Nano-Enabled Agrochemicals for Heavy Metal Remediation in Agriculture: Current Status, Mechanisms, and Future Prospects
Abstract
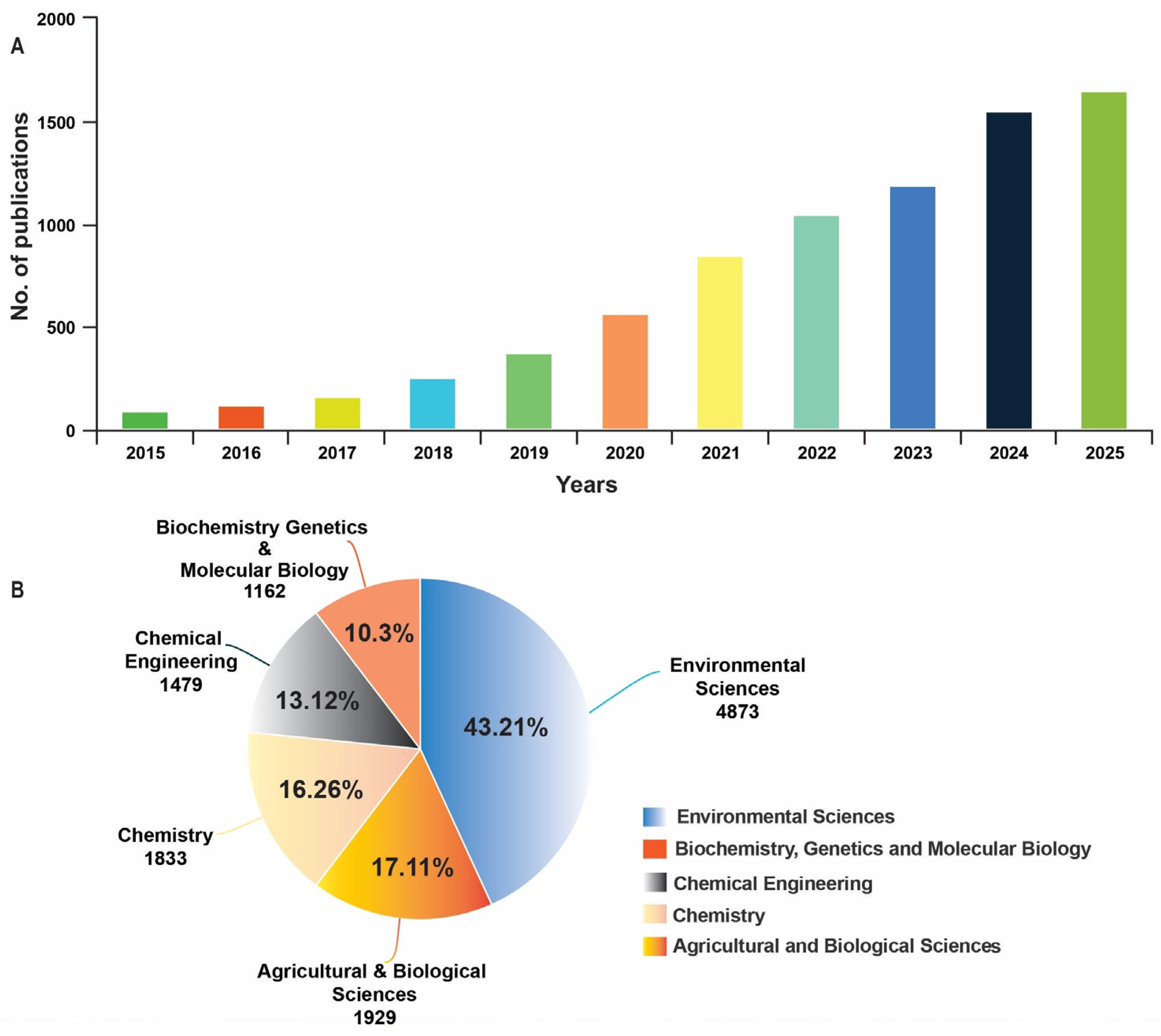
1. Introduction
Scope and Methodology of the Review
2. Types of Nano-Enabled Agrochemicals for Heavy Metal Remediation
2.1. Metal Oxide Nanomaterials
2.2. Carbon Nanomaterials
2.3. Polymeric Nanocomposites
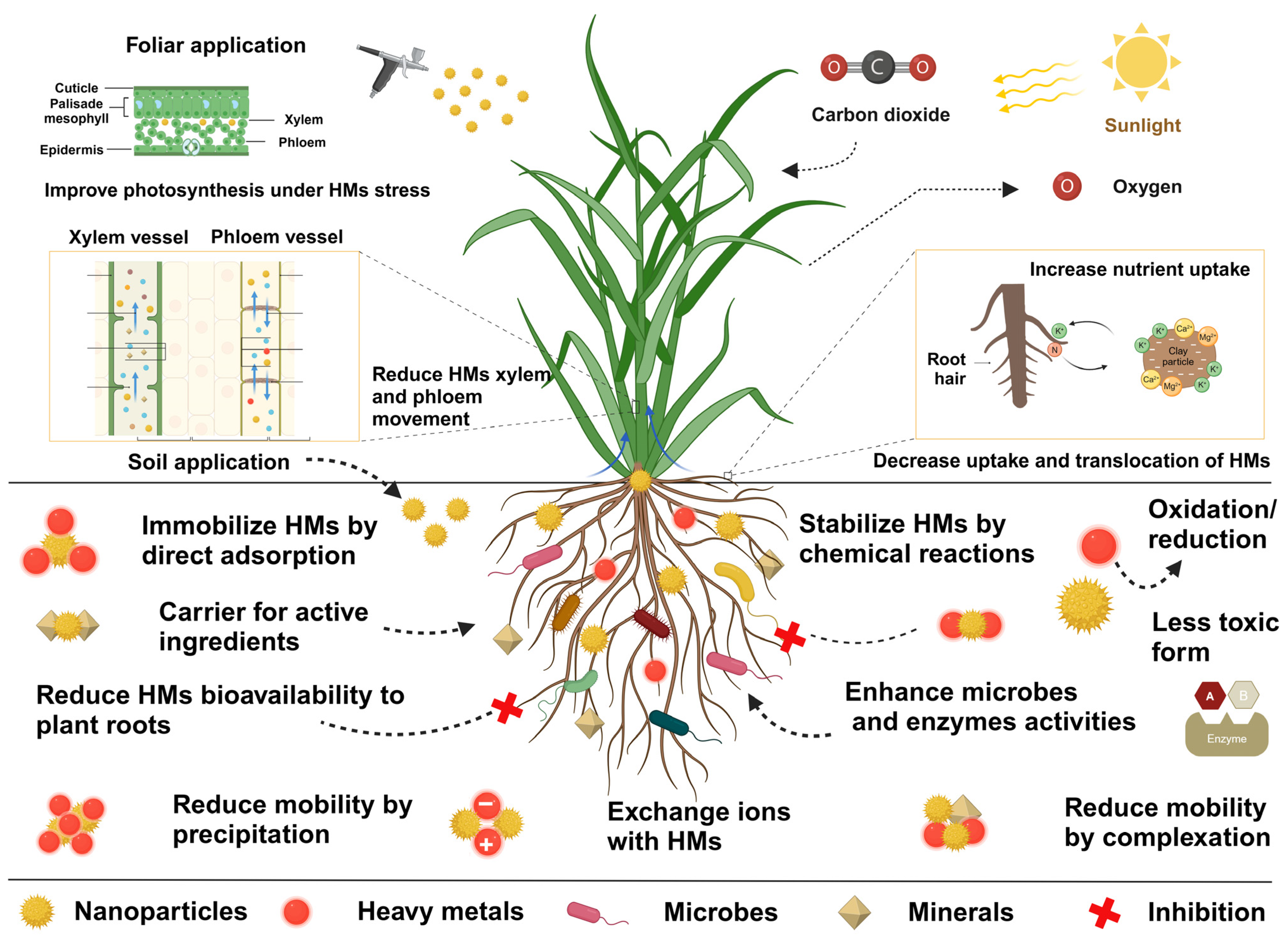
3. Mechanisms of Heavy Metal Remediation
3.1. Immobilization of Heavy Metals in Soil–Plant Systems
3.2. Mechanisms of Uptake and Translocation
3.3. Mechanisms of Regulation of Nutritional Profile and Photosynthesis
| Nanoparticles | Plant Species | Heavy Metals | Application Concentration | Impact on Plants | References |
|---|---|---|---|---|---|
| ZnO | Pea (Pisum sativum L.) | As | 100–200–300–400 mg L−1 | Improved growth, antioxidative system, reduced oxidative stress, As uptake, and increased yield | [86] |
| ZnO | Maize (Zea mays L.) | Cd | 25–50 mg L−1 | Improved chlorophyll pigments and enhanced the activity of antioxidant enzymes and decreased oxidative stress | [87] |
| ZnO | Wheat (Triticum aestivum) | Cd | 300 mg kg−1 | Increased growth attributes (root, shoot, husk, and grains dry weight) by alleviating Cd concentration in plants | [88] |
| ZnO | Solanum lycopersicum L. | Cd | 50 mg L−1 | The NPs help in maintaining photosynthesis efficiency and enhancing the plant’s antioxidant defense, which includes enzymes like SOD and CAT, which reduce oxidative damage | [89] |
| ZnO | Soybean (Glycine max) | As | 50–100 mg L−1 | Enhanced growth, increased photosynthetic pigments, antioxidant enzymes, and reduced ROS | [90] |
| ZnO | Rice (Oryza sativa L.) | As | 10–100 mg L−1 | Increased growth, phytochelatin content, anti-oxidative system, and decreased As accumulation | [91] |
| SiO2 | Wheat (Triticum aes-tivum) | Cd | 300–600 mg L−1 | Decreased oxidative stress, increased photosynthesis, antioxidant enzyme activities, reduced Cd concentrations in tissues, grains, and enhanced Si contents in plants | [79] |
| SiO2 | Soybean (Glycine max) | Hg | 500 mg L−1 | Increased growth, chlorophyll pigments, reduced Hg uptake and accumulation in both tissues | [92] |
| SiO | Brassica napus L. | Cd | 250 mg kg−1 | Increased biomass, chlorophyll pigments, carotenoids, photosynthetic rate, decreased cellular oxidative stress by improving antioxidative system and Cd translocation from root to shoot | [52] |
| SiO | Tomato (Solanum lycopersicum L.) | As | 250, 1000 mg L−1 | Increased growth, decreased As uptake, translocation and mitigated phytotoxicity, increased yield | [93] |
| FeO | Rice (Oryza sativa L.) | Cd | 10–15 mg L−1 | Enhanced growth, nutrient uptake photosynthetic parameters, and reduced oxidative stress by improving stress-responsive genetic mechanism | [94] |
| FeO | Wheat (Triticum aestivum) | Cd | 100 mg kg−1 | Immobilized Cd in soil, increased photosynthesis, nutrient uptake, growth, and enhanced antioxidative system, decreased oxidative stress | [34] |
| Fe3O4 | Coriander (Coriandrum sativum) | Cd, Pb | 100 mg L−1 | Improved growth, reduced uptake of Cd and Pb and oxidative stress | [95] |
| Fe | Wheat (Triticum aestivum L.) | Cd | 10 mg L−1 | Increased Fe uptake, plant growth parameters, photosynthesis, and strengthened antioxidative system | [96] |
| Ti | Vigna radiata L. | As | 10–50 mg L−1 | Increased growth and biomass, enhanced antioxidant activities, induced stress-responsive genes, decreased ROS | [71] |
| TiO2 | Glycine max | Cd | 100–300 mg kg−1 | Immobilized Cd in soil, reduced uptake, increased chlorophyll and net photosynthesis, RWC, growth parameters, reduced lipid peroxidation | [97] |
| TiO2 | Coriander (Coriandrum sativum L.) | Cd | 80 mg L−1 | Improved growth, chlorophyll pigments, photosynthetic rate, transpiration rate, stomatal conductance, total soluble sugars, and antioxidants enzymes activities | [98] |
| Ag | Moringa oleifera | Cd, Pb | 200 mg kg−1 | Immobilized Cd and Pb in soil, reduced their uptake, oxidative stress by increasing antioxidant activities | [99] |
| Ag | Lupinus luteus L. | Cd, Pb, Zn, Ni | 25 mg kg−1 | Improved growth, photosynthesis, GPX activity, and metallothioneins expression | [100] |
3.4. Antioxidant Defense System Activation
3.5. Effects of HMs on Protein, Osmolytes, and Synthesis of Signaling Molecules
3.6. Activation of Phytohormone and Antioxidant Enzymes Pathways
3.7. Activation of Stress-Responsive Transcription Factors
4. Environmental Impacts and Safety Considerations
4.1. Risk Assessment and Ecotoxicological Profiling
4.2. Challenges in Scale-Up and Field Application: Bridging the Lab-to-Field Gap
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of heavy metals in food and agricultural products by surface-enhanced Raman spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.a.; Xu, J.; Zhang, L.; Yang, X.; Zhang, X.; Zhang, C.; Gao, P.; Zhu, L. Environmental behaviors and toxic mechanisms of engineered nanomaterials in soil. Environ. Res. 2024, 242, 117820. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Dang, F.; Zhao, L.; Zhou, D.; Gu, X. Mixed effects and co-transfer of CeO2 NPs and arsenic in the pakchoi-snail food chain. J. Hazard. Mater. 2024, 462, 132770. [Google Scholar] [CrossRef]
