Synthesis, Electrochemistry, and Optoelectronic Properties of Biphenyl-EDOT-Based Electrochromic Polymers
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
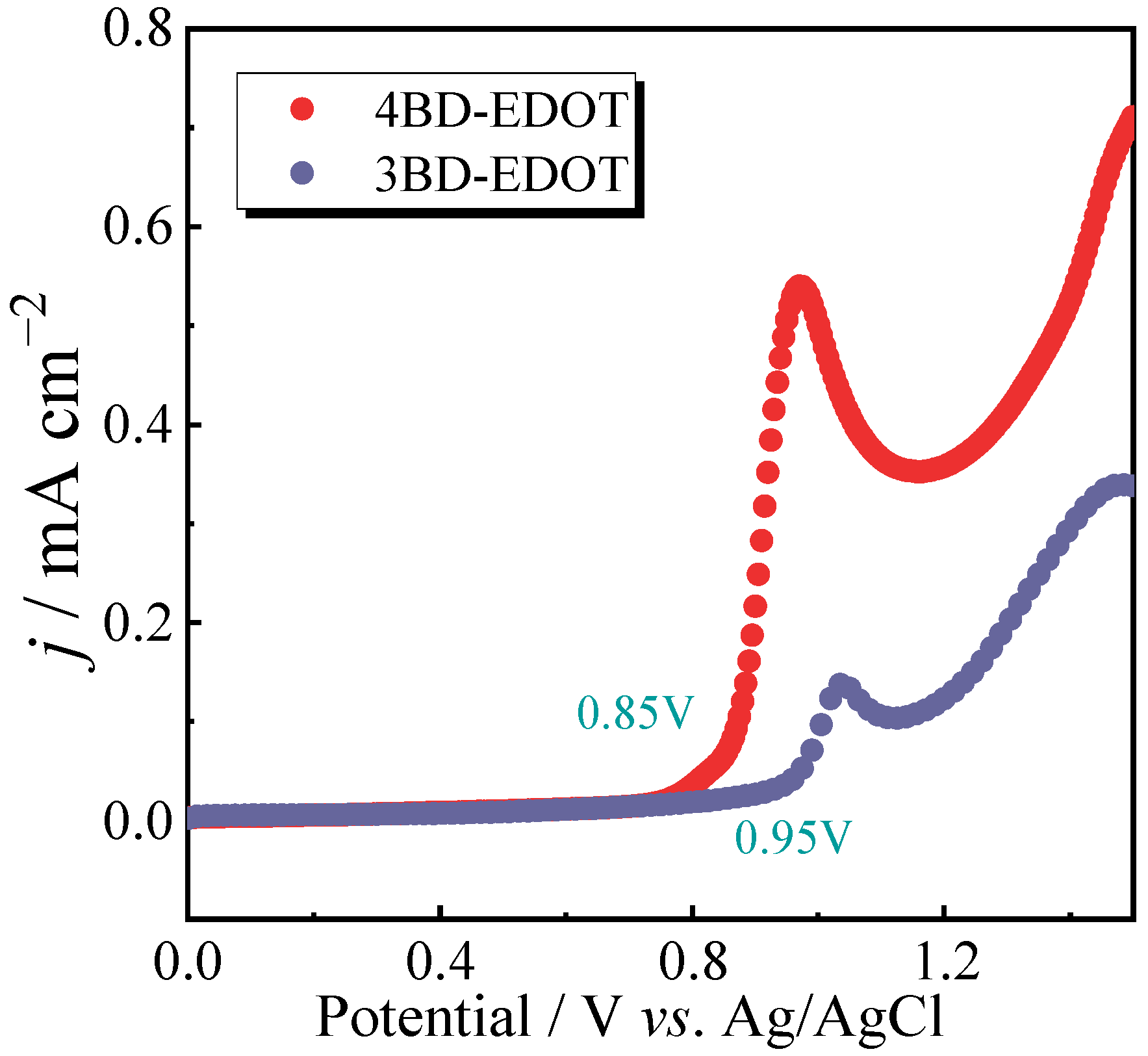
2.2. Electrochemical Polymerization of Monomers
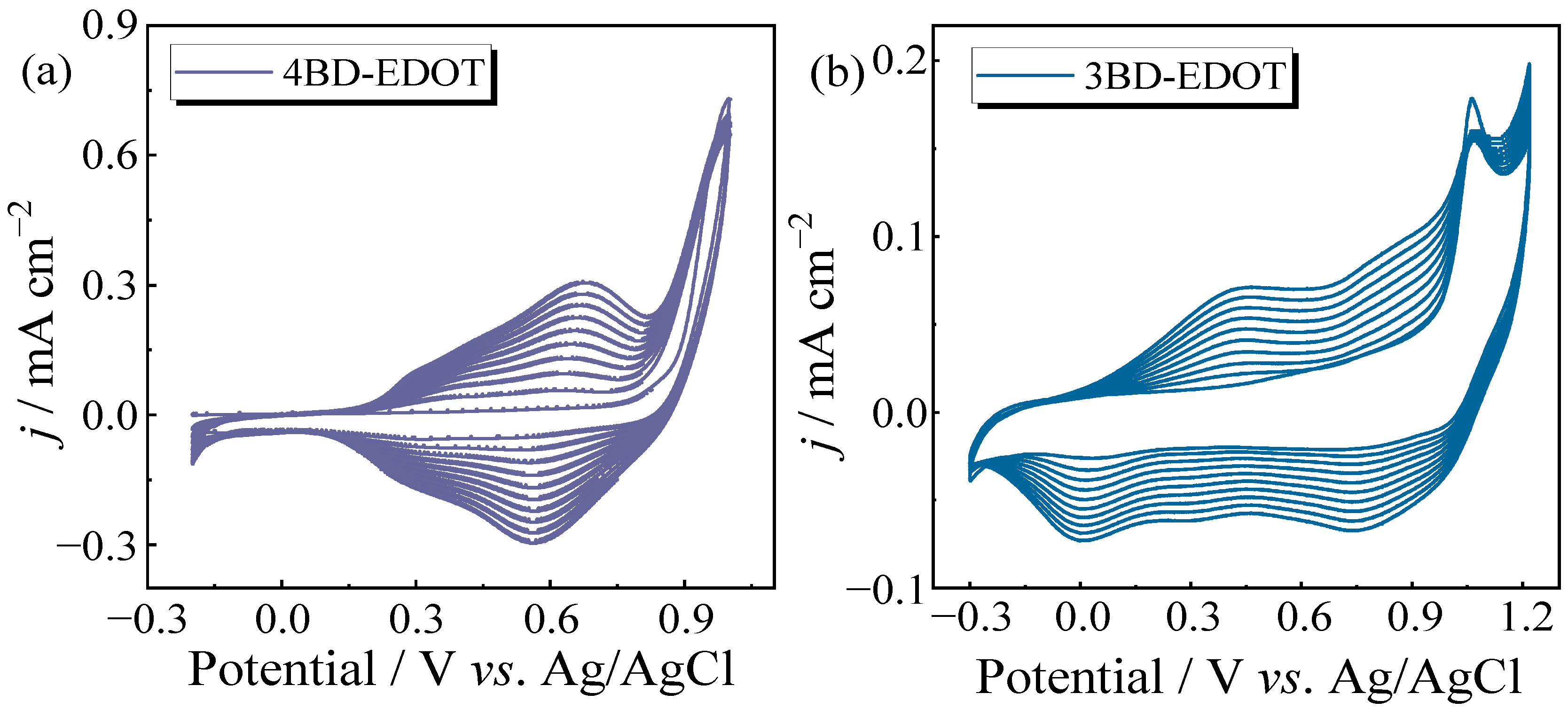
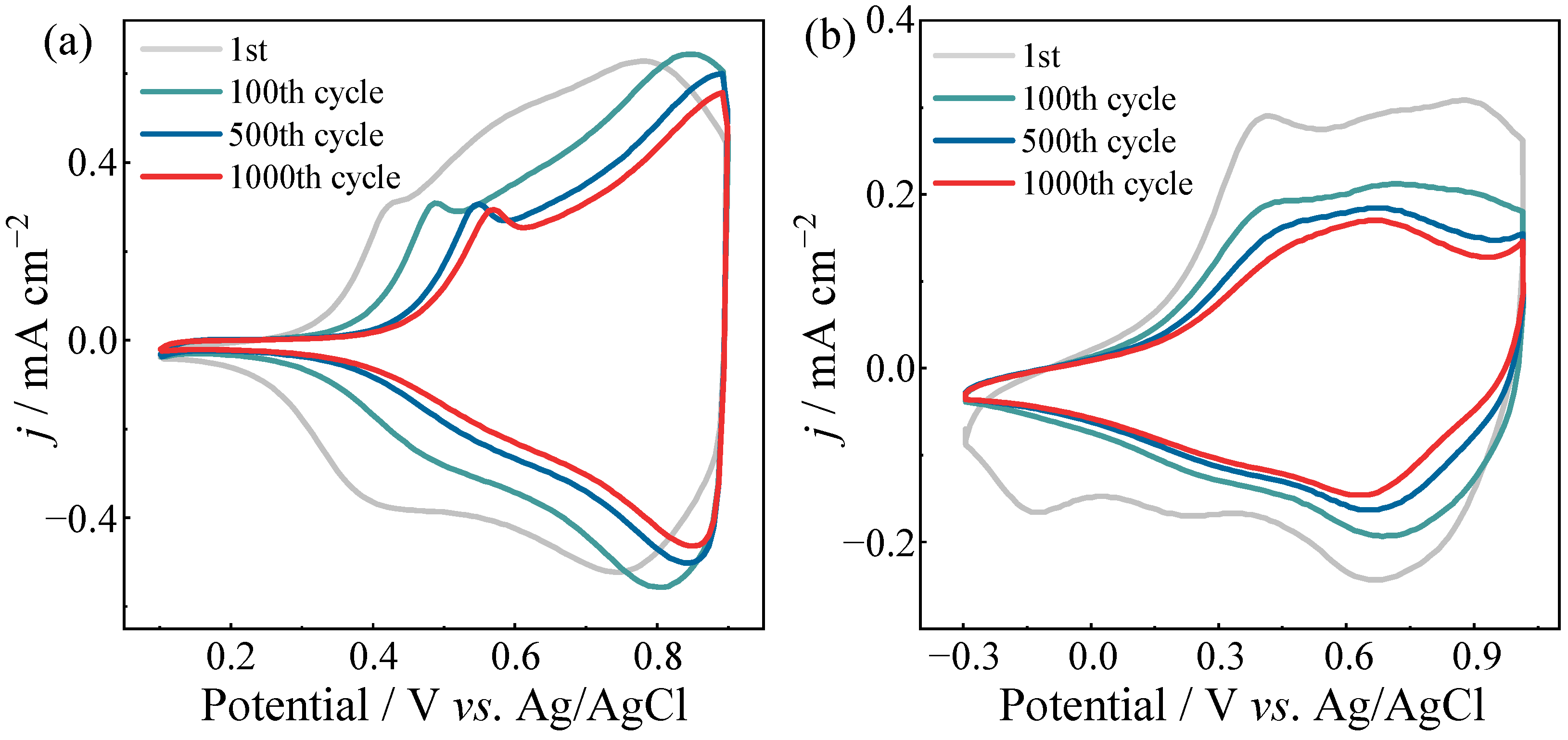
2.3. Electrochemistry of Polymers
2.4. FT-IR Spectra
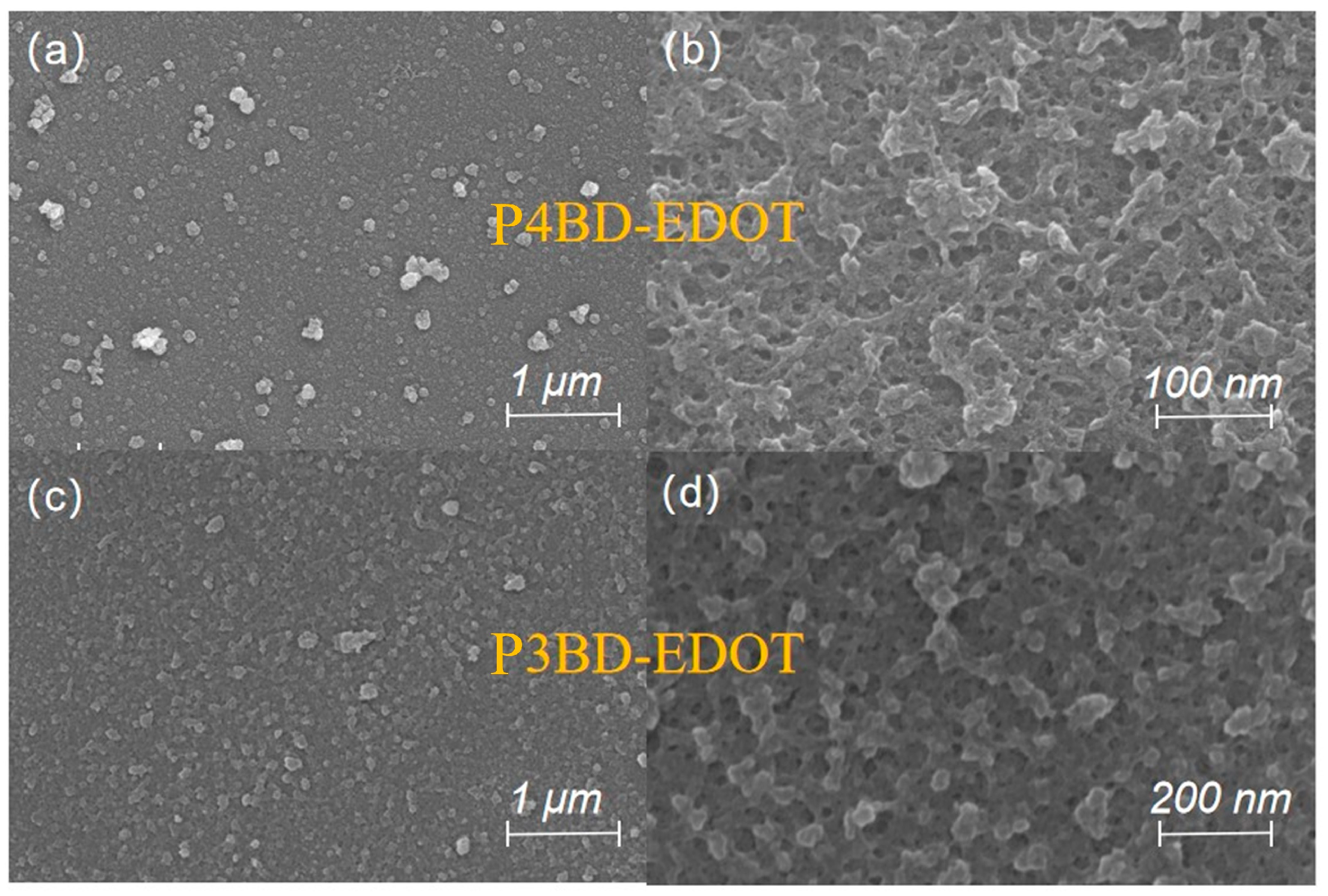
2.5. Surface Morphology
2.6. Spectroelectrochemistry
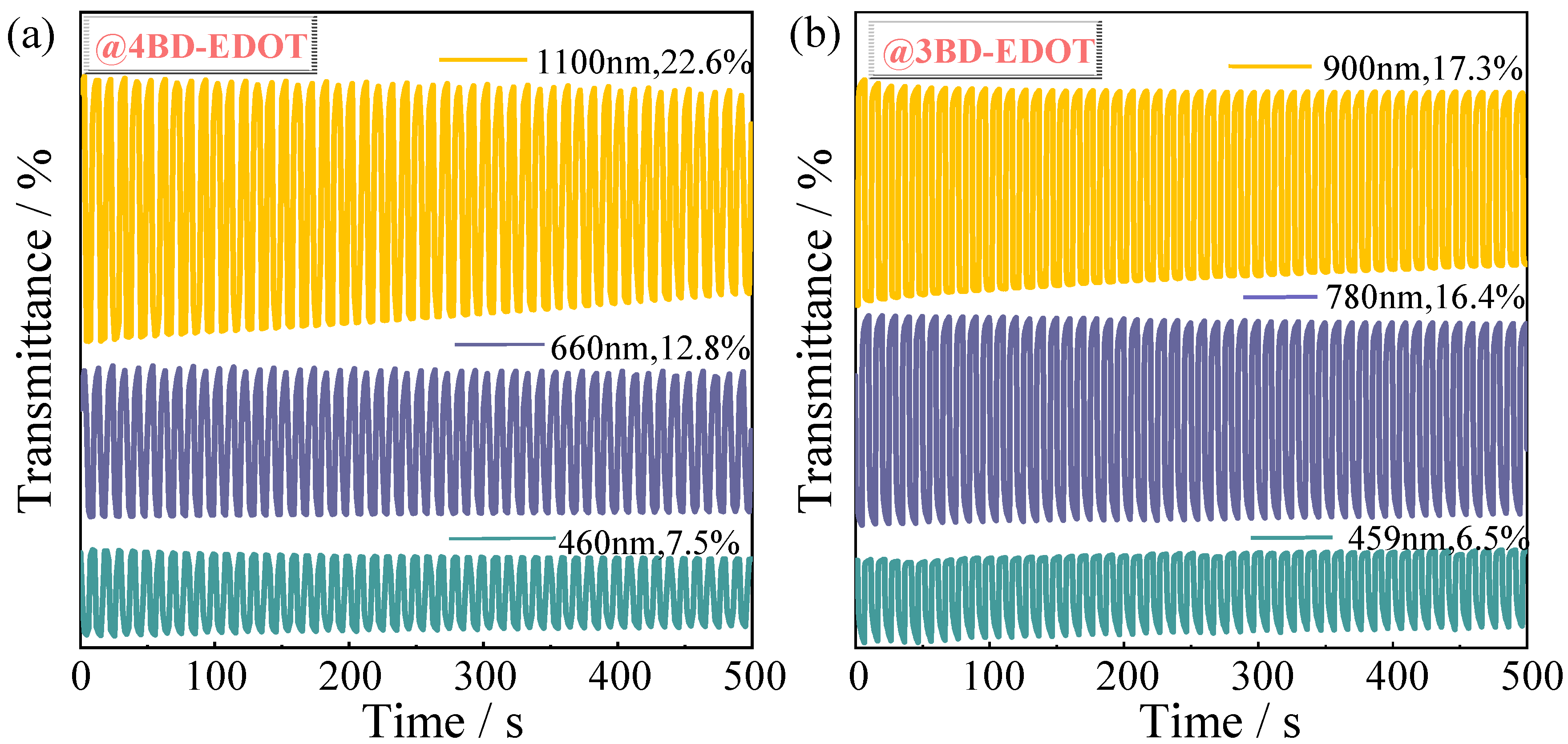
2.7. Electrochromic Properties of Polymer Films
2.8. Open Circuit Memory
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Zhao, J.S.; Cui, C.S.; Liu, J.F.; He, Q.P. Electrosynthesis and characterization of an electrochromic material from poly (1,4-bis (3-methylthiophen-2-yl) benzene) and its application in electrochromic device. Sol. Energy Mater. Sol. Cells 2012, 98, 161–167. [Google Scholar] [CrossRef]
- Wang, S.; Mo, D.Z.; Lin, K.W. EDOT substitution site effect on fluorenone acceptor units: Electrochemical polymerization and electrochromic properties of DA systems. Mater. Today Commun. 2024, 40, 109553. [Google Scholar] [CrossRef]
- Patra, B.C.; Khilari, S.; Manna, R.N.; Mondal, S.; Pradhan, D.; Pradhan, A.; Bhaumik, A. A metal-free covalent organic polymer for electrocatalytic hydrogen evolution. ACS Catal. 2017, 7, 6120–6127. [Google Scholar] [CrossRef]
- Liu, L.; Zha, D.W.; Wang, Y.; He, J.B. A nitrogen-and sulfur-rich conductive polymer for electrocatalytic evolution of hydrogen in acidic electrolytes. Int. J. Hydrogen Energy 2014, 39, 14712–14719. [Google Scholar] [CrossRef]
