Reusable BiOI-Modified CuWO4 Heterojunction Films and Their Excellent Photocatalytic Oxidation Activity of Nanoplastics and Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CuWO4 Films
2.2. Preparation of BiOI/CuWO4 Heterojunction Films
2.3. Characterization
2.4. Photocatalytic Activity Evaluation
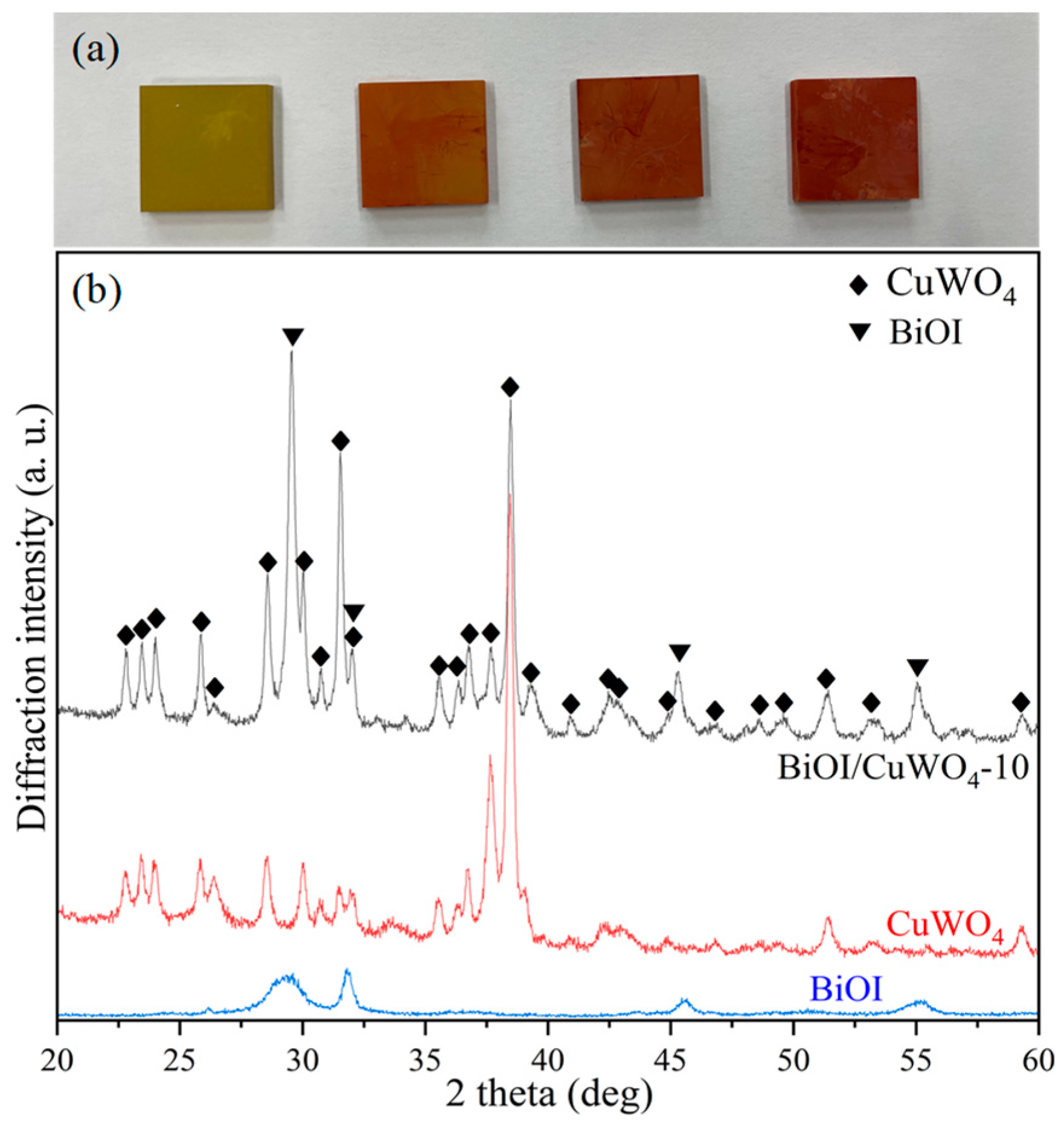
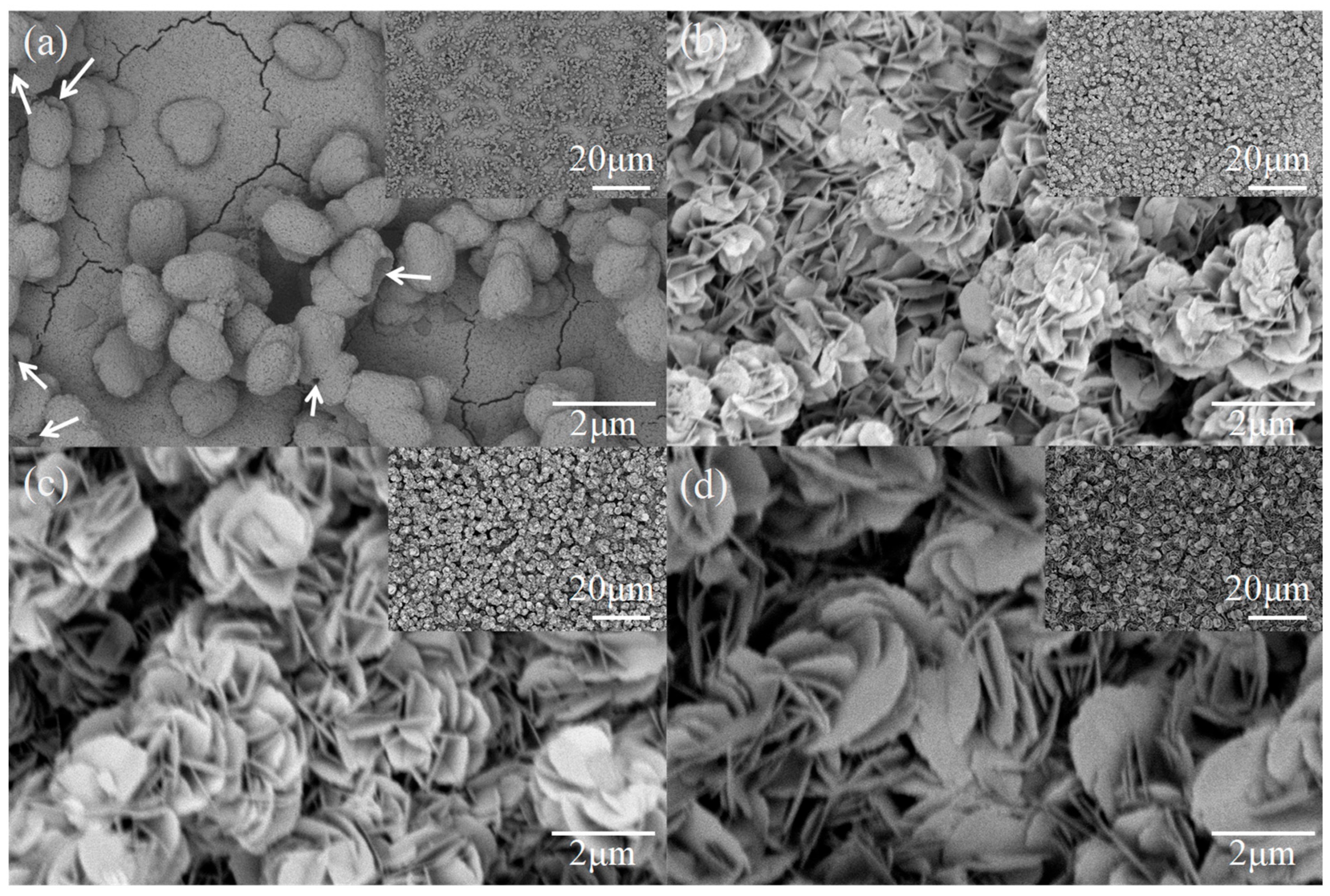
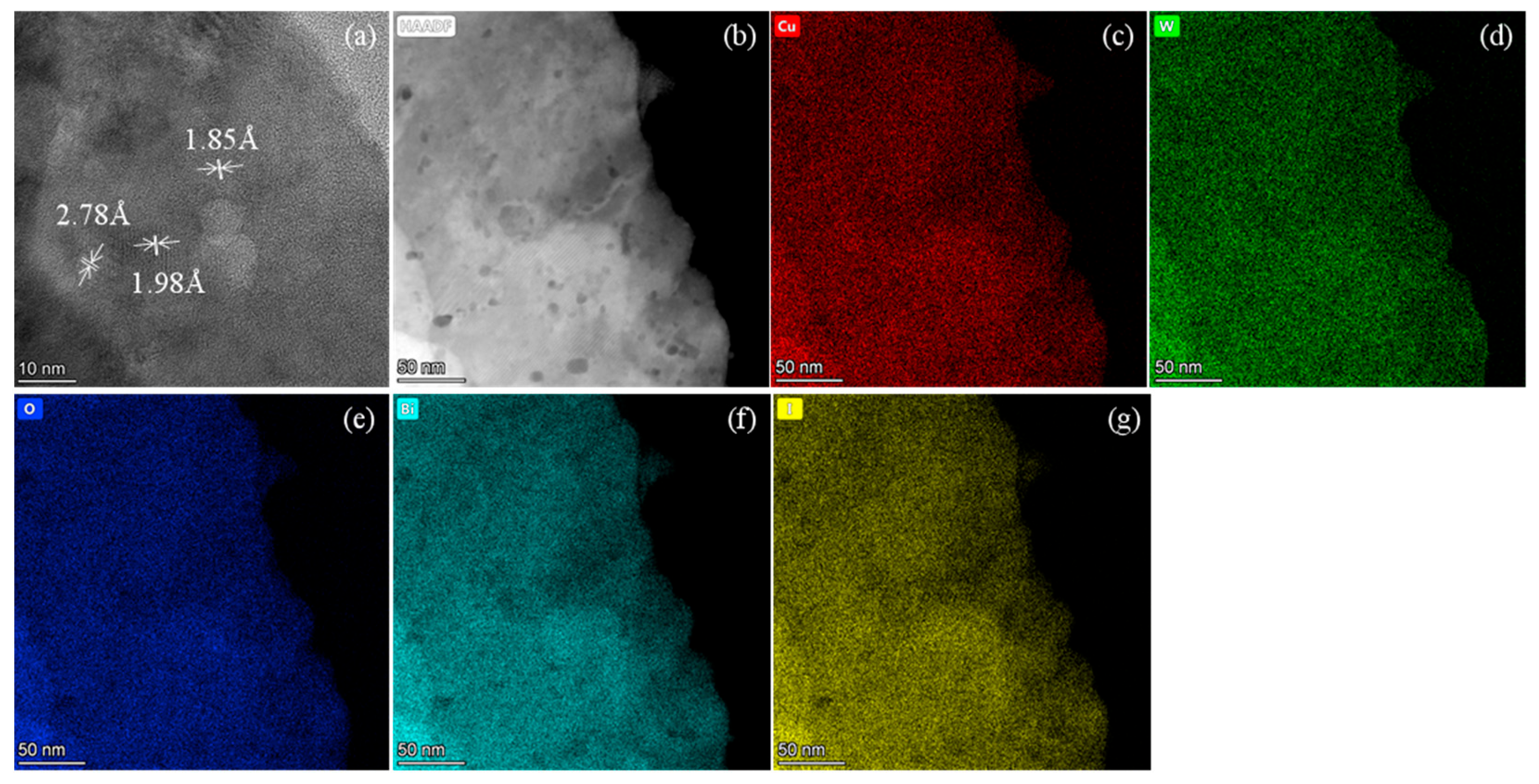
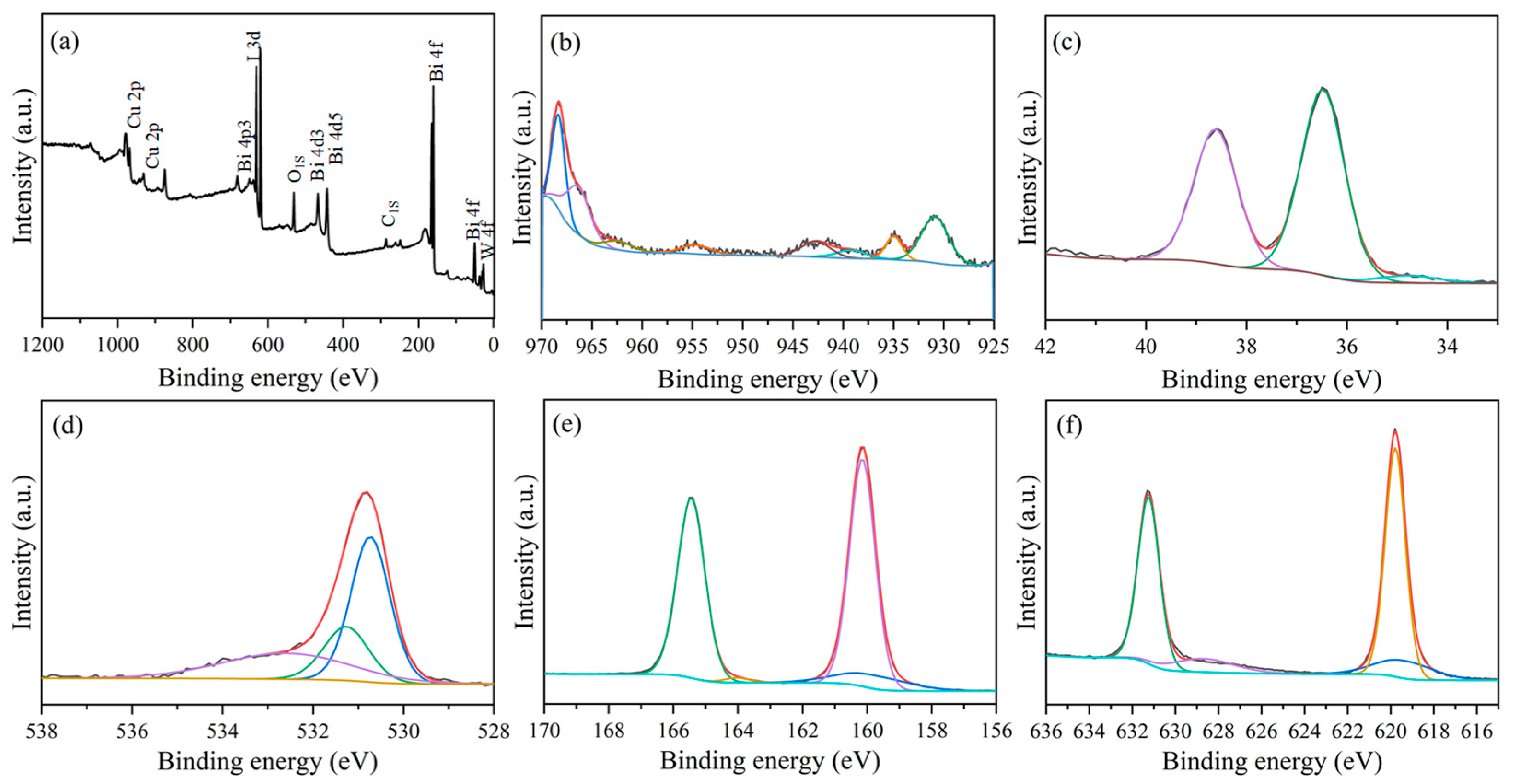
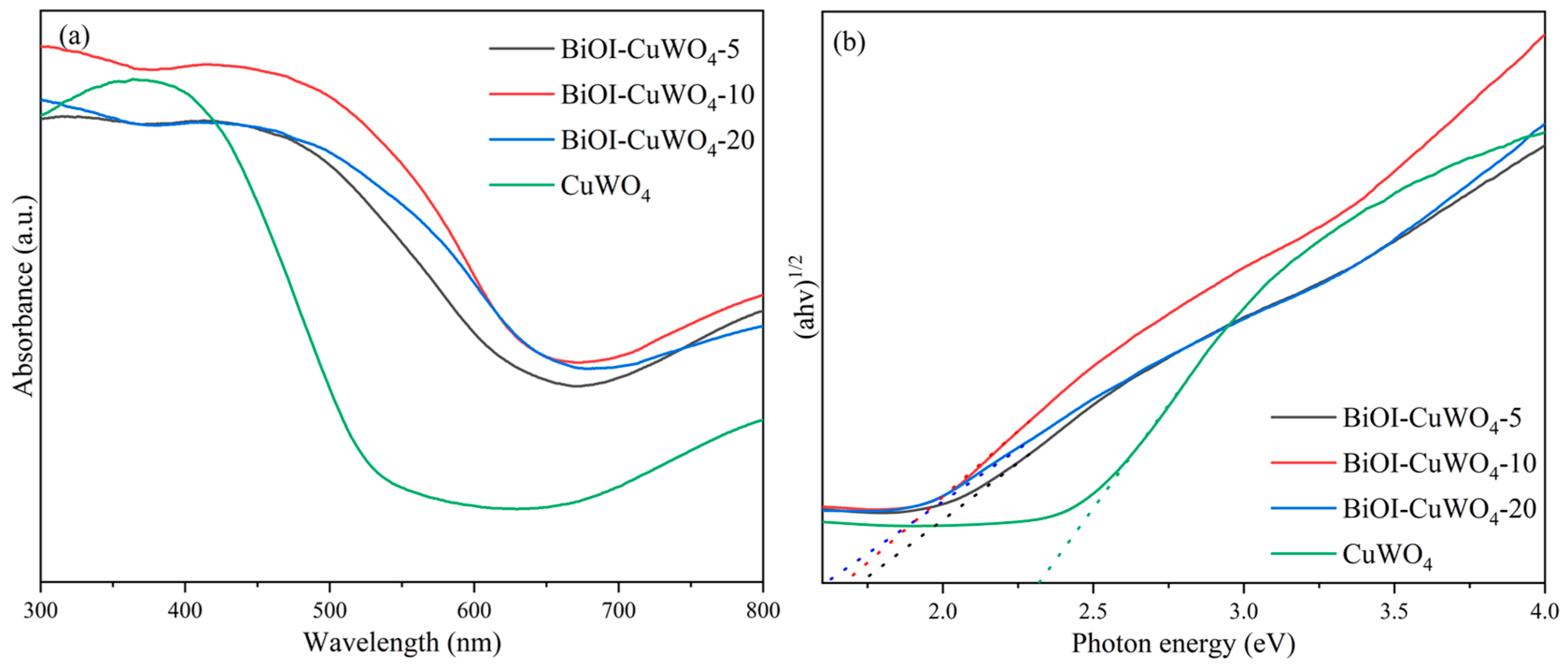
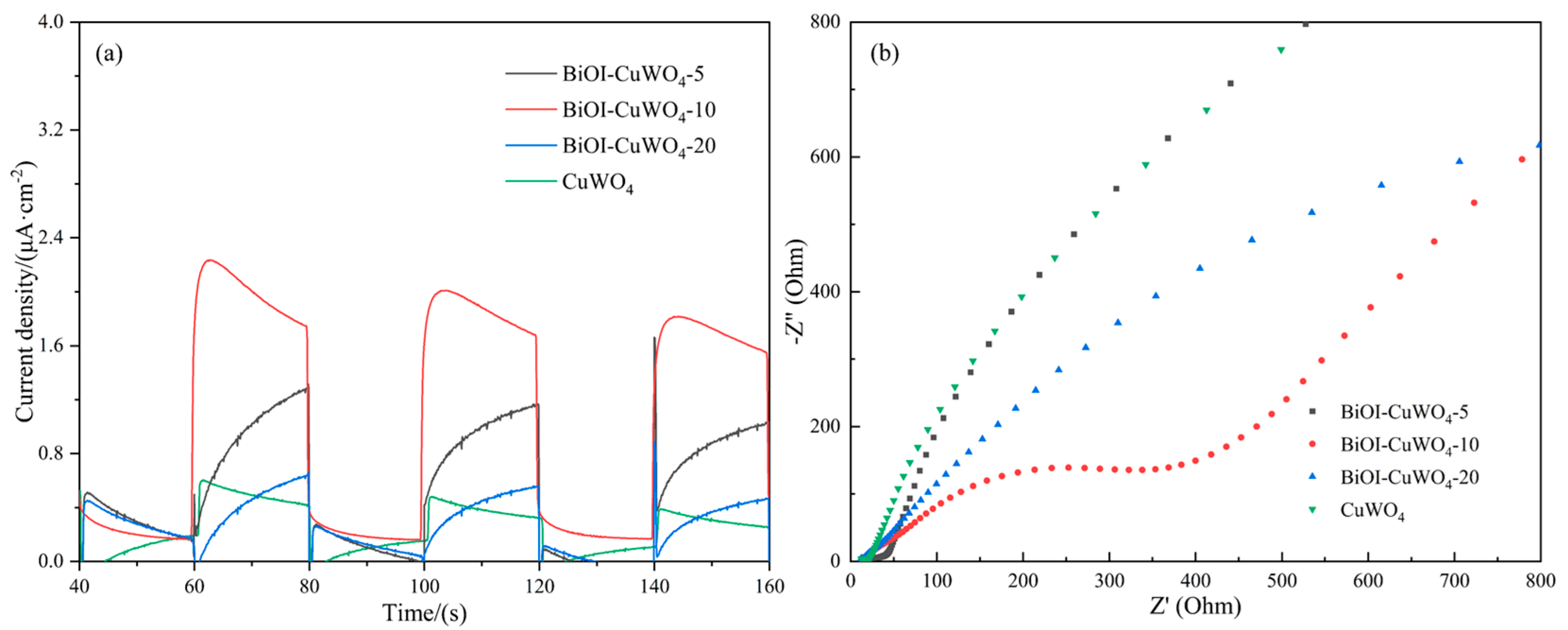
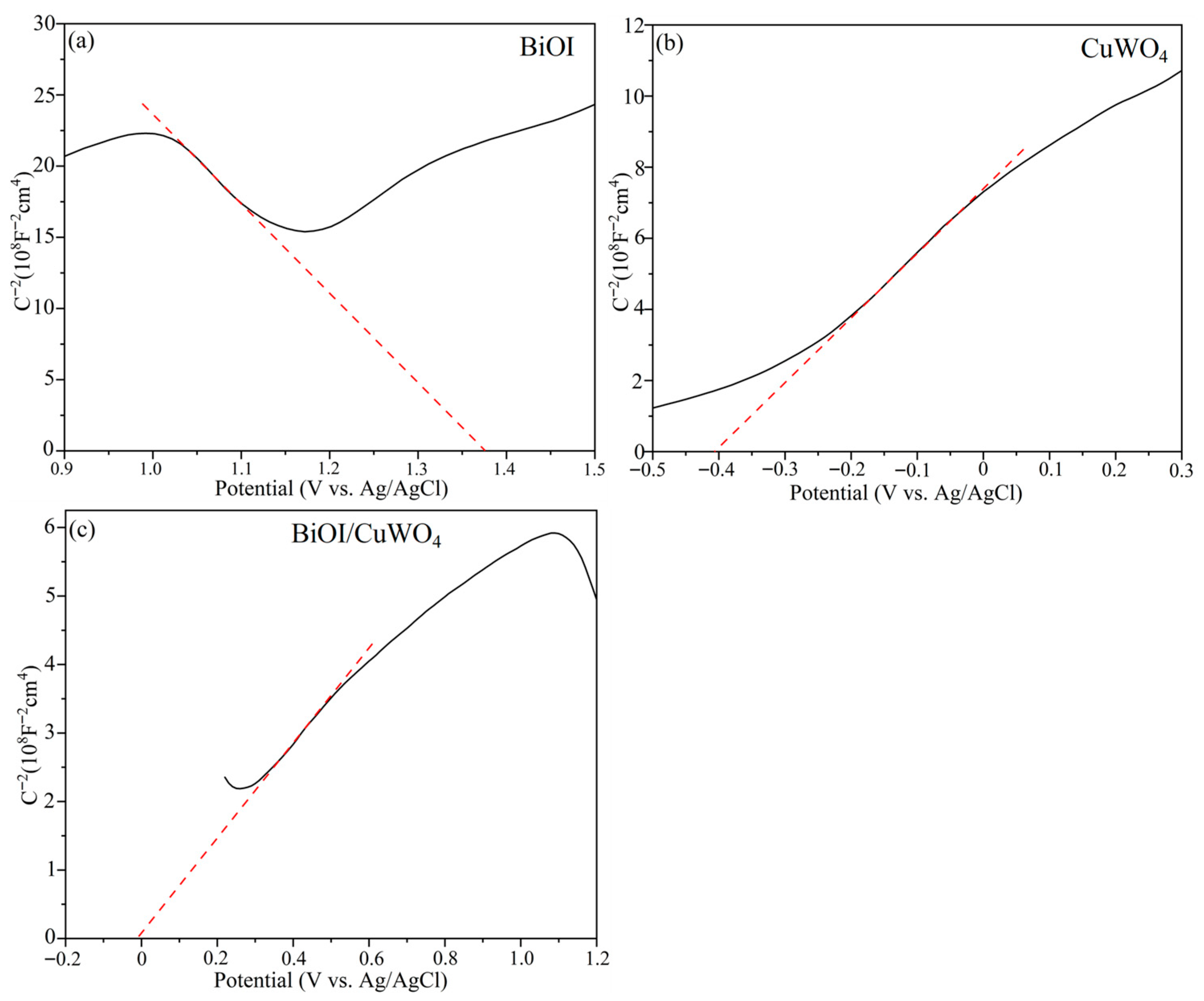
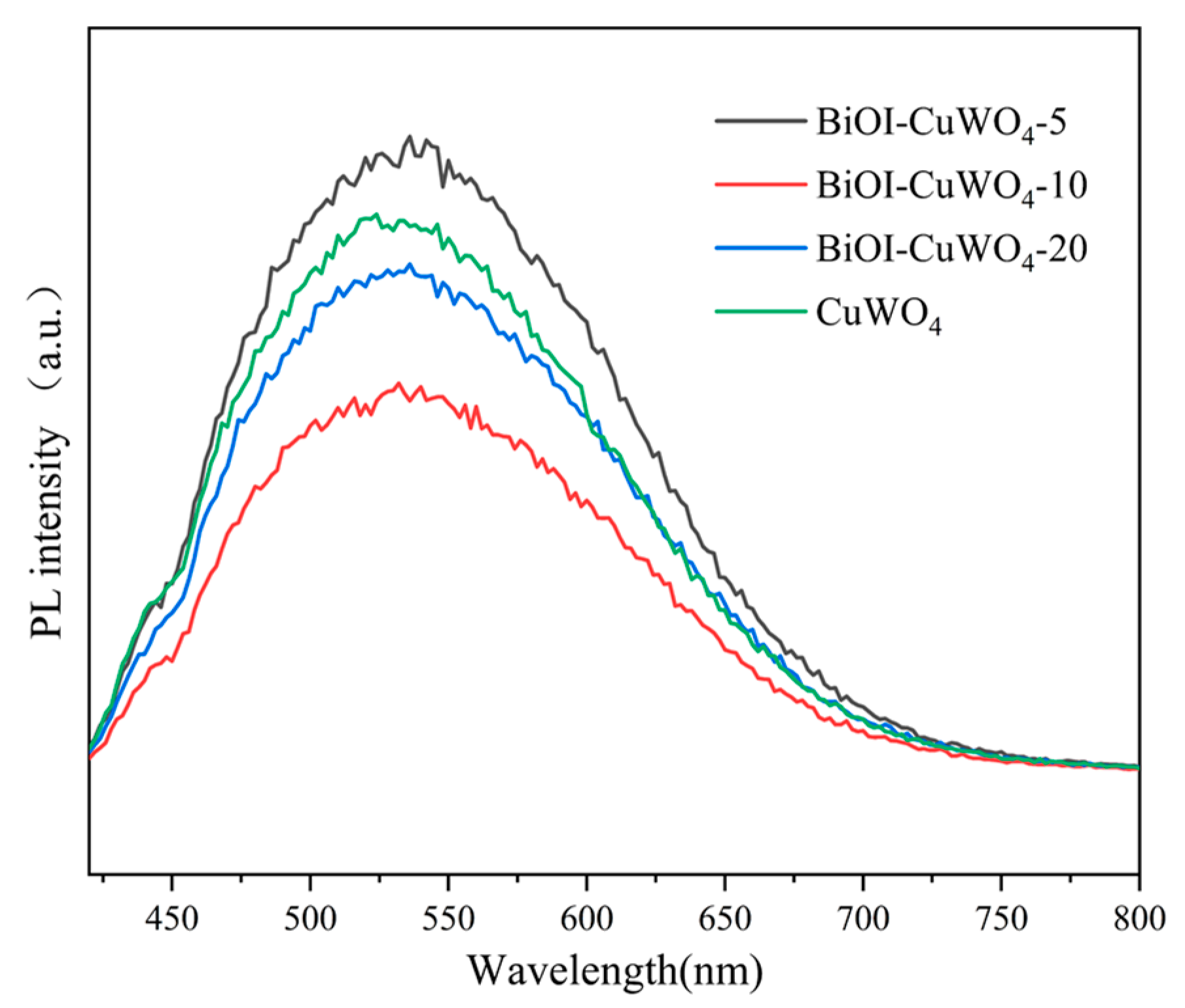
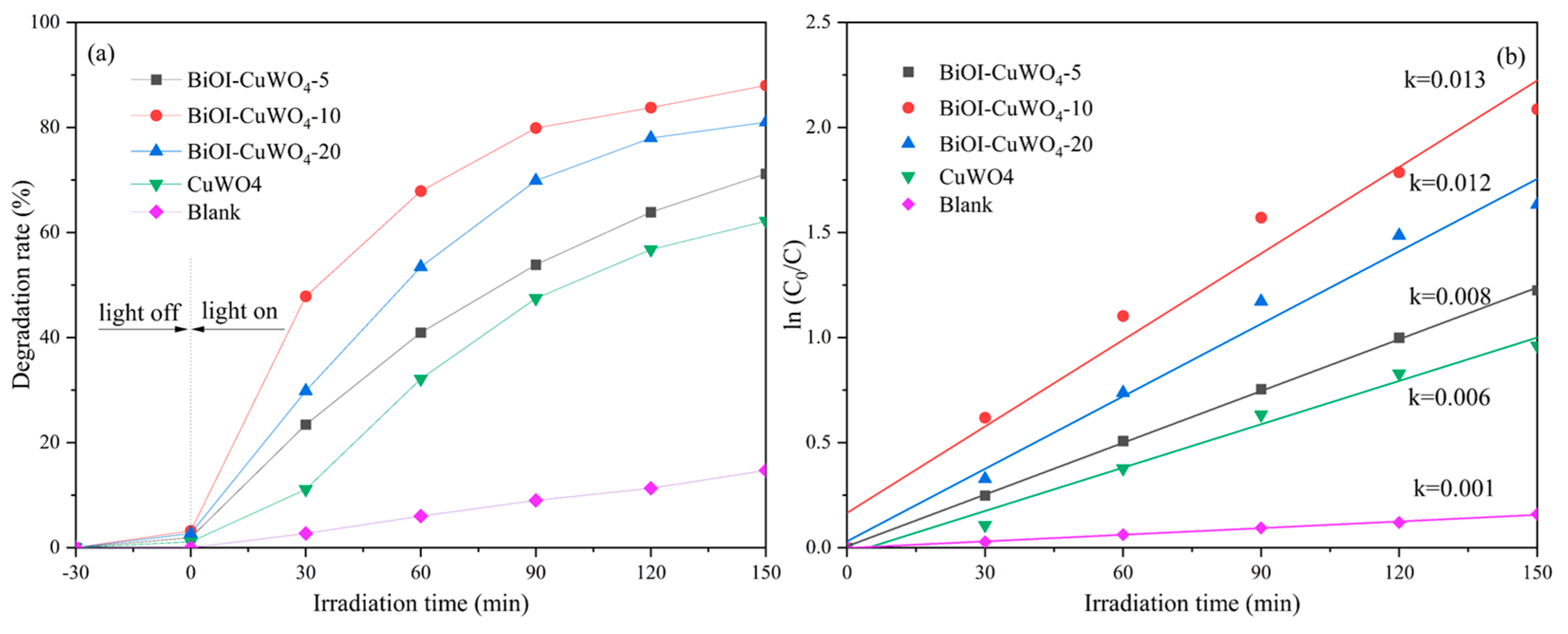
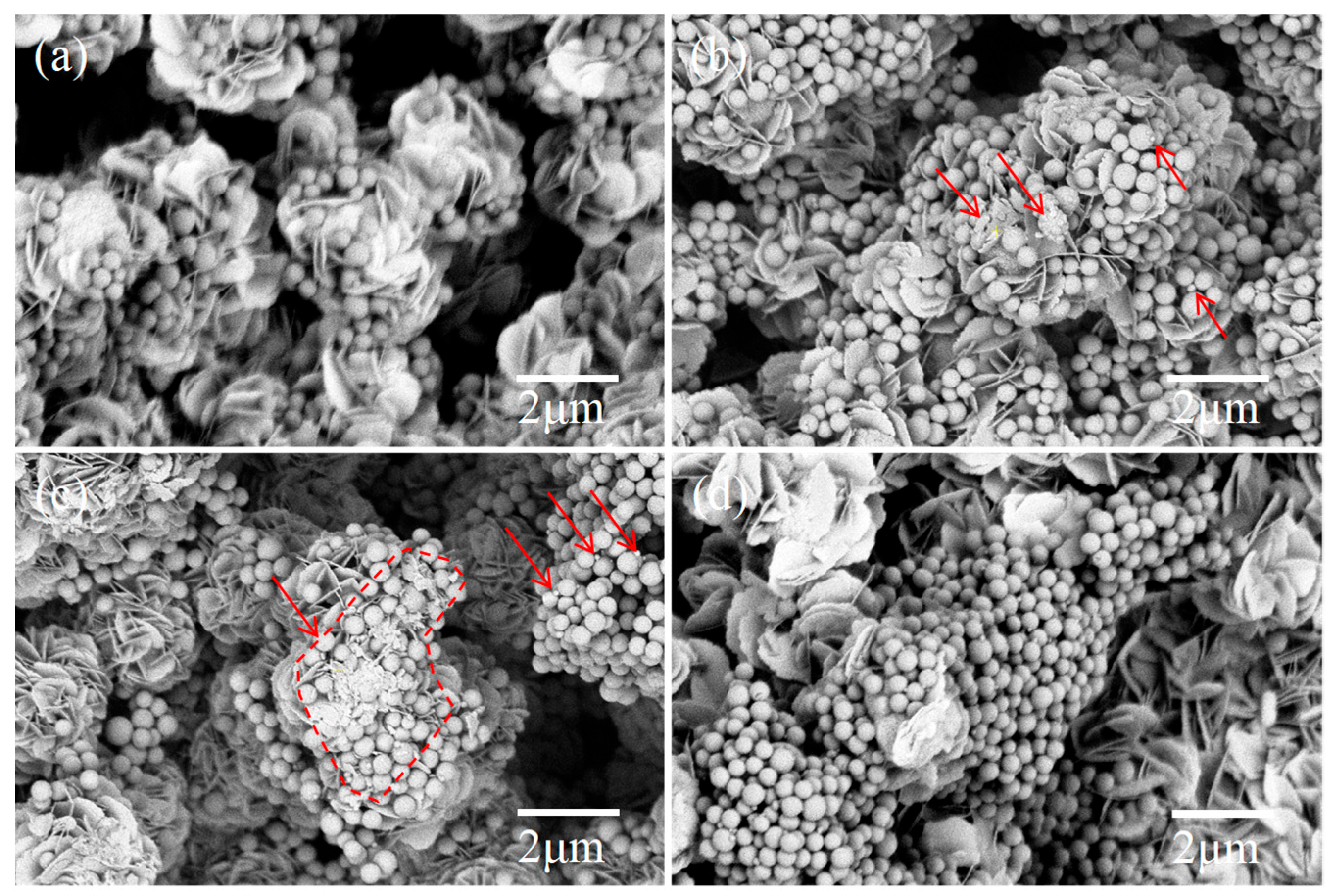
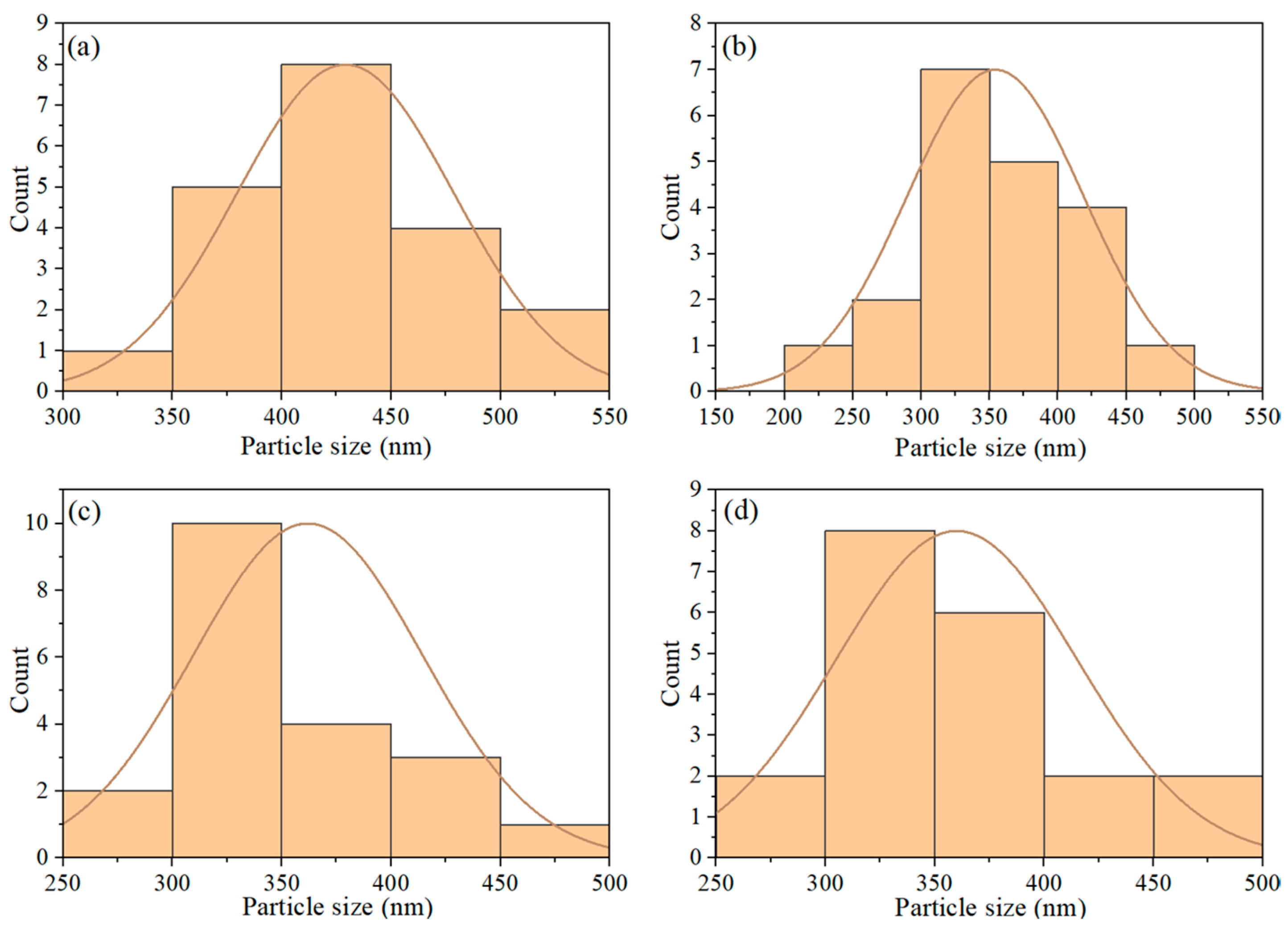
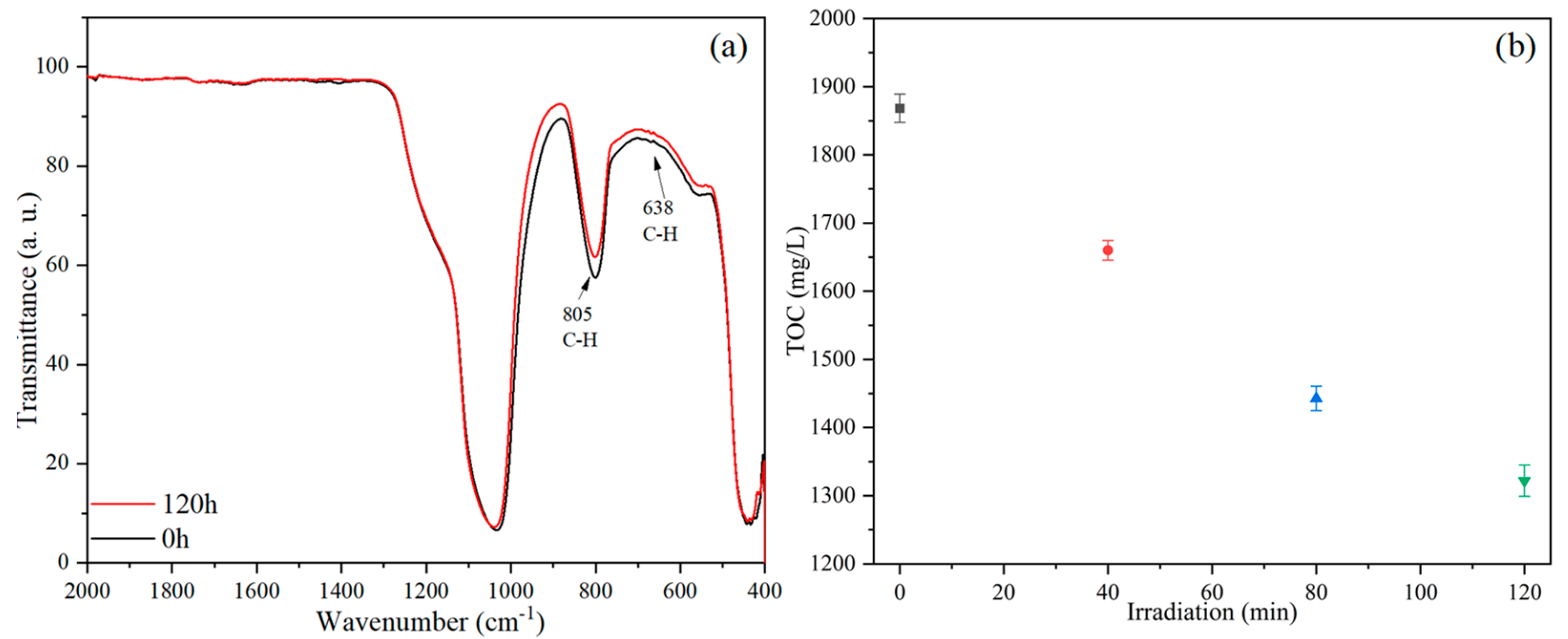
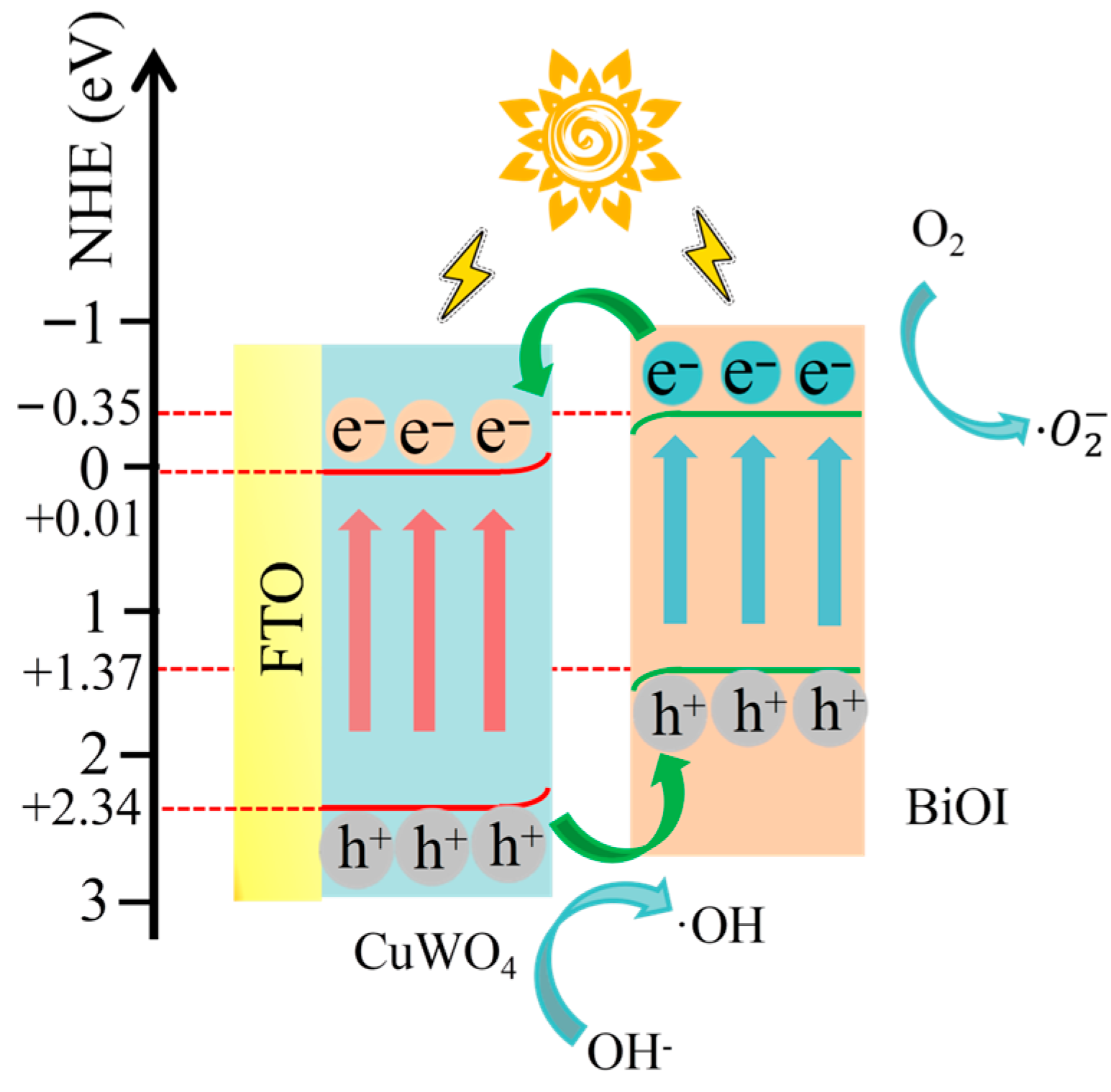
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, R.B.; Barros, F.A.; Sales, A.G.; Cruz-Filho, J.F.; Silva, R.M.; Caldeira, V.P.; Santos, A.G.; Santos, R.S.; Lima, A.E.; Luz, G.E., Jr. Enhanced photoelectrocatalytic performance of direct Z-scheme CuWO4|TiO2 heterojunction for moxifloxacin oxidation. Catal. Today 2025, 443, 114958. [Google Scholar] [CrossRef]
- Bai, X.; Fu, Z.; Ma, X.; Zhang, Z.; Fan, J.; Liu, E.; Li, J. Hydrophilic regulated photocatalytic converting phenol selectively over S-scheme CuWO4/TiO2. J. Clean. Prod. 2022, 369, 133099. [Google Scholar] [CrossRef]
- Chang, L.; Pu, Y.; Jing, P.; Ji, J.; Wei, X.; Cao, B.; Yu, Y.; Xu, S.; Xie, H. Z-Scheme CuWO4/BiOCl photocatalysts with oxygen vacancy as electron mediator for boosted photocatalytic degradation of norfloxacin. Surfaces Interfaces 2022, 31, 102010. [Google Scholar] [CrossRef]
- Shah, N.H.; Abbas, M.; Tariq, N.; Sulaman, M.; Imran, M.; Qasim, M.; Sandali, Y.; Cui, Y.; Wang, Y. Redeeming the photocatalytic potential of CuWO4 incorporating Ag6Si2O7 via S-scheme PN heterostructure. J. Alloy. Compd. 2024, 983, 173895. [Google Scholar] [CrossRef]
