Calcium Phosphate Nanoparticles Functionalized with a Cardio-Specific Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.2.1. Preparation of MP CaP NPs
2.2.2. Preparation of PAA MP CaP NPs
2.3. MP Quantification by HPLC
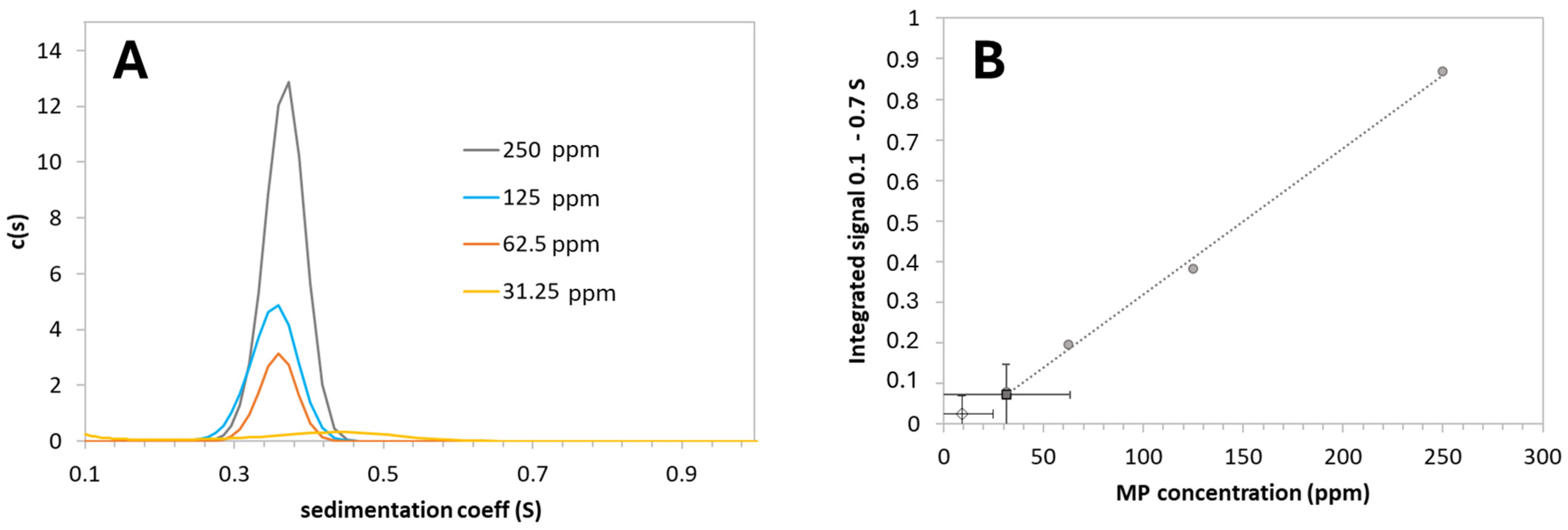
2.4. Analytical Ultracentrifugation