- Dong, Y.T.; Feng, J.X.; Li, G.R. Transition metal ion–induced high electrocatalytic performance of conducting polymer for oxygen and hydrogen evolution reactions. Macromol. Chem. Phys. 2017, 218, 1700359. [Google Scholar] [CrossRef]
- Zheng, H.H.; Yang, F.; Xiong, T.Z.; Adekoya, D.; Huang, Y.C.; Balogun, M.S.J.T. Polypyrrole hollow microspheres with boosted hydrophilic properties for enhanced hydrogen evolution water dissociation kinetics. ACS Appl. Mater. Interfaces 2020, 12, 57093–57101. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wu, X. Polypyrrole film decorated manganese oxide electrode materials for high-efficient aqueous zinc ion battery. Crystals 2023, 13, 1445. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, R.; Routh, P.; Shit, A.; Mondal, S.; Panja, A.; Nandi, A.K. Conductive MoS2 quantum dot/polyaniline aerogel for enhanced electrocatalytic hydrogen evolution and photoresponse properties. ACS Appl. Nano Mater. 2018, 1, 2306–2316. [Google Scholar] [CrossRef]
- Smie, A.; Synowczyk, A.; Heinze, J.; Alle, R.; Tschuncky, P.; Götz, G.; Bäuerle, P. β, β-Disubstituted oligothiophenes, a new oligomeric approach towards the synthesis of conducting polymers. J. Electroanal. Chem. 1998, 452, 87–95. [Google Scholar] [CrossRef]
- Wen, Y.-P.; Li, D.; Lu, Y.; He, H.-H.; Xu, J.-K.; Duan, X.-M.; Liu, M. Poly (3,4-ethylenedioxythiophene methanol)/ascorbate oxidase/nafion-single-walled carbon nanotubes biosensor for voltammetric detection of vitamin C. Chin. J. Polym. Sci. 2012, 30, 460–469. [Google Scholar] [CrossRef]
