A Review on ZnO Nanostructures for Optical Biosensors: Morphology, Immobilization Strategies, and Biomedical Applications
Abstract
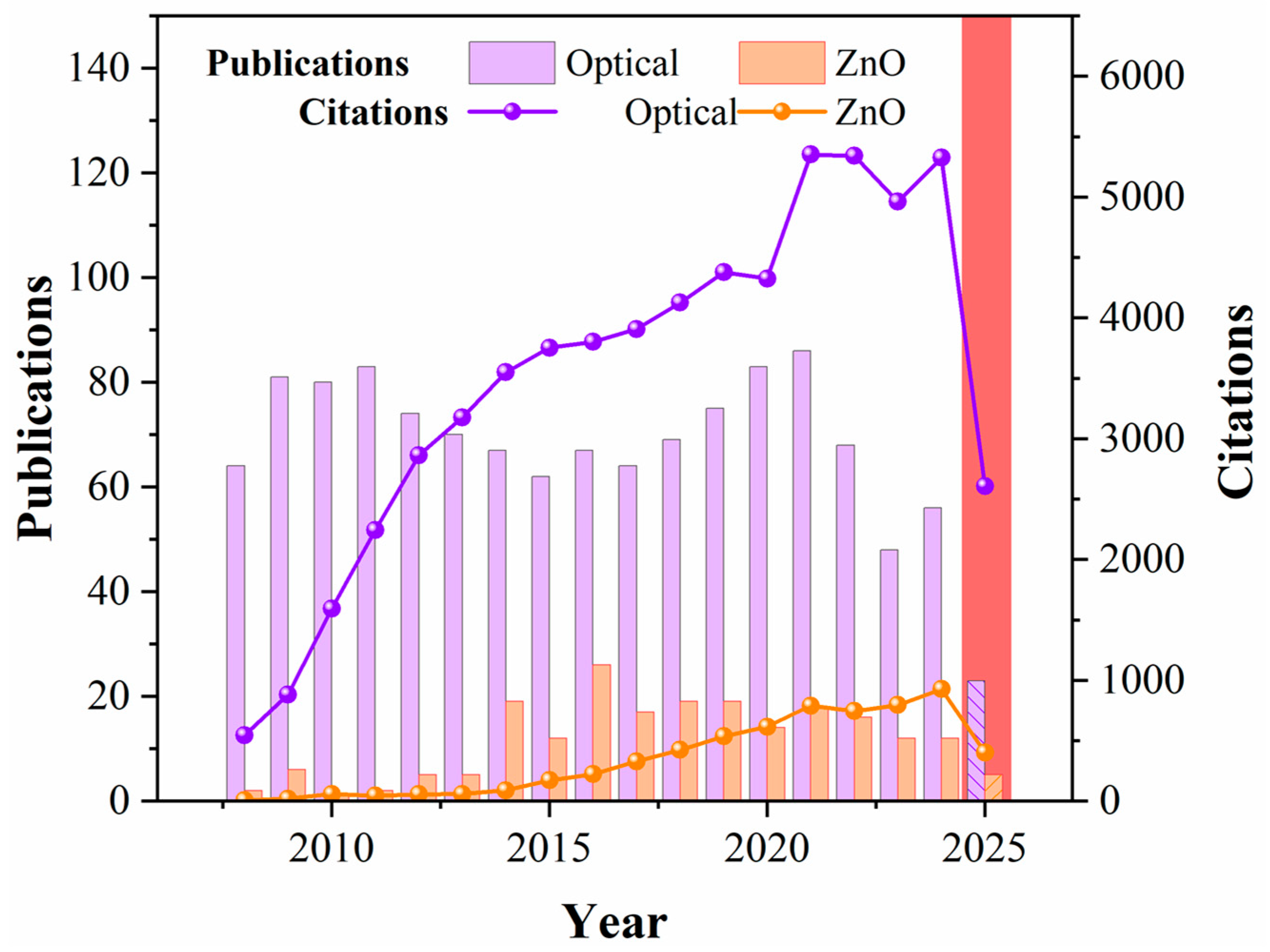
1. Introduction
- Nanostructuring flexibility: It can be fabricated through top-down (e.g., glancing-angle ion irradiation) or bottom-up (e.g., vapor-phase and chemical solution) approaches, yielding high-quality nanostructures.
- Broad substrate compatibility: It adheres to a wide range of substrates, from flexible polymers (e.g., polyimide, polyethylene naphthalate) to rigid ceramics (e.g., alumina), and from crystalline (e.g., quartz, silicon wafers) to amorphous (e.g., glass) materials.
- Superior physical properties: It exhibits enhanced electrical conductivity, optical transparency, and mechanical robustness compared with its bulk counterpart.
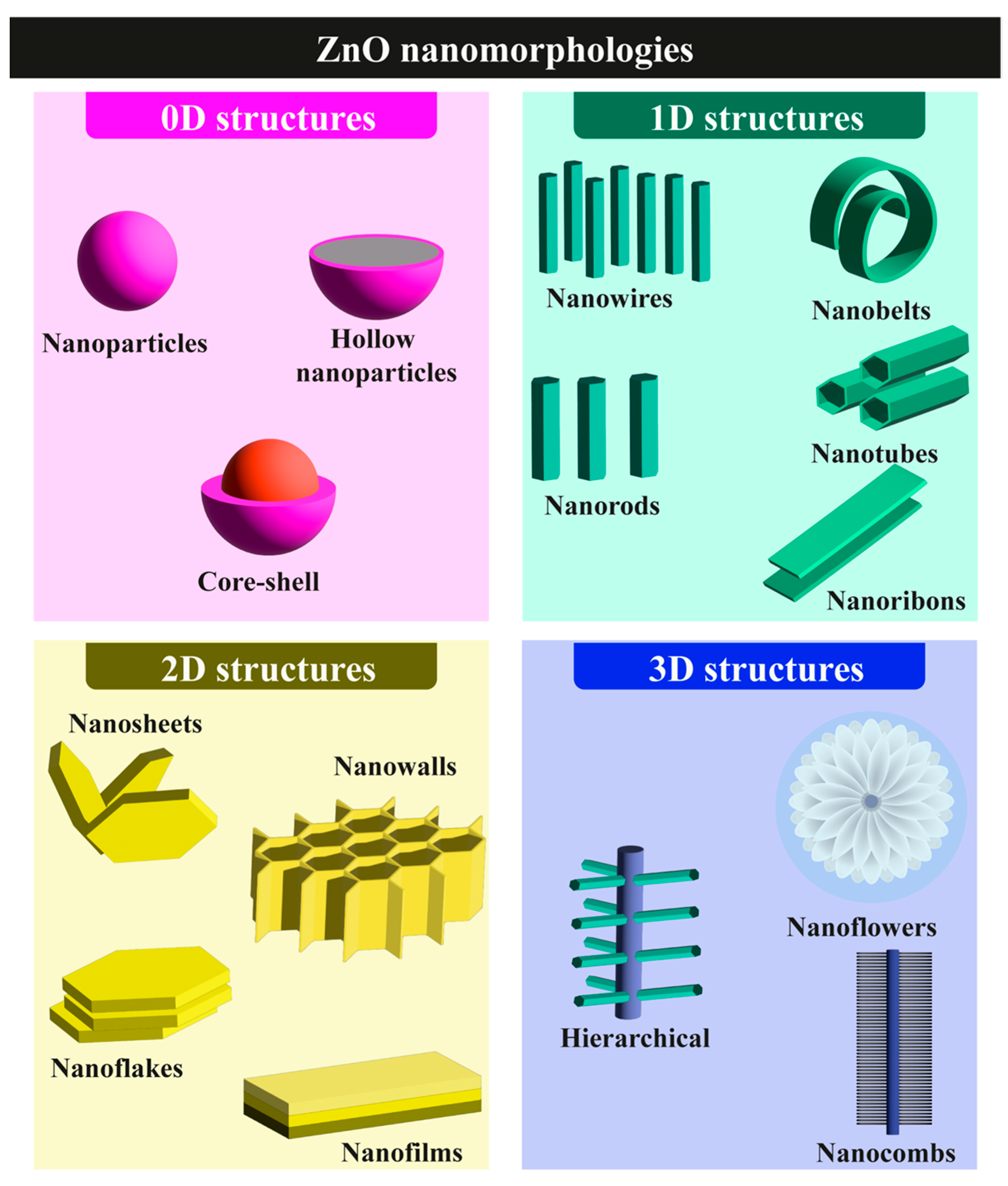
- Morphological diversity: It can be engineered into structures ranging from zero-dimensional (0D) nanoparticles to three-dimensional (3D) hierarchical architectures, allowing tailored biosensor configurations (Figure 3).
2. Strategies to Synthesize ZnO Nanostructures
2.1. Physical Methods

2.2. Wet-Chemical Solution Methods
2.3. ZnO Nanosystems: A Stable Platform for Biosensing Applications
2.3.1. 0D ZnO Nanostructures
2.3.2. One-Dimensional ZnO Nanostructures
2.3.3. Two-Dimensional ZnO Nanostructures
2.3.4. Three-Dimensional ZnO Nanostructures
3. Functionalization Strategies of ZnO Nanostructures
- Surface chemistry. Electrostatic interactions, surface area, loading capacity, and the number of active binding sites are governed by surface charge, porosity, crystalline orientation, and surface functional groups.
- Immobilization strategy. This encompasses covalent conjugation via silane agents (e.g., APTES), physical adsorption, electrochemical deposition, and other surface modification techniques.
3.1. Covalent Binding Methods
3.2. Direct Physical Adsorption Methods
3.3. Entrapment-Encapsulation Strategies
4. Optical Detection of Biomolecules in ZnO-Based Platforms
- Surface plasmon resonance (SPR) and localized SPR (LSPR);
- Fluorescence-based;
- Surface-enhanced Raman scattering (SERS);
- Photoluminescence (PL) spectroscopy biosensors.
- Label-free systems, such as SPR, which allow direct detection of analytes without additional tags.
- Labeled systems, which rely on fluorescence or chemiluminescence tags to amplify the detection signal.
