Chitosan-graft-poly(N-vinylcaprolactam) Nanoparticles Containing Crotalus atrox Snake Venom: Biological and Physicochemical Characterization †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethics Statement
2.3. Crotalus atrox Venom Extraction and Characterization
2.4. Synthesis of Chitosan-graft-poly(N-vinylcaprolactam) Copolymer
2.5. Preparation of Nanoparticles
2.6. Venom-Nanoparticle Entrapment Capacity
2.7. Physicochemical Characterization
2.8. Biological Tests
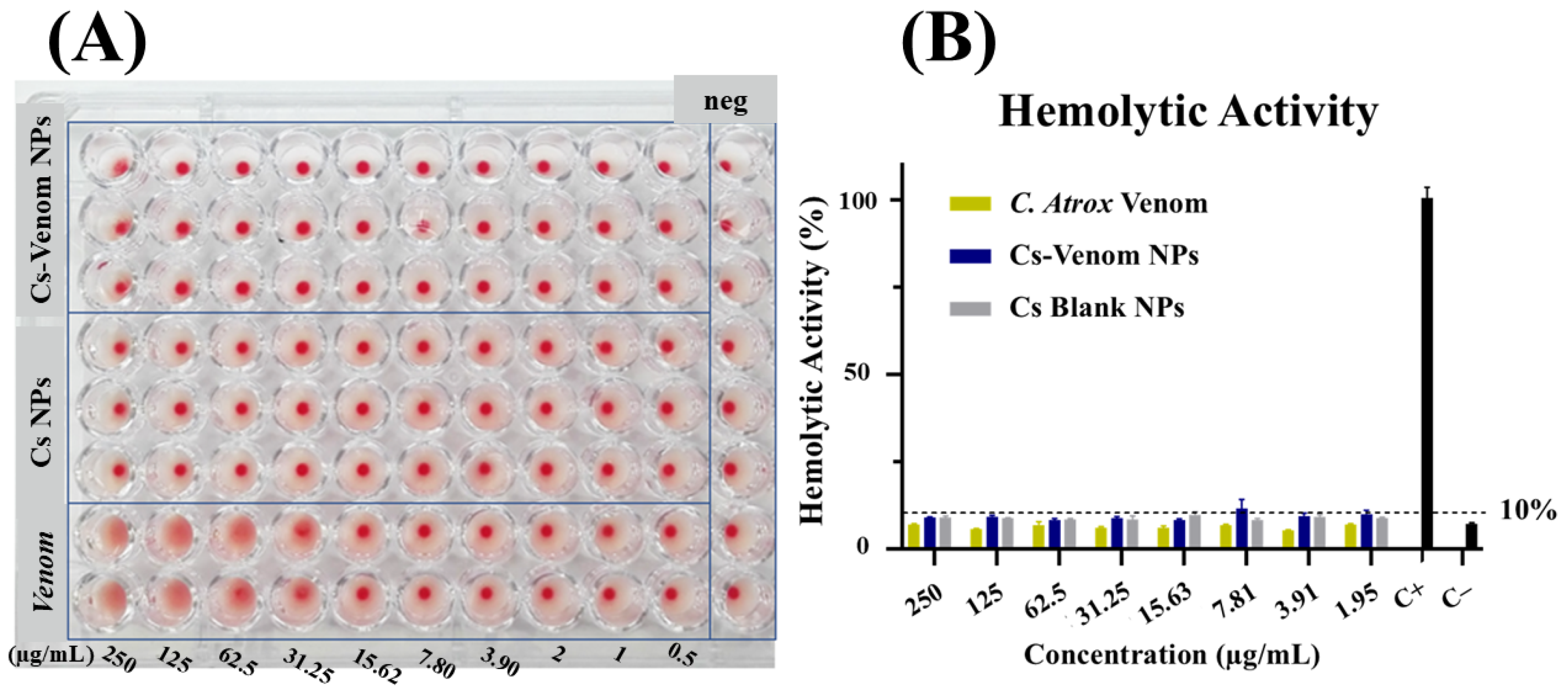
2.8.1. Hemagglutination Assay
2.8.2. Hemolytic Activity
2.8.3. Cytotoxicity Assay in Breast Cancer Cells
2.9. Intracellular Calcium in T47D Cell Cultures
2.10. Statistical Analysis
3. Results and Discussion
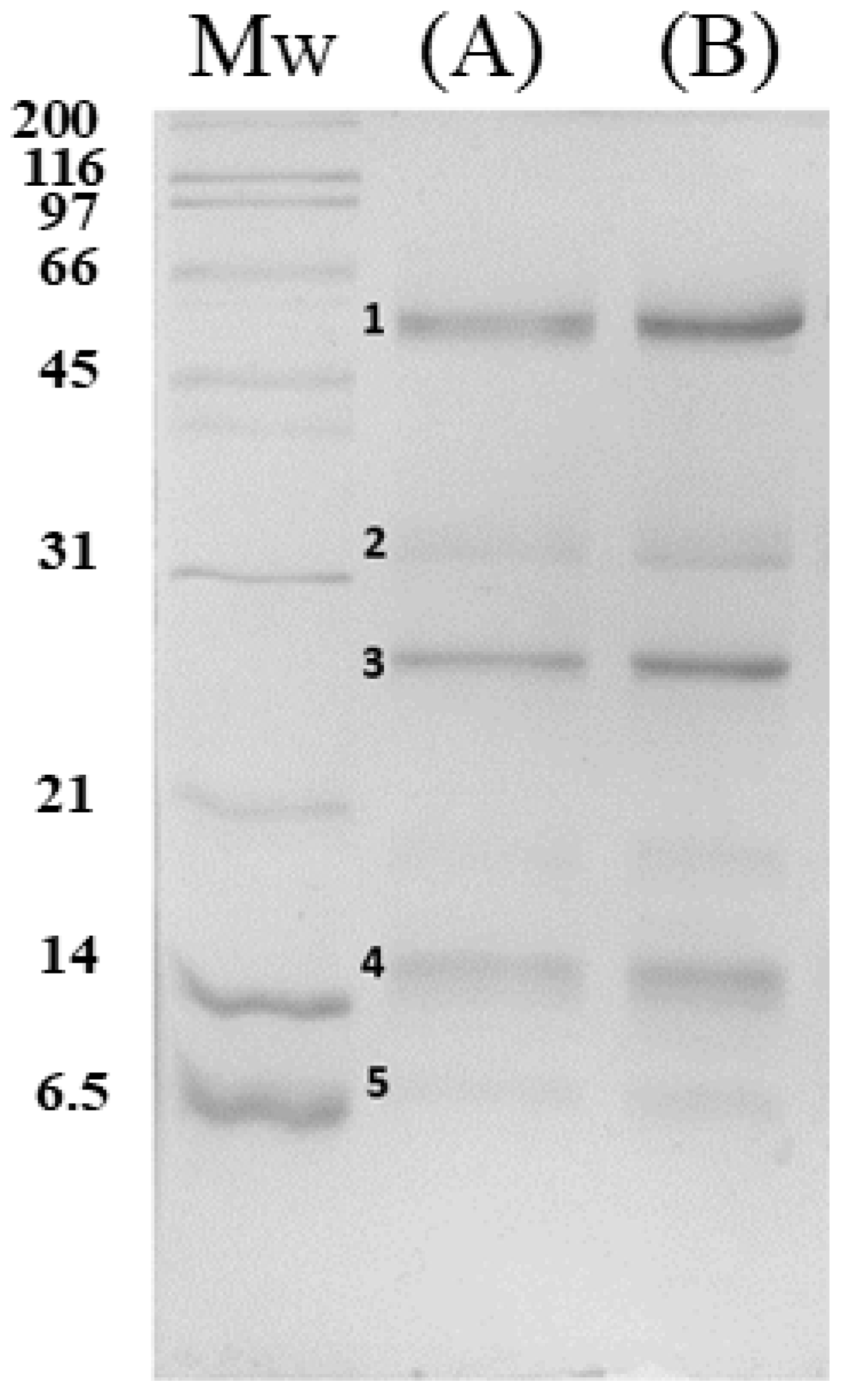
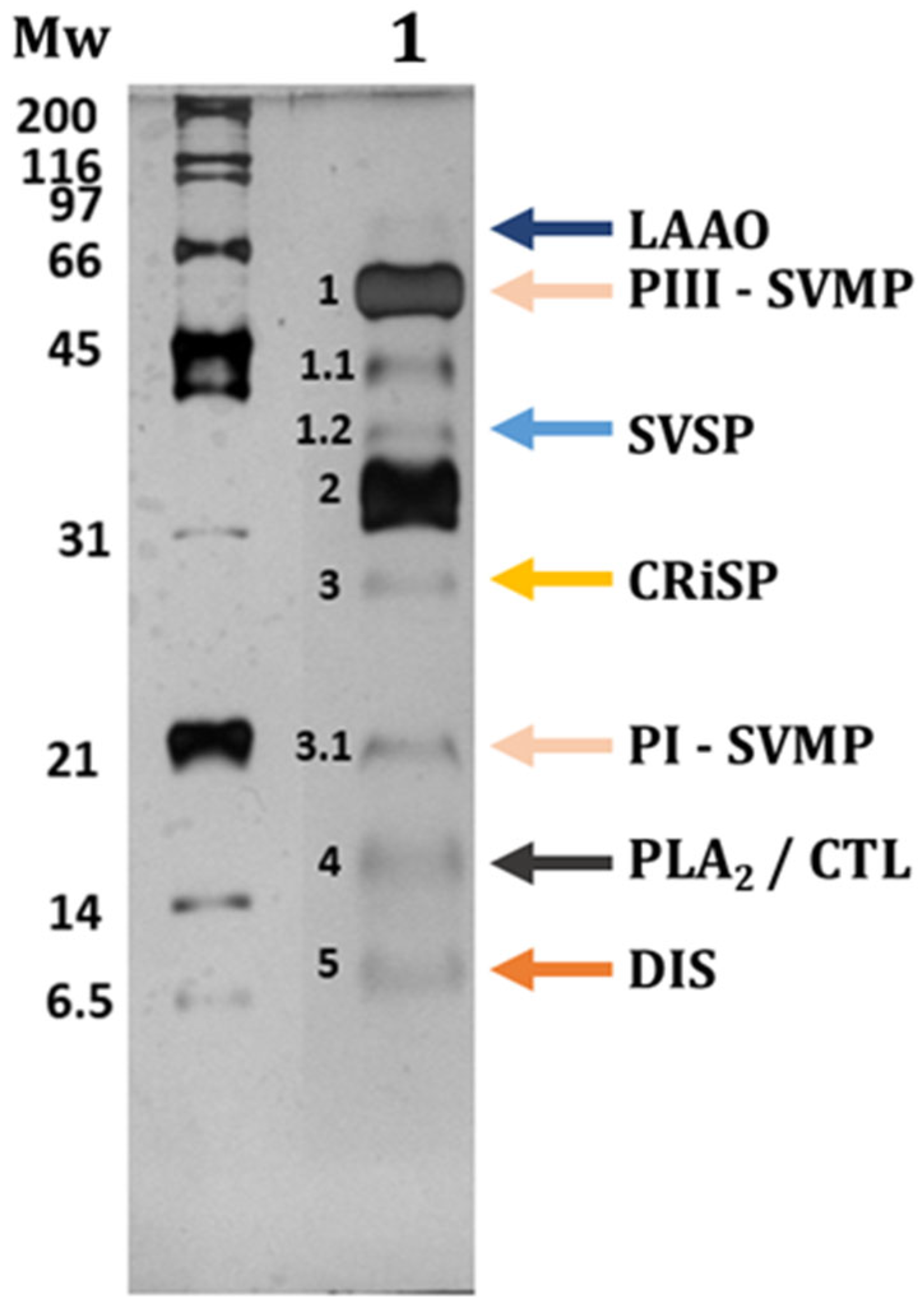
3.1. Characterization of C. atrox Venom
3.2. Physicochemical Characterization of Nanoparticles
3.3. Hemagglutination and Hemolytic Activity Assays
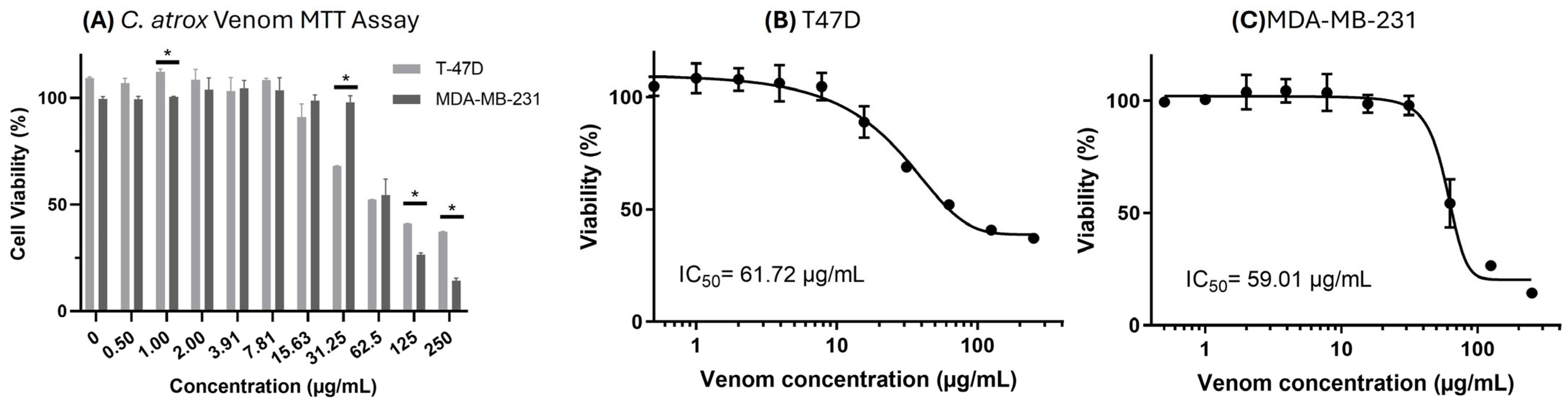
3.4. MTT Cell Viability Assays
3.5. Tumoral Cells Morphological Changes
3.6. Intracellular Calcium Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cs-g-PVCL | chitosan-graft-poly(N-vinylcaprolactam) copolymer |
| PVCL | poly(N-vinylcaprolactam) |
| Cs-g-PVCL NPs | chitosan-graft-poly(N-vinylcaprolactam) nanoparticles |
| Cs | Chitosan |
| Venom-loaded NPs | chitosan-graft-poly(N-vinylcaprolactam) nanoparticles loaded with C. atrox snake venom |
| NPs | nanoparticles |
| LCST | Lower critical solution temperature |
| C. atrox | Crotalus atrox snake |
| DH | Average hydrodynamic diameter |
| PDI | Polydispersity index |
| TPP | Sodium tripolyphosphate |
| RBC | Red blood cells |
References
- Goycoolea, F.M.; Lollo, G.; Remuñán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-Alginate Blended Nanoparticles as Carriers for the Transmucosal Delivery of Macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, P.; Mohan, N.; Srinivas, T.; Divya Dharshini, B.; Vaddadi, U.R.; Alle, M. Role of Carbohydrate-Based Nanomaterials in Therapeutic and Diagnostic Applications. In Carbohydrate Polymer Nanotechnologies: Design, Development, Utilization and Perspectives; Krishna Rao, K.S.V., Suresh Reddy, K.V.N., Alle, M., Eds.; Springer Nature: Singapore, 2025; pp. 147–170. ISBN 978-981-9621-71-2. [Google Scholar]
