Development of Tumor Microenvironment-Responsive Nanoparticles with Enhanced Tissue Penetration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Preparation of Liposomes and Lipid Nanoparticles
2.3. Spheroid Penetration of Liposomes
2.4. Immunofluorescent Staining of Neuropilin-1 in Tumors
2.5. Actin Staining in Cells and Tumor Tissue
2.6. ZO-1 Immunostaining
2.7. Measurement of Transepithelial Electrical Resistance (TEER) in Caco-2 Cell Monolayers
2.8. Biodistribution of Intravenously Administered Liposomes in Tumor-Bearing Mice
2.9. Cell Association and Intracellular Trafficking of Liposomes
2.10. Evaluation of Cell Death
2.11. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Spheroid Penetration Between SAPSp- and SAPSp-iRGD-lipo
3.2. Involvement of Neuropilin-1 in the Penetration of SAPSp-iRGD-lipo
3.3. Involvement of Actin Depolymerization in the Tissue Penetration of SAPSp-iRGD-lipo
3.4. Effect of SAPSp-iRGD-lipo on the Intercellular Barrier
3.5. Comparison of Biodistribution of Intravenously Administered Liposomes in Tumor-Bearing Mice
3.6. Cytosolic Delivery of Cargo by SAPSp-iRGD-Modified Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| lipo | Liposomes |
| SAPSp | Slightly acidic pH-sensitive peptides |
| ECM | Extracellular matrix |
| iRGD | Internalizing RGD |
| LNPs | Lipid nanoparticles |
| PEG | Polyethylene glycol |
| EPR | Enhanced permeability and retention |
| KIF11 | Kinesin family member 11 |
| CLSM | Confocal laser scanning microscopy |
| TEER | Transepithelial electrical resistance |
References
- Macewan, S.R.; Chilkoti, A. From Composition to Cure: A Systems Engineering Approach to Anticancer Drug Carriers. Angew. Chem. 2017, 56, 6712–6733. [Google Scholar] [CrossRef]
