Elucidating the Role of the Mo2C/MgO Catalyst Interface in the Mechanism of the Reverse Water Gas Shift Reaction
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Adsorption of Key Molecules on MgO(001) and Mo2C(001)
3.2. Adsorption of Key Molecules at the Mo2C/MgO Interface
3.3. Investigating the RWGS Pathway on Mo2C and Mo2C/MgO
3.3.1. Adsorption of CO2 and an Individual H Atom at the Mo2C/MgO Interface
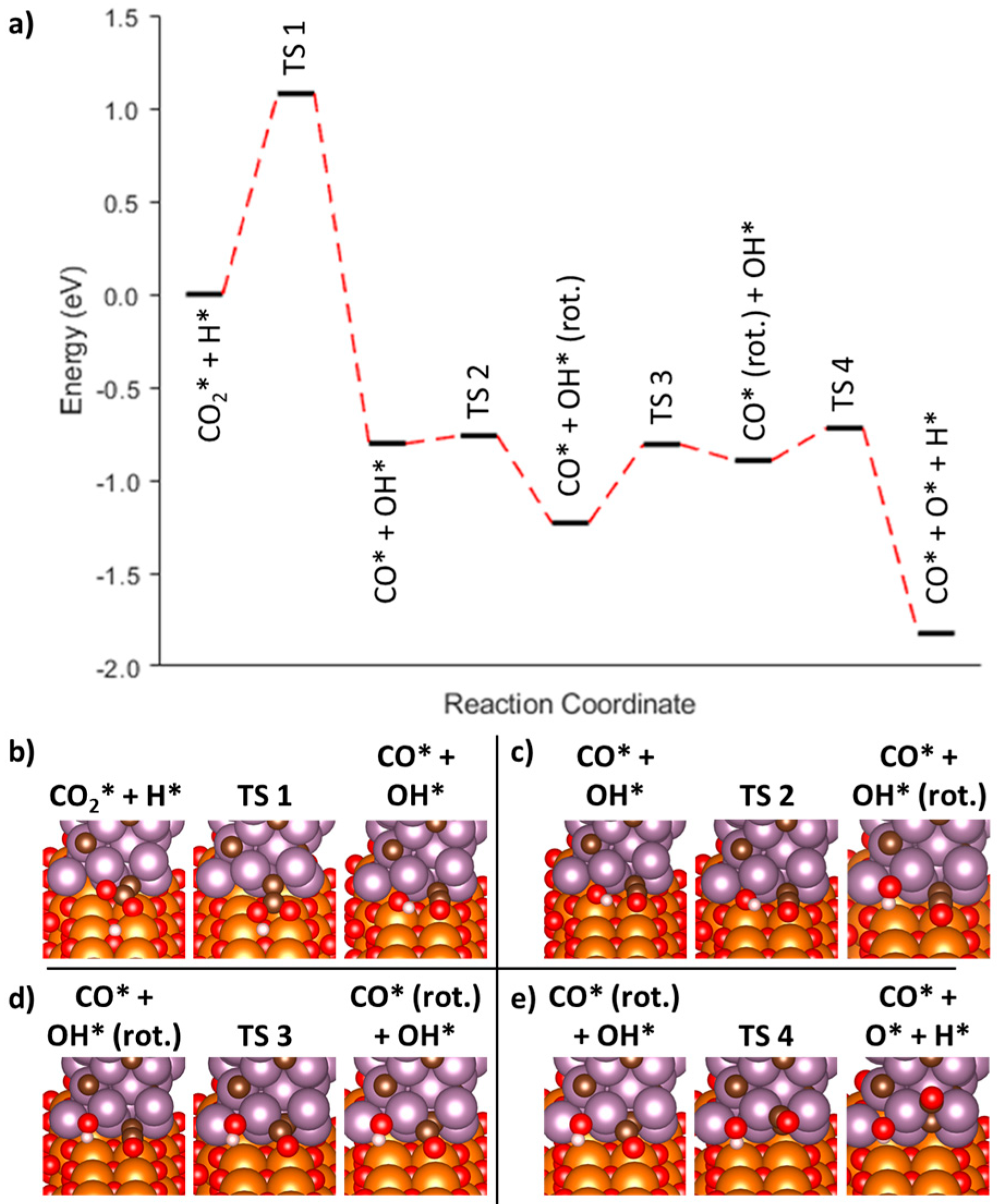
3.3.2. Associative Mechanism (*COOH) at the Mo2C/MgO Interface
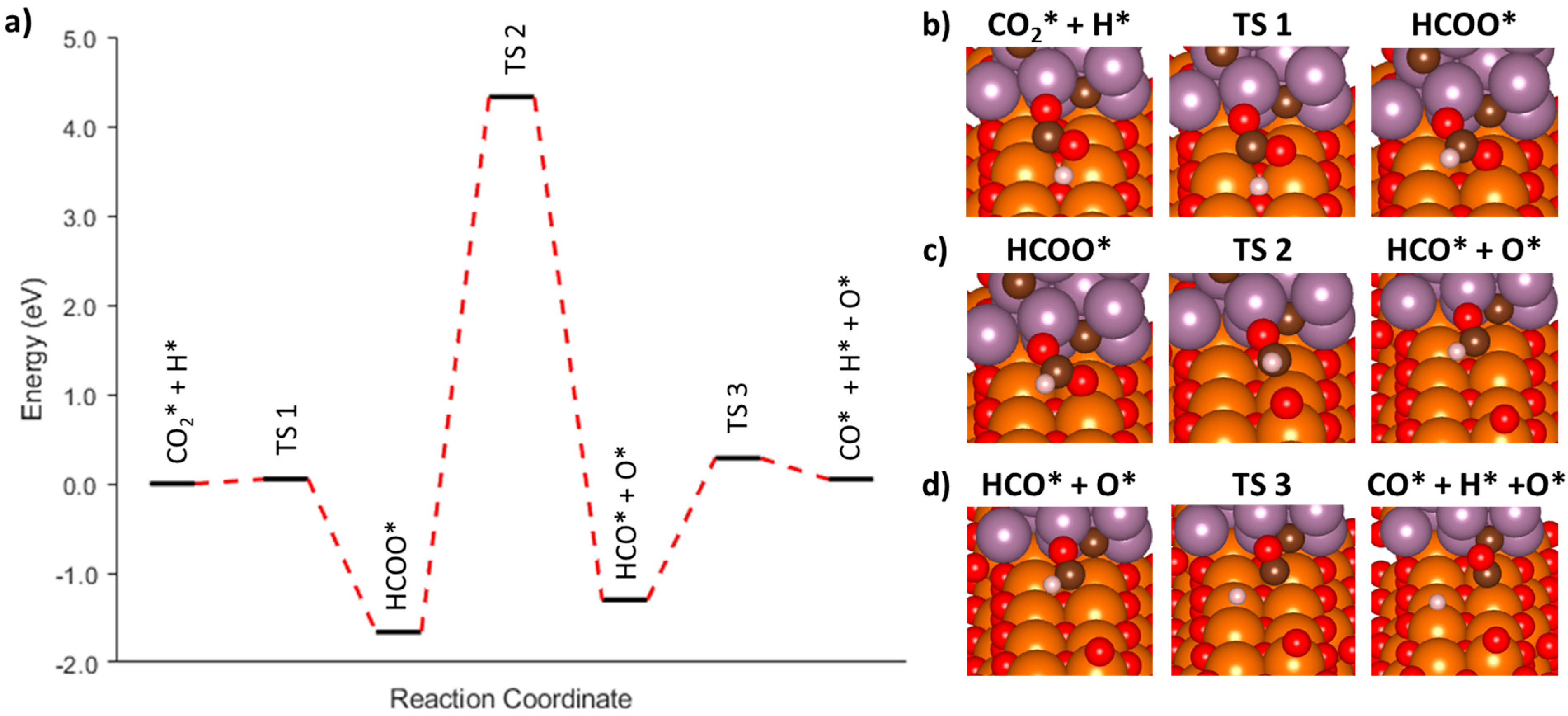
3.3.3. Associative Mechanism (*HCOO) at the Mo2C/MgO Interface
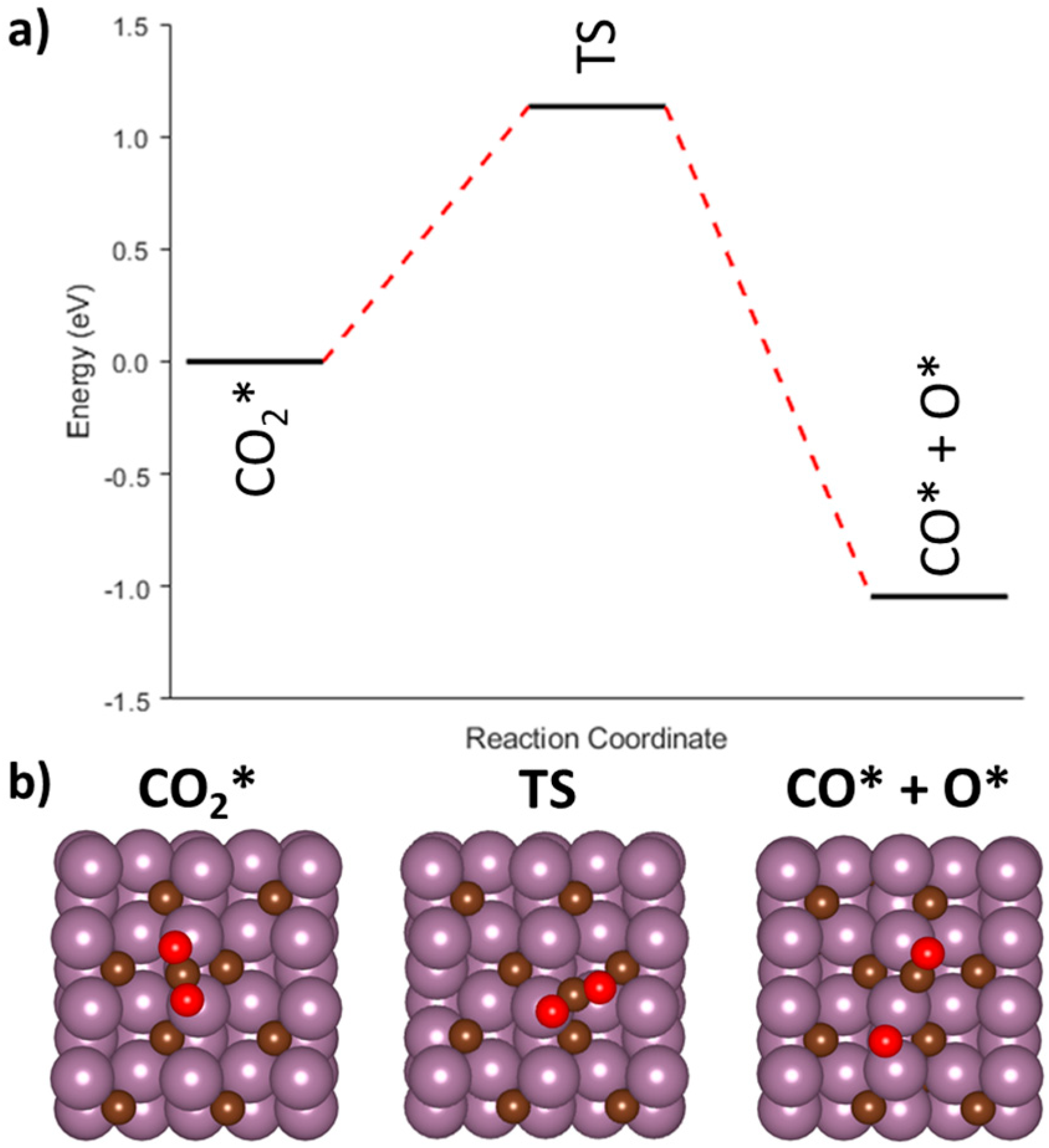
3.3.4. Redox Mechanism on the Mo2C(001) Surface
3.3.5. Associative Mechanism (HCOO) on the Mo2C(001) Surface
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DFT | Density Theory Functional |
| RWGS | Reverse Water Gas Shift |
| FT | Fischer–Tropsch Reaction |
| CINEB | Climbing Image Nudged Elastic Band |
References
- Turner, J.A. A Realizable Renewable Energy Future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef]
- Qiu, L.-Q.; Li, H.-R.; He, L.-N. Incorporating Catalytic Units into Nanomaterials: Rational Design of Multipurpose Catalysts for CO2 Valorization. Acc. Chem. Res. 2023, 56, 2225–2240. [Google Scholar] [CrossRef]
- Hu, X.-M.; Liang, H.-Q.; Rosas-Hernández, A.; Daasbjerg, K. Electrochemical Valorization of Captured CO2: Recent Advances and Future Perspectives. Chem. Soc. Rev. 2025, 54, 1216–1250. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Cao, Y.; Gao, J.; Song, C. Rational Design of Catalyst Supports for CO2 Hydrogenation to Light Olefins: Structural, Electronic, and Stabilization Strategies. Energy Fuels 2025, 39, 18350–18375. [Google Scholar] [CrossRef]
- Podder, J.; Patra, B.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K. A Review of Carbon Capture and Valorization Technologies. Energies 2023, 16, 2589. [Google Scholar] [CrossRef]
