Abstract
Addressing the challenges of energy production and environmental sustainability necessitates the development of advanced materials capable of facilitating both photocatalytic reduction and oxidation processes. Here, we report a Z-scheme Ag3PO4/CuBi2O4 heterojunction photocatalyst, which was fabricated via the in situ anisotropic growth of Ag3PO4 nanoparticles on the ends of CuBi2O4 microrods. The prepared heterojunction exhibits a low lattice mismatch (~3%) and features a covalently bonded interface, anchored by oxygen atoms, with the formation of P-O-Cu bonds. This interface synergizes with the built-in electric field to drive an efficient Z-scheme charge transfer mechanism, significantly enhancing the separation and migration of carriers. Furthermore, the interfacial chemical bonds induce electron redistribution that effectively weakens the Ag-O bond, thereby activating surface lattice oxygen. As a result, the photocatalyst shows remarkably improved performance for photocatalytic oxygen evolution synchronized with Cr(VI) reduction by enabling both the conventional adsorbate evolution mechanism and the lattice oxygen mechanism. This work provides critical insights into the design of efficient photocatalysts.
1. Introduction
Hexavalent chromium (Cr(VI)), identified as a highly soluble and carcinogenic heavy metal pollutant, is a prevalent contaminant in industrial effluents [1,2,3,4,5]. Its mutagenicity and bioaccumulation potential contribute to severe ecological and human health risks in aquatic environments [6,7]. Conventional methods like chemical reduction and adsorption face limitations, including secondary sludge generation, incomplete detoxification, and high operational costs [8,9]. Semiconductor photocatalysis has emerged as a highly attractive advanced oxidation/reduction technology for environmental remediation and solar energy conversion [10,11,12,13,14,15,16]. Current photocatalytic Cr(VI) reduction strategies require stoichiometric sacrificial electron donors (e.g., EDTA, methanol), which not only increase operational costs but also generate secondary pollutants, contradicting the principles of sustainable chemistry [3,17,18,19]. Synchronous coupling of Cr(VI) reduction with water oxidation to produce oxygen (O2) represents an ideal solution: 2H2O + Cr(VI) → O2 + Cr(III) + 4H+. However, achieving this requires the photocatalyst to simultaneously meet stringent thermodynamic and kinetic requirements. This includes possessing a conduction band potential sufficiently negative for Cr(VI) reduction and a valence band potential sufficiently positive for water oxidation. Furthermore, the catalyst must exhibit high electron–hole separation efficiency and provide ample reactive sites.
The Z-scheme heterojunction effectively preserves strong oxidation and reduction capabilities by mimicking natural photosynthesis and utilizing a unique Z-scheme charge transfer pathway [20,21,22]. The formation of a built-in electric field (BIEF) at the heterointerface is crucial for facilitating interfacial charge transfer [23,24,25]. Such a BIEF is readily induced by charge transfer mechanisms driven by diverse interactions utilized in heterojunction fabrication, such as hydrogen bonding [26,27], van der Waals forces [28,29], and electrostatic interactions [30,31,32,33,34]. However, the inherent instability of these interactions between constituent semiconductors frequently results in insufficient charge transfer. Heterojunction interfaces formed through chemical bonds, such as covalent, ionic, or coordination bonds, offer significant advantages over interfaces held together by simple physical contact or weak van der Waals forces [35,36,37,38,39,40]. On one hand, these strong chemical bonds provide robust interfacial adhesion, effectively resisting external stresses such as solution erosion and mechanical agitation, thereby preventing structural disintegration of the heterojunction. On the other hand, they establish continuous electronic pathways that lower interfacial energy barriers, significantly enhancing electron–hole transport across the interface [41,42,43,44]. Moreover, forming chemical bonds at the interface modifies the charge distribution, strengthening BIEF and further boosting charge separation efficiency. Therefore, designing a chemical bond-bridged heterointerface with a strong BIEF is highly desirable for synergistically optimizing photocatalytic Cr(VI) reduction coupled with O2 generation.
Herein, a P-O-Cu bond and IEF modulated Z-scheme heterojunction of Ag3PO4/CuBi2O4 with a small lattice mismatch is synthesized by in situ anisotropic growth of Ag3PO4 strategy on the end of the CuBi2O4 microrods for significantly enhanced photocatalytic O2 evolution and Cr(VI) reduction efficiency. Ag3PO4 and CuBi2O4 served as model materials due to interlaced band structures and distinct Fermi levels. An intimate covalent heterointerface was formed between Ag3PO4 and CuBi2O4 using the outermost O atoms as anchoring sites, resulting in a small interfacial mismatch of 3%. Systematic investigations reveal that the P-O-Cu bond and the IEF cooperatively induce the Z-scheme charge transfer mechanism, which in turn facilitates rapid charge separation and transfer [45,46]. More importantly, interfacial chemical bonding induces electron redistribution, significantly weakening the Ag-O bond energy and activating surface lattice oxygen. This, in turn, facilitates oxygen generation via the lattice oxygen pathway.
2. Materials and Methods
2.1. Chemicals
Sodium hydroxide (NaOH), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), dibasic sodium phosphate (Na2HPO4), copper nitrate trihydrate (Cu(NO3)2·3H2O), and silver nitrate (AgNO3) were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. These reagents were used without any treatment.
2.2. Synthesis of CuBi2O4 Microrods
CuBi2O4 microrods were synthesized by a one-step hydrothermal method. The specific process was as follows: First, 0.6 g of Cu(NO3)2·3H2O was sequentially dissolved in 80 mL of deionized water. Next, 2.42 g of Bi(NO3)3·5H2O and 0.87 g of NaOH were added to the above suspension under vigorous stirring, and stirred continuously at room temperature for 3 h. Finally, the resulting suspension was transferred to a 100 mL stainless steel autoclave and kept at 180 °C for 24 h of hydrothermal reaction. The final sample was washed several times in ionized water and ethanol, and was recorded as CuBi2O4 after drying at 70 °C for 12 h.
2.3. Synthesis of Ag3PO4/CuBi2O4 Composites
First, different contents of CuBi2O4 powder and 20 mL of deionized water were added to a beaker labeled as solution A. The sample was processed using an ultrasonic cell crusher for 1 h in order to achieve a uniform dispersion of the sample, which is conducive to obtaining CuBi2O4 microrods. Next, AgNO3 and Na2HPO4 were dissolved in 40 mL of deionized water, respectively, to obtain solution B and solution C. Subsequently, solution B was transferred to solution A, and solution C was instilled in a light-avoiding condition to change the color of the mixed solution from brown to bright yellow. Finally, the mixed solution was placed into a 100 mL Teflon-lined high-pressure cauldron and heated in an oven at 120 °C for 3 h. The precipitated product was washed with deionized water and anhydrous ethanol and dried under vacuum at 70 °C to obtain the desired yellow powder. The synthesized Ag3PO4/CuBi2O4 composite photocatalysts were named as ACBO-x, and x was 2, 5, 7, and 10, respectively (Here, x is the mass percentage of CuBi2O4 to Ag3PO4). Pure-phase Ag3PO4 nanoparticles were synthesized by the same method without the addition of CuBi2O4. The mass percentages of CuBi2O4 relative to Ag3PO4 in the ACBO-2, ACBO-5, ACBO-7, and ACBO-10 composite photocatalysts are 1.4%, 4.8%, 6.1%, and 8.9%, respectively, as determined by ICP analysis.