- Wu, J. Enhanced Permeability and Retention (EPR) Effect: Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Sebak, A.A. Limitations of Pegylated Nanocarriers: Unfavourable Physicochemical Properties, Biodistribution Patterns and Cellular and Subcellular Fates. Int. J. Appl. Pharm. 2018, 10, 6. [Google Scholar] [CrossRef]
- Ishida, T.; Kiwada, H. Accelerated Blood Clearance (ABC) Phenomenon upon Repeated Injection of PEGylated Liposomes. Int. J. Pharm. 2007, 354, 56–62. [Google Scholar] [CrossRef]
- Gautam, B.; Hsiao, J.-C.; Yu, H.-H.; Luo, C.-H.; Lee, H.-M.; Tseng, H.-R.; Gao, H.-D. Nano-On-Nano: Responsive Nanosubstrate-Mediated Liposome Delivery with High Cellular Uptake Efficiency. ACS Appl. Bio Mater. 2023, 6, 1611–1620. [Google Scholar] [CrossRef]
- Madni, A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Löbenberg, R. Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 2014, 17, 401–426. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-Based Drug Delivery Systems: From Laboratory Research to Industrial Production—Instruments and Challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Hama, S.; Itakura, S.; Nakai, M.; Nakayama, K.; Morimoto, S.; Suzuki, S.; Kogure, K. Overcoming the Polyethylene Glycol Dilemma via Pathological Environment-Sensitive Change of the Surface Property of Nanoparticles for Cellular Entry. J. Control. Release 2015, 206, 67–74. [Google Scholar] [CrossRef]
- Itakura, S.; Hama, S.; Matsui, R.; Kogure, K. Effective Cytoplasmic Release of SiRNA from Liposomal Carriers by Controlling the Electrostatic Interaction of SiRNA with a Charge-Invertible Peptide in Response to Cytoplasmic PH. Nanoscale 2016, 8, 10649–10658. [Google Scholar] [CrossRef]
- Suzuki, S.; Nakayama, K.; Kogure, K.; Hama, S.; Matsui, R.; Nishi, T.; Itakura, S.; Nishimoto, A. Tumor Microenvironment-Sensitive Liposomes Penetrate Tumor Tissue via Attenuated Interaction of the Extracellular Matrix and Tumor Cells and Accompanying Actin Depolymerization. Biomacromolecules 2017, 18, 535–543. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Biomaterials 2014, 35, 6829–6838. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Ruoslahti, E. Tumor-Penetrating Peptides. Adv. Healthc. Mater. 2015, 4, 2699–2707. [Google Scholar] [CrossRef]