- U.S. Department of Defense. Department of Defense Climate Adaption Plan; U.S. Department of Defense: Arlington, VA, USA, 2024.
- Willauer, H.D.; Hardy, D.R.; Schultz, K.R.; Williams, F.W. The Feasibility and Current Estimated Capital Costs of Producing Jet Fuel at Sea Using Carbon Dioxide and Hydrogen. J. Renew. Sustain. Energy 2012, 4, 33111. [Google Scholar] [CrossRef]
- Willauer, H.D.; DiMascio, F.; Hardy, D.R.; Williams, F.W. Development of an Electrolytic Cation Exchange Module for the Simultaneous Extraction of Carbon Dioxide and Hydrogen Gas from Natural Seawater. Energy Fuels 2017, 31, 1723–1730. [Google Scholar] [CrossRef]
- Kaiser, P.; Unde, R.B.; Kern, C.; Jess, A. Production of Liquid Hydrocarbons with CO2 as Carbon Source Based on Reverse Water-Gas Shift and Fischer-Tropsch Synthesis. Chem. Ing. Tech. 2013, 85, 489–499. [Google Scholar] [CrossRef]
- Wang, X.-G.; Qiao, L.-Y.; Xu, L.-Y.; Zeng, Y.-Y.; Huang, Z.-S.; Zong, S.-S.; Wang, J.-X.; Zhou, Z.-F.; Yao, Y.-G. Constructing Efficient Pd/Al2O3 Catalyst for Reverse Water-Gas Shift via Alkali-Modification. Chem. Eng. J. 2024, 500, 156937. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, M.; Ma, P.; Zheng, Y.; Chen, J.; Li, H.; Zhang, X.; Zheng, K.; Kuang, Q.; Xie, Z.-X. Atomically Dispersed Pt/CeO2 Catalyst with Superior CO Selectivity in Reverse Water Gas Shift Reaction. Appl. Catal. B Environ. 2021, 291, 120101. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Baldwin, J.W.; Peng, X.; Mpourmpakis, G.; Willauer, H.D. Potassium-Promoted Molybdenum Carbide as a Highly Active and Selective Catalyst for CO2 Conversion to CO. ChemSusChem 2017, 10, 2408–2415. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Lin, L.; Yao, S.; Zhang, M.; Liu, X.; Wang, X.; Li, Y.-W.; Shi, C.; Ma, D. Highly Dispersed Copper over β-Mo2C as an Efficient and Stable Catalyst for the Reverse Water Gas Shift (RWGS) Reaction. ACS Catal. 2017, 7, 912–918. