Waste Oyster Shell/Graphene Oxide Composite as a Dual-Functional Soil Conditioner and SRF: Impacts on Soil pH and Nutrient Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GO Sheet
2.2. Preparation of OSF-GO Particle
2.3. Characterization
2.4. Seedling Exposure
2.5. Pot Experiments
2.6. Statistical Analysis of Data
3. Results and Discussion
3.1. Morphology and Structure of GO Sheet
3.2. SEM-EDS Analysis of OSF-GO Particle
3.3. Evaluation of Growth Parameters in Hydroponic Systems
3.3.1. Effect of GO Concentration on Growth and Development of Loose-Leaf Lettuce Seedlings
3.3.2. Effect of GO Concentration on Root Growth
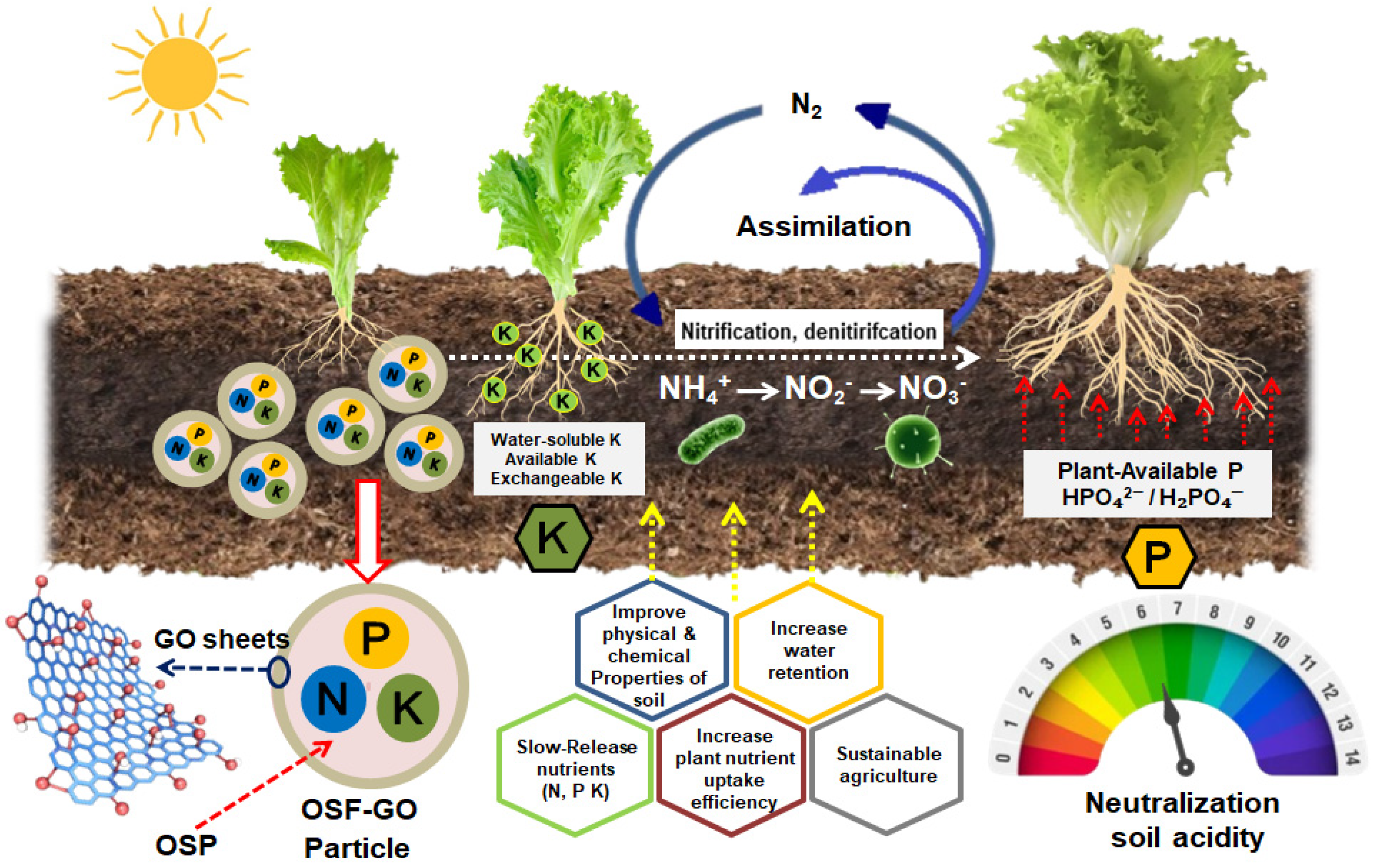
3.4. Functional Test of OSF-GO Particles in Pot Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Zhou, X.; Khashi u Rahman, M.; Liu, J.; Wu, F. Soil acidification mediates changes in soil bacterial community assembly processes in response to agricultural intensification. Environ. Microbiol. 2021, 23, 4741–4755. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tu, Q.; Zheng, S.; Deng, S.; Xia, Y.; Mao, W.; Gao, W.; Hu, L.; Kuzyakov, Y.; Hu, Y.; et al. Soil acidification induced by intensive agricultural use depending on climate. J. Soils Sediments 2022, 22, 2604–2607. [Google Scholar] [CrossRef]
- Xie, X.; Qiu, J.; Feng, X.; Hou, Y.; Wang, S.; Jia, S.; Liu, S.; Hou, X.; Dou, S. Spatial Distribution and Estimation Model of Soil pH in Coastal Eastern China. Int. J. Environ. Res. Public Health 2022, 19, 16855. [Google Scholar] [CrossRef] [PubMed]
- Guangdong Province Cultivated Land Fertilizer Station. Current situation of cultivated land in Guangdong Province and countermeasures for quality improvement. Agric. Compr. Dev. China 2020, 3, 56–57. [Google Scholar]
- Zheng, H.; Xiao, H.; Ye, X.; Zheng, X. Soil problems occurring in the economic development of Guangdong and the tactics of resolution. Soil. Environ. Sci. 2001, 10, 230–233. [Google Scholar]
- Yu, S.; Hou, X.; Huan, C.; Mu, Y. Comments on the oyster aquaculture industry in China: 1985–2020. Thalass. Int. J. Mar. Sci. 2023, 39, 875–882. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Laohavisuti, N.; Thompho, S.; Boonmee, W.; Mongkol, S.; Rungrojchaipon, P. Bio-green synthesis of calcium acetate from oyster shell waste at low cost and reducing the emission of greenhouse gases. Sustain. Environ. Res. 2023, 33, 26. [Google Scholar] [CrossRef]