- Ahmed, T.; Shou, L.; Guo, J.; Noman, M.; Qi, Y.; Yao, Y.; Masood, H.A.; Rizwan, M.; Ali, M.A.; Ali, H.M. Modulation of rhizosphere microbial community and metabolites by bio-functionalized nanoscale silicon oxide alleviates cadmium-induced phytotoxicity in bayberry plants. Sci. Total Environ. 2024, 933, 173068. [Google Scholar] [CrossRef]
- Mai, X.; Tang, J.; Tang, J.; Zhu, X.; Yang, Z.; Liu, X.; Zhuang, X.; Feng, G.; Tang, L. Research progress on the environmental risk assessment and remediation technologies of heavy metal pollution in agricultural soil. J. Environ. Sci. 2025, 149, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lakhiar, I.A.; Yan, H.; Zhang, J.; Wang, G.; Deng, S.; Bao, R.; Zhang, C.; Syed, T.N.; Wang, B.; Zhou, R. Plastic pollution in agriculture as a threat to food security, the ecosystem, and the environment: An overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.—Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Nazir, M.M.; Li, G.; Nawaz, M.; Noman, M.; Zulfiqar, F.; Ahmed, T.; Jalil, S.; Ijaz, M.; Kuzyakov, Y.; Du, D. Ionic and nano calcium to reduce cadmium and arsenic toxicity in plants: Review of mechanisms and potentials. Plant Physiol. Biochem. 2024, 216, 109169. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Hayat, K.; Zhang, D.; Zhou, P. Small structures with big impact: Multi-walled carbon nanotubes enhanced remediation efficiency in hyperaccumulator Solanum nigrum L. under cadmium and arsenic stress. Chemosphere 2021, 276, 130130. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Zhang, Y.; Tian, Y.; Yao, K.; Xu, M. Visualization of heavy metal cadmium in lettuce leaves based on wavelet support vector machine regression model and visible-near infrared hyperspectral imaging. J. Food Process Eng. 2021, 44, e13897. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Zhao, L.; Zhou, D. Nanozymes: An innovative approach to regulating heavy metal accumulation in plants and alleviating toxicity-a comprehensive review. Crit. Rev. Environ. Sci. Technol. 2025, 55, 732–756. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, H.; Li, J.; Zhang, W.; Zhao, F.; Wang, Q.; Shi, M.; Wang, Y.; Yan, X.; Chai, L. Advancements of heavy metals control in the environment from detection to separation and recovery: Emerging microfluidics strategy. Crit. Rev. Environ. Sci. Technol. 2025, 55, 1025–1045. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Yan, H.; Liu, C.; Zhu, X.; Chen, B. Microfluidics as an emerging platform for exploring soil environmental processes: A critical review. Environ. Sci. Technol. 2022, 56, 711–731. [Google Scholar] [CrossRef]
- Yadav, S.; Sawarni, N.; Dahiya, T.; Rana, J.; Sharma, M.; Batra, B. Nanoagriculture: Advantages and Drawbacks. In Agricultural and Environmental Nanotechnology: Novel Technologies and their Ecological Impact; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–42. [Google Scholar]
- Carmona, F.J.; Dal Sasso, G.; Ramírez-Rodríguez, G.B.; Pii, Y.; Delgado-López, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: Optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Rep. 2021, 11, 3419. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Zia-ur-Rehman, M.; Hussain, S.M.; Qayyum, M.F.; Rizwan, M.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Ali, S. Combined effects of zinc oxide nanoparticles and melatonin on wheat growth, chlorophyll contents, cadmium (Cd) and zinc uptake under Cd stress. Sci. Total Environ. 2023, 864, 161061. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Chen, M.; Tang, W.; Zeng, G.; Gong, J.; Zhang, P.; Ye, S. Using nanomaterials to facilitate the phytoremediation of contaminated soil. Crit. Rev. Environ. Sci. Technol. 2019, 49, 791–824. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-enabled agrochemicals: Mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 2024, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on natural, incidental, bioinspired, and engineered nanomaterials: History, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Faizan, M.; Singh, A.; Eren, A.; Sultan, H.; Sharma, M.; Djalovic, I.; Trivan, G. Small molecule, big impacts: Nano-nutrients for sustainable agriculture and food security. J. Plant Physiol. 2024, 301, 154305. [Google Scholar] [CrossRef]
