High-Performance Ethylene Glycol Room-Temperature Gas Sensor Based on Biomass-Derived Na-Doped Porous Carbon Microtubules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of CMH Sample
2.3. Materials Characterization and Tests
2.4. Preparation and Sensing Test of CMH Sensors
3. Results and Discussion
3.1. Characterization Results
3.2. Gas-Sensing Properties
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shimizu, T.; Inui, M. Novel aspects of ethylene glycol catabolism. Appl. Microbiol. Biotechnol. 2024, 108, 369. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.R.; Zhao, Y.J.; Ma, X.B.; Gong, J.L. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zong, Y.; Zhao, T.T.; Zhu, W.H.; Zhong, L.C.; Huang, Z.Q.; Xu, M.; Liu, H. Ag decorated CuGaO2 nanosheets for enhanced ethylene glycol detection. J. Alloys Compd. 2024, 970, 172563. [Google Scholar] [CrossRef]
- Leth, P.M.; Gregersen, M. Ethylene glycol poisoning. Forensic Sci. Int. 2005, 155, 179–184. [Google Scholar] [CrossRef]
- Kou, H.R.; Shao, T.T.; Dong, J.T.; Zhang, F.C.; Tian, S.W.; Wang, X.Y. Highly sensitive ethylene glycol gas sensor based on MIL-68(In)@ZIF-8 derivative. ACS Sens. 2024, 9, 6580–6591. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, W.L.; Wang, C.W.; Li, G. Au nanoparticles decorated ZnIn2S4/WO3 core-shell heterostructures as highly sensitive and selective ethylene glycol gas sensors. J. Alloys Compd. 2024, 998, 175027. [Google Scholar] [CrossRef]