- Sato, M.; Yamanaka, S.; Nakaya, J.; Hyodo, K. Electrochemical copolymerization of aniline with o-aminobenzonitrile. Electrochim. Acta 1994, 39, 2159–2167. [Google Scholar] [CrossRef]
- Tao, Y.J.; Zhou, Y.J.; Xu, X.Q.; Zhang, Z.Y.; Cheng, H.F.; Zheng, W.W. A multielectrochromic copolymer based on anthracene and thiophene via electrochemical copolymerization in boron trifluoride diethyl etherate. Electrochim. Acta 2012, 78, 353–358. [Google Scholar] [CrossRef]
- Zhang, C.; Hua, C.; Wang, G.H.; Ouyang, M.; Ma, C.N. A novel multichromic copolymer of 1,4-bis (3-hexylthiophen-2-yl) benzene and 3, 4-ethylenedioxythiophene prepared via electrocopolymerization. J. Electroanal. Chem. 2010, 645, 50–57. [Google Scholar] [CrossRef]
- Steckler, T.T.; Henriksson, P.; Mollinger, S.; Lundin, A.; Salleo, A.; Andersson, M.R. Very low band gap thiadiazoloquinoxaline donor–acceptor polymers as multi-tool conjugated polymers. J. Am. Chem. Soc. 2014, 136, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J.; Yassar, A.; Garnier, F. Electrochemical synthesis of highly conducting poly (thiophene) thin films. Synth. Met. 1989, 28, 275–280. [Google Scholar] [CrossRef]
- Turbiez, M.; Frère, P.; Roncali, J. Oligothienylenevinylenes incorporating 3, 4-ethylenedioxythiophene (EDOT) units. Tetrahedron 2005, 61, 3045–3053. [Google Scholar] [CrossRef]
- Ren, Z.; Shen, S.L.; Mo, D.Z.; Chao, P.J. Fluorinated Benzo [c][1,2,5] Selenadiazole-Based Electrochromic Polymers: Effects of Heteroatom Substitution and Fluorination on Performance. Polymer 2025, 338, 129056. [Google Scholar] [CrossRef]
- Mo, D.Z.; Tong, T. Tunable optoelectronic performances of fluorinated benzene-EDOT based hybrid electrochromic polymers. Polymer 2024, 303, 127117. [Google Scholar] [CrossRef]
