Phase-Controlled Synthesis of Alloyed (CdS)x(CuInS2)1−x Nanocrystals with Tunable Band Gap
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of WZ Alloyed (CdS)x(CuInS2)1−x Nanocrystals
2.3. Synthesis of ZB Alloyed (CdS)x(CuInS2)1−x Nanocrystals
2.4. Materials Characterizations
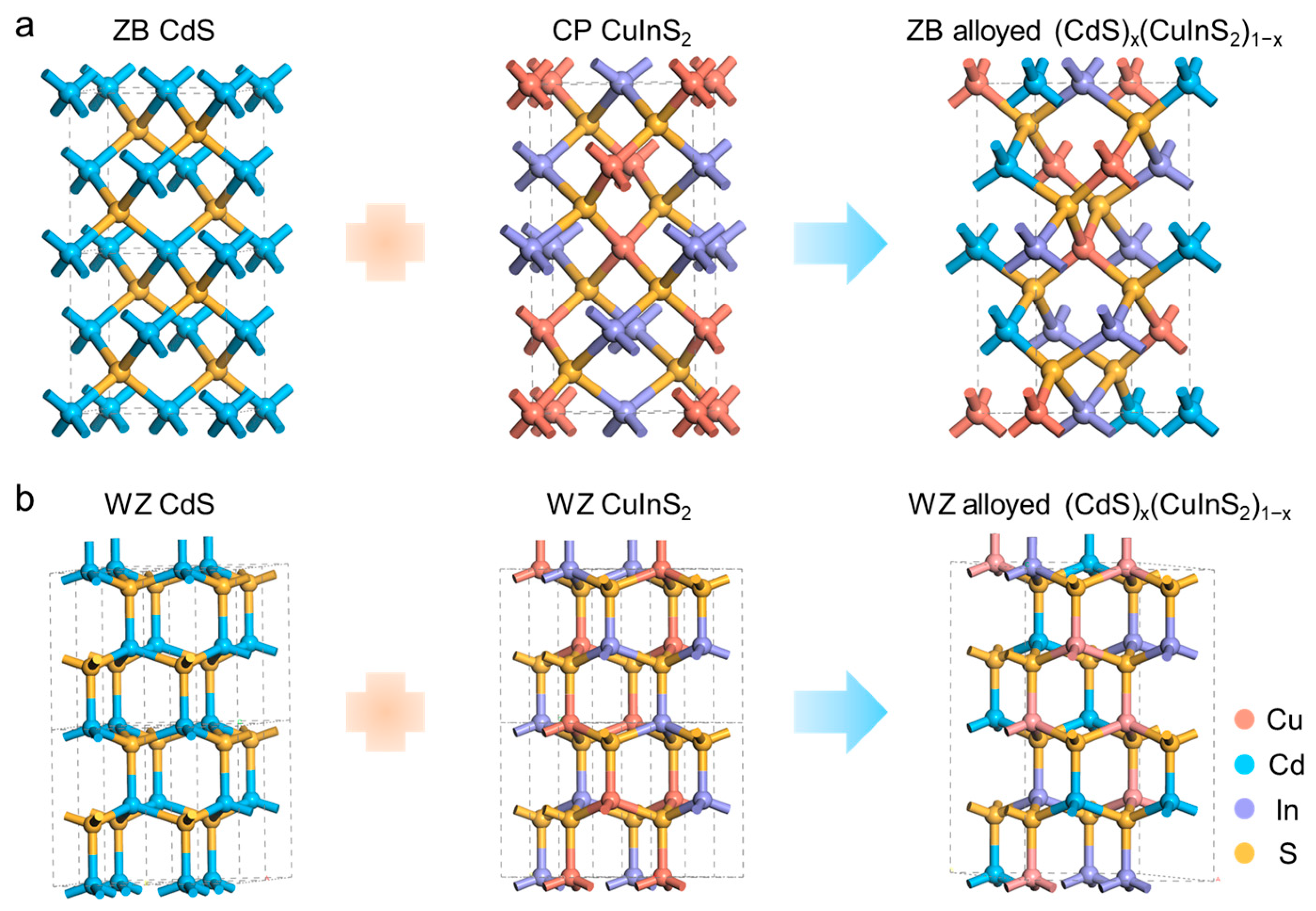
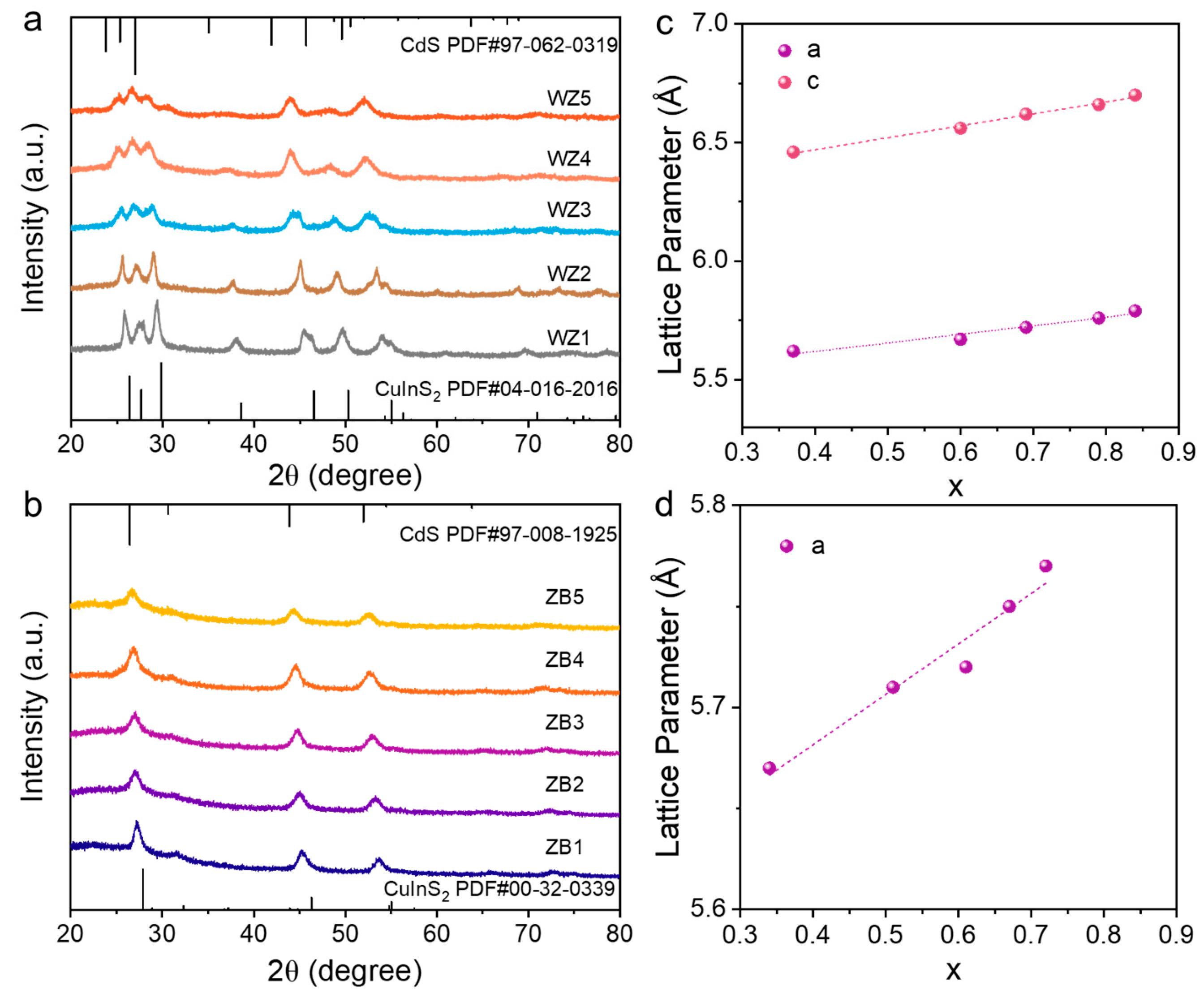
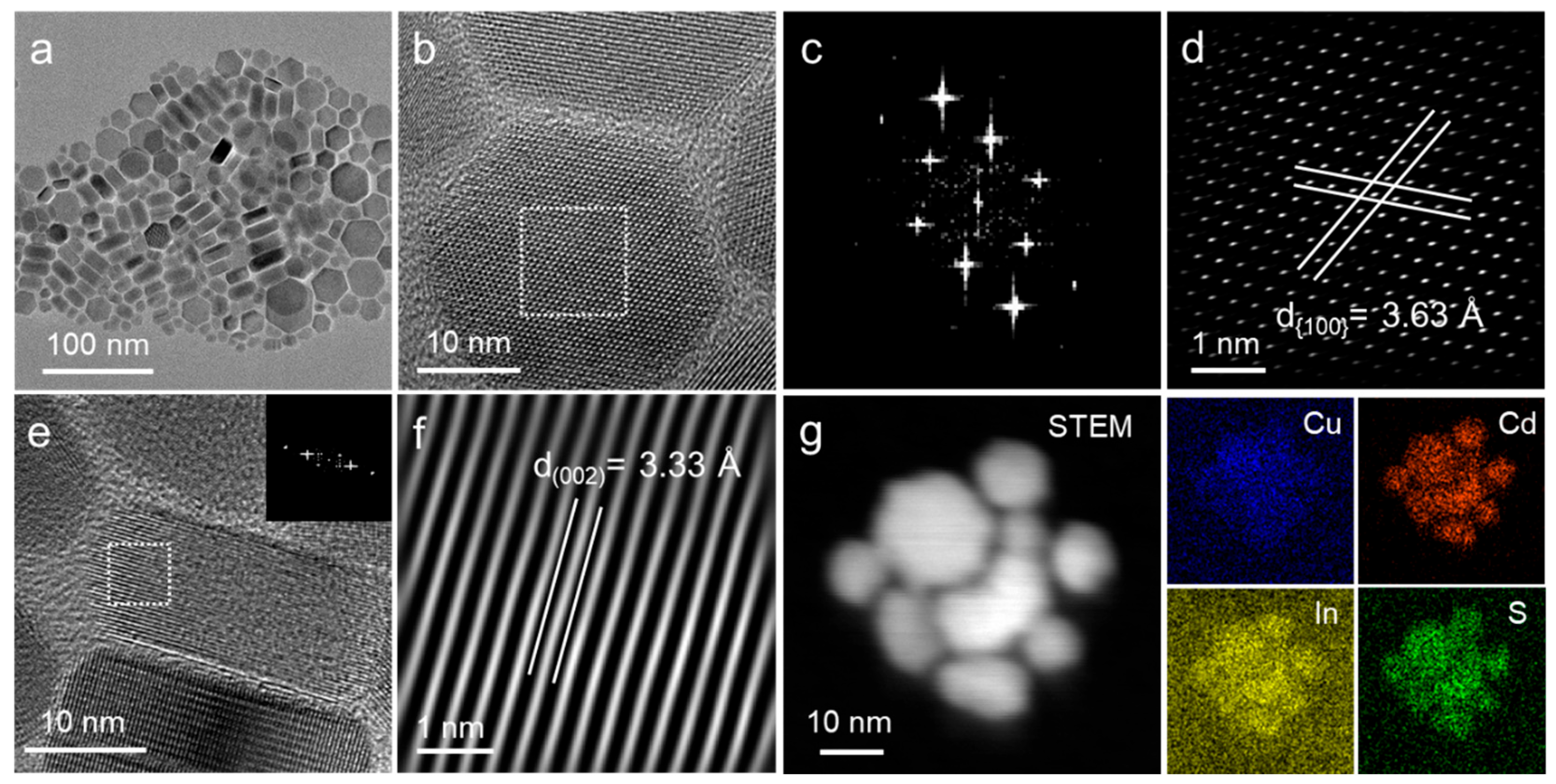
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Chalcopyrite |
| WZ | Wurtzite |
| ZB | Zinc-blende |
| 1-DDT | 1-dodecanethiol |
| OLA | Oleylamine |
| ODE | 1-Octadecene |
| VB | Valence band |
| CB | Conduction band |
| AC | Alternating-current |
| M-S | Mott−Schottky |
| Efb | Flat-band potentials |
| FFT | Fast Fourier transform |
References
- Jung, H.; Ahn, N.; Klimov, V.I. Prospects and challenges of colloidal quantum dot laser diodes. Nat. Photonics 2021, 15, 643–655. [Google Scholar] [CrossRef]
- Song, H.; Lin, Y.; Zhou, M.; Rao, H.; Pan, Z.; Zhong, X. Zn-Cu-In-S-Se Quinary “Green” Alloyed Quantum-Dot-Sensitized Solar Cells with a Certified Efficiency of 14.4 %. Angew. Chem. Int. Ed. 2021, 60, 6137–6144. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, R.; Marshall, A.R.; Wang, S.; Wieliczka, B.M.; Ni, J.; Zhang, J.J.; Yuan, J.Y.; Luther, J.M.; Hazarika, A.; et al. Colloidal Quantum Dot Solar Cells: Progressive Deposition Techniques and Future Prospects on Large-Area Fabrication. Adv. Mater. 2022, 34, 2107888. [Google Scholar] [CrossRef]
- Liang, W.F.; Nie, C.M.; Du, J.; Han, Y.Y.; Zhao, G.H.; Yang, F.; Liang, G.J.; Wu, K.F. Near-infrared photon upconversion and solar synthesis using lead-free nanocrystals. Nat. Photonics 2023, 17, 346–357. [Google Scholar] [CrossRef]
