Molecular Modelling of the Adsorption and Delivery of α-Pinene and Similar Terpenes of Essential Oils on Montmorillonite Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Models
2.2. Methodology
3. Results
3.1. Crystallographic and Spectroscopic Properties of α-Pinene’s Crystal Structure
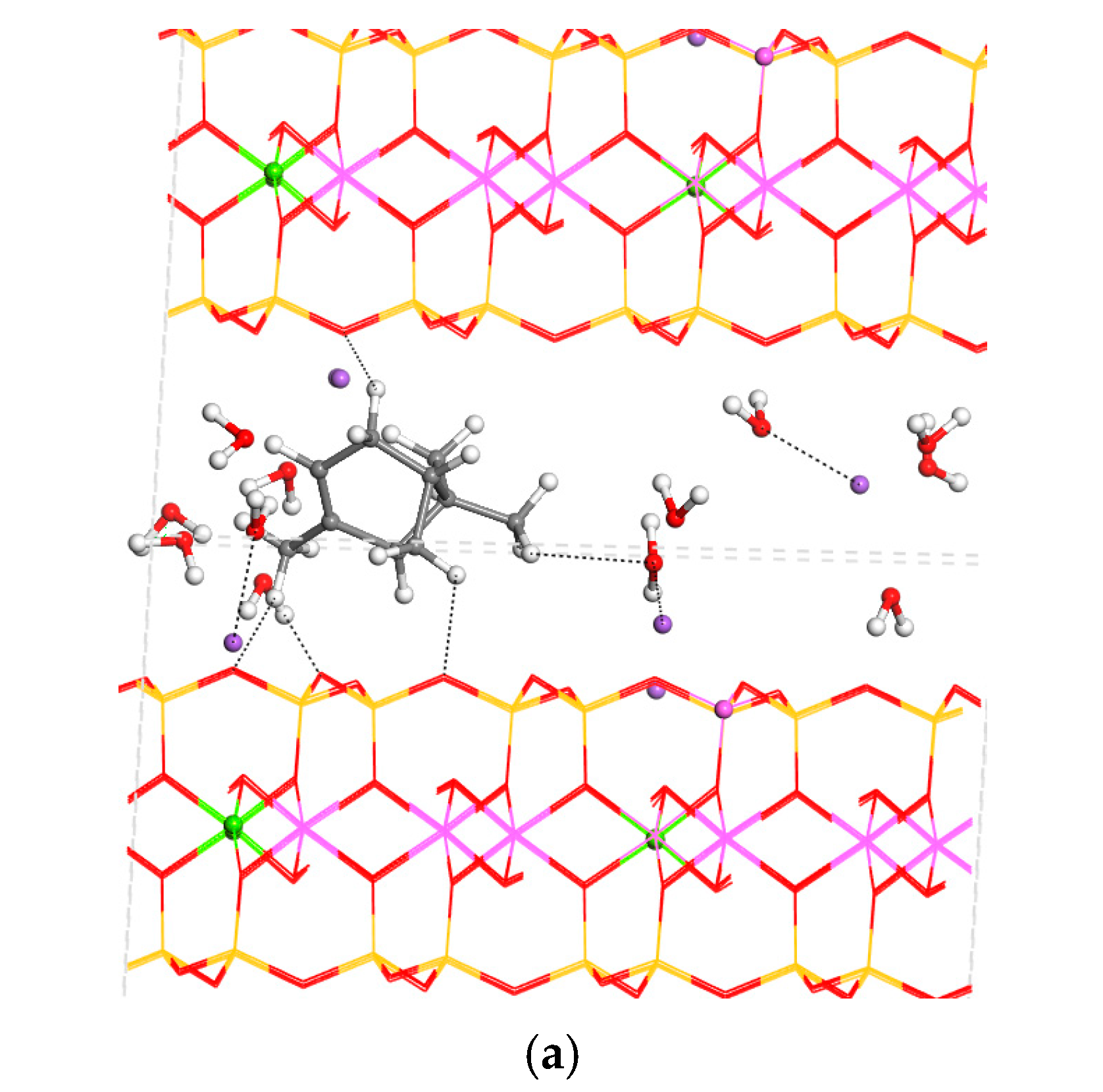
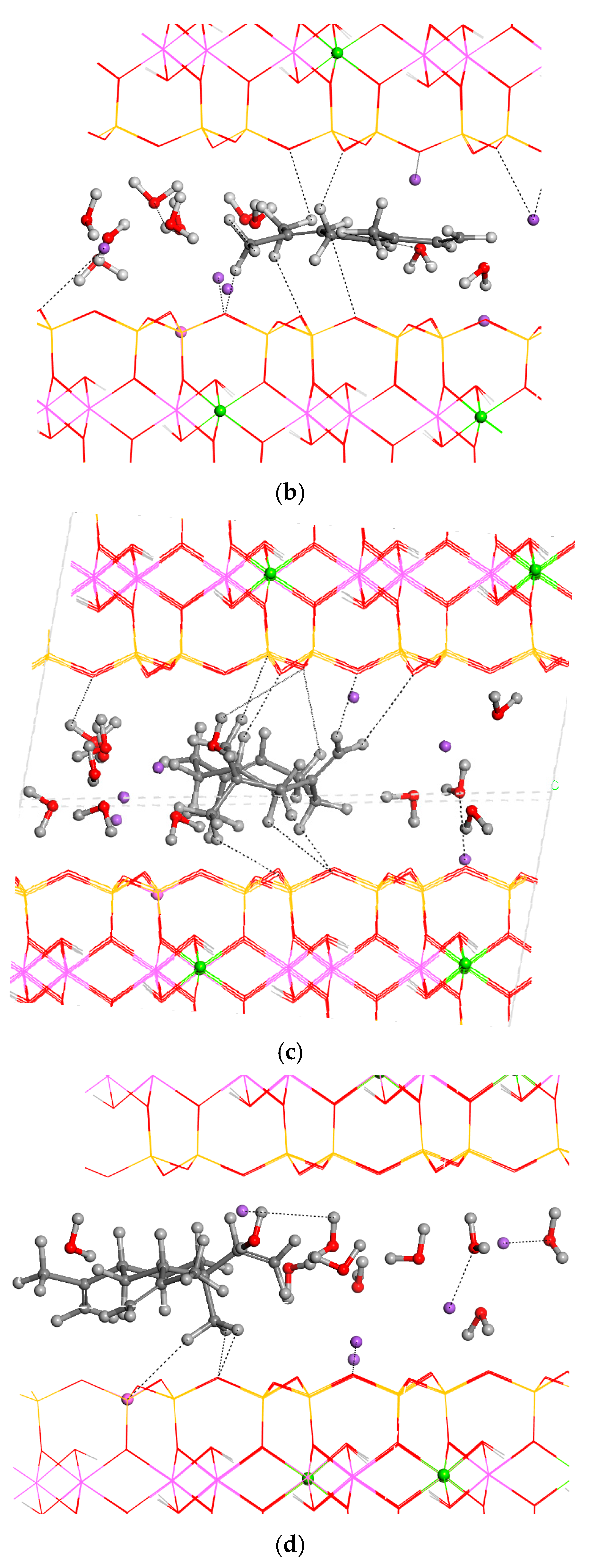
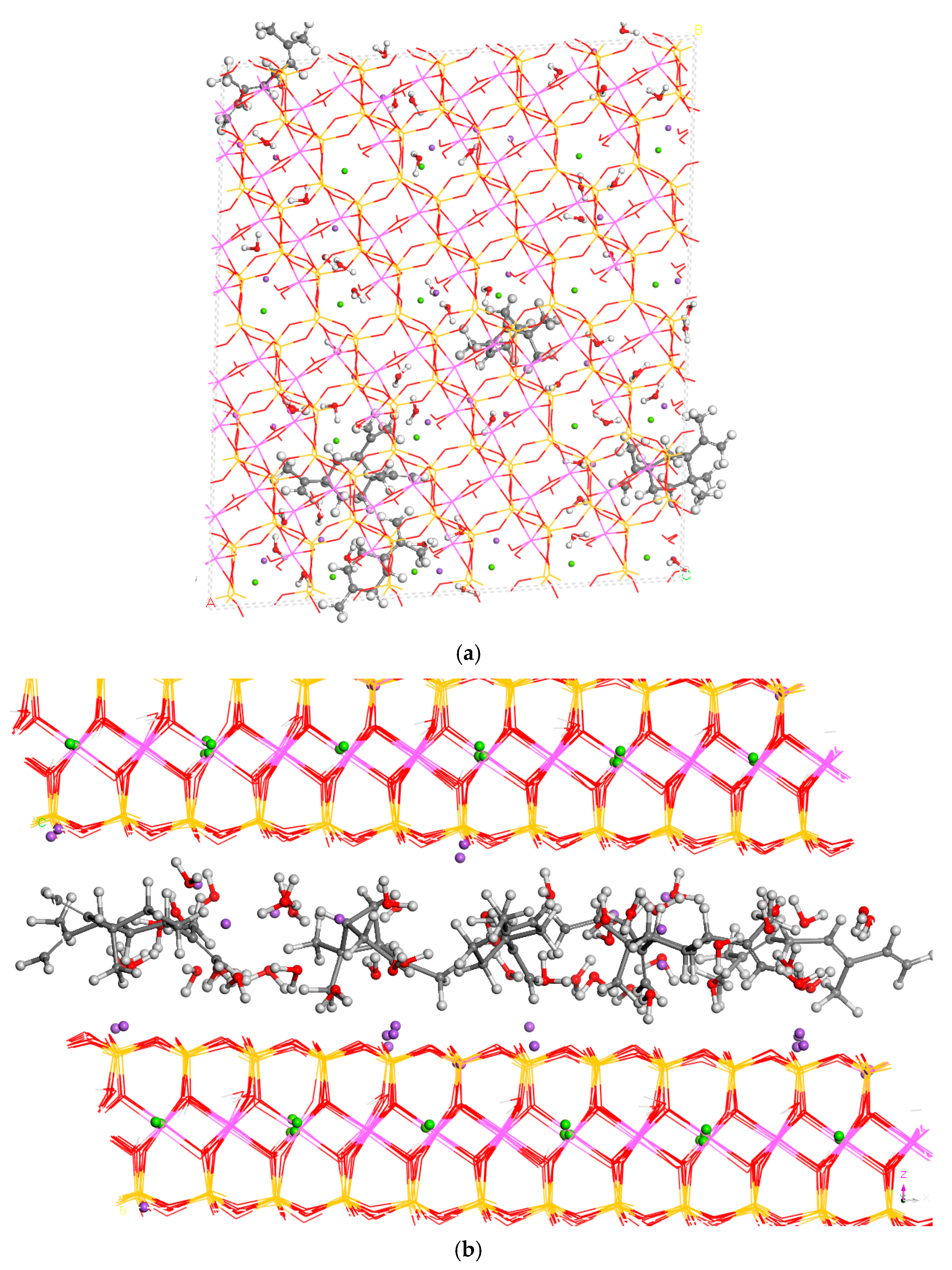
3.2. Intercalation of Organic Compounds in Clay Mineral Interlayer Space
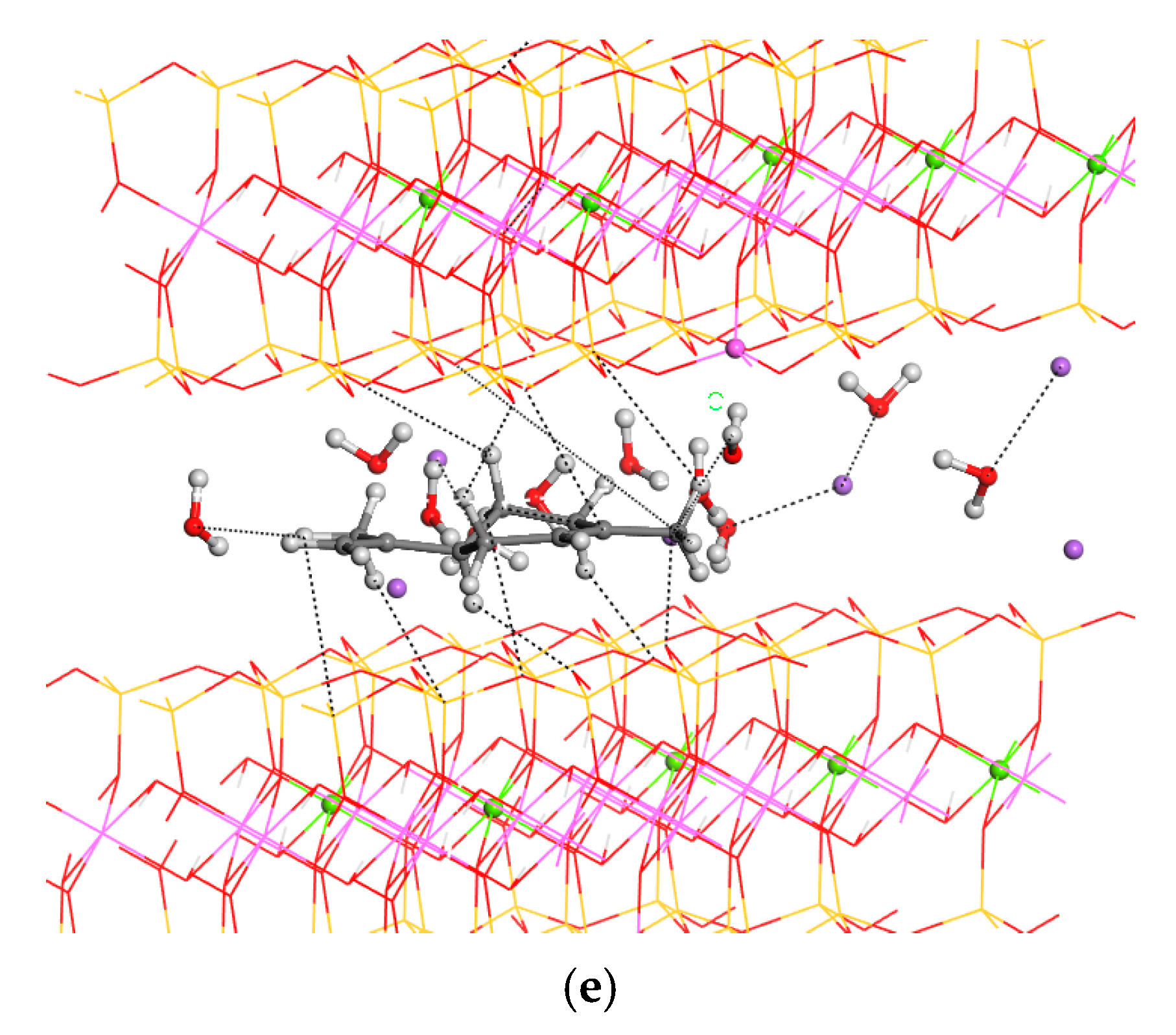
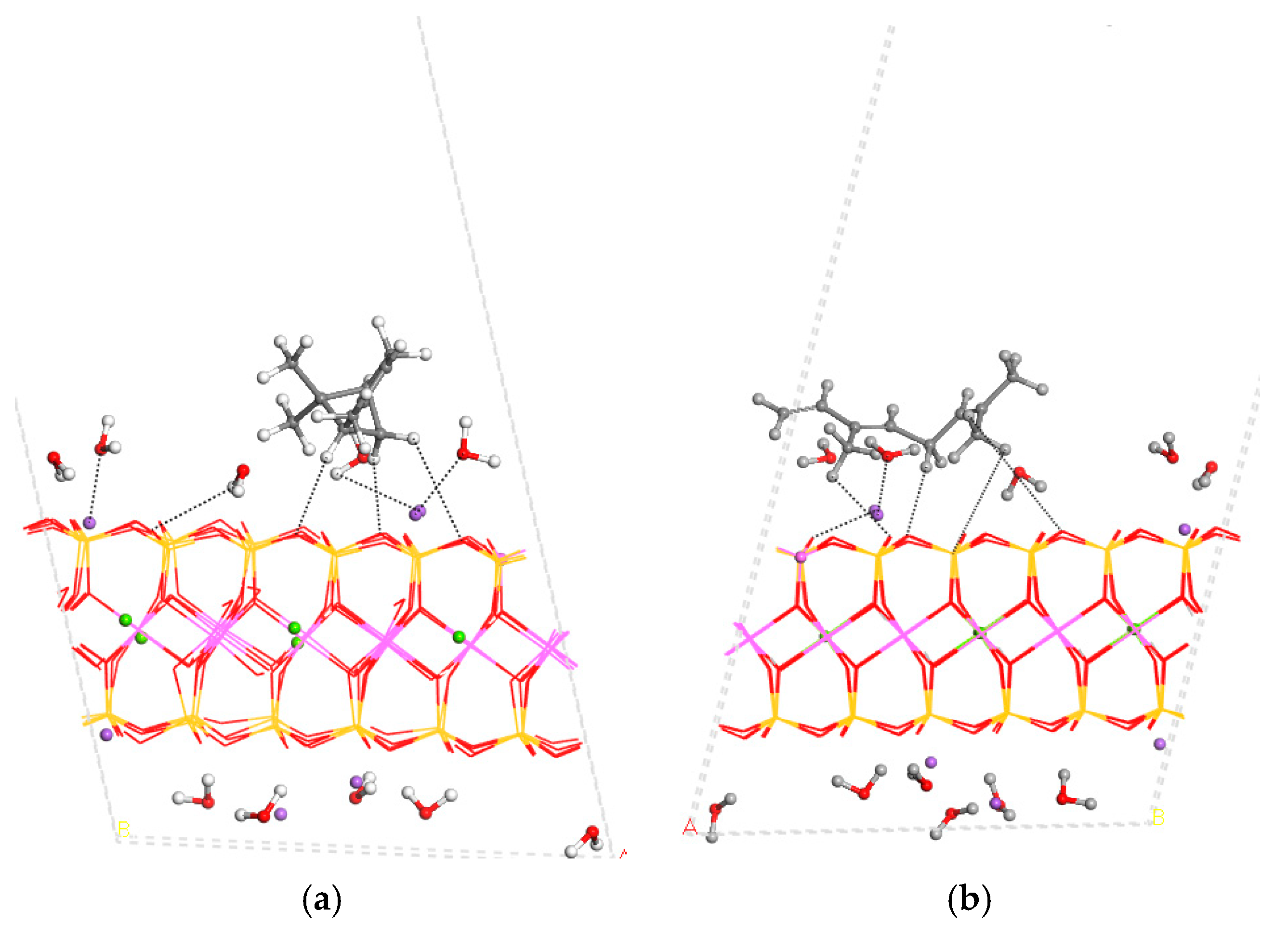
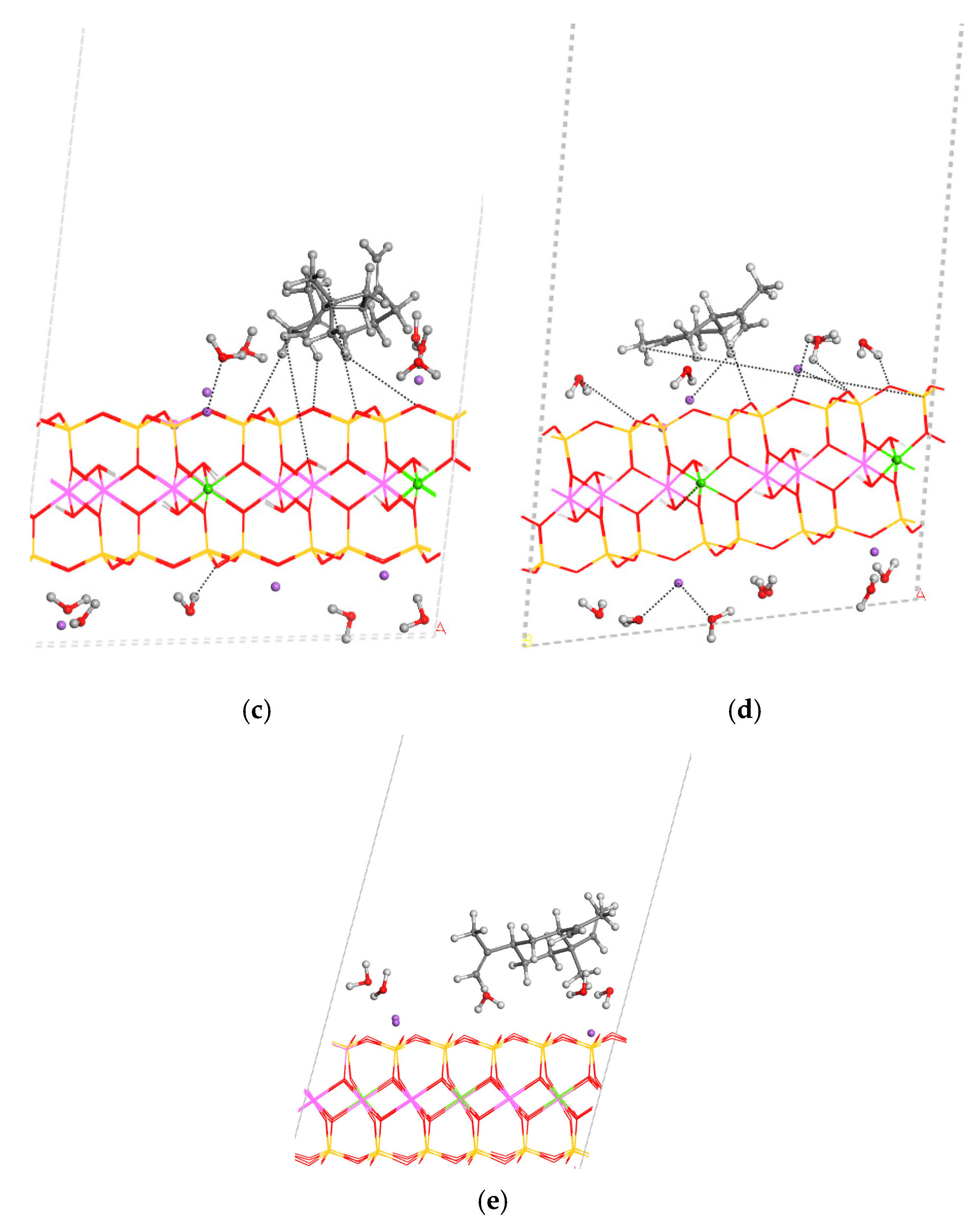
3.3. Adsorption on External Clay Surface
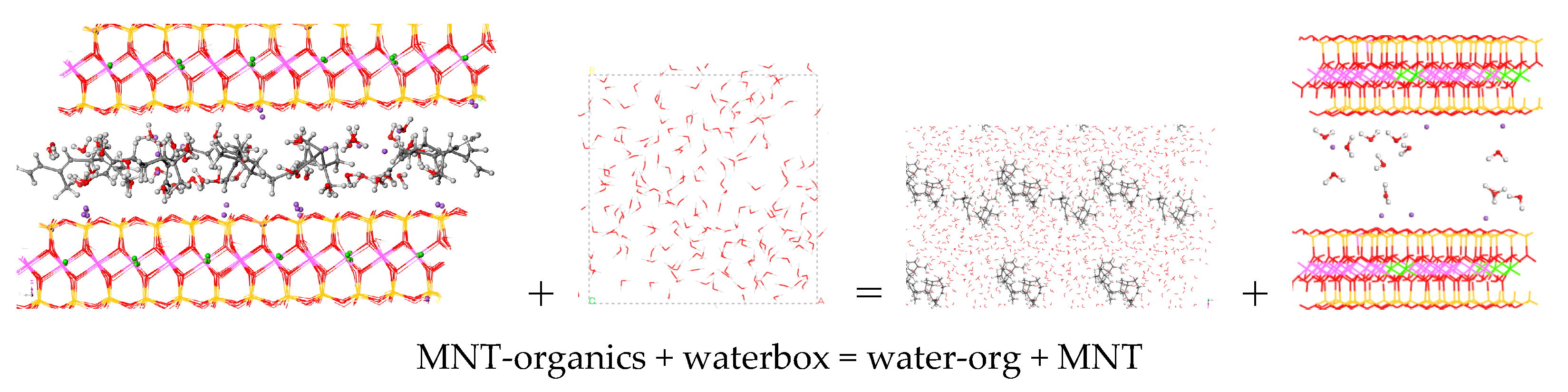
3.4. Desorption Process and Delivery of Essential Oil Components to External Media
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
- Santana, H.S.R.; De Carvalho, F.O.; Silva, E.R.; Santos, N.G.L.; Shanmugam, S.; Santos, D.N.; Wisniewski, J.O.; Junior, J.S.C.; Nunes, P.S.; Araujo, A.A.S.; et al. Anti-Inflammatory Activity of Limonene in the Prevention and Control of Injuries in the Respiratory System: A Systematic Review. Curr. Pharm. Des. 2020, 26, 2182–2191. [Google Scholar] [CrossRef]