2.4. Characterization
The X-ray diffraction (D/MAX2500PC, Riga Corporation, Beveren, Belgium) patterns were used to determine the C phase structure of the catalysts. Field-emission scanning electron microscopy and energy spectroscopy were used for observation of material geometry, geometries, and dispersion states. The morphology was studied using a scanning electron microscope (SEM, FEI NovaNano450, FEI, Hillsboro, OR, USA) and a transmission electron microscope (TEM, JEM-2800, JEOL, Tokyo, Japan). The light absorption range of the photocatalyst was analyzed on a UV–visible near infrared spectrophotometer (UV-2600, Shimadzu Corporation, Kyoto, Japan). Electron spin resonance (ESR) experiments were carried out with a Bruker A 300 (Bruker Corporation, Ettlingen, Germany) spectrometer to determine the electron spin resonance phenomenon of unpaired electrons in matter. Fourier transform infrared spectroscopy (FTIR) (Nicolet iS50, Thermo Fisher Scientific, Waltham, MA, USA) was used to identify the functional groups present in the identified molecules with a KBr background and scanning range set to 400–4000 cm−1. Laser Raman spectroscopy (LRS) model VERTEX 80 Raman microscope (Bruker Corporation, Germany) was used to provide information on the chemical structure, phases, and morphology of the samples, crystallinity, and molecular interactions. The X-ray photoelectron spectroscopy (XPS) model ESCALAB 250XI (Thermo Fisher Scientific, Waltham, MA, USA) was used to analyze the elemental composition, chemical state, and molecular structure of the samples. Evaluation of photoluminescence efficiency of photocatalysts was carried out with a Quanmanster TM40 fluorescence spectrometer (PL, Photon Technology International, Birmingham, NJ, USA), and fluorescence lifetime of photocatalysts was studied by time-resolved fluorescence spectroscopy (TRPL)
2.5. Photoelectricity Experiments
The electrochemical performance of the samples was evaluated on an electrochemical workstation (CHI660E, Shanghai Chenhua Instruments, Shanghai, China) using Na2SO4 (0.5 mol/L) solution as the working electrolyte solution and Pt and Ag/AgCl electrodes as the counter electrode and reference electrode, respectively. For the preparation of the working electrodes, 5 mg of the sample, 250 μL of ethylene glycol, 250 μL of ethanol, and 40 μL of the membrane solution were mixed well and subsequently added dropwise onto a 1 × 2 cm FTO conductive glass with a coverage area of 1 cm2 of the sample. Subsequently, the sample was placed in a 70 °C oven and dried underneath for 4 h for the preparation of the working electrodes.
2.6. Photocatalytic Experiments
25 mg photocatalysts were dispersed in a beaker containing 50 mL of AgNO3 (0.6 M) solution, ultrasonically mixed homogeneously, and poured into a photocatalytic quartz reactor and evacuated for 15 min to maintain a sealed environment. During the reaction, the reaction system was maintained at 10 °C using circulating water and irradiated with a 300 W Xenon lamp equipped with a cutoff filter (λ ≥ 420 nm). Finally, gaseous products were analyzed using a Shimadzu GC-2014 gas chromatograph equipped with a thermal conductivity detector (TCD) and a molecular sieve column, using argon as the carrier gas. In the experiments using potassium dichromate as the sacrificial agent, basically the same test conditions were used. The difference was that an additional 4 mL of the reaction system solution sample was taken before and after illumination. The corresponding Cr(VI) conversion rate was calculated by comparing the absorption intensity of the UV–visible spectrophotometer at 532 nm. The calculation formula of the reduction conversion rate is R = (C0 − C/C0) × 100%, where R denotes the Cr(VI) reduction rate, C0 represents the concentration before the start of the reaction, and C denotes the concentration after the end of the reaction.
3. Results
3.1. Structural and Morphological Characterization
Synthesis of Ag3PO4/CuBi2O4 heterojunction was conducted in a two-step process. First, pristine CuBi2O4 microrods were synthesized through the hydrothermal method. Subsequently, Ag3PO4 was in situ anchored on the surface of CuBi2O4 via a facile hydrothermal route to craft Ag3PO4/CuBi2O4 composites. For simplicity, Ag3PO4/CuBi2O4 is denoted as ACBO. The morphology and microstructural characteristics of the as-prepared photocatalysts were analyzed by SEM and TEM images. Figure S1 displays irregularly spherical Ag3PO4 particles, exhibiting slight agglomeration. CuBi2O4 clearly demonstrates a one-dimensional (1D) microrod structure, where the microrods possess lengths of approximately 2–5 μm (Figure 1a). The SEM images of the ACBO-5 sample indicate a strong interfacial interaction along the central portions of the CuBi2O4 microrods, whereas their extremities show a tendency towards divergence. Notably, anisotropic growth of the Ag3PO4 particles was observed, with preferential material deposition occurring at one terminal end (Figure 1b,e). The tetragonal CuBi2O4 microrod attaches to the (200) plane of body-centered cubic Ag3PO4 through its (210) plane, and the growth direction of CuBi2O4 microrods is along the [100] direction (Figure 1c,d). The observed intimate attachment is crucial for lowering charge transport barriers, resulting in enhanced ultrafast interfacial charge transfer. EDX-STEM elemental mapping (Figure 1f) clearly shows that Cu and Bi are preferentially localized in the stem region of the rod, whereas Ag and P are mainly distributed at the ends of the rod-like structure. Oxygen (O) is uniformly distributed across the entire microrod.
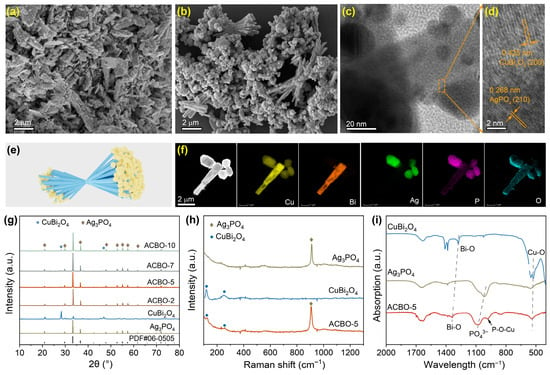
Figure 1.
(a) SEM image of CuBi2O4. SEM (b), TEM (c), and HRTEM (d) images of ACBO-5. (e) Schematic diagram of the ACBO-5 sample morphology. (f) Elements mapping of Cu, Bi, Ag, P, and O in ACBO-5. XRD patterns (g), Raman spectra (h), and FTIR spectra (i) of the as-synthesized samples.