- Han, Y.; Qi, X.-X.; Liu, Y.-C.; Wang, Y.; Xiang, Z.; Wang, X. Preparation of CuWO4/Bi2WO6 composite and its sonocatalytic removal of tetracycline by combined persulfate. J. Alloy. Compd. 2025, 1010, 177505. [Google Scholar] [CrossRef]
- Rangappa, H.S.; Cho, P.P.; Dash, P.; Meena, B.; Ghosal, P.; Lin, C.; Madras, G.; Subrahmanyam, C. Visible light-induced degradation of tetracycline using CaFe2O4/CuWO4 composite and its mechanistic insights. Surfaces Interfaces 2025, 62, 106160. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Liu, X.; Li, J.; Liu, Y.; Liu, M.; Qiu, X.; Li, W. Gradient surficial forward Ni and interior reversed Mo-doped CuWO4 films for enhanced photoelectrochemical water splitting. Chem. Eng. J. 2023, 471, 144730. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, Z.; Song, Q.; Li, X.; Liu, Z. Improved photoelectrochemical response of CuWO4/BiOI p-n heterojunction embedded with plasmonic Ag nanoparticles. Chem. Eng. J. 2019, 370, 218–227. [Google Scholar] [CrossRef]
- Jatav, N.; Kuntail, J.; Khan, D.; De, A.K.; Sinha, I. AgI/CuWO4 Z-scheme photocatalyst for the degradation of organic pollutants: Experimental and molecular dynamics studies. J. Colloid Interface Sci. 2021, 599, 717–729. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Fan, L.; Xiong, X.; Zhu, C.; Wu, C.; Dai, G. Electrospun CuWO4 nanofibers for visible light photocatalysis. Mater. Lett. 2019, 251, 23–25. [Google Scholar] [CrossRef]
- Ding, W.; Wu, X.; Lu, Q. Structure and photocatalytic activity of thin-walled CuWO4 nanotubes: An experimental and DFT study. Mater. Lett. 2019, 253, 323–326. [Google Scholar] [CrossRef]
- ReddyPrasad, P.; Naidoo, E.B. Ultrasonic synthesis of high fluorescent C-dots and modified with CuWO4 nanocomposite for effective photocatalytic activity. J. Mol. Struct. 2015, 1098, 146–152. [Google Scholar] [CrossRef]