- Laraib, I.; Qasim, S.; Uttra, A.M.; Al-Joufi, F.A. Anti-Inflammatory, Antihyperalgesic, and Gastric Safety Profiling of Ocimene: Attenuation of Nonsteroidal Anti-Inflammatory Drug-Induced Gastric Ulcers by Modulating Toll-like Receptor 4 and Pyroptosis Pathways. ACS Pharmacol. Transl. Sci. 2025, 8, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-Y.; Yan, L.-S.; Kang, J.-Y.; Gu, C.Y.; Cheng, B.C.-Y.; Wang, Y.-W.; Luo, G.; Zhang, Y. Eucalyptol, Limonene and Pinene Enteric Capsules Attenuate Airway Inflammation and Obstruction in Lipopolysaccharide-Induced Chronic Bronchitis Rat Model via TLR4 Signaling Inhibition. Int. Immunopharmacol. 2024, 129, 111571. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, C.; Yang, X.; Lin, Z.; Huang, H.; Shu, Y.; Shentu, J.; Xu, Y. A Mechanistic Review on the Anti-Inflammatory Effects of β-Caryophyllene. Mini-Rev. Org. Chem. 2025, 22, 671–684. [Google Scholar] [CrossRef]
- Liu, M.; Mao, L.; Daoud, A.; Hassan, W.; Zhou, L.; Lin, J.; Liu, J.; Shang, J. β-Elemene Inhibits Monocyte–Endothelial Cells Interactions via Reactive Oxygen Species/MAPK/NF-κB Signaling Pathway in Vitro. Eur. J. Pharmacol. 2015, 766, 37–45. [Google Scholar] [CrossRef]
- Xie, Q.; Li, F.; Fang, L.; Liu, W.; Gu, C. The Antitumor Efficacy of β-Elemene by Changing Tumor Inflammatory Environment and Tumor Microenvironment. BioMed Res. Int. 2020, 6892961. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The Progress of Essential Oils as Potential Therapeutic Agents: A Review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of Clays as Drug Delivery Systems: Possibilities and Limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Yang, Y.; Narayanan Nair, A.K.; Sun, S. Layer Charge Effects on Adsorption and Diffusion of Water and Ions in Interlayers and on External Surfaces of Montmorillonite. ACS Earth Space Chem. 2019, 3, 2635–2645. [Google Scholar] [CrossRef]
- Tenci, M.; Rossi, S.; Aguzzi, C.; Carazo, E.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Viseras, C.; Caramella, C.M.; Ferrari, F. Carvacrol/clay hybrids loaded into in situ gelling films. Int. J. Pharm. 2017, 531, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Berraaouan, D.; Tirinsi, M.; Mothana, R.A.; Al-Yousef, H.M.; Fauconnier, M.-L.; Salhi, S.; Tahani, A. Investigation into the role of layered nanoporous modified clays for adsorption/desorption of rosemary essential oil in the gas phase and the ability of this system as an antioxidant and preservation agent in food applications. Bioresour. Bioprocess. 2025, 12, 56. [Google Scholar] [CrossRef]
- Ghrab, S.; Balme, S.; Cretin, M.; Bouaziz, S.; Benzina, M. Adsorption of terpenes from Eucalyptus globulus onto modified beidellite. Appl. Clay Sci. 2018, 156, 169–177. [Google Scholar] [CrossRef]