- Inobeme, A.; Mathew, J.T.; Adetunji, C.O.; Ajai, A.I.; Inobeme, J.; Maliki, M.; Okonkwo, S.; Adekoya, M.A.; Bamigboye, M.O.; Jacob, J.O. Recent advances in nanotechnology for remediation of heavy metals. Environ. Monit. Assess. 2023, 195, 111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, F.; Jia, W.; Jiang, Y.; Wu, X.; Song, S.; Shen, H.; Shen, J. Nanomaterials and nanotechnology in agricultural pesticide delivery: A review. Langmuir 2024, 40, 18806–18820. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zaidi, S.J. Recent developments in the application of nanomaterials in agroecosystems. Nanomaterials 2020, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Bhat, M.P.; Lee, J.-H.; Kurkuri, M.D.; Kim, C.S.; Lee, K.-H. Carbon-based nanomaterials: Multifaceted role in agrochemical recognition, remediation, and release. Nano Today 2024, 57, 102388. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Xu, D.; Yang, W.; Bai, S. Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review. Nanotechnol. Rev. 2020, 9, 736–750. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, F.; Wang, W.; Zhang, S.; Wang, F. Remediation of Cr (VI)-contaminated soil by nano-zero-valent iron in combination with biochar or humic acid and the consequences for plant performance. Toxics 2020, 8, 26. [Google Scholar] [CrossRef]
- Guo, S.; Yang, H.; Sun, Q.; Zhang, G.; Zhao, T.; Zhou, Y.; Li, X.; Gao, P. Evaluation of a novel carbon-based micro-nano zero-valent iron composite for immobilization of heavy metals in soil. J. Environ. Chem. Eng. 2023, 11, 109740. [Google Scholar] [CrossRef]
- Kumari, N.; Tiwari, A.; Nandulal, I.S.; Das, S.P.; Mondal, B.P.; Bihari, B.; Ganguly, P.; Chandini; Kumar, R. Impacts of Nanomaterials on Soil Microbial Communities. In Nanotechnology-Based Sustainable Agriculture; John Wiley & Sons: Hoboken, NJ, USA, 2025; pp. 135–158. [Google Scholar]
- De Silva, M.; Cao, G.; Tam, M.K. Nanomaterials for the removal and detection of heavy metals: A review. Environ. Sci. Nano 2025, 12, 2154–2176. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Lian, M.; Zhang, C.; Ruan, X.; Li, T. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 2020, 197, 110600. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Q.; Lu, D.; Wang, C.; Cheng, T.; Fu, S. Reducing Cd uptake and translocation in wheat seedlings through integrated approaches using nanosilicon and extracellular polymeric substances. J. Agric. Food Chem. 2025, 73, 18617–18631. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef]
- Lyu, Y.; Yu, Y.; Li, T.; Cheng, J. Rhizosphere effects of Loliumperenne L. and Beta vulgaris var. cicla L. on the immobilization of Cd by modified nanoscale black carbon in contaminated soil. J. Soils Sediments 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Dhlamini, B.; Paumo, H.K.; Kamdem, B.P.; Katata-Seru, L.; Bahadur, I. Nano-engineering metal-based fertilizers using biopolymers: An innovative strategy for a more sustainable agriculture. J. Environ. Chem. Eng. 2022, 10, 107729. [Google Scholar] [CrossRef]
- Wang, A.; Kang, F.; Wang, Z.; Shao, Q.; Li, Z.; Zhu, G.; Lu, J.; Li, Y.Y. Facile Synthesis of nitrogen-rich carbon dots as fertilizers for mung bean sprouts. Adv. Sustain. Syst. 2019, 3, 1800132. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Lu, F.; Liu, Y.; Song, Y.; Sun, Y.; Zhong, J.; Huang, H.; Wang, Y.; Li, S. Impacts of carbon dots on rice plants: Boosting the growth and improving the disease resistance. ACS Appl. Bio Mater. 2018, 1, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tao, S.; Wang, X. Interactions between organic pollutants and carbon nanomaterials and the associated impact on microbial availability and degradation in soil: A review. Environ. Sci. Nano 2020, 7, 2486–2508. [Google Scholar] [CrossRef]
- Rezaei Cherati, S.; Shanmugam, S.; Pandey, K.; Khodakovskaya, M.V. Whole-transcriptome responses to environmental stresses in agricultural crops treated with carbon-based nanomaterials. ACS Appl. Bio Mater. 2021, 4, 4292–4301. [Google Scholar] [CrossRef]
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.-O.; Kim, J. Engineering plants with carbon nanotubes: A sustainable agriculture approach. J. Nanobiotechnol. 2022, 20, 275. [Google Scholar] [CrossRef]
- Hasaneen, M.; Abdel-Aziz, H.M.M.; Omer, A.M. Characterization of carbon nanotubes loaded with nitrogen, phosphorus and potassium fertilizers. Am. J. Nano Res. Appl. 2017, 5, 12–18. [Google Scholar] [CrossRef]
- Xin, X.; Zhao, F.; Judy, J.D.; He, Z. Copper stress alleviation in corn (Zea mays L.): Comparative efficiency of carbon nanotubes and carbon nanoparticles. NanoImpact 2022, 25, 100381. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.T.; Oates, R.; Subbiah, S.; Payton, P.R.; Singh, K.P.; Shah, S.A.; Green, M.J.; Klein, D.M.; Cañas-Carrell, J.E. Carbon nanotubes affect early growth, flowering time and phytohormones in tomato. Chemosphere 2020, 256, 127042. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Giroto, A.S.; Guimarães, G.G.; Foschini, M.; Ribeiro, C. Role of slow-release nanocomposite fertilizers on nitrogen and phosphate availability in soil. Sci. Rep. 2017, 7, 46032. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, W.; Li, X.-L.; Shen, S.-G.; Liang, S.-X.; Liu, C.; Shan, L. Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol. Eng. 2016, 95, 25–29. [Google Scholar] [CrossRef]
