Innovative Application of Nanomaterials in Vegetable Cultivation: Recent Advances in Growth Promotion and Stress Tolerance
Abstract
1. Introduction
2. Agricultural Applications of NMs in Vegetable Cultivation
2.1. Nanofertilizers
2.2. Nanopesticides
| Nanopesticides | Concentration | Size (nm) | Vegetable Species | References |
|---|---|---|---|---|
| Fe3O4 | 10 mg/L | N/A | Coriander | [26] |
| Ag | 1 g | 415 nm | Carrot | [56] |
| Ag | N/A | 43–74 nm | N/A | [62] |
| Cu | 1 g | 339 nm | Carrot | [56] |
| Graphene-Cu | 100 mg/L | 22–97 nm | Tomato | [67] |
| CH@CuO | 100 mg/L | 32.74 ± 2.3 nm | Tomato | [54] |
| S | 100 mg/L | N/A | Tomato | [57] |
| CoFe2O4 and NiFe2O4 | 500 ppm | 25 nm | Pepper | [58] |
| CuO | 3 mg/mL | 3.59–6.05 nm | Potato | [53] |
| MgO | 3 mg/mL | 3.71–6.58 nm | Potato | [53] |
| Fe2O3 NM-B, NM-H | 0.25 mM, 0.125 mM | 23.45 nm, 15.8 nm | Cucumber | [70] |
| La2O3 | 500 mg/L | 19.93 ± 6.95 nm | Cucumber | [55] |
| Cu3(PO4)2·3H2O | 10 mg/L | N/A | Watermelon | [71] |
| Bio-Mn | 100 µg/mL | 27.0–65.7 nm | Watermelon | [68] |
| Cu(OH) | 2.5 mg | N/A | Cucumber | [72] |
| GO | 1000 ppm | 100 nm | N/A | [60] |
| S | 100 mg/L | 70–150 nm | Lettuce | [73] |
| CNF | 1 mg/mL | 10–20 nm | Cabbage | [64] |
| Biochar-iron | 500 mg/kg | N/A | Chinese cabbage | [31] |
| Mn3O4 | N/A | 9.7 ± 1.1 nm | Rape | [74] |
| CeO2 | N/A | 6.8 ± 0.7 nm | Rape | [74] |
| MON@CeO | 200 mg/L | 45.4 nm | N/A | [61] |
| Glycine betain-ZnO | 100 mg/L | 17.34 nm | Coriander | [75] |
| CMC-Nar (Naringenin based nanocomposites) | N/A | 65 nm | Cumin | [76] |
| ZnO | 50 mg/L | N/A | Tomato | [18] |
| Cu | 400 mg/kg | 40 nm | Cucumber | [65] |
| Bio-Mn | 100 µg/mL | 27.0–65.7 nm | Watermelon | [68] |
| Mn3O4 | N/A | 8.9 ± 1.4 nm | Cucumber, Lettuce | [28] |
| SiO2 | 600 mg/L | N/A | Lilium | [77] |
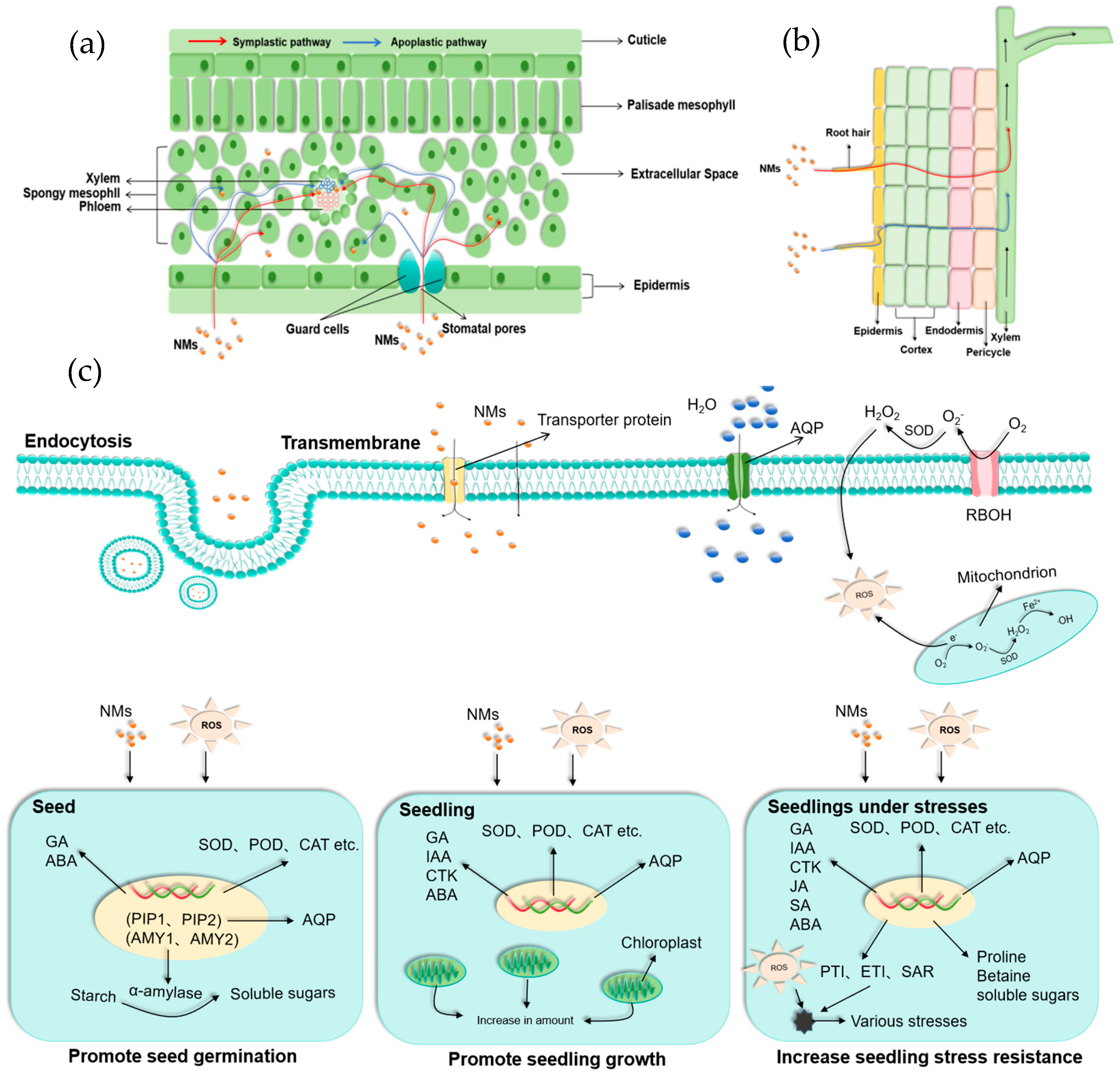
3. Uptake and Transportation of NMs in Plants
3.1. Pathways for Foliar Uptake
3.2. Pathways for Root Uptake
4. The Roles of NMs in Plant Growth and Development
4.1. NMs Promote Seed Germination
| NMs | Seed Germination (%) | Seedling Growth (%) | Yield (%) | References |
|---|---|---|---|---|
| Se | N/A | N/A | 19.8% | [20] |
| CNTs | N/A | N/A | 63% | [96] |
| Se | N/A | N/A | 67.6% | [21] |
| Biochar-iron | N/A | 34–200% (Shoot length), 16–118% (Root length), 5–150% (fresh biomass), 6–195% (Dry biomass) | N/A | [31] |
| Mn3O4 | N/A | 62% (Root length) | N/A | [27] |
| Fe3O4 | N/A | 27–109% (Shoot length) | N/A | [26] |
| ZnO-CaO | 100% | N/A | N/A | [35] |
| Fe3O4-SiO2 | N/A | 144% (root dry weight), 243% (shoot dry weight), 34.4% (leaf area) | N/A | [37] |
| Zinc-Molybdenum | 25% | N/A | N/A | [38] |
| GO | N/A | N/A | 67% (fresh biomass), 65% (Dry biomass) | [44] |
| CuO | 700%, 40% | 33%, 43% (Shoot length) | N/A | [39] |
| TiO2 | 36% | 129.27% (Shoot length), 252% (Root length), 56.10% (fresh biomass), 162.30% (Dry biomass) | N/A | [43] |
| Se | N/A | 17% (Shoot length), 25% (leaves), | 55%, 79% | [41] |
| ZnO | 107.4% | N/A | N/A | [42] |
| MgO | 9% | 50% (Shoot length), 18% (Root length), 26% (Seedling length), | N/A | [40] |
4.2. NMs Promote Plant Growth
5. The Roles of NMs in Mitigating Biotic and Abiotic Stresses
5.1. Roles of NMs in Plant Disease Control
5.2. Roles of NMs in Plant Pest Control
5.3. Roles of NMs in Plants Facing Abiotic Stresses
6. Challenges and Safety Considerations for NMs Applications
6.1. Soil Environmental Pollution and Food Safety Risks Resulting from NMs
6.2. Methods for Assessing the Safety Performance of NMs
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manzoor, J.; Sharma, M.; Wani, K. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Wang, H.; Li, Y.; Chen, B.; Gou, R.; Wang, D.; Jiang, Y.; Zheng, Y.; Hamed, K.E. Nanomaterials: Cross-disciplinary applications in ornamental plants. Nanotechnol. Rev. 2024, 13, 20240049. [Google Scholar] [CrossRef]
- Liu, D.; Chen, T.; Gong, Y.; Chen, X.; Zhang, W.; Xiao, R.; Yang, Y.; Zhang, T. Deciphering the key factors affecting pesticide residue risk in vegetable ecosystem. Environ. Res. 2024, 258, 119452. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- Li, F.; Liu, P.; Yi, L.S. Rehabilitation Treatment and Monitoring of Ankle Achilles Tendon Ligament Injury in Athletes Repaired by Nanomaterials. J. Nanomater. 2022, 2022, 5156292. [Google Scholar] [CrossRef]
- Shweta; Sonia, S.; Akhilesh, S.; Sanjay, C.; Vishal, G. Nanotechnology: A cutting-edge technology in vegetable production. J. Hortic. Sci. Biotechnol. 2021, 96, 682–695. [Google Scholar] [CrossRef]
- Demir, E. A review on nanotoxicity and nanogenotoxicity of different shapes of nanomaterials. J. Appl. Toxicol. 2020, 41, 118–147. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Wang, Y.; Ye, W.; Yin, K.Z. Small particles, big effects: How nanoparticles can enhance plant growth in favorable and harsh conditions. J. Integr. Plant Biol. 2024, 66, 1274–1294. [Google Scholar] [CrossRef]