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of Highly Selective and High-Temperature Endurable RWGS Heterogeneous Catalysts: Recent Advances and the Future Directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Holder, C.F.; Morse, J.R.; Barboun, P.M.; Shabaev, A.R.; Baldwin, J.W.; Willauer, H.D. Evaluating Metal Oxide Support Effects on the RWGS Activity of Mo2C Catalysts. Catal. Sci. Technol. 2023, 13, 2685–2695. [Google Scholar] [CrossRef]
- Morse, J.R.; Juneau, M.; Baldwin, J.W.; Porosoff, M.D.; Willauer, H.D. Alkali Promoted Tungsten Carbide as a Selective Catalyst for the Reverse Water Gas Shift Reaction. J. CO2 Util. 2020, 35, 38–46. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef]
- Morse, J.R.; Holder, C.F.; Baldwin, J.W.; Willauer, H.D. Optimization of Potassium Promoted Molybdenum Carbide Catalyst for the Low Temperature Reverse Water Gas Shift Reaction. Energies 2022, 15, 7109. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yang, X.; Boscoboinik, J.A.; Chen, J.G. Molybdenum Carbide as Alternative Catalysts to Precious Metals for Highly Selective Reduction of CO2 to CO. Angew. Chem. Int. Ed. Engl. 2014, 53, 6705–6709. [Google Scholar] [CrossRef] [PubMed]
- Juneau, M.; Pope, C.; Liu, R.; Porosoff, M.D. Support Acidity as a Descriptor for Reverse Water-Gas Shift over Mo2C-Based Catalysts. Appl. Catal. A Gen. 2021, 620, 118034. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent Advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Huma, T.; Hakimi, N.; Younis, M.; Huma, T.; Ge, Z.; Feng, J. MgO Heterostructures: From Synthesis to Applications. Nanomaterials 2022, 12, 2668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-J.; Li, Z.; Cui, Y.; Zhu, H.; Schneider, W.F.; Delgass, W.N.; Ribeiro, F.; Greeley, J. Importance of Metal-Oxide Interfaces in Heterogeneous Catalysis: A Combined DFT, Microkinetic, and Experimental Study of Water-Gas Shift on Au/MgO. J. Catal. 2017, 345, 157–169. [Google Scholar] [CrossRef]