4.1. Surface Plasmon Resonance (SPR) and Localized-SPR (LSPR) Biosensors
4.2. Fluorescence-Based Biosensors
4.3. Surface-Enhanced Raman Scattering (SERS) Spectroscopy
4.4. Photoluminescence Spectroscopy
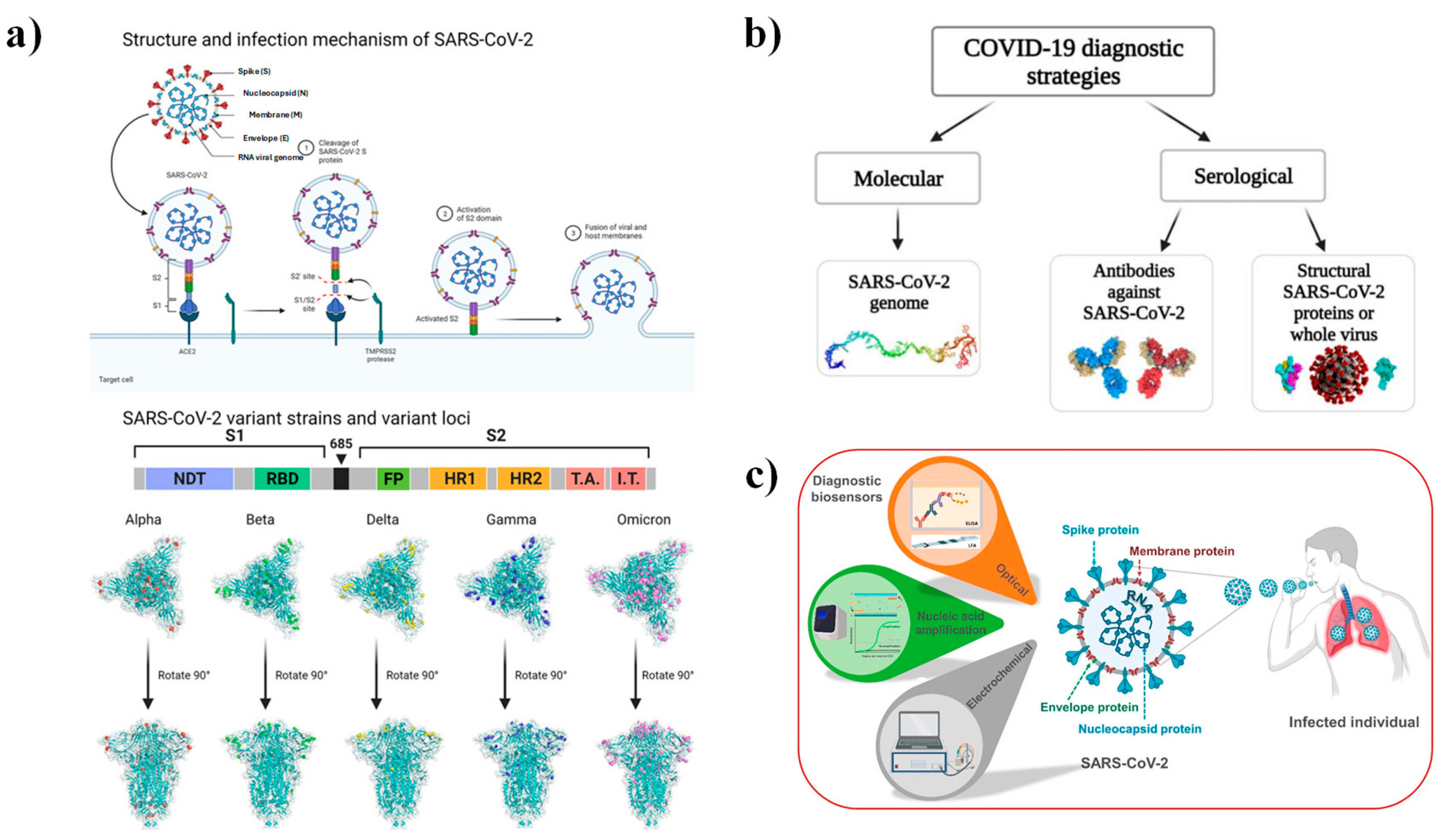
5. ZnO-Based Biosensors Targeting SARS-CoV-2: Perspectives and Future Directions
6. Future Directions
- 0D nanostructures provide label-free detection via quantum confinement and strong fluorescence signals. Their integration with metal NPs further enhances optical responses and lowers the limit of detection.
- 1D nanostructures offer high surface areas for dense biomolecule immobilization and efficient charge transport, enhancing sensitivity and reducing signal noise. Techniques like hydrothermal synthesis, CVD, or template-assisted growth enable their integration onto various substrates with controlled orientation and density.
- 2D nanostructures enable uniform biomolecule coverage and superior electron mobility, often forming hybrid heterostructures with synergistic properties.
- 3D morphologies provide ultra-high surface areas, ideal for multiplexed detection and rapid response times, often within minutes or in real-time.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Rab, S.; Pratap Singh, R.; Suman, R. Sensors for Daily Life: A Review. Sens. Int. 2021, 2, 100121. [Google Scholar] [CrossRef]
- Varshney, S.; Kumar Rajput, P.; Singhal, A.; Varshney, G. A Review on the Recent Developments in the Materials Used for Sensors. Mater. Today Proc. 2022, 62, 6679–6683. [Google Scholar] [CrossRef]
- Ghanbarlou, S.; Kahforoushan, D.; Abdollahi, H.; Zarrintaj, P.; Alomar, A.; Villanueva, C.; Davachi, S.M. Advances in Quantum Dot-Based Fluorescence Sensors for Environmental and Biomedical Detection. Talanta 2025, 294, 128176. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent Advances in ZnO Nanostructures and Thin Films for Biosensor Applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef]
- Viter, R.; Savchuk, M.; Iatsunskyi, I.; Pietralik, Z.; Starodub, N.; Shpyrka, N.; Ramanaviciene, A.; Ramanavicius, A. Analytical, Thermodynamical and Kinetic Characteristics of Photoluminescence Immunosensor for the Determination of Ochratoxin A. Biosens. Bioelectron. 2018, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Zhou, N.; Li, M.; Huang, C.; Mao, H. Recyclable 3D SERS Devices Based on ZnO Nanorod-Grafted Nanowire Forests for Biochemical Sensing. Appl. Surf. Sci. 2022, 582, 152336. [Google Scholar] [CrossRef]
- Rodrigues, J.; Pereira, S.O.; Zanoni, J.; Rodrigues, C.; Brás, M.; Costa, F.M.; Monteiro, T. ZnO Transducers for Photoluminescence-Based Biosensors: A Review. Chemosensors 2022, 10, 39. [Google Scholar] [CrossRef]
- Zhao, Z.; Lei, W.; Zhang, X.; Wang, B.; Jiang, H. ZnO-Based Amperometric Enzyme Biosensors. Sensors 2010, 10, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-Based Biosensor and Their Applications: A Review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and Applications of ZnO Nanostructures (ZONSs): A Review. Crit. Rev. Solid. State Mater. Sci. 2022, 47, 99–141. [Google Scholar] [CrossRef]
- Portillo-Cortez, K.; Martínez, A.; Bizarro, M.; García-Sánchez, M.F.; Güell, F.; Dutt, A.; Santana, G. ZnO Nanowires/N719 Dye with Different Aspect Ratio as a Possible Photoelectrode for Dye-Sensitized Solar Cells. Front. Chem. 2021, 8, 604092. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Lázaro, A.; Verdín-Betancourt, F.A.; Jayaraman, V.K.; Hernández-Gordillo, A.; de Lourdes López-González, M.; Sierra-Santoyo, A.; Santana, G.; Bizarro, M. Tracing the Degradation Pathway of Temephos Pesticide Achieved with Photocatalytic ZnO Nanostructured Films. Environ. Sci. Nano 2022, 9, 3538–3550. [Google Scholar] [CrossRef]
- Chou, H.T.; Huang, W.H.; Wu, T.M.; Yu, Y.K.; Hsu, H.C. LSPR Effects of Au Nanoparticles/ZnO Nano-Composite Films. Sens. Biosensing Res. 2017, 14, 17–20. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Lee, S.K. Fiber Optic Sensor Based on ZnO Nanowires Decorated by Au Nanoparticles for Improved Plasmonic Biosensor. Sci. Rep. 2019, 9, 15605. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. Current Challenges and Developments in Perovskite-Based Electrochemical Biosensors for Effective Theragnostics of Neurological Disorders. ACS Omega 2022, 7, 39491–39497. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Conejo-Dávila, A.S.; Estrada-Monje, A.; Maldonado-Orozco, M.C.; Reyes-López, S.Y.; Zaragoza-Contreras, E.A. Electrochemical Perovskite-Based Sensors for the Detection of Relevant Biomarkers for Human Kidney Health. Chemosensors 2023, 11, 507. [Google Scholar] [CrossRef]
- Krishna, M.S.; Singh, S.; Batool, M.; Fahmy, H.M.; Seku, K.; Shalan, A.E.; Lanceros-Mendez, S.; Zafar, M.N. A Review on 2D-ZnO Nanostructure Based Biosensors: From Materials to Devices. Mater. Adv. 2022, 4, 320–354. [Google Scholar] [CrossRef]
- Cong, S.; Liu, X.; Jiang, Y.; Zhang, W.; Zhao, Z. Surface Enhanced Raman Scattering Revealed by Interfacial Charge-Transfer Transitions. Innovation 2020, 1, 100051. [Google Scholar] [CrossRef] [PubMed]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical Biosensors Based on ZnO Nanostructures: Advantages and Perspectives. A Review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Colniță, A.; Toma, V.A.; Brezeștean, I.A.; Tahir, M.A.; Dina, N.E. A Review on Integrated ZnO-Based SERS Biosensors and Their Potential in Detecting Biomarkers of Neurodegenerative Diseases. Biosensors 2023, 13, 499. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured Metal Oxide-Based Biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Krishna, S.B.N.; Jakmunee, J.; Mishra, Y.K.; Prakash, J. ZnO Based 0-3D Diverse Nano-Architectures, Films and Coatings for Biomedical Applications. J. Mater. Chem. B 2024, 12, 2950–2984. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Javed, S.; Usman, M. A Review on Synthesis and Optoelectronic Applications of Nanostructured ZnO. Front. Mater. 2021, 8, 613825. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Ahmad Noorden, A.F.; Loong Peng Tan, M.; Jamaluddin, H.; Hamid, F.A.; Ahmad, M.K.; Hashim, U.; Ahmad, M.R.; Sultan, S.M. Three Dimensional Zinc Oxide Nanostructures as an Active Site Platform for Biosensor: Recent Trend in Healthcare Diagnosis. J. Electrochem. Soc. 2020, 167, 137501. [Google Scholar] [CrossRef]
- Viter, R.; Tepliakova, I.; Drobysh, M.; Zbolotnii, V.; Rackauskas, S.; Ramanavicius, S.; Grundsteins, K.; Liustrovaite, V.; Ramanaviciene, A.; Ratautaite, V.; et al. Photoluminescence-Based Biosensor for the Detection of Antibodies against SARS-CoV-2 Virus Proteins by ZnO Tetrapod Structure Integrated within Microfluidic System. Sci. Total Environ. 2024, 939, 173333. [Google Scholar] [CrossRef]
- Xin, Z.; He, Q.; Wang, S.; Han, X.; Fu, Z.; Xu, X.; Zhao, X. Recent Progress in ZnO-Based Nanostructures for Photocatalytic Antimicrobial in Water Treatment: A Review. Appl. Sci. 2022, 12, 7910. [Google Scholar] [CrossRef]
- Portillo-Cortez, K.; Islas, S.R.; Serrano-Lázaro, A.; Ortiz, A.; García-Sánchez, M.F.; Alonso, J.C.; Martínez, A.; Ramos, C.; Dutt, A.; Santana, G. A Novel Soft Deposition Methodology for Textured ZnO:Al Thin Films as Efficient Transparent Conductive Oxide Layers. Appl. Surf. Sci. Adv. 2022, 9, 100255. [Google Scholar] [CrossRef]
- Serrano-Lázaro, A.; Bizarro, M.; Kanakkillam, S.S.; Segura-Zavala, J.A.; Sánchez-Aké, C. Photocatalytic Activity of ZnO Films with Laser-Induced Dewetted Bimetallic NPs: Impact of Au/Pd Ratio. Mater. Sci. Semicond. Process. 2024, 179, 108530. [Google Scholar] [CrossRef]