- Wong, K.H.; Lu, A.; Chen, X.; Yang, Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules 2020, 25, 3620. [Google Scholar] [CrossRef]
- Aguilar, M.R.; San Román, J. Chapter 1—Introduction to Smart Polymers and Their Applications. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing in Materials; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–11. ISBN 978-0-08-102416-4. [Google Scholar]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-Responsive Polymers: Applications of Smart Materials in Drug Delivery and Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; San Román, J.; Argüelles-Monal, W.M. Effect of the Molecular Architecture on the Thermosensitive Properties of Chitosan-g-Poly(N-Vinylcaprolactam). Carbohydr. Polym. 2015, 134, 92–101. [Google Scholar] [CrossRef]
- Prabaharan, M.; Grailer, J.J.; Steeber, D.A.; Gong, S. Stimuli-Responsive Chitosan-Graft-Poly(N-Vinylcaprolactam) as a Promising Material for Controlled Hydrophobic Drug Delivery. Macromol. Biosci. 2008, 8, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quiroz, D.; González-Gómez, Á.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; Román, J.S.; Argüelles-Monal, W.M. Conformational Study on the Thermal Transition of Chitosan-g-Poly(N-Vinylcaprolactam) in Aqueous Solution. Colloid Polym. Sci. 2016, 294, 555–563. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Thomas, R.G.; Muthiah, M.; Lee, H.J.; Jeong, Y.Y.; Park, I.-K.; Jayakumar, R. Breast Tumor Targetable Fe3O4 Embedded Thermo-Responsive Nanoparticles for Radiofrequency Assisted Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sanoj Rejinold, N.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-Loaded Biocompatible Thermoresponsive Polymeric Nanoparticles for Cancer Drug Delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biodegradable and Thermo-Sensitive Chitosan-g-Poly(N-Vinylcaprolactam) Nanoparticles as a 5-Fluorouracil Carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Argüelles-Monal, W.; Sarabia-Sainz, A.-í.; Lucero-Acuña, A.; Castillo-Castro, T.d.; Román, J.S.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature Stimuli-Responsive Nanoparticles from Chitosan-Graft-Poly(N-Vinylcaprolactam) as a Drug Delivery System. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Thomas, R.G.; Muthiah, M.; Chennazhi, K.P.; Manzoor, K.; Park, I.