- Cherwoo, L.; Gupta, I.; Bhatia, R.; Setia, H. Improving agricultural practices: Application of polymers in agriculture. Energy Ecol. Environ. 2024, 9, 25–41. [Google Scholar] [CrossRef]
- Jalil, S.; Nazir, M.M.; Ali, Q.; Zulfiqar, F.; Moosa, A.; Altaf, M.A.; Zaid, A.; Nafees, M.; Yong, J.W.H.; Jin, X. Zinc and nano zinc mediated alleviation of heavy metals and metalloids in plants: An overview. Funct. Plant Biol. 2023, 50, 870–888. [Google Scholar] [CrossRef]
- Ahmed, T.; Masood, H.A.; Noman, M.; Al-Huqail, A.A.; Alghanem, S.M.; Khan, M.M.; Muhammad, S.; Manzoor, N.; Rizwan, M.; Qi, X. Biogenic silicon nanoparticles mitigate cadmium (Cd) toxicity in rapeseed (Brassica napus L.) by modulating the cellular oxidative stress metabolism and reducing Cd translocation. J. Hazard. Mater. 2023, 459, 132070. [Google Scholar] [CrossRef]
- Majumdar, A.; Upadhyay, M.K.; Ojha, M.; Afsal, F.; Giri, B.; Srivastava, S.; Bose, S. Enhanced phytoremediation of Metal (loid) s via spiked ZVI nanoparticles: An urban clean-up strategy with ornamental plants. Chemosphere 2022, 288, 132588. [Google Scholar] [CrossRef]
- Cao, P.; Qiu, K.; Zou, X.; Lian, M.; Liu, P.; Niu, L.; Yu, L.; Li, X.; Zhang, Z. Mercapto propyltrimethoxysilane-and ferrous sulfate-modified nano-silica for immobilization of lead and cadmium as well as arsenic in heavy metal-contaminated soil. Environ. Pollut. 2020, 266, 115152. [Google Scholar] [CrossRef]
- Lian, M.; Wang, L.; Feng, Q.; Niu, L.; Zhao, Z.; Wang, P.; Song, C.; Li, X.; Zhang, Z. Thiol-functionalized nano-silica for in-situ remediation of Pb, Cd, Cu contaminated soils and improving soil environment. Environ. Pollut. 2021, 280, 116879. [Google Scholar] [CrossRef]
- Vítková, M.; Puschenreiter, M.; Komárek, M. Effect of nano zero-valent iron application on As, Cd, Pb, and Zn availability in the rhizosphere of metal (loid) contaminated soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhou, H.; Gu, J.-F.; Huang, F.; Yang, W.-J.; Wang, S.-L.; Yuan, T.-Y.; Liao, B.-H. Effects of nano-Fe3O4-modified biochar on iron plaque formation and Cd accumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113970. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-T.; Liu, T.-X.; Wang, X.-Q.; Li, F.-B.; Lv, Y.-H.; Cui, J.-H.; Zeng, X.-D.; Yuan, Y.-Z.; Liu, C.-P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Q.; Gao, B.; Li, M.; Fan, Z.; Sang, W.; Hao, H.; Wei, X. Removal mechanisms of Cr (VI) and Cr (III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation. Environ. Pollut. 2020, 265, 115018. [Google Scholar] [CrossRef]
- Liu, Y.; Persson, D.P.; Li, J.; Liang, Y.; Li, T. Exposure of cerium oxide nanoparticles to the hyperaccumulator Sedum alfredii decreases the uptake of cadmium via the apoplastic pathway. J. Hazard. Mater. 2021, 417, 125955. [Google Scholar] [CrossRef]
- Fox, J.-P.; Capen, J.D.; Zhang, W.; Ma, X.; Rossi, L. Effects of cerium oxide nanoparticles and cadmium on corn (Zea mays L.) seedlings physiology and root anatomy. NanoImpact 2020, 20, 100264. [Google Scholar] [CrossRef]
- Rossi, L.; Sharifan, H.; Zhang, W.; Schwab, A.P.; Ma, X. Mutual effects and in planta accumulation of co-existing cerium oxide nanoparticles and cadmium in hydroponically grown soybean (Glycine max (L.) Merr.). Environ. Sci. Nano 2018, 5, 150–157. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, P.; He, M.; Cao, Y.; Adeel, M.; Shakoor, N.; Jiang, Y.; Zhao, W.; Li, Y.; Li, M. Iron-based nanomaterials reduce cadmium toxicity in rice (Oryza sativa L.) by modulating phytohormones, phytochelatin, cadmium transport genes and iron plaque formation. Environ. Pollut. 2023, 320, 121063. [Google Scholar] [CrossRef] [PubMed]
- Guha, T.; Barman, S.; Mukherjee, A.; Kundu, R. Nano-scale zero valent iron modulates Fe/Cd transporters and immobilizes soil Cd for production of Cd free rice. Chemosphere 2020, 260, 127533. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef]
- Jiang, M.; Dai, S.; Wang, B.; Xie, Z.; Li, J.; Wang, L.; Li, S.; Tan, Y.; Tian, B.; Shu, Q. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano 2021, 8, 1042–1056. [Google Scholar] [CrossRef]
- Kiany, T.; Pishkar, L.; Sartipnia, N.; Iranbakhsh, A.; Barzin, G. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ. Sci. Pollut. Res. 2022, 29, 34725–34737. [Google Scholar] [CrossRef]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Nazir, M.M.; Li, Q.; Noman, M.; Ulhassan, Z.; Ali, S.; Ahmed, T.; Zeng, F.; Zhang, G. Calcium oxide nanoparticles have the role of alleviating arsenic toxicity of barley. Front. Plant Sci. 2022, 13, 843795. [Google Scholar] [CrossRef]
- Katiyar, P.; Yadu, B.; Korram, J.; Satnami, M.L.; Kumar, M.; Keshavkant, S. Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J. Environ. Sci. 2020, 92, 18–27. [Google Scholar] [CrossRef]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Zhou, W.; Gill, R.A. Lead toxicity in cereals: Mechanistic insight into toxicity, mode of action, and management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Kumari, N.; Sharma, V. Zinc Oxide Nanoparticles Improve Photosynthesis by Modulating Antioxidant System and psb A Gene Expression Under Arsenic Stress in Different Cultivars of Vigna radiata. BioNanoScience 2025, 15, 242. [Google Scholar] [CrossRef]