- Cun, X.; Chen, J.; He, Q.; Gao, H.; Zhang, L.; Ruan, S.; Wan, J. A Novel Strategy Combining IRGD Peptide with Tumor-Microenvironment-Responsive and Multistage Nanoparticles for Deep Tumor Penetration. ACS Appl. Mater. Interfaces 2015, 7, 27458–27466. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.; Park, S. IRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 1906. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Nel, A.E.; Meng, H. Major Effect of Transcytosis on Nanodrug Delivery to Pancreatic Cancer. Mol. Cell. Oncol. 2017, 4, e1335273. [Google Scholar] [CrossRef]
- Mamnoon, B.; Loganathan, J.; Sathish, V.; Froberg, J.; Choi, Y.; Confeld, M.I.; De Fonseka, N.; Mallik, S.; Tuvin, D.M.; Feng, L. Targeted Polymeric Nanoparticles for Drug Delivery to Hypoxic, Triple-Negative Breast Tumors. ACS Appl. Bio Mater. 2020, 4, 1450–1460. [Google Scholar] [CrossRef]
- Hama, S.; Sakai, M.; Itakura, S.; Majima, E.; Kogure, K. Rapid Modification of Antibodies on the Surface of Liposomes Composed of High-Affinity Protein A-Conjugated Phospholipids for Selective Drug Delivery. Biochem. Biophys. Rep. 2021, 27, 101067. [Google Scholar] [CrossRef]
- Shirane, D.; Nakai, Y.; Akita, H.; Tanaka, H.; Yoshioka, H. Development of an Alcohol Dilution-Lyophilization Method for Preparing Lipid Nanoparticles Containing Encapsulated siRNA. Biol. Pharm. Bull. 2018, 41, 1291–1294. [Google Scholar] [CrossRef]
