Advancing Arsenic Water Treatment Using UiO-66 and Its Functionalized Metal–Organic Framework Analogs
Abstract
1. Introduction
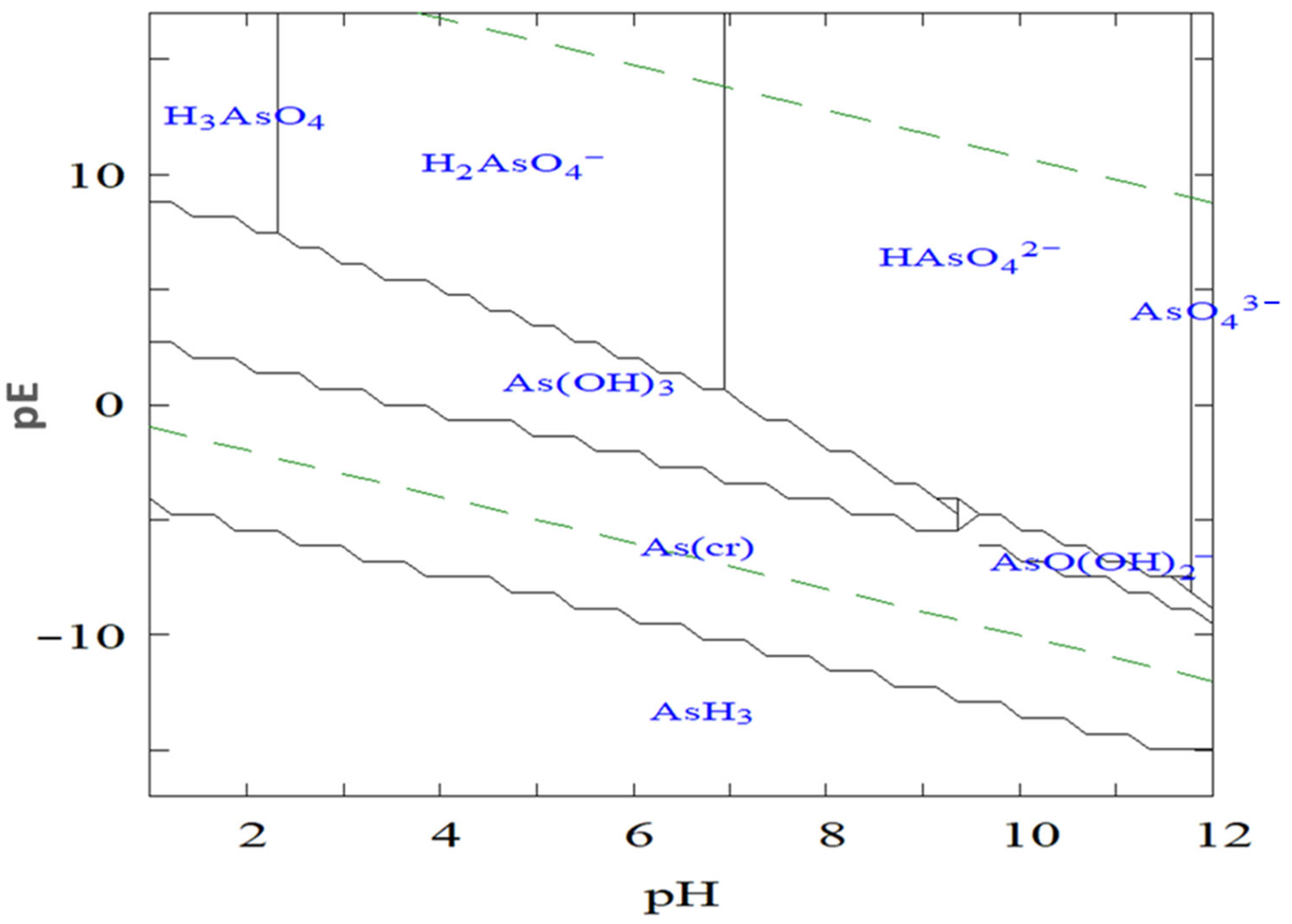
2. Arsenic Contamination
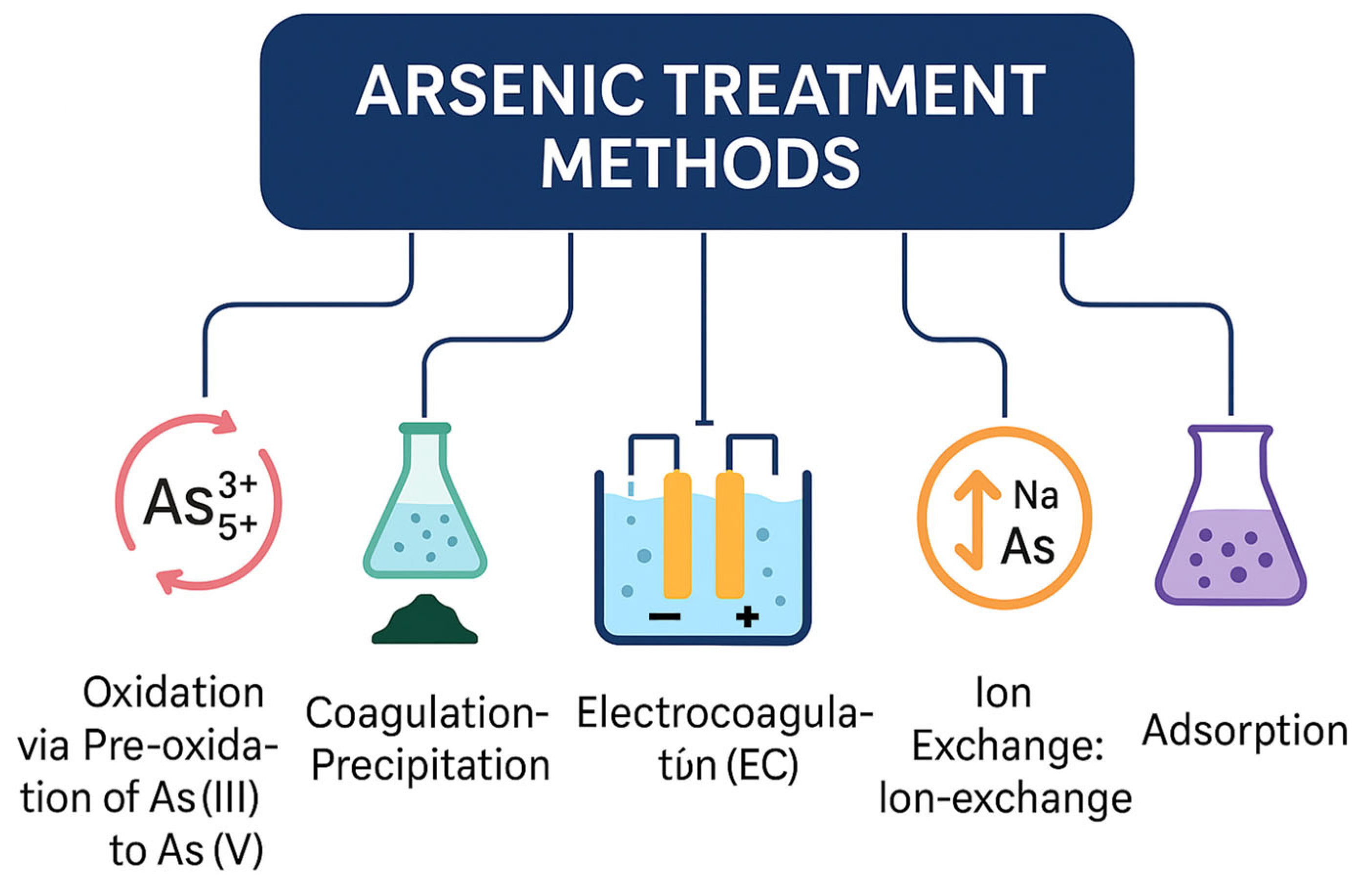
3. Arsenic Treatment Methods
| Adsorbents | Pollutants | Surface Area (BET) ** (m2 g−1) | *** (mg g−1) | References |
|---|---|---|---|---|
| Zero-valent Iron (ZVI) | As(V) | 2.53 | 0.73 | Su and Plus (2001) [53] |
| ZVI | As(V) | 187 | 66.8 | Peng et al. (2017) [49] |
| ZVI | As(V) | 997 | 1.42 | Zhang et al. (2021) [54] |
| Activated Carbon (AC) | As(V) | - | 30.5 | Mohan and Pittman (2007) [13] |
| AC (Coconut shell with 3% ash) | As(V) | 1150–1250 | 2.4 | Lorenzen et al. (1995) [55] |
| AC (Fe3O4-loaded) | As(V) | 349 | 204.2 | Liu et al. (2010) [56] |
| Organic biochar | As(V) | - | 16.2 | Zhu et al. (2016) [50] |
| TiO2 | As(III)/As(V) | - | 32.4/41.4 | Bang et al. (2005) [57] |
| Laterite | As(III)/As(V) | 181 | 8.0/24.8 | Maiti et al. (2010) [58] |
| Hydrous ferric oxide/zeolite | As(V) | - | 44.4 | Habuda-Stanic et al. (2008) [59] |
| Fe-Mn-Al oxides/oxyhydroxides | As(III)/As(V) | 456 | 34.3/21.2 | Jian and Maiti (2022) [60] |
| Activated alumina (Al2O3-CeO2) | As(III)/As(V) | - | 10.5/13.6 | Nakamoto and Kobayashi (2019) [61] |
| Magnetite | As(III)/As(V) | 60 | 29.1/11.4 | Yean et al. (2005) [62] |
| Magnetite | As(III)/As(V) | - | 18.7/17.2 | Liu et al., (2015) [63] |
| Zr-VBZ * | As(III)/As(V) | 421 | 6.5/7.0 | Gupta et al. (2022) [64] |
| Amorphous ZrO | As(III)/As(V) | 327 | 83/32.4 | Cui et al. (2012) [65] |
| ZrO2-sawdust | As(III)/As(V) | - | 29.0/12.0 | Setyono and Valiyaveettil (2014) [66] |
| Fe-Mn binary oxide coating sands | As(III)/As(V) | 117.4 | 22.07/11.94 | Han et al. (2022) [67] |
| Fe3O4 coated wheat straw | As(III)/As(V) | - | 3.9/8.1 | Hao et al. (2015) [68] |
| Fe3O4 nanoparticles | As(III)/As(V) | 179 | 16.56/46.06 | Feng et al. (2012) [69] |
| Zr doped β-FeOOH nanoparticles | As(V) | 330 | 120 | Sun et al. (2013) [51] |
4. The MOFs for Water Treatment
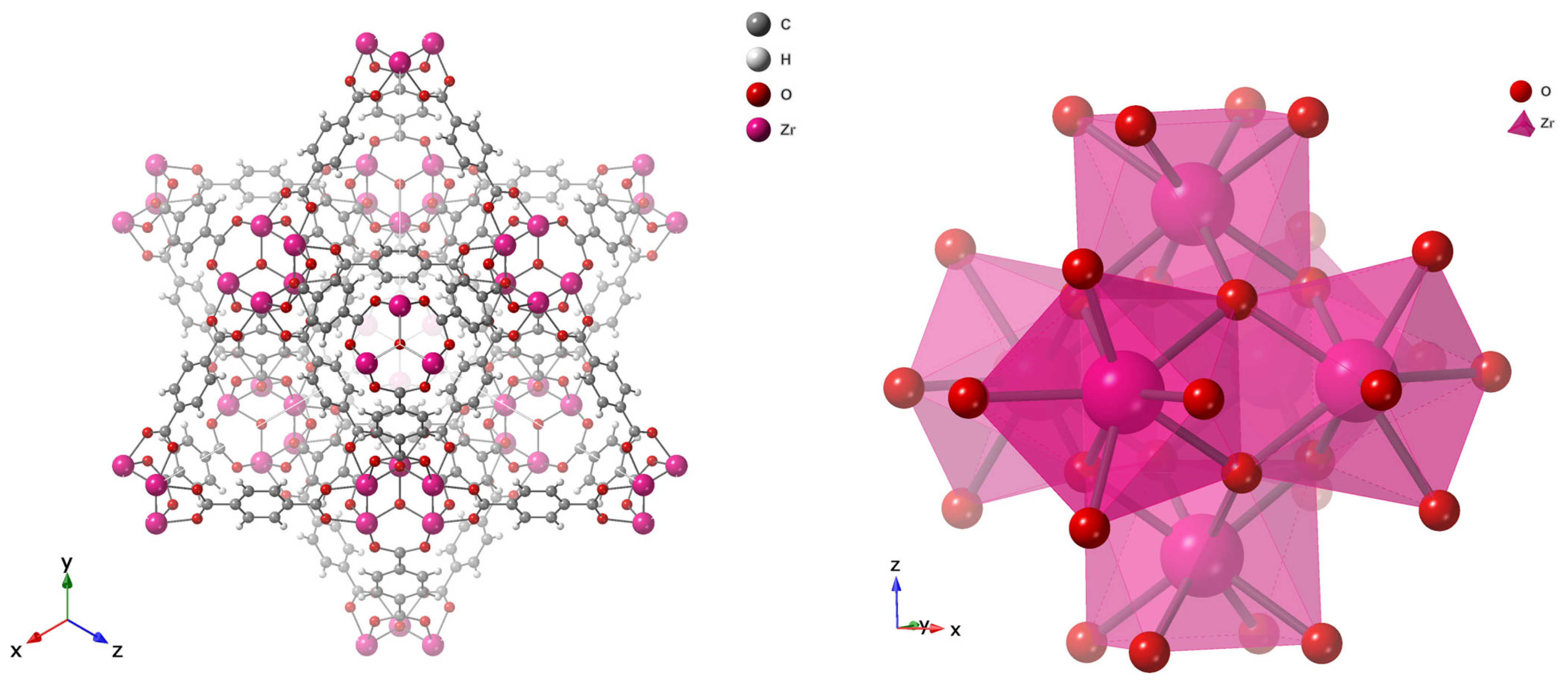
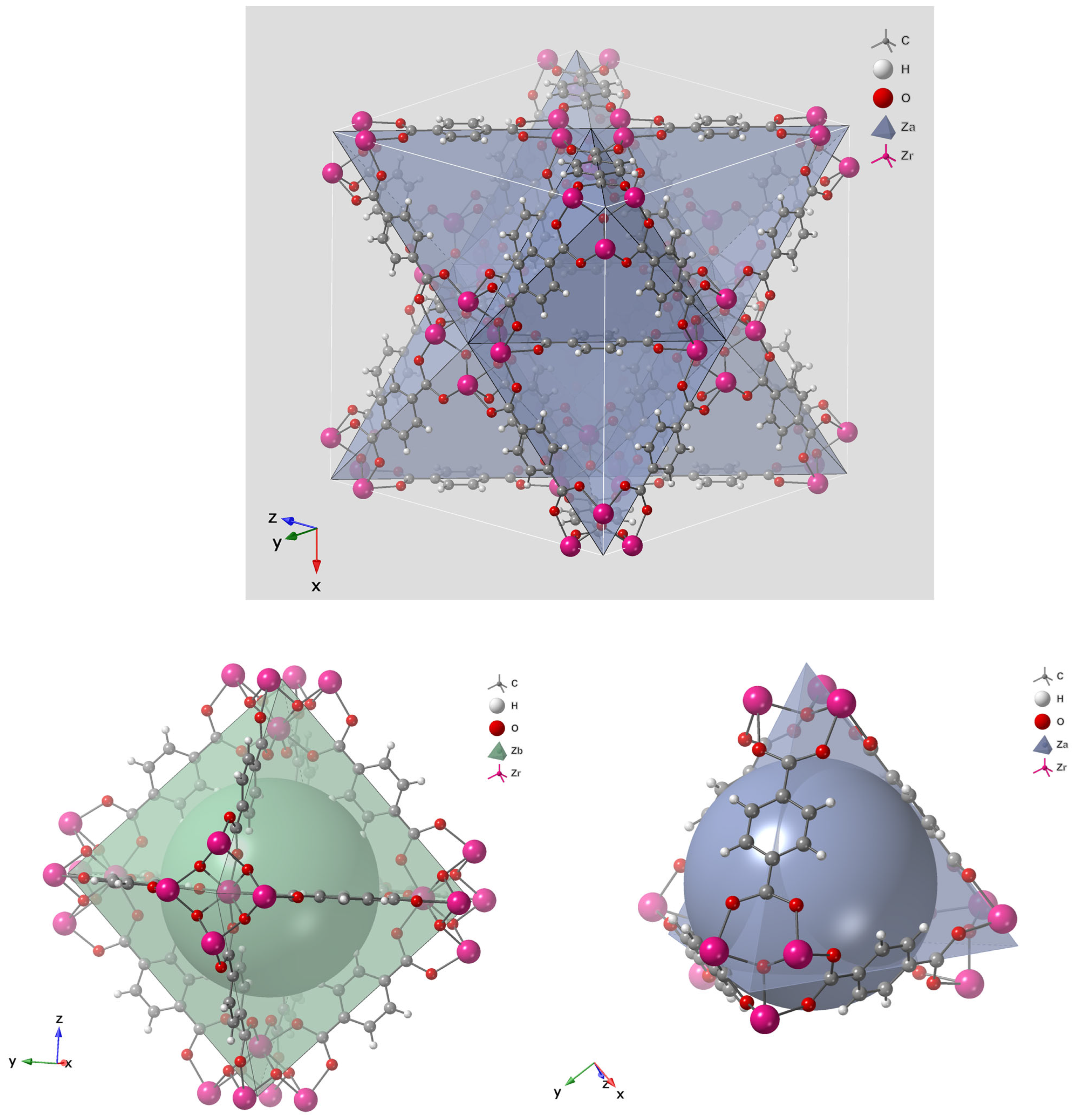
5. MOFs for Treatment of Arsenic
6. Strategies for Enhancement Arsenic Removal of UiO-66
| Adsorbents | Pollutants | BET Surface Area (m2 g−1) | (mg g−1) | References |
|---|---|---|---|---|
| γ-Fe2O3@ZrO2 | As(III)/As(V) | 62.2/18.3 | Feng et al. (2017) [137] | |
| CeO2/Fe3O4@UiO-66 & CeO2/Fe3O4@UiO-66-(SH)2 | various metal | 597, 539 | Real River As 86~99% | Boix et al. (2020) [149] |
| Ce-MOF-66 | As(III)/As(V) | 5.51/355.66 | Pervez et al. (2022) [139] | |
| Ce-MOF-808 | As(III)/As(V) | 402.09/217.79 | Pervez et al. (2022) [139] | |
| S-CuLa@UiO-66 | As(III) | 171 | Jiang et al. (2021) [150] | |
| UiO-66 (Fe/Zr) | As(III)/As(V) | 498.33 | 102/204 | Guo et al. (2023) [151] |
| ZrFc-MOF/ ZrFc-MOF/PMS 1) | As(S) | 59.59/111.34 | Li et al. (2023) [152] | |
| NZVI 2)@UiO-66 | As(III) | 360.6 | Liu et al. (2019) [140] | |
| Fe3O4@UiO-66-NH2 | 76.3 | 36 times higher than the UiO-66-NH2 | Chen et al. (2019) [143] | |
| Fe3O4@UiO-66 | As(V) | 124.8 | 73.2 | Huo et al. (2019) [145] |
| Fe3O4@TA 3)@UiO-66 | Sb(III)/As(III) | 130.3 | 49.51/97.82 | Qi et al. (2019) [144] |
| BSMM 4) | Sb(III) | 55 | 18.43 | Zhu et al. (2021) [153] |
| UiO-66@PGC 5) | As(III)/As(V) | 1312.37 | 185.38/201.02 | Pandi et al. (2020) [114] |
| UiO-66-NDC 6)/GO 7) | As(V) | 229.78 | 147.06 | Singh et al. (2022) [148] |
7. Green Synthesis of UiO-66: Transitioning from DMF to Sustainable Solvents
8. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part I: Occurence, Toxicity, Speciation, Mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Payne, K.; Abdel-Fattah, T.M. Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: Effects of pH, temperature, and ionic strength. J. Environ. Sci. Health 2005, 40, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Siddique, T.; Dutta, N.K.; Roy Choudhury, N. Nanofiltration for Arsenic Removal: Challenges, Recent Developments, and Perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef]
- Kanel, S.R.; Grenèche, J.-M.; Choi, H. Arsenic(V) Removal from Groundwater Using Nano Scale Zero-Valent Iron as a Colloidal Reactive Barrier Material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Nicomel, R.N.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Gavis, J. Review of Arsenic Cycle in Natural Waters. Water Res. 1972, 6, 1259–1274. [Google Scholar] [CrossRef]
- Sorlini, S.; Gialdini, F. Conventional oxidation treatments for the removal of arsenic with chlorine dioxide, hypochlorite, potassium permanganate and monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef]
- Meng, X.; Bang, S.; Korfiatis, G.P. Effects of silicate, sulfate, and carbonate on arsenic removal by ferric chloride. Water Res. 2000, 34, 1255–1261. [Google Scholar] [CrossRef]
- Sato, Y.; Kang, M.; Kamei, T.; Magara, Y. Performance of nanofiltration for arsenic removal. Water Res. 2002, 36, 3371–3377. [Google Scholar] [CrossRef]