- Koleva, L.; Umar, A.; Yasin, N.A.; Shah, A.A.; Siddiqui, M.H.; Alamri, S.; Riaz, L.; Raza, A.; Javed, T.; Shabbir, Z. Iron oxide and silicon nanoparticles modulate mineral nutrient homeostasis and metabolism in cadmium-stressed Phaseolus vulgaris. Front. Plant Sci. 2022, 13, 806781. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; ur Rehman, M.Z.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Hussain, S.; Niazi, M.B.K.; Shahid, M.; Song, F. Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J. Hazard. Mater. 2020, 398, 123175. [Google Scholar] [CrossRef]
- Nazir, M.M.; Noman, M.; Ahmed, T.; Ali, S.; Ulhassan, Z.; Zeng, F.; Zhang, G. Exogenous calcium oxide nanoparticles alleviate cadmium toxicity by reducing Cd uptake and enhancing antioxidative capacity in barley seedlings. J. Hazard. Mater. 2022, 438, 129498. [Google Scholar] [CrossRef]
- Jalil, S.; Alghanem, S.M.; Al-Huqail, A.A.; Nazir, M.M.; Zulfiqar, F.; Ahmed, T.; Ali, S.; Abeed, A.H.; Siddique, K.H.; Jin, X. Zinc oxide nanoparticles mitigated the arsenic induced oxidative stress through modulation of physio-biochemical aspects and nutritional ions homeostasis in rice (Oryza sativa L.). Chemosphere 2023, 338, 139566. [Google Scholar] [CrossRef]
- Silva, S.; Dias, M.C.; Silva, A.M. Titanium and zinc based nanomaterials in agriculture: A promising approach to deal with (a) biotic stresses? Toxics 2022, 10, 172. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S.; Wang, X. Heavy metal accumulation in different rice cultivars as influenced by foliar application of nano-silicon. Water Air Soil Pollut. 2016, 227, 228. [Google Scholar] [CrossRef]
- Banerjee, S.; Mondal, S.; Islam, J.; Sarkar, R.; Saha, B.; Sen, A. Rhizospheric nano-remediation salvages arsenic genotoxicity: Zinc-oxide nanoparticles articulate better oxidative stress management, reduce arsenic uptake, and increase yield in Pisum sativum (L.). Sci. Total Environ. 2024, 913, 169493. [Google Scholar] [CrossRef]
- Hussain, M.; Kaousar, R.; Haq, S.I.U.; Shan, C.; Wang, G.; Rafique, N.; Shizhou, W.; Lan, Y. Zinc-oxide nanoparticles ameliorated the phytotoxic hazards of cadmium toxicity in maize plants by regulating primary metabolites and antioxidants activity. Front. Plant Sci. 2024, 15, 1346427. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zia-ur-Rehman, M.; Rizwan, M.; Abbas, T.; Ayub, M.A.; Naeem, A.; Alharby, H.F.; Alabdallah, N.M.; Alharbi, B.M.; Qamar, M.J. Effect of soil texture and zinc oxide nanoparticles on growth and accumulation of cadmium by wheat: A life cycle study. Environ. Res. 2023, 216, 114397. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, R.; Ju, Q.; Xing, M.; Li, R.; Li, W.; Li, W.; Wang, W.; Deng, Y.; Xu, J. Mitigation mechanism of zinc oxide nanoparticles on cadmium toxicity in tomato. Front. Plant Sci. 2023, 14, 1162372. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 150. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Bai, X.; Zheng, L.; Zhao, J.; Li, Y.-f.; Zhang, Z.; Gao, Y. Silica nanoparticles alleviate mercury toxicity via immobilization and inactivation of Hg (ii) in soybean (Glycine max). Environ. Sci. Nano 2020, 7, 1807–1817. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2022, 29, 34147–34163. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef]
- Fahad; Balouch, A.; Agheem, M.H.; Memon, S.A.; Baloch, A.R.; Tunio, A.; Abdullah; Pato, A.H.; Jagirani, M.S.; Panah, P. Efficient mitigation of cadmium and lead toxicity in coriander plant utilizing magnetite (Fe3O4) nanofertilizer as growth regulator and antimicrobial agent. Int. J. Environ. Anal. Chem. 2022, 102, 3868–3879. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Lee, B.-K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Ahmed, S.; Yasin, N.A. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ. Pollut. 2022, 292, 118373. [Google Scholar] [CrossRef] [PubMed]
- Azeez, L.; Adejumo, A.L.; Lateef, A.; Adebisi, S.A.; Adetoro, R.O.; Adewuyi, S.O.; Tijani, K.O.; Olaoye, S. Zero-valent silver nanoparticles attenuate Cd and Pb toxicities on Moringa oleifera via immobilization and induction of phytochemicals. Plant Physiol. Biochem. 2019, 139, 283–292. [Google Scholar] [CrossRef]
- Jaskulak, M.; Rorat, A.; Grobelak, A.; Chaabene, Z.; Kacprzak, M.; Vandenbulcke, F. Bioaccumulation, antioxidative response, and metallothionein expression in Lupinus luteus L. exposed to heavy metals and silver nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 16040–16052. [Google Scholar] [CrossRef]
