Preparation and Formation Mechanism of Carbon Nanotubes via Coal Pyrolysis Using Alkaline Potassium Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Catalyst Loading Method
2.3. Experimental Method
2.4. Sample Characterization
3. Results and Discussion
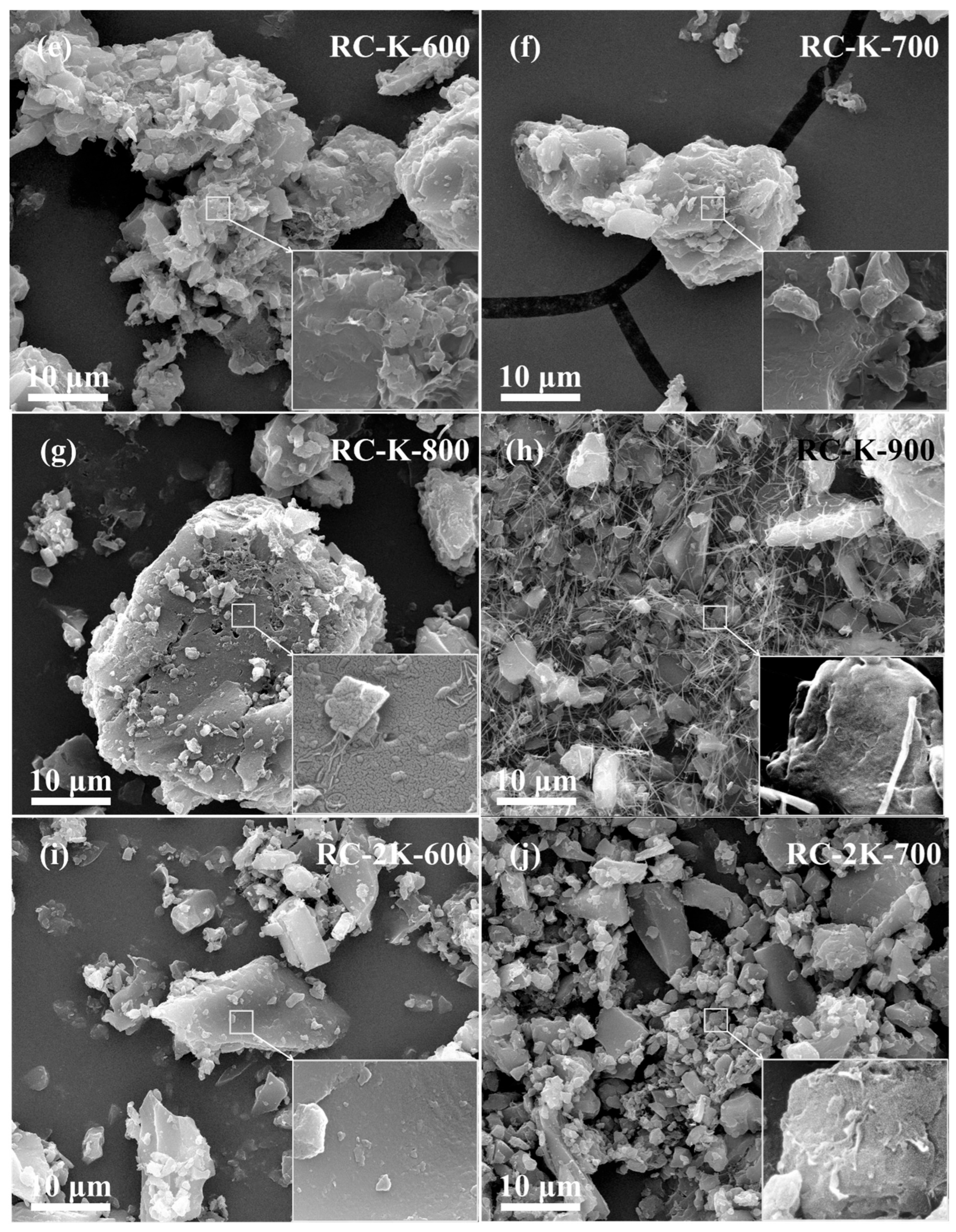
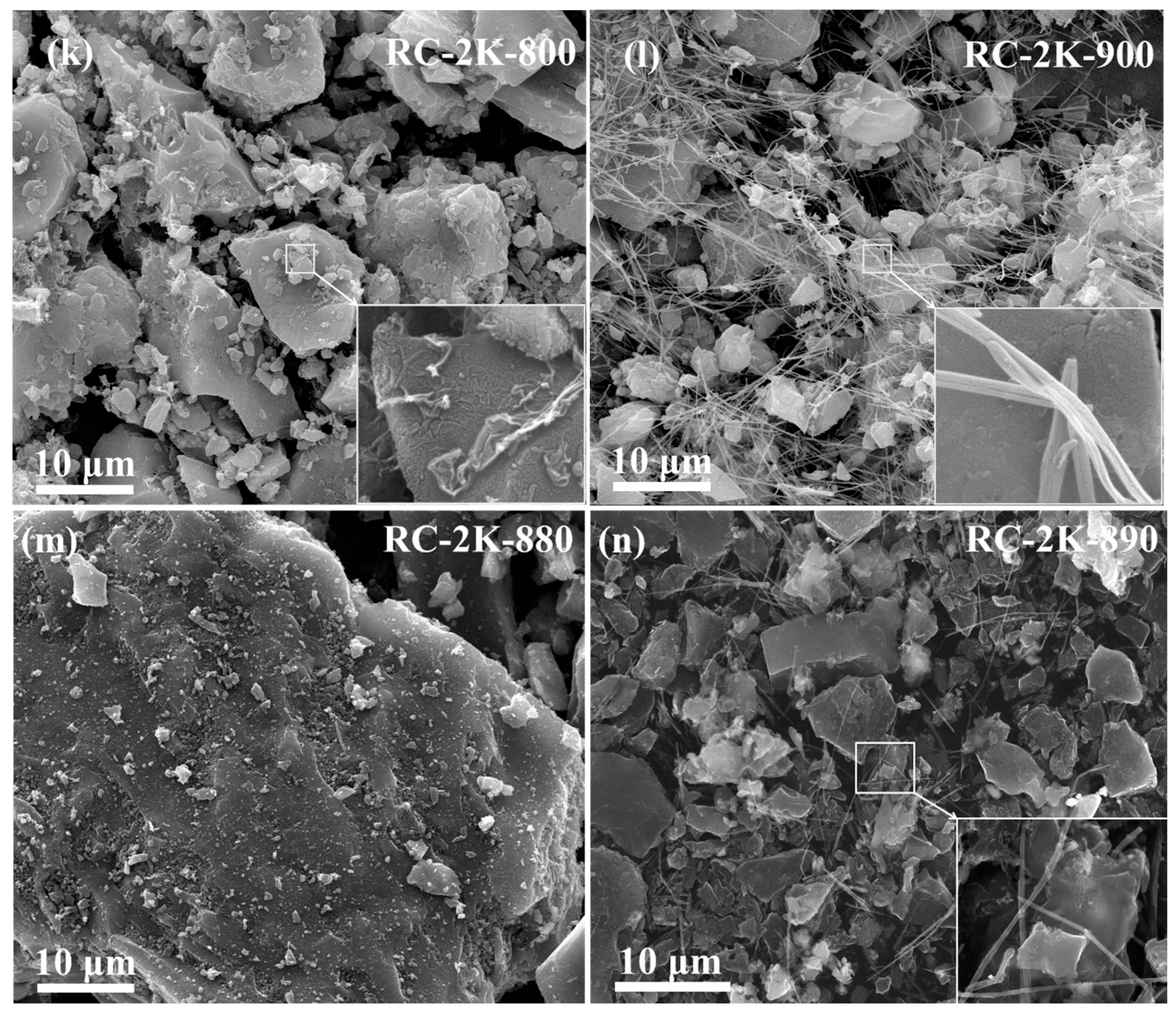
3.1. SEM Analysis
3.2. TG-DTG Analysis
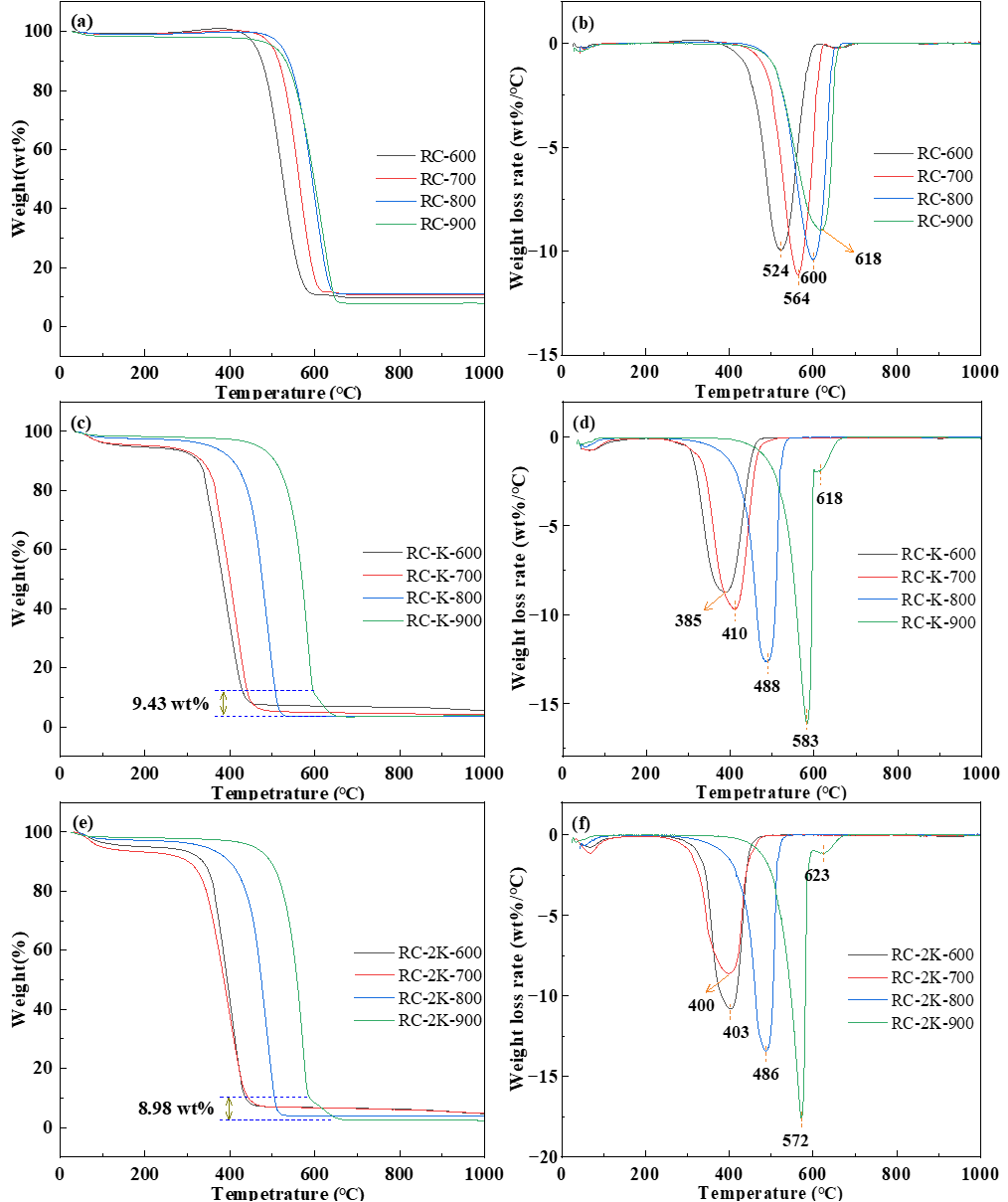
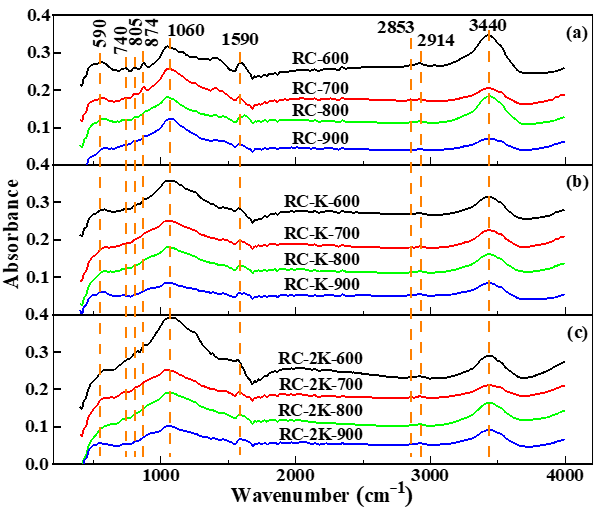
3.3. FTIR Spectroscopy Analysis
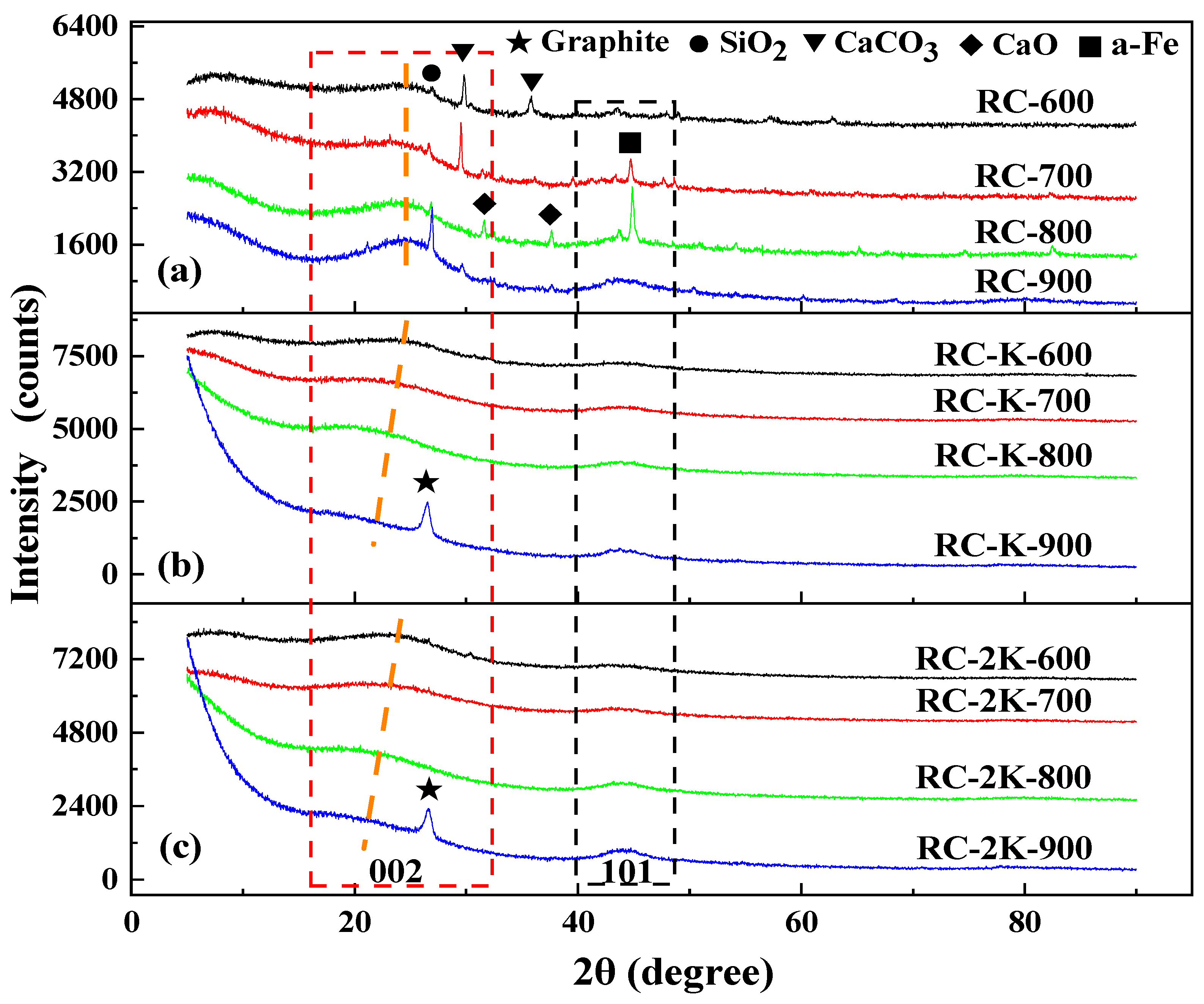
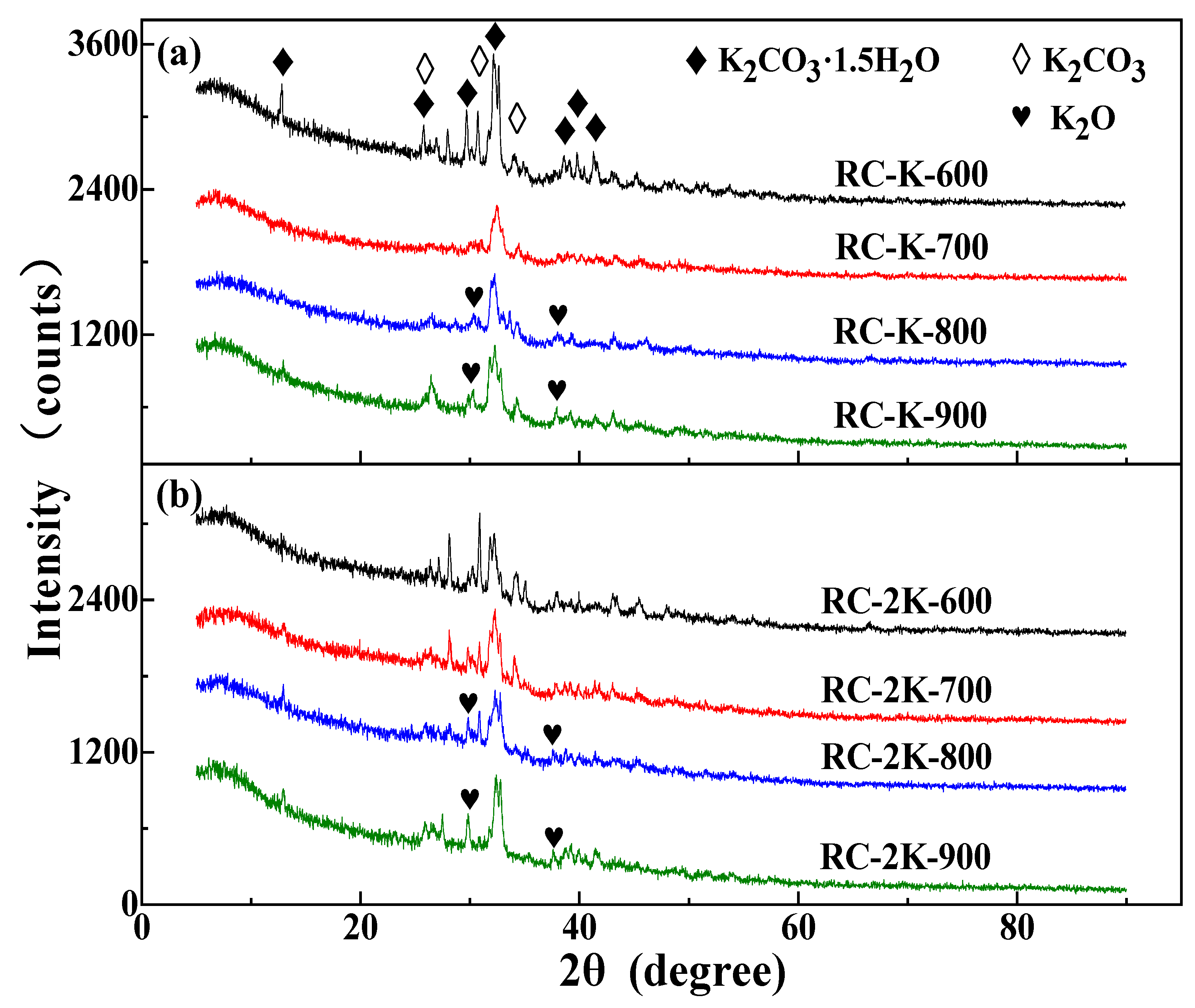
3.4. XRD Analysis
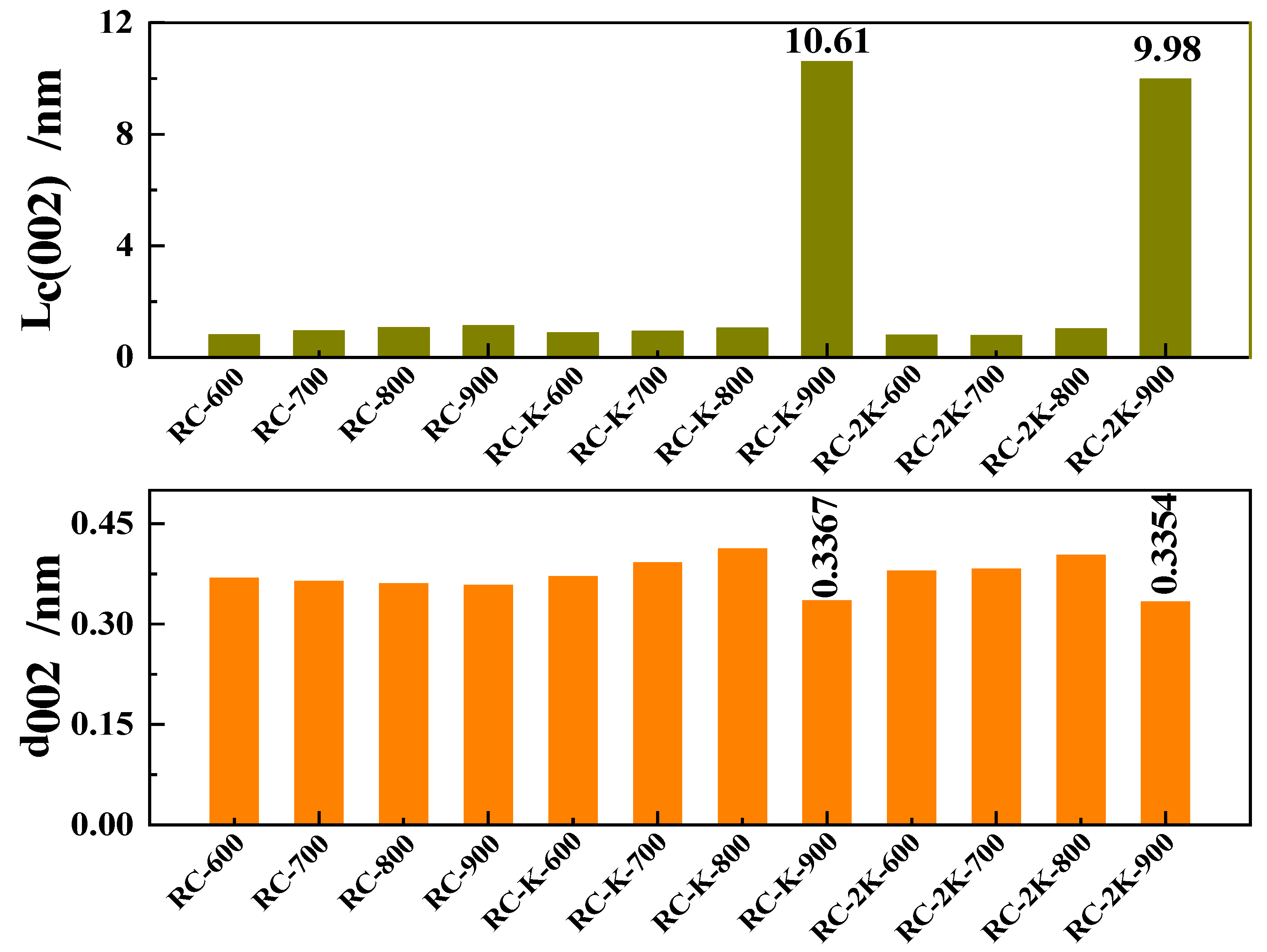
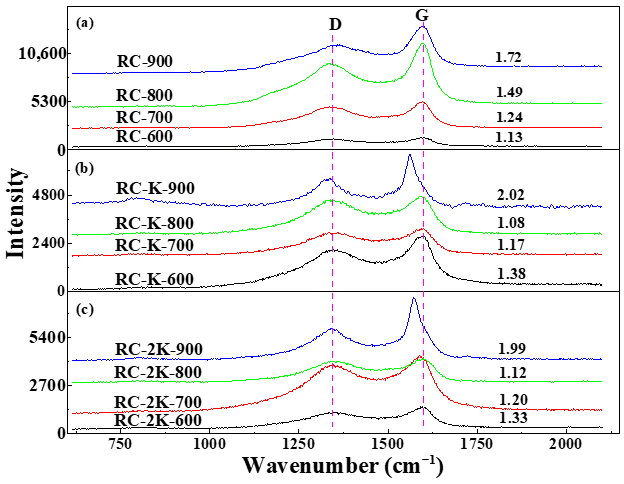
3.5. Raman Spectroscopy Analysis
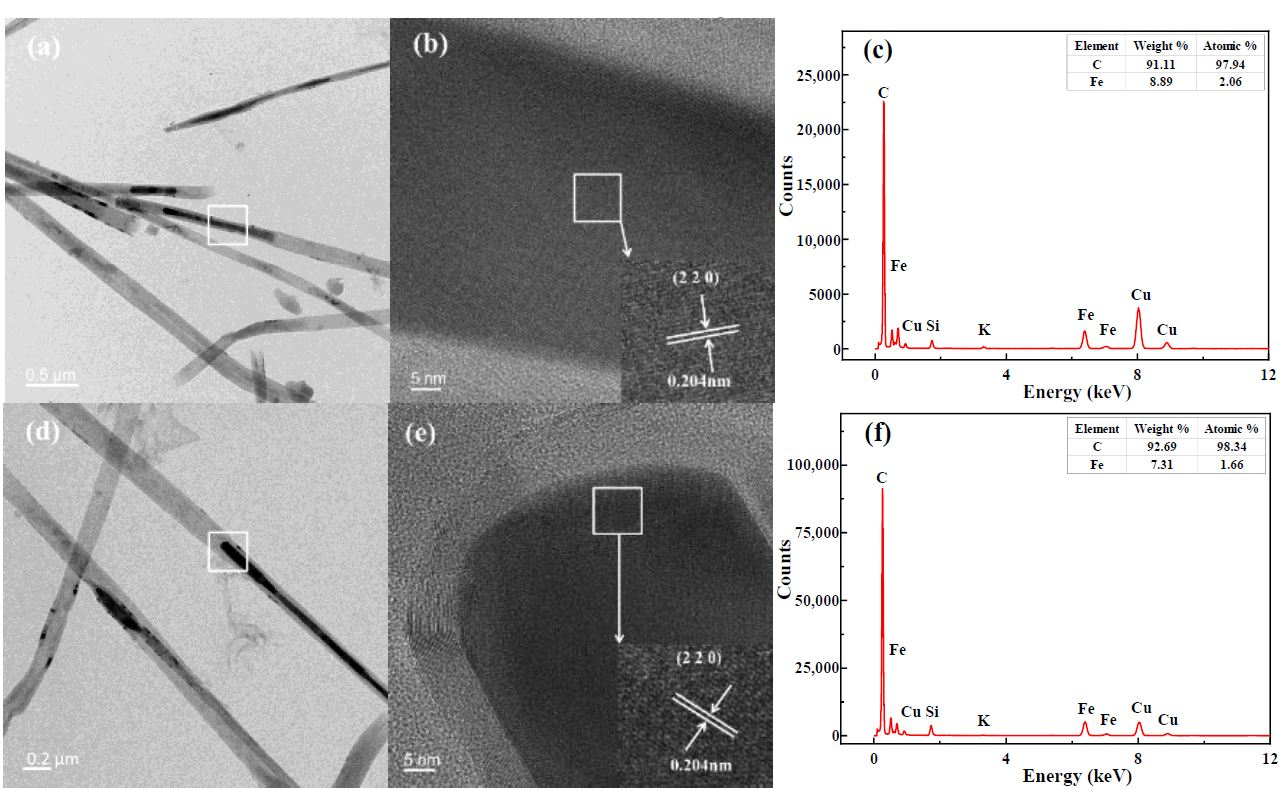
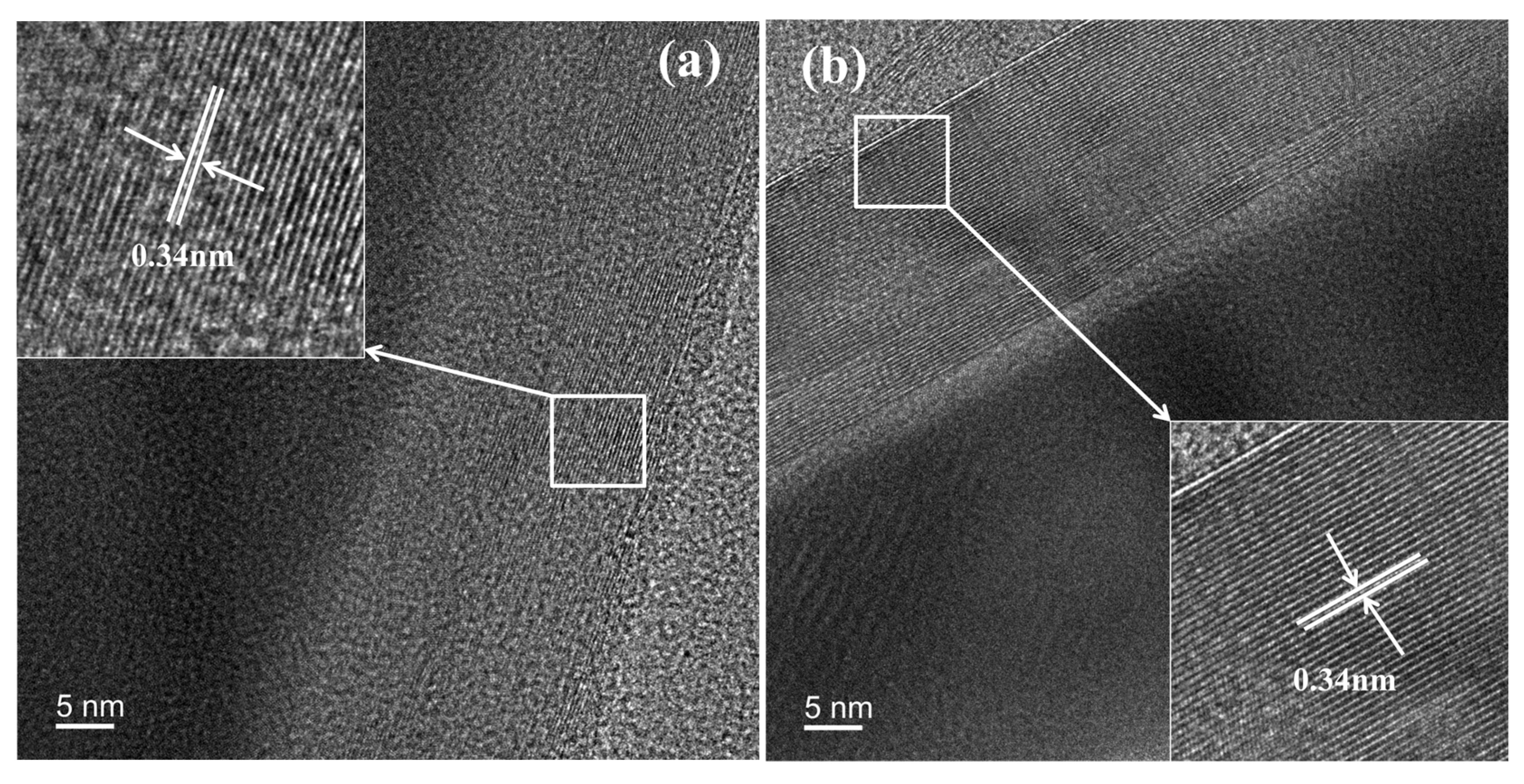
3.6. TEM-EDS Analysis
3.7. Mechanism of Carbon Nanotube Growth
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Y. Enhanced mechanical and thermal performance of high-strength engineered geopolymer composites reinforced by hybrid polyethylene fibres and carbon nanotubes. Constr. Build. Mater. 2025, 472, 140884. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Estaji, S.; Kiaei, H.; Mansourian-Tabaei, M.; Nouranian, S.; Jafari, S.H.; Ruckdäschel, H.; Arjmand, M.; Khonakdar, H.A. A review of electrical and thermal conductivities of epoxy resin systems reinforced with carbon nanotubes and graphene-based nanoparticles. Polym. Test. 2022, 112, 107645. [Google Scholar] [CrossRef]
- Soni, S.K.; Thomas, B.; Kar, V.R. A Comprehensive Review on CNTs and CNT-Reinforced Composites: Syntheses, Characteristics and Applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, G.; Miao, R.; Deng, J.; Wang, L.; Shao, Q.; Shao, C. The electronic transport characteristics subsequent to linear doping with nitrogen or boron in (8,0) single-walled carbon nanotubes. Mater. Sci. Semicond. Process. 2025, 185, 109000. [Google Scholar] [CrossRef]
- Jiang, N.; Shao, Y.; Zhao, X.; Zhang, Y.; Lu, P.; Ye, L.; Yang, Q.; Qiu, J. Two-dimensional carbon-based nanomaterials convoying dendrite-free Zn/Li metal batteries. Chem. Eng. Sci. 2025, 309, 121499. [Google Scholar] [CrossRef]