- Kim, J.; Benjamin, M.M. Modeling a novel ion exchange process for arsenic and nitrate removal. Water Res. 2004, 38, 2053–2062. [Google Scholar] [CrossRef]
- Tokunaga, S. Removal of arsenic(III) and arsenic(V) ions from aqueous solutions with lanthanum(III) salt and comparison with aluminum(III), calcium(II), and iron(III) salts. Water Environ. Res. 1999, 71, 299–306. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable metal–organic frameworks: Design, synthesis, and applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Kalmutzki, M.J.; Diercks, C.S. Introduction to Reticular Chemistry: Metal-Organic Frameworks and Covalent Organic Frameworks; Wiley--VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; p. 509. [Google Scholar]
- Wang, C.; Luan, J.; Wu, C. Metal-organic frameworks for aquatic arsenic removal. Water Res. 2019, 158, 370–382. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Zhang, L.; Li, Y.-H.; Wang, C.-C.; Wang, P.; Zhao, C.; Fu, H. Defective NH2-UiO-66 for effective Pb(II) removal: Facile fabrication strategy, performances and mechanisms. Prog. Nat. Sci. Mater. Int. 2024, 34, 420–428. [Google Scholar] [CrossRef]
- Chen, W.; Lin, S.-Z.; Song, Z.; Huang, G.-B.; Zhang, M. Construction of S-scheme UiO-66-NH2/Zn0.4Cd0.6S hybrid architectures with strong interfacial interactions triggering efficient photocatalytic H2O2 production, nitrogen fixation and water splitting. J. Mater. Sci. Technol. 2025; in press. [Google Scholar] [CrossRef]
- Liang, K.; Guo, W.; Li, L.; Cai, H.; Zhang, H.; Li, J.; Xu, F.; Yan, J.; Lv, D.; Xi, H.; et al. Defect-induced synthesis of nanoscale hierarchically porous metal–organic frameworks with tunable porosity for enhanced volatile organic compound adsorption. Nano Mater. Sci. 2024, 6, 467–474. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X. Single-Atom Cu Anchored on a UiO-66 Surface-Enhanced Raman Scattering Sensor for Trace and Rapid Detection of Volatile Organic Compounds. Research 2025, 8, 0841. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, J.; Zhong, Y.; Li, Z.; Qiu, H.; Wu, H.B.; Wang, J.; Wang, X.; Gu, C.; et al. Quasi-Solid Electrolyte Interphase Boosting Charge and Mass Transfer for Dendrite-Free Zinc Battery. Nano-Micro Lett. 2023, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.R. Is Arsenic an Aphrodisiac? The Sociochemistry of an Element; RSC Publishing: Cambridge, UK, 2008; 412p. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Bardach, A.E.; Ciapponi, A.; Soto, N.; Chaparro, M.R.; Calderon, M.; Briatore, A.; Cadoppi, N.; Tassara, R.; Litter, M.I. Epidemiology of chronic disease related to arsenic in Argentina: A systematic review. Sci. Total Environ. 2015, 538, 802–816. [Google Scholar] [CrossRef]
- Chen, C.J.; Chuang, Y.C.; You, S.L.; Lin, T.M.; Wu, H.Y. A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. J. Cancer 1986, 53, 399. [Google Scholar] [CrossRef]
- Berg, M.; Tran, H.C.; Nguyen, T.C.; Pham, H.V.; Schertenleib, R.; Giger, W. Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environ. Sci. Technol. 2001, 35, 2621–2626. [Google Scholar] [CrossRef]
- Nickson, R.; McArthur, J.; Burgess, W.; Ahmed, K.M.; Ravenscroft, P.; Rahman, M. Arsenic poisoning of Bangladesh groundwater. Nature 1998, 395, 338. [Google Scholar] [CrossRef]
- Fendorf, S.; Michael, H.A.; van Geen, A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific opinion on arsenic in food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- Chakraborti, D.; Das, B.; Rahman, M.M.; Chowdhury, U.K.; Biswas, B.; Goswami, A.B.; Nayak, B.; Pal, A.; Sengupta, M.K.; Ahamed, S.; et al. Status of groundwater arsenic contamination in the state of West Bengal, India: A 20-year study repor. Mol. Nutr. Food Res. 2011, 55, 1626–1634. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Arsenic in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: https://iris.who.int/server/api/core/bitstreams/ac5cc702-7983-4b6a-8094-869f4fe95593/content (accessed on 22 September 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR), Addendum to the Toxicological Profile for Arsenic, Agency for Toxic Substances and Disease Registry Division of Toxicology and Human Health Sciences Atlanta. Available online: https://www.atsdr.cdc.gov/toxprofiles/Arsenic_addendum.pdf (accessed on 22 September 2025).
- USEPA (U.S. Environmental Protection Agency). National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 1 November 2023).
- Sorg, T.J.; Logsdon, G.S. Treatment Technology to Meet Interim Primary Drinking-Water Regulations for Inorganics: Part. 2. J. Am. Water Work. Assoc. 1978, 70, 379–393. [Google Scholar] [CrossRef]
- Ghurye, G.; Clifford, D. As(III) oxidation using chemical and solid-phase oxidants. J. Am. Water Work. Assoc. 2004, 96, 84–96. [Google Scholar] [CrossRef]