- Serrano-Lázaro, A.; Verdín-Betancourt, F.A.; Jayaraman, V.K.; de Lourdes López-González, M.; Hernández-Gordillo, A.; Sierra-Santoyo, A.; Bizarro, M. Efficient Photocatalytic Elimination of Temephos Pesticide Using ZnO Nanoflowers. J. Photochem. Photobiol. A Chem. 2020, 393, 112414. [Google Scholar] [CrossRef]
- Maafa, I.M. Potential of Zinc Oxide Nanostructures in Biosensor Application. Biosensors 2025, 15, 61. [Google Scholar] [CrossRef]
- Galdamez, A.; Serrano, A.; Santana, G.; Arjona, N.; Arriaga, L.G.; Tapia Ramirez, J.; Oza, G.; Dutt, A. DNA Probe Functionalization on Different Morphologies of ZnO/Au Nanowire for Bio-Sensing Applications. Mater. Lett. 2019, 235, 250–253. [Google Scholar] [CrossRef]
- Sytu, M.R.C.; Hahm, J.I. Principles and Applications of ZnO Nanomaterials in Optical Biosensors and ZnO Nanomaterial-Enhanced Biodetection. Biosensors 2024, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Verma, M.; Acharya, A. Biomolecules Immobilized Nanomaterials and Their Biological Applications. In Nanomaterial-Based Biomedical Applications in Molecular Imaging, Diagnostics and Therapy; Springer: Singapore, 2020; pp. 79–101. [Google Scholar] [CrossRef]
- Zhao, D.; Song, H.; Hao, L.; Liu, X.; Zhang, L.; Lv, Y. Luminescent ZnO Quantum Dots for Sensitive and Selective Detection of Dopamine. Talanta 2013, 107, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.C.; Asif, M.H.; Bacher, G.; Danielsson, B.; Willander, M.; Bhand, S. Nanoimmunosensor Based on ZnO Nanorods for Ultrasensitive Detection of 17β-Estradiol. Biosens. Bioelectron. 2019, 126, 15–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Chen, M.; Yu, X.; Chang, Y.; Chen, A.; Zhu, H.; Tang, Z. Growth of Aligned ZnO Nanowires via Modified Atmospheric Pressure Chemical Vapor Deposition. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2016, 380, 3993–3997. [Google Scholar] [CrossRef]
- Cesini, I.; Kowalczyk, M.; Lucantonio, A.; D’alesio, G.; Kumar, P.; Camboni, D.; Massari, L.; Pingue, P.; De Simone, A.; Morgera, A.F.; et al. Seedless Hydrothermal Growth of ZnO Nanorods as a Promising Route for Flexible Tactile Sensors. Nanomaterials 2020, 10, 977. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, W.; Kang, K.; Kim, I.; Kang, D.; Oh, D.K.; Kim, M.C.; Choi, H.; Kim, K.; Kim, M.; et al. Low-Temperature Large-Area Fabrication of ZnO Nanowires on Flexible Plastic Substrates by Solution-Processible Metal-Seeded Hydrothermal Growth. Nano. Converg. 2020, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, J.; Li, L.; Wang, W. Direct Synthesis of Vertically Aligned ZnO Nanowires on FTO Substrates Using a CVD Method and the Improvement of Photovoltaic Performance. Nanoscale Res. Lett. 2012, 7, 293. [Google Scholar] [CrossRef]
- Scalisi, A.A.; Toro, R.G.; Malandrino, G.; Fragalà, M.E.; Pezzotti, G. Growth of ZnO Nanostructures Produced by MOCVD: A Study of the Effect of the Substrate. Chem. Vap. Depos. 2008, 14, 115–122. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Prokhorov, I.; Vshivtsev, M.; Kudryashov, M.; Kudryashova, Y.; Malyshev, V.; Spivak, Y.; Greshnyakov, E.; Knyazev, A.; et al. Variety of ZnO Nanostructured Materials Prepared by PECVD. Opt. Quantum Electron. 2022, 54, 646. [Google Scholar] [CrossRef]
- Bui, Q.C.; Ardila, G.; Sarigiannidou, E.; Roussel, H.; Jiménez, C.; Chaix-Pluchery, O.; Guerfi, Y.; Bassani, F.; Donatini, F.; Mescot, X.; et al. Morphology Transition of ZnO from Thin Film to Nanowires on Silicon and Its Correlated Enhanced Zinc Polarity Uniformity and Piezoelectric Responses. ACS Appl. Mater. Interfaces 2020, 12, 29583–29593. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Boarino, L.; Valov, I.; Ricciardi, C. Memristive Devices Based on Single ZnO Nanowires-from Material Synthesis to Neuromorphic Functionalities. Semicond. Sci. Technol. 2022, 37, 034002. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Shimpi, N.G.; Jain, S.; Karmakar, N.; Shah, A.; Kothari, D.C.; Mishra, S. Synthesis of ZnO Nanopencils Using Wet Chemical Method and Its Investigation as LPG Sensor. Appl. Surf. Sci. 2016, 390, 17–24. [Google Scholar] [CrossRef]
- Toma, F.T.Z.; Rahman, M.S.; Maria, K.H. A Review of Recent Advances in ZnO Nanostructured Thin Films by Various Deposition Techniques. Discov. Mater 2025, 5, 60. [Google Scholar] [CrossRef]
- Tiwari, C.; Pandey, A.; Dixit, A. Precursor Mediated and Defect Engineered ZnO Nanostructures Using Thermal Chemical Vapor Deposition for Green Light Emission. Thin. Solid Films 2022, 762, 139539. [Google Scholar] [CrossRef]
- Mohammad, S.N. Vapor–Solid Growth Mechanism. Synth. Nanomater. 2020, 307, 121–136. [Google Scholar] [CrossRef]
- Oh, D.K.; Choi, H.; Shin, H.; Kim, K.; Kim, M.; Ok, J.G. Tailoring Zinc Oxide Nanowire Architectures Collectively by Catalytic Vapor-Liquid-Solid Growth, Catalyst-Free Vapor-Solid Growth, and Low-Temperature Hydrothermal Growth. Ceram. Int. 2021, 47, 2131–2143. [Google Scholar] [CrossRef]
- Menzel, A.; Subannajui, K.; Bakhda, R.; Wang, Y.; Thomann, R.; Zacharias, M. Tuning the Growth Mechanism of ZnO Nanowires by Controlled Carrier and Reaction Gas Modulation in Thermal CVD. J. Phys. Chem. Lett. 2012, 3, 2815–2821. [Google Scholar] [CrossRef]
- Hedrich, C.; Haugg, S.; Pacarizi, L.; Furlan, K.P.; Blick, R.H.; Zierold, R. Low-Temperature Vapor-Solid Growth of ZnO Nanowhiskers for Electron Field Emission. Coatings 2019, 9, 698. [Google Scholar] [CrossRef]
- Campos, L.C.; Tonezzer, M.; Ferlauto, A.S.; Grillo, V.; Magalhães-Paniago, R.; Oliveira, S.; Ladeira, L.O.; Lacerda, R.G. Vapor-Solid-Solid Growth Mechanism Driven by Epitaxial Match between Solid AuZn Alloy Catalyst Particles and ZnO Nanowires at Low Temperatures. Adv. Mater. 2008, 20, 1499–1504. [Google Scholar] [CrossRef]
- Huang, J.; Chen, F.; Zhang, Q.; Zhan, Y.; Ma, D.; Xu, K.; Zhao, Y. 3D Silver Nanoparticles Decorated Zinc Oxide/Silicon Heterostructured Nanomace Arrays as High-Performance Surface-Enhanced Raman Scattering Substrates. ACS Appl. Mater. Interfaces 2015, 7, 5725–5735. [Google Scholar] [CrossRef]
- Serrano, A.; Arana, A.; Galdámez, A.; Dutt, A.; Monroy, B.M.; Güell, F.; Santana, G. Effect of the Seed Layer on the Growth and Orientation of the ZnO Nanowires: Consequence on Structural and Optical Properties. Vacuum 2017, 146, 509–516. [Google Scholar] [CrossRef]
- Udom, I.; Ram, M.K.; Stefanakos, E.K.; Hepp, A.F.; Goswami, D.Y. One Dimensional-ZnO Nanostructures: Synthesis, Properties and Environmental Applications. Mater. Sci. Semicond. Process 2013, 16, 2070–2083. [Google Scholar] [CrossRef]
- Labis, J.P.; Al-Anazi, A.Q.; Al-Brithen, H.A.; Hezam, M.; Alduraibi, M.A.; Algarni, A.; Alharbi, A.A.; Al-Awadi, A.S.; Khan, A.; El-Toni, A.M. Designing Zinc Oxide Nanostructures (Nanoworms, Nanoflowers, Nanowalls, and Nanorods) by Pulsed Laser Ablation Technique for Gas-Sensing Application. J. Am. Ceram. Soc. 2019, 102, 4367–4375. [Google Scholar] [CrossRef]
- Saha, S.; Arya, S.K.; Singh, S.P.; Sreenivas, K.; Malhotra, B.D.; Gupta, V. PLD Grown ZnO-K3[Fe(CN)6] Composite Thin Film for Biosensing Application. Thin Solid. Film. 2010, 519, 1184–1186. [Google Scholar] [CrossRef]
- Simon, H.; Krekeler, T.; Schaan, G.; Mader, W. Metal-Seeded Growth Mechanism of ZnO Nanowires. Cryst. Growth Des. 2013, 13, 572–580. [Google Scholar] [CrossRef]
- Rahmanian, R.; Mozaffari, S.A.; Amoli, H.S.; Abedi, M. Development of Sensitive Impedimetric Urea Biosensor Using DC Sputtered Nano-ZnO on TiO2 Thin Film as a Novel Hierarchical Nanostructure Transducer. Sens. Actuators B Chem. 2018, 256, 760–774. [Google Scholar] [CrossRef]
- Singh, S.P.; Arya, S.K.; Pandey, P.; Malhotra, B.D.; Saha, S.; Sreenivas, K.; Gupta, V. Cholesterol Biosensor Based on Rf Sputtered Zinc Oxide Nanoporous Thin Film. Appl. Phys. Lett. 2007, 91, 2768302. [Google Scholar] [CrossRef]