- Saleem, S.; Rizvi, A.; Khan, M. Microbiome-mediated nano-bioremediation of heavy metals: A prospective approach of soil metal detoxification. Int. J. Environ. Sci. Technol. 2023, 20, 12823–12846. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Basit, F.; Nazir, M.M.; Shahid, M.; Abbas, S.; Javed, M.T.; Naqqash, T.; Liu, Y.; Yajing, G. Application of zinc oxide nanoparticles immobilizes the chromium uptake in rice plants by regulating the physiological, biochemical and cellular attributes. Physiol. Mol. Biol. Plants 2022, 28, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Zhao, S.; Kamran, M.; Ur Rehman, N.; Mora-Poblete, F.; Maldonado, C.; Hamzah Saleem, M.; Parveen, A.; Ahmed Al-Ghamdi, A.; Al-Hemaid, F.M. Effect of nano-silicon on the regulation of ascorbate-glutathione contents, antioxidant defense system and growth of copper stressed wheat (Triticum aestivum L.) seedlings. Front. Plant Sci. 2022, 13, 986991. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Q.; Guo, Z.; Fu, J.; Sun, Y.; Gu, C.; Xing, B.; Dhankher, O.P. Sulfur nanoparticles improved plant growth and reduced mercury toxicity via mitigating the oxidative stress in Brassica napus L. J. Clean. Prod. 2021, 318, 128589. [Google Scholar] [CrossRef]
- Qi, W.-Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.-F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.-G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Raja, V.; Singh, K.; Qadir, S.U.; Singh, J.; Kim, K.-H. Alleviation of cadmium-induced oxidative damage through application of zinc oxide nanoparticles and strigolactones in Solanum lycopersicum L. Environ. Sci. Nano 2024, 11, 2633–2654. [Google Scholar] [CrossRef]
- Zeeshan, M.; Hu, Y.X.; Iqbal, A.; Salam, A.; Liu, Y.X.; Muhammad, I.; Ahmad, S.; Khan, A.H.; Hale, B.; Wu, H.Y. Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol. Environ. Saf. 2021, 225, 112738. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, Y.; Guo, Z.; Han, M.; Yan, X. Zinc oxide nanoparticles alleviate cadmium toxicity and promote tolerance by modulating programmed cell death in alfalfa (Medicago sativa L.). J. Hazard. Mater. 2024, 469, 133917. [Google Scholar] [CrossRef]
- Upadhyay, R.; Saini, R.; Shukla, P.; Tiwari, K. Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 2025, 24, 953–983. [Google Scholar] [CrossRef]
- Shah, A.A.; Aslam, S.; Akbar, M.; Ahmad, A.; Khan, W.U.; Yasin, N.A.; Ali, B.; Rizwan, M.; Ali, S. Combined effect of Bacillus fortis IAGS 223 and zinc oxide nanoparticles to alleviate cadmium phytotoxicity in Cucumis melo. Plant Physiol. Biochem. 2021, 158, 1–12. [Google Scholar] [CrossRef]
- Zeeshan, M.; Sun, C.; Wang, X.; Hu, Y.; Wu, H.; Li, S.; Salam, A.; Zhu, S.; Khan, A.H.; Holford, P. Insights into the ameliorative effect of ZnONPs on arsenic toxicity in soybean mediated by hormonal regulation, transporter modulation, and stress responsive genes. Front. Plant Sci. 2024, 15, 1427367. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and response of plants to heavy metals action. Front. Plant Sci. 2016, 7, 629. [Google Scholar] [CrossRef]
- Sultana, R.; Imam, Z.; Kumar, R.R.; Banu, V.S.; Nahakpam, S.; Bharti, R.; Bharadwaj, C.; Singh, A.K.; Pasala, R.K.; Singh, D.R. Signaling and defence mechanism of jasmonic and salicylic acid response in pulse crops: Role of WRKY transcription factors in stress response. J. Plant Growth Regul. 2025, 44, 5–21. [Google Scholar] [CrossRef]
- Guo, K.; Hu, A.; Wang, K.; Wang, L.; Fu, D.; Hao, Y.; Wang, Y.; Ali, A.; Adeel, M.; Rui, Y. Effects of spraying nano-materials on the absorption of metal (loid) s in cucumber. IET Nanobiotechnol. 2019, 13, 712–719. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Liao, W. Calmodulin-Binding Transcription Factors: Roles in Plant Response to Abiotic Stresses. Plants 2025, 14, 532. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L. Transcription factors involved in plant responses to cadmium-induced oxidative stress. Front. Plant Sci. 2024, 15, 1397289. [Google Scholar] [CrossRef]
- Liu, P.; Sun, L.; Zhang, Y.; Tan, Y.; Zhu, Y.; Peng, C.; Wang, J.; Yan, H.; Mao, D.; Liang, G. The metal tolerance protein OsMTP11 facilitates cadmium sequestration in the vacuoles of leaf vascular cells for restricting its translocation into rice grains. Mol. Plant 2024, 17, 1733–1752. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Hu, Y.X.; Guo, X.H.; Sun, C.Y.; Salam, A.; Ahmad, S.; Muhammad, I.; Nasar, J.; Jahan, M.S.; Fahad, S. Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L.(Merr.) roots. Environ. Pollut. 2023, 317, 120637. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, C.; White, J.C.; Cao, Y.; Zhang, F.; Tong, R.; Yu, H.; Hao, Y.; Yan, W.; Kah, M. Engineered nanomaterials reduce metal (loid) accumulation and enhance staple food production for sustainable agriculture. Nat. Food 2024, 5, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.O.; Amber, I.; Officer, S.; Oluyemi, G.F. Transport of nanoparticles in porous media and associated environmental impact: A review. J. Eng. Res. 2024, 12, 275–284. [Google Scholar] [CrossRef]
- Konwarh, R.; Workie, A.; Moges, A.; Seifu, D.; Elias, D.; Kebede, N.; Tsigemariam, Y.; Singh, A.P. Preliminary investigation into prospective applications of nanosilver, produced using teff (Eragrostis tef) flour extract. J. Microbiol. Biotechnol. Food Sci. 2024, 13, e9892. [Google Scholar] [CrossRef]