- Ding, W.; Zheng, H.; Sun, Y.; Zhao, Z.; Zheng, X.; Wu, Y.; Xiao, W. Activation of MnFe2O4 by sulfite for fast and efficient removal of arsenic (III) at circumneutral pH: In-volvement of Mn(III). J Hazard. Mater. 2021, 403, 123623. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.C.; Edwards, M. Coagulation With Hydrolyzing Metal Salts: Mechanisms and water Quality Impacts. Crit. Rev. Environ. Sci. Technol. 2014, 44, 303–347. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Clifford, D.; Samanta, G. Arsenic removal by coagulation with aluminum, iron, titanium, and zirconium. J. Am. Water Work. Assoc. 2008, 100, 76–88. [Google Scholar] [CrossRef]
- Bandaru, S.R.S.; van Genuchten, C.M.; Kumar, A.; Glade, S.; Hermandez, D.; Nahata, M.; Gadgil, A. Rapid and Efficient Arsenic Removal by Iron Electrocoagulation Enabled with in Situ Generation of Hydrogen Peroxide. Environ. Sci. Technol. 2020, 54, 6094–6103. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Anantha Singh, A.T.S. Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef]
- Kumar, P.R.; Chaudhari, S.; Khilar, K.C.; Mahajan, S. Removal of arsenic from water by electrocoagulation. Chemosphere 2004, 55, 1245–1252. [Google Scholar] [CrossRef]
- Algieri, C.; Pugliese, V.; Coppola, G.; Curcio, S.; Calabro, V.; Chakraborty, S. Arsenic removal from groundwater by membrane technology: Advantages, disadvantages, and effect on human health. Groundw. Sustain. Dev. 2022, 19, 100815. [Google Scholar] [CrossRef]
- Berg, M.; Luzi, S.; Trang, P.T.K.; Viet, P.H.; Giger, W.; Stüben, D. Arsenic removal from groundwater by household sand filters: Comparative field study, model calculations, and health benefits. Environ. Sci. Technol. 2006, 40, 5567–5573. [Google Scholar] [CrossRef]
- Voegelin, A.; Kaegi, R.; Berg, M.; Nitzsche, K.; Kappler, A.; Lan, V.M.; Trang, P.T.K.; Göttlicher, J.; Steininger, R. Solid-phase characterisation of an effective household sand filter for As, Fe and Mn removal from groundwater in Vietnam. Environ. Chem. 2014, 11, 566–578. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K. Arsenic removal from aqueous solutions by adsorption on magnetite nanoparticles. Water Environ. J. 2011, 25, 429–437. [Google Scholar] [CrossRef]
- Peng, X.; Xi, B.; Zhao, Y.; Shi, Q.; Meng, X.; Mao, X.; Jiang, Y.; Ma, Z.; Tan, W.; Liu, H.; et al. Effect of Arsenic on the Formation and Adsorption Property of Ferric Hydroxide Precipitates in ZVI Treatment. Environ. Sci. Technol. 2017, 51, 10100–10108. [Google Scholar] [CrossRef]
- Zhu, N.Y.; Yan, T.M.; Qiao, J.; Cao, H.L. Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Hu, C.; Hu, X.X.; Qu, J.H.; Yang, M. Characterization and adsorption performance of Zr-doped akaganéite for efficient arsenic removal. J. Chem. Technol. Biotechnol. 2013, 88, 629–635. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.Ö.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Puls, R. Arsenate and arsenite removal by zero-valent iron: Kinetics, redox transformation and implications for in situ groundwater remediation. Environ. Sci. Technol. 2001, 35, 1487–1492. [Google Scholar] [CrossRef]
- Zhang, N.; Eric, M.; Zhang, C.; Zhang, J.; Feng, K.; Li, Y.; Wang, S. ZVI impregnation altered arsenic sorption by ordered mesoporous carbon in presence of Cr(VI): A mechanistic investigation. J. Hazard. Mater. 2021, 414, 125507. [Google Scholar] [CrossRef]
- Lorenzen, L.; van Deventer, J.S.J.; Land, W.M. Factors affecting the mechanism of the adsorption of arsenic species on activated carbon. Miner. Eng. 1995, 8, 557–569. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S.; Sasai, R. Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass. Chem. Eng. J. 2010, 160, 57–62. [Google Scholar] [CrossRef]
- Bang, S.; Patel, M.; Lippincott, L.; Meng, X. Removal of arsenic from ground water by granular titanium dioxide adsorbent. Chemosphere 2005, 60, 389–397. [Google Scholar] [CrossRef]
- Maiti, A.; Basu, J.K.; De, S. Development of a Treated Laterite for Arsenic Adsorption: Effects of Treatment Parameters. Ind. Eng. Chem. Res. 2010, 49, 4873–4886. [Google Scholar] [CrossRef]
- Habuda-Stanic, M.; Kalajdžić, B.; Kuleš, M.; Velić, N. Arsenite and arsenate sorption by hydrous ferric oxide/polymeric material. Desalination 2008, 229, 1–9. [Google Scholar] [CrossRef]
- Jian, N.; Maiti, A. Fe-Mn-Al metal oxides/oxyhydroxides as As(III) oxidant under visible light and adsorption of total arsenic in the groundwater environment. Sep. Purif. Technol. 2022, 302, 122170. [Google Scholar] [CrossRef]
- Nakamoto, K.; Kobayashi, T. Arsenate and arsenite adsorbents composed of nano-sized cerium oxide deposited on activated alumina. Sep. Sci. Technol. 2019, 54, 523–534. [Google Scholar] [CrossRef]
- Yean, S.; Cong, L.; Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Kan, A.T.; Colvin, V.L.; Tomson, M.B. Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. J. Mater. Res. 2005, 20, 3255–3264. [Google Scholar] [CrossRef]
- Liu, C.H.; Chuang, Y.H.; Chen, T.Y.; Tian, Y.; Wang, M.-K.; Zhamg, W. Mechanism of arsenic adsorption on magnetite nanoparticles from water: Thermodynamic and spectroscopic studies. Environ. Sci. Technol. 2015, 49, 7726–7734. [Google Scholar] [CrossRef]