- Lawal, A.T. Recent application of carbon nanotubes in energy storage and conversion devices. Carbon Trends 2025, 19, 100470. [Google Scholar] [CrossRef]
- Li, C.; Zhong, J.; Sun, Y. Novel approach to enhance the damping performance of cement-based materials through polymer/carbon nanotube composite coating and gradation of aggregates. Constr. Build. Mater. 2025, 471, 140708. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sensors Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diamond Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Pant, M.; Singh, R.; Negi, P.; Tiwari, K.; Singh, Y. A comprehensive review on carbon nano-tube synthesis using chemical vapor deposition. Mater. Today Proc. 2021, 46, 11250–11253. [Google Scholar] [CrossRef]
- Kwon, O.; Park, J.; Park, Y.T. Current Progress of Carbon Nanotubes Applied to Proton Exchange Membrane Fuel Cells: A Comprehensive Review. Int. J. Precis. Eng. Manuf. Green Technol. 2024, 11, 659–684. [Google Scholar] [CrossRef]
- Shifa, M.; Toor, Z.S.; Tariq, F. Arc Discharge Synthesis and Multistep Purification of Multiwall Carbon Nanotubes. Nano 2024, 19, 2450007. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, H.; Deng, Y.; Miao, R.; Lai, D.; Deng, J.; Zhang, J.; Chen, Q.; Shao, Q.; Shao, C. Synthesis of high-quality multi-walled carbon nanotubes by arc discharge in nitrogen atmosphere. Vacuum 2024, 225, 113198. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Nelson, P.F. Environmental Issues: Emissions, Pollution Control, Assessment, and Management; Woodhead Publishing: Cambridge, UK, 2023; Volume 2, pp. 31–76. [Google Scholar]
- Zhao, Y.; Mu, W.; Yang, J.; Li, G.; Zhang, H.; Wang, Y. Synergistic optimization and control of coal-based impregnating pitch performance by refining and thermal modification. J. Ind. Eng. Chem. 2025, 150, 771–782. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Wu, C.; Wu, W.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. Green synthesis of nitrogen and oxygen enriched porous carbon from lignite humate for high-performance supercapacitors. J. Energy Storage 2024, 101, 113955. [Google Scholar] [CrossRef]