- Wang, H.; Yao, L.; Zhang, Q.; Wang, L.; Wu, Y.; Cai, K.; Shen, C.; Lin, C.; Huang, Y.; Chen, H. DLVO interaction energies between hollow nanoparticles and fractal surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 135923. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Ahmad, Z.; Yang, X.; Huo, L. Transport of engineered nanoparticles in porous media and its enhancement for remediation of contaminated groundwater. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2301–2378. [Google Scholar] [CrossRef]
- Islam, S. Toxicity and transport of nanoparticles in agriculture: Effects of size, coating, and aging. Front. Nanotechnol. 2025, 7, 1622228. [Google Scholar] [CrossRef]
- Macůrková, A.; Maryška, L.; Jindřichová, B.; Drobníková, T.; Vrchotová, B.; Pospíchalová, R.; Záruba, K.; Hubáček, T.; Siegel, J.; Burketová, L. Effect of round-shaped silver nanoparticles on the genetic and functional diversity of soil microbial community in soil and “soil-plant” systems. Appl. Soil Ecol. 2021, 168, 104165. [Google Scholar] [CrossRef]
- Leroy, M.; Jassey, V.E.; Silvestre, J.; Barret, M.; Flahaut, E.; Larue, C. Carbon nanotubes alter agrosystem multifunctionality. Environ. Sci. Nano 2024, 11, 4126–4137. [Google Scholar] [CrossRef]
- Hamada, A.M.; Radi, A.A.; Al-Kahtany, F.A.; Farghaly, F.A. A review: Zinc oxide nanoparticles: Advantages and disadvantages. J. Plant Nutr. 2024, 47, 656–679. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Tabatabaei, F.; Ahadi, A.-m. Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol. Environ. Saf. 2015, 113, 259–270. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Shi, Y.; Li, Z.; Zhang, X.; Liu, T.; Farooq, T.H.; Pan, Y.; Chen, X.; Yan, W. Combined toxicity of zinc oxide nanoparticles and cadmium inducing root damage in Phytolacca americana L. Sci. Total Environ. 2022, 806, 151211. [Google Scholar] [CrossRef]
- Karmous, I.; Tlahig, S.; Loumerem, M.; Lachiheb, B.; Bouhamda, T.; Mabrouk, M.; Debouba, M.; Chaoui, A. Assessment of the risks of copper-and zinc oxide-based nanoparticles used in Vigna radiata L. culture on food quality, human nutrition and health. Environ. Geochem. Health 2022, 44, 4045–4061. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Kohatsu, M.Y.; Seabra, A.B.; Monteiro, L.R.; Gomes, D.G.; Oliveira, H.C.; Rolim, W.R.; De Jesus, T.A.; Batista, B.L.; Lange, C.N. Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Environ. Monit. Assess. 2020, 192, 232. [Google Scholar] [CrossRef]
- Saleh, A.M.; Hassan, Y.M.; Selim, S.; AbdElgawad, H. NiO-nanoparticles induce reduced phytotoxic hazards in wheat (Triticum aestivum L.) grown under future climate CO2. Chemosphere 2019, 220, 1047–1057. [Google Scholar] [CrossRef]
- Chung, I.-M.; Venkidasamy, B.; Thiruvengadam, M. Nickel oxide nanoparticles cause substantial physiological, phytochemical, and molecular-level changes in Chinese cabbage seedlings. Plant Physiol. Biochem. 2019, 139, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ghoto, K.; Simon, M.; Shen, Z.-J.; Gao, G.-F.; Li, P.-F.; Li, H.; Zheng, H.-L. Physiological and root exudation response of maize seedlings to TiO2 and SiO2 nanoparticles exposure. BioNanoScience 2020, 10, 473–485. [Google Scholar] [CrossRef]
- Alsiary, W.A.; Abdelgawad, H.; Madany, M.M. How could actinobacteria augment the growth and redox homeostasis in barley plants grown in TiO2NPs-contaminated soils? A growth and biochemical study. Plant Physiol. Biochem. 2023, 202, 107943. [Google Scholar] [CrossRef]
- Tan, W.; Du, W.; Darrouzet-Nardi, A.J.; Hernandez-Viezcas, J.A.; Ye, Y.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of the exposure of TiO2 nanoparticles on basil (Ocimum basilicum) for two generations. Sci. Total Environ. 2018, 636, 240–248. [Google Scholar] [CrossRef]
- Falco, W.F.; Scherer, M.D.; Oliveira, S.L.; Wender, H.; Colbeck, I.; Lawson, T.; Caires, A.R. Phytotoxicity of silver nanoparticles on Vicia faba: Evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci. Total Environ. 2020, 701, 134816. [Google Scholar] [CrossRef]
- Hatami, M. Toxicity assessment of multi-walled carbon nanotubes on Cucurbita pepo L. under well-watered and water-stressed conditions. Ecotoxicol. Environ. Saf. 2017, 142, 274–283. [Google Scholar] [CrossRef]
- Anjum, N.A.; Singh, N.; Singh, M.K.; Sayeed, I.; Duarte, A.C.; Pereira, E.; Ahmad, I. Single-bilayer graphene oxide sheet impacts and underlying potential mechanism assessment in germinating faba bean (Vicia faba L.). Sci. Total Environ. 2014, 472, 834–841. [Google Scholar] [CrossRef]