- Wang, C.; Gai, D.; Fan, J.; Lin, H.; Yuan, R.; Long, J.; Lin, Q. CuWO4 doped with Se for enhanced photocatalytic antibacterial activity. New J. Chem. 2025, 49, 10065–10079. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Diao, P. Molybdenum doped CuWO4 nanoflake array films as an efficient photoanode for solar water splitting. Electrochim. Acta 2019, 308, 195–205. [Google Scholar] [CrossRef]
- Salimi, R.; Alvani, A.A.S.; Mei, B.T.; Naseri, N.; Du, S.F.; Mul, G. Ag-functional CuWO4/WO3 nanocomposites for solar water splitting. New J. Chem. 2019, 43, 2196–2203. [Google Scholar] [CrossRef]
- Salimi, R.; Alvani, A.A.S.; Naseri, N.; Du, S.F.; Poelman, D. Visible-enhanced photocatalytic performance of CuWO4/WO3 hetero-structures: Incorporation of plasmonic Ag nanostructures. New J. Chem. 2018, 42, 11109–11116. [Google Scholar] [CrossRef]
- Chen, Z.A.; Yan, X.; Zhi, M. Reduced CuWO4 photocatalysts for photocatalytic non-oxidative coupling of methane reaction. Colloids Surfaces A 2024, 680, 132712. [Google Scholar] [CrossRef]
- Xiang, D.; Jin, X.; Sun, G.; Zhong, C.; Gao, S. Oxygen vacancy engineering of ultra-small CuWO4 nanoparticles for boosting photocatalytic organic pollutant degradation. Dalton Trans. 2024, 53, 7839–7847. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y. Photocatalytic organic degradation over W-rich and Cu-rich CuWO4 under UV and visible light. RSC Adv. 2015, 5, 8108–8113. [Google Scholar] [CrossRef]
- Aloni, P.; Venkatesan, P.; Sundaresan, A.P.; Roy, D.; Ranjan, R.K.; Sharma, A.; Doong, R.-A.; Selvam, N.C.S. Unveiling the impact of nitrogen-doped graphene quantum dots on improving the photocatalytic performance of CuWO4 nanocomposite. Appl. Surf. Sci. 2024, 686, 162130. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.-F.; Liao, P.-Y.; Zeng, Y.-S.; Wang, Z.; Liu, Z.-Q. Cascaded electron transition in CuWO4/CdS/CDs heterostructure accelerating charge separation towards enhanced photocatalytic activity. Chin. Chem. Lett. 2020, 31, 1516–1519. [Google Scholar] [CrossRef]
- Dashtian, K.; Ghaedi, M.; Shirinzadeh, H.; Hajati, S.; Shahbazi, S. Achieving enhanced blue-light-driven photocatalysis using nanosword-like VO2/CuWO4 type II n–n heterojunction. Chem. Eng. J. 2018, 339, 189–203. [Google Scholar] [CrossRef]