- Lakshmi Narayanan, M.; Prabhu, K.; Ponpandian, N.; Viswanathan, C. Cu Encrusted RF Sputtered ZnO Thin Film Based Electrochemical Immunosensor for Highly Sensitive Detection of IL-6 in Human Blood Serum. Microchem. J. 2024, 199, 110061. [Google Scholar] [CrossRef]
- Sigallon, M.C.; Baillard, A.; Consonni, V.; Aubrit, F.; Potrzebowska, N.; Grasset, R.; Tabellout, M.; Gogneau, N.; Sarrey, E.; Wegrowe, J.E.; et al. Flexible Piezoelectric Energy Harvester Made of Vertically-Aligned ZnO Nanowires Hydrothermally-Grown by Template-Assisted Synthesis in Poled PVDF. Nano Trends 2025, 10, 100112. [Google Scholar] [CrossRef]
- Torres, F.D.; Lopez, J.L.; Rodriguez, A.S.; Gallardo, P.S.; Morales, E.R.; Hernandez, G.P.; Guillen, J.C.; Flores, L.L. Sol–Gel/Hydrothermal Synthesis of Well-Aligned ZnO Nanorods. Boletín Soc. Española Cerámica Vidr. 2023, 62, 348–356. [Google Scholar] [CrossRef]
- Feng, W.; Wang, B.; Huang, P.; Wang, X.; Yu, J.; Wang, C. Wet Chemistry Synthesis of ZnO Crystals with Hexamethylenetetramine(HMTA): Understanding the Role of HMTA in the Formation of ZnO Crystals. Mater. Sci. Semicond. Process 2016, 41, 462–469. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-Gel Synthesis of Thorn-like ZnO Nanoparticles Endorsing Mechanical Stirring Effect and Their Antimicrobial Activities: Potential Role as Nano-Antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef]
- Bernal-Díaz, A.; Hernández-Gordillo, A.; Alonso, J.C.; Rodil, S.E.; Bizarro, M. Strong Thickness Dependence in Thin Film Photocatalytic Heterojunctions: The ZnO-Bi2O3 Case Study. Dalton Trans. 2024, 53, 7081–7092. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, M.; Tominaga, H.; Yoshino, K.; Ogomi, Y.; Shen, Q.; Toyoda, T.; Minemoto, T.; Hayase, S. Growth Mechanism of ZnO Thin Films Grown by Spray Pyrolysis Using Diethylzinc Solution. Phys. Status Solidi A Appl. Mater. Sci. 2018, 215, 1700406. [Google Scholar] [CrossRef]
- Marouf, S.; Beniaiche, A.; Kardarian, K.; Mendes, M.J.; Sanchez-Sobrado, O.; Águas, H.; Fortunato, E.; Martins, R. Low-Temperature Spray-Coating of High-Performing ZnO:Al Films for Transparent Electronics. J. Anal. Appl. Pyrolysis 2017, 127, 299–308. [Google Scholar] [CrossRef]
- Gu, B.; Xu, C.; Yang, C.; Liu, S.; Wang, M. ZnO Quantum Dot Labeled Immunosensor for Carbohydrate Antigen 19-9. Biosens. Bioelectron. 2011, 26, 2720–2723. [Google Scholar] [CrossRef]
- Sarangi, S.N.; Nozaki, S.; Sahu, S.N. ZnO Nanorod-Based Non-Enzymatic Optical Glucose Biosensor. J. Biomed. Nanotechnol. 2015, 11, 988–996. [Google Scholar] [CrossRef]
- Gu, B.X.; Xu, C.X.; Zhu, G.P.; Liu, S.Q.; Chen, L.Y.; Li, X.S. Tyrosinase Immobilization on ZnO Nanorods for Phenol Detection. J. Phys. Chem. B 2009, 113, 377–381. [Google Scholar] [CrossRef]
- Iyer, M.A.; Oza, G.; Velumani, S.; Maldonado, A.; Romero, J.; Muñoz, M.D.L.; Sridharan, M.; Asomoza, R.; Yi, J. Scanning Fluorescence-Based Ultrasensitive Detection of Dengue Viral DNA on ZnO Thin Films. Sens. Actuators B Chem. 2014, 202, 1338–1348. [Google Scholar] [CrossRef]
- Kaur, G.; Paliwal, A.; Tomar, M.; Gupta, V. Detection of Neisseria Meningitidis Using Surface Plasmon Resonance Based DNA Biosensor. Biosens. Bioelectron. 2016, 78, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yan, X.; Luo, N.; Song, Y.; Zhang, Y. ZnO Nanotetrapod Network as the Adsorption Layer for the Improvement of Glucose Detection via Multiterminal Electron-Exchange. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 169–173. [Google Scholar] [CrossRef]
- Qurashi, A.; Rather, J.A.; De Wael, K.; Merzougui, B.; Tabet, N.; Faiz, M. Rapid Microwave Synthesis of High Aspect-Ratio ZnO Nanotetrapods for Swift Bisphenol A Detection. Analyst 2013, 138, 4764–4768. [Google Scholar] [CrossRef]
- Prabhakar, T.; Giaretta, J.; Zulli, R.; Rath, R.J.; Farajikhah, S.; Talebian, S.; Dehghani, F. Covalent Immobilization: A Review from an Enzyme Perspective. Chem. Eng. J. 2025, 503, 158054. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163. [Google Scholar] [CrossRef]
- Tiwari, S.; Vinchurkar, M.; Rao, V.R.; Garnier, G. Zinc Oxide Nanorods Functionalized Paper for Protein Preconcentration in Biodiagnostics. Sci. Rep. 2017, 7, 43905. [Google Scholar] [CrossRef]
- Shukla, M.; Palani, I.A.; Singh, V. Effect of Immobilization Technique on Performance ZnO Nanorods Based Enzymatic Electrochemical Glucose Biosensor. J. Phys. Conf. Ser. 2017, 924, 012013. [Google Scholar] [CrossRef]
- Feng, L.X.; Tang, C.; Han, X.X.; Zhang, H.C.; Guo, F.N.; Yang, T.; Wang, J.H. Simultaneous and Sensitive Detection of Multiple Small Biological Molecules by Microfluidic Paper-Based Analytical Device Integrated with Zinc Oxide Nanorods. Talanta 2021, 232, 122499. [Google Scholar] [CrossRef] [PubMed]
- Usha, S.P.; Shrivastav, A.M.; Gupta, B.D. FO-SPR Based Dextrose Sensor Using Ag/ZnO Nanorods/GOx for Insulinoma Detection. Biosens. Bioelectron. 2016, 85, 986–995. [Google Scholar] [CrossRef]
- Tamashevski, A.; Harmaza, Y.; Viter, R.; Jevdokimovs, D.; Poplausks, R.; Slobozhanina, E.; Mikoliunaite, L.; Erts, D.; Ramanaviciene, A.; Ramanavicius, A. Zinc Oxide Nanorod Based Immunosensing Platform for the Determination of Human Leukemic Cells. Talanta 2019, 200, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Fidal, V.T.; Chandra, T.S. New Approach to Biosensing of Co-Enzyme Nicotinamide Adenine Dinucleotide (NADH) by Incorporation of Neutral Red in Aluminum Doped Nanostructured ZnO Thin Films. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1559–1565. [Google Scholar] [CrossRef]
- Ramesh, T.; Foo, K.L.; Haarindraprasad, R.; Sam, A.J.; Solayappan, M. Gold-Hybridized Zinc Oxide Nanorods as Real-Time Low-Cost NanoBiosensors for Detection of Virulent DNA Signature of HPV-16 in Cervical Carcinoma. Sci. Rep. 2019, 9, 17039. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Bechelany, M.; Iatsunskyi, I. Photoluminescence Label-Free Immunosensor for the Detection of Aflatoxin B1 Using Polyacrylonitrile/Zinc Oxide Nanofibers. Mater. Sci. Eng. C 2021, 118, 111401. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Fedorenko, V.; Smyntyna, V.; Konup, I.; Konup, A.; Eriksson, M.; Yakimova, R.; Ramanavicius, A.; Balme, S.; Bechelany, M. ZnO Films Formed by Atomic Layer Deposition as an Optical Biosensor Platform for the Detection of Grapevine Virus A-Type Proteins. Biosens. Bioelectron. 2017, 92, 763–769. [Google Scholar] [CrossRef]
- Dai, Z.; Shao, G.; Hong, J.; Bao, J.; Shen, J. Immobilization and Direct Electrochemistry of Glucose Oxidase on a Tetragonal Pyramid-Shaped Porous ZnO Nanostructure for a Glucose Biosensor. Biosens. Bioelectron. 2009, 24, 1286–1291. [Google Scholar] [CrossRef]
- Wang, D.; Tan, Y. Electrodeposition of Enzymes-Integrated Mesoporous Composite Films by Interfacial Templating: A Paradigm for Electrochemical Biosensors. Electrochim. Acta 2014, 116, 495–503. [Google Scholar] [CrossRef]
- Sanguino, P.; Monteiro, T.; Bhattacharyya, S.R.; Dias, C.J.; Igreja, R.; Franco, R. ZnO Nanorods as Immobilization Layers for Interdigitated Capacitive Immunosensors. Sens. Actuators B Chem. 2014, 204, 211–217. [Google Scholar] [CrossRef]