- Asmatulu, E.; Andalib, M.N.; Subeshan, B.; Abedin, F. Impact of nanomaterials on human health: A review. Environ. Chem. Lett. 2022, 20, 2509–2529. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Rui, Y. The impact of nanomaterials on plant health: A review of exposure, toxicity, and control. Environ. Sci. Nano 2025, 12, 2965–2982. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Fawzy, Z.F.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Faizy, S.E.-D.; Abowaly, M.; El-Henawy, A.; Kiss, A. Nano-restoration for sustaining soil fertility: A pictorial and diagrammatic review article. Plants 2022, 11, 2392. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Engineered nanomaterials and associated threats in the environment risk assessment strategies. In Biotechnological Interventions in the Removal of Emerging Pollutants; Springer: Berlin/Heidelberg, Germany, 2025; pp. 515–537. [Google Scholar]
- Kim, S.H.; Bae, S.; Hwang, Y.S. Comparative bioaccumulation, translocation, and phytotoxicity of metal oxide nanoparticles and metal ions in soil-crop system. Sci. Total Environ. 2023, 856, 158938. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, A.; Ma, P.; Wu, Y.; Zhu, F.; Du, D.; Okoye, C.O.; Chen, X.; Deng, Q. Interaction between airway inflammation and gut microbiota dysbiosis caused by high temperatures (40 °C) and traffic-PM2.5 in mouse model. Environ. Res. 2025, 285, 122731. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Chi, Y.; Ma, X.; Chu, S.; You, Y.; Chen, X.; Wang, J.; Wang, R.; Zhang, X.; Zhang, D.; Zhao, T.; et al. Nitrogen cycle induced by plant growth-promoting rhizobacteria drives “microbial partners” to enhance cadmium phytoremediation. Microbiome 2025, 13, 113. [Google Scholar] [CrossRef]
- Chen, X.; Chu, S.; Chi, Y.; Wang, J.; Wang, R.; You, Y.; Hayat, K.; Khalid, M.; Zhang, D.; Zhou, P.; et al. Unraveling the role of multi-walled carbon nanotubes in a corn-soil system: Plant growth, oxidative stress and heavy metal(loid)s behavior. Plant Physiol. Biochem. 2023, 200, 107802. [Google Scholar] [CrossRef] [PubMed]



| Nanoparticles | Plant Species | Concentration | Negative Effects on Plants | References |
|---|---|---|---|---|
| ZnO NPs | Phytolacca americana L. | 500 mg kg−1 | Reduced root and shoot growth, increased lipid peroxidation, and severely damaged root cells | [130] |
| Cu NPs, ZnO NPs | Mung bean (Vigna radiata L.) | 1000, 2000 mg L−1 | Inhibited seed germination, embryo growth, disturbed micronutrients (Fe, Mn, Cu, Zn, K) and macronutrients (Ca, Na, Mg,), increased antioxidants | [131] |
| CuO | Lettuce | 40 μg mL−1 | Decreased seed germination and reduced radicle growth | [132] |
| NiO2 | Wheat | 120 mg kg−1 | Reduced plant growth, inhibited photosynthesis, and increased antioxidant activities | [133] |
| NiO NPs | Chinese cabbage | 50, 250, and 500 mg L−1 | Reduced root growth, chlorophyll content, and carotenoid, increased lipid peroxidation and ROS production, caused molecular and metabolic changes | [134] |
| SiO2 | Maize | 1000 mg L−1 | Adversely affected early growth parameters, reduced chlorophyll and carotenoid pigments, and triggered oxidative stress | [135] |
| TiO2 NPs | Barley (Hordeum vulgare) | 2000 mg kg−1 | Decreased biomass, photosynthesis, increased antioxidants (SOD, CAT), and induced oxidative stress | [136] |
| TiO2 | Basil (Ocimum basilicum). | 750 mg kg−1 | Decreased chlorophyll b (52%), total chlorophyll (30%), reduced Mg contents in root by 115% | [137] |
| AgNPs | Vicia faba | 100 mg L−1 | Reduced photochemical efficacy of photosystem II (PSII), increased ROS | [138] |
| MWCNTs | Cucurbita pepo L. | 125, 250, 500 mg L−1 | Decreased germination percentage, shoot growth, biomass, increased oxidative damage. | [139] |
| GO NPs | Faba bean (Vicia faba L.) | 100, 200, 400, 800, 1600 mg L−1 | Reduced growth, CAT, and APX activity, increased electrolyte leakage | [140] |
| AgNP | Brassica nigra | 200, 400, 800, 1600 mg L−1 | Decreased soluble sugars, reduced sugars, inhibited seed germinating and seedling growth. Inhibited stress-responsive signaling pathways and key metabolic enzymes | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, M.M.; Li, G.; Nawaz, M.; Ahmed, T.; Noman, M.; Jalil, S.; Zheng, X.; Chen, X.; Du, D. Nano-Enabled Agrochemicals for Heavy Metal Remediation in Agriculture: Current Status, Mechanisms, and Future Prospects. Nanomaterials 2025, 15, 1588. https://doi.org/10.3390/nano15201588
Nazir MM, Li G, Nawaz M, Ahmed T, Noman M, Jalil S, Zheng X, Chen X, Du D. Nano-Enabled Agrochemicals for Heavy Metal Remediation in Agriculture: Current Status, Mechanisms, and Future Prospects. Nanomaterials. 2025; 15(20):1588. https://doi.org/10.3390/nano15201588
Chicago/Turabian StyleNazir, Muhammad Mudassir, Guanlin Li, Mohsin Nawaz, Temoor Ahmed, Muhammad Noman, Sanaullah Jalil, Xiaojun Zheng, Xunfeng Chen, and Daolin Du. 2025. "Nano-Enabled Agrochemicals for Heavy Metal Remediation in Agriculture: Current Status, Mechanisms, and Future Prospects" Nanomaterials 15, no. 20: 1588. https://doi.org/10.3390/nano15201588
APA StyleNazir, M. M., Li, G., Nawaz, M., Ahmed, T., Noman, M., Jalil, S., Zheng, X., Chen, X., & Du, D. (2025). Nano-Enabled Agrochemicals for Heavy Metal Remediation in Agriculture: Current Status, Mechanisms, and Future Prospects. Nanomaterials, 15(20), 1588. https://doi.org/10.3390/nano15201588