- Alexeev, O.; Kawi, S.; Shelef, M.; Gates, B.C. Synthesis and Characterization of Model MgO-Supported Catalysts with Pt−Mo Interactions. J. Phys. Chem. 1996, 100, 253–264. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Z.; Zhao, Z.; Cybulskis, V.J.; Sabnis, K.D.; Han, C.W.; Ortalan, V.; Schneider, W.F.; Greeley, J.; Delgass, W.N.; et al. Participation of Interfacial Hydroxyl Groups in the Water-Gas Shift Reaction over Au/MgO Catalysts. Catal. Sci. Technol. 2017, 7, 5257–5266. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Z.; Shen, H.; Li, X.; Zhao, S.; Xie, B.; Xia, S. Density Functional Theory Study of CO2 Adsorption on Metal (M=Li, Al, K, Ca) Doped MgO. Mol. Catal. 2024, 553, 113708. [Google Scholar] [CrossRef]
- Ruhaimi, A.H.; Aziz, M.A.A.; Jalil, A.A. Magnesium Oxide-Based Adsorbents for Carbon Dioxide Capture: Current Progress and Future Opportunities. J. CO2 Util. 2021, 43, 101357. [Google Scholar] [CrossRef]
- Lv, G.; Li, S.; Zhang, H.; Qian, W.; Cheng, J.; Qian, P. CO2 Adsorption on a K-Promoted MgO Surface: A DFT Theoretical Study. Surf. Sci. 2024, 749, 122575. [Google Scholar] [CrossRef]
- Wu, S.; Tan, B.T.; Senevirathna, H.L.; Wu, P. Polarization of CO2 for Improved CO2 Adsorption by MgO and Mg(OH)2. Appl. Surf. Sci. 2021, 562, 150187. [Google Scholar] [CrossRef]
- Jensen, M.B.; Pettersson, L.G.M.; Swang, O.; Olsbye, U. CO2 Sorption on MgO and CaO Surfaces: A Comparative Quantum Chemical Cluster Study. J. Phys. Chem. B 2005, 109, 16774–16781. [Google Scholar] [CrossRef]
- Karlsen, E.J.; Nygren, M.A.; Pettersson, L.G.M. Comparative Study on Structures and Energetics of NOx, SOx, and COx Adsorption on Alkaline-Earth-Metal Oxides. J. Phys. Chem. B 2003, 107, 7795–7802. [Google Scholar] [CrossRef]
- Thomele, D.; Baumann, S.O.; Schneider, J.; Sternig, A.K.; Shulda, S.; Richards, R.M.; Schwab, T.; Zickler, G.A.; Bourret, G.R.; Diwald, O. Cubes to Cubes: Organization of MgO Particles into One-Dimensional and Two-Dimensional Nanostructures. Cryst. Growth Des. 2021, 21, 4674–4682. [Google Scholar] [CrossRef] [PubMed]
