mayne-L’Hermite, M.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of Nanomaterials in Aqueous Media: Towards Protocol Optimization. J. Vis. Exp. JoVE 2017, 130, 56074. [Google Scholar] [CrossRef]
- Rastogi, A.; Singh, P.; Haraz, F.A.; Barhoum, A. Biological Synthesis of Nanoparticles: An Environmentally Benign Approach. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 571–604. ISBN 978-0-323-51255-8. [Google Scholar]
- Pourali, P.; Baserisalehi, M.; Afsharnezhad, S.; Behravan, J.; Ganjali, R.; Bahador, N.; Arabzadeh, S. The Effect of Temperature on Antibacterial Activity of Biosynthesized Silver Nanoparticles. Biometals 2013, 26, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, E.; Li, J.; Qiu, Y.; Chen, R. Rapid Ultrasonic-Microwave Assisted Synthesis of Spindle-like Ag/ZnO Nanostructures and Their Enhanced Visible-Light Photocatalytic and Antibacterial Activities. Catal. Today 2020, 339, 391–402. [Google Scholar] [CrossRef]
- Connolly, M.; Fernandez-Cruz, M.-L.; Quesada-Garcia, A.; Alte, L.; Segner, H.; Navas, J. Comparative Cytotoxicity Study of Silver Nanoparticles (AgNPs) in a Variety of Rainbow Trout Cell Lines (RTL-W1, RTH-149, RTG-2) and Primary Hepatocytes. Int. J. Environ. Res. Public Health 2015, 12, 5386–5405. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
| Condition | Temperature (°C) | Sonication Time (min) |
|---|---|---|
| 1.1 | 30 | 30 |
| 1.2 | 30 | 60 |
| 1.3 | 30 | 90 |
| 1.4 | 45 | 30 |
| 1.5 | 45 | 60 |
| 1.6 | 45 | 90 |
| 1.7 | 60 | 30 |
| 1.8 | 60 | 60 |
| 1.9 | 60 | 90 |
| Kinetic Model | Kinetic Parameters | R2 | |
|---|---|---|---|
| qe,calc. (mg g−1) | 91.5 | ||
| PFO 1 | qe,exp. (mg g−1) | 81.9 | 0.91 |
| K1 (min−1) | 0.144 | ||
| PSO 1 | qe,exp. (mg g−1) | 90.8 | 0.993 |
| K2 (g mg−1 min−1) | 4.47 × 10−3 | ||
| IPD 1 | Kp (mg g−1 min−1/2) | 5.73 | 0.898 |
| C | 44.4 | ||
| Sample | 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 |
|---|---|---|---|---|---|---|---|---|---|
| L. monocyogenes ATCC 19117 (mm) | 11.75 ± 0.52 aAB | 12.00 ± 0.55 aB | 12.00 ± 0.5 aB | 12.00 ± 0.55 aB | 12.00 ± 0.5 aB | 12.00 ± 0.55 aB | 12.00 ± 0.5 aB | 12.00 ± 0.55 aB | 12.00 ± 0.5 aB |
| S. enterica ATCC 14028 (mm) | 12.33 ± 0.59 aAB | 15.0 ± 0.71 bC | 16.66 ± 0.78 cD | 15.0 ± 0.71 bC | 16.66 ± 0.78 cD | 15.0 ± 0.71 bC | 16.66 ± 0.78 cD | 15.0 ± 0.71 bC | 16.66 ± 0.78 cD |
| E. coli ATCC 8739 (mm) | 12.50 ± 0.62 aA | 12.33 ± 0.57 aA | 13.83 ± 0.65 bA | 12.33 ± 0.57 aA | 13.83 ± 0.65 bA | 12.33 ± 0.57 aA | 13.83 ± 0.65 bA | 12.33 ± 0.57 aA | 13.83 ± 0.65 bA |
| P. aeruginosa ATCC 9027 (mm) | 15.83 ± 0.7 bC | 15.75 ± 0.72 bC | 16.75 ± 0.75 cD | 15.75 ± 0.72 bC | 16.75 ± 0.75 cD | 15.75 ± 0.72 bC | 16.75 ± 0.75 cD | 15.75 ± 0.72 bC | 16.75 ± 0.75 cD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayari, A.; Chouayekh, H.; Smaoui, S.; Ayadi, W.; Ali Zainy, F.M.; Badr El-din, A.S.; Aljadaani, A.H.; Hmida-Sayari, A.; Yakout, A.A. Structural, Adsorptive, and Antibacterial Properties of a Novel Silver (Diethyldithiocarbamate)-Decorated Reduced Graphene Oxide Nanocomposite for Sustainable Wastewater Treatment. Nanomaterials 2025, 15, 1709. https://doi.org/10.3390/nano15221709
Sayari A, Chouayekh H, Smaoui S, Ayadi W, Ali Zainy FM, Badr El-din AS, Aljadaani AH, Hmida-Sayari A, Yakout AA. Structural, Adsorptive, and Antibacterial Properties of a Novel Silver (Diethyldithiocarbamate)-Decorated Reduced Graphene Oxide Nanocomposite for Sustainable Wastewater Treatment. Nanomaterials. 2025; 15(22):1709. https://doi.org/10.3390/nano15221709
Chicago/Turabian StyleSayari, Adel, Hichem Chouayekh, Slim Smaoui, Wajdi Ayadi, Faten M. Ali Zainy, Ahmed S. Badr El-din, Abeer H. Aljadaani, Aida Hmida-Sayari, and Amr A. Yakout. 2025. "Structural, Adsorptive, and Antibacterial Properties of a Novel Silver (Diethyldithiocarbamate)-Decorated Reduced Graphene Oxide Nanocomposite for Sustainable Wastewater Treatment" Nanomaterials 15, no. 22: 1709. https://doi.org/10.3390/nano15221709
APA StyleSayari, A., Chouayekh, H., Smaoui, S., Ayadi, W., Ali Zainy, F. M., Badr El-din, A. S., Aljadaani, A. H., Hmida-Sayari, A., & Yakout, A. A. (2025). Structural, Adsorptive, and Antibacterial Properties of a Novel Silver (Diethyldithiocarbamate)-Decorated Reduced Graphene Oxide Nanocomposite for Sustainable Wastewater Treatment. Nanomaterials, 15(22), 1709. https://doi.org/10.3390/nano15221709