The typical powder XRD patterns for the Ag3PO4, CuBi2O4, and ACBO-5 heterojunction with different amounts of Ag3PO4 are displayed in Figure 1g. The diffraction peaks of Ag3PO4 mainly located at 2θ = 20.8°, 29.7°, 33.3°, 36.6°, 46.8°, 52.7°, 55.0°, 57.3 °, and 71.9°, corresponding to the (110), (200), (210), (211), (310), (222), (320), (321), and (421) crystal planes of Ag3PO4 (JCPDS no. 06-0505), respectively [47]. For the pure CuBi2O4, four remarkable diffraction peaks were observed at 2θ = 21.0°, 28.2°, 33.4°, and 46.3°, corresponding to the (200), (131), (130), and (141) crystal planes of CuBi2O4 (JCPDS no. 720493), which were in agreement with the literature report [48]. It is worth noting that the diffraction peaks of the Ag3PO4/CuBi2O4 composite material can simultaneously exhibit the characteristic diffraction peaks of Ag3PO4 and CuBi2O4. As the CuBi2O4 content increased from 2 to 10%, the main characteristic peaks of the samples still existed, but the characteristic peaks at 28.2° gradually strengthened. All these XRD results confirmed that Ag3PO4 and CuBi2O4 successfully combined together. As shown in Figure 1h, the Raman spectra of CuBi2O4 were consistent with those reported in the literature, indicating the successful preparation of CuBi2O4. For the Raman spectra of Ag3PO4, the peak located at 909.8 cm−1 can be severally assigned to the symmetric stretching vibration of PO43− of Ag3PO4 (Figure 1i). The peaks at 121.2 cm−1 correspond to lattice vibrations involving the heavier Bi and Cu cations, while the peak at 253.6 cm−1 is associated with the stretching and contraction of the Bi-O bonds in CuBi2O4. The Raman spectra of the composite material displayed characteristic peaks corresponding to both Ag3PO4 and CuBi2O4. This observation provides strong corroboration for the successful formation of the Ag3PO4/CuBi2O4 heterojunction. This observation provides strong corroboration for the successful formation of the Ag3PO4/CuBi2O4 heterojunction, further supported by the complementary XRD analysis.
Figure 1i presents the Fourier transform infrared (FTIR) spectra of the as-obtained samples. All the samples showed a significant absorption peak at 1627 cm−1, which was caused by the bending vibration modes of the O-H groups of adsorbed water on the catalyst surface. For CuBi2O4, infrared absorption peaks were observed at 1398 cm−1 and 477 cm−1, corresponding to the characteristic vibrations of Bi-O and Cu-O bonds, respectively [49]. Furthermore, the Ag3PO4 materials exhibited an absorption peak at 1094 cm−1, which corresponds to the P=O stretching vibration mode of PO43− [50]. The P=O stretching vibration in the composites experienced a shift to a higher wavenumber relative to that of pristine Ag3PO4. This observation not only confirms the successful formation of the heterojunction but also suggests a significant interaction at the interface between CuBi2O4 and Ag3PO4. Notably, a new characteristic peak observed at 982 cm−1 in the composites can be indexed to a P-O-Cu bonding state [51], which provides direct evidence for the intimate interface between CuBi2O4 and Ag3PO4 formed through the creation of P-O-Cu bonds.
The lattice mismatch (δ) at the interface of two dissimilar semiconductors is a crucial, yet often underappreciated, factor that plays a significant role in determining photocatalytic performance [52,53]. The lattice constants for Ag3PO4 (210) and CuBi2O4 (200) are b1 = 6.00 Å (Figure 2a) and b2 = 5.81 Å (Figure 2b), respectively. This results in a δ value of only 3% between the Ag3PO4 and CuBi2O4 planes (Figure 2c). Heterointerfaces can be broadly categorized into three types based on the degree of δ: incoherent interfaces (δ > 20%, Figure 2d), semicoherent interfaces (5% ≤ δ ≤ 20%, Figure 2e), and coherent interfaces (δ < 5%, Figure 2f). Heterostructures with a large δ often hinder photocatalytic activity due to the formation of extensive grain boundaries, inefficient heterointerfaces, and slow charge carrier migration. In contrast, heterostructures with a small δ are considered optimal photocatalysts, as they minimize grain boundaries, promote effective heterointerfaces, and facilitate rapid charge transfer across the junction. The resulting Ag3PO4 and CuBi2O4 heterojunction, linked via a covalent P-O-Cu bridge, was thus meticulously engineered with a small interfacial lattice mismatch, which is well below the 5% threshold for a coherent interface. Theoretically, the proposed structure is designed to facilitate rapid charge transfer, which is expected to be the basis for enhanced photocatalytic performance.
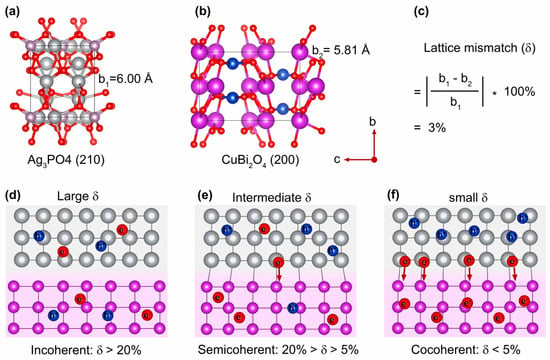
Figure 2.
Crystal structures and lattice constants of Ag3PO4 (210) (a) and CuBi2O4 (200) (b). (c) The calculation of interfacial lattice mismatch. Schematic illustration of heterojunctions with large δ (d), intermediate δ (e), and small δ (f).
3.2. Photocatalytic Activity of the Ag3PO4/CuBi2O4 Photocatalyst
In order to investigate the photocatalytic activity of Ag3PO4/CuBi2O4 composite, the water oxidation reaction was conducted under visible light irradiation. Figure 3a,b illustrate that pure Ag3PO4 displayed a modest O2 evolution rate of only 92.5 µmol·g−1·h−1, with AgNO3 serving as an electron scavenger. This limited activity is attributed to the facile recombination of photogenerated carriers. In comparison, a significant enhancement in photocatalytic O2 evolution activity was achieved through the incorporation of CuBi2O4. The O2 evolution rate of the ACBO composite was found to increase with the mass ratio of AgNO3 to CuBi2O4, reaching an optimal rate of up to 371.6 µmol·g−1·h−1 at a 5.0% AgNO3 loading. This represents an impressive enhancement, being approximately 4.1 times higher than that of pristine Ag3PO4. When potassium dichromate (K2Cr2O7) was employed as the electron scavenger instead of AgNO3, the photocatalytic O2 evolution rate was significantly enhanced (Figure 3c,d). The optimal ACBO-5 composite demonstrated robust photocatalytic activity, achieving an O2 evolution rate of 652.7 µmol·g−1·h−1 and a Cr(VI) reduction efficiency of 82.1%. The ACBO-5 demonstrates superior photocatalytic oxygen evolution activity, outperforming a range of reported catalysts (Table S2). This highlights the great potential of our design strategy for advancing solar-driven water splitting applications. The enhanced performance can be attributed to the rod-like structure of CuBi2O4, which minimizes charge migration pathways, drives efficient photoinduced carrier separation, and provides abundant surface active sites. Excessive loading of AgNO3 (e.g., in ACBO-7 and ACBO-9) was observed to be counterproductive for photocatalytic activity, primarily attributed to the excessive AgNO3 shielding the light absorption of CuBi2O4. The above results indicate that the loading of CuBi2O4 has a positive effect on enhancing the photocatalytic activity of Ag3PO4, and the ACBO composite is a catalyst with high photocatalytic reaction activity. The stability of a catalyst is a critical factor in determining its successful application in photocatalysis. It has been previously reported that the photocatalytic efficiency of Ag3PO4 is often compromised due to the favorable reduction of Ag+ to Ag0 nanoparticles [54,55]. While the ACBO-5 heterojunction system demonstrates expected photocatalytic activity, its long-term photochemical stability has yet to be fully established. To assess its long-term stability, cyclic photocatalytic tests were performed on ACBO-5. The photocatalytic activity of ACBO-5 remained stable, showing no significant decrease over five cycles (Figure 3e). The concentrations of Cu, Ag, and Bi ions in the solution were determined by ICP after the photocatalytic reaction (Table S3). The results show that the ion concentrations of Cu, Ag, and Bi in the solution were minimal, indicating that the material remains stable and that ion release is negligible under the tested conditions. As shown in Figure 3f, the consistent positions of the XRD diffraction peaks and the absence of any diffraction peaks corresponding to precipitated Ag particles before and after the reaction conclusively demonstrate that the ACBO-5 sample maintains high reactivity and stability throughout the photocatalytic synchronous reaction [56,57,58].