- Chen, H.; Leng, W.; Xu, Y. Enhanced visible-light photoactivity of CuWO4 through a surface-deposited CuO. J. Phys. Chem. C 2014, 118, 9982–9989. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Mousavi, M. Deposition of CuWO4 nanoparticles over g-C3N4/Fe3O4 nanocomposite: Novel magnetic photocatalysts with drastically enhanced performance under visible-light. Adv. Powder Technol. 2018, 29, 1379–1392. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, J.; Hou, K.; Zhu, Z.; Zheng, Y. Preparation of CuWO4@Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem. Eng. J. 2017, 307, 803–811. [Google Scholar] [CrossRef]
- Eghbali-Arani, M.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Pourmasoud, S. Ultrasound-assisted synthesis of YbVO4 nanostructure and YbVO4/CuWO4 nanocomposites for enhanced photocatalytic degradation of organic dyes under visible light. Ultrason. Sonochem. 2018, 43, 120–135. [Google Scholar] [CrossRef]
- Sagadevan, S.; Mathanmohun, M.; Le, M.-V.; Hessel, V. CuWO4-based nanocomposites as efficient photocatalysts for antibiotic degradation. Mater. Sci. Eng. B 2025, 315, 118102. [Google Scholar] [CrossRef]
- Moura, J.P.; Reis, R.Y.; Lima, A.E.; Santos, R.S.; Luz, G.E. Improved photoelectrocatalytic properties of ZnO/CuWO4 heterojunction film for RhB degradation. J. Photochem. Photobiol. A 2020, 401, 112778. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z. S-scheme CuWO4@g-C3N4 core-shell microsphere for CO2 photoreduction. Mater. Sci. Semicond. Process. 2023, 153, 107177. [Google Scholar] [CrossRef]
- Basyach, P.; Deb, J.; Sk, S.; Pal, U.; Gogoi, M.; Sastry, G.N.; Saikia, L. Controlled Ni doping on a g-C3N4/CuWO4 photocatalyst for improved hydrogen evolution. Phys. Chem. Chem. Phys. 2023, 25, 23033–23046. [Google Scholar] [CrossRef]