- Nakamura, I.; Tabuchi, Y.; Itakura, S.; Hama, S.; Nishi, T.; Kogure, K.; Takasaki, I. Lipocalin2 as a Plasma Marker for Tumors with Hypoxic Regions. Sci. Rep. 2014, 4, 7235. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Utsumi, S.; Fukuda, Y.; Nakayama, K.; Okamura, Y.; Tsuchiya, H.; Fukuzawa, K.; Harashima, H.; Kogure, K. Development of a Novel Drug Delivery System Consisting of an Antitumor Agent, Tocopheryl Succinate. J. Control. Release 2012, 161, 843–851. [Google Scholar] [CrossRef]
- Zhang, S.; Hosaka, M.; Negishi, K.; Iida, Y.; Tobita, S.; Yoshihara, T.; Takeuchi, T. Phosphorescent Light–Emitting Iridium Complexes Serve as a Hypoxia-Sensing Probe for Tumor Imaging in Living Animals. Cancer Res. 2010, 70, 4490–4498. [Google Scholar] [CrossRef]
- de Jong, O.G.; van Balkom, B.W.M.; Gremmels, H.; Verhaar, M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J. Cell. Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Marusak, C.; Thakur, V.; Thakur, A.; Beier, U.H.; Nussenzweig, A.; Khanna, C.; Camphausen, K.A.; Citrin, D.E.; O’Connell, M.P.; Weeraratna, A.T.; et al. Matrix Metalloproteinase-Mediated ECM Remodeling Drives Melanoma Resistance to BRAF Inhibition. Clin. Cancer Res. 2020, 26, 6039–6051. [Google Scholar] [CrossRef]
- Shoval, H.; Kalo, E.; Benayahu, D.; Zipori, D.; Gazit, Z. Melanoma Cells Affect the Fibroblastic Microenvironment and Alter Extracellular Matrix Organization. Sci. Rep. 2017, 7, 7690. [Google Scholar]
- Teesalu, T.; Kotamraju, V.R.; Ruoslahti, E.; Sugahara, K.N. C-End Rule Peptides Mediate Neuropilin-1-Dependent Cell, Vascular, and Tissue Penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Chu, D.; Mao, G.; Paterson, D.J.; Tsao, S.C.; Xie, Z.; Sun, B.; Wang, W.; Centurion, F.; et al. Size-Dependent Penetration of Nanoparticles in Tumor Spheroids: A Multidimensional and Quantitative Study of Transcellular and Paracellular Pathways. Small 2023, 20, e2304693. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhang, Z.; Shi, J.; Yang, M.; Zeng, X.; Yang, Y.; Li, A.; Liu, H.; Liu, J.; Zhao, F.; et al. Bioactive Nanomotors Enable Efficient Intestinal Barrier Penetration for Colorectal Cancer Therapy. Nat. Commun. 2025, 16, 1678. [Google Scholar] [CrossRef]