-K.; Jeong, Y.Y.; Jayakumar, R. Anti-Cancer, Pharmacokinetics and Tumor Localization Studies of pH-, RF- and Thermo-Responsive Nanoparticles. Int. J. Biol. Macromol. 2015, 74, 249–262. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Wagner Angela, M.; Spencer David, S.; Peppas Nicholas, A. Advanced Architectures in the Design of Responsive Polymers for Cancer Nanomedicine. J. Appl. Polym. Sci. 2018, 135, 46154. [Google Scholar] [CrossRef]
- Abbasi Kajani, A.; Haghjooy Javanmard, S.; Asadnia, M.; Razmjou, A. Recent Advances in Nanomaterials Development for Nanomedicine and Cancer. ACS Appl. Bio Mater. 2021, 4, 5908–5925. [Google Scholar] [CrossRef]
- Aftab, S.; Shah, A.; Nadhman, A.; Kurbanoglu, S.; Aysıl Ozkan, S.; Dionysiou, D.D.; Shukla, S.S.; Aminabhavi, T.M. Nanomedicine: An Effective Tool in Cancer Therapy. Int. J. Pharm. 2018, 540, 132–149. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Al Shaer, D.; Albericio, F.; de la Torre, B.G. 2020 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2021, 14, 145. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Wang, B.; Xie, L.; Chen, W. New FDA Drug Approvals for 2024: Synthesis and Clinical Application. Eur. J. Med. Chem. 2025, 285, 117241. [Google Scholar] [CrossRef]
- Jimenez-Canale, J.; Fernandez-Quiroz, D.; Teran-Saavedra, N.G.; Diaz-Galvez, K.R.; Gallegos-Tabanico, A.; Burgara-Estrella, A.J.; Sarabia-Sainz, H.M.; Guzman-Partida, A.M.; Robles-Burgueño, M.d.R.; Vazquez-Moreno, L.; et al. Cytotoxic Activity of Crotalus Molossus Molossus Snake Venom-Loaded in Chitosan Nanoparticles against T-47D Breast Carcinoma Cells. Acta Biochim. Pol. 2022, 69, 233–243. [Google Scholar] [CrossRef]
- Boundy, J. Snakes of the World: A Supplement; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-46135-4. [Google Scholar]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the Venom Proteome of the Western Diamondback Rattlesnake, Crotalus atrox, via Snake Venomics and Combinatorial Peptide Ligand Library Approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef]
- Rex, C.J.; Mackessy, S.P. Venom Composition of Adult Western Diamondback Rattlesnakes (Crotalus atrox) Maintained under Controlled Diet and Environmental Conditions Shows Only Minor Changes. Toxicon 2019, 164, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.C.; Zuliani, J.P.; et al. Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy. BioMed Res. Int. 2014, 2014, e203639. [Google Scholar] [CrossRef]