- Qu, X.H.; Li, M.C.; Mu, H.L.; Jin, B.B.; Song, M.G.; Zhang, K.L.; Wu, Y.S.; Li, L.S.; Yu, Y. Facile fabrication of lilac-like multiple self-supporting WO3 nanoneedle arrays with cubic/hexagonal phase junctions for highly sensitive ethylene glycol gas sensors. ACS Sens. 2024, 9, 3604–3615. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, W.H.; Zhong, L.C.; Guo, A.Y. Highly sensitive and selective ethylene glycol sensor based on Mg doped delafossite AgCrO2. Ceram. Int. 2024, 50, 16489–16498. [Google Scholar] [CrossRef]
- Wei, J.S.; Ma, S.Y.; Cai, Y.H.; Xu, C.Y.; Liu, J.M.; Jiang, H.T. A high-performance ethylene glycol sensor based on fibrous ErFeO3 prepared by electrostatic spinning. Ceram. Int. 2023, 49, 32611–32618. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Yang, L.X.; Li, W.K.; Li, X.B.; Liu, X.B.; Sun, S.; Hu, W.Y.; Liu, D.N.; Wang, Y.J.; Ma, S.Y. Study of high-performance glycol gas sensor based on BMO/In2O3 heterostructure. Ceram. Int. 2025, 51, 4661–4676. [Google Scholar] [CrossRef]
- Su, C.; Li, M.Y.; Zhang, Y.F.; Liu, T.Q.; Ren, C.; Li, P.P.; Yin, X.Q.; Zhang, L.; Zhang, M.; Wu, W.W. Boosting ethylene glycol sensing performance with dendritic hierarchical CuO/Co3O4 heterojunction nanowire. ACS Appl. Nano Mater. 2023, 6, 19249–19256. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Luo, K.; Peng, H.R.; Zhang, B.; Chen, L.M.; Zhang, P.P.; Peng, Z.J.; Fu, X.L. Advances in carbon nanotube-based gas sensors: Exploring the path to the future. Coord. Chem. Rev. 2024, 518, 216049. [Google Scholar] [CrossRef]
- Hughes, K.J.; Iyer, K.A.; Bird, R.E.; Ivanov, J.; Banerjee, S.; Georges, G.; Zhou, Q.A. Review of carbon nanotube research and development: Materials and emerging applications. ACS Appl. Nano Mater. 2024, 7, 18695–18713. [Google Scholar] [CrossRef]