- Bennett, E.; Greenberg, M.W.; Jordan, A.J.; Hamachi, L.S.; Banerjee, S.; Billinge, S.J.L.; Owen, J.S. Size Dependent Optical Properties and Structure of ZnS Nanocrystals Prepared from a Library of Thioureas. Chem. Mater. 2022, 34, 706–717. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, X. Facile synthesis of ZnS-CuInS2-alloyed nanocrystals for a color-tunable fluorchrome and photocatalyst. Inorg. Chem. 2011, 50, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-J.; Wu, L.; Gong, M.; Liu, G.; Wang, Y.-X.; Yu, S.-H.; Chen, S.; Wang, L.-W.; Gong, X.-G. Composition-and Band-Gap-Tunable Synthesis of Wurtzite-Derived Cu2ZnSn(S1–xSex)4 Nanocrystals: Theoretical and Experimental Insights. ACS Nano 2013, 7, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, Z.; Lin, Y.; Guo, P.; Li, S.; Pan, D.; Ji, X. Alloyed (ZnS)x(Cu2SnS3)1–x and (CuInS2)x(Cu2SnS3)1–x nanocrystals with arbitrary composition and broad tunable band gaps. Chem. Commun. 2011, 47, 964–966. [Google Scholar] [CrossRef]
- Ning, C.-Z.; Dou, L.; Yang, P. Bandgap engineering in semiconductor alloy nanomaterials with widely tunable compositions. Nat. Rev. Mater. 2017, 2, 17070. [Google Scholar] [CrossRef]
- Filho, M.A.M.; Farmer, W.; Hsiao, C.-L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Ankit, K.; Gueorguiev, G.K. Density Functional Theory-Fed Phase Field Model for Semiconductor Nanostructures: The Case of Self-Induced Core–Shell InAlN Nanorods. Cryst. Growth Des. 2024, 24, 4717–4727. [Google Scholar] [CrossRef]
- Pela, R.R.; Hsiao, C.-L.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Electronic and optical properties of core–shell InAlN nanorods: A comparative study via LDA, LDA-1/2, mBJ, HSE06, G0W0 and BSE methods. PCCP 2024, 26, 7504–7514. [Google Scholar] [CrossRef]
- Grechenkov, J.; Gopejenko, A.; Bocharov, D.; Isakoviča, I.; Popov, A.I.; Brik, M.G.; Piskunov, S. Ab Initio Modeling of CuGa1−xInxS2, CuGaS2(1−x)Se2x and Ag1−xCuxGaS2 Chalcopyrite Solid Solutions for Photovoltaic Applications. Energies 2023, 16, 4823. [Google Scholar] [CrossRef]