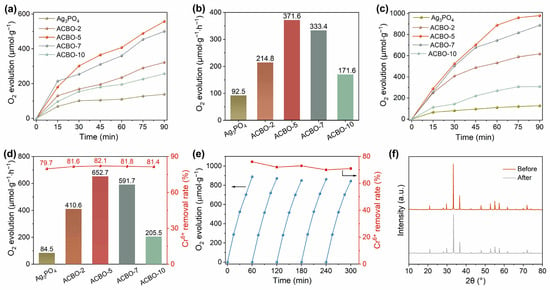
Figure 3.
(a,b) Photocatalytic oxygen evolution performance of Ag3PO4 and Ag3PO4/CuBi2O4 composite with AgNO3 serving as an electron scavenger. (c,d) Photocatalytic performance of oxygen evolution synchronously with Cr(VI) reduction. (e) Cycling experiment of ACBO-5. (f) XRD patterns of ACBO-5 before and after the cyclic experiment.
3.3. Insights into Z-Scheme Heterojunction Formation
The surface elemental compositions and corresponding chemical valence of Ag3PO4, CuBi2O4, and ACBO-5 were analyzed by XPS. The XPS survey spectrum of the ACBO-5 composite confirms the presence of Ag, P, O, Cu, and Bi elements, thereby providing evidence for the successful coupling of Ag3PO4 and CuBi2O4 (Figure 4a). As shown in Figure 4b, the Ag 3d XPS spectra for Ag3PO4 exhibit two characteristic peaks at 368.0 and 374.1 eV, which are characteristic of Ag 3d5/2 and Ag 3d3/2, respectively, indicating the Ag+ oxidation state. Whereas in the ACBO-5 sample, a slight negative shift of the Ag 3d5/2 and Ag 3d3/2 peaks to 367.9 eV and 373.9 eV, respectively, was observed. The main XPS peak of P 2p is located at 133.4 eV, corresponding to P-O (Figure 4c). Additionally, the ACBO-5 exhibits a more negative binding energy (BE) compared to the Ag3PO4. As depicted in Figure 4d, the Cu 2p core-level spectrum of monomeric CuBi2O4 shows characteristic peaks for Cu 2p1/2 and Cu 2p3/2 at 954.3 and 934.5 eV, respectively. The resulting spin-orbit splitting of 19.8 eV is consistent with the presence of the Cu2+. The other pair of peaks at 940.7 and 943.6 eV was assigned to the satellite peak of Cu2+. In the ACBO-5 sample, the BEs of the Cu 2p core levels showed a negative shift relative to those of CuBi2O4. As shown in Figure 4e, the Bi 4f XPS spectrum of CuBi2O4 reveals two characteristic peaks at 158.6 eV and 163.9 eV, respectively. These spectral features are consistent with the Bi 4f7/2 and Bi 4f5/2, respectively, unequivocally indicating the Bi3+ oxidation state. When coupled with Ag3PO4, the Bi 4f peaks were observed to shift to higher binding energies, specifically to 159.6 eV and 164.9 eV. The O 1s spectrum (Figure 4f) can be deconvoluted into three distinct components at binding energies of 530.6, 532.3, and 533.5 eV, which are assigned to lattice oxygen, surface hydroxyl/adsorbed oxygen species, and oxygen involved in interfacial P-O-Cu bonding, respectively [59,60,61]. Those results above suggest electron accumulation from CuBi2O4 on the surface of Ag3PO4. Moreover, the ACBO material exhibits a lower electron density at the Ag sites, resulting in a more ionic metal–oxygen bond, which may facilitate lattice oxygen activation.
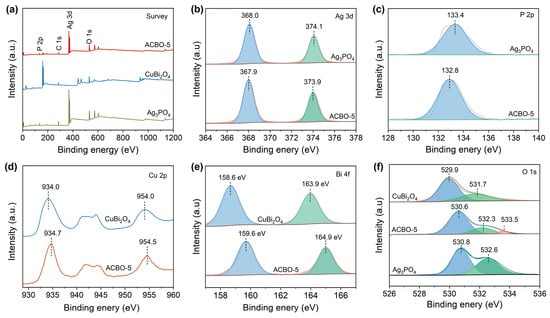
Figure 4.
(a) Survey, (b) Ag 3d, (c) P 2p, (d) Cu 2p, (e) Bi 4f, and (f) O 1s XPS spectra.
3.4. Photophysical and Electrochemical Properties
UV–visible diffuse reflectance spectroscopy (DRS) was employed to characterize the light absorption properties of the samples, as depicted in Figure 5a. The as-prepared Ag3PO4 samples displayed limited absorption in the visible light region, characterized by an absorption edge at 550 nm. The coupling of CuBi2O4 with Ag3PO4 resulted in a significant enhancement in the light absorption intensity of the ACBO heterostructure. The increased light absorption capability contributes to more efficient photocarrier generation, which is highly beneficial for improving photocatalytic performance. The band gaps of Ag3PO4 and CuBi2O4 were estimated to be 2.40 eV and 1.75 eV, respectively, using the Tauc equation (Figure 5b). Figure S2 illustrates the n-type semiconductor behavior of Ag3PO4 (positive slope) and the p-type semiconductor behavior of CuBi2O4 (negative slope). Their respective flat band potentials were determined to be −0.32 V and 0.82 V (vs. Ag/AgCl, pH = 6.8), respectively. The conduction band potentials for Ag3PO4 and CuBi2O4 are thus determined to be −0.27 V and −0.55 V (vs. NHE, pH = 0), respectively. The valence band positions (EVB) of Ag3PO4 and CuBi2O4 were subsequently derived as 2.67 V and 1.22 V (vs. NHE, pH = 0), respectively, by incorporating their determined band gap (Eg) into Eg = EVB − ECB.
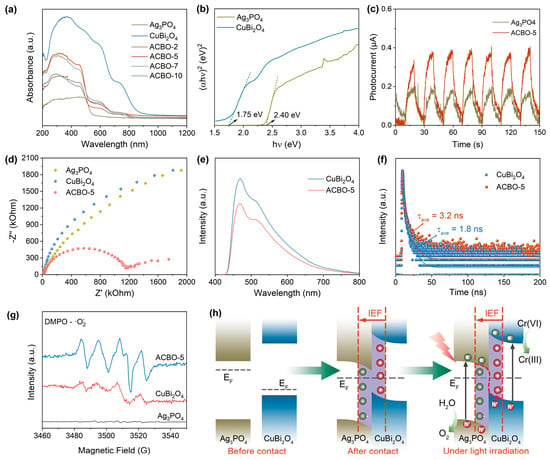
Figure 5.
UV-vis DRS (a) of the prepared photocatalysts and the corresponding Tauc plots (b). Transient photocurrent curve density curves (c), EIS Nyquist plots (d), PL spectra (e), and TRPL spectra (f) of the different samples. (g) EPR spectra of DMPO-·O2− over Ag3PO4, CuBi2O4, and ACBO-5. (h) The schematic of the Z-scheme heterojunction before and after hybridization, and under light illumination.