- Gupta, A.R.; Joshi, V.C.; Yadav, A.; Sharma, S. Synchronous Removal of Arsenic and Fluoride from Aqueous Solution: A Facile Approach to Fabricate Novel Functional Metallopolymer Microspheres. ACS Omega 2022, 7, 4879–4891. [Google Scholar] [CrossRef]
- Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Ind. Eng. Chem. 2012, 18, 1418–1427. [Google Scholar] [CrossRef]
- Setyono, D.; Valiyaveettil, S. Chemically modified sawdust as renewable adsorbent for arsenic removal from water. ACS Sustain. Chem. Eng. 2014, 2, 2722–2729. [Google Scholar] [CrossRef]
- Han, Y.S.; Kim, S.H.; Jang, J.Y.; Ji, S. Arsenic removal characteristics of natural Mn-Fe binary coating on waste filter sand from a water treatment facility. Environ. Sci. Pollut. Res. 2022, 29, 2136–2145. [Google Scholar] [CrossRef]
- Hao, L.; Zheng, T.; Jiang, J.; Hu, Q.; Li, X.; Wang, P. Removal of As (III) from water using modified jute fibres as a hybrid adsorbent. RSC Adv. 2015, 5, 10723–10732. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.K.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 44, 15606–15613. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016, 45, 5107. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Matsubara, I.; Higuchi, T.; Saito, Y. The crystal structure of bis(adiponitrilo) copper(I) nitrate. Bull. Chem. Soc. Jpn. 1959, 32, 1221–1226. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective Binding and Removal of Guests in a Microporous Metal-Organic Framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Sun, Z.; Richardson, D.A.; Groy, T.L. Directed Transformation of Molecules to Solids: Synthesis of a Microporous Sulfide from Molecular Germanium Sulfide Cages. J. Am. Chem. Soc. 1994, 116, 807–808. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular. Channels J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.; Michael, O.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef]
- Bennett, T.D.; Tan, J.C.; Yue, Y.; Baxter, E.; Ducati, C.; Terrill, N.J.; Yeung, H.H.; Zhou, Z.; Chen, W.; Henke, S.; et al. Hybrid glasses from strong and fragile metal-organic framework liquids. Nat. Commun. 2015, 6, 8079. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surblé, S. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem. 2004, 116, 6456–6461. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Alsalme, A.; Xiang, S.; Zhang, Z.; Chen, B. Design and applications of water-stable metal-organic frameworks: Status and challenges. Coord. Chem. Rev. 2020, 423, 213507. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Ghosh, P.; Colón, Y.J.; Snurr, R.Q. Water Adsorption in UiO-66: The Importance of Defects. Chem. Commun. 2014, 50, 11329–11331. [Google Scholar] [CrossRef]
- Chen, Z.; Hanna, S.L.; Redfern, L.R.; Alezi, D.; Islamoglu, T.; Farha, O.K. Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs. Coord. Chem. Rev. 2019, 386, 32–49. [Google Scholar] [CrossRef]
- Zou, D.; Liu, D. Understanding the modifications and applications of highly stable porous frameworks via UiO-66. Mater. Today Chem. 2019, 12, 139–165. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2017, 5, 1141–1152. [Google Scholar] [CrossRef]
- Homaee, M.; Hamadi, H.; Nobakht, V.; Javaherian, M.; Salahshournia, B. Ultrasound-assisted synthesis of UiO-66-NHSO3H via post-synthetic modification as a heterogeneous Brønsted acid catalyst. Polyhedron 2019, 165, 152–161. [Google Scholar] [CrossRef]
- Moghadaskhou, F.; Eivazzadeh-Keihan, R.; Sadat, Z.; Tadjarodi, A.; Maleki, A. Fabrication and characterization of a novel catalyst based on modified zirconium metal-organic-framework for synthesis of polyhydroquinolines. Sci. Rep. 2023, 13, 16584. [Google Scholar] [CrossRef]
- Edubilli, S.; Gumma, S. A systematic evaluation of UiO-66 metal organic framework for CO2/N2 separation. Sep. Purif. Technol. 2019, 224, 85–94. [Google Scholar] [CrossRef]
- Kazemi, A.; Moghadaskhou, F.; Pordsari, M.A.; Manteghi, F.; Tadjarodi, A.; Ghaemi, A. Enhanced CO2 capture potential of UiO-66-NH2 synthesized by sonochemical method: Experimental findings and performance evaluation. Sci. Rep. 2023, 13, 19891. [Google Scholar] [CrossRef]
- Chavan, S.; Vitillo, J.G.; Gianolio, D. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, W.; Liang, J.; Li, J. Synthesis and hydrogen storage studies of metal–organic framework UiO-66. Int. J. Hydrogen Energy 2013, 38, 13104–13109. [Google Scholar] [CrossRef]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M.; Bessarabov, D. Modulated synthesis of zirconium-metal organic framework (Zr-MOF) for hydrogen storage applications. Int. J. Hydrogen Energy 2014, 39, 890–895. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, W.; Han, X.; Chen, Y.; da Silva, I.; Lee, D.; Sheveleva, A.M.; Wang, Z.; Li, J.; Li, W.; et al. Direct Observation of Ammonia Storage in UiO-66 Incorporating Cu(II) Binding Sites. J. Am. Chem. Soc. 2022, 144, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, I.A.; Wells, C.J.R.; Forgan, R.S. Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery. Angew. Chem. 2020, 132, 5249–5255. [Google Scholar] [CrossRef]