- Zhan, J.; Lu, J.; Wang, D. Review of shell waste reutilization to promote sustainable shellfish aquaculture. Rev. Aquac. 2022, 14, 477–488. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, M.; Wu, M.; Weng, L.; Wang, Y.; Hu, L.; Cao, M.J. Toward green farming technologies: A case study of oyster shell application in fruit and vegetable production in Xiamen. Sustainability 2022, 15, 663. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Huang, Y.; Zhuang, X.; Xu, S.; Zhuang, S.; Fang, H.; Cao, M. Improvement effect on acidic soil and the quality of guanxi pomelo by calcined oyster shell powder. J. Jimei Univ. Nat. Sci. 2020, 25, 256–264. [Google Scholar]
- Choi, J.S.; Lee, H.J.; Jin, S.K.; Lee, H.J.; Choi, Y.I. Effect of oyster shell calcium powder on the quality of restructured pork ham. Korean J. Food Sci. Anim. Resour. 2014, 34, 372. [Google Scholar] [CrossRef]
- Lian, W.; Li, H.; Yang, J.; Joseph, S.; Bian, R.; Liu, X.; Zheng, J.; Drosos, M.; Zhang, X.; Li, L.; et al. Influence of pyrolysis temperature on the cadmium and lead removal behavior of biochar derived from oyster shell waste. Bioresour. Technol. Rep. 2021, 15, 100709. [Google Scholar] [CrossRef]
- Yang, X.; Liu, K.; Wen, Y.; Huang, Y.; Zheng, C. Application of Natural and Calcined Oyster Shell Powders to Improve Latosol and Manage Nitrogen Leaching. Int. J. Environ. Res. Public Health 2023, 20, 3919. [Google Scholar] [CrossRef]
- Bolan, N.; Srivastava, P.; Rao, C.S.; Satyanaraya, P.V.; Anderson, G.C.; Bolan, S.; Nortjé, G.P.; Kronenberg, R.; Bardhan, S.; Abbott, L.K.; et al. Distribution, characteristics and management of calcareous soils. Adv. Agron. 2023, 182, 81–130. [Google Scholar]
- Raiesi Ardali, T.; Ma’mani, L.; Chorom, M.; Motamedi, E.; Fathi Gharebaba, M. A biocompatible NPK+ Fe+ Zn slow release fertilizer: Synthesis and its evaluation in tomato plant growth improvement. Sci. Rep. 2024, 14, 4640. [Google Scholar] [CrossRef]