- Sergeyev, D.; Zhanturina, N.; Aizharikov, K.; Popov, A.I. Influence of “Productive” Impurities (Cd, Na, O) on the Properties of the Cu2ZnSnS4 Absorber of Model Solar Cells. Latv. J. Phys. Tech. Sci. 2021, 58, 13–23. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, D.; Kondamareddy, K.K.; He, Y.; Gu, W.; Li, J.; Fan, H.; Wang, H.; Ho, W. Recent Advances and Insights in Designing ZnxCd1–xS-Based Photocatalysts for Hydrogen Production and Synergistic Selective Oxidation to Value-Added Chemical Production. ACS Appl. Mater. Interfaces 2024, 16, 48895–48926. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Q.; Zhuang, T.-T.; Li, Y.; Zhang, G.; Liu, G.-Q.; Fan, F.-J.; Shi, L.; Yu, S.-H. Single crystalline quaternary sulfide nanobelts for efficient solar-to-hydrogen conversion. Nat. Commun. 2020, 11, 5194. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, A.; Ciston, J.; Bustillo, K.; Nordlund, D.; Milliron, D.J. Synergistic role of dopants on the morphology of alloyed copper chalcogenide nanocrystals. J. Am. Chem. Soc. 2015, 137, 6464–6467. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chen, H.; Wang, R.; Shang, Y.; Zhang, Q.; Li, W.; Zhang, G.; Su, J.; Dinh, C.T.; de Arquer, F.P.G.; et al. 0D–2D Quantum Dot: Metal Dichalcogenide Nanocomposite Photocatalyst Achieves Efficient Hydrogen Generation. Adv. Mater. 2017, 29, 1605646. [Google Scholar] [CrossRef]
- Zu, B.; Chen, S.; Jin, Q.; Xu, Z.; Wu, X.; Wu, L. Wurtzite CuIn(SxSe1–x)2 Nanocrystals: Colloidal Synthesis and Band-Gap Engineering. Inorg. Chem. 2024, 63, 21816–21821. [Google Scholar] [CrossRef]
- Duda, M.; Joshi, P.; Borodziuk, A.; Sobczak, K.; Sikora-Dobrowolska, B.; Maćkowski, S.; Dennis, A.M.; Kłopotowski, Ł. Multimodal Temperature Readout Boosts the Performance of CuInS2/ZnS Quantum Dot Nanothermometers. ACS Appl. Mater. Interfaces 2024, 16, 60008–60017. [Google Scholar] [CrossRef]
- Lim, L.; Zhao, X.F.; Tan, Z.K. Non-Toxic CuInS/ZnS Colloidal Quantum Dots for Near-Infrared Light-Emitting Diodes. Adv. Mater. 2023, 35, 2301887. [Google Scholar] [CrossRef]
