Enhancing Structural and Interfacial Stability of NaNi1/3Mn1/3Fe1/3O2 Cathodes via Sb3+ Doping for Sodium Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization and Electrochemical Analysis
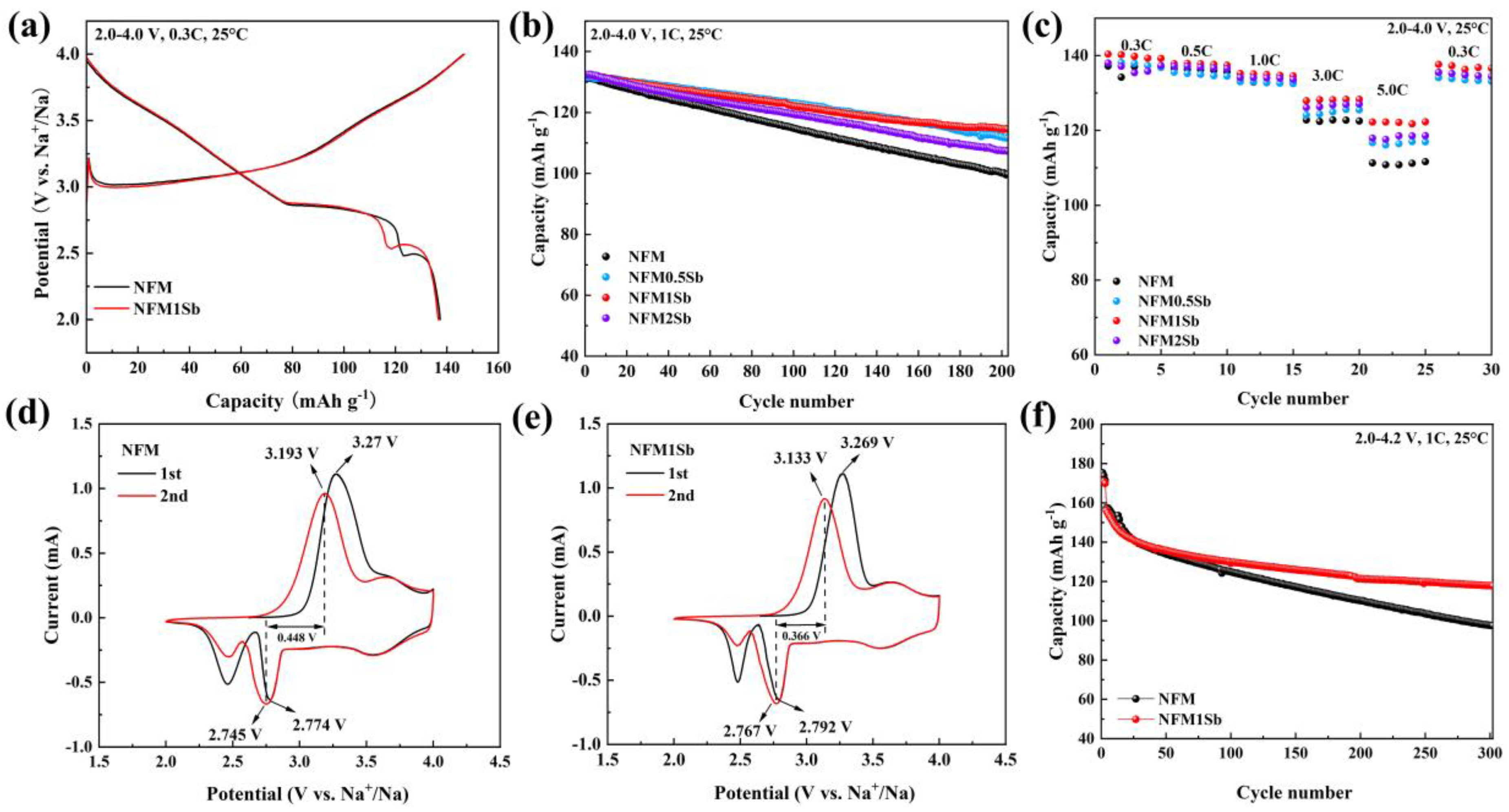
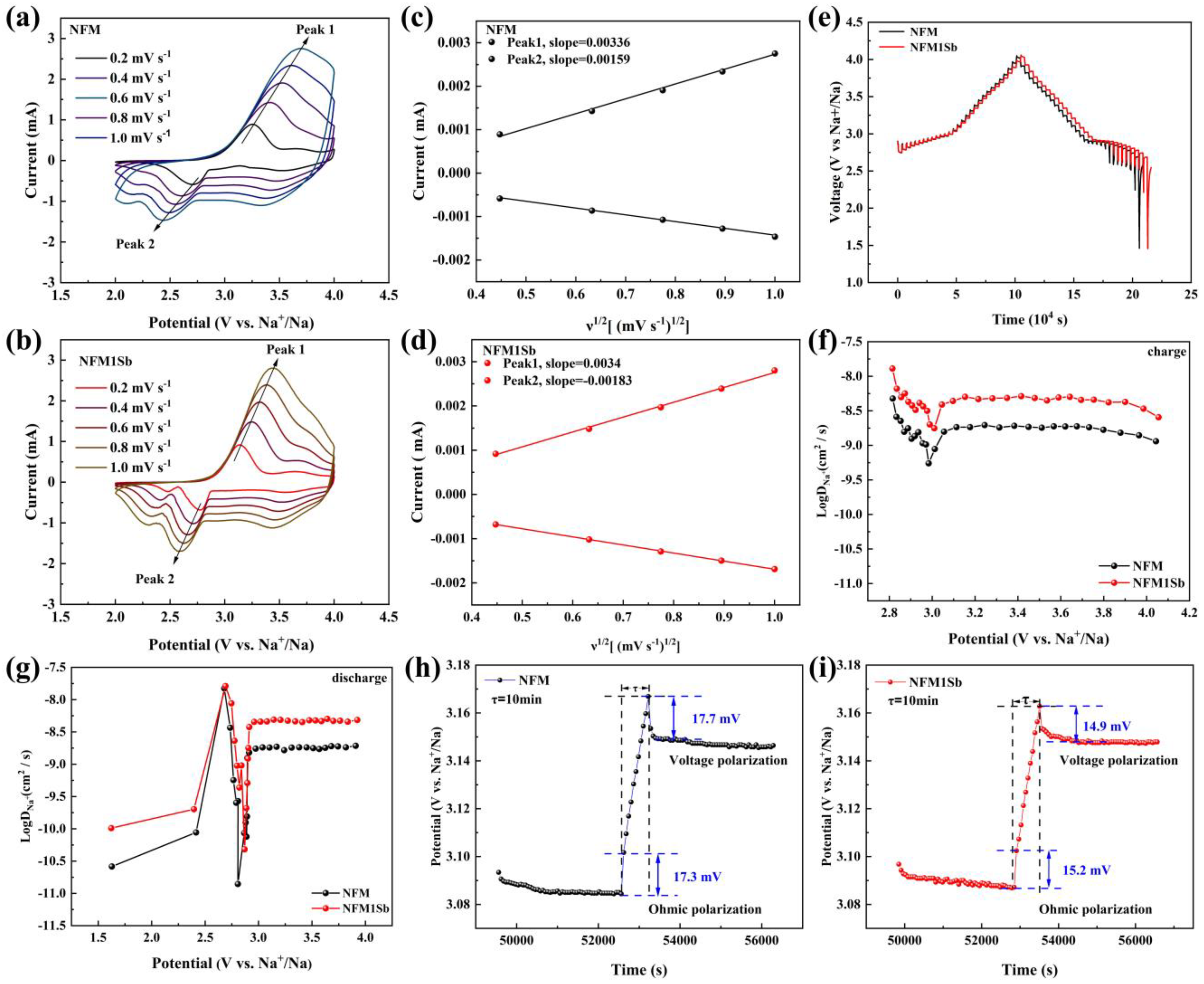
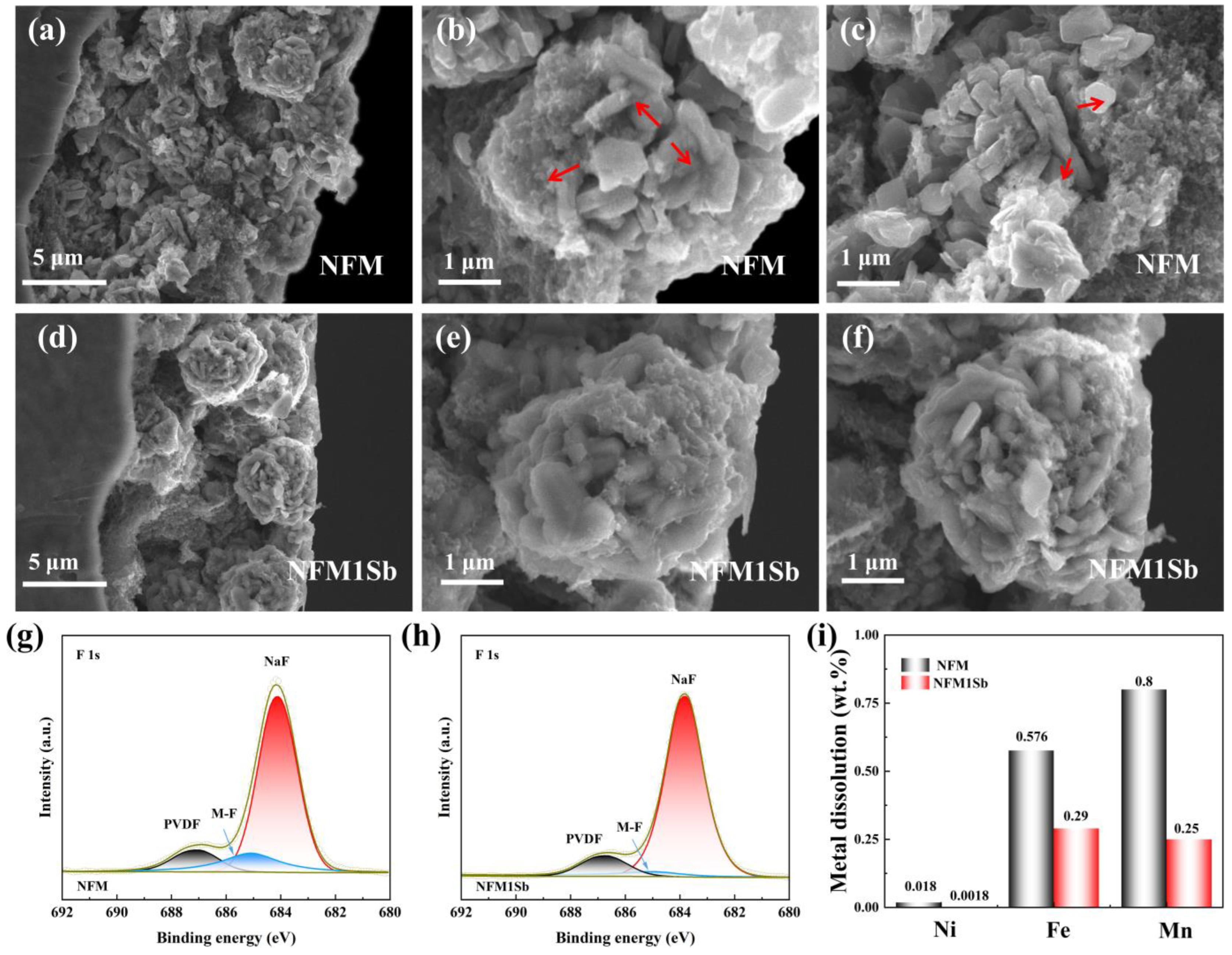
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, S.; Zhu, L.; Fu, L.; Wang, M.; Zhang, X.; Tang, Y.; Sun, D.; Wang, H. Cryogenic and in situ characterization techniques for electrode interphase analysis. eScience 2024, 5, 100291. [Google Scholar]
- Fang, H.; Gao, S.; Ren, M.; Huang, Y.; Cheng, F.; Chen, J.; Li, F. Dual-Function Presodiation with Sodium Diphenyl Ketone towards Ultra-stable Hard Carbon Anodes for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2022, 62, e202214717. [Google Scholar]
- Guo, Y.-J.; Jin, R.-X.; Fan, M.; Wang, W.-P.; Xin, S.; Wan, L.-J.; Guo, Y.-G. Sodium layered oxide cathodes: Properties, practicality and prospects. Chem. Soc. Rev. 2024, 53, 7828–7874. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Yao, Z.; Wang, J.; Sanchez, B.; Ding, F.; Qi, X.; Lu, Y.; Bai, X.; Li, B.; et al. Rational design of layered oxide materials for sodium-ion batteries. Science 2020, 370, 708–711. [Google Scholar] [CrossRef]
- Xie, C.; Wu, H.; Kang, L.; Ding, Z.; Jiawen, D.; Zhang, R.; Zhang, Q.; Sun, D.; Ren, Y.; Li, Y.; et al. Microalloying induced stable welded interfaces for highly reversible zero-excess sodium metal batteries. Energy Environ. Sci. 2024, 17, 4228–4237. [Google Scholar] [CrossRef]
- Liang, X.; Hwang, J.Y.; Sun, Y.-K. Practical Cathodes for Sodium-Ion Batteries: Who Will Take The Crown? Adv. Energy Mater. 2023, 13, 2301975. [Google Scholar]
- Li, C. Research of Cathode Materials for Sodium-Ion Batteries. Highlights Sci. Eng. Technol. 2024, 116, 283–289. [Google Scholar] [CrossRef]