- Gasparotto, J.; Da Boit Martinello, K. Coal as an energy source and its impacts on human health. Energy Geosci. 2021, 2, 113–120. [Google Scholar] [CrossRef]
- Alhassan, A.; Ozturk, I.; Al-Zyoud, M.F.; Bekun, F.V. Coal consumption-environmental sustainability nexus in developed and developing major coal-consuming economies. Heliyon 2024, 10, e25619. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Li, Y.; Yang, H.; Jin, L.; Li, D.; Hu, H. Removal of elemental mercury from coal combustion flue gas by sodium halides impregnated red mud. J. Fuel Chem. Technol. 2025, 53, 53–67. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Pan, Z.; Cui, J.; Wang, B.; Zhang, D.; Guo, Y.; Cheng, F. Exploring the mechanisms of enhanced activated carbon’s toluene adsorption and regeneration by utilizing inherent pyrite in coal. Fuel 2025, 386, 134224. [Google Scholar] [CrossRef]
- Pang, L.S.; Wilson, M.A. Nanotubes from coal. Energy Fuels 1993, 7, 436–437. [Google Scholar] [CrossRef]
- Williams, K.A.; Tachibana, M.; Allen, J.L.; Grigorian, L.; Cheng, S.C.; Fang, S.L.; Sumanasekera, G.U.; Loper, A.L.; Williams, J.H.; Eklund, P.C. Single-wall carbon nanotubes from coal. Chem. Phys. Lett. 1999, 310, 31–37. [Google Scholar] [CrossRef]
- Qiu, J.S.; Zhang, F.; Zhou, Y.; Han, H.M.; Hu, D.S.; Tsang, S.C.; Harris, P.J.F. Carbon nanomaterials from eleven caking coals. Fuel 2002, 81, 1509–1514. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Qiu, J. Synthesis of branched carbon nanotubes from coal. Carbon 2006, 44, 1321–1324. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, Z.; Zhao, Z.; Wang, T. Synthesis of double-walled carbon nanotubes from coal in hydrogen-free atmosphere. Fuel 2007, 86, 282–286. [Google Scholar] [CrossRef]
- Awasthi, S.; Awasthi, K.; Ghosh, A.K.; Srivastava, S.K.; Srivastava, O.N. Formation of single and multi-walled carbon nanotubes and graphene from Indian bituminous coal. Fuel 2015, 147, 35–42. [Google Scholar] [CrossRef]
- Wilson, M.A.; Patney, H.K.; Kalman, J. New developments in the formation of nanotubes from coal. Fuel 2002, 81, 5–14. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Li, G.; Zhao, Y.; Lv, X.; Luo, Y.; Zhang, Y. Formation of carbon nanotubes from potassium catalyzed pyrolysis of bituminous coal. Fuel 2019, 239, 230–238. [Google Scholar] [CrossRef]