- Strauss, I.; Chakarova, K.; Mundstock, A.; Mihaylov, M.; Hadjiivanov, K.; Guschanski, N.; Caro, J. UiO-66 and UiO-66-NH2 based sensors: Dielectric and FTIR investigations on the effect of CO2 adsorption. Microporous Mesoporous Mater. 2020, 302, 110227. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, X.; Wang, J.; Li, H.; Sun, Y.; Hao, X.; Qin, Y.; Niu, B.; Li, C. Washable and flexible gas sensor based on UiO-66-NH2 nanofibers membrane for highly detecting SO2. Chem. Eng. J. 2022, 428, 131720. [Google Scholar] [CrossRef]
- Min, X.; Wu, X.; Shao, P.; Ren, Z.; Ding, L.; Luo, X. Ultrahigh capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number. Chem. Eng. J. 2019, 358, 321–330. [Google Scholar] [CrossRef]
- Athari, M.; Fattahi, M.; Khosravi-Nikou, M.; Hajhariri, A. Adsorption of different anionic and cationic dyes by hybrid nanocomposites of carbon nanotube and graphene materials over UiO-66. Sci. Rep. 2022, 12, 20415. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.-R.; Arjmand, M. UiO-66 metal–organic frameworks in water treatment: A critical review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- Cheng, S.; Xie, P.; Yu, Z.; Gu, R.; Su, Y. Enhanced Adsorption Performance of UiO-66 via Modification with Functional Groups and Integration into Hydrogels. Environ. Res. 2022, 212 Pt C, 113354. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-Benzenetricarboxylic Metal–Organic Coordination Polymers Prepared by Solvothermal Method and Their Application in Efficient As(V) Removal from Aqueous Solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Li, Z.; Zhang, B.; Zhu, M.; Hu, X.; Zhang, Y.; Li, F. Zeolitic Imidazolate Framework-8 with High Efficiency in Trace Arsenate Adsorption and Removal from Water. J. Phys. Chem. C 2014, 118, 27382–27387. [Google Scholar] [CrossRef]
- Tarboush, B.J.; Chouman, A.; Jonderian, A.; Ahmad, M.; Hmadeh, M.; Al-Ghoul, M. Metal–Organic Framework-74 for Ultratrace Arsenic Removal from Water: Experimental and Density Functional Theory Studies. ACS Appl. Nano Mater. 2018, 1, 3283–3292. [Google Scholar] [CrossRef]
- Wang, D.; Gilliland, S.E., 3rd; Yi, X.; Logan, K.; Heitger, D.R.; Lucas, H.R.; Wang, W.-N. Iron Mesh-Based Metal Organic Framework Filter for Efficient Arsenic Removal. Environ. Sci. Technol. 2018, 52, 4275–4284. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.K.; Duong, Q.T.; Nguyen, T.V.; Lee, G.D. Arsenic removal from aqueous solution by adsorption using novel MiL-53(Fe) as highly efficient adsorbent. RSC Adv. 2015, 7, 5261–5268. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.; Zhou, Y.; Jiang, L.; Wang, C. Selective adsorption of arsenate and the reversible structure transformation of the mesoporous metal-organic framework MIL-100(Fe). Phys. Chem. Chem. Phys. 2016, 18, 10864–10867. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Li, Z.; Zhu, M.; Li, F. Characteristics of arsenate removal from water by metal-organic frameworks (MOFs). Water Sci. Technol. 2014, 70, 2014. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Yang, J.-C.; Sui, K.-W.; Yin, N. Facile synthesis of metal-organic framework MOF-808 for arsenic removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Pandi, K.; Lee, D.W.; Choi, J. Facile synthesis of zirconium-organic frameworks@biomass-derived porous graphitic nanocomposites: Arsenic adsorption performance and mechanism. J. Mol. Liq. 2020, 314, 113552. [Google Scholar] [CrossRef]
- He, X.; Deng, F.; Shen, T.; Yang, L.; Chen, D.; Luo, J.; Luo, X.; Min, X.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid. Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef]
- Islamoglu, T.; Otake, K.-i.; Li, P.; Buru, C.T.; Peters, A.W.; Akpinar, I.; Garibay, S.J.; Farha, O. Revisiting the structural homogeneity of NU-1000, a Zr-based metal–organic framework. Cryst. Eng. Comm. 2018, 20, 5913–5918. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal-Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.-W.; Lee, Y.-J.; Lee, D.-J. Adsorption of hydrogen arsenate and dihydrogen arsenate ions from neutral water by UiO-66-NH2. J. Environ. Manag. 2019, 247, 263–268. [Google Scholar] [CrossRef]
- Xu, R.; Ji, Q.; Zhao, P.; Jian, M.; Xiang, C.; Hu, C.; Zhang, G.; Tang, C.; Liu, R.; Zhang, X.; et al. Hierarchically porous UiO-66 with tunable mesopores and oxygen vacancies for enhanced arsenic removal. J. Mater. Chem. A 2020, 8, 7870. [Google Scholar] [CrossRef]
- Somjit, V.; Thinsoongnoen, P.; Sriphumrat, K.; Pimu, S.; Arayachukiat, S.; Kongpatpanich, K. Metal-Organic Framework Aerogel for Full pH Range Operation and Trace Adsorption of Arsenic in Water. ACS Appl. Mater. Interfaces 2022, 14, 40005–40013. [Google Scholar] [CrossRef]
- Audu, C.O.; Nguyen, H.G.T.; Chang, C.Y.; Katz, M.J.; Mao, L.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. The dual capture of As(V) and As(III) by UiO-66 and analogues. Chem. Sci. 2016, 7, 6492–6498. [Google Scholar] [CrossRef]