- Zhao, Q.; Dou, S.; Liu, H.-K.; Hu, H.; Zhou, L.; Chen, M. Recent Progress and Advances of High-entropy Polyanionic Cathodes in Lithium-ion and Sodium-ion batteries. Chem. Commun. 2025, 61, 12419–12430. [Google Scholar]
- Xiang, J.; Wei, Y.; Zhong, Y.; Yang, Y.; Cheng, H.; Yuan, L.; Xu, H.; Huang, Y. Building Practical High-Voltage Cathode Materials for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2200912. [Google Scholar]
- Zhu, L.; Xiang, S.; Wang, M.; Sun, D.; Huang, X.; Li, Y.; Tang, Y.; Peng, Z.; Zhang, Q.; Wang, H. Heterogeneous NASICON-Type Cathode With Reversible Multielectron Reaction for High-Performance Sodium-Ion Batteries. Adv. Mater. 2024, 37, 2408918. [Google Scholar]
- Zhu, L.; Wang, M.; Xiang, S.; Fu, L.; Sun, D.; Huang, X.; Li, Y.; Tang, Y.; Zhang, Q.; Wang, H. Exceeding Three-Electron Reactions in Polyanionic Cathode To Achieve High-Energy Density for Sodium-Ion Batteries. ACS Nano 2024, 18, 13073–13083. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.; Popovic-Neuber, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, M.; Qin, L.; Chen, M.; Chen, Z.; Guo, S.; Wang, L.; Guozhao, F.; Liang, S. Simultaneous regulation of cations and anions in an electrolyte for high-capacity, high-stability aqueous zinc–vanadium batteries. eScience 2022, 2, 209–218. [Google Scholar] [CrossRef]
- Gonzalo, E.; Zarrabeitia, M.; Drewett, N.; del Amo, J.M.; Rojo, T. Sodium manganese-rich layered oxides: Potential candidates as positive electrode for Sodium-ion batteries. Energy Storage Mater. 2020, 34, 682–707. [Google Scholar] [CrossRef]
- Pfeiffer, L.; Axmann, P.; Wohlfahrt-Mehrens, M. NaxMnyNi1-YO2 Cathode Materials for Sodium-Ion Batteries: Structure, Synthesis, Electrochemistry and Influence of Ambient Storage. ECS Meet. Abstr. 2022, MA2022-02, 448. [Google Scholar] [CrossRef]