- Hama, S.; Kimura, Y.; Mikami, A.; Shiota, K.; Toyoda, M.; Tamura, A.; Nagasaki, Y.; Kanamura, K.; Kajimoto, K.; Kogure, K. Electric Stimulus Opens Intercellular Spaces in Skin. J. Biol. Chem. 2014, 289, 2450–2456. [Google Scholar] [CrossRef]
- Yu, D.; Weber, C.R.; Raleigh, D.R.; Shen, L.; Marchiando, A.M.; Wang, Y.; Turner, J.R. MLCK-Dependent Exchange and Actin Binding Region-Dependent Anchoring of ZO-1 Regulate Tight Junction Barrier Function. Proc. Natl. Acad. Sci. USA 2010, 107, 8237–8241. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Bridges, A.; Fanning, A.S.; Anderson, J.M. ZO-1 Stabilizes the Tight Junction Solute Barrier through Coupling to the Perijunctional Cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef]
- Romberg, B.; Oussoren, C.; Snel, C.J.; Carstens, M.G.; Hennink, W.E.; Storm, G. Pharmacokinetics of poly(hydroxyethyl-L-asparagine)-coated liposomes is superior to PEG-liposomes at low lipid dose and upon repeated administration. Biochim. Biophys. Acta Biomembr. 2007, 1768, 737–743. [Google Scholar] [CrossRef]
- Qi, J.; Yao, P.; He, F.; Yu, D.; Guo, Y. Blood clearance and biodistribution of cationic and anionic liposomes after intravenous injection in mice. Colloids Surf. B Biointerfaces 2016, 141, 93–100. [Google Scholar] [CrossRef]
- Tao, W.; South, V.J.; Zhang, Y.; Davide, J.P.; Farrell, L.; Kohl, N.E.; Sepp-Lorenzino, L.; Lobell, R.B. Induction of Apoptosis by an Inhibitor of the Mitotic Kinesin KSP Requires Both Activation of the Spindle Assembly Checkpoint and Mitotic Slippage. Cancer Cell 2005, 8, 49–59. [Google Scholar] [CrossRef]