- Cao, S.; Wu, Z.F.; Sun, Q.H.; Zhang, W.Y.; Beysen, S.; Wang, S.Y.; Shaymurat, T.; Zhang, M.; Duan, H.M. Gas sensing properties of cotton-based carbon fibers and ZnO/carbon fibers regulated by changing carbonization temperatures. Sens. Actuators B Chem. 2021, 337, 129818. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, M.C.; Shen, X.Y.; Wang, H.M.; Wang, H.M.; Xia, K.L.; Yin, Z.; Zhang, Y.Y. Biomass-derived carbon materials: Controllable preparation and versatile applications. Small 2021, 17, 2008079. [Google Scholar] [CrossRef]
- Zou, Y.H.; Chen, S.; Sun, J.; Liu, J.Q.; Che, Y.K.; Liu, X.H.; Zhang, J.; Yang, D.J. Highly efficient gas sensorusing a hollow SnO2 microfiber for triethylamine detection. ACS Sens. 2017, 2, 897–902. [Google Scholar] [CrossRef]
- Sun, J.M.; Wu, Z.W.; Ma, C.H.; Xu, M.C.; Luo, S.; Li, W.; Liu, S.X. Biomass-derived tubular carbon materials: Progress in synthesis and applications. J. Mater. Chem. A 2021, 9, 13822–13850. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, W.Y.; Sun, Q.H.; Tian, N.; Sun, J.; Wu, Z.F. High-performance room temperature N2H4 sensor based on tree pathogen derived self-doped N porous carbon. Mater. Today Chem. 2025, 43, 102499. [Google Scholar] [CrossRef]
- Juang, T.Y.; Kao, J.C.; Wang, J.C.; Hsu, S.Y.; Chen, C.P. Carbonized bamboo-derived carbon nanodots as efficient cathode interfacial layers in high-performance organic photovoltaics. Adv. Mater. Interfaces 2018, 5, 1800031. [Google Scholar] [CrossRef]
- Qin, Z.J.; Wu, Z.F.; Sun, Q.H.; Sun, J.; Zhang, M.; Chen, F.J.; Zhang, D.Z.; Lv, C.W.; Duan, H.M. Dog nose-inspired high-performance ammonia sensor based on biochar/SnO2 composite. Carbon 2023, 213, 118297. [Google Scholar] [CrossRef]