- Zhao, J.; Minegishi, T.; Kaneko, H.; Ma, G.; Zhong, M.; Nakabayashi, M.; Hisatomi, T.; Katayama, M.; Shibata, N.; Yamada, T.; et al. Efficient hydrogen evolution on (CuInS2)x(ZnS)1−x solid solution-based photocathodes under simulated sunlight. Chem. Commun. 2019, 55, 470–473. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.M.; Zhang, L.Y.; Chen, J.; Wang, S.B.; Ma, M.M.; Yin, Z.; Man, Z.W.; Yi, D.; Wang, Z.J.; et al. Boosting Photocatalytic Hydrogen Evolution of 2D Multinary Copper Chalcogenide Nanocrystals Enabled by Tuning Metal Precursors. Small 2025, 21, 2501503. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; An, L.; Sun, Z.; Hou, W.; Yang, Y.; Yang, Z.; Lu, Y. Synthesis of Cu−In−S ternary nanocrystals with tunable structure and composition. J. Am. Chem. Soc. 2008, 130, 5620–5621. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qu, J.; Han, P.; Hülsey, M.J.; Zhang, G.; Wang, Y.; Wang, S.; Chen, D.; Lu, J.; Yan, N. Visible-light-driven amino acids production from biomass-based feedstocks over ultrathin CdS nanosheets. Nat. Commun. 2020, 11, 4899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Poli, I.; Meggiolaro, D.; De Angelis, F.; Petrozza, A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 2021, 6, 986–1002. [Google Scholar] [CrossRef]
- Wang, J.-J.; Hu, J.-S.; Guo, Y.-G.; Wan, L.-J. Eco-friendly visible-wavelength photodetectors based on bandgap engineerable nanomaterials. J. Mater. Chem. 2011, 21, 17582. [Google Scholar] [CrossRef]
- Li, H.; Wang, X. Phase Control in Inorganic Nanocrystals through Finely Tuned Growth at an Ultrathin Scale. Acc. Chem. Res. 2019, 52, 780–790. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, S.; Zu, B.; Wu, X.; Liu, G.; Wu, L. Phase-Controlled Synthesis of Single-Crystalline Tin Sulfide Nanosheets for PEC Water Oxidation. Inorg. Chem. 2025, 64, 12112–12119. [Google Scholar] [CrossRef]
- McKeever, H.; Kapuria, N.; Nicolson, A.; Sen, S.; Scanlon, D.; Ryan, K.M.; Singh, S. Ligand-Assisted Colloidal Synthesis of Alkali Metal-Based Ternary Chalcogenide: Nanostructuring and Phase Control in Na–Cu–S System. Nano Lett. 2025, 25, 4652–4658. [Google Scholar] [CrossRef]