- Deng, C.; Xu, J.; Tong, W.; Liu, Y.; Hunt, R.; Skinner, P.; Xiong, H. Understanding the Effect of Li Substitution in NaNi0.4Fe0.2Mn0.4O2 Cathode Material for Sodium Ion Batteries. ECS Meet. Abstr. 2017, MA2017-02, 212. [Google Scholar] [CrossRef]
- Yuan, X.G.; Guo, Y.-J.; Gan, L.; Yang, X.A.; He, W.H.; Zhang, X.S.; Yin, Y.X.; Xin, S.; Yao, H.R.; Huang, Z.; et al. A Universal Strategy toward Air-Stable and High-Rate O3 Layered Oxide Cathodes for Na-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2111466. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, Z.; Wang, Q.; Li, H.; Wang, J.; Liu, M.; Ganapathy, S.; Lu, Y.; Cabana, J.; Li, B.; et al. Revealing High Na-Content P2-Type Layered Oxides as Advanced Sodium-Ion Cathodes. J. Am. Chem. Soc. 2020, 142, 5742–5750. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Zou, P.; Lin, R.; Ma, L.; Yin, L.; Li, T.; Xu, W.; Jia, H.; Li, Q.; et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 2022, 610, 67–73. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, M.; Huang, Y.; Li, F.; Hua, W.; Indris, S.; Li, F. Negative Lattice Expansion in an O3-Type Transition-Metal Oxide Cathode for Highly Stable Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202316949. [Google Scholar] [CrossRef]
- Zhao, S.; Ning, F.; Yu, X.; Guo, B.; Teófilo, R.; Huang, J.; Shi, Q.; Wu, S.; Feng, W.; Zhao, Y. Inhomogeneous Coordination in High-Entropy O3-Type Cathodes Enables Suppressed Slab Gliding and Durable Sodium Storage. Angew. Chem. Int. Ed. 2024, 64, e202416290. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, Y.; Zhao, X.; Hu, Y.; Li, T.; Liu, H.; Zhong, Y.; Jiao, L.; Liu, Y.; Chen, J. Intrinsic Distortion against Jahn-Teller Distortion: A New Paradigm for High-Stability Na-Ion Layered Mn-Rich Oxide Cathodes. Angew. Chem. Int. Ed. 2025, 64, e202423728. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Mariyappan, S.; Ramesha, K.; Abakumov, A.; Rousse, G.; Gonbeau, D.; Doublet, M.-L.; Annigere, P.; Tendeloo, G. Invited Presentation: Present Understanding of the High Capacity Layered Oxide Electrodes. ECS Meet. Abstr. 2014, MA2014-04, 103. [Google Scholar] [CrossRef]
- Li, M.; Zhuo, H.; Lei, J.; Guo, Y.; Yuan, Y.; Wang, K.; Liao, Z.; Xia, W.; Geng, D.; Sun, X.; et al. Unravelling the structure-stability interplay of O3-type layered sodium cathode materials via precision spacing engineering. Nat. Commun. 2025, 16, 2010. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Yao, L.; Wang, Y.; Jiang, N.; Liu, Y. Anion/Cation Solvation Engineering for a Ternary Low-Concentration Electrolyte toward High-Voltage and Long-Life Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2315007. [Google Scholar]
- Kuang, J.; Liu, Z.; Fu, L.; Shi, Y.; Zhang, M.; Wang, Y.; Ding, N.; Sun, D.; Tang, Y.; Wang, H. Charge Tuning and Anchor Effect Achieving Stable High-Voltage Layered Metal Oxides for Sodium-Ion Battery. Angew. Chem. Int. Ed. 2025, 64, e202500715. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, Z.; Gan, Q.; Mai, Y.W. A review on the ionic conductivity and mechanical properties of composite polymer electrolytes for lithium batteries: Insights from the perspective of polymer/filler composites. Mater. Sci. Eng. R Rep. 2024, 160, 100815. [Google Scholar] [CrossRef]
- Wang, X.; Yin, L.; Ronne, A.; Zhang, Y.; Hu, Z.; Tan, S.; Wang, Q.; Song, B.; Li, M.; Rong, X.; et al. Stabilizing lattice oxygen redox in layered sodium transition metal oxide through spin singlet state. Nat. Commun. 2023, 14, 7665. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jiang, P.; Wang, S.; Chen, W.; Wei, B.; Lu, X.; Qian, G.; Kan, W.; Chen, H.; Yin, W.; et al. Slight compositional variation-induced structural disorder-to-order transition enables fast Na+ storage in layered transition metal oxides. Nat. Commun. 2022, 13, 7888. [Google Scholar] [CrossRef]