- Sinha, R.; Kiran, P.S.; Kumar, K.V.; Pandit, N.; Satish, C.; Keshri, S.; Keshri, A.K. MXene/Biomass-derived activated carbon composite for supercapacitor applications. Carbon 2025, 236, 120101. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, R.; Wang, H.; Zhou, S.; Pan, Z.; Huang, Y.; Sun, D.; Tang, Y.; Ji, X.; Amine, K.; et al. Revealing the closed pore formation of waste wood-derived hard carbon for advanced sodium-ion battery. Nat. Commun. 2023, 14, 6024. [Google Scholar] [CrossRef] [PubMed]
- Reshma, R.P.; Abishek, N.S.; Gopalakrishna, K.N. Synthesis and characterization of graphene oxide, tin oxide, and reduced graphene oxide-tin oxide nanocomposites. Inorg. Chem. Commun. 2024, 165, 112451. [Google Scholar] [CrossRef]
- Sun, T.; Gao, C.; Tan, X.; Zhang, N.; Xie, G.; Shi, Z.; Yang, Z.; Wang, T.; Wu, Y. High-value utilization of corn plants derived biomass carbon materials for potassium ion storage. Sustain. Mater. Technol. 2025, 44, e01359. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, L.; Wan, S.; Liu, M.; Niu, X.; Wang, K.; Li, H. Wedelia chinensis-derived biomass porous carbon as anode material for high performance sodium/potassium-ion batteries. Ionics 2024, 30, 4655–4664. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.T.; Ren, C.; Zeng, M.; Zhou, Z.H.; Su, Y.J.; Hu, N.T.; Wei, H.; Yang, Z. Controllable synthesis of heterostructured CuO-NiO nanotubes and their synergistic effect for glycol gas sensing. Sens. Actuators B Chem. 2020, 304, 127347. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Li, M.C.; Li, H.F.; Ping, Z.H.; Wang, P.J.; Wu, Y.S.; Li, L.S. La-doped micro-angular cube ZnSnO3 with nano-La2O3 decoration for enhanced ethylene glycol sensing performance at low temperature. Sens. Actuators A Phys. 2023, 362, 114649. [Google Scholar] [CrossRef]