- Sandroni, M.; Wegner, K.D.; Aldakov, D.; Reiss, P. Prospects of Chalcopyrite-Type Nanocrystals for Energy Applications. ACS Energy Lett. 2017, 2, 1076–1088. [Google Scholar] [CrossRef]
- Ning, J.J.; Kershaw, S.V.; Rogach, A.L. Synthesis and Optical Properties of Cubic Chalcopyrite/Hexagonal Wurtzite Core/Shell Copper Indium Sulfide Nanocrystals. J. Am. Chem. Soc. 2019, 141, 20516–20524. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Dong, B.; Hou, L.; Yu, D.; Wang, W.; Xie, X.; de Mello Donega, C.; Cao, L.; Xia, C. Zn-alloying induced spectral narrowing in wurtzite CuInS2 quantum dots for deep-red light-emitting diodes. Chem. Eng. J. 2025, 516, 164062. [Google Scholar] [CrossRef]
- Fan, F.-J.; Wu, L.; Yu, S.-H. Energetic I–III–VI 2 and I 2–II–IV–VI 4 nanocrystals: Synthesis, photovoltaic and thermoelectric applications. Energy Environ. Sci. 2013, 7, 190–208. [Google Scholar] [CrossRef]
- Pan, D.; Weng, D.; Wang, X.; Xiao, Q.; Chen, W.; Xu, C.; Yang, Z.; Lu, Y. Alloyed semiconductor nanocrystals with broad tunable band gaps. Chem. Commun. 2009, 45, 4221–4223. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, W.; Fang, F.; Zhang, K.; Yu, X.; Chang, K. Gel-assisted synthesis of CIZS for visible-light photocatalytic reduction reaction. Chem. Eng. J. 2022, 429, 132364. [Google Scholar] [CrossRef]
- Singh, A.; Coughlan, C.; Milliron, D.J.; Ryan, K.M. Solution Synthesis and Assembly of Wurtzite-Derived Cu–In–Zn–S Nanorods with Tunable Composition and Band Gap. Chem. Mater. 2015, 27, 1517–1523. [Google Scholar] [CrossRef]
- Hsieh, P.-Y.; Kameyama, T.; Takiyama, T.; Masuoka, K.; Yamamoto, T.; Hsu, Y.-J.; Torimoto, T. Controlling the visible-light driven photocatalytic activity of alloyed ZnSe–AgInSe2 quantum dots for hydrogen production. J. Mater. Chem. A 2020, 8, 13142–13149. [Google Scholar] [CrossRef]