- Yang, T.; Yin, Z. Probing the Structure Evolution of Na-Cu-Mn-O Based Layered Oxide Cathode Materials in Sodium Ion Batteries. ECS Meet. Abstr. 2023, MA2023-02, 3108. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Zou, C.; Jin, H.; Wang, S.; Chou, S.-L.; Liu, Y.; Dou, S.X. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar]
- Zuo, W.; Qiu, J.; Liu, X.; Ren, F.; Liu, H.; He, H.; Luo, C.; Li, J.; Ortiz, G.; Duan, H.; et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres. Nat. Commun. 2020, 11, 3544. [Google Scholar] [CrossRef]
- Zuo, W.; Xu, G.-L.; Amine, K. The Air Stability of Sodium Layered Oxide Cathodes. ECS Meet. Abstr. 2022, MA2022-02, 2594. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Shi, Y.; Liu, H.; Sun, D.; Huang, X.; Ding, N.; Xu, Y.; Tang, Y.; Wang, H.-Y. One-step Dual Modification Strategy for Enhancing Sodium Storage in O3-type NaNi1/3Fe1/3Mn1/3O2 Cathodes. J. Mater. Chem. A 2025, 13, 20737–20745. [Google Scholar] [CrossRef]
- Sundaram, M.; Appadoo, D. Traditional salt-in-water electrolyte vs. water-in-salt electrolyte with binary metal oxide for symmetric supercapacitors: Capacitive vs. Faradaic. Dalton Trans. 2020, 49, 11743–11755. [Google Scholar] [CrossRef] [PubMed]
- Weppner, W.; Huggins, R. Electrochemical investigation of the chemical diffusion, partial ionic conductivities, and other kinetic parameters in Li3Sb and Li3Bi. J. Solid State Chem. 1977, 22, 297–308. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, X.; Weng, J.; Wang, L.; Wu, X.; Miao, Z.; Zhao, J.; Zhou, J.; Zhuo, S. Synthesis, Structure, and Electrochemical Properties of O’3-Type Monoclinic NaNi0.8Co0.15Al0.05O2 Cathode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2018, 7, 657–663. [Google Scholar] [CrossRef]