- Agougui, C.; Cecilia, J.A.; Saad, H.; Franco-Duro, F.; Essid, R.; Khabbouchi, M.; Frini-Srasra, N. Adsorption of Carvone and Limonene from Caraway Essential Oil onto Tunisian Montmorillonite Clay for Pharmaceutical Application. Sci. Rep. 2022, 12, 19814. [Google Scholar] [CrossRef]
- Essifi, K.; Hammani, A.; Berraaouan, D.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Montmorillonite Nanoclay Based Formulation for Controlled and Selective Release of Volatile Essential Oil Compounds. Mater. Chem. Phys. 2022, 277, 125569. [Google Scholar] [CrossRef]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-deValle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite/Essential Oil Composites in a Multilayer Film Reservoir. Micropor. Mesopor. Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Loukidi, A.; Chatzimichailidou, S.; Salmas, C.E.; Giannakas, A.E.; Achilias, D.S. Effect of Na-and Organo-Modified Montmorillonite/Essential Oil Nanohybrids on the Kinetics of the in Situ Radical Polymerization of Styrene. Nanomaterials 2021, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Márquez, M.; Soriano-Correa, C.; Sainz-Díaz, C.I. Adsorption of Sulfonamides on Phyllosilicate Surfaces by Molecular Modeling Calculations. J. Phys. Chem. C 2017, 121, 2905–2914. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Meruvia-Rojas, Y.V.; Molina-Montes, E.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Intercalation of the Anticancer Drug Lenalidomide into Montmorillonite for Bioavailability Improvement: A Computational Study. J. Mol. Model. 2025, 31, 5. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 20 February 2025).