- Cui, K.; Mo, D.Z.; Chao, P.J. The tunable optoelectronic performances of EDOT-benzene-based hybrid electrochromic polymers with different methoxy groups. Electrochim. Acta 2025, 525, 146108. [Google Scholar] [CrossRef]
- Nie, M.; Xia, Y.F.; Wang, Z.B.; Yu, F.D.; Zhang, Y.; Wu, J.; Wu, B. Effects of precursor particle size on the performance of LiNi0.5Co0.2Mn0.3O2 cathode material. Ceram. Int. 2015, 41, 15185–15192. [Google Scholar] [CrossRef]
- Yang, J.; Giuso, V.; Hou, M.C.; Remadna, E.; Forté, J.; Su, H.C.; Gourlaouen, C.; Mauro, M.; Bertrand, B. Biphenyl Au (III) complexes with phosphine ancillary ligands: Synthesis, optical properties, and electroluminescence in Light-Emitting electrochemical cells. Inorg. Chem. 2023, 62, 4903–4921. [Google Scholar] [CrossRef]
- Jain, Z.J.; Gide, P.S.; Kankate, R.S. Biphenyls and their derivatives as synthetically and pharmacologically important aromatic structural moieties. Arabian J. Chem. 2017, 10, S2051–S2066. [Google Scholar] [CrossRef]
- Ge, C.Y.; Lee, J.J. Simultaneous and interference-free detection of hydroquinone and catechol on poly (Evans Blue)-modified glassy carbon electrode. J. Electrochem. Soc. 2016, 163, B556. [Google Scholar] [CrossRef]
- Orritt, K.M.; Feng, L.P.; Newell, J.F.; Sutton, J.N.; Grossman, S.; Germe, T.; Abbott, L.R.; Jackson, H.L.; Bury, B.K.L.; Maxwell, A.; et al. De novo design of type II topoisomerase inhibitors as potential antimicrobial agents targeting a novel binding region. RSC Med. Chem. 2022, 13, 831–839. [Google Scholar] [CrossRef]