- Jaison, J.; Ahmed, B.; Chan, Y.S.; Alain, D.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity, and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, R.; Rajeswari, E.; Harish, S.; Sivakumar, V.; Gangai, S.R.; Jaya, S.D. Role of chitosan nanoparticles in sustainable plant disease management. J. Nanopart. Res. 2025, 27, 13. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Abhilash, M.R.; Nataraj, K.; Amruthesh, K.N.; Ansari, M.A.; Alomary, M.N.; Murali, M. Toxicological effects of nanoparticles in plants: Mechanisms involved at morphological, physiological, biochemical and molecular levels. Plant Physiol. Biochem. 2024, 210, 108604. [Google Scholar] [CrossRef]
- Muzammil, S.; Ashraf, A.; Siddique, M.H.; Aslam, B.; Rasul, I.; Abbas, R.; Afzal, M.; Faisal, M.; Hayat, S. A review on toxicity of nanomaterials in agriculture: Current scenario and future prospects. Sci. Prog. 2023, 106, 00368504231221672. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Special Issue: Agricultural Nanotechnology. Plants 2024, 13, 489. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Tripathi, S.; Omar, R.A.; Chauhan, P.; Sinhal, V.K.; Singh, A.; Srivastava, A.; Garg, S.K.; Singh, V.P. Next-generation fertilizers: The impact of bionanofertilizers on sustainable agriculture. Microb. Cell Fact. 2024, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, W.; Zhu, G.; Wang, Q.; Zhang, P.; Rui, Y. Recent Trends in Foliar Nanofertilizers: A Review. Nanomaterials 2023, 13, 2906. [Google Scholar] [CrossRef]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.H.; Chakrabartty, I. Nanofertilizers: A Smart and Sustainable Attribute to Modern Agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef]
- Naderi, M.R.; Danesh-Shahraki, A. Nanofertilizers and their roles in sustainable agriculture. Int. J. Agric. Crop Sci. 2013, 5, 2229–2232. [Google Scholar]
- Mohammad, F.; Akhter, B.J.; Chen, C.; Nasser, A.M.; Leonard, W.; Parvaiz, A.; Fangyuan, Y. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Zhao, M.J.; Chen, F.R.; Li, X.N.; Wang, C.X.; Cao, X.S.; Jiao, L.Y.; Yue, L.; Wang, Z.Y. Rhizosphere regulation with cerium oxide nanomaterials promoted carrot taproot thickening. Environ. Sci. Nano. 2024, 11, 3359–3373. [Google Scholar] [CrossRef]
- Wang, C.; Yue, L.; Cheng, B.; Chen, F.; Zhao, X.; Wang, Z.; Xing, B. Mechanisms of growth-promotion and Se-enrichment in Brassica chinensis L. by selenium nanomaterials: Beneficial rhizosphere microorganisms, nutrient availability, and photosynthesis. Environ. Sci. Nano 2022, 9, 302–312. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, C.; Chen, F.; Yue, L.; Cao, X.; Liu, X.; Yao, Y.; Wang, Z.; Xing, B. Multiomics understanding of improved quality in cherry radish (Raphanus sativus L. var. radculus pers) after foliar application of selenium nanomaterials. Sci. Total Environ. 2022, 824, 153712. [Google Scholar] [CrossRef]
- Ko, J.A.; Hwang, Y.S. Effects of nanoTiO2 on tomato plants under different irradiances. Environ. Pollut. 2019, 255, 113141. [Google Scholar] [CrossRef]
- Jahan, S.; Alias, Y.B.; Bakar, A.F.B.A.; Yusoff, I.B. Toxicity evaluation of ZnO and TiO2 nanomaterials in hydroponic red bean (Vigna angularis) plant: Physiology, biochemistry and kinetic transport. J. Environ. Sci. 2018, 13, 140–152. [Google Scholar] [CrossRef] [PubMed]
- López-Vargas, E.R.; González-García, Y.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A.; Cabrera, R.I.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials to Modify the Germination, Growth, and Antioxidant Status of Tomato Seedlings. Agronomy 2020, 10, 639. [Google Scholar] [CrossRef]
- Neelam, R.; Kusum; Vinita, H. Chitosan/ZnO nanocomposites for improving the growth and reducing the toxicity of Zn in Sorghum bicolor (L.) Moench plants. Acta Physiol. Plant. 2024, 46, 67. [Google Scholar] [CrossRef]
- Fahad; Balouch, A.; Agheem, M.H.; Memon, S.A.; Qasim, S. Efficient mitigation of cadmium and lead toxicity in coriander plant utilizing magnetite (Fe3O4) nanofertilizer as growth regulator and antimicrobial agent. Int. J. Environ. Anal. Chem. 2020, 102, 3868–3879. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, T.; Zhou, P.; Wu, Z.; Tan, Z.; Rui, Y. Insights into the effect of manganese-based nanomaterials on the distribution trait and nutrition of radish (Raphanus sativus L.). Plant Physiol. Biochem. 2024, 207, 108428. [Google Scholar] [CrossRef]
- Lu, L.; Huang, M.; Huang, Y.; Corvini, P.F.-X.; Ji, R.; Zhao, L. Mn3O4 nanozymes boost endogenous antioxidant metabolites in cucumber (Cucumis sativus) plant and enhance resistance to salinity stress. Environ. Sci. Nano 2020, 7, 1692–1703. [Google Scholar] [CrossRef]
- Shoukat, A.; Pitann, B.; Hossain, M.S.; Saqib, Z.A.; Nawaz, A.; Mühling, K.H. Zinc and silicon fertilizers in conventional and nano-forms: Mitigating salinity effects in maize (Zea mays L.). J. Plant Nutr. Soil Sci. 2024, 187, 678–689. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Bacchu, M.S.; Ali, M.R.; Khan, M.Z.H. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innovation. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Aborisade, M.A.; Oba, B.T.; Kumar, A.; Liu, J.; Chen, D.; Okimiji, O.P.; Zhao, L. Remediation of metal toxicity and alleviation of toxic metals-induced oxidative stress in Brassica chinensis L. using biochar-iron nanocomposites. Plant Soil 2023, 493, 629–645. [Google Scholar] [CrossRef]