- Leus, K.; Perez, J.P.H.; Folens, K.; Meledina, M.; Van Tendeloo, G.; Du Laing, G.; Van Der Voort, P. UiO-66-(SH)2 as stable, selective and regenerable adsorbent for the removal of mercury from water under environmentally-relevant conditions. Faraday Discuss. 2017, 201, 145–161. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, Q.; Tong, T.; Sun, K.; Lin, L.-C.; Tang, C.Y.; Yang, F.; Guiver, M.D.; Quan, X.; Dong, Y. Robust ultrathin nanoporous MOF membrane with intra-crystalline defects for fast water transport. Nat. Commun. 2022, 13, 6. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, J.; Zhang, S.; Alsaedi, A.; Hayat, T.; Li, J.; Song, Y. Efficient removal of metal contaminants by EDTA modified MOF from aqueous solutions. J. Colloid. Interface Sci. 2019, 555, 403–412. [Google Scholar] [CrossRef]
- Shao, P.; Ding, P.L.; Luo, J.; You, D.; Zhang, Q.; Luo, X. Lattice-Defect-Enhanced Adsorption of Arsenic on Zirconia Nanospheres: A Combined Experimental and Theoretical Study. ACS Appl. Mater. Interfaces 2019, 11, 29736–29745. [Google Scholar] [CrossRef]
- Assaad, N.; Sabeh, G.; Hmadeh, M. Defect Control in Zr-Based Metal−Organic Framework Nanoparticles for Arsenic Removal from Water. ACS Appl. Nano Mater. 2020, 3, 8997–9008. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonio, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Qu, G.; Jia, P.; Zhang, T.; Li, Z.; Chen, C.; Zhao, Y. UiO-66(Zr)-derived t-zirconia with abundant lattice defect for remarkably enhanced arsenic removal. Chemosphere 2022, 288 Pt 2, 132594. [Google Scholar] [CrossRef]
- Somjit, V.; Thinsoongnoen, P.; Pila, T.; Boekfa, B.; Wannapaiboon, S.; Kongpatpanich, K. Hydroxylation of UiO-66 Metal–Organic Frameworks for High Arsenic(III) Removal Efficiency. Inorg. Chem. 2022, 61, 11342–11348. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic effects of ligand substitution on metaleorganic framework photocatalysts: The case study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, G.; Zhang, S.; He, X.; Zhong, Y.; Gao, X. Enhanced Breathing Effect of Nanoporous UIO-66-DABA Metal–Organic Frameworks with Coordination Defects for High Selectivity and Rapid Adsorption of Hg(II). ACS Appl. Nano Mater. 2023, 6, 18372–18380. [Google Scholar] [CrossRef]
- Davydiuk, T.; Chen, X.; Huang, L.; Shuai, Q.; Le, X.C. Removal of inorganic arsenic from water using metal organic frameworks. J. Environ. Sci. 2020, 97, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; James, J.B.; Armstrong, M.R.; Close, E.C.; Letham, P.A.; Nikkhah, K.; Lin, Y.S.; Mu, B. Influences of Deprotonation and Modulation on Nucleation and Growth of UiO-66: Intergrowth and Orienta-tion. J. Phys. Chem. C 2018, 122, 2200–2206. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; Li, Y.; Zhang, R.; Lu, G. Large-Scale Synthesis of Monodisperse UiO-66 Crystals with Tunable Sizes and Missing Linker Defects via Acid/Base Co-Modulation. ACS Appl. Mater. Interfaces 2017, 9, 15079–15085. [Google Scholar] [CrossRef]
- Vermoortele, F.; Bueken, B.; Le Bars, G.; Vande Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; VanSpeybroeck, V.; et al. Synthesis Modulation as a Tool To Increase the Catalytic Activity of Metal-Organic Frameworks: The Unique Case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Zhang, A.; Liao, C. Preparation of Fe–Co based MOF-74 and its effective adsorption of arsenic from aqueous solution. J. Environ. Sci. 2019, 80, 197–207. [Google Scholar] [CrossRef]
- Feng, C.; Aldrich, C.; Eksteen, J.J.; Arrigan, D.W.M. Removal of arsenic from alkaline process waters of gold cyanidation by use of γ-Fe2O3@ZrO2 nanosorbents. Hydrometallurgy 2017, 174, 71–77. [Google Scholar] [CrossRef]
- Yang, J.C.; Yin, X.B. CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity. Sci. Rep. 2017, 7, 40955. [Google Scholar] [CrossRef] [PubMed]
- Pervez, N.; Chen, C.; Li, Z.; Naddeo, V.; Zhao, Y. Tuning the structure of cerium-based metal-organic frameworks for efficient removal of arsenic species: The role of organic ligands. Chemosphere 2022, 303 Pt 1, 134934. [Google Scholar] [CrossRef]
- Liu, T.Y.; Zhang, Z.C.; Wang, Z.H.; Wang, Z.L.; Bush, R. Highly efficient and rapid removal of arsenic(iii) from aqueous solutions by nanoscale zero-valent iron supported on a zirconium 1,4-dicarboxybenzene metal–organic framework (UiO-66 MOF). RSC Adv. 2019, 9, 39475–39487. [Google Scholar] [CrossRef]
- Picchi, D.F.; Biglione, C.; Horcajada, P. Nanocomposites Based on Magnetic Nanoparticles and Metal-Organic Frame works for Therapy, Diagnosis, and Theragnostics. ACS Nanosci. Au 2024, 4, 85–114. [Google Scholar] [CrossRef]
- Wu, M.-X.; Wang, Y.; Zhou, G.; Liu, X. Core–Shell MOFs@MOFs: Diverse Designability and Enhanced Selectivity. ACS Appl. Mater. Interfaces 2020, 12, 54285–54305. [Google Scholar] [CrossRef]
- Chen, R.; Tao, C.; Zhang, Z.; Chen, X.; Liu, Z.; Wang, J. Layer-by-Layer Fabrication of Core-Shell Fe3O4@UiO-66-NH2 with High Catalytic Reactivity toward the Hydrolysis of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2019, 11, 43156–43165. [Google Scholar] [CrossRef]