- Ehlert, J.; Kronemann, J.; Zumbrägel, N.; Preller, M. Lipase-catalyzed chemoselective ester hydrolysis of biomimetically coupled aryls for the synthesis of unsymmetric biphenyl Esters. Molecules 2019, 24, 4272. [Google Scholar] [CrossRef]
- Kwong, H.C.; Kumar, C.S.C.; Mah, S.H.; Chia, T.S.; Quah, C.K.; Loh, Z.H.; Chandraju, S.; Lim, G.K. Novel biphenyl ester derivatives as tyrosinase inhibitors: Synthesis, crystallographic, spectral analysis and molecular docking studies. PLoS ONE 2017, 12, e0170117. [Google Scholar] [CrossRef] [PubMed]
- Zade, S.S.; Bendikov, M. Twisting of Conjugated Oligomers and Polymers: Case Study of Oligo-and Polythiophene. Chem. Eur. J. 2007, 13, 3688–3700. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.; Jeong, H.; Jeon, S. Optoelectrochemical properties of copolymer of terthiophene with 3, 4-ethlenedioxypyrrole. J. Electroanal. Chem. 2009, 636, 107–112. [Google Scholar] [CrossRef]
- Xue, Y.; Xue, Z.X.; Zhang, W.W.; Zhang, W.N.; Chen, S.A.; Lin, K.W.; Xu, J.K. Enhanced electrochromic performances of Polythieno [3, 2-b] thiophene with multicolor conversion via embedding EDOT segment. Polymer 2018, 159, 150–156. [Google Scholar] [CrossRef]
- Yigitsoy, B.; Karim, S.M.A.; Balan, A.; Baran, D.; Toppare, L. Benzyl substituted benzotriazole containing conjugated polymers: Effect of position of the substituent on electrochromic properties. Synth. Met. 2010, 160, 2534–2539. [Google Scholar] [CrossRef]
- Hwang, J.; Son, J.I.; Shim, Y.B. Electrochromic and electrochemical properties of 3-pyridinyl and 1, 10-phenanthroline bearing poly (2,5-di (2-thienyl)-1H-pyrrole) derivatives. Sol. Energy Mater. Sol. Cells 2010, 94, 1286–1292. [Google Scholar] [CrossRef]