To further clarify the transfer pathway of photogenerated electrons, EPR spectroscopy was employed under illumination to capture superoxide (·O2−) radicals using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin-trapping agent. As depicted in Figure 5g, significant DMPO-·O2− signals are detected for CuBi2O4, whereas no such signals are observed for Ag3PO4. This difference is attributed to the relative positions of their conduction bands (CB) with respect to the standard reduction potential of O2/O2− (−0.33 V vs. NHE). The CB of CuBi2O4 is sufficiently negative to reduce adsorbed O2 to ·O2−. Conversely, the CB of Ag3PO4 is significantly positive, preventing this reduction reaction from occurring. Compared to CuBi2O4, ACBO-5 exhibits a significantly stronger DMPO-·O2− signal, indicating a more efficient accumulation of photogenerated electrons on the surface of CuBi2O4 under irradiation. Those results further provide strong evidence that the photogenerated charge transfer in the ACBO heterojunction follows a Z-scheme mechanism.
Based on the aforementioned characterization results, the photocatalytic reaction mechanism of ACBO can be displayed in Figure 5h. Upon contact between the two materials, free electrons from CuBi2O4 spontaneously migrate to Ag3PO4 until their Fermi energy levels equilibrate. This results in downward band bending at the Ag3PO4 interface (due to electron aggregation) and upward band bending at the CuBi2O4 (due to electron depletion). Consequently, a built-in electric field (IEF) pointing from CuBi2O4 to Ag3PO4 was established, thereby preventing further electron transfer between the materials. Under illumination, electrons within both Ag3PO4 and CuBi2O4 undergo excitation, promoting their transition from the VB to the CB. Consequently, under the influence of the IEF, energy band bending, and Coulombic attraction, the photogenerated electrons in Ag3PO4 preferentially recombined with photogenerated holes in CuBi2O4. This process preserves photo-induced carriers exhibiting the highest redox potential, which then contribute to subsequent photocatalytic reactions.
To better understand the high photocatalytic activity of the ACBO-5 heterojunction system, we employed transient photocurrent response, electrochemical impedance spectroscopy (EIS), photoluminescence (PL), and time-resolved photoluminescence (TRPL) to investigate the separation and transfer characteristics of photogenerated charge carriers. In the photocurrent test (Figure 5c), the photoresponsivity of ACBO-5 was significantly higher than that of the individual monomers, Ag3PO4 and CuBi2O4. This compelling result unequivocally demonstrates that the ACBO-5 composite photocatalyst possesses superior internal and interfacial separation efficiency of photogenerated charge carriers, which directly contributes to its excellent photocatalytic activity [62,63]. Figure 5d presents the Nyquist curve obtained from the EIS analysis of the samples. The charge transfer resistance, as indicated by the semicircle radii, decreased in the order CuBi2O4 > Ag3PO4 > ACBO-5, suggesting that the interfacial charge transfer is significantly accelerated upon the binding of Ag3PO4 to CuBi2O4. Furthermore, a substantial decrease in the PL emission intensity was observed for the ACBO-5 photocatalyst relative to pristine CuBi2O4, indicating that the heterojunction formation plays a crucial role in suppressing carrier recombination (Figure 5e). In addition, TRPL decay spectra revealed that ACBO-5 exhibited a longer average lifetime compared with that of pure CuBi2O4, indicating that the fabricated ACBO heterojunction facilitates more efficient charge separation (Figure 5f).
3.5. Mechanism of Light-Driven O2 Evolution Coupled with Cr(VI) Reduction
To gain in-depth insights into the photocatalytic reaction mechanism of ACBO-5, a series of free radical capture experiments was conducted (Figure 6a,b). 1,4-benzoquinone (BQ), Tert-butyl alcohol (TBA), and ethylenediaminetetraacetic acid disodium (EDTA-2Na) were employed as capture agents for ·O2−, ·OH, and h+, respectively. The introduction of BQ significantly suppressed the photocatalytic activity of ACBO-5. Specifically, the oxygen evolution rate decreased from 652.7 µmol·g−1·h−1 to 106.1 µmol·g−1·h−1, while the corresponding Cr(VI) reduction efficiency fell from 82.1% to merely 7.3%. Given that BQ (E0 ≈ +0.1 V vs. NHE) exhibits a much higher electron capture efficiency than Cr(VI), the reduction efficiency of Cr(VI) consequently decreased significantly due to this competition. The semiquinone free radicals (BQ·), generated by BQ capturing electrons, can further consume crucial reactive oxygen intermediates, peroxyl radicals (·OOH). This consumption interrupts the four-electron reaction pathway of OER, leading to a significant deterioration in OER performance. Upon the addition of TBA as a scavenger, the oxygen evolution activity of the composite photocatalyst was reduced by 26%, indicating that the formation of ·OH is a significant, but not dominant, step in the photocatalytic oxidation pathways. These findings indiscreetly demonstrate the direct involvement of surface lattice oxygen in the O2 evolution reaction, with a remarkable contribution rate of about 84%. Additionally, the O2 evolution activity significantly dropped to 48.7 µmol·g−1·h−1 after the addition of EDTA-2Na scavenger, indicating the crucial role of h+ in the photocatalytic water oxidation process. Conversely, the Cr(VI) reduction efficiency remained largely unaffected by the addition of either EDTA-2Na or TBA, consistently staying above 80%, suggesting a negligible role for ·OH and h+ in driving the Cr(VI) reduction process [64].
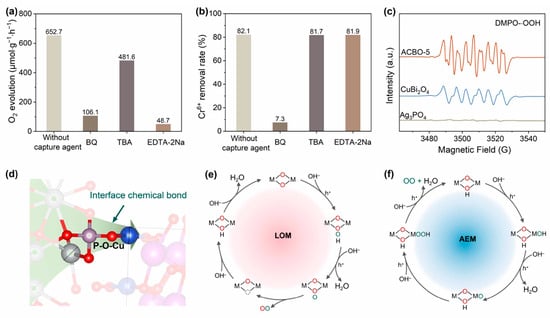
Figure 6.
Photocatalytic O2 evolution (a) synchronously with Cr(VI) reduction (b) performance of ACBO-5 under various experimental conditions. (c) In situ EPR spectra of DMPO-·OOH. (d) Schematic diagram of the P-O-Cu interfacial bond formation. Schematic illustration of the LOM (e) and AEM (f) pathway on ACBO-5.
To gain deeper insights into the mechanism of light-driven O2 evolution, in situ EPR was employed to monitor the intermediates (Figure 6c). The photocatalytic systems of CuBi2O4 and ACBO-5 exhibited characteristic ·OOH radical peaks; however, these peaks were absent in the Ag3PO4 systems, which is consistent with the radical quenching results. Quantitative analysis revealed a 1.4-fold amplification of DMPO-·OOH adduct signals in the ACBO-5 system compared to pure CuBi2O4. These findings underscore the enhanced ·OOH generation driven by the heterojunction formation, which is consistent with the results of activity tests.
Based on the results above, two mainstream reaction pathways are involved in the oxygen evolution reaction (OER) mechanism: the lattice oxygen mechanism (LOM, Figure 6e) and the adsorbate evolution mechanism (AEM, Figure 6f). The earlier mechanism dominates under these conditions. The formation of interfacial chemical bonds serves a dual role: it enhances charge carrier separation while also inducing a rearrangement of interfacial electrons that consequently weakens the Ag-O bond, which in turn promotes increased lattice oxygen activity (Figure 6d). Consequently, the rate of oxygen generation increases via a mechanism that facilitates lattice oxygen participation.