- Tombuloglu, H.; Alsaeed, M.; Slimani, Y.; Demir-Korkmaz, A.; Tombuloglu, G.; Sozeri, H.; Almessiere, M.A.; Baykal, A.; Kayed, T.S.; Ercan, I. Formulation of Manganese Zinc Spinel Ferrite (Mn0.5Zn0.5Fe2O4) Nanoparticles for the Growth Promotion of Plants. J. Plant Nutr. Soil Sci. 2023, 23, 3561–3574. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Hu, C.; Lei, B.; Zhang, X.; Li, Y.; Zhan, Q.; Liu, Y.; Zhuang, J. Promoting the Growth of Mung Bean Plants through Uptake and Light Conversion of NaYF4:Yb,Er@CDs Nanocomposites. ACS Sustain. Chem. Eng. 2020, 8, 9751–9762. [Google Scholar] [CrossRef]
- Raju, D.; Mehta, U.J.; Beedu, S.R. Biogenic green synthesis of monodispersed gum kondagogu (Cochlospermum gossypium) iron nanocomposite material and its application in germination and growth of mung bean (Vigna radiata) as a plant model. IET Nanobiotechnol. 2016, 10, 141–146. [Google Scholar] [CrossRef]
- Anand, K.V.; Keerthika, S.; Vasantharaja, R.; Kannan, M.; Preetha, S.; Selvan, S.M.; Chaturvedi, S.; Govindaraju, K. Biogenic preparation of ZnO, CaO, and ZnO-CaO nanocomposites and its influence on agro-morphological characteristics of mung bean. Environ. Sci. Pollut. Res. 2022, 29, 22251–22259. [Google Scholar] [CrossRef]
- Awal, S.A.; Shinya, M.; Ahmad, F.; Toshiyuki, F.; Takeshi, S.; Wuled, L.I. The Role of Silica Nanoparticles in Promoting the Germination of Tomato (Solanum lycopersicum) Seeds. Nanomaterials 2023, 13, 2110. [Google Scholar] [CrossRef]
- Irshad, M.K.; Ansari, J.R.; Noman, A.; Javed, W.; Lee, J.C.; Aqeel, M.; Waseem, M.; Lee, S.S. Seed priming with Fe3O4-SiO2 nanocomposites simultaneously mitigate Cd and Cr stress in spinach (Spinacia oleracea L.): A way forward for sustainable environmental management. Ecotox. Environ. Saf. 2024, 286, 117195. [Google Scholar] [CrossRef]
- Chaparro, E.H.O.; Estrada, C.A.R.; Páez, J.C.A.; Sánchez, E.; Álvarez, S.P.; Esparza, L.U.C.; Márquez, E.M.; Mendoza, C.C.; Cruz, J.J.P.; Lagos, C.L.F. Nanopriming with Zinc–Molybdenum in Jalapeño Pepper on Imbibition, Germination, and Early Growth. Agronomy 2024, 14, 1609. [Google Scholar] [CrossRef]
- Rocha, J.D.G.D.; Rodrigues, G.A.G.; Tirroni, J.; Keller, P.E.; Mioto, P.T.; Soares, C.; Padoin, N.; Riella, H.G. Effect of CuO nanoparticles from yerba mate on germination and initial growth of lettuce and tomato seeds. J. Environ. Chem. Eng. 2025, 13, 118852. [Google Scholar] [CrossRef]
- Anand, K.V.; Anugraga, A.R.; Kannan, M.; Singaravelu, G.; Govindaraju, K. Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.). Mater. Lett. 2020, 271, 127792. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Perfileva, A.I. Mushroom-Derived Novel Selenium Nanocomposites’ Effects on Potato Plant Growth and Tuber Germination. Molecules 2022, 27, 4438. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc Oxide and Zinc Oxide Nanoparticles Impact on In Vitro Germination and Seedling Growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Abdelmagid, S.Y.; Gharib, F.A.E.L.; Ahmed, E.Z. Impact of titanium nanoparticles on germination and early growth of faba bean (Vicia faba L.). Sci. Rep. 2025, 15, 32450. [Google Scholar] [CrossRef]
- Shafiq, M.F.; Huma, A.Z.e.; Danish, A.M.; Arusa, A.; Tehmina, A.; Hamza, R.; Guihua, L. Green synthesis and application of GO nanoparticles to augment growth parameters and yield in mungbean (Vigna radiata L.). Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Lai, S.-H.; Chye, M.-L. Plant Acyl-CoA-Binding Proteins-Their Lipid and Protein Interactors in Abiotic and Biotic Stresses. Cells 2021, 10, 1064. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Chhipa, H. Nano-based pesticides: Challenges for pest and disease management. Euro-mediterr. J. Environ. 2021, 6, 69. [Google Scholar] [CrossRef]
- Chaudhary, P.; Khati, P.; Chaudhary, A.; Maithani, D.; Kumar, G.; Sharma, A. Cultivable and metagenomic approach to study the combined impact of nanogypsum and Pseudomonas taiwanensis on maize plant health and its rhizospheric microbiome. PLoS ONE 2021, 16, e0250574. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Corrigendum: Biopesticides as an alternative to synthetic pesticides: A case for nanopesticides, phytopesticides and microbial pesticides. Front. Microbiol. 2024, 14, 1258968. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, G.; Jagathambal, M.; Kumar, G.S.; Kolesnikov, E. Hylotelephium telephium Flower Extract-Mediated Biosynthesis of CuO and ZnO Nanoparticles with Promising Antioxidant and Antibacterial Properties for Healthcare Applications. JOM 2020, 72, 1264–1272. [Google Scholar] [CrossRef]
- Yu, W.X.; Ming, C.Y.; Bo, N.X.; Li, Z.L. Optimized wet-chemical synthesis of ultra-small CuO nanoparticles with high antibacterial activity. J. Nanopart. Res. 2024, 26, 119. [Google Scholar] [CrossRef]
- Juan-Ni, C.; Lin-Tong, W.U.; Kun, S.; Yun-Song, Z.; Wei, D. Nonphytotoxic copper oxide nanoparticles are powerful “nanoweapons” that trigger resistance in tobacco against the soil-borne fungal pathogen Phytophthora nicotianae. J. Integr. Agric. 2022, 21, 3245–3262. [Google Scholar] [CrossRef]
- Shuaikang, L.; Weiqiang, T.; Zhongwei, L.; Xuefeng, W.; Kai, Y.; Wei, D.; Siang, C.; Shuhan, C.; Dong, Z.; Lin, C. Biosynthesis of cupric oxide nanoparticles: Its antiviral activities against TMV by directly destroying virion and inducing plant resistance. Phytopathol. Res. 2024, 6, 30. [Google Scholar] [CrossRef]