- Chen, L.C.; Ho, K.C. Design equations for complementary electrochromic devices: Application to the tungsten oxide–Prussian blue system. Electrochim. Acta 2001, 46, 2151–2158. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Facchetti, A.; Mushrush, M.; Yoon, M.H.; Hutchison, G.R.; Ratner, M.A.; Marks, T.J. Building blocks for N-type molecular and polymeric electronics. perfluoroalkyl-versus alkyl-functionalized oligothiophenes (NTs; n = 2–6). systematic synthesis, spectroscopy, electrochemistry, and solid-state organization. J. Am. Chem. Soc. 2004, 126, 13480–13501. [Google Scholar] [CrossRef] [PubMed]
- Perepichka, I.F.; Perepichka, D.F. Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 2. [Google Scholar]
- Becke, A.D. Density–functional thermochemistry. I. The effect of the exchange–only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Ginder, J.; Epstein, A.; MacDiarmid, A. Torsional Defects and Photoinduced Charge Transfer in Ring-Containing Polymers. Mol. Cryst. Liq. Cryst. 1991, 194, 13–21. [Google Scholar] [CrossRef]
- Roncali, J.; Blancharda, P.; Frère, P. 3,4-Ethylenedioxythiophene (EDOT) as a versatile building block for advanced functional π-conjugated systems. J. Mater. Chem. 2005, 15, 1589–1610. [Google Scholar] [CrossRef]
- Wang, S.; Mo, D.Z. Adjustable optoelectronic properties of triphenylamine-ethylenedioxythiophene based hybrid electrochromic polymer with different electron-donating substituents. Appl. Mater. Today 2024, 40, 102357. [Google Scholar] [CrossRef]