4. Conclusions
We successfully fabricated an innovative Ag3PO4/CuBi2O4 composite, in which Ag3PO4 nanoparticles are loaded onto one end of CuBi2O4 microrods, synthesized through an in situ anisotropic growth strategy. This composite features a tightly bonded covalent heterointerface formed by P-O-Cu bonds, which synergistically interacts with the internal electric field (IEF) to induce a Z-type charge transfer mechanism. This unique charge flow dramatically improves charge separation and migration, leading to significant enhancements in both Cr(VI) reduction and oxygen generation. Furthermore, our analysis reveals that the interfacial chemical bonding triggers electron redistribution, which reduces the Ag-O bond energy and activates surface lattice oxygen. This activation is key to accelerating oxygen evolution through a highly efficient lattice oxygen pathway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15201592/s1, Figure S1: SEM image of Ag3PO4; Figure S2: Mott-Schottky (M-S) plot of (a) Ag3PO4 and (b) CuBi2O4. Table S1. Mass ratio of CuBi2O4 to Ag3PO4 in the composite photocatalyst determined by ICP analysis. Table S2. Comparison of the photocatalytic oxygen evolution performance. Table S3. Concentration of Cu, Ag, and Bi ions in solution determined by ICP after photocatalytic reaction.
Author Contributions
Conceptualization, Y.W., Z.S., and B.W.; methodology, Y.W. and Z.S.; formal analysis, Y.W., Z.S., and B.W.; investigation, Y.W. and Z.S.; resources, Y.W. and B.W.; data curation, Y.W. and B.W.; writing—original draft preparation, Z.S.; writing—review and editing, Y.W. and B.W.; visualization, Y.W., Z.S., and B.W.; supervision, B.W.; project administration, B.W.; funding acquisition, Y.W. and B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22302079), the Natural Science Foundation of Jiangsu Province (BK20230521), the Young Scientific and Technological Talents Support Project of Zhenjiang Association for Science and Technology, Zhejiang Provincial Postdoctoral Science Foundation (2024-00004), and Academic Degree and Postgraduate Education Reform Project of Jiangsu Province (KYCX25_4168).
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Materials. Additional raw data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Duan, L.; Cheng, T.; Zhu, Y.; Wang, Y.; Gao, Y.; Bi, J. Lanthanide-Porphyrin MOF as a Multifunctional Platform for Detection and Integrated Elimination of Cr(VI) and Ciprofloxacin. Inorg. Chem. 2025, 64, 1983–1993. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.H.; Han, X.K.; Ma, X.X.; Zhang, L.; Zhu, H.J.; Kong, X.J.; Li, X.; Yan, H.; Zhou, H.W.; et al. Enhancing Photoreduction of Cr(VI) through a Multivalent Manganese(II)-Organic Framework Incorporating Anthracene Moieties. Inorg. Chem. 2024, 63, 16897–16907. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, P.; Zhang, T.; Zhu, Y.; Qiu, F. Fabrication of Recyclable Magnetic Double-Base Aerogel with Waste Bioresource Bagasse as the Source of Fiber for the Enhanced Removal of Chromium Ions from Aqueous Solution. Food Bioprocess. 2020, 119, 257–267. [Google Scholar] [CrossRef]
- Li, L.; Liu, G.; Dong, J.; Zhang, Y.; Cao, S.; Wang, K.; Wang, B.; She, Y.; Xia, J.; Li, H. In Situ Construction of CuTCPP/Bi4O5Br2 Hybrids for Improved Photocatalytic CO2 and Cr(VI) Reduction. Inorg. Chem. 2024, 63, 9753–9762. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Jayan, H.; Cai, J.; El-Seedi, H.R.; Guo, Z.; Zou, X. Development of a Sensitive SERS Method for Label-Free Detection of Hexavalent Chromium in Tea Using Carbimazole Redox Reaction. Foods 2023, 12, 2673. [Google Scholar] [CrossRef]
- Qing, Y.; Gao, W.; Long, Y.; Kang, Y.; Xu, C. Functionalized Titanium-Based MOF for Cr(VI) Removal from Wastewater. Inorg. Chem. 2023, 62, 6909–6919. [Google Scholar] [CrossRef]
- Wang, J.; Ahmad, W.; Hassan, M.M.; Zareef, M.; Viswadevarayalu, A.; Arslan, M.; Li, H.; Chen, Q. Landing Microextraction Sediment Phase onto Surface Enhanced Raman Scattering to Enhance Sensitivity and Selectivity for Chromium Speciation in Food and Environmental Samples. Food Chem. 2020, 323, 126812. [Google Scholar] [CrossRef]
- Buccato, D.G.; Ullah, H.; De Lellis, L.F.; Morone, M.V.; Larsen, D.S.; Di Minno, A.; Cordara, M.; Piccinocchi, R.; Baldi, A.; Greco, A.; et al. Efficacy and Tolerability of a Food Supplement Based on Zea mays L., Gymnema sylvestre (Retz.) R. br. ex Sm, Zinc and Chromium for the Maintenance of Normal Carbohydrate Metabolism: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 2459. [Google Scholar] [CrossRef]
- Han, S.; Xie, H.; Zhang, L.; Wang, X.; Zhong, Y.; Shen, Y.; Wang, H.; Hao, C. High-Performance Polyethylenimine-Functionalized Lignin/Silica Porous Composite Microsphere for the Removal of Hexavalent Chromium, Phosphate, and Congo Red from Aqueous Solutions. Ind. Crop. Prod. 2023, 194, 116289. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Z.; He, L.; Han, L.; Li, B.; Xu, Y. Polyoxometalate Functionalized Cyclic Trinuclear Copper Compounds for Bifunctional Electrochemical Detection and Photocatalytic Reduction of Cr(VI). Inorg. Chem. 2024, 63, 12564–12571. [Google Scholar] [CrossRef]
- Du, Y.; Li, Y.; Huang, G.; Pu, H.; Li, Q.; Lu, C.; Tan, L.; Dong, L.; Zhou, C. CdBi2S4-Decorated Aminated Polyacrylonitrile Nanofiber for Photocatalytic Treatment of Cr(VI) and Tetracycline Wastewater. Inorg. Chem. 2024, 63, 5611–5622. [Google Scholar] [CrossRef]
- Ghaedrahmati, L.; Jafarzadeh, M. One-Pot Synthesis of a CoSx@Ag2S/CdS Z-Scheme Photocatalyst for RhB Photodegradation and Cr(VI) Photoreduction. Langmuir 2025, 41, 15461–15473. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Yan, W.; Xie, T.; Chen, Y.; Wei, Y. Optimizing Electronic Density at Active W Sites for Boosting Photocatalytic H2 Evolution. Inorg. Chem. 2024, 63, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Costantino, F.; Kamat, P.V. Do sacrificial donors donate H2 in photocatalysis? ACS Energy Lett. 2021, 7, 242–246. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Thomas, A.; Janáky, C. Photocatalytic activity of inorganic semiconductor surfaces: Myths, hype, and reality. J. Phys. Chem. Lett. 2015, 6, 139–147. [Google Scholar] [CrossRef]