2.5. NPs Characterization
2.6. Biological Evaluation
3. Results and Discussion
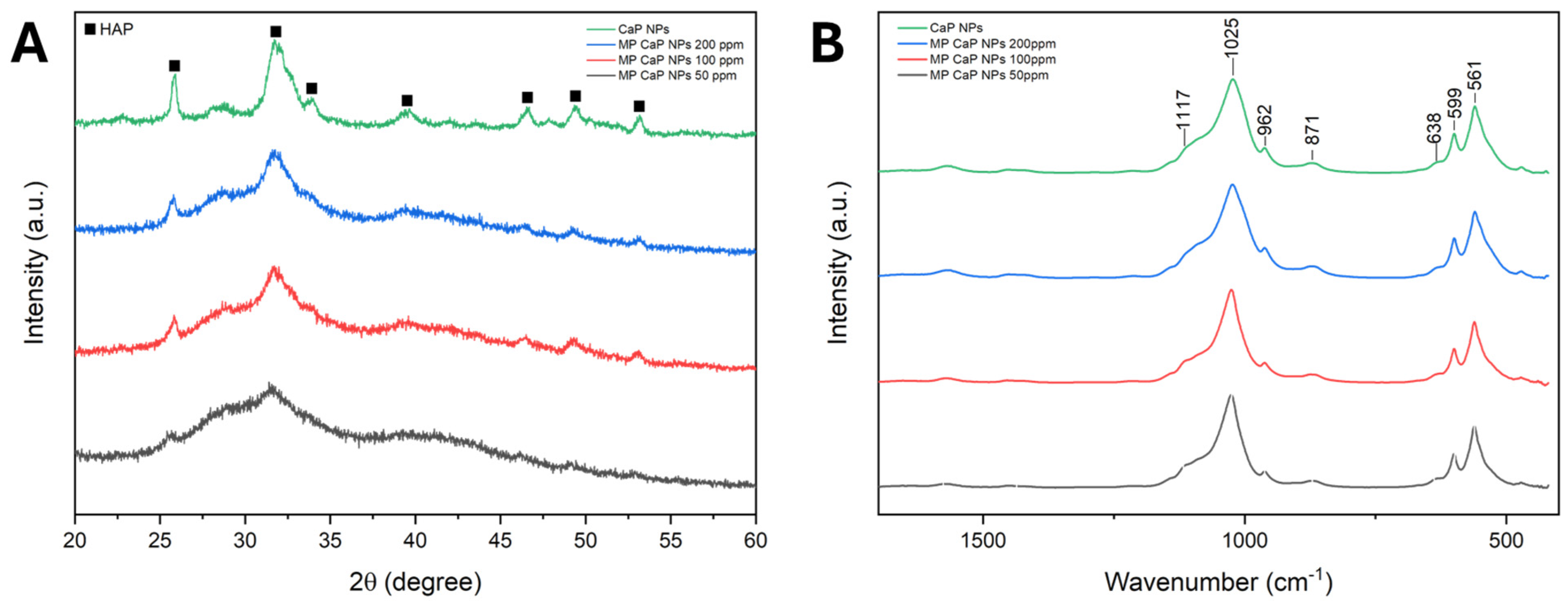