- Mutch, G.A.; Shulda, S.; McCue, A.J.; Menart, M.J.; Ciobanu, C.V.; Ngo, C.; Anderson, J.A.; Richards, R.M.; Vega-Maza, D. Carbon Capture by Metal Oxides: Unleashing the Potential of the (111) Facet. J. Am. Chem. Soc. 2018, 140, 4736–4742. [Google Scholar] [CrossRef] [PubMed]
- Baddour, F.G.; Roberts, E.J.; To, A.T.; Wang, L.; Habas, S.E.; Ruddy, D.A.; Bedford, N.M.; Wright, J.; Nash, C.P.; Schaidle, J.A.; et al. An Exceptionally Mild and Scalable Solution-Phase Synthesis of Molybdenum Carbide Nanoparticles for Thermocatalytic CO2 Hydrogenation. J. Am. Chem. Soc. 2020, 142, 1010–1019. [Google Scholar] [CrossRef]
- Figueras, M.; Gutiérrez, R.A.; Viñes, F.; Ramírez, P.J.; Rodriguez, J.A.; Illas, F. Supported Molybdenum Carbide Nanoparticles as an Excellent Catalyst for CO2 Hydrogenation. ACS Catal. 2021, 11, 9679–9687. [Google Scholar] [CrossRef]
- Karadaghi, L.R.; To, A.T.; Habas, S.E.; Baddour, F.G.; Ruddy, D.A.; Brutchey, R.L. Activating Molybdenum Carbide Nanoparticle Catalysts under Mild Conditions Using Thermally Labile Ligands. Chem. Mater. 2022, 34, 8849–8857. [Google Scholar] [CrossRef]
- Jurado, A.; Morales-García, Á.; Viñes, F.; Illas, F. Molecular Mechanism and Microkinetic Analysis of the Reverse Water Gas Shift Reaction Heterogeneously Catalyzed by the Mo2C MXene. ACS Catal. 2022, 12, 15658–15667. [Google Scholar] [CrossRef]
- Dolz, D.; De Armas, R.; Lozano-Reis, P.; Morales-García, Á.; Viñes, F.; Sayós, R.; Illas, F. Understanding the Reverse Water Gas Shift Reaction over Mo2C MXene Catalyst: A Holistic Computational Analysis. ChemCatChem 2024, 16, e202400122. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry Reforming of Methane by Stable Ni–Mo Nanocatalysts on Single-Crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Efstathiou, A.M.; Zhang, Z.L.; Verykios, X.E. Reforming of Methane with Carbon Dioxide to Synthesis Gas over Supported Rh Catalysts. Catal. Today 1994, 21, 579–587. [Google Scholar] [CrossRef]