- Bond, A.D.; Davies, J.E. (1S)-(–)-α-Pinene. Acta Cryst. E 2001, 57, o1039–o1040. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Viseras, C.; Sainz-Díaz, C.I. Praziquantel–Clays as Accelerated Release Systems to Enhance the Low Solubility of the Drug. Pharmaceutics 2020, 12, 914. [Google Scholar] [CrossRef]
- Sainz-Díaz, C.I.; Escamilla-Roa, E.; Hernández-Laguna, A. Quantum Mechanical Calculations of Trans-Vacant and Cis-Vacant Polymorphism in Dioctahedral 2: 1 Phyllosilicates. Am. Mineralog. 2005, 90, 1827–1834. [Google Scholar] [CrossRef]
- Heinz, H.; Lin, T.-J.; Kishore Mishra, R.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An Ab Initio Force-Field Optimized for Condensed-Phase Applications. Overview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Jorge, N.L.; Garrafa, M.V.; Romero, J.M.; Jorge, M.J.; Jorge, L.C.; Delfino, M.R.; Meruvia-Rojas, Y.V.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Adsorption of Ciprofloxacin on Clay Minerals in Argentinian Santa Rosa-Corrientes Soils. Molecules 2024, 29, 1760. [Google Scholar] [CrossRef] [PubMed]
- Materials Studio, Version 2020. Software for Matrials Modelling, Biovia. Dassault Inc.: Waltham, MA, USA, 2020.