- Guo, J.; Feng, F.; Jiang, X.; Wang, R.; Chu, D.; Ren, Y.; Chen, F.; He, P.; Ma, Z.-F.; Chen, S.; et al. Boosting Selective Na Migration Kinetics in Structuring Composite Polymer Electrolyte Realizes Ultrastable All-Solid-State Sodium Batteries. Adv. Funct. Mater. 2024, 34, 2313496. [Google Scholar] [CrossRef]
- Yoon, M.; Dong, Y.; Huang, Y.; Wang, B.; Kim, J.; Park, J.-S.; Hwang, J.; Park, J.; Cho, J.; Li, J. Eutectic salt-assisted planetary centrifugal deagglomeration for single-crystalline cathode synthesis. Nat. Energy 2023, 8, 482–491. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shi, Y.; Zhang, M.; Sun, D.; Li, H.; Wang, H.; Tang, Y. Enhancing Structural and Interfacial Stability of NaNi1/3Mn1/3Fe1/3O2 Cathodes via Sb3+ Doping for Sodium Ion Batteries. Nanomaterials 2025, 15, 1575. https://doi.org/10.3390/nano15201575
Liu Y, Shi Y, Zhang M, Sun D, Li H, Wang H, Tang Y. Enhancing Structural and Interfacial Stability of NaNi1/3Mn1/3Fe1/3O2 Cathodes via Sb3+ Doping for Sodium Ion Batteries. Nanomaterials. 2025; 15(20):1575. https://doi.org/10.3390/nano15201575
Chicago/Turabian StyleLiu, Yong, You Shi, Mengjie Zhang, Dan Sun, Huanhuan Li, Haiyan Wang, and Yougen Tang. 2025. "Enhancing Structural and Interfacial Stability of NaNi1/3Mn1/3Fe1/3O2 Cathodes via Sb3+ Doping for Sodium Ion Batteries" Nanomaterials 15, no. 20: 1575. https://doi.org/10.3390/nano15201575
APA StyleLiu, Y., Shi, Y., Zhang, M., Sun, D., Li, H., Wang, H., & Tang, Y. (2025). Enhancing Structural and Interfacial Stability of NaNi1/3Mn1/3Fe1/3O2 Cathodes via Sb3+ Doping for Sodium Ion Batteries. Nanomaterials, 15(20), 1575. https://doi.org/10.3390/nano15201575