- Nagaoka, K.; Okamura, M.; Aika, K. Titania Supported Ruthenium as a Coking-Resistant Catalyst for High Pressure Dry Reforming of Methane. Catal. Commun. 2001, 2, 255–260. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Usui, M.; Takezawa, N. Mechanism of the Reverse Water Gas Shift Reaction over Cu/ZnO Catalyst. J. Catal. 1992, 134, 220–225. [Google Scholar] [CrossRef]
- Ginés, M.J.L.; Marchi, A.J.; Apesteguía, C.R. Kinetic Study of the Reverse Water-Gas Shift Reaction over CuO/ZnO/Al2O3 Catalysts. Appl. Catal. A Gen. 1997, 154, 155–171. [Google Scholar] [CrossRef]
- Pahija, E.; Panaritis, C.; Gusarov, S.; Shadbahr, J.; Bensebaa, F.; Patience, G.; Boffito, D.C. Experimental and Computational Synergistic Design of Cu and Fe Catalysts for the Reverse Water–Gas Shift: A Review. ACS Catal. 2022, 12, 6887–6905. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, Y.; Wang, X.; Xu, H.; Guo, H.; Liu, L.; Xu, H.; Cui, W.; Liu, X. Research Progress on the Optimization of RWGS Catalytic Systems and Reactors and the Integrated Technology of CO2 Capture and Conversion. Carbon Capture Sci. Technol. 2025, 16, 100476. [Google Scholar] [CrossRef]
- Ahmadi Khoshooei, M.; Wang, X.; Vitale, G.; Formalik, F.; Kirlikovali, K.O.; Snurr, R.Q.; Pereira-Almao, P.; Farha, O.K. An Active, Stable Cubic Molybdenum Carbide Catalyst for the High-Temperature Reverse Water-Gas Shift Reaction. Science 2024, 384, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B-Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A Climbing Image Nudged Elastic Band Method for Finding Saddle Points and Minimum Energy Paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Wei, J.; Qin, S.-N.; Liu, J.-L.; Ruan, X.-Y.; Guan, Z.; Yan, H.; Wei, D.-Y.; Zhang, H.; Cheng, J.; Xu, H.; et al. In Situ Raman Monitoring and Manipulating of Interfacial Hydrogen Spillover by Precise Fabrication of Au/TiO2/Pt Sandwich Structures. Angew. Chem. Int. Ed. Engl. 2020, 59, 10343–10347. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, H.; Yang, Z.; Li, C. Magic of Hydrogen Spillover: Understanding and Application. Green Energy Environ. 2022, 7, 1161–1198. [Google Scholar] [CrossRef]