- Rabea, A.; Naeem, E.; Balabel, N.M.; Daigham, G.E. Management of potato brown rot disease using chemically synthesized CuO-NPs and MgO-NPs. Bot. Stud. 2023, 64, 20. [Google Scholar] [CrossRef]
- Ismail, A.M.; Mosa, M.A.; El-Ganainy, S.M. Chitosan-Decorated Copper Oxide Nanocomposite: Investigation of Its Antifungal Activity against Tomato Gray Mold Caused by Botrytis cinerea. Polymers 2023, 15, 1099. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Wang, C.; Yue, L.; Tao, M.; Elmer, W.H.; White, J.C.; Cao, X.; Xing, B. Nanomaterial Size and Surface Modification Mediate Disease Resistance Activation in Cucumber (Cucumis sativus). ACS Nano 2023, 17, 4871–4885. [Google Scholar] [CrossRef]
- Usman, O.; Baig, M.M.M.; Ikram, M.; Iqbal, T.; Islam, S.; Syed, W.; Al-Rawi, M.B.A. Green synthesis of metal nanoparticles and study their anti-pathogenic properties against pathogens effect on plants and animals. Sci. Rep. 2024, 14, 11354. [Google Scholar] [CrossRef]
- Cao, X.; Wang, C.; Luo, X.; Yue, L.; White, J.C.; Elmer, W.; Dhankher, O.P.; Wang, Z.; Xing, B. Elemental Sulfur Nanoparticles Enhance Disease Resistance in Tomatoes. ACS Nano 2021, 15, 11817–11827. [Google Scholar] [CrossRef] [PubMed]
- Parul, S.; Adikshita, S.; Monica, S.; Nikhil, B.; Pedro, E.; Aditya, J.; Preeti, T.; Atul, T. Nanomaterial Fungicides: In Vitro and In Vivo Antimycotic Activity of Cobalt and Nickel Nanoferrites on Phytopathogenic Fungi. Glob. Chall. 2017, 1, 1700041. [Google Scholar] [CrossRef]
- Bouqellah, N.A.; Abdulmajeed, A.M.; Alharbi, F.K.R.; Mattar, E.; Al-Sarraj, F.; Abdulfattah, A.M.; Hassan, M.M.; Baazeem, A.; Al-Harthi, H.F.; Musa, A. Optimizing encapsulation of garlic and cinnamon essential oils in silver nanoparticles for enhanced antifungal activity against Botrytis cinerea pathogenic disease. Physiol. Mol. Plant Pathol. 2025, 136, 102522. [Google Scholar] [CrossRef]
- Lampiri, E.; Yap, P.L.; Berillis, P.; Athanassiou, C.G.; Losic, D. Graphene powders as new contact nanopesticides: Revealing key parameters on their insecticidal activity for stored product insects. Chemosphere 2024, 364, 143200. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yu, C.; Chang, X.; Wan, Y.; Ba, Y.; Li, C.; Lv, H.; Guo, Z.; Cai, T.; Ren, Z. CeO2 nanohybrid as a synergist for insecticide resistance management. Chem. Eng. J. 2022, 446, 137074. [Google Scholar] [CrossRef]
- Aziz, A.T.; Alshehri, M.A.; Panneerselvam, C.; Murugan, K.; Trivedi, S.; Mahyoub, J.A.; Hassan, M.A.M.; Maggi, F.; Sut, S.; Dall’Acqua, S. The desert wormwood (Artemisia herba-alba)—From Arabian folk medicine to a source of green and effective nanoinsecticides against mosquito vectors. J. Photochem. Photobiol. B Biol. 2018, 180, 225–234. [Google Scholar] [CrossRef]
- Yang, Z.; Maofeng, J.; Amit, L.; Hezhong, W.; Shijun, J.; Daolong, D. Molecular mechanism of nanochitin whisker elicits plant resistance against Phytophthora and the receptors in plants. Int. J. Biol. Macromol. 2020, 165, 2660–2667. [Google Scholar] [CrossRef]
- Parada, R.Y.; Mayumi, E.; Aklog, Y.F.; Chihiro, M.; Shinsuke, I.; Hironori, K. Optimization of nanofibrillation degree of chitin for induction of plant disease resistance: Elicitor activity and systemic resistance induced by chitin nanofiber in cabbage and strawberry. Int. J. Biol. Macromol. 2018, 165, 2660–2667. [Google Scholar] [CrossRef]
- Huang, Y.; Adeleye, A.S.; Zhao, L.; Minakova, A.S.; Anumol, T.; Keller, A.A. Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 2019, 270, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yolanda, G.; Rubisela, L.E.; Marissa, P.; Gregorio, C.; Adalberto, B.; Jesús, V.; Fabián, P.; Antonio, J. Seed Priming with Carbon Nanomaterials Improves the Bioactive Compounds of Tomato Plants under Saline Stress. Plants 2022, 11, 1984. [Google Scholar] [CrossRef] [PubMed]
- Cota-Ungson, D.; González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum, Increase Antioxidant Activity, and Decrease Stress in Tomato Plants. Plants 2023, 12, 2270. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Ijaz, U.; Shahid, M.; Nazir, M.M.; Azizullah White, J.C.; Li, D.; Song, F. Bio-Functionalized Manganese Nanoparticles Suppress Fusarium Wilt in Watermelon (Citrullus lanatus L.) by Infection Disruption, Host Defense Response Potentiation, and Soil Microbial Community Modulation. Small 2022, 19, 2205687. [Google Scholar] [CrossRef]
- Shangguan, W.; Chen, H.; Zhao, P.; Cao, C.; Yu, M.; Huang, Q.; Cao, L. Scenario-oriented nanopesticides: Shaping nanopesticides for future agriculture. Adv. Agrochem 2024, 3, 265–278. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Al-shammari, B.M.; Abdelaziz, A.M.; Nofel, M.M.; Gobara, M.; Elkhatib, W.F.; Eid, N.A.; Salem, M.S.; Attia, M.S. Protective role of iron oxide nanocomposites on disease index, and biochemical resistance indicators against Fusarium oxysporum induced-cucumber wilt disease: In vitro, and in vivo studies. Microb. Pathog. 2023, 180, 106131. [Google Scholar] [CrossRef] [PubMed]