- Tao, W.; South, V.J.; Diehl, R.E.; Davide, J.P.; Sepp-Lorenzino, L.; Fraley, M.E.; Arrington, K.L.; Lobell, R.B. An Inhibitor of Kinesin Spindle Protein Activates the Intrinsic Apoptotic Pathway Independently of P53 and de Novo Protein Synthesis. Mol. Cell. Biol. 2007, 27, 689–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Chen, X. Engineering pH-Responsive Charge-Reversal Nanoparticles for Enhanced Tumor-Targeted Drug Delivery. Biomaterials 2021, 276, 120737. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Li, Z.; Shen, S.; Li, J.; Ye, J.; Zhao, Z.; Guo, S.; Zhang, Y.; Liu, Y. Tumor Microenvironment-Activated Charge-Reversal Nanocarriers for Improved Cancer Therapy. Bioact. Mater. 2021, 6, 3610–3622. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, K.; Jiang, B.; Li, L.; Wang, X.; Ji, J.; Liu, Y. A comprehensive study of iRGD-modified liposomes with improved chemotherapeutic efficacy on B16 melanoma. J. Pharm. Pharm. Sci. 2015, 22, 10–20. [Google Scholar] [CrossRef]
- Du, R.; Jiang, P.; Li, H.; Kong, F.; Kong, D.; Li, Y.; Sun, X.; Zhao, Y. Antitumor effect of iRGD-modified liposomes containing conjugated linoleic acid–paclitaxel. Int. J. Nanomed. 2014, 9, 5179–5195. [Google Scholar] [CrossRef]
- Nikitovic, D.; Berdiaki, A.; Spyridaki, I.; Papoutsidakis, A.; Tsatsakis, A.; Tzanakakis, G.N. Enhancing Tumor Targeted Therapy: The Role of iRGD Peptide in Nanocarriers. Cancers 2024, 16, 3768. [Google Scholar] [CrossRef]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef]
- Amin, M.; Mansourian, M.; Burgers, P.C.; Amin, B.; Jaafari, M.R.; ten Hagen, T.L.M. Increased Targeting Area in Tumors by Dual-Ligand Modification of Liposomes with RGD and TAT Peptides. Pharmaceutics 2022, 14, 458. [Google Scholar] [CrossRef]
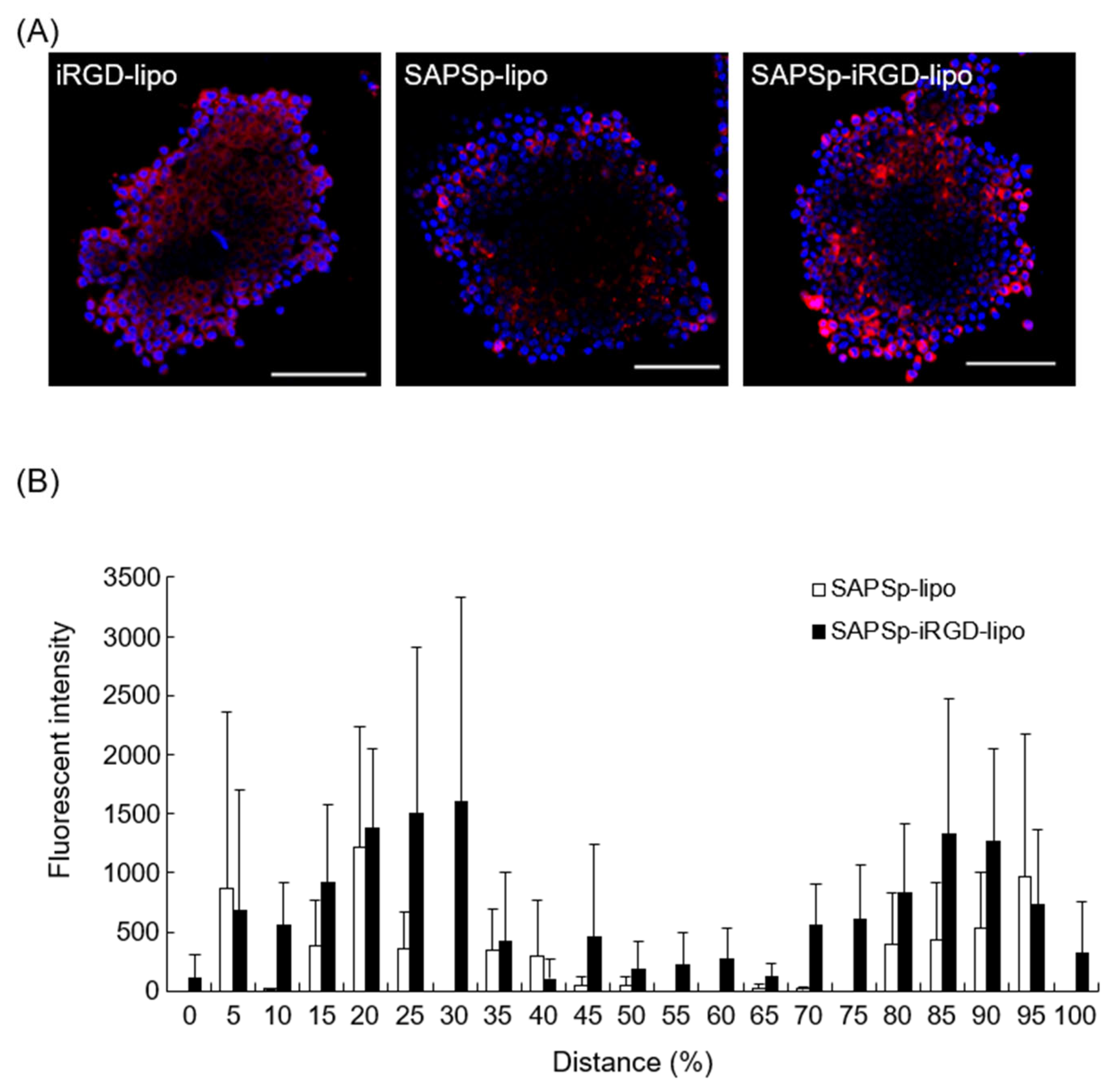


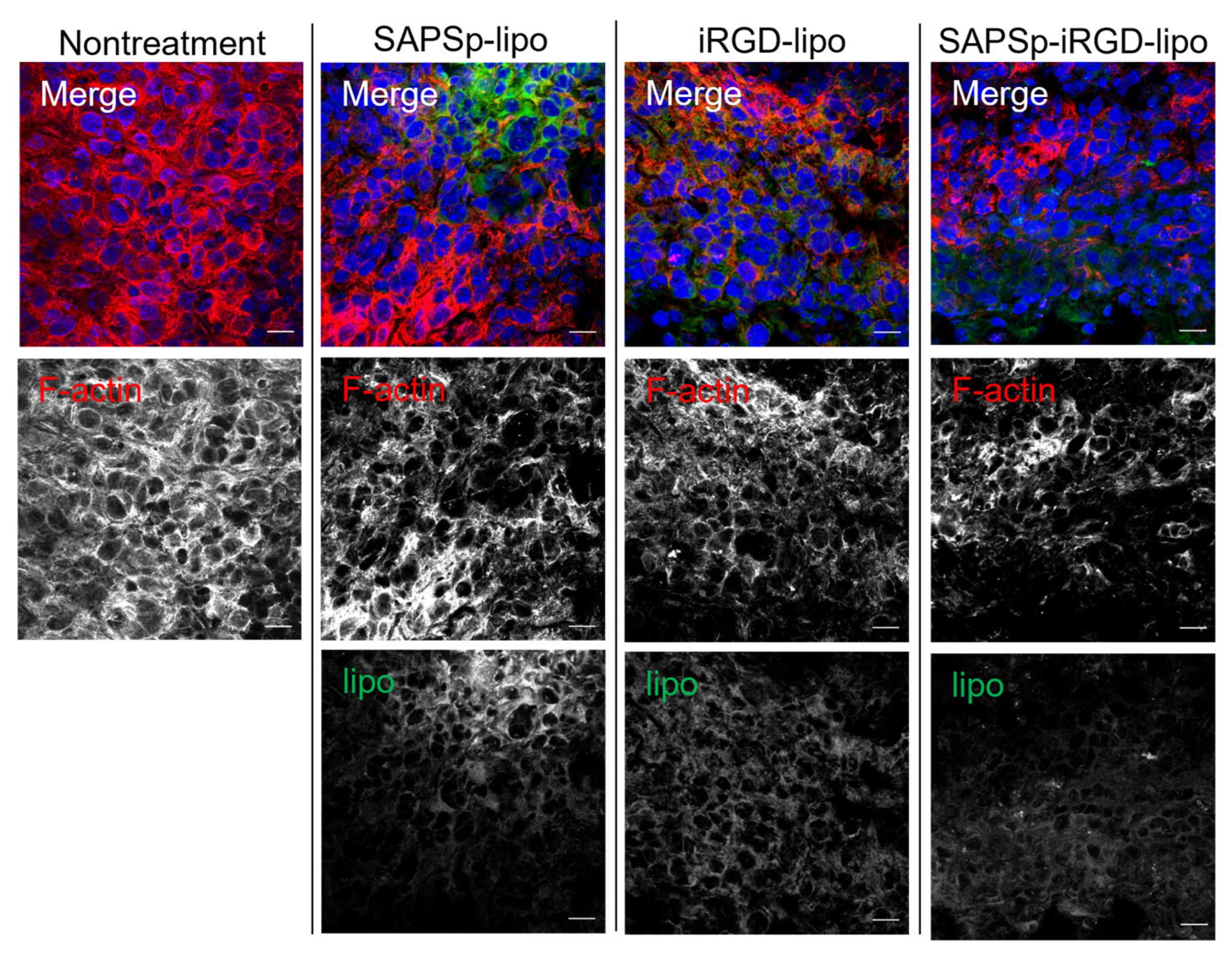


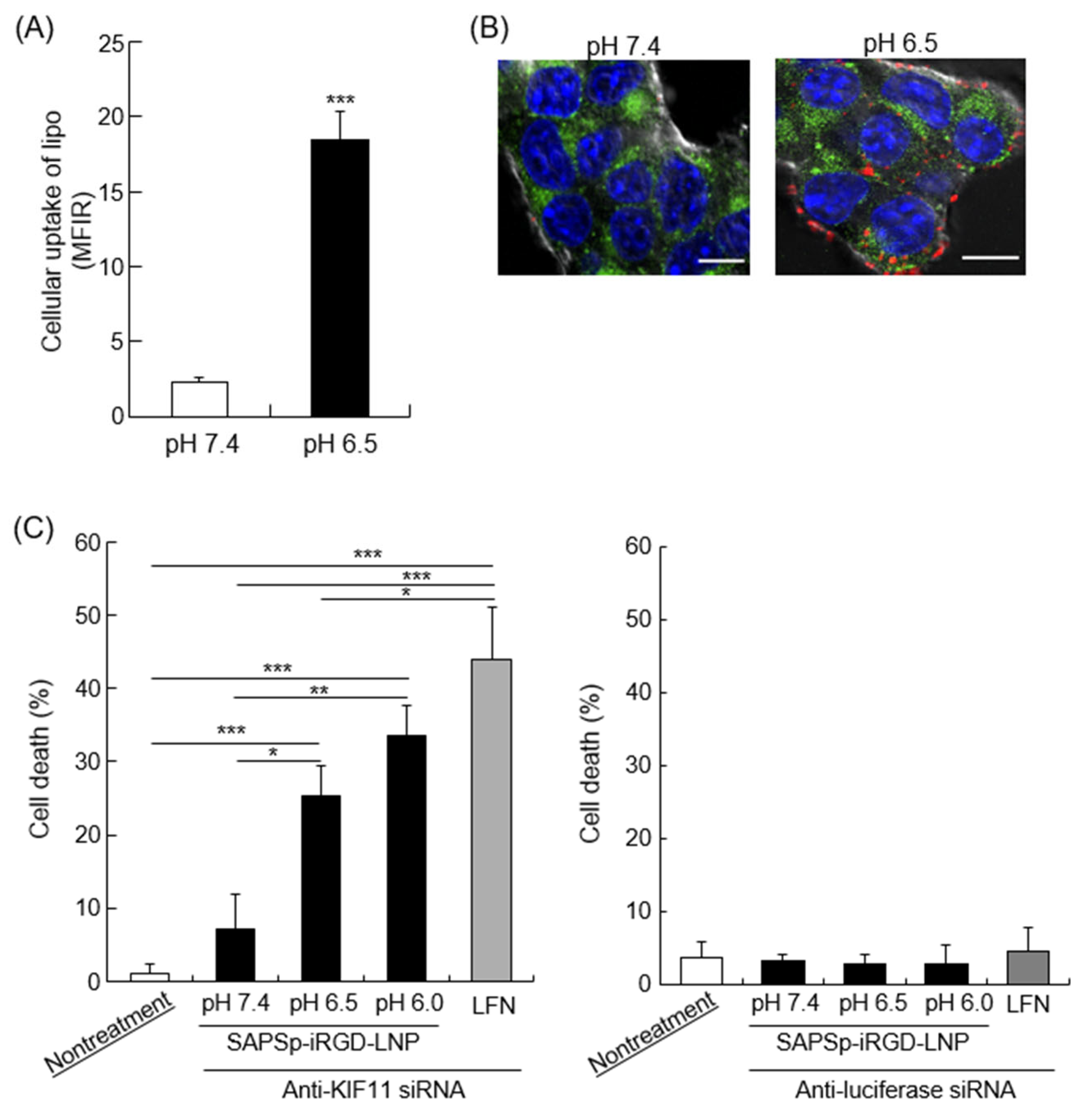
| pH | Particle Size (nm) | Polydispersity Index | ζ-potential (mV) |
|---|---|---|---|
| 7.4 | 103 ± 12 | 0.262 ± 0.001 | −15 ± 1.8 |
| 6.5 | 103 ± 11 | 0.341 ± 0.016 | −5.5 ± 1.3 |