- Khoobiar, S. Particle to Particle Migration of Hydrogen Atoms on Platinum—Alumina Catalysts from Particle to Neighboring Particles. J. Phys. Chem. 1964, 68, 411–412. [Google Scholar] [CrossRef]
- Yao, X.; Wei, Z.; Mei, J.; Guo, X.; Tian, X. The Reverse Water Gas Shift Reaction (RWGS) Mechanism Study on the γ-MoC(100) Surface. RSC Adv. 2025, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Peng, X.; Porosoff, M.D.; Willauer, H.D.; Mpourmpakis, G. Elucidating the Role of Oxygen Coverage in CO2 Reduction on Mo2C. Catal. Sci. Technol. 2017, 7, 5521–5529. [Google Scholar] [CrossRef]
- Meixner, D.L.; Arthur, D.A.; George, S.M. Kinetics of Desorption, Adsorption, and Surface Diffusion of CO2 on MgO(100). Surf. Sci. 1992, 261, 141–154. [Google Scholar] [CrossRef]
- Belgamwar, R.; Verma, R.; Das, T.; Chakraborty, S.; Sarawade, P.; Polshettiwar, V. Defects Tune the Strong Metal–Support Interactions in Copper Supported on Defected Titanium Dioxide Catalysts for CO2 Reduction. J. Am. Chem. Soc. 2023, 145, 8634–8646. [Google Scholar] [CrossRef]
- Du, H.; Shao, Z. A Review on Modulating Oxygen Vacancy Defect of Catalysts to Promote CO2 Reduction Reaction to CO. Energy Fuels 2025, 39, 5672–5690. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, B.; Dun, W.; Chen, P.; Zhang, T.; Dong, H.; Qing, Y.; Liu, W.; He, Y.; Wang, H. Revealing the Oxidative Stress Mechanism Induced by Defect Engineering of Magnesium Oxide Nanoparticles under Dark Conditions. J. Environ. Chem. Eng. 2025, 13, 119192. [Google Scholar] [CrossRef]
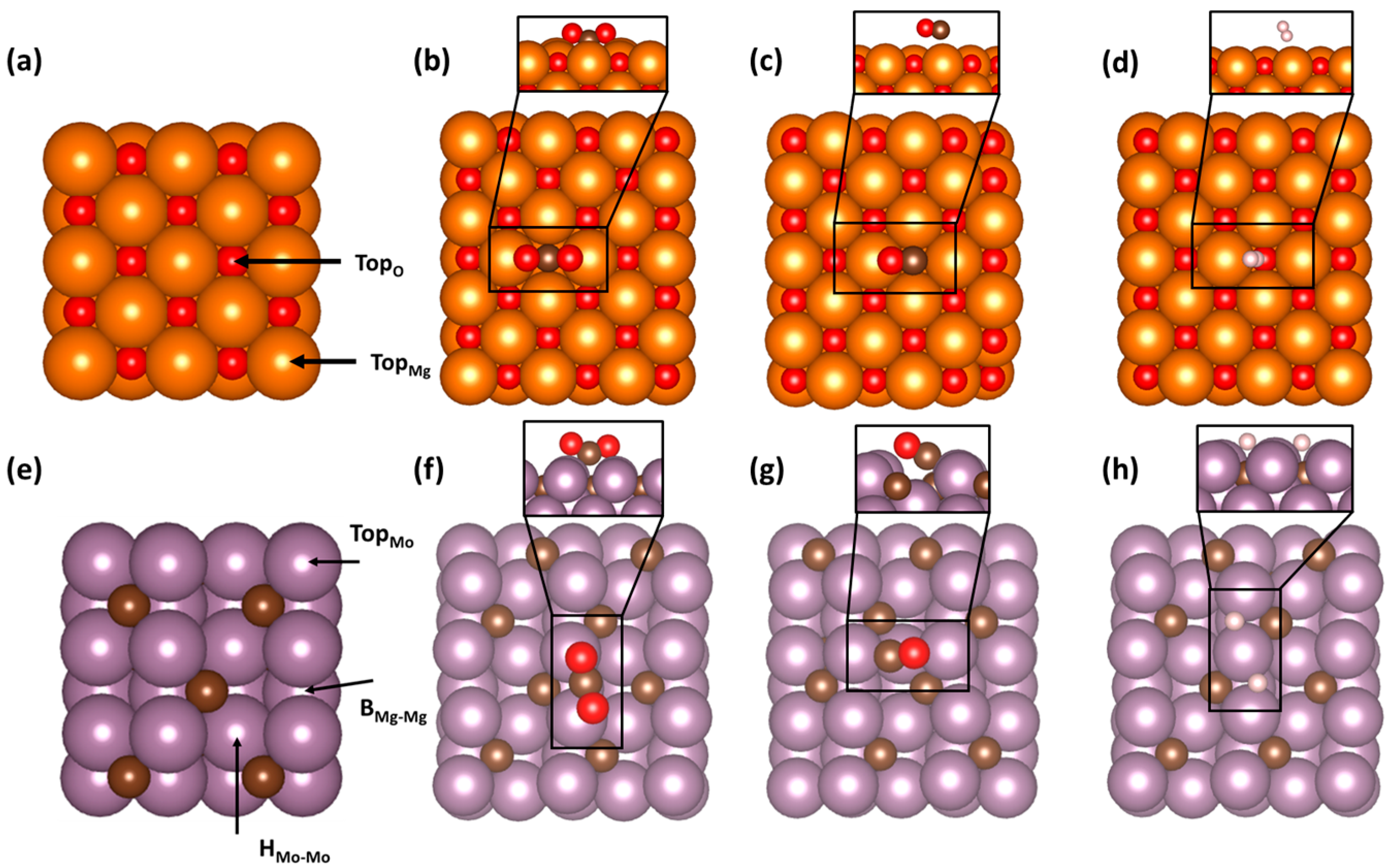
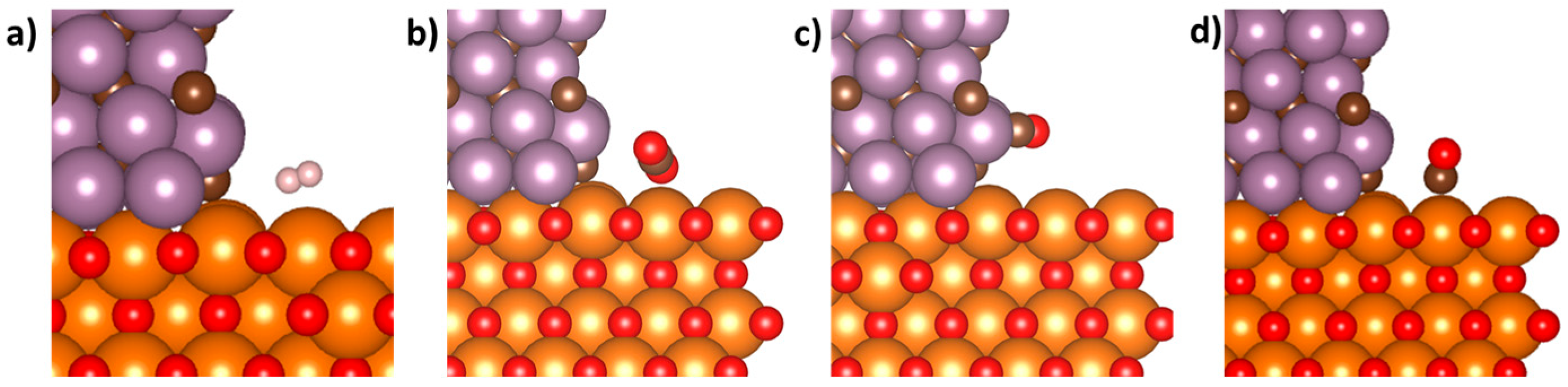
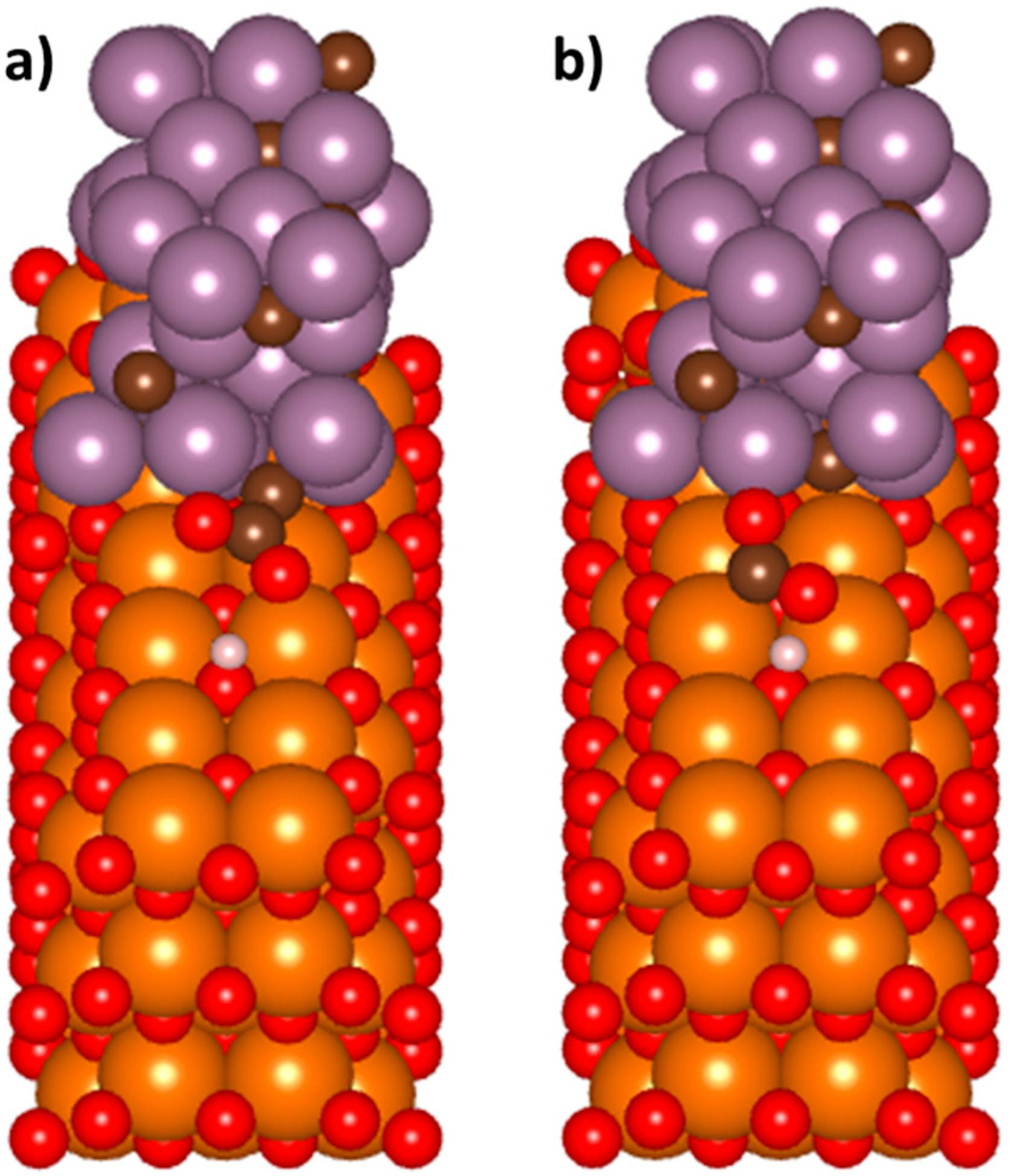

| Molecule and Surface | Adsorption Energy (eV) |
|---|---|
| CO @ Mo2C(001) | −2.23 |
| H2 @ Mo2C(001) | −1.93 |
| CO2 @ Mo2C(001) | −2.37 |
| CO @ MgO(001) | −0.17 |