- Xu, M.; Zai, J.; Yuan, Y.; Qian, X. Band gap-tunable (CuIn)xZn2(1−x)S2 solid solutions: Preparation and efficient photocatalytic hydrogen production from water under visible light without noble metals. J. Mater. Chem. 2012, 22, 23929. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Liu, X.; Xu, J.; Chu, X.; Chen, G.; Li, D.; Wang, M.; Wang, X.; Naisa, C.; et al. Designing multi-metal-site nanosheet catalysts for CO2 photoreduction to ethylene. Nat. Commun. 2025, 16, 6500. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Ma, S.; Zhang, Y.; Wang, L.; Zhu, G.; Huang, W.; Wang, S. Induced dipole moments in amorphous ZnCdS catalysts facilitate photocatalytic H2 evolution. Nat. Commun. 2024, 15, 2600. [Google Scholar] [CrossRef]
- Wu, L.; Su, F.; Liu, T.; Liu, G.-Q.; Li, Y.; Ma, T.; Wang, Y.; Zhang, C.; Yang, Y.; Yu, S.-H. Phosphorus-Doped Single-Crystalline Quaternary Sulfide Nanobelts Enable Efficient Visible-Light Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2022, 144, 20620–20629. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Levcenko, S.; Unold, T.; Laffir, F.; Ryan, K.M. Compositionally tunable photoluminescence emission in Cu2ZnSn(S(1−x)Se(x))4 nanocrystals. Angew. Chem. Int. Ed. 2013, 52, 9120–9124. [Google Scholar] [CrossRef]
- Li, X.D.; Sun, Y.F.; Xu, J.Q.; Shao, Y.J.; Wu, J.; Xu, X.L.; Pan, Y.; Ju, H.X.; Zhu, J.F.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 Evolution Reaction from Aqueous Solutions over Band Structure-Controlled (AgIn)xZn2(1−x)S2 Solid Solution Photocatalysts with Visible-Light Response and Their Surface Nanostructures. J. Am. Chem. Soc. 2004, 126, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ngaw, C.K.; Yuan, Y.; Bassi, P.S.; Joachim Loo, S.C.; Yang Tan, T.T. Bandgap engineering of ternary sulfide nanocrystals by solution proton alloying for efficient photocatalytic H2 evolution. Nano Energy 2016, 26, 577–585. [Google Scholar] [CrossRef]
- Tian, L.; Bi, E.; Yavuz, I.; Deger, C.; Tian, Y.; Zhou, J.; Zhang, S.; Liu, Q.; Shen, J.; Yao, L.; et al. Divalent cation replacement strategy stabilizes wide-bandgap perovskite for Cu(In,Ga)Se2 tandem solar cells. Nat. Photonics 2025, 19, 479–485. [Google Scholar] [CrossRef]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 Evolution under Visible-Light Irradiation over Band-Structure-Controlled (CuIn)xZn2(1−x)S2 Solid Solutions. J. Phys. Chem. B 2005, 109, 7323–7329. [Google Scholar] [CrossRef]