- Trim, S.A.; Trim, C.M. Venom: The Sharp End of Pain Therapeutics. Br. J. Pain 2013, 7, 179–188. [Google Scholar] [CrossRef]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef]
- Duro-Castano, A.; Talelli, M.; Rodríguez-Escalona, G.; Vicent, M.J. Chapter 13—Smart Polymeric Nanocarriers for Drug Delivery. In Smart Polymers and Their Applications, 2nd ed.; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing in Materials; Woodhead Publishing: Cambridge, UK, 2019; pp. 439–479. ISBN 978-0-08-102416-4. [Google Scholar]
- Mohammadpourdounighi, N.; Behfar, A.; Ezabadi, A.; Zolfagharian, H.; Heydari, M. Preparation of Chitosan Nanoparticles Containing Naja Naja Oxiana Snake Venom. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 137–143. [Google Scholar] [CrossRef]
- Saber, A.E.S.; Abdelwahab, A.K.; El Amir, A.M.; Nassar, M.I.; Zohdi, H.F. Bee venom loaded chitosan nanoparticles as treatment for amoebiasis in mice. J. Egypt. Soc. Parasitol. 2017, 47, 443–458. [Google Scholar] [CrossRef]
- Shambayati, M.H.; Mehrabi, M.; Mohammadpour Dounighi, N.; Ramazani, A.; Zare Mirakabadi, A.; Ahmadi, E. Characterizing and Controlling the Loading of Vipera Albicornuta Venom in Chitosan Nanoparticles as an Adjuvant and Vaccine Delivery System. Nanomed. Res. J. 2019, 4, 220–227. [Google Scholar] [CrossRef]
- Rocha Soares, K.S.; Oliveira, A.R.; Daniele-Silva, A.; Glaucia-Silva, F.; Caroni, A.L.P.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Self-Assembled Scorpion Venom Proteins Cross-Linked Chitosan Nanoparticles for Use in the Immunotherapy. J. Mol. Liq. 2017, 241, 540–548. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Guimarães-Gomes, V.; Oliveira-Carvalho, A.L.; Junqueira-de-Azevedo, I.d.L.M.; Dutra, D.L.S.; Pujol-Luz, M.; Castro, H.C.; Ho, P.L.; Zingali, R.B. Cloning, Characterization, and Structural Analysis of a C-Type Lectin from Bothrops Insularis (BiL) Venom. Arch. Biochem. Biophys. 2004, 432, 1–11. [Google Scholar] [CrossRef]
- Meléndez-Martínez, D.; Macías-Rodríguez, E.; Vázquez-Briones, R.; López-Vera, E.; Sandra Cruz-Pérez, M.; Vargas-Caraveo, A.; Gatica-Colima, A.; Fernando Plenge-Tellechea, L. In Vitro Hemotoxic, α-Neurotoxic and Vasculotoxic Effects of the Mexican Black-Tailed Rattlesnake (Crotalus Molossus Nigrescens) Venom. J. Venom Res. 2017, 8, 1–8. [Google Scholar]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current Understanding of Interactions between Nanoparticles and the Immune System. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef]