- Mo, D.; Tong; Lin, K. Chlorinated benzothiadiazole-based donor-acceptor polymers with tunable optoelectronic performances. Electrochim. Acta 2023, 473, 143506. [Google Scholar] [CrossRef]
- Mo, D.; Tong, T.; Chao, P.; Deng, K.; Zhang, Q. Effects of polymer precursor conjugation length on the optoelectronic properties of fluorinated benzothiadiazole-based D–A systems. New J. Chem. 2024, 48, 7590–7598. [Google Scholar] [CrossRef]
- Ren, Z.; Mo, D.; Wang, S.; Tong, T.; Deng, K.; Chao, P. Effects of fluorine atom numbers on electrochromic properties of the benzothiadiazole-based D-A polymers. Polymer 2024, 312, 127655. [Google Scholar] [CrossRef]
- Mo, D.; Ren, Z.; Deng, K.; Chao, P. Difluorinated benzoselenadiazole: A new promising electron withdrawing acceptor unit for building efficient D-A type electrochromic polymers. Polymer 2025, 319, 128068. [Google Scholar] [CrossRef]
- Ren, Z.; Zhou, J.; Mo, D.; Chao, P. Difluorinated benzothiadiazole based donor-acceptor electrochromic polymers with tunable optoelectronic properties by varying the thiophene donor units. Synth. Met. 2025, 314, 117946. [Google Scholar] [CrossRef]
- Li, M.; Sheynin, Y.; Patra, A.; Bendikov, M. Tuning the Electrochromic Properties of Poly(alkyl-3,4-ethylenedioxyselenophenes) Having High Contrast Ratio and Coloration Efficiency. Chem. Mater. 2009, 21, 2482–2488. [Google Scholar] [CrossRef]
- Sonmez, G.; Meng, H.; Wudl, F. Organic Polymeric Electrochromic Devices: Polychromism with Very High Coloration Efficiency. Chem. Mater. 2004, 16, 574–580. [Google Scholar] [CrossRef]




| Polymer | Redox Stability | λmax (nm) | λonset (nm) | Eg,opt (eV) | Reduction | Oxidation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |||||||