- Kabiri, S.; Degryse, F.; Tran, D.N.; da Silva, R.C.; McLaughlin, M.; Losic, D. Graphene oxide: A new carrier for slow release of plant micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325–43335. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Jin, L.; Yang, K.; Yao, K.; Zhang, S.; Tao, H.; Lee, S.T.; Peng, R. Functionalized graphene oxide in enzyme engineering: A selective modulator for enzyme activity and thermostability. ACS Nano 2012, 6, 4864–4875. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.G.; Costa, V.O.; Martinez, A.H.; Régnier, B.M.; Gomes, G.C.; Zarbin, A.J.; Orth, E.S. Functionalization of graphene oxide via epoxide groups: A comprehensive review of synthetic routes and challenges. Front. Carbon 2024, 3, 1393077. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, X.; Bao, H.; Yang, Z.; Ma, F. The effects of hydroxide and epoxide functional groups on the mechanical properties of graphene oxide and its failure mechanism by molecular dynamics simulations. RSC Adv. 2020, 10, 29610–29617. [Google Scholar] [CrossRef]
- Das, P.; Penton, C.R.; Westerhoff, P.; Perreault, F. Prospects of 2D graphene nanomaterials in plant-based agriculture and their fate in terrestrial soils: A critical review. Environ. Sci. Nano 2023, 10, 2936–2956. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Zhang, X.; Chen, Z.; Wang, H.; Li, P.C.H. Effects of graphene oxide on plant growth: A review. Plants 2022, 11, 2826. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Xing, W.; Sitharaman, B. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J. Mater. Chem. B 2014, 2, 6354–6362. [Google Scholar] [CrossRef] [PubMed]
- Shekari, F.; Abbasi, A.; Mustafavi, S.H. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J. Saudi Soc. Agric. Sci. 2017, 16, 367–374. [Google Scholar] [CrossRef]
- Cheng, H.; He, Y.; Xian, Y.; Hao, X. Performance and Evaluation of Slow-Release Fertilizer Encapsulated by Waterless Synthesized GO Sheets. Coatings 2024, 14, 1215. [Google Scholar] [CrossRef]
- DB64/T 1734–2020; Determination of Soil Hydrolyzable Nitrogen by Kjeldahl Method. Ningxia Hui Autonomous Region Department of Agriculture and Rural Affairs: Yinchuan, China, 2020.
- NY/T 1121.7-2014; Soil Testing—Part 7: Method for Determination of Available Phosphorus in Soil. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2014.
- NY/T 889-2004; Determination of Exchangeable Potassium Andnon—Exchangeable Potassium Content in Soil. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2004.