- Mirzaei, F.; Mohammadpour Dounighi, N.; Avadi, M.R.; Rezayat, M. A New Approach to Antivenom Preparation Using Chitosan Nanoparticles Containing EchisCarinatus Venom as A Novel Antigen Delivery System. Iran. J. Pharm. Res. 2017, 16, 858–867. [Google Scholar]
- Soares, K.S.R.; Gláucia-Silva, F.; Daniele-Silva, A.; Torres-Rêgo, M.; de Araújo, N.K.; Menezes, Y.A.S.d.; Damasceno, I.Z.; Tambourgi, D.V.; Da Silva-Júnior, A.A.; Fernandes-Pedrosa, M.D.F. Antivenom Production against Bothrops Jararaca and Bothrops Erythromelas Snake Venoms Using Cross-Linked Chitosan Nanoparticles as an Immunoadjuvant. Toxins 2018, 10, 158. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Mohammadur Dounighi, N.; Mehrabi, M.; Avadi, M.R.; Zolfagharian, H.; Rezayat, M. Preparation, Characterization and Stability Investigation of Chitosan Nanoparticles Loaded with the Echis Carinatus Snake Venom as a Novel Delivery System. Arch. Razi Inst. 2015, 70, 269–277. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kusunoki, T.; Kasai, K. Complete Primary Structure of a Galactose-Specific Lectin from the Venom of the Rattlesnake Crotalus atrox. Homologies with Ca2(+)-Dependent-Type Lectins. J. Biol. Chem. 1991, 266, 2320–2326. [Google Scholar] [CrossRef]
- Lomonte, B.; Rojas, G.; Gutiérrez, J.; Ramírez, G. Isolation of a Galactose-Binding Lectin from the Venom of the Snake Bothrops Godmani (Godmann’s Pit Viper). Toxicon 1990, 28, 75–81. [Google Scholar] [CrossRef]
- Carvalho, D.D.; Marangoni, S.; Oliveira, B.; Novello, J.C. Isolation and Characterization of a New Lectin from the Venom of the Snake Bothrops Jararacussu. IUBMB Life 1998, 44, 933–938. [Google Scholar] [CrossRef]
- Havt, A.; Toyama, M.H.; do Nascimento, N.R.F.; Toyama, D.O.; Nobre, A.C.L.; Martins, A.M.C.; Barbosa, P.S.F.; Novello, J.C.; Boschero, A.C.; Carneiro, E.M.; et al. A New C-Type Animal Lectin Isolated from Bothrops pirajai Is Responsible for the Snake Venom Major Effects in the Isolated Kidney. Int. J. Biochem. Cell Biol. 2005, 37, 130–141. [Google Scholar] [CrossRef]