- Shen, S.K.; Xin, Y.Y.; Jiang, H.Y.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Biomass-derived graphitic carbon/MoO3 nanosheets-crosslinked tubes for high response and rapid detection of butyl mercaptan at low temperatures. Sens. Actuators B Chem. 2025, 435, 137655. [Google Scholar] [CrossRef]
- He, H.L.; Liu, J.J.; Liu, H.D.; Pan, Q.J.; Zhang, G. The development of high-performance room temperature NOX one-dimensional Na0.23TiO2/TiO2 compound gas sensor. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 648, 129444. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Polyakov, A.Y.; Eriksson, J.; Antosiewicz, T.J.; Shegai, T.O. Humidity-enhanced NO2 gas sensing using atomically sharp edges in multilayer MoS2. Small Struct. 2025, 6, 2400409. [Google Scholar] [CrossRef]
- Cao, N.; Zhao, Y.; Chen, H.; Huang, J.; Yu, M.; Bao, Y.; Wang, D.; Cui, S. Poly(ethylene glycol) becomes a supra-polyelectrolyte by capturing hydronium ions in water. Macromolecules 2022, 55, 4656–4664. [Google Scholar] [CrossRef]
- Ding, J.J.; Dai, H.F.; Chen, H.X.; Jin, Y.X.; Fu, H.W.; Xiao, B. Highly sensitive ethylene glycol gas sensor based on ZnO/rGO nanosheets. Sens. Actuators B Chem. 2022, 372, 132655. [Google Scholar] [CrossRef]
- Liu, M.M.; Ma, S.Y.; Wang, L.; Cai, Y.H.; Ma, N.N. Highly sensitive and selective glycol gas sensor based on SmFeO3 microspheres. Ceram. Int. 2023, 49, 1108–1113. [Google Scholar] [CrossRef]