- Das, T.; Saikia, B.K.; Baruah, B.P. Formation of carbon nano-balls and carbon nano-tubes from northeast Indian Tertiary coal: Value added products from low grade coal. Gondwana Res. 2016, 31, 295–304. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Lv, X.; Luo, Y.; Zhang, Y. Transformation of primary siderite during coal catalytic pyrolysis and its effects on the growth of carbon nanotubes. Fuel Process. Technol. 2020, 198, 106235. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Tang, M.; Hao, X.; Liu, J.; Zhang, G.; Zhang, Y. Preparation of N, O co-doped carbon nanotubes and activated carbon composites with hierarchical porous structure for CO2 adsorption by coal pyrolysis. Fuel 2023, 333, 126465. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Zhao, Y.; Zhang, G. Ultrahigh-capacity adsorption of Rhodamine B by N, S co-doped carbon nanotube composites derived from coal. Sep. Purif. Technol. 2025, 376, 134045. [Google Scholar] [CrossRef]
- Tao, Z.; Zhao, Y.; Wang, Y.; Zhang, G. Recent advances in carbon nanotube technology: Bridging the gap from fundamental science to wide applications. J. Carbon Res. 2024, 10, 69. [Google Scholar] [CrossRef]
- Rashidi, A.; Akbarnejad, M.; Khodadadi, A.; Mortazavi, Y.; Ahmadpourd, A. Single-wall carbon nanotubes synthesized using organic additives to Co–Mo catalystssupported on nanoporous MgO. Nanotechnology 2007, 18, 315605. [Google Scholar] [CrossRef]
- Li, R.; Antunes, E.F.; Kalfon-Cohen, E.; Kudo, A.; Acauan, L.; Yang, W.C.D.; Wang, C.; Cui, K.; Liotta, A.H.; Rajan, A.G. Low-temperature growth of carbon nanotubes catalyzed by sodium-based ingredients. Angew. Chem. Int. Ed. 2019, 58, 9204–9209. [Google Scholar] [CrossRef]
- Modekwe, H.U.; Moothi, K.; Daramola, M.O.; Mamo, M.A. Corn cob char as catalyst support for developing carbon nanotubes from waste polypropylene plastics: Comparison of activation techniques. Polymers 2022, 14, 2898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ge, L.; Li, X.; Zuo, M.; Xu, C.; Chen, S.; Li, Q.; Wang, Y.; Xu, C. Effects of the carbonization temperature and intermediate cooling mode on the properties of coal-based activated carbon. Energy 2023, 273, 127177. [Google Scholar] [CrossRef]