- Wu, D.; Dong, W.; Yin, T.; Jie, G.; Zhou, H. PCN-224/Nano-Zinc Oxide Nanocomposite-Based Electrochemiluminescence Biosensor for HPV-16 Detection by Multiple Cycling Amplification and Hybridization Chain Reaction. Sens. Actuators B Chem. 2022, 372, 132659. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Tolubayeva, D.B.; Gritsenko, L.V.; Kedruk, Y.Y.; Aitzhanov, M.B.; Nemkayeva, R.R.; Abdullin, K.A. Effect of Hydrogen Plasma Treatment on the Sensitivity of ZnO Based Electrochemical Non-Enzymatic Biosensor. Biosensors 2023, 13, 793. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaba, Y.; Abe, T.; Nakagawa, A.; Niikura, I.; Kashiwaba, Y.; Daibo, M.; Fujiwara, T.; Osada, H. Formation of a ZnO2 Layer on the Surface of Single Crystal ZnO Substrates with Oxygen Atoms by Hydrogen Peroxide Treatment. J. Appl. Phys. 2013, 113, 113501. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ikram, S. Zinc Oxide Nanoparticles-Impregnated Chitosan Surfaces for Covalent Immobilization of Trypsin: Stability & Kinetic Studies. Int. J. Biol. Macromol. 2022, 207, 205–221. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Liu, X.; Xian, Y.; Shi, G.; Jin, L. ZnO Nanorods/Au Hybrid Nanocomposites for Glucose Biosensor. Biosens. Bioelectron. 2010, 26, 275–278. [Google Scholar] [CrossRef]
- Ghaffari, S.B.; Sarrafzadeh, M.H.; Fakhroueian, Z.; Shahriari, S.; Khorramizadeh, M.R. Functionalization of ZnO Nanoparticles by 3-Mercaptopropionic Acid for Aqueous Curcumin Delivery: Synthesis, Characterization, and Anticancer Assessment. Mater. Sci. Eng. C 2017, 79, 465–472. [Google Scholar] [CrossRef]
- Han, Z.; Weng, Q.; Lin, C.; Yi, J.; Kang, J. Development of CdSe–Zno Flower-Rod Core-Shell Structure Based Photoelectrochemical Biosensor for Detection of Norovirous RNA. Sensors 2018, 18, 2980. [Google Scholar] [CrossRef]
- Sandhyarani, N. Surface Modification Methods for Electrochemical Biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–75. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, P.; Nie, Y.; Wang, P.; Zhang, Y.; Xing, L.; Xue, X. Biomolecule-Adsorption-Dependent Piezoelectric Output of ZnO Nanowire Nanogenerator and Its Application as Self-Powered Active Biosensor. Biosens. Bioelectron. 2014, 57, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, S.; Mozaffari, S.A.; Ebrahimi, F. Polyvinyl Alcohol as a Crucial Omissible Polymer to Fabricate an Impedimetric Glucose Biosensor Based on Hierarchical 3D-NPZnO/Chitosan. Carbohydr. Polym. 2021, 266, 118105. [Google Scholar] [CrossRef]
- Yen, Y.K.; Yang, C.M.; Kao, C.T.; Yen, T.H.; Shanmugam, R.; Chen, Y.L.; Lin, H.E. A ZnO-Nanorod/PEDOT:PSS Nanocomposite Functionalized Bridge-like Membrane Type Nanomechanical Sensing Device for Ultrasensitive Blood Lead Detection. Anal. Chim. Acta 2024, 1331, 343317. [Google Scholar] [CrossRef]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef] [PubMed]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable Synthesis of ZnO Nanoparticles and Their Morphology-Dependent Antibacterial and Optical Properties. J. Photochem. Photobiol. B 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Guo, R.; Zheng, W.; He, K.; Geng, Y.; Liu, L.; Ai, Q.; He, Y.; Zhang, Y.N.; Zhao, Y. ZnO-Nafion Assisted Optical Fiber Dual-SPR Biosensor for Simultaneous Detection of Urea and Uric Acid Concentrations. Biosens. Bioelectron. 2025, 271, 117076. [Google Scholar] [CrossRef]
- Meeseepong, M.; Ghosh, G.; Shrivastava, S.; Lee, N.E. Fluorescence-Enhanced Microfluidic Biosensor Platform Based on Magnetic Beads with Highly Stable ZnO Nanorods for Biomarker Detection. ACS Appl. Mater. Interfaces 2023, 15, 21754–21765. [Google Scholar] [CrossRef]
- Maiolo, L.; Maita, F.; Del Rio De Vicente, J.I.; Lucarini, I.; Strisciullo, G.; Sablone, S.; Liscio, A.; Petrone, G.; Mussi, V. Silver-Coated ZnO Disordered Nanostructures as Low-Cost and Label-Free Raman Biosensing Platform for Fast Detection of Complex Organic Profiles. Biosens. Bioelectron. X 2023, 13, 100309. [Google Scholar] [CrossRef]
- Tamashevski, A.; Harmaza, Y.; Slobozhanina, E.; Viter, R.; Iatsunskyi, I. Photoluminescent Detection of Human T-Lymphoblastic Cells by ZnO Nanorods. Molecules 2020, 25, 3168. [Google Scholar] [CrossRef] [PubMed]
- Włodarski, M.; Nowak, M.P.; Putkonen, M.; Nyga, P.; Norek, M. Surface Modification of ZnO Nanotubes by Ag and Au Coatings of Variable Thickness: Systematic Analysis of the Factors Leading to UV Light Emission Enhancement. ACS Omega 2024, 9, 1670–1682. [Google Scholar] [CrossRef]
- Naorem, B.D.; Singh, J.P.; Sharma, B.; Garg, S.; Sherin, H.; Momaliya, M.; Sahu, S.; Chowdhuri, A.; Verma, M.; Tomar, M.; et al. Mn-Doped ZnO Thin Films as a Platform for Reagentless Uric Acid Biosensor. Chem. Phys. Impact 2025, 10, 100823. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, T.; Tao, T. ZnO Quantum Dots for Fluorescent Detection of Environmental Contaminants. J. Environ. Chem. Eng. 2021, 9, 106800. [Google Scholar] [CrossRef]
- Dorfman, A.; Kumar, N.; Hahm, J.I. Nanoscale ZnO-Enhanced Fluorescence Detection of Protein Interactions. Adv. Mater. 2006, 18, 2685–2690. [Google Scholar] [CrossRef]
- Ahn, K.Y.; Kwon, K.; Huh, J.; Kim, G.T.; Lee, E.B.; Park, D.; Lee, J. A Sensitive Diagnostic Assay of Rheumatoid Arthritis Using Three-Dimensional ZnO Nanorod Structure. Biosens. Bioelectron. 2011, 28, 378–385. [Google Scholar] [CrossRef]
- Sang, C.H.; Chou, S.J.; Pan, F.M.; Sheu, J.T. Fluorescence Enhancement and Multiple Protein Detection in ZnO Nanostructure Microfluidic Devices. Biosens. Bioelectron. 2016, 75, 285–292. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced Fluorescence Detection of Proteins Using ZnO Nanowires Integrated inside Microfluidic Chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef]
- Soundharraj, P.; Dhinasekaran, D.; Rajendran, A.R.; Prakasarao, A.; Ganesan, S. N-Doped Zinc Oxide as an Effective Fluorescence Sensor for Urea Detection. New J. Chem. 2021, 45, 6080–6090. [Google Scholar] [CrossRef]
- Fujisawa, J.I.; Kaneko, N.; Hanaya, M. Interfacial Charge-Transfer Transitions in ZnO Induced Exclusively by Adsorption of Aromatic Thiols. Chem. Commun. 2020, 56, 4090–4093. [Google Scholar] [CrossRef]
- Barbillon, G. Fabrication and SERS Performances of Metal/Si and Metal/ZnO Nanosensors: A Review. Coatings 2019, 9, 86. [Google Scholar] [CrossRef]
- Deng, W.; Yuan, L.; Wan, P.; Sun, J.; Kan, C.; Shi, D.; Xu, C.; Lu, J. Piezoelectric-Enhanced Ultrasensitive ZnO/Ag Microcavity SERS Substrate for Dopamine Detection. Nano Energy 2025, 133, 110449. [Google Scholar] [CrossRef]
- Picciolini, S.; Castagnetti, N.; Vanna, R.; Mehn, D.; Bedoni, M.; Gramatica, F.; Villani, M.; Calestani, D.; Pavesi, M.; Lazzarini, L.; et al. Branched Gold Nanoparticles on ZnO 3D Architecture as Biomedical SERS Sensors. RSC Adv. 2015, 5, 93644–93651. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Yang, X.; Yuan, R.; Chai, Y. A SERS Biosensor Constructed by Calcined ZnO Substrate with High-Efficiency Charge Transfer for Sensitive Detection of Pb2+. Sens. Actuators B Chem. 2021, 343, 130142. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghosh, R.; Giri, P.K. Tuning the Visible Photoluminescence in Al Doped ZnO Thin Film and Its Application in Label-Free Glucose Detection. Sens. Actuators B Chem. 2018, 254, 681–689. [Google Scholar] [CrossRef]
- Viter, R.; Khranovskyy, V.; Starodub, N.; Ogorodniichuk, Y.; Gevelyuk, S.; Gertnere, Z.; Poletaev, N.; Yakimova, R.; Erts, D.; Smyntyna, V.; et al. Application of Room Temperature Photoluminescence from ZnO Nanorods for Salmonella Detection. IEEE Sens. J. 2014, 14, 2028–2034. [Google Scholar] [CrossRef]
- Myndrul, V.; Yanovska, A.; Babayevska, N.; Korniienko, V.; Diedkova, K.; Jancelewicz, M.; Pogorielov, M.; Iatsunskyi, I. 1D ZnO–Au Nanocomposites as Label-Free Photoluminescence Immunosensors for Rapid Detection of Listeria Monocytogenes. Talanta 2024, 271, 125641. [Google Scholar] [CrossRef]
- World Health Organization. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Interim Guidance; World Health Organization: Geneva, Switzerland, 2021.