- Liu, M.M.; Ma, S.Y.; Cai, Y.H.; Ma, N.N.; Wang, L.; Sheng, H. ZnO/ZnCo2O4 composite prepared by one-step hydrothermal method for high-performance ethylene glycol sensor. Ceram. Int. 2022, 48, 22305–22312. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ma, S.Y.; Li, X.B.; Wang, D.; Hu, W.Y.; Li, S.Y.; Liu, D.N.; Wang, Y.J. Sm3+doped Bi2MoO6 microspheres with enhanced ethylene glycol sensing performance. Ceram. Int. 2024, 50, 2788–2799. [Google Scholar] [CrossRef]
- Zhou, T.T.; Cao, S.; Zhang, R.; Tu, J.C.; Fei, T.; Zhang, T. Effect of cation substitution on the gas-sensing performances of ternary spinel MCo2O4 (M = Mn, Ni, and Zn) multishelled hollow twin spheres. ACS Appl. Mater. Interfaces 2019, 11, 28023–28032. [Google Scholar] [CrossRef]
- Li, X.; Tang, X.; Wang, Z.; Xu, Y.; Wei, W.; He, Y.; Li, H. N/p-type doping modulation of the adsorption and selection behavior of harmful gas molecules on the surface of SWCNTs for enhanced gas-sensing performance. ACS Sens. 2025, 10, 2790–2801. [Google Scholar] [CrossRef]
- Ran, B.; Hu, P.; Sun, J.; Fang, J.; Sun, Q.; Wang, J.; Zhu, Y.; Tian, N.; Wu, Z.; Duan, H. Self-doped Na-carbon materials derived from a lyocell fiber for a high-performance trimethylamine gas sensor at room temperature. J. Hazard. Mater. 2024, 480, 136289. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Hu, J.; Rong, X.; Zhang, W.; Wang, Y.; Li, S.; Li, G.; Wang, D. Facile engineering of metal–organic framework derived SnO2@NiO core–shell nanocomposites based gas sensor toward superior VOCs sensing performance. Chem. Eng. J. 2024, 501, 157692. [Google Scholar] [CrossRef]