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Mei, X.; Gao, M.; Wang, K.; Zhao, D. Direct laser writing of MnO2 decorated graphene as flexible supercapacitor electrodes. J. Mater. Sci. 2020, 55, 17108–17119. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Lamberti, A.; Perrucci, F.; Caprioli, M.; Serrapede, M.; Fontana, M.; Bianco, S.; Ferrero, S.; Tresso, E. New insights on laser-induced graphene electrodes for flexible supercapacitors: Tunable morphology and physical properties. Nanotechnology 2017, 28, 174002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Ren, J.; Qiao, J.; Zhao, J.G.; Li, J.W.; Liu, Z.H.; Li, W.; Xing, B.; Zhang, J.; Nie, H. Influence of functionalized graphene on the bacterial and fungal diversity of Vicia faba rhizosphere soil. New Carbon. Mater. 2024, 39, 1227–1242. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y.; Creamer, A.E.; Chen, H. Slow-release fertilizer encapsulated by graphene oxide films. Chem. Eng. J. 2014, 255, 107–113. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Xiao, K.; Xu, Y.; Cao, X.; Xu, H.; Li, Y. Advanced characterization of membrane surface fouling. In 60 Years of the Loeb-Sourirajan Membrane; Elsevier: Amsterdam, The Netherlands, 2022; pp. 499–532. [Google Scholar]
- Zhang, M.; Parajuli, R.R.; Mastrogiovanni, D.; Dai, B.; Lo, P.; Cheung, W.; Brukh, R.; Chiu, P.L.; Zhou, T.; Liu, Z.; et al. Production of graphene sheets by direct dispersion with aromatic healing agents. Small 2010, 6, 1100–1107. [Google Scholar] [CrossRef]