- Liu, R.; Fu, A.; Deng, Z.; Li, Y.; Liu, T. Promising Methods for Detection of Novel Coronavirus SARS-CoV-2. View 2020, 1, e4. [Google Scholar] [CrossRef]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-Care Testing Detection Methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Z.; Zhu, J.; Li, C.; Fang, Z.; Chen, K.; Zhang, Y. An Updated Review of SARS-CoV-2 Detection Methods in the Context of a Novel Coronavirus Pandemic. Bioeng. Transl. Med. 2023, 8, e10356. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Loconsole, D.; Sallustio, A.; Picca, R.A.; Felici, R.; Chironna, M.; Cioffi, N. On the Efficacy of ZnO Nanostructures against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3040. [Google Scholar] [CrossRef]
- Phan, L.M.; Tieu, M.V.; Pham, T.T.; Cho, S. Clinical Utility of Biosensing Platforms for Confirmation of Sars-CoV-2 Infection. Biosensors 2021, 11, 167. [Google Scholar] [CrossRef]
- Tekin, Y.S.; Kul, S.M.; Sagdic, O.; Rodthongkum, N.; Geiss, B.; Ozer, T. Optical Biosensors for Diagnosis of COVID-19: Nanomaterial-Enabled Particle Strategies for Post Pandemic Era. Microchim. Acta 2024, 191, 320. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.; Rini, J.M.; Liu, X. Enhancing the Performance of Paper-Based Electrochemical Impedance Spectroscopy Nanobiosensors: An Experimental Approach. Biosens Bioelectron 2021, 177. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.K.; Lee, J.H.; Kim, H.Y.; Kim, N.H.; Lee, C.H.; Lee, C.S.; Kim, H.G. ZnO Nanowire-Based Early Detection of SARS-CoV-2 Antibody Responses in Asymptomatic Patients with COVID-19. Adv. Mater. Interfaces 2022, 9, 2102046. [Google Scholar] [CrossRef]
- Nunez, F.A.; Castro, A.C.; Daher, I.P.; Cunha-Neto, E.; Kalil, J.; Boscardin, S.B.; Lanfredi, A.J.; Oliveira, V.L.; Alves, W.A. ZnO-Based Electrochemical Immunosensor to Assess Vaccine-Induced Antibody-Mediated Immunity against Wild-Type and Gamma SARS-CoV-2 Strains. Biosensors 2023, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Vafabakhsh, M.; Dadmehr, M.; Kazemi Noureini, S.; Es’haghi, Z.; Malekkiani, M.; Hosseini, M. Paper-Based Colorimetric Detection of COVID-19 Using Aptasenor Based on Biomimetic Peroxidase like Activity of ChF/ZnO/CNT Nano-Hybrid. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2023, 301, 122980. [Google Scholar] [CrossRef] [PubMed]
- Nunez, F.A.; Oliveira, V.L.; Remuzgo, C.; Silva, M.R.A.; Daher, I.; Oliveira, J.R.; Silva, T.L.; Cunha-Neto, E.; Kalil, J.; Santos, K.S.; et al. Electrochemical Immunosensor for Antibody Recognition Against SARS-CoV-2 B-Cell Epitope: Impact of RBD Mutations on Antigen-Antibody Binding. J. Mater. Chem. B 2025, 13, 9925–9936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, Z.; Zhou, J.; Jia, X.; Li, H.; Wang, X.; Chen, Y.; Sun, Z.; He, X.; Li, H.; et al. Nano-Biosensor for SARS-CoV-2/COVID-19 Detection: Methods, Mechanism and Interface Design. RSC Adv. 2023, 13, 17883–17906. [Google Scholar] [CrossRef]
- Yari, P.; Liang, S.; Chugh, V.K.; Rezaei, B.; Mostufa, S.; Krishna, V.D.; Saha, R.; Cheeran, M.C.J.; Wang, J.P.; Gómez-Pastora, J.; et al. Nanomaterial-Based Biosensors for SARS-CoV-2 and Future Epidemics. Anal. Chem. 2023, 95, 15419–15449. [Google Scholar] [CrossRef]

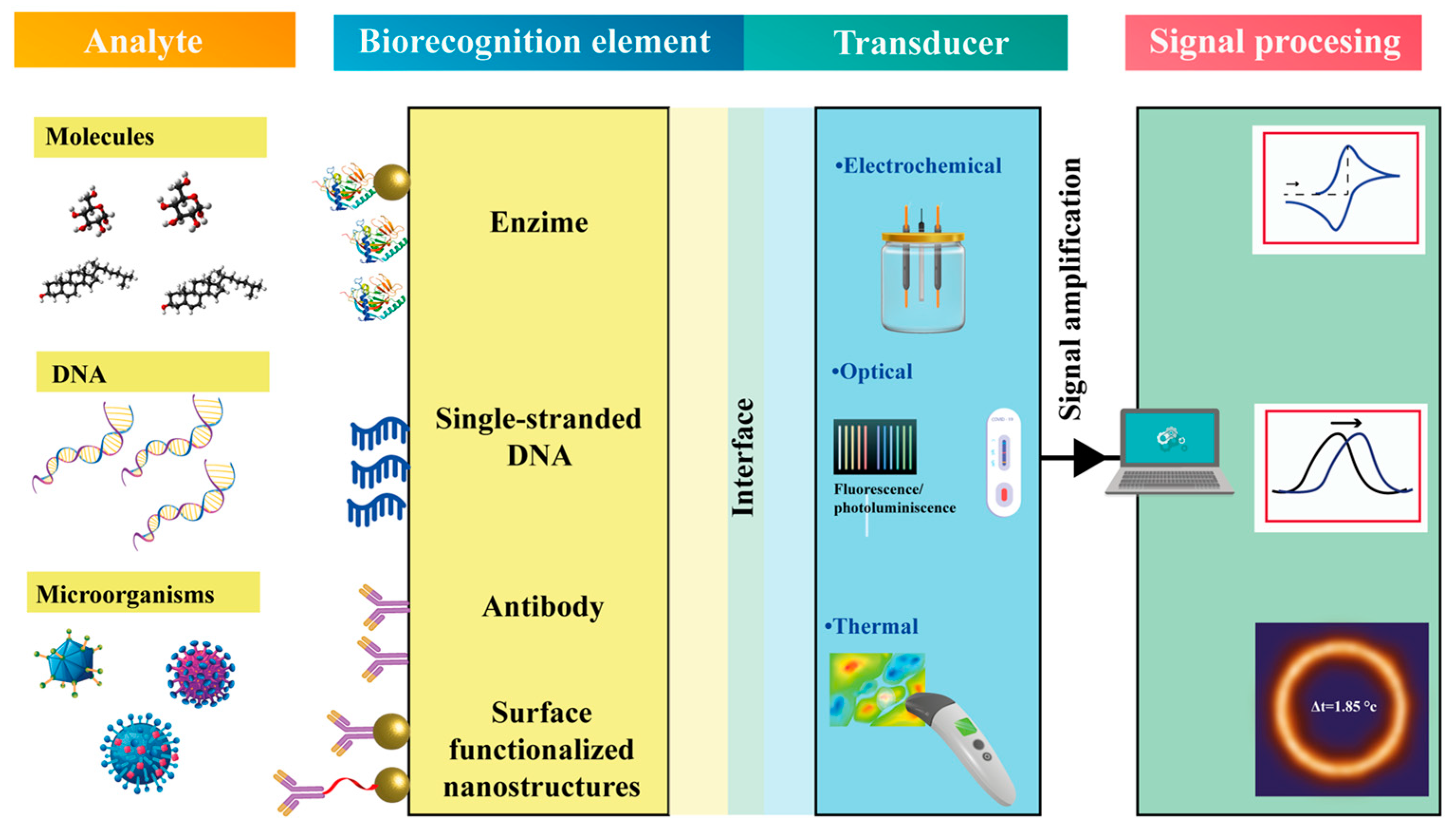


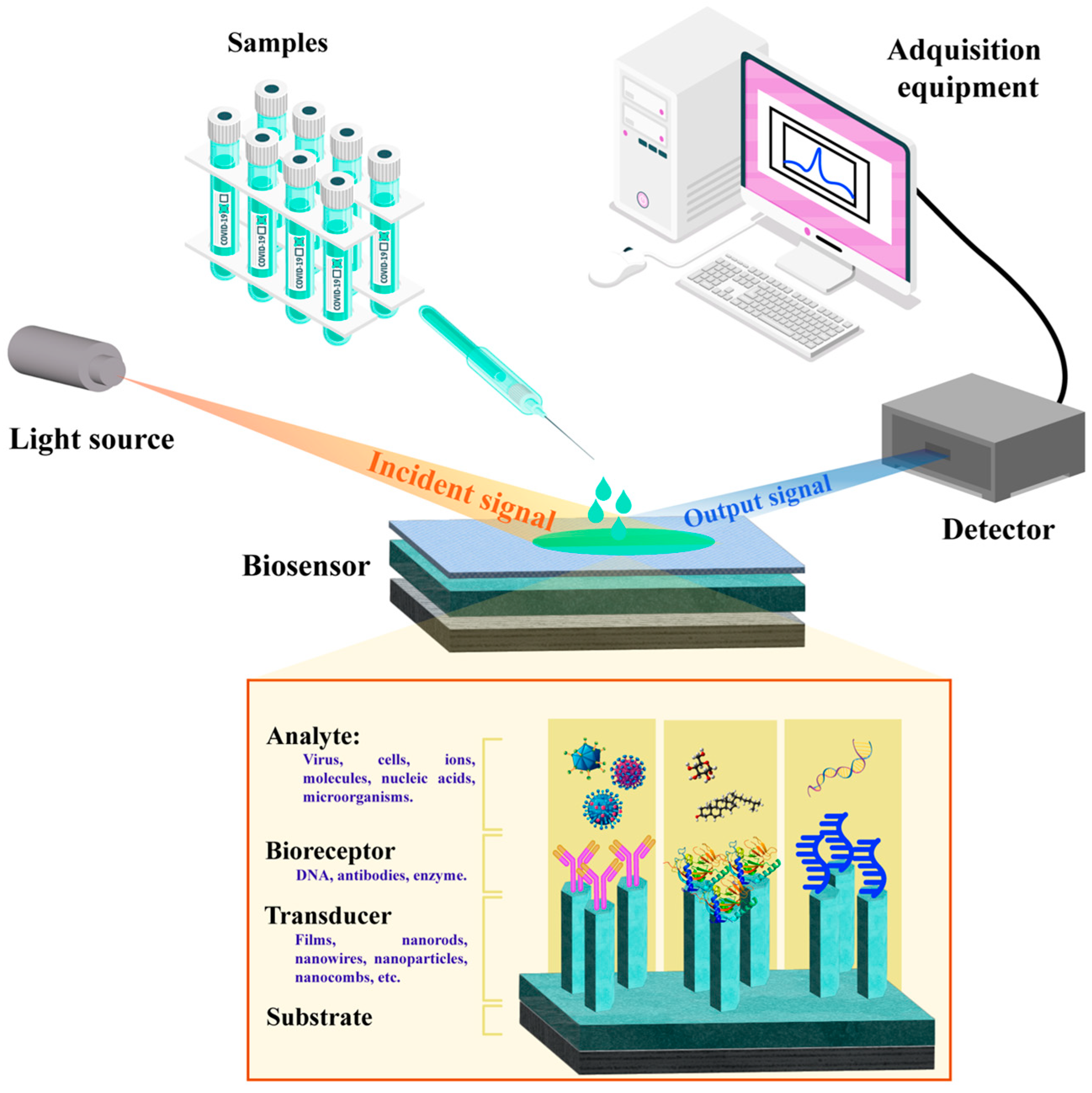
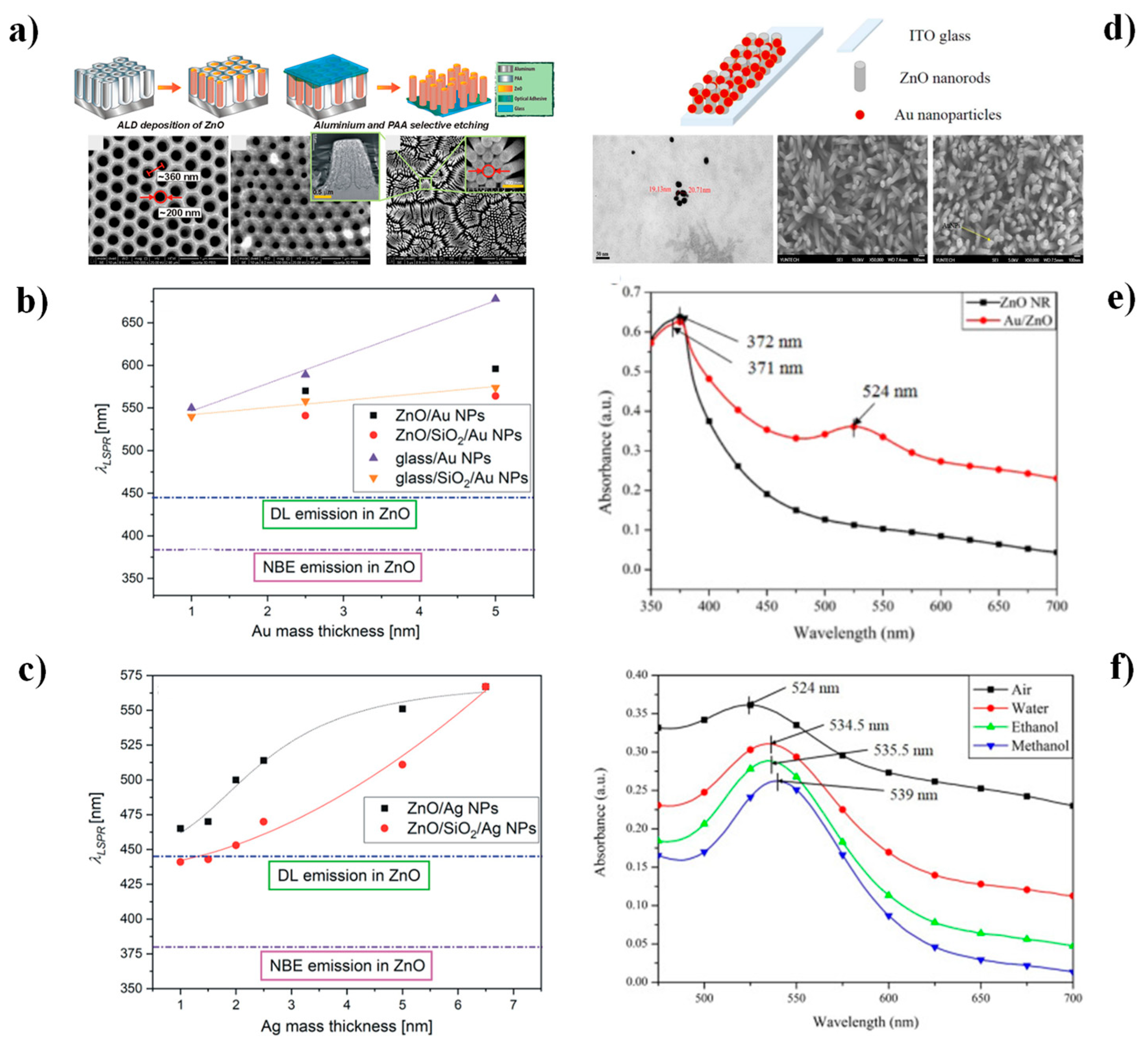

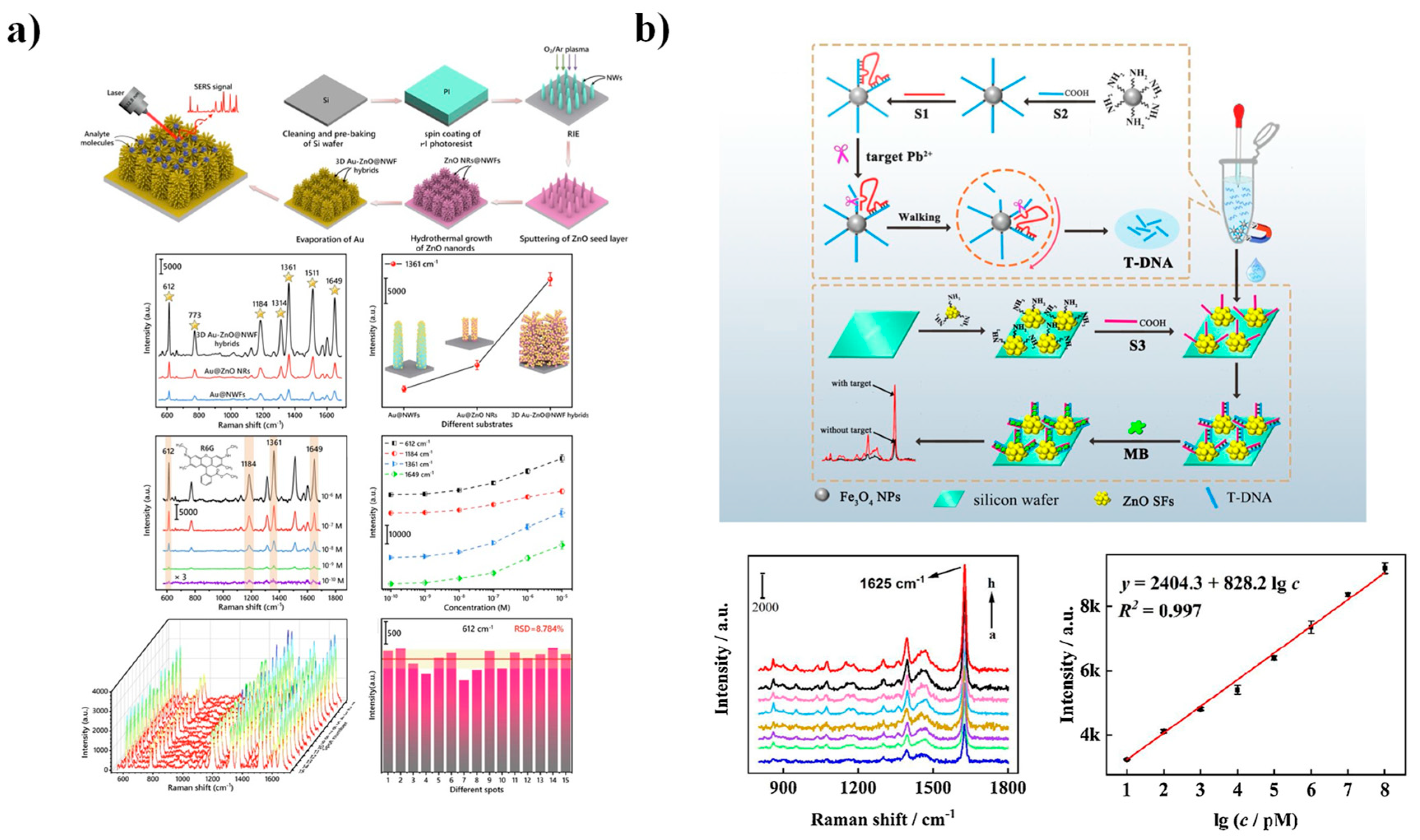
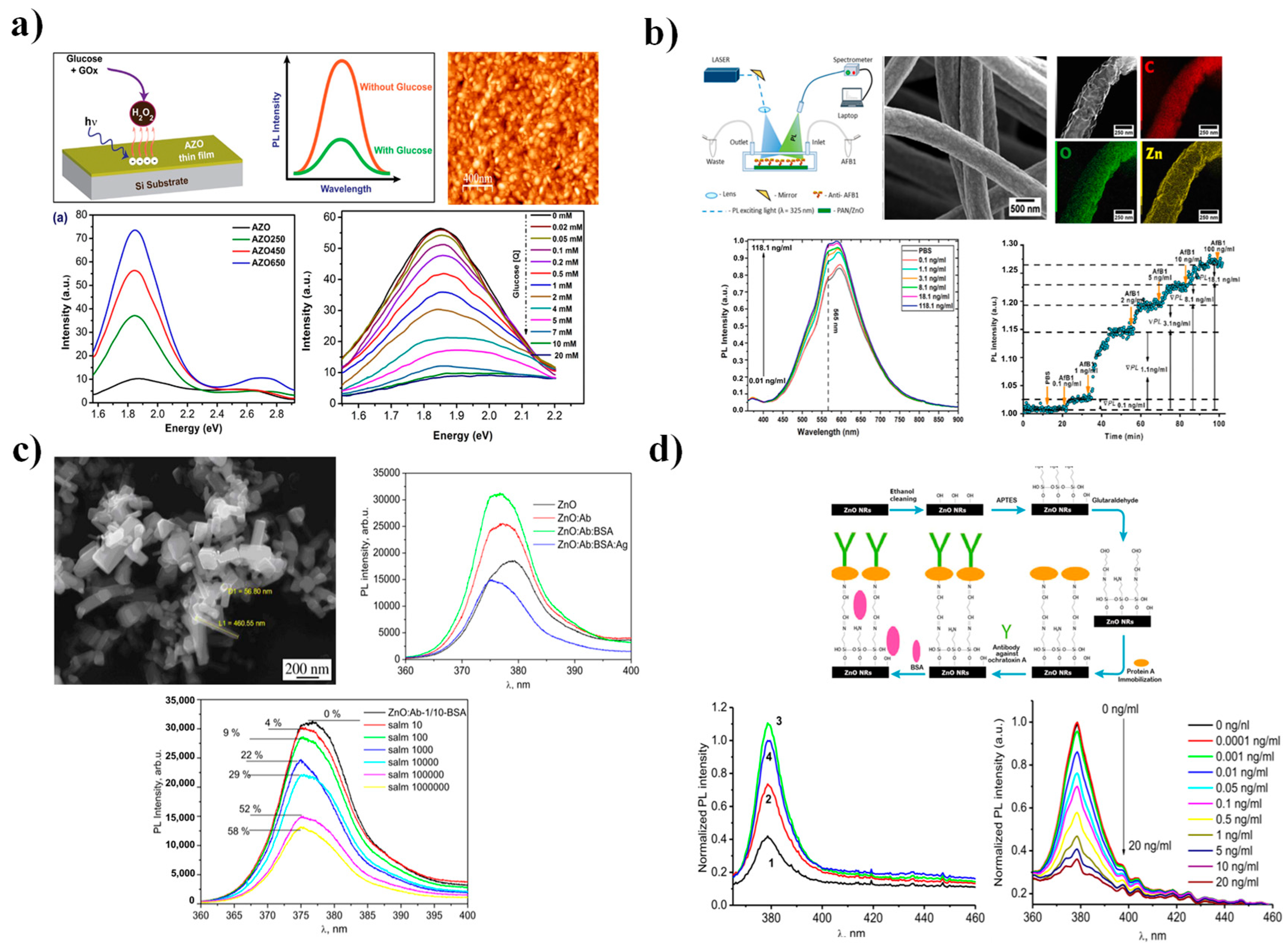
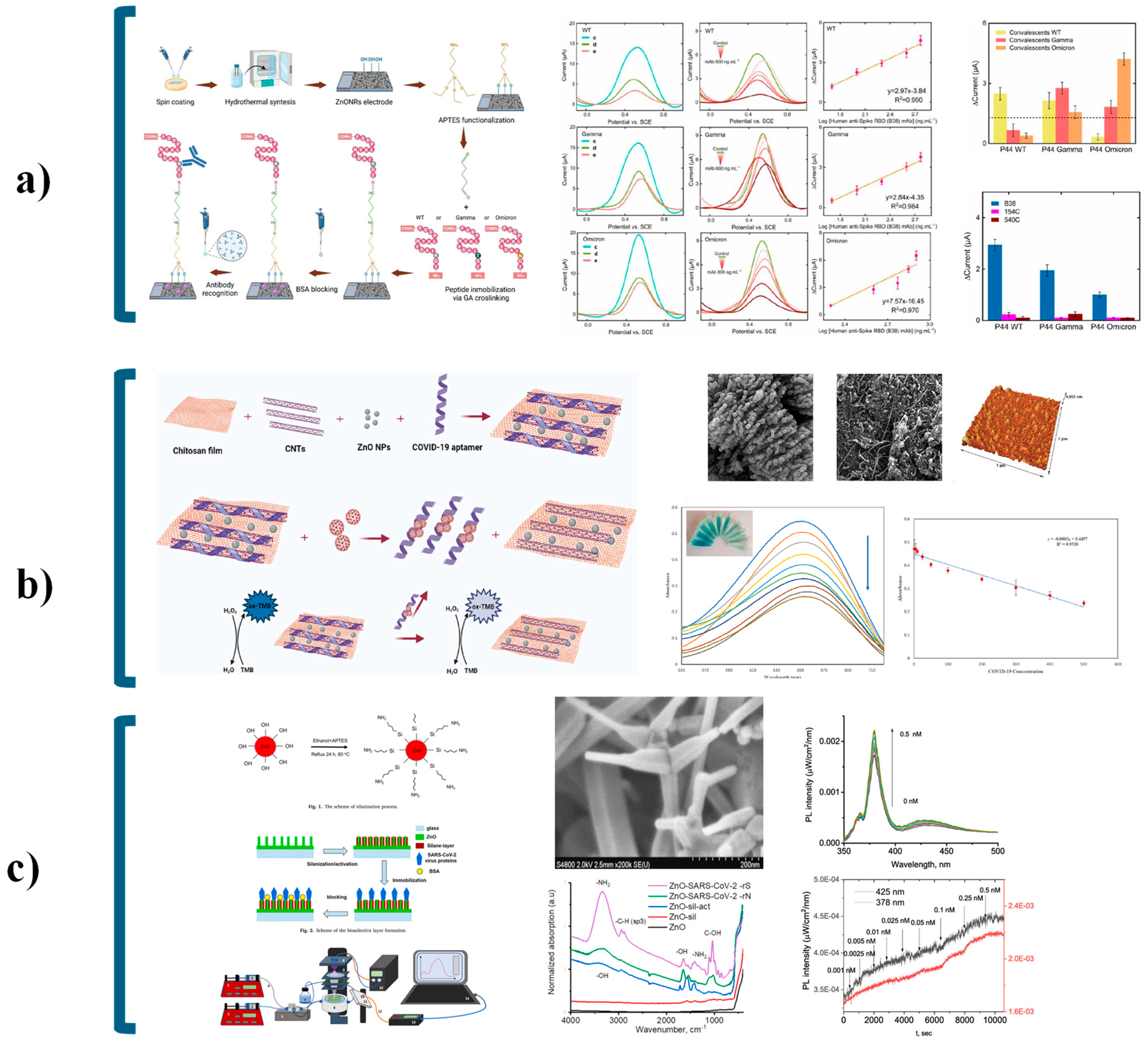