- Askari, N.; Beheshti, M.; Mowla, D.; Farhadian, M. Synthesis of CuWO4/Bi2S3 Z-scheme heterojunction with enhanced cephalexin photodegradation. J. Photochem. Photobiol. A 2020, 394, 112463. [Google Scholar] [CrossRef]
- Cui, Y.; Lin, C.; Li, M.; Zhu, N.; Meng, J.; Zhao, J. CuWO4/CuS heterojunction photocatalyst for the application of visible-light-driven photodegradation of dye pollutions. J. Alloy. Compd. 2022, 893, 162181. [Google Scholar] [CrossRef]
- Obiedallah, F.M.; Zeid, E.F.A.; Abu-Sehly, A.-H.; Aboraia, A.M.; El-Ghaffar, S.A.; El-Aal, M.A. Hydrothermal synthesis of CuWO4/Co3O4 nanocomposites for water remediation and antibacterial activity applications. Ceram. Int. 2025, 51, 13478–13492. [Google Scholar] [CrossRef]
- Chen, R.; Dou, X.; Xia, J.; Chen, Y.; Shi, H. Boosting peroxymonosulfate activation over Bi2MoO6/CuWO4 to rapidly degrade tetracycline: Intermediates and mechanism. Sep. Purif. Technol. 2022, 296, 121345. [Google Scholar] [CrossRef]
- Jatav, N.; Shrivastava, A.; De, A.K.; Sinha, I. Experimental and molecular dynamics investigations on Z-scheme visible light Ag3PO4/CuWO4 photocatalysts for antibiotic degradation. J. Environ. Chem. Eng. 2022, 10, 107975. [Google Scholar] [CrossRef]
- Luo, J.; Wu, Y.; Chen, X.; He, T.; Zeng, Y.; Wang, G.; Wang, Y.; Zhao, Y.; Chen, Z. Synergistic adsorption-photocatalytic activity using Z-scheme based magnetic ZnFe2O4/CuWO4 heterojunction for tetracycline removal. J. Alloy. Compd. 2022, 910, 164954. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, L.; Zhao, Q.; Guan, S.; Lu, Y. Fabrication and photocatalytic performance of BiOI-CuWO4 heterojunction flexible films. J. Mater. Sci. Mater. Electron. 2025, 36, 212. [Google Scholar] [CrossRef]
- Sun, Z.; Amrillah, T. Potential application of bismuth oxyiodide (BiOI) when it meets light. Nanoscale 2024, 16, 5079–5106. [Google Scholar] [CrossRef]
- Bharagav, U.; Reddy, N.R.; Rao, V.N.; Ravi, P.; Sathish, M.; Shankar, M.; Kumari, M.M. CuWO4 as a novel Z-scheme partner to construct TiO2 based stable and efficient heterojunction for photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 40391–40406. [Google Scholar] [CrossRef]