- Park, S.; Choi, K.S.; Kim, S.; Gwon, Y.; Kim, J. Graphene oxide-assisted promotion of plant growth and stability. Nanomaterials 2020, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Hua, S.; Zhu, L.; Nie, P.; Yang, C.; Xu, F.; Wu, D.; Dong, W. Application of graphene oxide in Agrobacterium-mediated genetic transformation and construction of a novel DNA delivery system for watermelon. Sci. Rep. 2025, 15, 5465. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, W.; Chen, X.; Gao, Y.; Wu, X.; Ding, M.; Duo, L. Graphene oxide affected root growth, anatomy, and nutrient uptake in alfalfa. Ecotoxicol. Environ. Saf. 2023, 250, 114483. [Google Scholar] [CrossRef]
- Shen, S.; Liu, Y.; Wang, F.; Yao, G.; Xie, L.; Xu, B. Graphene oxide regulates root development and influences IAA concentration in rice. J. Plant Growth Regul. 2019, 38, 241–248. [Google Scholar] [CrossRef]
- Gao, M.; Chang, X.; Yang, Y.; Song, Z. Foliar graphene oxide treatment increases photosynthetic capacity and reduces oxidative stress in cadmium-stressed lettuce. Plant Physiol. Biochem. 2020, 154, 287–294. [Google Scholar] [CrossRef]
- O’Kennedy, S. Soil pH and its impact on nutrient availability and crop growth. Int. J. Geogr. Geol. Environ. 2022, 4, 236–238. [Google Scholar]