| Strategy | Advantages | Disadvantages | Sensing Application Areas | Ref. |
|---|---|---|---|---|
| Covalent attachment | Strong and long-lasting binding High stability under harsh conditions. Minimize non-specific adsorption. | Irreversible immobilization. Complex optimization, dependent on temperature, pH, and reaction time. Risk of biomolecule denaturation. | Long-term biosensors (e.g., implantable). Optical biosensors. | [26,35,78] |
| Physical adsorption | Simple and rapid process, no need for reducing agents or modified molecules Preserves biomolecule conformation. Suitable for a wide range of biomolecules. | Weak binding (sensitive to ionic strength). Possible non-specific adsorption, which may affect sensor specificity. Low reproducibility. | Short-term or disposable sensors. Protein and antibody sensors. | [35] |
| Entrapment/encapsulation | Protects biomolecules from harsh environments. In porous ZnO (e.g., nanotubes), biomolecule immobilization density is maximized. | Slow analyte diffusion due to dense matrices. Requires optimization of polymer-ZnO ratios. Reduced signal-to-noise ratio. | Enzymatic sensors (e.g., cholesterol). | [79] |
| Cross-linking | Facilitate multi-enzyme system stability. Strong attachment and reduced leaching. Allows biomolecule orientation for accessibility. | Blocks active sites. Risk of aggregation. | Multi-analyte detection systems. | [79] |
| ZnO Classification | Nanoarchitecture Configuration | Target Analyte | Bioselective Layer | Linear Range | Limit of Detection (LOD) | Sensitivity | Immobilization Strategy | Biosensing Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 0D | APTES-capped ZnO QDs | Dopamine | NM | 0.05–10 µM | 12 nM | NM | Physical adsorption | Optical biosensor (Fluorescent mechanism) | [36] |
| Si/CA 19-9 Ab/CA 19-9/ZnO QDs | antigen CA19-9 | CA 19-9 Ab | 0.1–180 U mL−1 1–180 U mL−1 | 0.04 U mL−1 0.25 U mL−1 | 0.47 µA/U ml−1 | Covalent bonding | Electrochemical mechanism Optical mechanism | [71] | |
| 1D | GaN/ZnO NRs/β-D- glucose | Glucose | Glucose oxidase | 0.5–30 mM | 0.5–30 mM | 1.4%/mM | Covalent bonding | Optical sensing | [72] |
| Au/ZnO NRs | Phenol | Tyrosinase | >20 µM <20 µM | 0.623 µM | 103.08 µA mM−1 40.76 µA mM−1 | Physical adsorption | Optical sensing | [73] | |
| Ag-ZnO NRs-mAb-E2 | 17β-Estradiol (E2) | Monoclonal antibody of E2 (mAb-E2) | 0.1–200 pg mL−1 | 0.01 pg mL−1 | 0.01 pg mL−1 | Covalent bonding | Electrochemical sensing | [37] | |
| ZnO NRs on Pt film | Glucose | Glucose oxidase | 0.5–3 mM (physical adsorption) 0.5–8.5 mM (cross-linking) | NM | 17.7 mA cm−2 M−1 | Physical adsorption and cross-linking | Electrochemical sensing | [81] | |
| Whatman filter/ZnO NRs | Glucose (GO) and uric acid (UA) | Glucose oxidase uricase | 0.01–10 mM (GO) 0.01–5 mM (UA) | 3 µM (GO) 4 µM (UA) | NM | Physical adsorption | Colorimetric method | [82] | |
| Ag/ZnO NRs | Dextrose | Glucose oxidase | 0–10 mM | 0.012 mM | 26.29 nm mM−1 | Physical adsorption | Optical sensing | [83] | |
| Glass/ZnO NRs | Human leukemic cells B-lymphoblastoid cell line IM9 | Anti-CD19-FITC* | 10 to 500 cells | NM | 10 till 1000 cells per mm2 | Physical adsorption | Optical sensing | [84] | |
| Neutral Red-nanostructured ZnO (NR-AZO) | Nicotinamide adenine dinucleotide (NADH) | Lactate dehydrogenase (LDH) | 0.1–1 mM | 22 µM | 0.45 µA cm−2 | Physical adsorption | Electrochemical sensing | [85] | |
| Au hybridized ZnO NRs | HPV-16 DNA | Thiolated ssDNA (HPV-16 E6) | 10−7–10−15 M | 1 fM | 1 fM | Covalent bonding | Electrochemical biosensor | [86] | |