- Gabal, M.; Hoff, D.; Kasper, G. Influence of the atmosphere on the thermal decomposition kinetics of the CaCO3 content of PFBC coal flying ash. J. Therm. Anal. Calorim. 2007, 89, 109–116. [Google Scholar] [CrossRef]
- Niu, J.; Luo, L.; Cui, J.; Zhang, H.; Guo, Y.; Li, L.; Cheng, F. Impact of inherent calcium in coal on the structure and performance of activated carbon in flue gas activation: The enhanced mechanism of calcite on the methylene blue adsorption. J. Clean. Prod. 2023, 428, 139374. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Sun, Y.; Xu, B.; Jiang, Z.; Qu, X.; Zhang, C. An effective strategy to synthesize well-designed activated carbon derived from coal-based carbon dots via oxidation before activation with a low koh content as supercapacitor electrodes. Nanomaterials 2023, 13, 2909. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.S.; Zaini, M.A.A. Potassium hydroxide activation of activated carbon: A commentary. Carbon Lett. 2015, 16, 275–280. [Google Scholar] [CrossRef]
- Wang, S.; Nam, H.; Nam, H. Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J. Environ. Chem. Eng. 2020, 8, 103683. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour. Technol. Rep. 2019, 7, 100266. [Google Scholar] [CrossRef]
- Okolo, G.N.; Neomagus, H.W.; Everson, R.C.; Roberts, M.J.; Bunt, J.R.; Sakurovs, R.; Mathews, J.P. Chemical–structural properties of South African bituminous coals: Insights from wide angle XRD–carbon fraction analysis, ATR–FTIR, solid state 13C NMR, and HRTEM techniques. Fuel 2015, 158, 779–792. [Google Scholar] [CrossRef]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P.K. FT-IR and XRD analysis of coal from Makum coalfield of Assam. J. Earth Syst. Sci. 2007, 116, 575–579. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, T.K.; Zhao, Y.Q.; He, S.Q.; Zhang, Y.F. Structural evolution and formation mechanisms of caking components of modified lignite in subcritical H2O-CO systems. Energy Fuels 2019, 33, 12073–12082. [Google Scholar] [CrossRef]