- GB15618-2018; National Standard Soil Environmental Quality Risk Control Standard for Soil Contamination of Agri-cultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Khaled, F.; Sayed, A. Soil pH and its influence on nutrient availability and plant health. Int. J. Adv. Chem. Res. 2023, 5, 68–70. [Google Scholar] [CrossRef]
- Oshunsanya, S.O. Introductory chapter: Relevance of soil pH to agriculture. Soil PH Nutr. Availab. Crop Perform. 2018, 3–6. [Google Scholar]
- Keen, G.A.; Prosser, J.I. Interrelationship between pH and surface growth of Nitrobacter. Soil. Biol. Biochem. 1987, 19, 665–672. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Halli, H.M.; Sujayananad, G.K.; Vadivel, R.; Das, T.K.; Raj, R.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Kvakić, M.; Tzagkarakis, G.; Pellerin, S.; Ciais, P.; Goll, D.; Mollier, A.; Ringeval, B. Carbon and phosphorus allocation in annual plants: An optimal functioning approach. Front. Plant Sci. 2020, 11, 149. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Yi, C.; Zhu, J.; Chen, L.; Huang, X.; Wu, R.; Zhang, H.; Dai, X.; Liang, J. Speciation of iron and aluminum in relation to phosphorus sorption and supply characteristics of soil aggregates in subtropical forests. Forests 2023, 14, 1804. [Google Scholar] [CrossRef]
- Szczerba, M.W.; Britto, D.T.; Kronzucker, H.J. K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 2009, 166, 447–466. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Syarifah, R.N.K.; Purwanto, P.; Sarjito, A.; Hanifa, H.; Bayyinah, L.N.I. The interaction of nitrogen and potassium nutrients in increasing growth, yield, and quality of sweet corn products. Kultivasi 2025, 24. [Google Scholar] [CrossRef]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Li, C.; Zameer, R.; Liu, L.; Wen, Q.; Zheng, Y.; Zheng, J.; Yu, C.; Song, G.; Song, C.P.; Li, Z.; et al. Harnessing the acid growth theory to optimize apoplastic acidification for enhancing cotton fiber elongation. Plant Commun. 2025, 6, 101390. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Han, T.F.; Huang, J.; Asad, S.; Li, D.M.; Yu, X.C.; Huang, Q.H.; Ye, H.C.; Hu, H.W.; Hu, Z.H.; et al. Links between potassium of soil aggregates and pH levels in acidic soils under long-term fertilization regimes. Soil. Tillage Res. 2020, 197, 104480. [Google Scholar] [CrossRef]


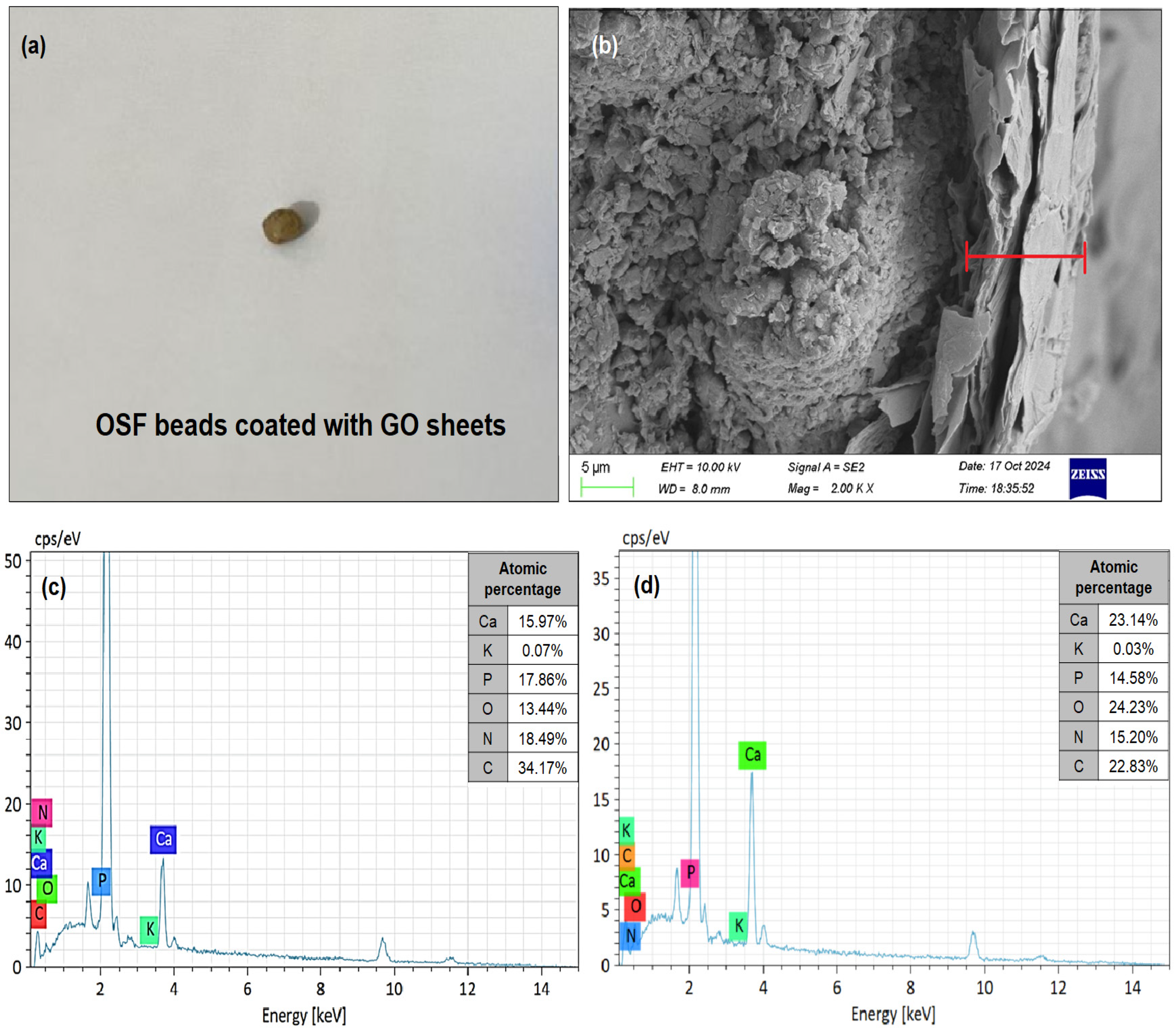
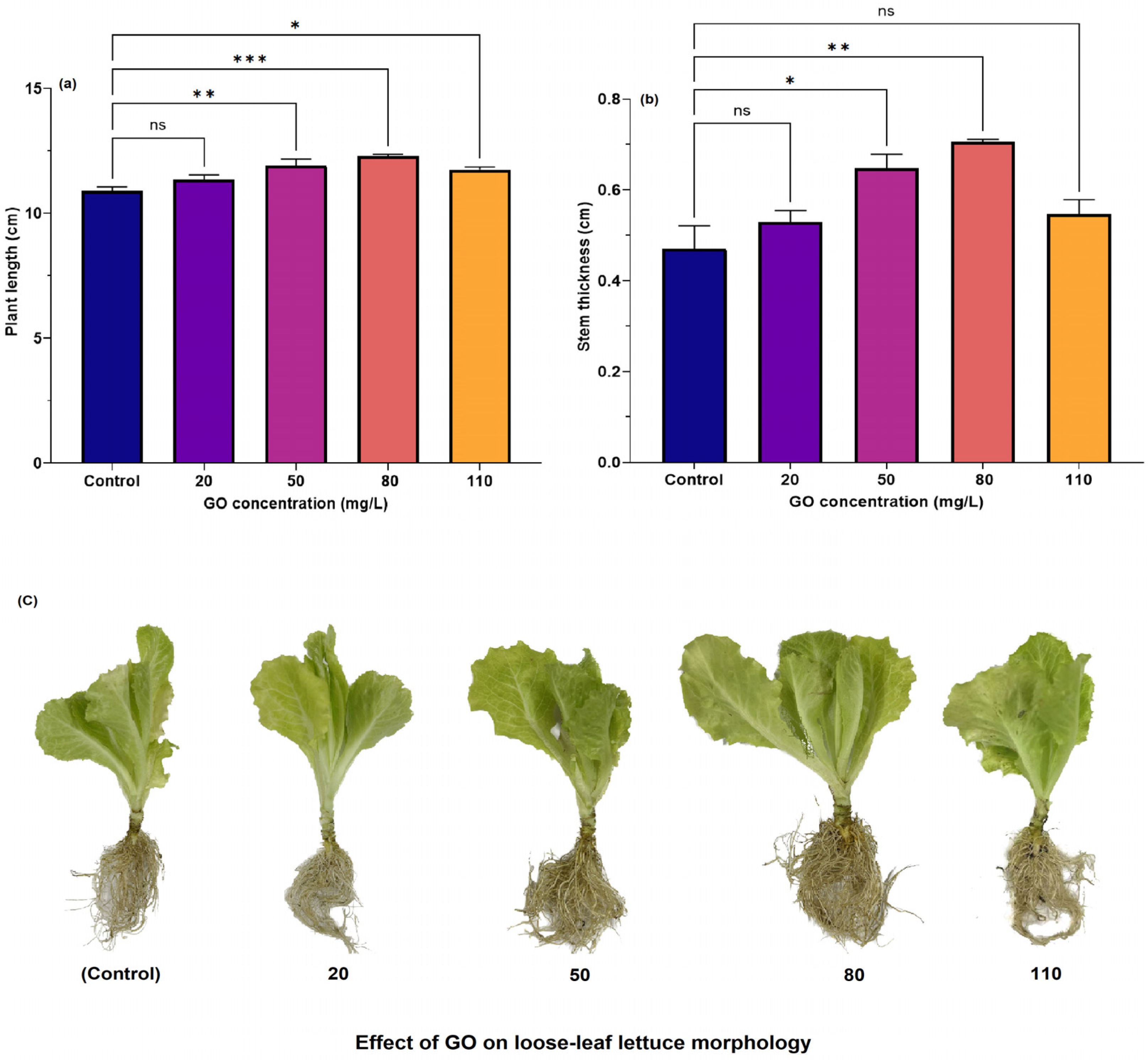
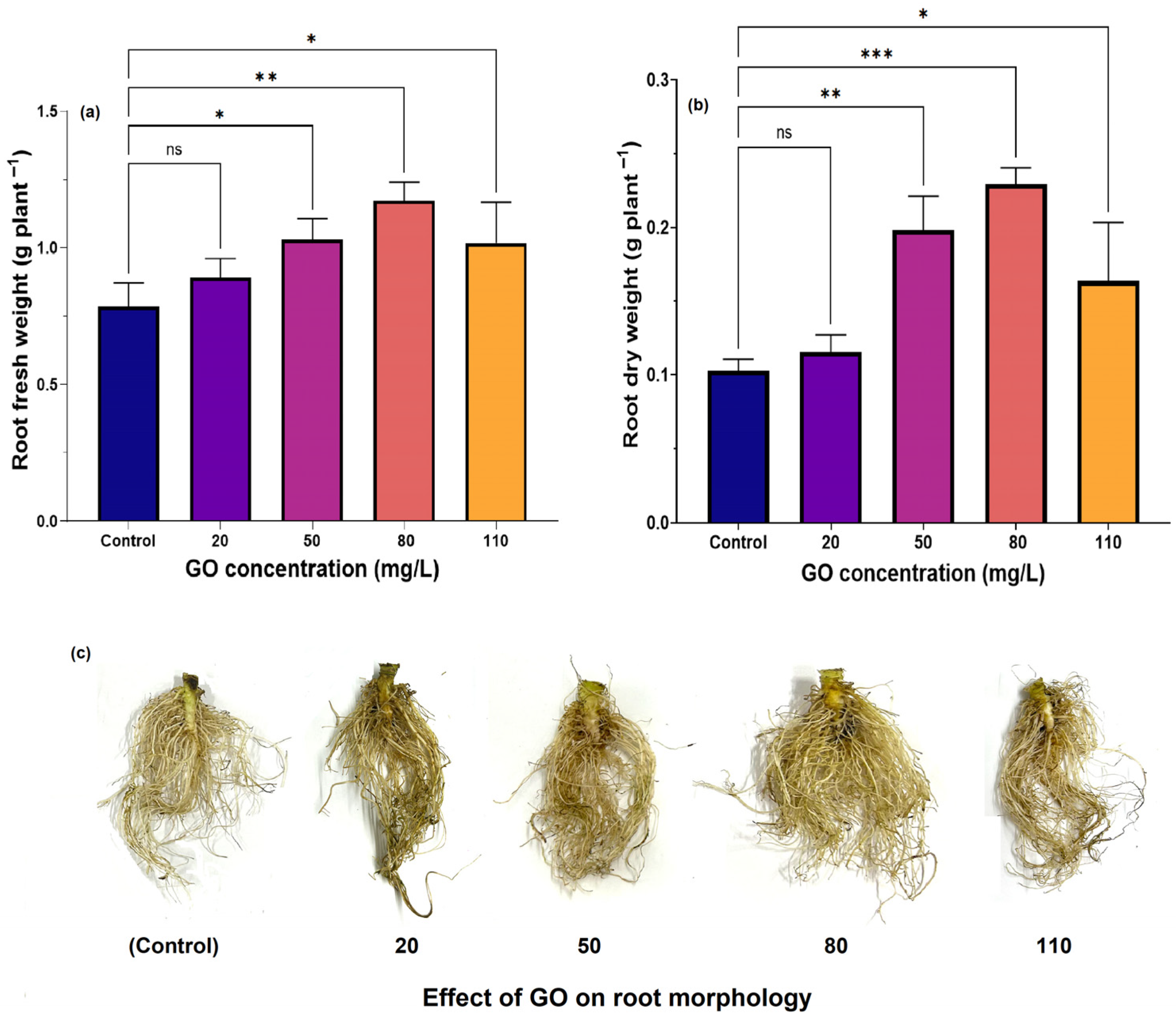
| Treatment Samples | Particle Constitute | |||
|---|---|---|---|---|
| GF (mg) | CaO (mg) (from Calcined OSP) | GO as a Coating on Metals (mg/L) | GF:OSF Ratio | |
| GF | 60 | 0 | 0 | -- |
| OSF-GO1 | 40 | 20 | 2.5 | 2:1 |
| OSF-GO2 | 30 | 30 | 2.5 | 1:1 |
| Treatment Samples | Plant Morphology | Plant Height | Stem Thickness | Root Length | pH | Alkli Hydrolysable N | Available P | Available K |
|---|---|---|---|---|---|---|---|---|
| cm | mg/kg | |||||||