- Segall, M.D.; Lindan, P.J.; Probert, M.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-Principles Simulation: Ideas, Illustrations and the CASTEP code. J. Physics Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type Density Functional Constructed with a Long-range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational Density-Functional Perturbation Theory for Dielectrics and Lattice Dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Molecular Modeling and Infrared and Raman Spectroscopy of the Crystal Structure of the Chiral Antiparasitic Drug Praziquantel. J. Mol. Model. 2017, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.L.F.; Ricca, A.; da Silveira, E.F. Infrared spectroscopy of α-pinene ices irradiated by energetic ions at temperatures relevant to astronomical environments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 329, 125504. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.L.F.; Doreste, D.V.; Ricca, A.; Murhej, Y.; da Silveira, E.F.; Boduch, P.; Rothard, H.; Domaracka, A. Physicochemical Properties of α-Pinene in Water Ice Analogs under Energetic Heavy-Ion Irradiation. ACS Earth Space Chem. 2025, 9, 2180–2198. [Google Scholar] [CrossRef]
- An, P.; Yuan, C.-Q.; Liu, X.-H.; Xiao, D.-B.; Luo, Z.-X. Vibrational Spectroscopic Identification of Isoprene, Pinenes and Their Mixture. Chin. Chem. Lett. 2016, 27, 527–534. [Google Scholar] [CrossRef]
- Pradhita, M.; Masruri, M.; Rahman, M.F. Study Catalytic Oxidation Ofa-Pinene Using Hydrogen Peroxide-Iron (III) Chloride. In Proceedings of the IConSSE FSM SWCU, Salatiga, Indonesia, 1 August 2015; pp. 90–96. [Google Scholar]