- Huang, B.; Sun, S.; Li, X.; Li, X.; Wang, N.; Li, X. Layered MoTe2/ZnO heterojunctions for sensitive TEA sensors at room temperature. Sens. Actuators B Chem. 2025, 439, 137862. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Chen, Y.; Bai, Y.; Ye, S.; Zhang, S.; Chen, X.; Wang, P.; Li, A.; Li, L.; et al. MOF derived Pt-In2O3 hollow microtube for ultrasensitive ppb-level detection of p-xylene and aging characteristics analysis. Chin. Chem. Lett. 2025, in press. [Google Scholar] [CrossRef]
- Liang, Y.; Ru, C.; Tang, H.; Zhao, X.; Tang, L.; Yao, S.; Yang, Y. Enhanced spatial charge separation and gas adsorption in Bi2WO6 facet junction for efficient visible light-excited gas detection at room temperature. J. Materiomics. 2025, 11, 101062. [Google Scholar] [CrossRef]
- Shi, Z.; Qiao, L.; Ma, M.; Jia, Z.; Liu, B.; Gao, L. N-doped clay-like Ti3C2Tx MXene/N-doped CNTs composites for ethylene glycol detection in ambient air and exhaled breath. Electrochem. Commun. 2024, 164, 107738. [Google Scholar] [CrossRef]
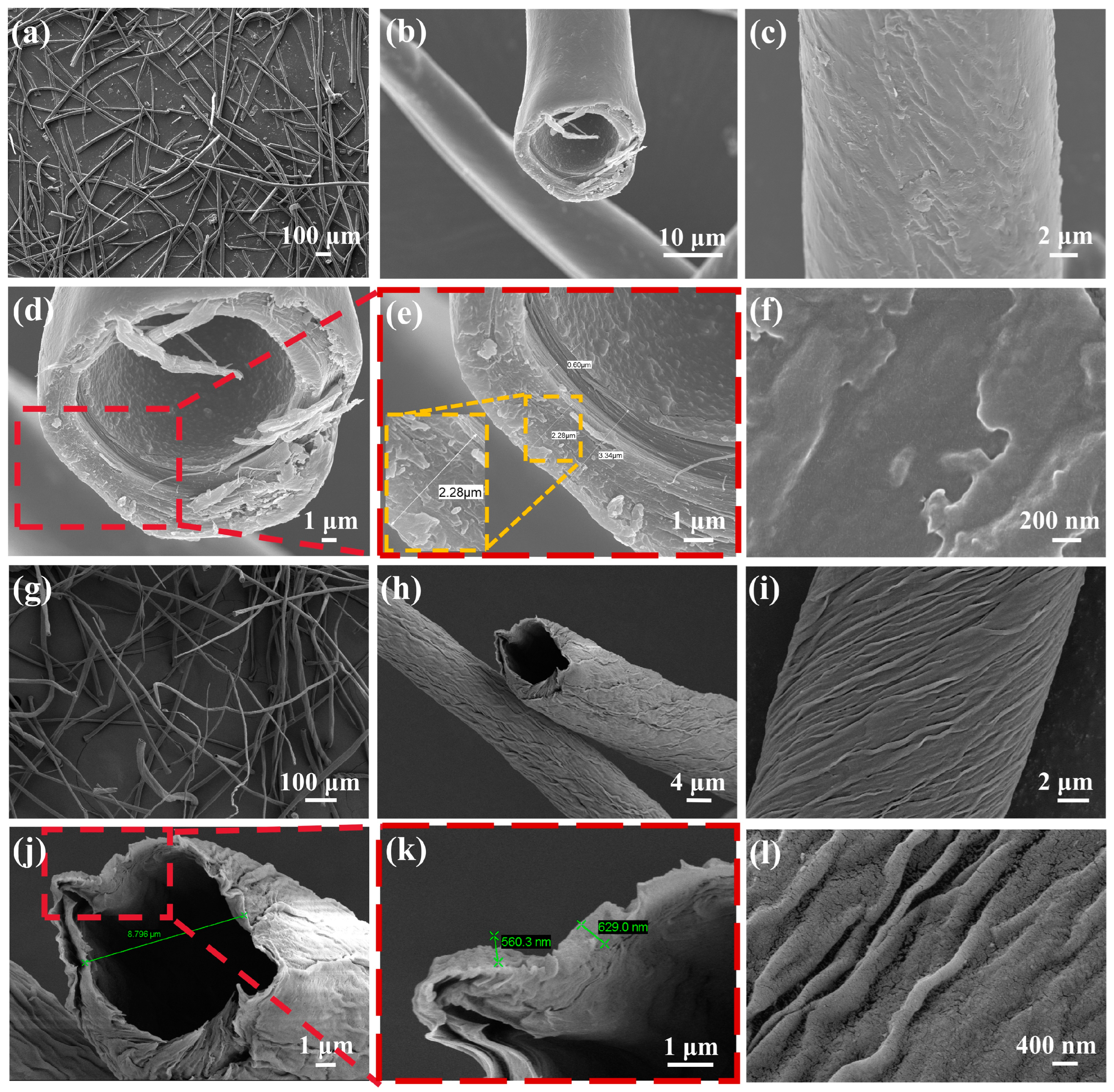
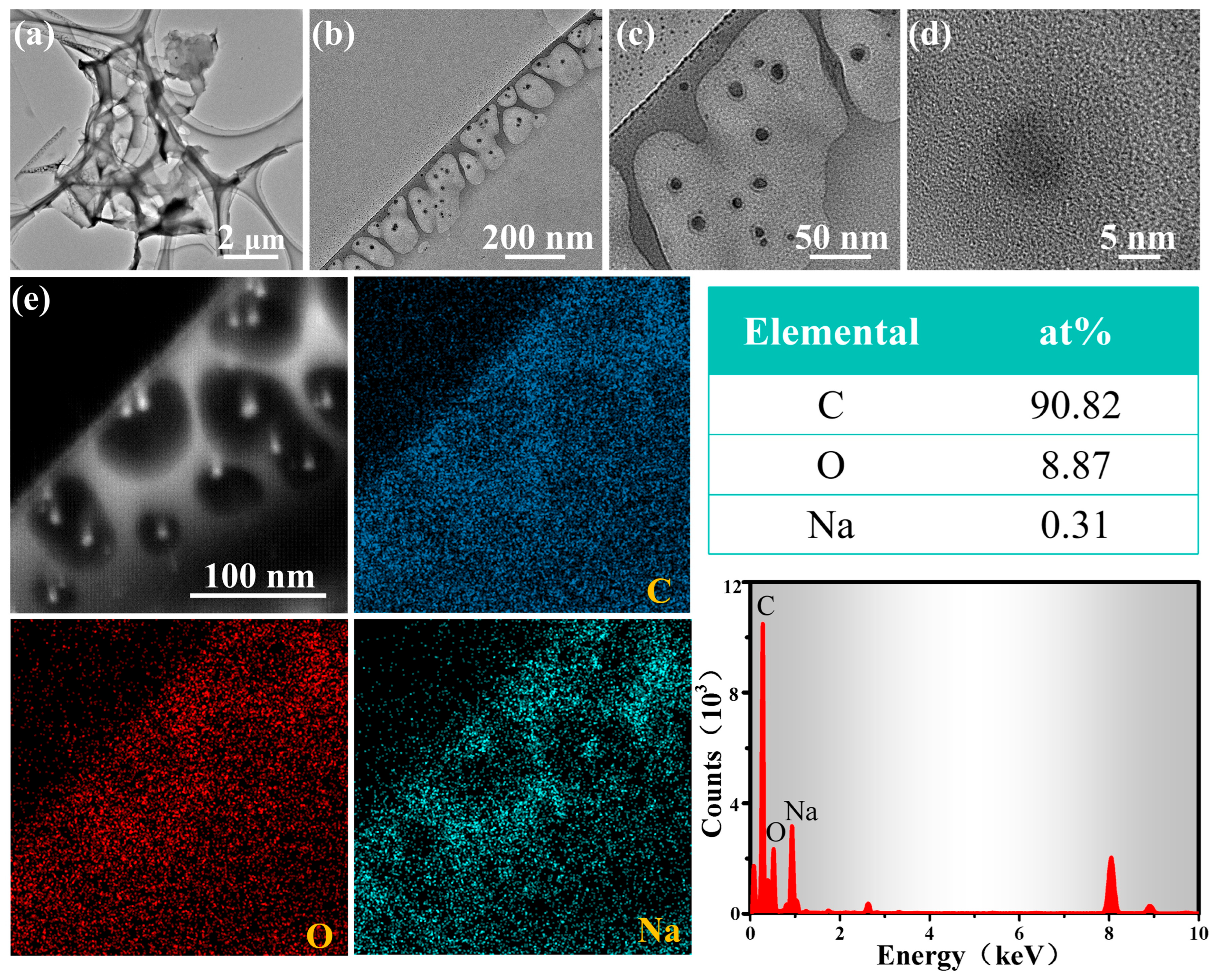
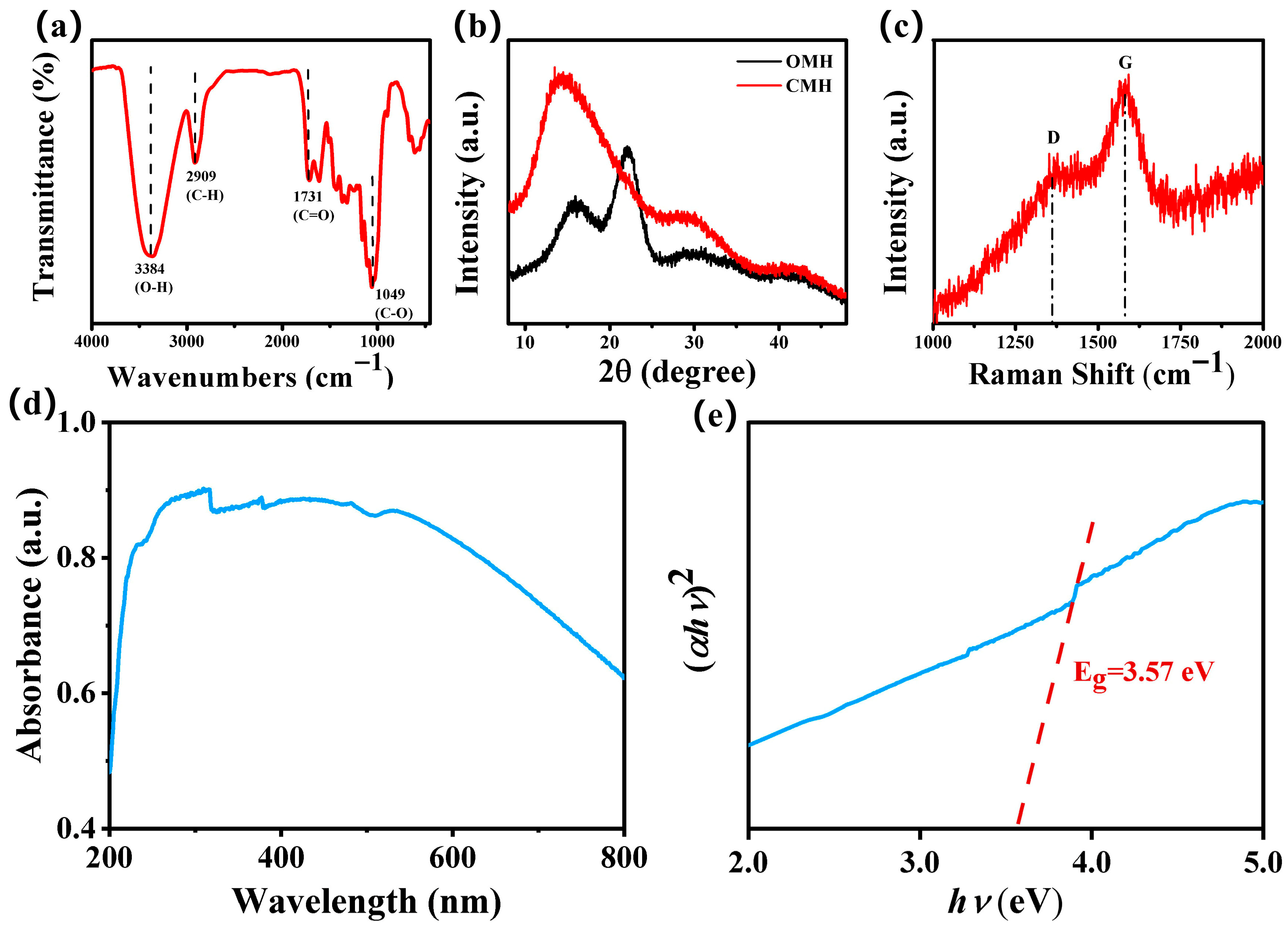
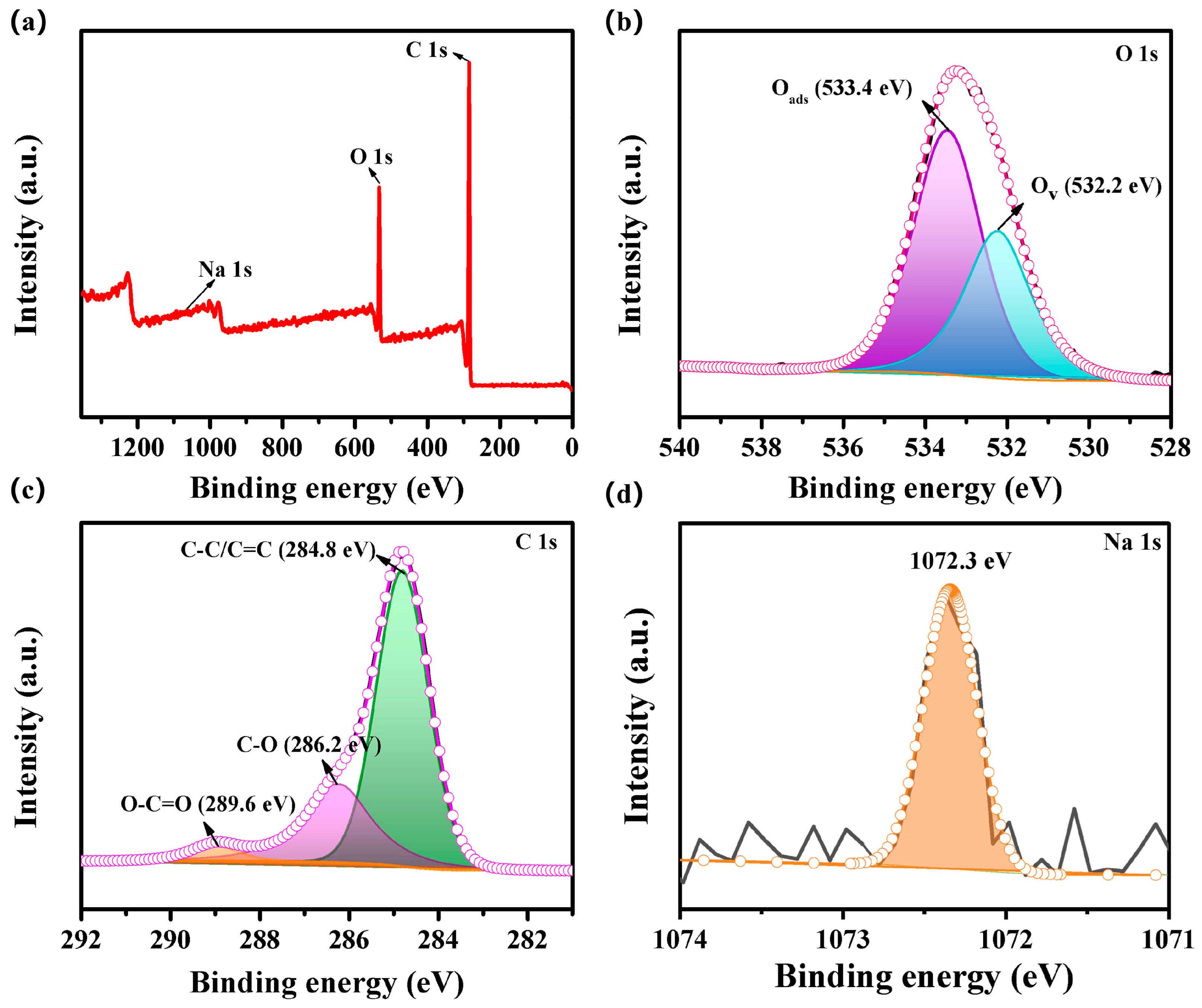
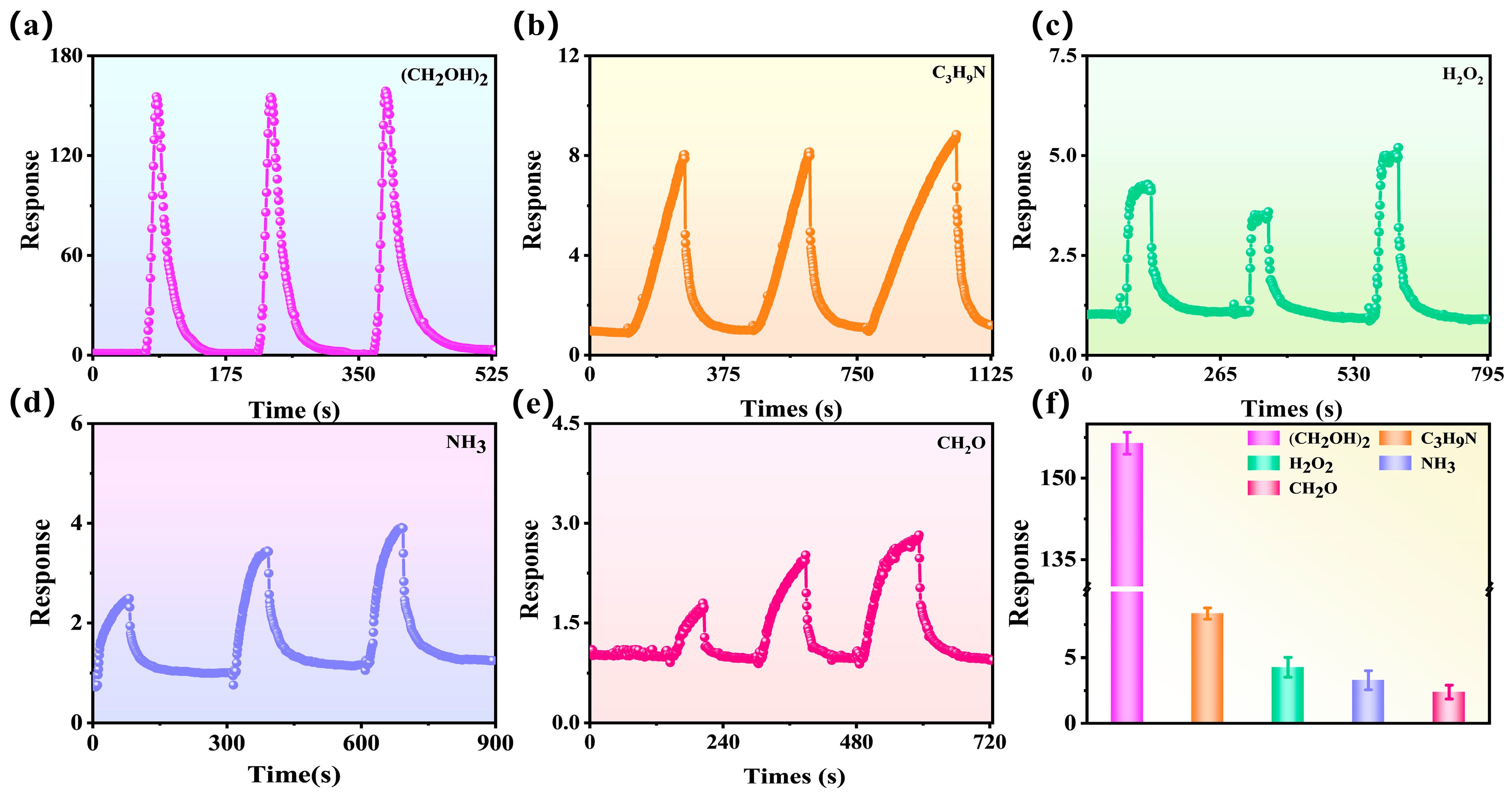
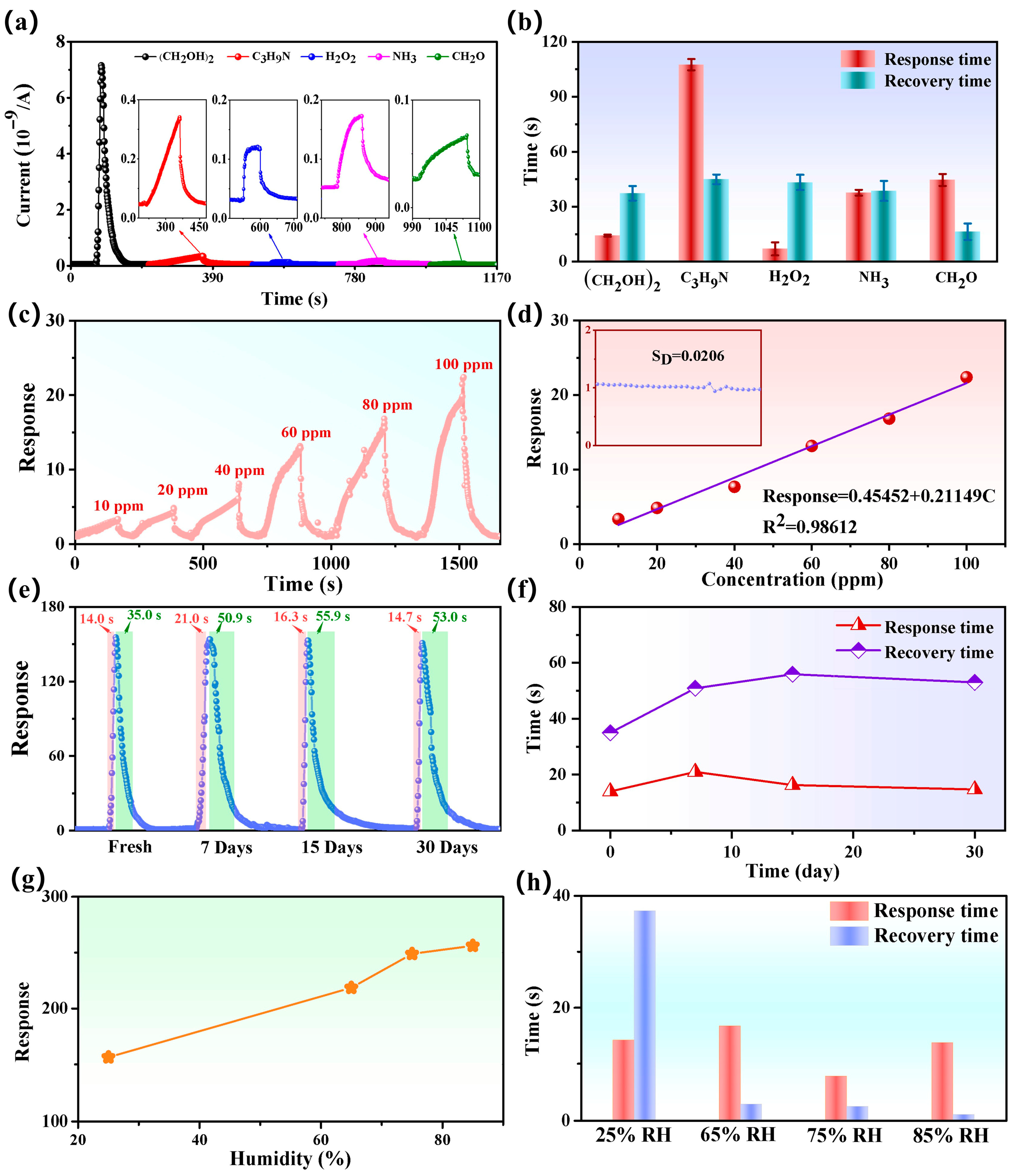
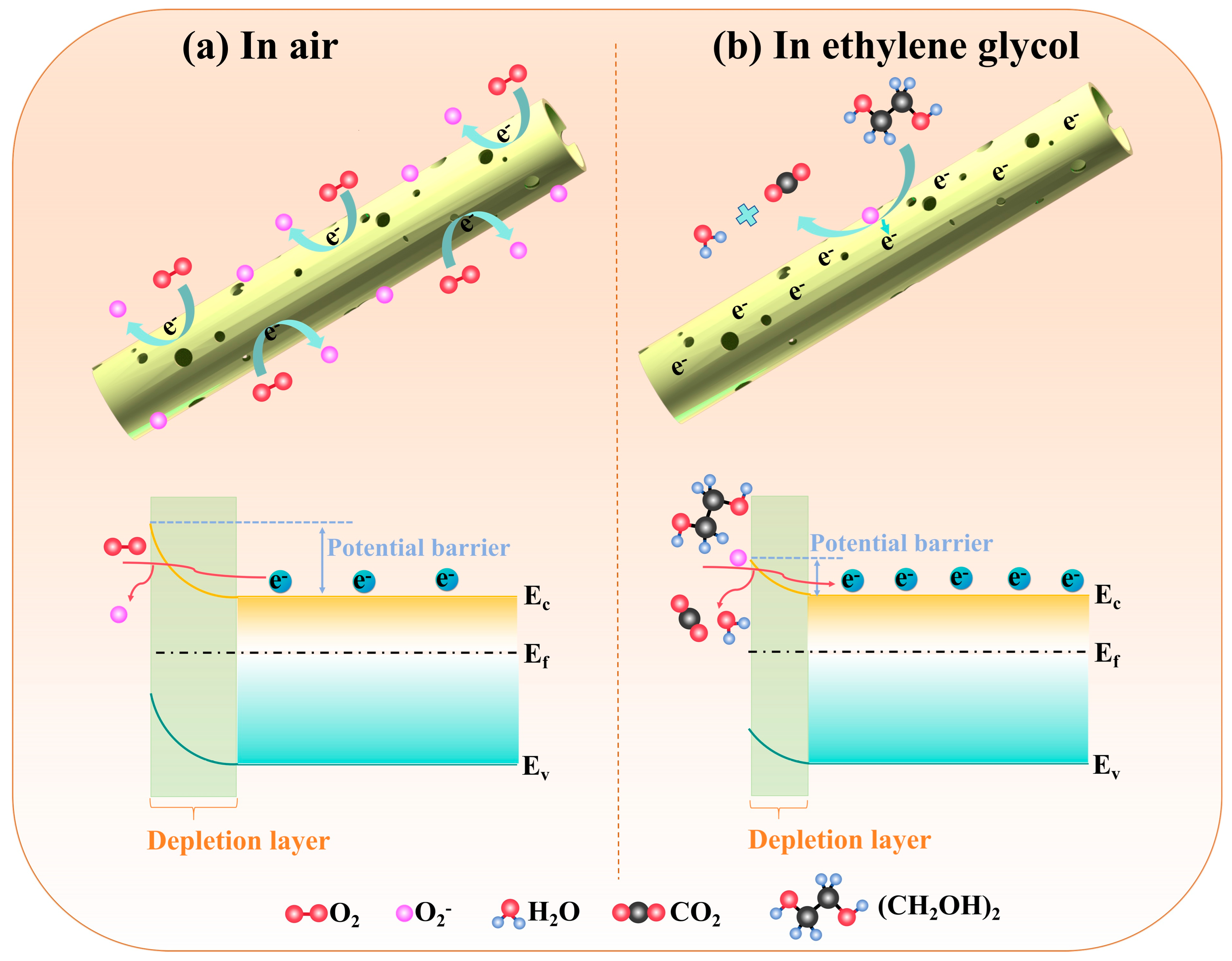

| Sensing Materials | Temp. (°C) | Con. (ppm) | Res. (Ra/Rg) | Tres/Trec (s) | LoD (ppm) | Ref. |
|---|---|---|---|---|---|---|
| ZnO/rGO | 220 | 100 | 277 | 38/26 | 1 a | [33] |
| ErFeO3 | 230 | 100 | 15.8 | 61/39 | 0.035 b | [9] |
| BMO/In2O3 | 220 | 100 | 38 | 26/129 | 1 a | [10] |
| SmFeO3 | 180 | 100 | 117 | 80/95 | 5 a | [34] |