| P4BD-EDOT | 55% (1000 cycles) | 457 | 805 | 1.54 |  | 56.66 | −0.50 | 10.72 |  | 54.46 | 8.30 | −6.08 |
| P3BD-EDOT | 49% (1000 cycles) | 460 | 714 | 1.73 |  | 70.25 | 1.31 | 8.76 |  | 88.23 | −2.51 | 3.52 |
| Polymers | Wavelength (nm) | ∆T (%) | Response Time/s | CE/cm2 C−1 | |
|---|---|---|---|---|---|
| Reduction | Oxidation | ||||
| P4BD-EDOT | 460 | 7.5 | 3.2 | 1.5 | 189.6 |
| 660 | 12.8 | 3.0 | 1.7 | 162.0 | |
| 1100 | 22.6 | 0.5 | 0.5 | 189.6 | |
| P3BD-EDOT | 459 | 6.5 | 0.3 | 0.2 | 141.1 |
| 780 | 16.4 | 0.3 | 0.2 | 250.4 | |
| 900 | 17.3 | 0.4 | 0.2 | 190.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Deng, Y.; Mo, D.; Xu, M.; Deng, K. Synthesis, Electrochemistry, and Optoelectronic Properties of Biphenyl-EDOT-Based Electrochromic Polymers. Nanomaterials 2025, 15, 1643. https://doi.org/10.3390/nano15211643
Shen S, Deng Y, Mo D, Xu M, Deng K. Synthesis, Electrochemistry, and Optoelectronic Properties of Biphenyl-EDOT-Based Electrochromic Polymers. Nanomaterials. 2025; 15(21):1643. https://doi.org/10.3390/nano15211643
Chicago/Turabian StyleShen, Shuanglai, Yaoteng Deng, Daize Mo, Mengze Xu, and Kuirong Deng. 2025. "Synthesis, Electrochemistry, and Optoelectronic Properties of Biphenyl-EDOT-Based Electrochromic Polymers" Nanomaterials 15, no. 21: 1643. https://doi.org/10.3390/nano15211643
APA StyleShen, S., Deng, Y., Mo, D., Xu, M., & Deng, K. (2025). Synthesis, Electrochemistry, and Optoelectronic Properties of Biphenyl-EDOT-Based Electrochromic Polymers. Nanomaterials, 15(21), 1643. https://doi.org/10.3390/nano15211643