- Nguemtchouin, M.G.M.; Ngassoum, M.B.; Kamga, R.; Deabate, S.; Lagerge, S.; Gastaldi, E.; Chalier, P.; Cretin, M. Characterization of inorganic and organic clay modified materials: An approach for adsorption of an insecticidal terpenic compound. Appl. Clay Sci. 2015, 104, 110–118. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A novel method for the preparation of inorganic and organo-modified montmorillonite essential oil hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
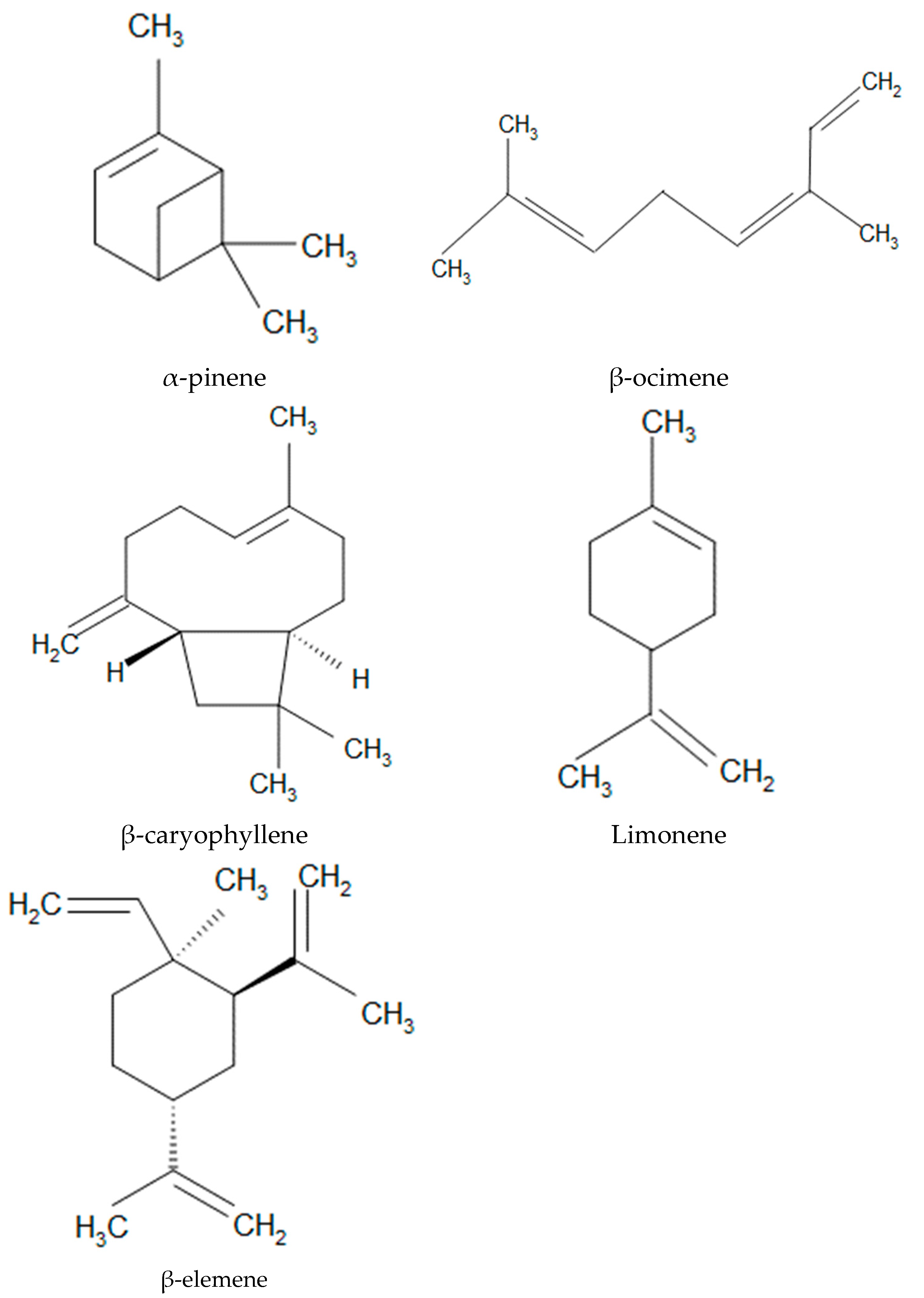
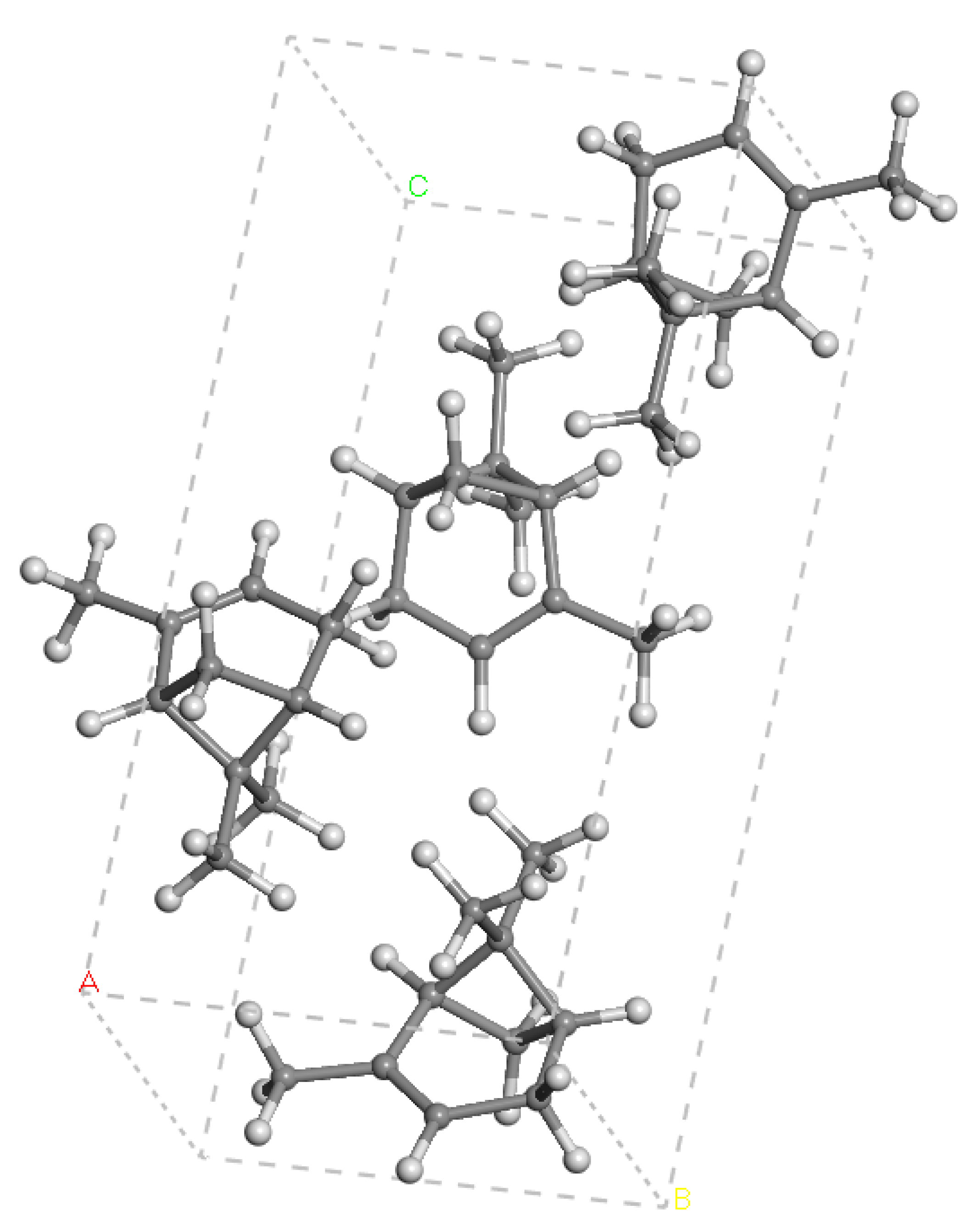
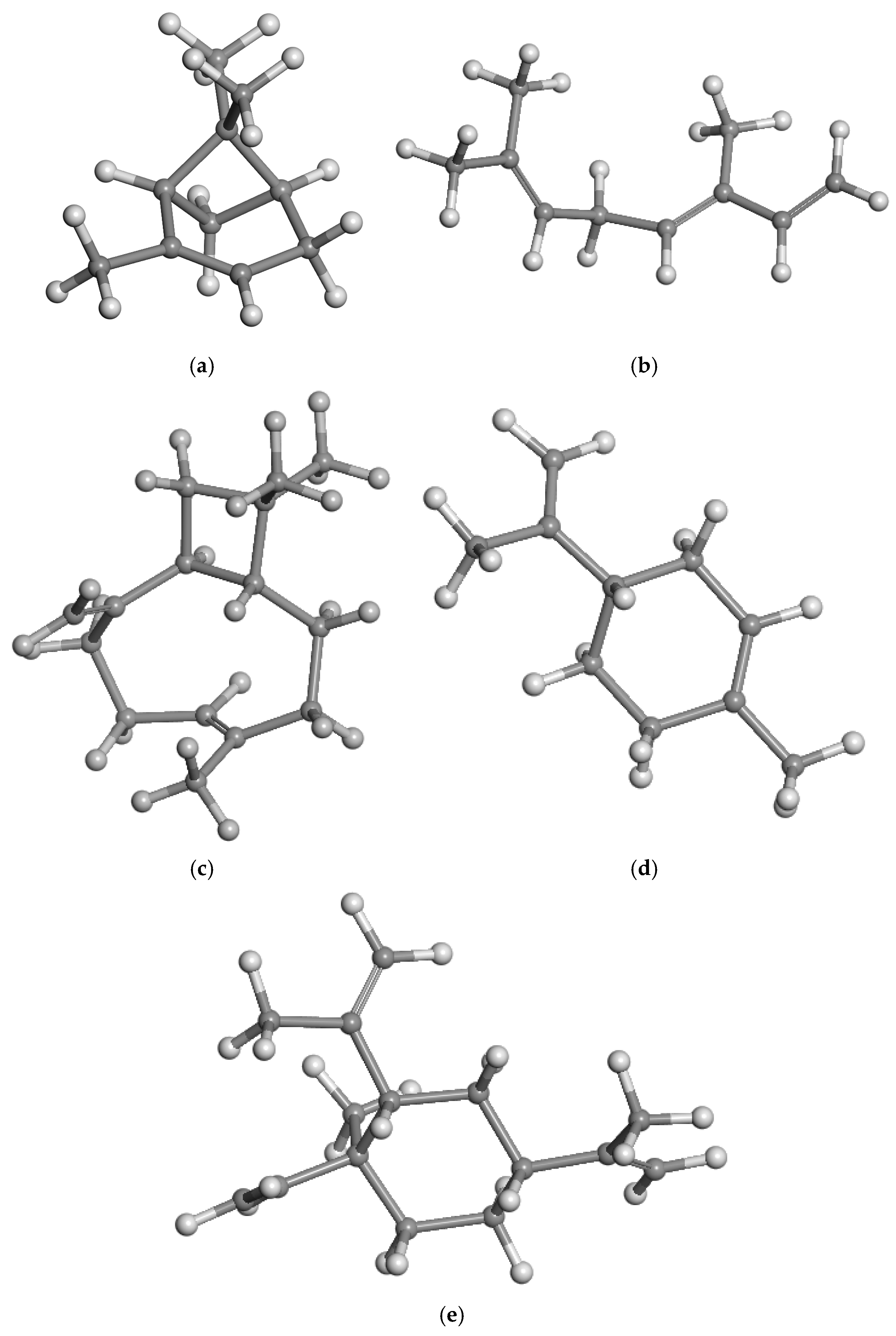
| Geometric Features | a | b | c | d(C-C) | d(C=C) |
|---|---|---|---|---|---|
| Experimental | 7.19 | 7.59 | 15.92 | 1.506 | 1.320 |
| Calculated INTERFACE FF | 7.10 | 7.59 | 15.73 | 1.520 | 1.341 |
| Calculated COMPASS FF | 7.19 | 7.46 | 15.34 | 1.545 | 1.543 |
| Calculated DFT | 7.13 | 7.57 | 15.79 | 1.554 | 1.345 |
| Mode a | Exp. b | Calculated |
|---|---|---|
| ν(C-H) (HC=C) | 3022, 3023 f | 3079, 3078 |
| ν(C-H) CH2 c | 2990 f | 3050 as, 2985 s |
| ν(C-H) CH3 | 2968-2938 as f, 2874 s f | 3025-2964 as, 2925-2922 s c, 2906 s d |
| ν(C-H) cyclic | 2918 f | 2943 |
| ν(C-H) CH2 d | 2982-2837, 2832 s f | 2888 s |
| ν(C=C) | 1653, 1659 e, 1661 f | 1651 |
| δ(C-H) CH2 | 1443, 1469-1443 e 1472-1442 s f, 1265-1204 e, 1337-1205 f | 1470-1465 s d, 1409-1406 s c, 1190 |
| δ(C-H) CH3 | 1371, 1333, 1380-1367 umb e, 1449 f, 1381-1363 umb f | 1452-1416 as, 1354-1349 umb |
| δ(C-H) CH | 1261, 1211, 1124 e, 1249-1126 f | 1308-1200, 1171-1029 |
| Cell Parameters | a | b | c | α | β | γ | d(001) |
|---|---|---|---|---|---|---|---|
| MNT | 15.46 | 17.87 | 12.0 | 97.4 | 105.4 | 90.1 | 11.6 |
| α-pinene–MNT | 15.48 | 17.88 | 14.26 | 87.9 | 103.0 | 90.1 | 13.90 |
| β-ocimene–MNT | 15.48 | 17.88 | 13.26 | 106.3 | 103.3 | 90.0 | 12.90 |
| β-caryophyllene–MNT | 15.48 | 17.89 | 13.87 | 100.7 | 98.9 | 90.1 | 13.87 |