| CF |  | 14.3 ± 0.60 | 1.4 ± 0.08 | 6.3 ± 0.43 | 5.38 ± 0.07 | 229.7 ± 6.18 | 185.4 ± 7.22 | 117.8 ± 4.62 |
| OSF-GO1 |  | 28.9 ± 1.91 | 2.8 ± 0.17 | 9.2 ± 0.88 | 6.41 ± 0.11 | 200.4 ± 8.36 | 159.9 ± 6.35 | 91.1 ± 5.95 |
| OSF-GO2 |  | 26.1 ± 2.23 | 2.1 ± 0.33 | 8.1 ± 1.07 | 6.30 ± 0.06 | 194.4 ± 4.68 | 152.3 ± 4.79 | 99.9 ± 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Xian, Y.; Lu, Y.; Zhang, Z.; He, Y.; Hao, X. Waste Oyster Shell/Graphene Oxide Composite as a Dual-Functional Soil Conditioner and SRF: Impacts on Soil pH and Nutrient Availability. Nanomaterials 2025, 15, 1666. https://doi.org/10.3390/nano15211666
Cheng H, Xian Y, Lu Y, Zhang Z, He Y, Hao X. Waste Oyster Shell/Graphene Oxide Composite as a Dual-Functional Soil Conditioner and SRF: Impacts on Soil pH and Nutrient Availability. Nanomaterials. 2025; 15(21):1666. https://doi.org/10.3390/nano15211666
Chicago/Turabian StyleCheng, Hsuhui, Yuxing Xian, Yetong Lu, Ziying Zhang, Yishi He, and Xiangying Hao. 2025. "Waste Oyster Shell/Graphene Oxide Composite as a Dual-Functional Soil Conditioner and SRF: Impacts on Soil pH and Nutrient Availability" Nanomaterials 15, no. 21: 1666. https://doi.org/10.3390/nano15211666
APA StyleCheng, H., Xian, Y., Lu, Y., Zhang, Z., He, Y., & Hao, X. (2025). Waste Oyster Shell/Graphene Oxide Composite as a Dual-Functional Soil Conditioner and SRF: Impacts on Soil pH and Nutrient Availability. Nanomaterials, 15(21), 1666. https://doi.org/10.3390/nano15211666