| In2O3@ZnO | 200 | 200 | 200.12 | 53/50 | 1 a | [5] |
| AgCrO2 | 120 | 10 | 16.2 | 32/130 | 1 a | [8] |
| ZnO/ZnCo2O4 | 160 | 100 | 15.63 | 190/45 | 1.59 b | [35] |
| WO3 | 160 | 100 | 2305 | 107/215 | 1 a | [7] |
| Au/ZnIn2S4/WO3 | 220 | 50 | 98.92 | 57/136 | 0.006 b | [6] |
| La-doped ZnSnO3 | 140 | 100 | 1488.79 | — | 0.2 a | [28] |
| Sm3+ doped Bi2MoO6 | 240 | 100 | 60.1 | 20/78 | 5 a | [36] |
| CuO/Co3O4 | 130 | 100 | 6.3 | 33/54 | 5 a | [11] |
| Ag decorated CuGaO2 | 165 | 10 | 3.46 | 15/30 | 0.067 b | [3] |
| CMH | 25 | 100 | 22.4 c | 135.6/56.9 | 0.292 b | This work |
| 500 | 155.4 c | 14.7/37.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Sun, Q.; Li, J.; Wu, Z.; Duan, H. High-Performance Ethylene Glycol Room-Temperature Gas Sensor Based on Biomass-Derived Na-Doped Porous Carbon Microtubules. Nanomaterials 2025, 15, 1686. https://doi.org/10.3390/nano15221686
Xu Y, Sun Q, Li J, Wu Z, Duan H. High-Performance Ethylene Glycol Room-Temperature Gas Sensor Based on Biomass-Derived Na-Doped Porous Carbon Microtubules. Nanomaterials. 2025; 15(22):1686. https://doi.org/10.3390/nano15221686
Chicago/Turabian StyleXu, Yan, Qihua Sun, Jialin Li, Zhaofeng Wu, and Haiming Duan. 2025. "High-Performance Ethylene Glycol Room-Temperature Gas Sensor Based on Biomass-Derived Na-Doped Porous Carbon Microtubules" Nanomaterials 15, no. 22: 1686. https://doi.org/10.3390/nano15221686
APA StyleXu, Y., Sun, Q., Li, J., Wu, Z., & Duan, H. (2025). High-Performance Ethylene Glycol Room-Temperature Gas Sensor Based on Biomass-Derived Na-Doped Porous Carbon Microtubules. Nanomaterials, 15(22), 1686. https://doi.org/10.3390/nano15221686