- Komori, Y.; Nikai, T.; Tohkai, T.; Sugihara, H. Primary Structure and Biological Activity of Snake Venom Lectin (APL) from Agkistrodon p. Piscivorus (Eastern Cottonmouth). Toxicon 1999, 37, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An Attractive Biocompatible Polymer for Microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Biswas, A.; Gomes, A.; Sengupta, J.; Datta, P.; Singha, S.; Dasgupta, A.K.; Gomes, A. Nanoparticle-Conjugated Animal Venom-Toxins and Their Possible Therapeutic Potential. J. Venom Res. 2012, 3, 15–21. [Google Scholar]
- Lima, J.M.d.; Sarmento, R.R.; Souza, J.R.d.; Brayner, F.A.; Feitosa, A.P.S.; Padilha, R.; Alves, L.C.; Porto, I.J.; Batista, R.F.B.D.; de Oliveira, J.E.; et al. Evaluation of Hemagglutination Activity of Chitosan Nanoparticles Using Human Erythrocytes. BioMed Res. Int. 2015, 2015, e247965. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Tuo, W.-W.; Wang, D.; Kang, L.-L.; Chen, X.-Y.; Luo, M. Restoring the Youth of Aged Red Blood Cells and Extending Their Lifespan in Circulation by Remodelling Membrane Sialic Acid. J. Cell. Mol. Med. 2016, 20, 294–301. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvătescu, C.A.; Ifteni, P.; Pleş, L. Anticancer Activity of Toxins from Bee and Snake Venom—An Overview on Ovarian Cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Zhou, X.; Kini, R.M. Snake Venom Phospholipase A2 Enzymes. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010; p. 548. ISBN 978-0-8493-9165-1. [Google Scholar]
- Bradshaw, M.J.; Saviola, A.J.; Fesler, E.; Mackessy, S.P. Evaluation of Cytotoxic Activities of Snake Venoms toward Breast (MCF-7) and Skin Cancer (A-375) Cell Lines. Cytotechnology 2016, 68, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Yalcın, H.T.; Ozen, M.O.; Gocmen, B.; Nalbantsoy, A. Effect of Ottoman Viper (Montivipera Xanthina (Gray, 1849)) Venom on Various Cancer Cells and on Microorganisms. Cytotechnology 2014, 66, 87–94. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, Á.; Sanz, B.; Román, J.S.; Goya, G.F.; Hernández, R.; Mijangos, C. Chitosan Nanoparticles for Combined Drug Delivery and Magnetic Hyperthermia: From Preparation to in Vitro Studies. Carbohydr. Polym. 2017, 157, 361–370. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Maeda, H. The 35th Anniversary of the Discovery of EPR Effect: A New Wave of Nanomedicines for Tumor-Targeted Drug Delivery-Personal Remarks and Future Prospects. J. Pers. Med. 2021, 11, 229. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Skrzydlewska, E.; Figaszewski, Z.A. Changes in Electric Properties of Human Breast Cancer Cells. J. Membr. Biol. 2013, 246, 161–166. [Google Scholar] [CrossRef]