- Borgatta, J.; Ma, C.; Hudson-Smith, N.; Elmer, W.; Plaza Perez, C.D.; Roberto, T.R.; Zuverza-Mena, N.; Haynes, C.L.; White, J.C.; Hamers, R.J. Copper Based Nanomaterials Suppress Root Fungal Disease in Watermelon (Citrullus lanatus): Role of Particle Morphology, Composition and Dissolution Behavior. ACS Sustain. Chem. Eng. 2018, 6, 14847–14856. [Google Scholar] [CrossRef]
- Lijuan, Z.; Yuxiong, H.; Keller, A.A. Comparative Metabolic Response between Cucumber (Cucumis sativus) and Corn (Zea mays) to a Cu(OH)2 Nanopesticide. J. Agric. Food Chem. 2017, 66, 6628–6636. [Google Scholar] [CrossRef]
- Xuesong, C.; Yulin, L.; Xing, L.; Chuanxi, W.; Le, Y.; Wade, E.; Parkash, D.O.; White, J.C.; Zhenyu, W.; Baoshan, X. Mechanistic investigation of enhanced bacterial soft rot resistance in lettuce (Lactuca sativa L.) with elemental sulfur nanomaterials. Sci. Total Environ. 2023, 884, 163793. [Google Scholar] [CrossRef]
- Yanhui, L.; Jin, H.; Jie, Q.; Fameng, Z.; Jiahao, L.; Linlin, C.; Lu, C.; Jiangjiang, G.; Honghong, W.; Zhaohu, L. Improvement of leaf K+ retention is a shared mechanism behind CeO2 and Mn3O4 nanoparticles improved rapeseed salt tolerance. Stress Biol. 2022, 2, 46. [Google Scholar] [CrossRef]
- Saad, H.; Anila, S.; Muhammad, Z. Synergistic effect of glycine betain-ZnO nanocomposite in vitro for the amelioration of drought stress in coriander. Plant Cell Tiss. Organ Cult. 2023, 155, 505–519. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.H.; Mehdi, R.S. Effect of naringenin based nanocomposites and pure naringenin on cumin (Cuminum cyminum L.) under drought stress. Physiol. Mol. Biol. Plants 2024, 30, 791–805. [Google Scholar] [CrossRef]
- Francisco, S.J.; Yolanda, G.; Adalberto, B.; Berenice, M.A.; Susana, G.; Gregorio, C.; Socorro, G.M.D.; Antonio, J. Silicon Nanoparticles Improve the Shelf Life and Antioxidant Status of Lilium. Plants 2021, 10, 2338. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano. 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Y.; Zhang, F.; Chen, B.; Hu, X.; Weir, M.D.; Schneider, A.; Jia, L.; Gu, N.; Xu, H.H.K. Iron Oxide Nanoparticles In Liquid or Powder Form Enhanced Osteogenesis Via Stem Cells on Injectable Calcium Phosphate Scaffold. News Sci. 2020, 2974, 102069. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.J.; Goh, N.S.; Demirer, G.S.; Matos, J.L.; Landry, M.P. Nanoparticle-Mediated Delivery towards Advancing Plant Genetic Engineering. Trends Biotechnol. 2018, 36, 882–897. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, Translocation, and Consequences of Nanomaterials on Plant Growth and Stress Adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Xueran, W.; Hongguo, X.; Pei, W.; Heng, Y. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Lenggoro, I.W. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials 2024, 14, 131. [Google Scholar] [CrossRef]
- Milewska-Hendel, A.; Zubko, M.; Stróż, D.; Kurczyńska, E.U. Effect of Nanoparticles Surface Charge on the Arabidopsis thaliana (L.) Roots Development and Their Movement into the Root Cells and Protoplasts. Int. J. Mol. Sci. 2019, 20, 1650. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Ren, M.; Varela-Ramirez, A.; Li, C.; Hernandez-Viezcas, J.A.; Aguilera, R.J.; Gardea-Torresdey, J.L. Transport of Zn in a sandy loam soil treated with ZnO NPs and uptake by corn plants: Electron microprobe and confocal microscopy studies. Chem. Eng. J. 2012, 184, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, F.; Vardhanabhuti, B.; Mustapha, A.; Lin, M. Detection of Engineered Silver Nanoparticle Contamination in Pears. J. Agric. Food Chem. 2012, 60, 10762. [Google Scholar] [CrossRef]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Zhang, Q.; Wei, J.; Zhao, X.; Zheng, Z.; Chen, B.; Xu, Z. Nanopriming boost seed vigor: Deeper insights into the effect mechanism. Plant Physiol. Biochem. 2024, 214, 108895. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ashraf, U.; Duan, M.; Ren, Y.; Xing, P.; Yan, Z.; Tang, X. Ultrasonic seed treatment improved seed germination, growth, and yield of rice by modulating associated physio-biochemical mechanisms. Ultrason. Sonochem. 2024, 104, 106821. [Google Scholar] [CrossRef]
- Singh, Y.; Kaushal, S.; Sodhi, R.S. Biogenic synthesis of silver nanoparticles using cyanobacterium: Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination. Nanoscale Adv. 2020, 2, 3972–3982. [Google Scholar] [CrossRef]
- Kaur, R.; Yadu, B.; Chauhan, N.S.; Parihar, A.S.; Keshavkant, S. Nano zinc oxide mediated resuscitation of aged Cajanus cajan via modulating aquaporin, cell cycle regulatory genes and hormonal responses. Plant Cell Rep. 2024, 43, 110. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.M.; Baksi, S.; Rani, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. NanoBoost: Maximizing crop resilience and yield via nanopriming under salt stress. Environ. Exp. Bot. 2024, 226, 105937. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Z.; Lao, B.; Li, C.; Zhu, J.; Yu, R.; Liu, M. Phytotoxicity of halloysite nanotubes using wheat as a model: Seed germination and growth. Environ. Sci. Nano 2021, 10, 3015–3027. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef] [PubMed]