- Weng, B.; Zhang, M.; Lin, Y.; Yang, J.; Lv, J.; Han, N.; Xie, J.; Jia, H.; Su, B.-L.; Roeffaers, M.; et al. Photo-assisted technologies for environmental remediation. Nat. Rev. Clean Technol. 2025, 1, 201–215. [Google Scholar] [CrossRef]
- Santarcangelo, C.; Baldi, A.; Ciampaglia, R.; Dacrema, M.; Di Minno, A.; Pizzamiglio, V.; Daglia, M. Long-Aged Parmigiano Reggiano PDO: Trace Element Determination Targeted to Health. Foods 2022, 11, 172. [Google Scholar] [CrossRef]
- Ahmed, S.; Razzaq, I.; Sardar, R.; Ahmad, M.N.; Aziz, T.; Shami, A.; Lin, L. Food Security through Triacontanol Priming: Mitigating Chromium Stress and Boosting Yield in Raphanus sativus L. Ital. J. Food Sci. 2025, 37, 276–294. [Google Scholar] [CrossRef]
- Zarekarizi, F.; Ghasempour, H.; Habibi, B.; Morsali, A.; Ramazani, A. Development of a Novel Mixed-Metal-Organic Framework: An Innovative Photocatalyst for Simultaneous Cr(VI) Reduction and Phenol Degradation. Inorg. Chem. 2024, 63, 24363–24373. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wu, X.; Zhao, Y.; Li, R.; Li, C. Unlocking the Key to Photocatalytic Hydrogen Production Using Electronic Mediators for Z-Scheme Water Splitting. J. Am. Chem. Soc. 2025, 147, 3641–3649. [Google Scholar] [CrossRef]
- Song, W.; Chong, K.C.; Qi, G.; Xiao, Y.; Chen, G.; Li, B.; Tang, Y.; Zhang, X.; Yao, Y.; Lin, Z.; et al. Unraveling the Transformation from Type-II to Z-Scheme in Perovskite-Based Heterostructures for Enhanced Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2024, 146, 3303–3314. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-Doped Nitrogen-Deficient Carbon Nitride-Based Z-Scheme Heterostructures for Photocatalytic Overall Water Splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, X.; Shen, J.; Zhang, Z.; Wang, D.; Lin, J.; Wu, J.C.S.; Fu, X.; Wang, X.; Li, C. Direct and Indirect Z-Scheme Heterostructure-Coupled Photosystem Enabling Cooperation of CO2 Reduction and H2O Oxidation. Nat. Commun. 2020, 11, 3043. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.M.; Zhang, X.; Wang, L.; Shen, J.; Zhou, M.; Shen, L. Data-Driven Discovery of Transition Metal Dichalcogenide-Based Z-Scheme Photocatalytic Heterostructures. ACS Catal. 2023, 13, 9936–9945. [Google Scholar] [CrossRef]
- Li, N.; Shi, M.; Sun, G.; Wu, M.; Li, Q.; Shen, W.; Ma, J. Z-Scheme CdIn2S4/Bi2WO6 Heterojunction for High Piezo-Photo Synergetic Performance. Inorg. Chem. 2023, 62, 8261–8270. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhu, Z.; Tian, N.; Zhang, Y.; Huang, H. Hydrogen Bonds and In Situ Photoinduced Metallic Bi0/Ni0 Accelerating Z-Scheme Charge Transfer of BiOBr@NiFe-LDH for Highly Efficient Photocatalysis. Angew. Chem. Int. Ed. 2024, 63, e202408862. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Lin, Y.; Zhang, X.; Yu, Y.; Zhang, R. Z-Scheme Cu2(OH)3F Nanosheets-Decorated 3D Bi2WO6 Heterojunction with an Intimate Hetero-Surface Contact through a Hydrogen Bond for Enhanced Photoinduced Charge Separation and Transfer. Chem. Eng. J. 2022, 427, 131704. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Chen, L.; Xiong, Y.; Xu, H. Van der Waals Heterostructures Comprised of Ultrathin Polymer Nanosheets for Efficient Z-Scheme Overall Water Splitting. Angew. Chem. Int. Ed. 2018, 5, 3454–3458. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, B.; Wang, Z.; Xu, T.; Pan, C.; Zou, J.; Zhang, X.; Qi, Z.; Liu, H.; Feng, Y.; et al. Van der Waals Epitaxial Growth and Optoelectronics of Large-Scale WSe2/SnS2 Vertical Bilayer p-n Junctions. Nat. Commun. 2017, 8, 1906. [Google Scholar] [CrossRef]
- Canossa, S.; Wuttke, S. Functionalization chemistry of porous materials. Adv. Funct. Mater. 2020, 30, 2003875. [Google Scholar] [CrossRef]
- Zellner, R. Biological responses to nanoscale particles Beilstein. J. Nanotechnol. 2015, 6, 380–382. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, S.; Puente-Santiago, A.R.; Wu, C.; Weng, B. Zinc mediated electronic structure of CoP toward photocatalytic H2 evolution. Appl. Catal. B Environ. Energy 2025, 367, 125098. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, T.; He, D.; Chen, P.; Liu, H.; Lv, H.; Wu, H.; Su, D.; Pang, H.; Wang, C. Efficient Photocatalytic Desulfurization in Air through Improved Photogenerated Carriers Separation in MIL-101/Carbon Dots-g-C3N4 Nanocomposites. Angew. Chem. 2024, 136, e202408989. [Google Scholar] [CrossRef]
- Xing, C.; Yu, G.; Zhou, J.; Liu, Q.; Chen, T.; Liu, H.; Li, X. Solar Energy-Driven Upcycling of Plastic Waste on Direct Z-Scheme Heterostructure of V-Substituted Phosphomolybdic Acid/g-C3N4 Nanosheets. Appl. Catal. B Environ. 2022, 315, 121496. [Google Scholar] [CrossRef]
- Yue, H.; Guo, Z.; Zhou, Z.; Zhang, X.; Guo, W.; Zhen, S.; Wang, P.; Wang, K.; Yuan, W. S-S Bond Strategy at Sulfide Heterointerface: Reversing Charge Transfer and Constructing Hydrogen Spillover for Boosted Hydrogen Evolution. Angew. Chem. Int. Ed. 2024, 63, e202409465. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Zhang, J.; Shao, C.; Dai, K.; Fan, K.; Liang, C. Interfacial Chemical Bond and Oxygen Vacancy-Enhanced In2O3/CdSe-DETA S-Scheme Heterojunction for Photocatalytic CO2 Conversion. Adv. Funct. Mater. 2023, 33, 2214470. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Wang, W.; Li, Y.; He, H.; Deng, L.; Zhang, Y.; Huang, J.; Zhao, N.; Yu, G.; et al. Rational Design of Defect Metal Oxide/Covalent Organic Frameworks Z-Scheme Heterojunction for Photoreduction CO2 to CO. Appl. Catal. B Environ. 2023, 327, 122419. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, H.; Liu, L.; Chen, F.; Tian, N.; Zhang, Y.; Yu, H. Chemically Bonded α-Fe2O3/Bi4MO8Cl Dot-on-Plate Z-Scheme Junction with Strong Internal Electric Field for Selective Photo-Oxidation of Aromatic Alcohols. Angew. Chem. Int. Ed. 2022, 61, e202203519. [Google Scholar] [CrossRef]
- Ono, L.K.; Park, N.G.; Zhu, K.; Huang, W.; Qi, Y. Perovskite Solar Cells—Towards Commercialization. ACS Energy Lett. 2017, 2, 1749–1751. [Google Scholar] [CrossRef]