| Treatment | Mean % | Bliss-Predicted | Bliss Excess | Interaction |
|---|---|---|---|---|
| SAPSp-lipo | 37.2 | - | - | - |
| iRGD-lipo | 31.1 | - | - | - |
| SAPSp-iRD-lipo | 62.3 | 56.7 | +5.6 | Mild synergy |
| SAPSp-lipo | iRGD-lipo | SAPSp-iRGD-lipo | |
|---|---|---|---|
| Pearson’s correlation coefficient | 0.218 ± 0.021 | 0.247 ± 0.028 | 0.257 ± 0.029 |
| siRNA Encapsulated in LNPs | Encapsulation (%) | pH | Particle Size (nm) | Polydispersity Index | ζ-Potential (mV) |
|---|---|---|---|---|---|
| Anti-KIF11 siRNA | 85.0 | 7.4 | 73.4 ± 9.2 | 0.353 ± 0.080 | −16 ± 2.5 |
| 6.5 | 74.5 ± 11 | 0.353 ± 0.053 | −6.7 ± 1.9 | ||
| Control siRNA | 72.7 | 7.4 | 70.0 ± 5.1 | 0.297 ± 0.061 | −13 ± 3.1 |
| 6.5 | 72.9 ± 21 | 0.312 ± 0.119 | −6.4 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, K.; Matsui, R.; Itagaki, N.; Takeuchi, Y.; Fukuda, H.; Tanaka, K.-I.; Hama, S. Development of Tumor Microenvironment-Responsive Nanoparticles with Enhanced Tissue Penetration. Nanomaterials 2025, 15, 1695. https://doi.org/10.3390/nano15221695
Kitamura K, Matsui R, Itagaki N, Takeuchi Y, Fukuda H, Tanaka K-I, Hama S. Development of Tumor Microenvironment-Responsive Nanoparticles with Enhanced Tissue Penetration. Nanomaterials. 2025; 15(22):1695. https://doi.org/10.3390/nano15221695
Chicago/Turabian StyleKitamura, Karin, Ryo Matsui, Nagisa Itagaki, Yuka Takeuchi, Hana Fukuda, Ken-Ichiro Tanaka, and Susumu Hama. 2025. "Development of Tumor Microenvironment-Responsive Nanoparticles with Enhanced Tissue Penetration" Nanomaterials 15, no. 22: 1695. https://doi.org/10.3390/nano15221695
APA StyleKitamura, K., Matsui, R., Itagaki, N., Takeuchi, Y., Fukuda, H., Tanaka, K.-I., & Hama, S. (2025). Development of Tumor Microenvironment-Responsive Nanoparticles with Enhanced Tissue Penetration. Nanomaterials, 15(22), 1695. https://doi.org/10.3390/nano15221695