- Qi, P.; Luo, R.; Pichler, T.; Zeng, J.; Wang, Y.; Fan, Y.; Sui, K. Development of a magnetic core-shell Fe3O4@TA@UiO-66 microsphere for removal of arsenic(III) and antimony(III) from aqueous solution. J. Hazard. Mater. 2019, 378, 120721. [Google Scholar] [CrossRef]
- Huo, J.-B.; Xu, L.; Chen, X.; Zhang, Y.; Yang, J.-C.E.; Yuan, B.; Fu, M.-L. Direct epitaxial synthesis of magnetic Fe3O4@UiO-66 composite for efficient removal of arsenate from water. Microporous Mesoporous Mater. 2019, 276, 68–75. [Google Scholar] [CrossRef]
- Ahmadipouya, S.; Haris, M.H.; Ahmadijokani, F.; Jarahiyan, A.; Molavi, H.; Moghaddam, F.M.; Rezakazemi, M.; Arjmand, M. Magnetic Fe3O4@UiO-66 nanocomposite for rapid adsorption of organic dyes from aqueous solution. J. Mol. Liq. 2021, 322, 114910. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Tajahmadi, S.; Haris, M.H.; Bahi, A.; Rezakazemi, M.; Molavi, H.; Ko, F.; Arjmand, M. Fe3O4@PAA@UiO-66-NH2 magnetic nanocomposite for selective adsorption of Quercetin. Chemosphere 2021, 275, 130087. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Naik, T.S.S.K.; U, B.; Khan, N.A.; Wani, A.B.; Behera, S.K.; Nath, B.; Bhati, S.; Sigh, J.; Ramamurthy, P.C. A systematic study of arsenic adsorption and removal from aqueous environments using novel graphene oxide functionalized UiO-66-NDC nanocomposites. Sci. Rep. 2022, 12, 15802. [Google Scholar] [CrossRef] [PubMed]
- Boix, G.; Troyano, J.; Garzon-Tovar, L.; Camur, C.; Bermejo, N.; Yazdi, A.; Piella, J.; Bastus, N.G.; Puntes, V.F.; Imaz, I.; et al. MOF-Beads Containing Inorganic Nanoparticles for the Simultaneous Removal of Multiple Heavy Metals from Water. ACS Appl. Mater. Interfaces 2020, 12, 10554–10562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-R.; Li, Y.; Zhang, D.; Zhou, Y.-X.; Xu, G.; Wang, C.; Lan, Y.; Guo, J. Decorating S-doped Cu-La bimetallic oxides with UIO-66 to increase the As(III) adsorption capacity via synchronous oxidation and adsorption. J. Hazard. Mater. 2021, 418, 126238. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Zheng, L.-W.; Xu, Y.; Shen, Y.-W.; Zhang, K.-G.; Yuan, C.-G. Facile fabrication of Fe/Zr binary MOFs for arsenic removal in water: High capacity, fast kinetics and good reusability. J. Environ. Sci. 2023, 128, 213–223. [Google Scholar] [CrossRef]
- Li, Z.; Ma, S.; Sang, L.; Qu, G.; Zhang, T.; Xu, B.; Jin, W.; Zhao, Y. Enhanced Arsenite Removal from Water Using Zirconium-Ferrocene MOFs Coupled with Peroxymono sulfate: Oxidation and Multi-sites Adsorption Mechanism. Chemosphere 2023, 319, 138044. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, J.; Yuan, Q.; Wang, X.; Zhao, Z.; Hursthouse, A.S.; Wang, Z.; Li, Q. A biochar supported magnetic metal organic framework for the removal of trivalent antimony. Chemosphere 2021, 282, 131068. [Google Scholar] [CrossRef]
- Obeidli, A.; Salah, H.B.; Murisi, M.; Sabouni, R. Recent advancements in MOFs synthesis and their green applications. Int. J. Hydrogen Energy 2022, 47, 2561–2593. [Google Scholar] [CrossRef]
- Hao, F.; Yan, X.-P. Nano-sized zeolite-like metal-organic frameworks induced hematological effects on red blood cell. J. Hazard. Mater. 2022, 424, 127353. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Haponiuk, J.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Anand, B.; Tsang, Y.F.; Kim, K.-H.; Khullar, S.; Wang, B. Regeneration, degradation, and toxicity effect of MOFs: Opportunities and challenges. Environ. Res. 2019, 176, 108488. [Google Scholar] [CrossRef] [PubMed]
- Ashour, R.M.; Abdel-Magied, A.F.; Wu, Q.; Olsson, R.T.; Forsberg, K. Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers 2020, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- An, H.T.; Zhang, X.; Dong, C.; Lu, M.-Y.; Li, R.; Xie, Y.; Xie, L.-H.; Li, J.-R. Seed-aided green synthesis of metal-organic frameworks in water. Green. Chem. Eng. 2023, 4, 64–72. [Google Scholar] [CrossRef]
- Venturi, D.M.; Campana, F.; Marmottini, F.; Costantino, F.; Vaccaro, L. Extensive Screening of Green Solvents for Safe and Sustainable UiO-66 Synthesis. ACS Sustain. Chem. Eng. 2020, 8, 17154–17164. [Google Scholar] [CrossRef]
- Biehler, E.; Pagola, S.; Stam, D.; Merkelbach, J.; Jandl, C.; Abdel-Fattah, T.M. A Comparison of Microcrystal Electron Diffraction and X-ray Powder Diffraction for the Structural Analysis of Metal–Organic Frameworks. Appl. Crystallogr. 2025, 58, 398–411. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T. Fabrication of metal-organic framework architectures with macroscopic size: A review. Co-ord. Chem. Rev. 2022, 462, 214520. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly Water-Stable Zirconium Metal–Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Fang, J.; Ding, L.-X.; Wang, H. A nano-silica modified polyimide nanofiber separator with enhanced thermal and wetting properties for high safety lithium-ion batteries. J. Membr. Sci. 2017, 537, 248–254. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Mamdouh, I.M.; El-Monaem, E.M.A.; El-Subruiti, G.M. Highly Efficient Removal for Methylene Blue and Cu2+ onto UiO-66 Metal–Organic Framework/Carboxylated Graphene Oxide-Incorporated Sodium Alginate Beads. ACS Omega 2021, 6, 23528–23541. [Google Scholar] [CrossRef]