- Ji, S.; Shi, T.; Qiu, X.; Zhang, J.; Xu, G.; Chen, C.; Jiang, Z.; Ye, C. A route to phase controllable Cu2ZnSn(S(1−x)Se(x))4 nanocrystals with tunable energy bands. Sci. Rep. 2013, 3, 2733. [Google Scholar] [CrossRef]
- Frick, J.J.; Cava, R.J.; Bocarsly, A.B. Chalcopyrite CuIn(S1−xSex)2 for Photoelectrocatalytic H2 Evolution: Unraveling the Energetics and Complex Kinetics of Photogenerated Charge Transfer in the Semiconductor Bulk. Chem. Mater. 2018, 30, 4422–4431. [Google Scholar] [CrossRef]
- Zheng, J.; Lei, Z. Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl. Catal. B 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Chen, S.; Zu, B.; Jin, Q.; Wu, X.; Xu, Z.; Wu, L. General Synthesis of Wurtzite Cu-Based Quaternary Selenide Nanocrystals via the Colloidal Method. ACS Appl. Mater. Interfaces 2025, 17, 24382–24389. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, B.; Chen, S.; Bao, L.; Liu, Y.; Wu, L. Phase-Controlled Synthesis of Alloyed (CdS)x(CuInS2)1−x Nanocrystals with Tunable Band Gap. Nanomaterials 2025, 15, 1661. https://doi.org/10.3390/nano15211661
Zu B, Chen S, Bao L, Liu Y, Wu L. Phase-Controlled Synthesis of Alloyed (CdS)x(CuInS2)1−x Nanocrystals with Tunable Band Gap. Nanomaterials. 2025; 15(21):1661. https://doi.org/10.3390/nano15211661
Chicago/Turabian StyleZu, Bingqian, Song Chen, Liping Bao, Yingjie Liu, and Liang Wu. 2025. "Phase-Controlled Synthesis of Alloyed (CdS)x(CuInS2)1−x Nanocrystals with Tunable Band Gap" Nanomaterials 15, no. 21: 1661. https://doi.org/10.3390/nano15211661
APA StyleZu, B., Chen, S., Bao, L., Liu, Y., & Wu, L. (2025). Phase-Controlled Synthesis of Alloyed (CdS)x(CuInS2)1−x Nanocrystals with Tunable Band Gap. Nanomaterials, 15(21), 1661. https://doi.org/10.3390/nano15211661