- Wuttipong, M.; Sarmah, A.K.; Santi, M.; Piyada, T. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Muyao, R.; Biao, T.; Jiayi, X.; Zhengpeng, Y.; Huabin, Z.; Qiyuan, T.; Xiaoli, Z.; Weiqin, W. Priming methods affected deterioration speed of primed rice seeds by regulating reactive oxygen species accumulation, seed respiration and starch degradation. Front. Plant Sci. 2023, 14, 1267103. [Google Scholar] [CrossRef]
- Shi, F.; Cao, Y.; Gao, Y.; Qiu, Y.; Lu, Y.; Han, B.; Shen, Y. The Impact of Magnetic Field and Gibberellin Treatment on the Release of Dormancy and Internal Nutrient Transformation in Tilia miqueliana Maxim. Seeds. For. 2024, 15, 311. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Z.; Sun, D.W. Bioinspired Nanomodification Strategies: Moving from Chemical-Based Agrosystems to Sustainable Agriculture. ACS Nano 2021, 15, 12655–12686. [Google Scholar] [CrossRef]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of Hormonal Regulation in Rice Seed Germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.M.M.; Rizwan, M. Chemically synthesized silver nanoparticles induced physio-chemical and chloroplast ultrastructural changes in broad bean seedlings. Chemosphere 2019, 235, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotox. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Nie, L.; Song, S.; Yin, Q.; Zhao, T.; Liu, H.; He, A.; Wang, W. Enhancement in Seed Priming-Induced Starch Degradation of Rice Seed Under Chilling Stress via GA-Mediated α-Amylase Expression. Rice 2022, 15, 19. [Google Scholar] [CrossRef]
- Salem, M.A.; Ismail, M.A.; Radwan, K.H.; Elhalim, H.M.A. Unlocking the Potential of Plant Growth-Promoting Rhizobacteria to Enhance Drought Tolerance in Egyptian Wheat (Triticum aestivum). Sustainability 2024, 16, 4605. [Google Scholar] [CrossRef]
- Imran, H.H.; Imran, Z.; Ul, A.Q.; Asifa, N.; Aamna, N.; Rida, J.; Arslan, S.S.; Ali, K.A.; Mominur, R.M.; Summya, R.; et al. Synthesis and characterization of copper oxide nanoparticles: Its influence on corn (Z. mays) and wheat (Triticum aestivum) plants by inoculation of Bacillus subtilis. Environ. Sci. Pollut. Res. 2022, 30, 37370–37385. [Google Scholar] [CrossRef]
- Jochum, M.D.; Mcwilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Jo, Y.K. Bioprospecting Plant Growth-Promoting Rhizobacteria That Mitigate Drought Stress in Grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef]
- Qin, H.; Wang, Z.; Sha, W.; Song, S.; Qin, F.; Zhang, W. Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification. Microorganisms 2024, 12, 700. [Google Scholar] [CrossRef]
- Su, C.; Chen, A.; Liang, W.; Xie, W.; Xu, X.; Zhan, X.; Zhang, W.; Peng, C. Copper-based nanomaterials: Opportunities for sustainable agriculture. Sci. Total Environ. 2024, 926, 171948. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hashmi, S.S.; Palma, J.M.; Corpas, F.J. Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 2022, 290, 133329. [Google Scholar] [CrossRef]
- Swarnali, D.; Shreya, N.; Tauhid, A.A.; Ankita, B.; Falguni, B.; Saikat, M.; Geetha, G.; Arindam, B.; Amitava, M.; Rita, K.; et al. Application of green synthesized bimetallic nZVI-Cu nanoparticle as a sustainable alternative to chemical fertilizers to enhance growth and photosynthetic efficiency of rice seedlings. Plant Physiol. Biochem. 2023, 201, 107837. [Google Scholar] [CrossRef]
- Liu, A.; Xiao, W.; Lai, W.; Wang, J.; Li, X.; Yu, H.; Zha, Y. Potential Application of Selenium and Copper Nanoparticles in Improving Growth, Quality, and Physiological Characteristics of Strawberry under Drought Stress. Agriculture 2024, 14, 1172. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Hayat, S.; Alam, P. Foliar Application of Copper Oxide Nanoparticles Increases the Photosynthetic Efficiency and Antioxidant Activity in Brassica juncea. J. Food Qual. 2022, 2022, 5535100. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, X.; Xin, C.; Si, J.; Li, S.; Li, Y.; Zheng, X.; Li, H.; Wei, X.; et al. Nano-ZnO priming induces salt tolerance by promoting photosynthetic carbon assimilation in wheat. Arch. Agron. Soil. Sci. 2020, 66, 1259–1273. [Google Scholar] [CrossRef]
- Borah, K.D.; Bhuyan, J. Magnesium porphyrins with relevance to chlorophylls. Dalton Trans. 2017, 46, 6497–6509. [Google Scholar] [CrossRef]
- Kong, W.; Yu, X.; Chen, H.; Liu, L.; Xiao, Y.; Wang, Y.; Wang, C.; Lin, Y.; Yu, Y.; Wang, C.; et al. The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice. Plant Mol. Biol. 2016, 92, 177–191. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Wang, W.; Cao, T.; Zhang, L.; Wang, Z.; Chi, X.; Shi, T.; Wang, H.; He, X.; et al. High-yield porphyrin production through metabolic engineering and biocatalysis. Nat. Biotechnol. 2024, 43, 1717–1727. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Han, J.; Li, J.; Lin, X.; Wang, X. Effect of graphene oxide-glyphosate nanocomposite on wheat and rape seedlings: Growth, photosynthesis performance, and oxidative stress response. Environ. Technol. Innov. 2022, 27, 102527. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Mir, M.M.; Sofi, S.A.; Shah, M.A.; Sidiq, T.; Sunooj, K.V.; Hamdani, A.M.; Khaneghah, A.M. Current strategies for the reduction of pesticide residues in food products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Alves, D.M.R.; de Oliveira, J.N.; de Mello Prado, R.; Ferreira, P.M. Silicon in the form of nanosilica mitigates P toxicity in scarlet eggplant. Sci. Rep. 2023, 13, 9190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, R.; Upadhyay, S.K.; Verma, K.K.; Tripathi, R.M.; Liu, H.; Dhankher, O.P.; Tripathi, R.D.; Sahi, S.V.; Seth, C.S. Review on interactions between nanomaterials and phytohormones: Novel perspectives and opportunities for mitigating environmental challenges. Plant Sci. 2023, 340, 111964. [Google Scholar] [CrossRef] [PubMed]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593–594, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, M.K.; Salehi, H. Silver Nanoparticles: An Influential Element in Plant Nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef]