| polyacrylonitrile/ZnO (PAN/ZnO) nanofibers | Aflatoxin B1 (AFB1) | anti-AFB1 | 0.1–100 ng mL−1 | 39 pg mL−1 | 0.1–20 ng ml−1 | Covalent bonding | Optical biosensor | [87] | |
| ZnO-NRs | Ochratoxin A (OTA) | Anti-OTA | 10−4–20 ng mL−1 | 0.0001 ng mL−1 | 0.1–1 ng ml−1 | Covalent bonding | Immunosensor | [7] | |
| 2D | Si/ZnO film | Grapevine virus A (GVA)-type | Anti-GVA | 1 pg mL−1 to 1 µg mL−1 | NM | 1–10 ng ml−1 | Physical adsorption | Optical sensing | [88] |
| ZnO films | DNA of the dengue virus | specific probe strand | NM | NM | 1 × 10−15 moles of DNA | Physical adsorption | Optical sensing | [74] | |
| Glass/Au/ZnO thin film/ssDNA | Neisseria meningitidis DNA | DNA strands | 10–180 ng µL−1 | 5 ng µL−1 | 0.03°/(ng µL−1) | Physical adsorption | Optical sensing | [75] | |
| 3D | ZnO/Si nanoneedles/AgNPs | Rhodamine 6G (R6G) | NA | 10−6 –10−12 M | 1.1 × 10−16 M | NM | Physical adsorption | Optical sensing | [55] |
| Bare and Nafion-modified ZnO pyramids | Glucose | Glucose oxidase | 0.05–8.2 mM | 0.01 mM | NM | Physical adsorption | Electrochemical sensing | [89] | |
| ZnO/DPGP/ADH ZnO/DPGP/GOx | Ethanol Glucose | Alcohol dehydrogenase Glucose oxidase | 10–650 µM 1–50 mM | 2.1 µM 3.6 µM | 7.6 µA cm−2 mM−1 53.9 µA cm−2 mM−1 | Covalent bonding | Electrochemical sensing | [90] | |
| ZnO/SARS-CoV-rS ZnO/SARS-CoV-rN (tetrapods) | SARS-CoV-2 proteins | SARS-CoV-2 spike/nucleocapsid proteins | 0.025–0.5 nM 0.3–1 nM | 0.01 nM 0.3 nM | NM | Covalent bonding (cross-linker) | Immunosensor | [91] | |
| PCN-224/nano-zinc oxide nanocomposite | Human papillomavirus (HPV-16) DNA | Thiolated Capture DNA + DNA-dopamine conjugates | 1 fM–1 nM | 0.13 fM | NM | Covalent bonding | Electrooptical biosensor | [92] |
| Optical Technique | Working Principle | Biosensing Application | Ref. |
|---|---|---|---|
| SPR/LSPR | Refractive index changes at metal interfaces | Label-free protein detection | [83,107] |
| Fluorescence | Emission from fluorophore-labeled analytes | High-specificity cellular imaging | [74,108] |
| Raman Spectroscopy | Molecular vibrational mode analysis | Pathogen identification via SERS | [55,109] |
| PL | NBE and DLE emissions (shift, intensity, quenching) | Label-free analyte detection with high sensitivity | [9,27,110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Lázaro, A.; Portillo-Cortez, K.; de la Mora Mojica, M.B.; Durán-Álvarez, J.C. A Review on ZnO Nanostructures for Optical Biosensors: Morphology, Immobilization Strategies, and Biomedical Applications. Nanomaterials 2025, 15, 1627. https://doi.org/10.3390/nano15211627
Serrano-Lázaro A, Portillo-Cortez K, de la Mora Mojica MB, Durán-Álvarez JC. A Review on ZnO Nanostructures for Optical Biosensors: Morphology, Immobilization Strategies, and Biomedical Applications. Nanomaterials. 2025; 15(21):1627. https://doi.org/10.3390/nano15211627
Chicago/Turabian StyleSerrano-Lázaro, Amauri, Karina Portillo-Cortez, María Beatriz de la Mora Mojica, and Juan C. Durán-Álvarez. 2025. "A Review on ZnO Nanostructures for Optical Biosensors: Morphology, Immobilization Strategies, and Biomedical Applications" Nanomaterials 15, no. 21: 1627. https://doi.org/10.3390/nano15211627
APA StyleSerrano-Lázaro, A., Portillo-Cortez, K., de la Mora Mojica, M. B., & Durán-Álvarez, J. C. (2025). A Review on ZnO Nanostructures for Optical Biosensors: Morphology, Immobilization Strategies, and Biomedical Applications. Nanomaterials, 15(21), 1627. https://doi.org/10.3390/nano15211627