- Khan, M.; Ahmed, M.M.; Akhtar, M.N.; Sajid, M.; Riaz, N.N.; Asif, M.; Kashif, M.; Shabbir, B.; Ahmad, K.; Saeed, M.; et al. Fabrication of CuWO4@MIL-101(Fe) nanocomposite for efficient OER and photodegradation of methylene blue. Heliyon 2024, 10, e40546. [Google Scholar] [CrossRef] [PubMed]
- Radoń, A.; Ciuraszkiewicz, A.; Łukowiec, D.; Toroń, B.; Baran, T.; Kubacki, J.; Włodarczyk, P. From micro to subnano scale: Insights into the dielectric properties of BiOI nanoplates. Mater. Today Nano 2025, 31, 100649. [Google Scholar] [CrossRef]
- Bai, Y.; Bai, H.; Qu, K.; Wang, F.; Guan, P.; Xu, D.; Fan, W.; Shi, W. In-situ approach to fabricate BiOI photocathode with oxygen vacancies: Understanding the N2 reduced behavior in photoelectrochemical system. Chem. Eng. J. 2019, 362, 349–356. [Google Scholar] [CrossRef]
- Náfrádi, M.; Hernadi, K.; Kónya, Z.; Alapi, T. Investigation of the efficiency of BiOI/BiOCl composite photocatalysts using UV, cool and warm white LED light sources—Photon efficiency, toxicity, reusability, matrix effect, and energy consumption. Chemosphere 2021, 280, 130636. [Google Scholar] [CrossRef]
- Liang, L.; Liu, H.; Tian, Y.; Hao, Q.; Liu, C.; Wang, W.; Xie, X. Fabrication of novel CuWO4 hollow microsphere photocatalyst for dye degradation under visible-light irradiation. Mater. Lett. 2016, 182, 302–304. [Google Scholar] [CrossRef]
- Tang, Y.; Rong, N.; Liu, F.; Chu, M.; Dong, H.; Zhang, Y.; Xiao, P. Enhancement of the photoelectrochemical performance of CuWO4 films for water splitting by hydrogen treatment. Appl. Surf. Sci. 2016, 361, 133–140. [Google Scholar] [CrossRef]
- Tian, C.M.; Jiang, M.; Tang, D.; Qiao, L.; Xiao, H.Y.; Oropeza, F.E.; Hofmann, J.P.; Hensen, E.J.M.; Tadich, A.; Li, W.; et al. Elucidating the electronic structure of CuWO4 thin films for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2019, 7, 11895–11907. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Qiu, W.; He, G.; Wang, K.; Liu, Y.; Wu, Q.; Li, J. Oriented CuWO4 films for improved photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2022, 14, 47737–47746. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, J.; Li, W.; Liu, Y.; Xie, R.; Yang, Y.; Li, Y.; Chen, Q. In situ formation of CuWO4/WO3 heterojunction plates array films with enhanced photoelectrochemical properties. Int. J. Hydrogen Energy 2015, 40, 6512–6520. [Google Scholar] [CrossRef]
- Lv, C.; Cheng, H.; Fan, R.; Sun, J.; Liu, X.; Ji, Y. Fabrication of rGO/BiOI photocathode and its catalytic performance in the degradation of 4-Fluoroaniline. Heliyon 2024, 10, e37024. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.; Vargas, B.; Mejía, D.; Cortés-Lagunes, S.; Serrano-Lázaro, A.; Ovalle-Encinia, O.; Zanella, R.; Rodríguez, C. Synthesis of highly crystalline BiOI thin films for the photocatalytic removal of antibiotics in tap water and secondary effluents: Assessing the potential hazard of treated water. J. Environ. Chem. Eng. 2024, 12, 114590. [Google Scholar] [CrossRef]
- Velusamy, P.; Liu, S.; Xing, R.; Sathiya, M.; Ahmad, A.; Albaqami, M.D.; Alotabi, R.G.; Elamurugu, E.; Pandian, M.S.; Ramasamy, P. Enhanced photo-electrocatalytic performance of the nano heterostructures based on Pr3+ modified g-C3N4 and BiOI. Int. J. Hydrogen Energy 2022, 47, 32903–32920. [Google Scholar] [CrossRef]
- Kumar, U.; Shrivastava, A.; De, A.K.; Pai, M.R.; Sinha, I. Fenton reaction by H2O2 produced on a magnetically recyclable Ag/CuWO4/NiFe2O4 photocatalyst. Catal. Sci. Technol. 2023, 13, 2432. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, W.-J.; Wang, K.; Chang, L.; Yan, R.-Q.; Xiong, X.; Huang, G.-B.; Han, D.-M. Oxygen vacancy-mediated CuWO4/CuBi2O4 samples with efficient charge transfer for enhanced catalytic activity toward photodegradation of pharmacologically active compounds. Langmuir 2023, 39, 17830–17843. [Google Scholar] [CrossRef] [PubMed]