- Ping, Y.; Cao, D.; Hu, J.; Lin, Y.; Dang, C.; Xue, D. The application, safety, and challenge of nanomaterials on plant growth and stress tolerance. Ind. Crops Prod. 2024, 222, 119691. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Vásquez-López, A.; Rojas-Chávez, H.; Valdés-Madrigal, M.A.; Cruz-Martínez, H.; Medina, D.I. Engineered Metal Oxide Nanoparticles as Fungicides for Plant Disease Control. Plants 2023, 12, 2461. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Belal, E.B.; Khateeb, N.M.M.E.; Shreef, B.A. Utilizing bio-synthesis of nanomaterials as biological agents for controlling soil-borne diseases in pepper plants: Root-knot nematodes and root rot fungus. BMC Plant Biol. 2024, 24, 110. [Google Scholar] [CrossRef]
- Ramekar, S.; Dutt, M. Leveraging next-generation technologies to enhance systemic acquired resistance (SAR) in fruit trees. Plant Cell Tiss. Organ Cult. 2025, 160, 15. [Google Scholar] [CrossRef]
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal Activity of Copper Oxide Nanoparticles against Root Rot Disease in Cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef]
- Derbalah, A.; Abdelsalam, I.; Behiry, S.I.; Abdelkhalek, A.; Abdelfatah, M.; Ismail, S.; Elsharkawy, M.M. Copper oxide nanostructures as a potential method for control of zucchini yellow mosaic virus in squash. Pest Manag. Sci. 2022, 78, 3587–3595. [Google Scholar] [CrossRef]
- Feng, Y.; Chuanxi, W.; Chen, F.; Cao, X.; Wang, J.; Yue, L.; Zhenyu, W. Cerium oxide nanomaterials improve cucumber flowering, fruit yield and quality: The rhizosphere effect. Environ. Sci. Nano 2023, 10, 2010–2021. [Google Scholar] [CrossRef]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in agricultural pest management and their environmental risks: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10507–10532. [Google Scholar] [CrossRef]
- Aa, E.H.; Hm, E.B.; As, A.W.; Mak, E.S. The silica-nano particles treatment of squash foliage and survival and development of Spodoptera littoralis (Bosid.) larvae. Entomol. Zool. Stud. 2016, 175, 175–180. [Google Scholar]
- Ibrahiem, S.A.; Reda, F.M.; ElAzeem, E.M.A.; Hashem, M.S.; Ammar, H.A. Mycosynthesis of chitosan-selenium nanocomposite and its activity as an insecticide against the cotton leafworm Spodoptera littoralis. Sci. Rep. 2025, 15, 1012. [Google Scholar] [CrossRef]
- El-Latef, E.A.A.; Wahba, M.N.; Mousa, S.; El-Bassyouni, G.T.; El-Shamy, A.M. Cu-doped ZnO-nanoparticles as a novel eco-friendly insecticide for controlling Spodoptera littoralis. Biocatal. Agric. Biotechnol. 2023, 52, 102823. [Google Scholar] [CrossRef]
- Hayat, F.; Khanum, F.; Li, J.; Iqbal, S.; Khan, U.; Javed, H.U.; Razzaq, M.K.; Altaf, M.A.; Peng, Y.; Ma, X.; et al. Nanoparticles and their potential role in plant adaptation to abiotic stress in horticultural crops: A review. Sci. Hortic. 2023, 321, 112285. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, J.; Bao, G.; Yan, B.; Qu, Y.; Zhang, M.; Tang, W. Physiological Responses of Highland Barley Seedlings to NaCl, Drought, and Freeze-Thaw Stress. J. Plant Growth. Regul. 2020, 40, 154–161. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of plants to overcome abiotic and biotic stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar exposure of Fe3O4 nanoparticles on Nicotiana benthamiana: Evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. Chem. Chem. 2020, 393, 122415. [Google Scholar] [CrossRef]
- Fang, J.; Peng, Y.; Zheng, L.; He, C.; Peng, S.; Huang, Y.; Wang, L.; Liu, H.; Feng, G. Chitosan-Se Engineered Nanomaterial Mitigates Salt Stress in Plants by Scavenging Reactive Oxygen Species. J. Agric. Food Chem. 2023, 72, 176–188. [Google Scholar] [CrossRef]
- Ayyaz, A.; Batool, I.; Zhang, K.N.; Hannan, F.; Sun, Y.Q.; Qin, T.J.; Athar, H.U.R.; Zafar, Z.U.; Farooq, M.A.; Zhou, W.J. Unravelling mechanisms of CaO nanoparticle-induced drought tolerance in: An analysis of metabolite and nutrient profiling. Environ. Sci. Nano. 2024, 11, 2550–2567. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Alhussaen, K.M.; El-Alosey, A.R.; Alomrani, M.A.M.; Kalaji, H.M. Titanium dioxide nanoparticles require K+ and hydrogen sulfide to regulate nitrogen and carbohydrate metabolism during adaptive response to drought and nickel stress in cucumber. Environ. Pollut. 2023, 334, 122008. [Google Scholar] [CrossRef]
- Khan, M.N.; Li, Y.; Khan, Z.; Chen, L.; Liu, J.; Hu, J.; Wu, H.; Li, Z. Nanoceria seed priming enhanced salt tolerance in rapeseed through modulating ROS homeostasis and α-amylase activities. J. Nanobiotechnol. 2021, 19, 276. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability 2022, 14, 723. [Google Scholar] [CrossRef]