- Zheng, K.; Pullerits, T. Two dimensions are better for perovskites. J. Phys. Chem. Lett. 2019, 10, 5881–5885. [Google Scholar] [CrossRef] [PubMed]
- Seigneur, N.; Mayer, K.U.; Steefel, C.I. Reactive transport in evolving porous media. Rev. Mineral. Geochem. 2019, 85, 197–238. [Google Scholar] [CrossRef]
- Lei, J.; Yang, H.; Weng, B.; Zheng, Y.M.; Chen, S.; Menezes, P.W.; Meng, S. Optimization of adsorption sites for selective hydrobenzoin and syngas production in a single photoredox cycle. Adv. Energy Mater. 2025, 15, 2500950. [Google Scholar] [CrossRef]
- Xia, X.; Pan, J.H.; Pan, X.; Hu, L.; Yao, J.; Ding, Y.; Wang, D.; Ye, J.; Dai, S. Photochemical conversion and storage of solar energy. ACS Energy Lett. 2019, 4, 405–410. [Google Scholar] [CrossRef]
- Li, S.; Meng, S.; Zhang, H.; Puente-Santiago, A.R.; Wang, Z.; Chen, S.; Muñoz-Batista, M.J.; Zheng, Y.M.; Weng, B. Tailoring Redox Active Sites with Dual-Interfacial Electric Fields for Concurrent Photocatalytic Biomass Valorization and H2 Production. Adv. Funct. Mater. 2025, e13682. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, Y.; Xue, Q.; Zhang, C.; Bao, T.; Zhang, X.; Yuan, L.; Qiao, S.; Song, L.; Zou, J.; et al. MOF-on-MOF Heterostructured Electrocatalysts for Efficient Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202409799. [Google Scholar] [CrossRef]
- He, B.; Xiao, P.; Wan, S.; Zhang, J.; Chen, T.; Zhang, L.; Yu, J. Rapid Charge Transfer Endowed by Interfacial Ni-O Bonding in S-Scheme Heterojunction for Efficient Photocatalytic H2 and Imine Production. Angew. Chem. Int. Ed. 2023, 62, e202313172. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Chen, W.J.; Jiang, H. Facile Synthesis of Ag/Ag3PO4/AMB Composite with Improved Photocatalytic Performance. Chem. Eng. J. 2017, 308, 889–896. [Google Scholar] [CrossRef]
- Gudipati, N.S.; Ramesh, A.; Vanjari, S.; Duvvuri, S.; Challapalli, S. MnO2 and CuBi2O4 Hybrid Microstructures for Efficient Nonenzymatic Hydroxylamine Detection. J. Chem. Sci. 2023, 135, 101. [Google Scholar] [CrossRef]
- Tortorelli, M.; Chakarova, K.; Lisi, L.; Hadjiivanov, K. Disproportionation of Associated Cu2+ Sites in Cu-ZSM-5 to Cu+ and Cu3+ and FTIR Detection of Cu3+(NO)x (x = 1,2) Species. J. Catal. 2014, 309, 376–385. [Google Scholar] [CrossRef]
- Liu, H.S.; Chin, T.S.; Yung, S.W. FTIR and XPS Studies of Low-Melting PbO-ZnO-P2O2 Glasses. Mater. Chem. Phys. 1997, 50, 1–10. [Google Scholar] [CrossRef]
- Sang, Y.; Song, G.; Gao, Z.; Zhang, X.; Wang, T.; Li, Y.; Zhang, L.; Guo, L. Sea Urchin-Like CuO Particles Prepared Using Cu3(PO4)2 Flowers as Precursor for High-Performance Ethanol Sensing. Nanotechnology 2020, 31, 165504. [Google Scholar] [CrossRef]
- Hu, J.; Li, B.; Li, X.; Yang, T.; Yang, X.; Qu, J.; Yang, H.; Lin, Z. Lattice Match-Enabled Covalent Heterointerfaces with Built-in Electric Field for Efficient Hydrogen Peroxide Photosynthesis. Adv. Mater. 2024, 36, 2412070. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J. Nanointerface Chemistry: Lattice-Mismatch-Directed Synthesis and Application of Hybrid Nanocrystals. Chem. Rev. 2020, 120, 2123–2170. [Google Scholar] [CrossRef]
- Pang, F.; Liu, X.; He, M.; Ge, J. Ag3PO4 Colloidal Nanocrystal Clusters with Controllable Shape and Superior Photocatalytic Activity. Nano Res. 2015, 8, 106–116. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, L.; Lu, H.; Li, Y.; Hu, C.; Yu, H. One-Pot Pyridine-Assisted Synthesis of Visible-Light-Driven Photocatalyst Ag/Ag3PO4. Appl. Catal. B Environ. 2012, 115, 245–252. [Google Scholar] [CrossRef]
- Chen, L.; Wang, C.; Wang, Z.; Li, G. Pivotal Role of Water Vapor–Mediated Defect Engineering on SrTiO3 Nanofiber Toward Efficient Photocatalytic Water Splitting. Mater. Today Energy 2024, 44, 101622. [Google Scholar] [CrossRef]
- Lv, H.; Suo, Z.; Zhang, F.; Wan, B.; Zhou, C.; Ng, X.; Wang, G.; Chen, Y.; Liu, Y. Electronic Structure Engineering and a Cascade Electron Transfer Channel in a Ni2P/1T-WS2/ZnIn2S4 Ternary Heterojunction for Enhanced Photocatalytic Hydrogen Evolution: Construction, Kinetics, and Mechanistic Insights. Inorg. Chem. Front. 2025, 12, 4223–4236. [Google Scholar] [CrossRef]
- Huang, K.; Feng, B.; Wen, X.; Hao, L.; Xu, D.; Liang, G.; Shen, R.; Li, X. Effective Photocatalytic Hydrogen Evolution by Ti3C2-Modified CdS Synergized with N-Doped C-Coated Cu2O in S-Scheme Heterojunctions. Chin. J. Struct. Chem. 2023, 42, 100204. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Gottardi, G.; Micheli, V.; Canteri, R.; Coy, E.; Bechelany, M. Atomic Layer Deposition of Palladium Coated TiO2/Si Nanopillars: ToF-SIMS, AES and XPS Characterization Study. Appl. Surf. Sci. 2021, 542, 148603. [Google Scholar] [CrossRef]
- Maslakov, K.I.; Teterin, Y.A.; Stefanovsky, S.V.; Kalmykov, S.N.; Teterin, A.Y.; Ivanov, K.E. XPS Study of Uranium-Containing Sodium-Aluminum-Iron-Phosphate Glasses. J. Alloys Compd. 2017, 712, 36–43. [Google Scholar] [CrossRef]
- Abouelnaga, A.M. Enhanced UV–Visible Optical Response of Cu2+-Doped ZnAl2O4 Spinel Thin Films. J. Solid State Sci. Technol. 2025, 14, 083006. [Google Scholar] [CrossRef]
- Gopannagari, M.; Reddy, K.A.J.; Inae, S.; Bae, H.S.; Lee, J.; Woo, T.G.; Rangappa, A.P.; Kumar, D.P.; Reddy, D.A.; Kim, T.K. High-Performance Silver-Doped Porous CuBi2O4 Photocathode Integrated with NiO Hole-Selective Layer for Improved Photoelectrochemical Water Splitting. Adv. Sustain. Syst. 2023, 7, 2300085. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, H.; Yang, H.; Zhu, L.; Ding, C.; Zhang, G.; Bi, J.; Yan, S.; Liu, G.; Hou, H. UV-Vis-NIR-Light-Driven Ag2O/Ag2S/CuBi2O4 Double Z-Scheme Configuration for Enhanced Photocatalytic Applications. Mater. Sci. Semicond. Process. 2021, 126, 105668. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Xu, X.; Qu, Y.; Ding, X.; Chen, H. Surface Plasmon Resonance-Induced Visible-Light Photocatalytic Performance of Silver/Silver Molybdate Composites. Chin. J. Catal. 2017, 38, 260–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).