- Kotov, N.; Keskitalo, M.M.; Johnson, C.M. Nano FTIR spectroscopy of liquid water in the –OH stretching region. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 330, 125640. [Google Scholar] [CrossRef]
- Suhasaria, A.; Senapati, R.; Rout, P.K.; Sengupta, S.; Ghosal, S.; Banerjee, P.; Mukherjee, D.; Dey, S.; Sukul, D. Electrochemical behavior of mild steel coated with long alkyl-chain benzothiazole derivatives in acid solution: Effect of aliphatic chain length. Colloids Surf. A 2024, 703, 135264. [Google Scholar] [CrossRef]
- Khairul, W.M.; Tagiling, A.A.; Muzaman, A.Q.; Rahamathullah, R.; Mohammed, M.; Saidin, S.; Arshad, S.; Razak, I.A.; Razak, F.I.A.; Sapari, S. The experimental and DFT approaches on electronic, thermal and conductivity properties of non-linear optical bearing fused aromatic chalcones towards prospective OLEDs. J. Mol. Struct. 2025, 1319, 139585. [Google Scholar] [CrossRef]
- Fathurrahman, N.A.; Alhaboudal, M.A.; Mohammed, S.; Bello, A.K.; Al-Saadi, A.A. The role of hydrogen bonding in the conformational stability of 2-methoxyresorcinol: Insights from theoretical calculations, SERS spectroscopy, and solvent effect. J. Mol. Liq. 2024, 414, 126201. [Google Scholar] [CrossRef]
- Islam Touahria, Y.; Chafai, N.; Moumeni, O.; Boublia, A.; Mehri, M.; Benguerba, Y. Synthesis, characterization, and comprehensive computational analysis of aromatic hydrazone compounds: Unveiling quantum parameters, evaluating antioxidant activity, and investigating molecular docking interactions. J. Mol. Liq. 2024, 403, 124897. [Google Scholar] [CrossRef]
- Sinha, A.; Wei, J. Phase evolution and mechanical-hydroscopic properties of alkali-silica reaction gels modified by magnesium nitrate. Cem. Concr. Compos. 2023, 144, 105283. [Google Scholar] [CrossRef]
- Spencer, W.; Senanayake, G.; Altarawneh, M.; Ibana, D.; Nikoloski, A.N. Review of the effects of coal properties and activation parameters on activated carbon production and quality. Miner. Eng. 2024, 212, 108712. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, G.; Park, J.E.; Kim, S.H. Limitation of K2CO3 as a chemical agent for upgrading activated carbon. Processes 2021, 9, 1000. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, T.; Ling, Y.; Yin, Y.; Nong, G. Solid waste of calcium lignin replaces fossil fuel power by gasification to reduce CO2 emissions. Process Saf. Environ. Prot. 2024, 182, 857–865. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, S.; Zheng, H.; Zuo, Z.; Liu, Y. Nanomechanical properties of anthracite and graphite: The role of heteroatom functional groups and structural evolution. Int. J. Coal Geol. 2025, 301, 104714. [Google Scholar] [CrossRef]
- Xiong, Z.; Syed-Hassan, S.S.A.; Hu, X.; Guo, J.; Chen, Y.; Liu, Q.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Effects of the component interaction on the formation of aromatic structures during the pyrolysis of bio-oil at various temperatures and heating rates. Fuel 2018, 233, 461–468. [Google Scholar] [CrossRef]
- Behera, S. Carbon: The Material and Its Characterization by Raman Spectroscopy; AIP Conference Proceedings; AIP: Melville, NY, USA, 2008; pp. 61–71. [Google Scholar]
- Sparavigna, A.C. Graphene, Graphene Oxide and Carbon Nanotubes in Raman Spectroscopy. Int. J. Sci. 2024, 13, 1–26. [Google Scholar] [CrossRef]
- Li, J.; Lan, H.; Liu, H.; Zhang, G.; An, X.; Liu, R.; Qu, J. Intercalation of nanosized Fe3C in iron/carbon to construct multifunctional interface with reduction, catalysis, corrosion resistance, and immobilization capabilities. ACS Appl. Mater. Interfaces 2019, 11, 15709–15717. [Google Scholar] [CrossRef]
- Jiang, W.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.; Wang, J.; Hu, J.; Wei, Z.; Wan, L. Understanding the high activity of Fe-N-C electrocatalysts in oxygen reduction: Fe/Fe3C nanoparticles boost the activity of Fe-Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Strydom, C.; Collins, A.; Bunt, J. The influence of various potassium compound additions on the plasticity of a high-swelling South African coal under pyrolyzing conditions. J. Anal. Appl. Pyrolysis 2015, 112, 221–229. [Google Scholar] [CrossRef]
- Sergeev, D.; Yazhenskikh, E.; Kobertz, D.; Müller, M. Vaporization behavior of Na2CO3 and K2CO3. Calphad 2019, 65, 42–49. [Google Scholar] [CrossRef]