- Yang, W.; Shi, C.; Hu, Q.; Wu, Y.; Fang, D.; Pei, F.; Mariga, A.M. Nanocomposite packaging regulate respiration and energy metabolism in Flammulina velutipes. Postharvest Biol. Technol. 2019, 151, 119–126. [Google Scholar] [CrossRef]
- Kusiak, M.; Oleszczuk, P.; Joko, I. Cross-examination of engineered nanomaterials in crop production: Application and related implications. J. Hazard. Mater. 2022, 424, 127374. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; Mckee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Hanif, M.N.; Aijaz, N.; Azam, K.; Akhtar, M.; Laftah, W.A.; Babur, M.; Abbood, N.K.; Benitez, I.B. Impact of microplastics on soil (physical and chemical) properties, soil biological properties/soil biota, and response of plants to it: A review. Int. J. Environ. Sci. Technol. 2024, 21, 10277–10318. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and CuO nanoparticles: A threat to soil organisms, plants, and human health. Environ. Geochem. Health. 2020, 42, 147–158. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.D.; Zerbi, G.; Lucotti, A.; Catelani, T.; Mantecca, P. Carbon nanotubes: Structural defects as stressors inducing lung cell toxicity. Chem. Biol. Interact. 2023, 382, 110613. [Google Scholar] [CrossRef]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.Y. Approach to using mechanism-based structure activity relationship (SAR) analysis to assess human health hazard potential of nanomaterials. Food Chem. Toxicol. 2015, 85, 120–126. [Google Scholar] [CrossRef]
- Drasler, B.; Sayre, P.; Steinh?user, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. Nanoimpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Dusinska, M.; Tulinska, J.; Yamani, N.E.; Kuricova, M.; Liskova, A.; Rollerova, E.; Ruden-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef]
- Varsou, D.D.; Kolokathis, P.D.; Antoniou, M.; Sidiropoulos, N.K.; Tsoumanis, A.; Papadiamantis, A.G.; Melagraki, G.; Lynch, I.; Afantitis, A. In silico assessment of nanoparticle toxicity powered by the Enalos Cloud Platform: Integrating automated machine learning and synthetic data for enhanced nanosafety evaluation. Comput. Struct. Biotechnol. J. 2024, 25, 47–60. [Google Scholar] [CrossRef]
- Mancardi, G.; Mikolajczyk, A.; Annapoorani, V.K.; Bahl, A.; Blekos, K.; Burk, J.; Çetin, Y.A.; Chairetakis, K.; Dutta, S.; Escorihuela, L.; et al. A computational view on nanomaterial intrinsic and extrinsic features for nanosafety and sustainability. Mater. Today 2023, 67, 344–370. [Google Scholar] [CrossRef]

| Nanofertilizers | Concentration | Size (nm) | Vegetable Species | References |
|---|---|---|---|---|
| Se | 0.5 mg/kg | 62.3 ± 14.6 nm | Chinese cabbage | [20] |
| Se | 10 mg/L | 61.9 ± 13.7 nm | Cherry radish | [21] |
| Biochar-iron | 500 mg/kg | N/A | Chinese cabbage | [31] |
| Mn3O4 | 10 mg/kg | 104.1 mm | Radish | [27] |
| Fe3O4 | 10 mg/L | N/A | Coriander | [26] |
| CeO2 | 50 mg/kg | 7.0 nm | Carrot | [19] |
| Mn0.5Zn0.5Fe2O4 | 100 mg/L, 200 mg/L | 14 nm | Squash | [32] |
| NaYF4:Yb,Er@CDs | 0.5 mg/mL | N/A | Mung bean | [33] |
| TiO2 | 100 mg/L | 5 nm | Tomato | [22] |
| TiO2 | 20–200 μg/mL | 80 ± 15 nm | Red bean | [23] |
| Fe | N/A | 2–6 nm | Mung bean | [34] |
| ZnO-CaO | 500 ppm | N/A | Mung bean | [35] |
| Graphene | 1000 mg/L | 8–12 nm | Tomato | [24] |
| Si | 100 mg/L, 1000 mg/L | 10–17 nm, 110–120 nm | Tomato | [36] |
| Fe3O4-SiO2 | 100 mg/L | N/A | Spinach | [37] |
| ZnMo | 124–10 mg/L | 200 nm | Pepper | [38] |
| CuO | 10 mg/L | 23.43 nm | Lettuce, Tomato | [39] |
| MgO | 100 mg/L | 15–20 nm | Green gram | [40] |
| Se | N/A | 150 nm | Potato | [41] |
| ZnO | 800 mg/L | 20–60 nm | Allium cepa L. | [42] |
| TiO2 | 10 μm | 12.8 nm | Faba bean | [43] |
| GO (Graphene oxide) | 1200 mg/L | 10–100,000 nm | Mung bean | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Bai, Y.; Zhu, D.; He, C.; Bu, F.; Luo, Y.; Zhao, P.; Qiu, Y.; Wei, Z.; Zhang, J.; et al. Innovative Application of Nanomaterials in Vegetable Cultivation: Recent Advances in Growth Promotion and Stress Tolerance. Nanomaterials 2025, 15, 1659. https://doi.org/10.3390/nano15211659
Lv W, Bai Y, Zhu D, He C, Bu F, Luo Y, Zhao P, Qiu Y, Wei Z, Zhang J, et al. Innovative Application of Nanomaterials in Vegetable Cultivation: Recent Advances in Growth Promotion and Stress Tolerance. Nanomaterials. 2025; 15(21):1659. https://doi.org/10.3390/nano15211659
Chicago/Turabian StyleLv, Wenxuan, Yixue Bai, Dongyang Zhu, Changzheng He, Fengjiao Bu, Yusong Luo, Ping Zhao, Yanhong Qiu, Zunzheng Wei, Jie Zhang, and et al. 2025. "Innovative Application of Nanomaterials in Vegetable Cultivation: Recent Advances in Growth Promotion and Stress Tolerance" Nanomaterials 15, no. 21: 1659. https://doi.org/10.3390/nano15211659
APA StyleLv, W., Bai, Y., Zhu, D., He, C., Bu, F., Luo, Y., Zhao, P., Qiu, Y., Wei, Z., Zhang, J., Guo, S., Yu, Y., Wang, J., Ren, Y., Gong, G., Zhang, H., Xu, Y., Liu, G., Dai, S., & Li, M. (2025). Innovative Application of Nanomaterials in Vegetable Cultivation: Recent Advances in Growth Promotion and Stress Tolerance. Nanomaterials, 15(21), 1659. https://doi.org/10.3390/nano15211659