- Salatin, S.; Yari Khosroushahi, A. Overviews on the Cellular Uptake Mechanism of Polysaccharide Colloidal Nanoparticles. J. Cell. Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Hayashi, H.; Araki, S. Two Vascular Apoptosis-Inducing Proteins from Snake Venom Are Members of the Metalloprotease/Disintegrin Family. Eur. J. Biochem. 1998, 253, 36–41. [Google Scholar] [CrossRef]
- Markland, F.S.; Swenson, S. Snake Venom Metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, E.; Nakamura, S.; Oshima, Y.; Shibuya, T.; Miao, J.Y.; Hayashi, H.; Nikai, T.; Araki, S. Hemorrhagic Activity of the Vascular Apoptosis-Inducing Proteins VAP1 and VAP2 from Crotalus atrox. Toxicon 2008, 52, 589–593. [Google Scholar] [CrossRef]
- Costa, T.R.; Burin, S.M.; Menaldo, D.L.; de Castro, F.A.; Sampaio, S.V. Snake Venom L-Amino Acid Oxidases: An Overview on Their Antitumor Effects. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 23. [Google Scholar] [CrossRef]
- Venkatesan, C.; Vimal, S.; Hameed, A.S.S. Synthesis and Characterization of Chitosan Tripolyphosphate Nanoparticles and Its Encapsulation Efficiency Containing Russell’s Viper Snake Venom. J. Biochem. Mol. Toxicol. 2013, 27, 406–411. [Google Scholar] [CrossRef]
- Tanjoni, I.; Weinlich, R.; Della-Casa, M.S.; Clissa, P.B.; Saldanha-Gama, R.F.; de Freitas, M.S.; Barja-Fidalgo, C.; Amarante-Mendes, G.P.; Moura-da-Silva, A.M. Jararhagin, a Snake Venom Metalloproteinase, Induces a Specialized Form of Apoptosis (Anoikis) Selective to Endothelial Cells. Apoptosis 2005, 10, 851–861. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of Snake Venom Metalloproteinases in Cell Biology: Effects on Platelets, Inflammatory and Endothelial Cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef]
- Wu, W.-B.; Huang, T.-F. Activation of MMP-2, Cleavage of Matrix Proteins, and Adherens Junctions during a Snake Venom Metalloproteinase-Induced Endothelial Cell Apoptosis. Exp. Cell Res. 2003, 288, 143–157. [Google Scholar] [CrossRef] [PubMed]


| Sample | DH (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| Crude venom | 1618 ± 112 | 0.60 | −4.2 ± 0.4 |
| Filtered venom | 175 ± 2.7 | 0.22 | −5.0 ± 0.2 |