- Singh, H.; Goyal, A.; Bhardwaj, S.K.; Khatri, M.; Bhardwaj, N. Highly robust UiO-66@PVDF metal–organic framework beads for tartrazine removal from aqueous solutions. Mater. Sci. Eng. B 2022, 288, 116165. [Google Scholar] [CrossRef]



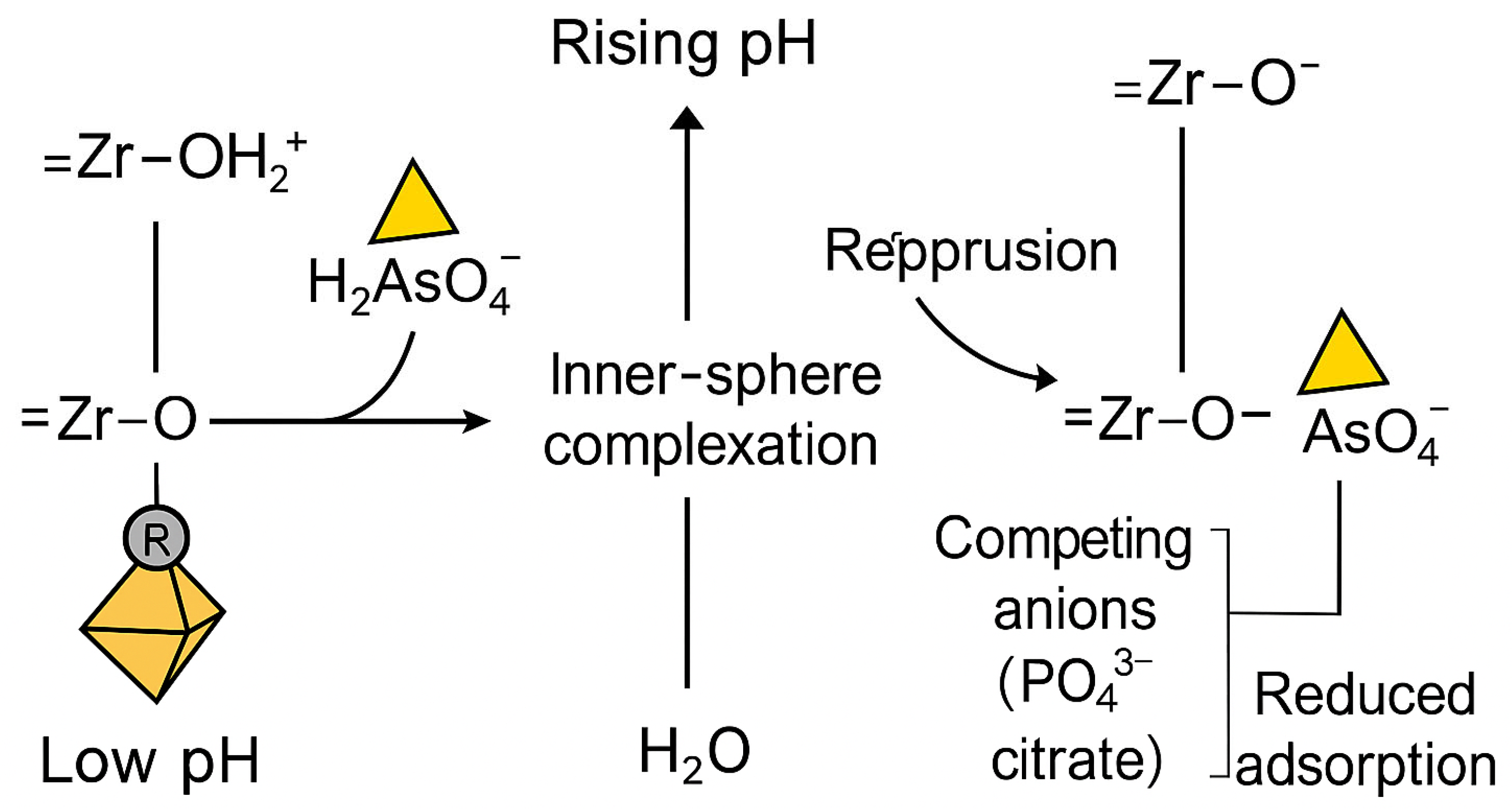
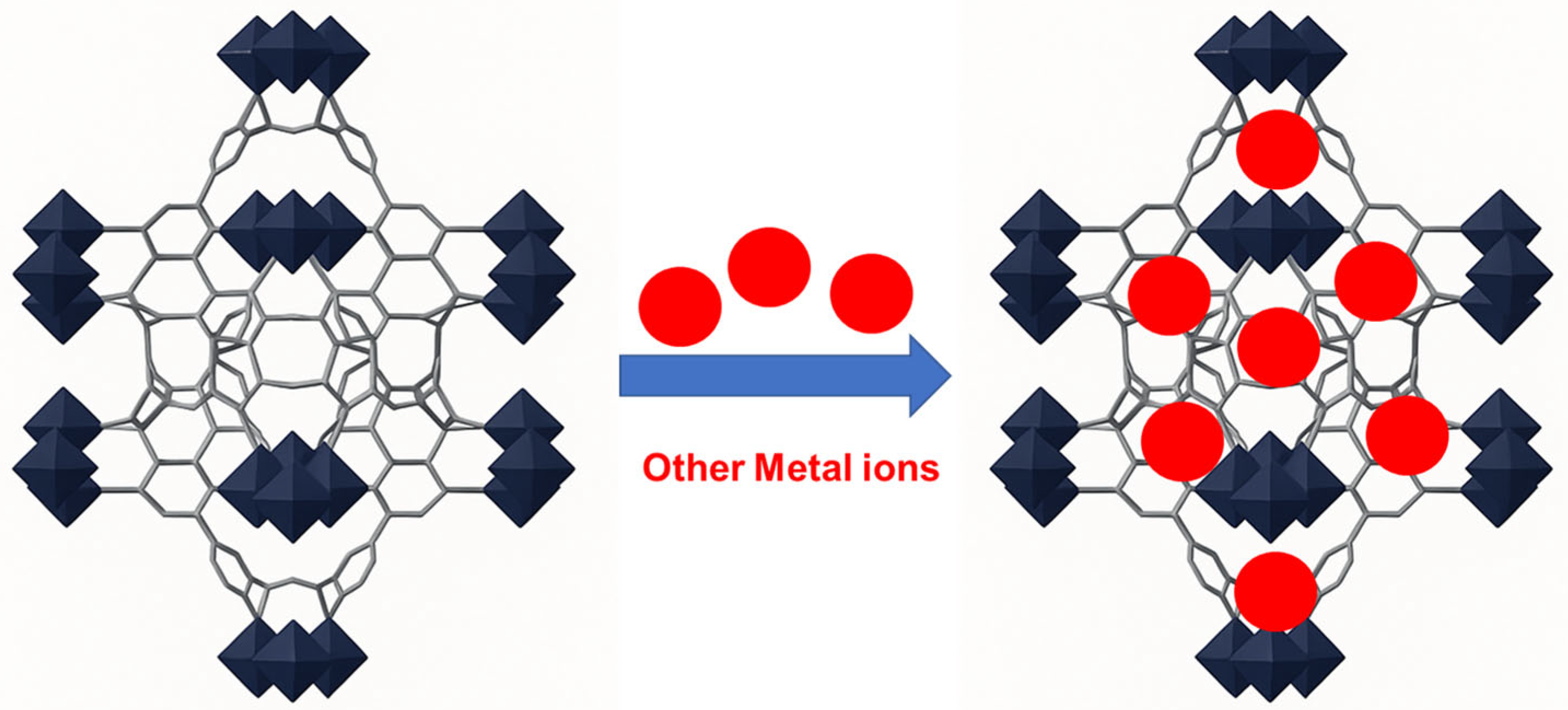
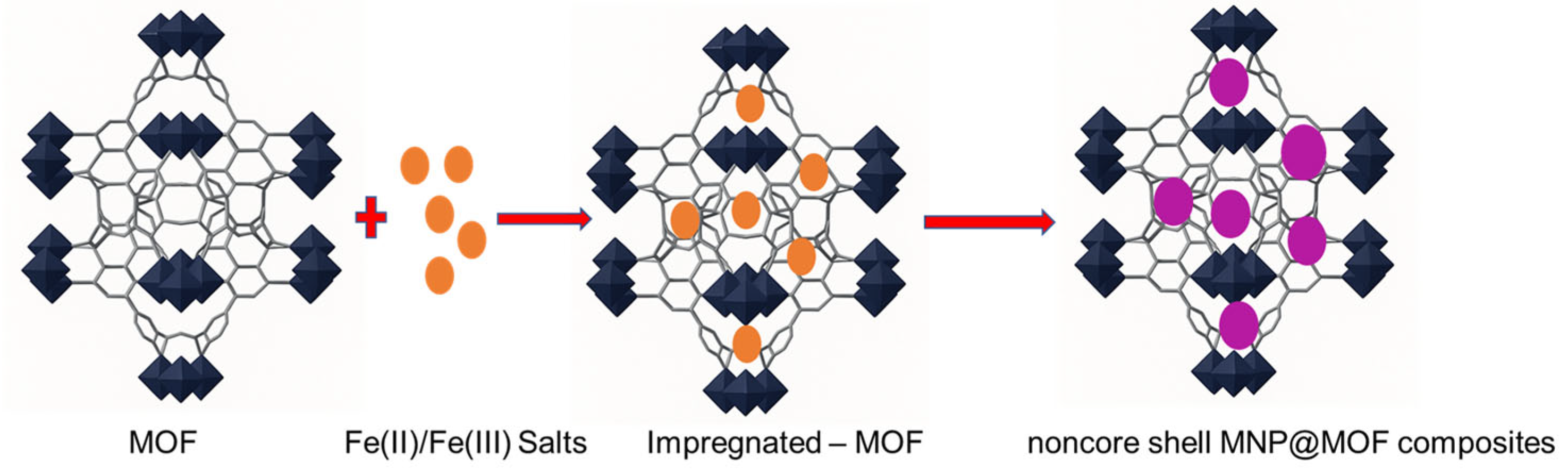
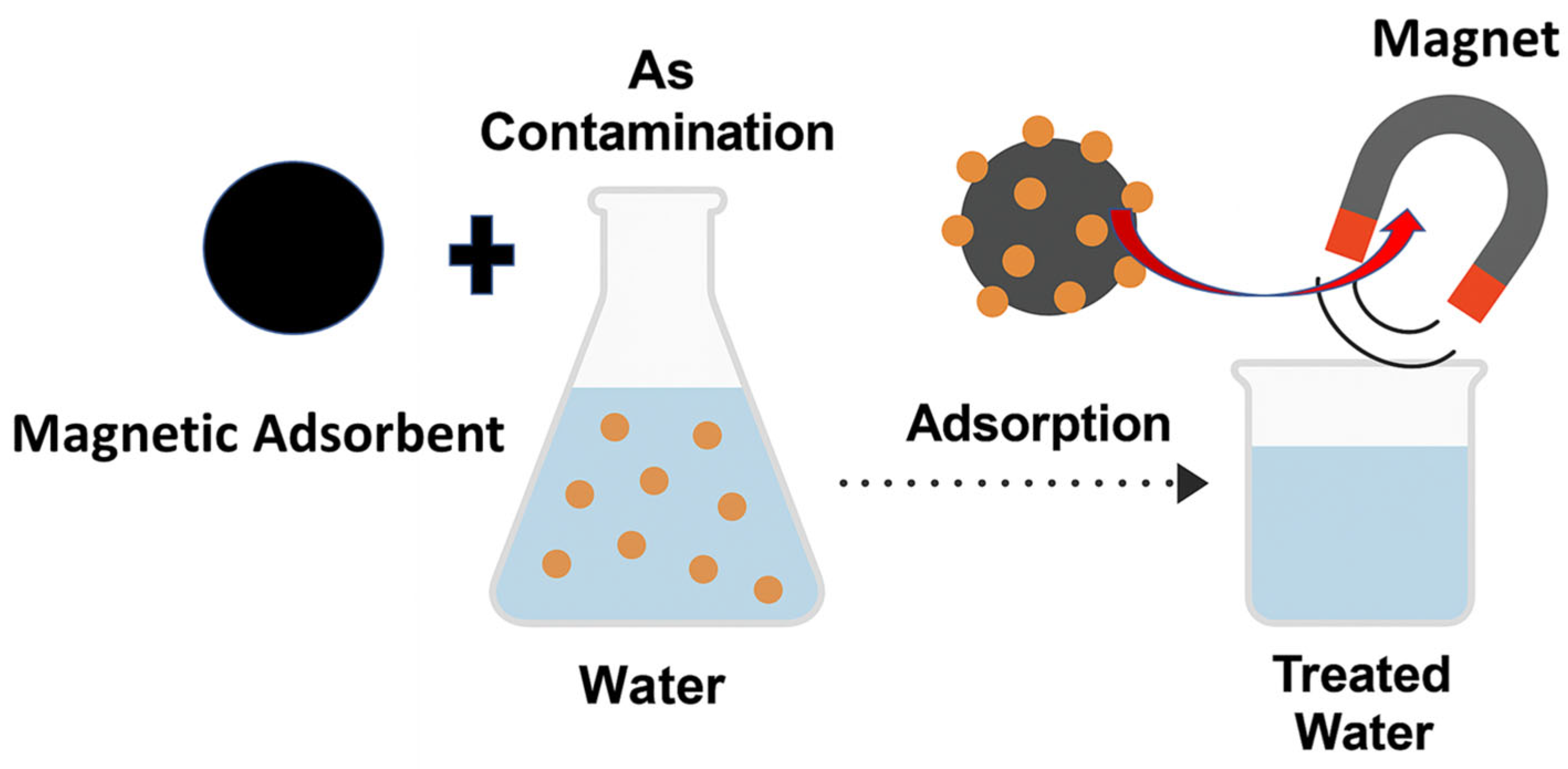
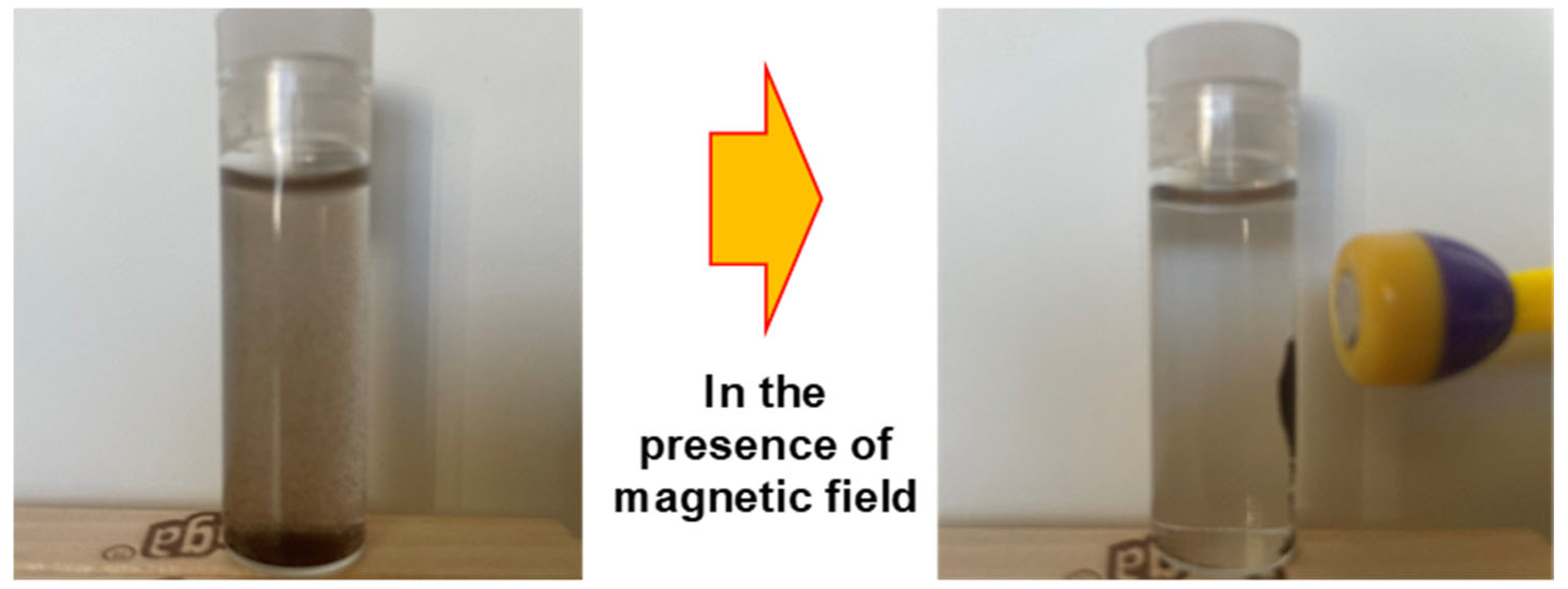
| Adsorbents | Pollutants | Surface Area (BET) (m2 g−1) | * (mg g−1) | References |
|---|---|---|---|---|
| Fe-BTC | As(V) | - | 12.3 | Zhu et al. (2012) [105] |
| ZIF-8 | As(V) | 1388 | 76.5 | Li et al. (2014a) [106] |
| ZIF-8 | As(III)/As(V) | 1064 | 49/60 | Jian et al. (2015) [109] |
| MOF-74 | As(V) | - | 99 | Tarboush et al. (2018) [107] |
| MIL-100(Fe) | As(III)/As(V) | 720 | 35.2/19.2 | Wang et al. (2018) [108] |
| MIL-53(Fe) | As(V) | 14 | 21.3 | Vu et al. (2015) [110] |
| MIL-101(Fe) | As(V) | 1370 | 110 | Cai et al. (2016) [111] |
| MIL-53(Al) | As(V) | 920 | 105.6 | Li et al. (2014b) [112] |
| MOF-808 | As(V) | - | 24.83 | Li et al. (2015) [113] |
| UiO-66 | As(V) | 570 | 303.4 (147.7~303.4) | Wang et al. (2015) [23] |
| UiO-66 | As(III)/As(V) | 1318.23 | 110.47/132.18 | Pandi et al. (2020) [114] |
| UiO-66 | As(III)/As(V) | 485.9 | 205/71.13 | He et al. (2019) [115] |
| Adsorbents | Pollutants | Surface Area (BET) (m2 g−1) | *** (mg g−1) | References |
|---|---|---|---|---|
| UiO-66-NH2 | As(III)/As(V) | 113.4 | 200/68.21 | He et al. (2019) [115] |
| UiO-66-NH2 | As(V) | 468 | 76.9 | Chang et al. (2019) [118] |
| * HP-UiO-66-NH2 | As(V) | 974.43 | 84.03 ~248.75 | Xu et al. (2020) [119] |
| UiO-66-NH2 | As(III)/As(V) | 300 | 104.8/131.6 | Somjit et al. (2022a) [120] |
| UiO-66-(SH) 2 | As(III)/As(V) | 1150 | 40.0/10.0 | Audu et al. (2016) [121] |
| UiO-66-(SH) 2 | Hg(II) | 499 | 236.4 | Leus et al. (2017) [122] |
| ** am-UiO-66-NO2 | As(V) | 531 | 85 | Wang et al. (2022) [123] |
| UiO-66-NO2 | As(V) | 660 | 68 | Wang et al. (2022) [123] |
| UiO-66-EDTA | 11 metals | 383.5 | Hg(II) 161.2 Pb(II) 153.8 | Wu et al. (2019) [124] |
| Adsorbent | Pollutant(s) | BET * Surface Area (m2 g−1) | ** (mg/g) | Regeneration (Cycles/Retention) % | PXRD After Cycling | N2 Sorption After Cycling (Retained) | Reference |
|---|---|---|---|---|---|---|---|
| UiO-66–NH2–HCl | As(V) | 468–855 | 76.9–161.3 | 5/90% | Peaks unchanged; minor intensity loss | 92% BET | Chang et al. (2019) [118] |
| UiO-66–SH–Al3+ | As(III)/As(V) | 30.2 | 90.9/98.8 | 4/80% | Slight broadening; Partial oxidation | 80% BET | Shao et al. (2019) [125] |
| UiO-66–TFA/AA | As(V) | 1041–1690 | 89.3–365.4 | 6/95% | Stable lattice reflections | 94% BET | Assaad et al. (2020) [126] |
| Lattice-Defected UiO-66 (t-ZrO2) | As(III)/As(V) | 133.7 | 352.1/147.5 | 4/88% | Minor shift; defect-related broadening | 87% BET | Qu et al. (2022) [128] |
| DU (Defected UiO-66) | As(III) | 946–1238 | 204 | 5/91% | Well-preserved peaks | 90% BET | Somjit et al. (2022b) [129] |
| UiO-66–DABA | Hg(II) | 753.9–1329.2 | 713 | 6/96% | No peak shift; high crystallinity | 95% BET | Zhao et al. (2023) [131] |
| Adsorbents | Pollutants | Surface Area (BET) (m2 g−1) | **** (mg g−1) | References |
|---|---|---|---|---|
| UiO-66-NH2-HCl | As(V) | 468–855 | 76.9–161.3 | Chang et al. (2019) [118] |
| UiO-66-SH-A * | As(III)/As(V) | 30.21 | 90.9/98.8 | Shao et al. (2019) [125] |
| UiO-66-TFA **, AA *** | As(V) | 1041–1690 | 89.3–365.4 | Assaad et al. (2020) [126] |
| Lattice defected UiO-66 (t-ZrO2) | As(III)/As(V) | 133.7 | 352.1/147.5 | Qu et al. (2022) [128] |
| DU (defected UiO-66) | As(III) | 946–1238 | 204 | Somjit et al., (2022b) [129] |
| UiO-66-DABA (DABA: 3,5-diaminobenzoic acid) | Hg(II) | 753.9– 1329.2 | 713 | Zhao et al. (2023) [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Abdel-Fattah, T.M. Advancing Arsenic Water Treatment Using UiO-66 and Its Functionalized Metal–Organic Framework Analogs. Nanomaterials 2025, 15, 1621. https://doi.org/10.3390/nano15211621
Ji S, Abdel-Fattah TM. Advancing Arsenic Water Treatment Using UiO-66 and Its Functionalized Metal–Organic Framework Analogs. Nanomaterials. 2025; 15(21):1621. https://doi.org/10.3390/nano15211621
Chicago/Turabian StyleJi, Sangwoo, and Tarek M. Abdel-Fattah. 2025. "Advancing Arsenic Water Treatment Using UiO-66 and Its Functionalized Metal–Organic Framework Analogs" Nanomaterials 15, no. 21: 1621. https://doi.org/10.3390/nano15211621
APA StyleJi, S., & Abdel-Fattah, T. M. (2025). Advancing Arsenic Water Treatment Using UiO-66 and Its Functionalized Metal–Organic Framework Analogs. Nanomaterials, 15(21), 1621. https://doi.org/10.3390/nano15211621