- Fazli, A.; Lauciello, S.; Brescia, R.; Carzino, R.; Athanassiou, A.; Fragouli, D. Synergistic degradation of polystyrene nanoplastics in water: Harnessing solar and water-driven energy through a Z-scheme SnO2/g-C3N4/PVDF-HFP piezo-photocatalytic system. Appl. Catal. B Environ. Energy 2024, 353, 124056. [Google Scholar] [CrossRef]
- Acuña-Bedoya, J.D.; Luévano-Hipólito, E.; Cedillo-González, E.I.; Domínguez-Jaimes, L.P.; Hurtado, A.M.; Hernández-López, J.M. Boosting visible-light photocatalytic degradation of polystyrene nanoplastics with immobilized CuxO obtained by anodization. J. Environ. Chem. Eng. 2021, 9, 106208. [Google Scholar] [CrossRef]
- Budnikova, Y.; Vasilyeva, M.; Lukiyanchuk, I.; Egorkin, V.; Tkachev, V.; Korochentsev, V.; Shlyk, D.; Arefieva, O.; Myagchilov, A. Plasma electrolytic preparation of film CoWO4/WO3 p-n heterostructures and their photocatalytic and electrochemical properties. J. Photochem. Photobiol. A 2025, 467, 116414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Hao, L.; Zhao, X.; Guan, S.; Lu, Y. Reusable BiOI-Modified CuWO4 Heterojunction Films and Their Excellent Photocatalytic Oxidation Activity of Nanoplastics and Methylene Blue. Nanomaterials 2025, 15, 1579. https://doi.org/10.3390/nano15201579
Hu T, Hao L, Zhao X, Guan S, Lu Y. Reusable BiOI-Modified CuWO4 Heterojunction Films and Their Excellent Photocatalytic Oxidation Activity of Nanoplastics and Methylene Blue. Nanomaterials. 2025; 15(20):1579. https://doi.org/10.3390/nano15201579
Chicago/Turabian StyleHu, Te, Liang Hao, Xiaohui Zhao, Sujun Guan, and Yun Lu. 2025. "Reusable BiOI-Modified CuWO4 Heterojunction Films and Their Excellent Photocatalytic Oxidation Activity of Nanoplastics and Methylene Blue" Nanomaterials 15, no. 20: 1579. https://doi.org/10.3390/nano15201579
APA StyleHu, T., Hao, L., Zhao, X., Guan, S., & Lu, Y. (2025). Reusable BiOI-Modified CuWO4 Heterojunction Films and Their Excellent Photocatalytic Oxidation Activity of Nanoplastics and Methylene Blue. Nanomaterials, 15(20), 1579. https://doi.org/10.3390/nano15201579