| Sample a | DH (nm) | PDI | ζ Potential (mV) | EE (%) | LC (%) | Yield (%) |
|---|---|---|---|---|---|---|
| Cs-g-PVCL NP | 244 ± 1.2 | 0.28 | + 30.8 ± 0.7 | - | - | 89.0 |
| Venom-loaded NP | 628 ± 2.8 | 0.90 | + 31.7 ± 0.8 | 88.0 ± 2.2 | 22 ± 1.6 | 86.5 |
| Cs-g-PVCL NP—0.4% NaCl | 198 ± 0.9 | 0.19 | + 21.8 ± 2.1 | - | - | 90.0 |
| Venom-loaded NP—0.4% NaCl | 222 ± 1.1 | 0.24 | + 32.0 ± 1.1 | 88.6 ± 0.6 | 22.3 ± 0.8 | 84.0 |
| Cs-g-PVCL NP—0.8% NaCl | 262 ± 1.2 | 0.15 | + 17.5 ± 2.2 | - | - | 88.2 |
| Venom-loaded NP—0.8% NaCl | 283 ± 1.6 | 0.47 | + 25.4 ± 1.4 | 85.1 ± 1.5 | 21.2 ± 0.6 | 85.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudy, S.S.; Jimenez-Canale, J.; Sarabia-Sainz, J.A.; Guzmán Partida, A.M.; Burgara-Estrella, A.J.; Silva-Campa, E.; Angulo Molina, A.; Montiel-Herrera, M.; Flores-Ramírez, N.; Zavala-Rivera, P.; et al. Chitosan-graft-poly(N-vinylcaprolactam) Nanoparticles Containing Crotalus atrox Snake Venom: Biological and Physicochemical Characterization. Nanomaterials 2025, 15, 1538. https://doi.org/10.3390/nano15191538
Rudy SS, Jimenez-Canale J, Sarabia-Sainz JA, Guzmán Partida AM, Burgara-Estrella AJ, Silva-Campa E, Angulo Molina A, Montiel-Herrera M, Flores-Ramírez N, Zavala-Rivera P, et al. Chitosan-graft-poly(N-vinylcaprolactam) Nanoparticles Containing Crotalus atrox Snake Venom: Biological and Physicochemical Characterization. Nanomaterials. 2025; 15(19):1538. https://doi.org/10.3390/nano15191538
Chicago/Turabian StyleRudy, Serena Sophia, Jorge Jimenez-Canale, Jose A. Sarabia-Sainz, Ana María Guzmán Partida, Alexel J. Burgara-Estrella, Erika Silva-Campa, Aracely Angulo Molina, Marcelino Montiel-Herrera, Nelly Flores-Ramírez, Paul Zavala-Rivera, and et al. 2025. "Chitosan-graft-poly(N-vinylcaprolactam) Nanoparticles Containing Crotalus atrox Snake Venom: Biological and Physicochemical Characterization" Nanomaterials 15, no. 19: 1538. https://doi.org/10.3390/nano15191538
APA StyleRudy, S. S., Jimenez-Canale, J., Sarabia-Sainz, J. A., Guzmán Partida, A. M., Burgara-Estrella, A. J., Silva-Campa, E., Angulo Molina, A., Montiel-Herrera, M., Flores-Ramírez, N., Zavala-Rivera, P., & Fernández-Quiroz, D. (2025). Chitosan-graft-poly(N-vinylcaprolactam) Nanoparticles Containing Crotalus atrox Snake Venom: Biological and Physicochemical Characterization. Nanomaterials, 15(19), 1538. https://doi.org/10.3390/nano15191538