| Limonene–MNT | 15.48 | 17.89 | 13.19 | 91.1 | 110.7 | 90.1 | 12.34 |
| β-elemene–MNT | 15.48 | 17.88 | 13.60 | 93.7 | 100.6 | 90.1 | 13.37 |
| Combined–MNT | 31.46 | 36.90 | 15.69 | 108.7 | 103.4 | 90.7 | 15.26 |
| Adsorbate | Interlayer Eads | External Surface Eads |
|---|---|---|
| α-pinene | 38.55 | −10.14 |
| β-ocimene | 23.04 | −7.42 |
| β-caryophyllene | 42.49 | −10.4 |
| Limonene | 34.13 | −14.72 |
| β-elemene | 53.54 | −9.6 |
| All combined | 112.7 |
| Organic Molecule | Interlayer Edes. | Surface Edes. |
|---|---|---|
| α-pinene | −63.49 | −14.8 |
| β-ocimene | −55.33 | −7.33 |
| β-caryophyllene | −90.64 | −21.28 |
| Limonene | −52.32 | −3.47 |
| β-elemene | −90.58 | −27.44 |
| Combined one | −231.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, S.; Hernández-Laguna, A.; Sainz-Díaz, C.I. Molecular Modelling of the Adsorption and Delivery of α-Pinene and Similar Terpenes of Essential Oils on Montmorillonite Surfaces. Nanomaterials 2025, 15, 1573. https://doi.org/10.3390/nano15201573
Kanwal S, Hernández-Laguna A, Sainz-Díaz CI. Molecular Modelling of the Adsorption and Delivery of α-Pinene and Similar Terpenes of Essential Oils on Montmorillonite Surfaces. Nanomaterials. 2025; 15(20):1573. https://doi.org/10.3390/nano15201573
Chicago/Turabian StyleKanwal, Shamsa, Alfonso Hernández-Laguna, and C. Ignacio Sainz-Díaz. 2025. "Molecular Modelling of the Adsorption and Delivery of α-Pinene and Similar Terpenes of Essential Oils on Montmorillonite Surfaces" Nanomaterials 15, no. 20: 1573. https://doi.org/10.3390/nano15201573
APA StyleKanwal, S., Hernández-Laguna, A., & Sainz-Díaz, C. I. (2025). Molecular Modelling of the Adsorption and Delivery of α-Pinene and Similar Terpenes of Essential Oils on Montmorillonite Surfaces. Nanomaterials, 15(20), 1573. https://doi.org/10.3390/nano15201573