| H2 @ MgO(001) | −0.12 |
| CO2 @ MgO(001) | −0.70 |
| CO2 @ Mo2C/MgO | −0.47 |
| H @ Mo2C/MgO | −0.57 |
| CO @ Mo2C/MgO | −2.16 |
| Step | Reaction | Mo2C/MgO | Mo2C(001) | ||
|---|---|---|---|---|---|
| EReact (eV) | Eact (eV) | EReact (eV) | Eact (eV) | ||
| Associative Carboxylate Mechanism | |||||
| AC1-1 | CO2* + H* → CO* + OH* | −0.80 | 1.08 | --- | --- |
| AC1-2 | CO* + OH* → CO* + OH* (rot.) | −0.43 | 0.04 | --- | --- |
| AC1-3 | CO* + OH* (rot.) → CO* (rot.) + OH* | 0.34 | 0.42 | --- | --- |
| AC2 | CO* (rot.) + OH* → CO* + O* + H* | −0.93 | 0.17 | --- | --- |
| Associative Formate Mechanism | |||||
| AF1-1 | CO2* + H* → HCOO* | −1.66 | 0.05 | 0.75 | 1.95 |
| AF1-2 | HCOO* → HCOO* (tilt) | --- | --- | −0.21 | 0.21 |
| AF2 | HCOO* → HCO* + O* | −1.30 | 5.99 | −0.27 | 1.02 |
| AF3 | HCO* + O* → CO* + O* + H* | 0.05 | 1.57 | −0.56 | 0.94 |
| Redox Mechanism | |||||
| R1 | CO2* → CO* + O* | --- | --- | −1.04 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holder, C.; Shabaev, A.; Baldwin, J.; Willauer, H. Elucidating the Role of the Mo2C/MgO Catalyst Interface in the Mechanism of the Reverse Water Gas Shift Reaction. Nanomaterials 2025, 15, 1591. https://doi.org/10.3390/nano15201591
Holder C, Shabaev A, Baldwin J, Willauer H. Elucidating the Role of the Mo2C/MgO Catalyst Interface in the Mechanism of the Reverse Water Gas Shift Reaction. Nanomaterials. 2025; 15(20):1591. https://doi.org/10.3390/nano15201591
Chicago/Turabian StyleHolder, Cameron, Andrew Shabaev, Jeffrey Baldwin, and Heather Willauer. 2025. "Elucidating the Role of the Mo2C/MgO Catalyst Interface in the Mechanism of the Reverse Water Gas Shift Reaction" Nanomaterials 15, no. 20: 1591. https://doi.org/10.3390/nano15201591
APA StyleHolder, C., Shabaev, A., Baldwin, J., & Willauer, H. (2025). Elucidating the Role of the Mo2C/MgO Catalyst Interface in the Mechanism of the Reverse Water Gas Shift Reaction. Nanomaterials, 15(20), 1591. https://doi.org/10.3390/nano15201591