- Tachimoto, H.; Abe, I. Activated Carbon Application Technology—Its Maintenance Management and Problems; Southeast University Press: Nanjing, China, 2002; pp. 39–40. [Google Scholar]


| Samples | Proximate Analyses (wad/%) | Ultimate Analyses (wdaf/%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | Volatile Matter | C | H | S | N | O * | |
| RC (raw coal) | 5.31 | 5.75 | 30.13 | 83.11 | 4.81 | 0.22 | 0.73 | 11.13 |
| RC-K-900 | 1.28 | 10.86 | 5.49 | 85.64 | 0.33 | 0.13 | 0.41 | 13.49 |
| RC-2K-900 | 1.36 | 11.07 | 5.76 | 85.19 | 0.37 | 0.15 | 0.38 | 13.91 |
| Samples | 600 °C | 700 °C | 800 °C | 900 °C |
|---|---|---|---|---|
| RC | RC-600 | RC-700 | RC-800 | RC-900 |
| RC-K | RC-K-600 | RC-K-700 | RC-K-800 | RC-K-900 |
| RC-2K | RC-2K-600 | RC-2K-700 | RC-2K-800 | RC-2K-900 |
| Wavenumber (cm−1) | Peak Assignment |
|---|---|
| 3440 | O–H stretching vibrations |
| 2914 | –CH2 stretching vibration of aliphatic |
| 2853 | –CH3 stretching vibration of aliphatic |
| 1590 | C=C stretching vibrations of aromatic rings |
| 1060 | C–C, C–H stretching vibration of aromatic rings, C–O–C stretching vibration |
| 874, 805, 740 | C–H stretching vibration of aromatic rings |
| 590 | Si–O stretching vibrations, Si–O–Al compounded stretching vibrations, quartz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, Q. Preparation and Formation Mechanism of Carbon Nanotubes via Coal Pyrolysis Using Alkaline Potassium Catalysts. Nanomaterials 2025, 15, 1691. https://doi.org/10.3390/nano15221691
Zhang T, Wang Q. Preparation and Formation Mechanism of Carbon Nanotubes via Coal Pyrolysis Using Alkaline Potassium Catalysts. Nanomaterials. 2025; 15(22):1691. https://doi.org/10.3390/nano15221691
Chicago/Turabian StyleZhang, Tiankai, and Qi Wang. 2025. "Preparation and Formation Mechanism of Carbon Nanotubes via Coal Pyrolysis Using Alkaline Potassium Catalysts" Nanomaterials 15, no. 22: 1691. https://doi.org/10.3390/nano15221691
APA StyleZhang, T., & Wang, Q. (2025). Preparation and Formation Mechanism of Carbon Nanotubes via Coal Pyrolysis Using Alkaline Potassium Catalysts. Nanomaterials, 15(22), 1691. https://doi.org/10.3390/nano15221691