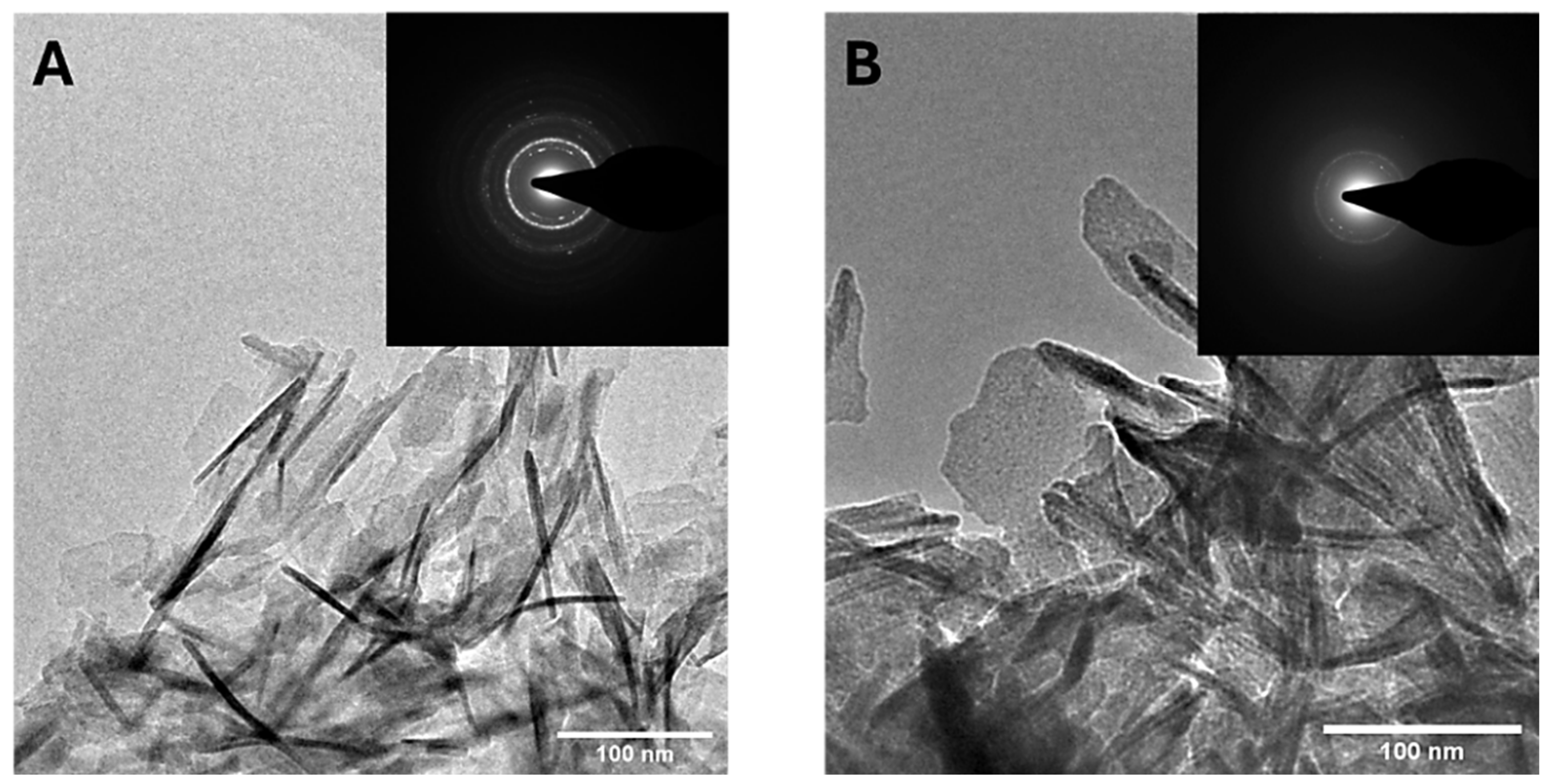
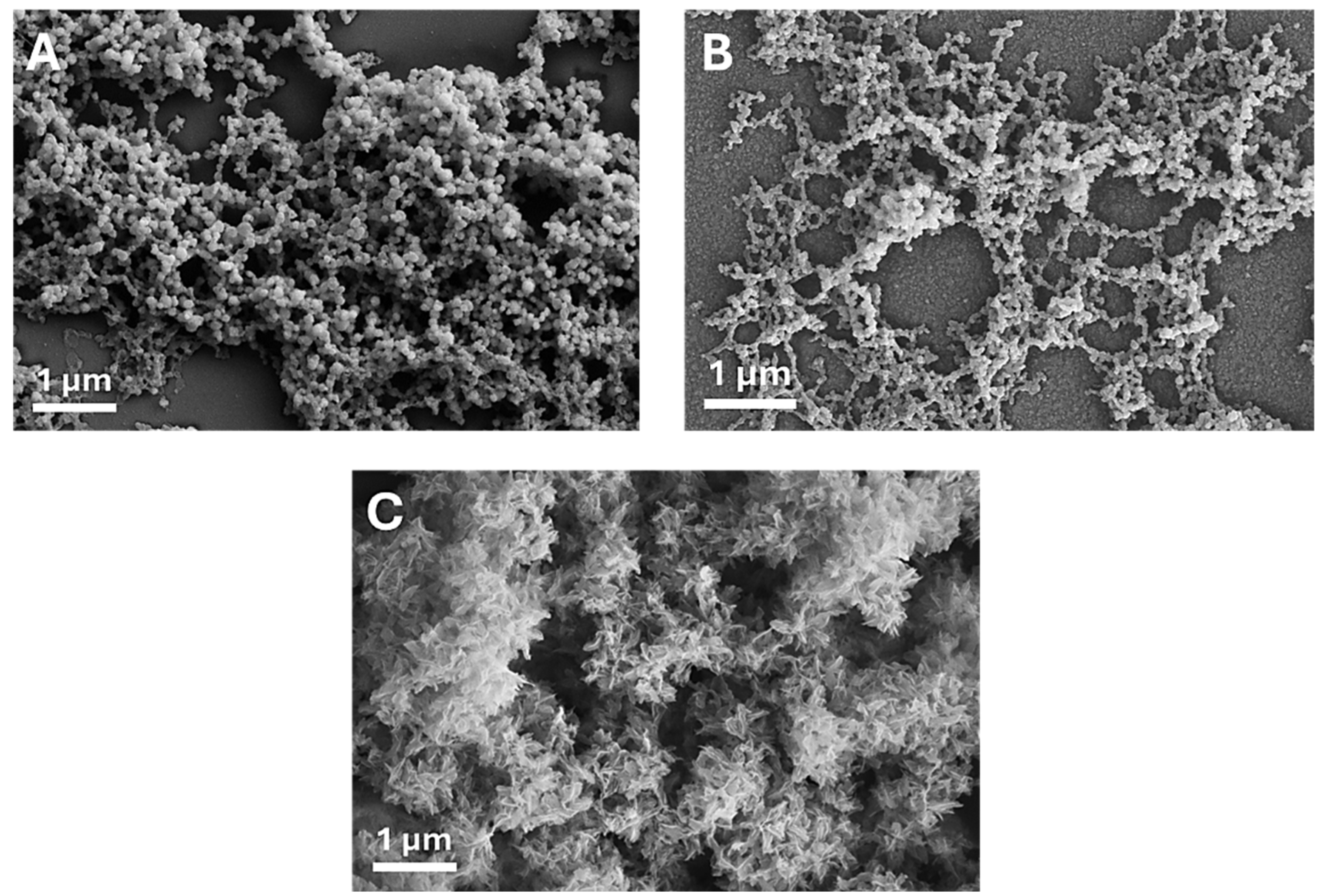
3.1. MP CaP NPs Characterization
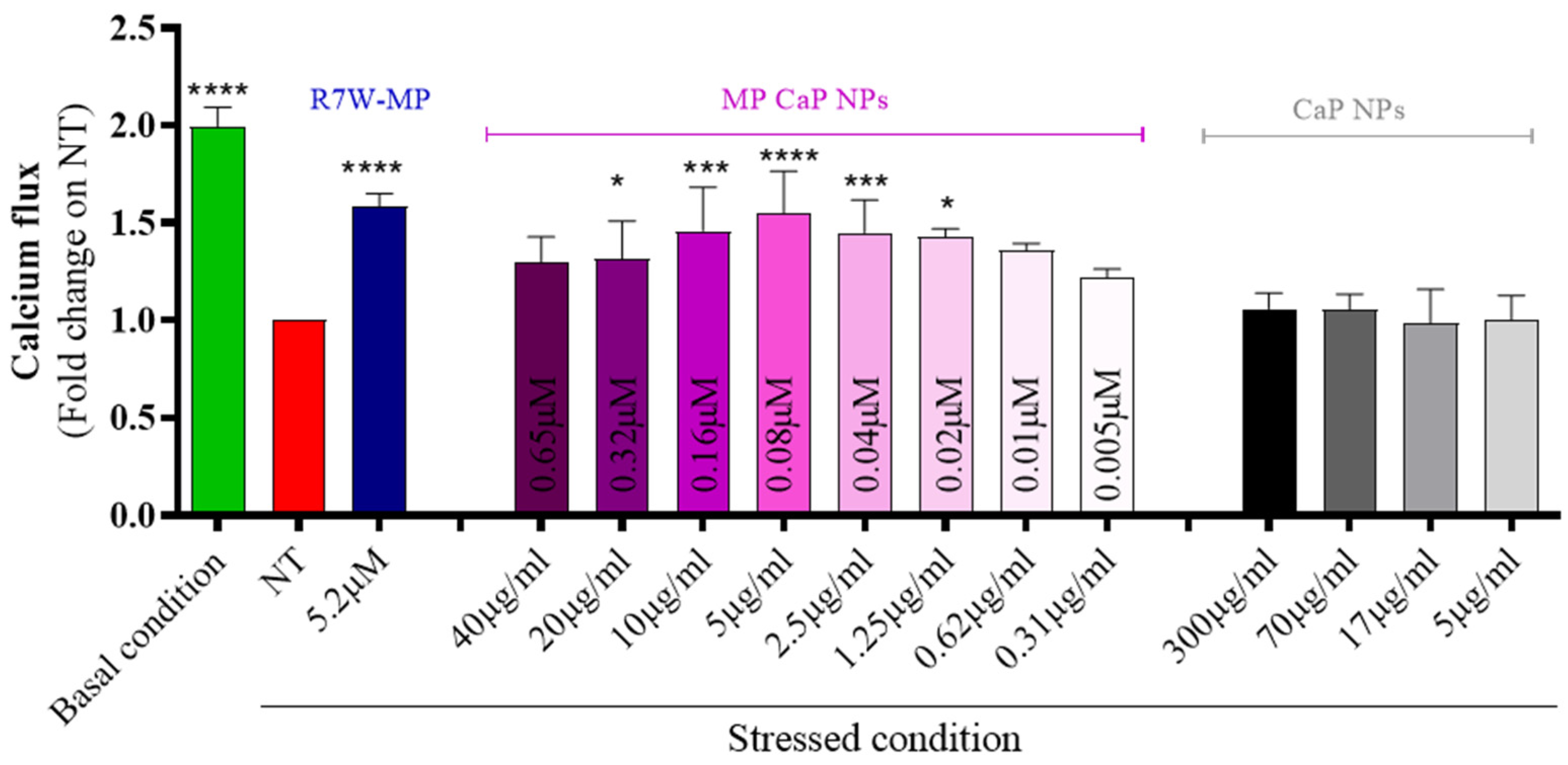
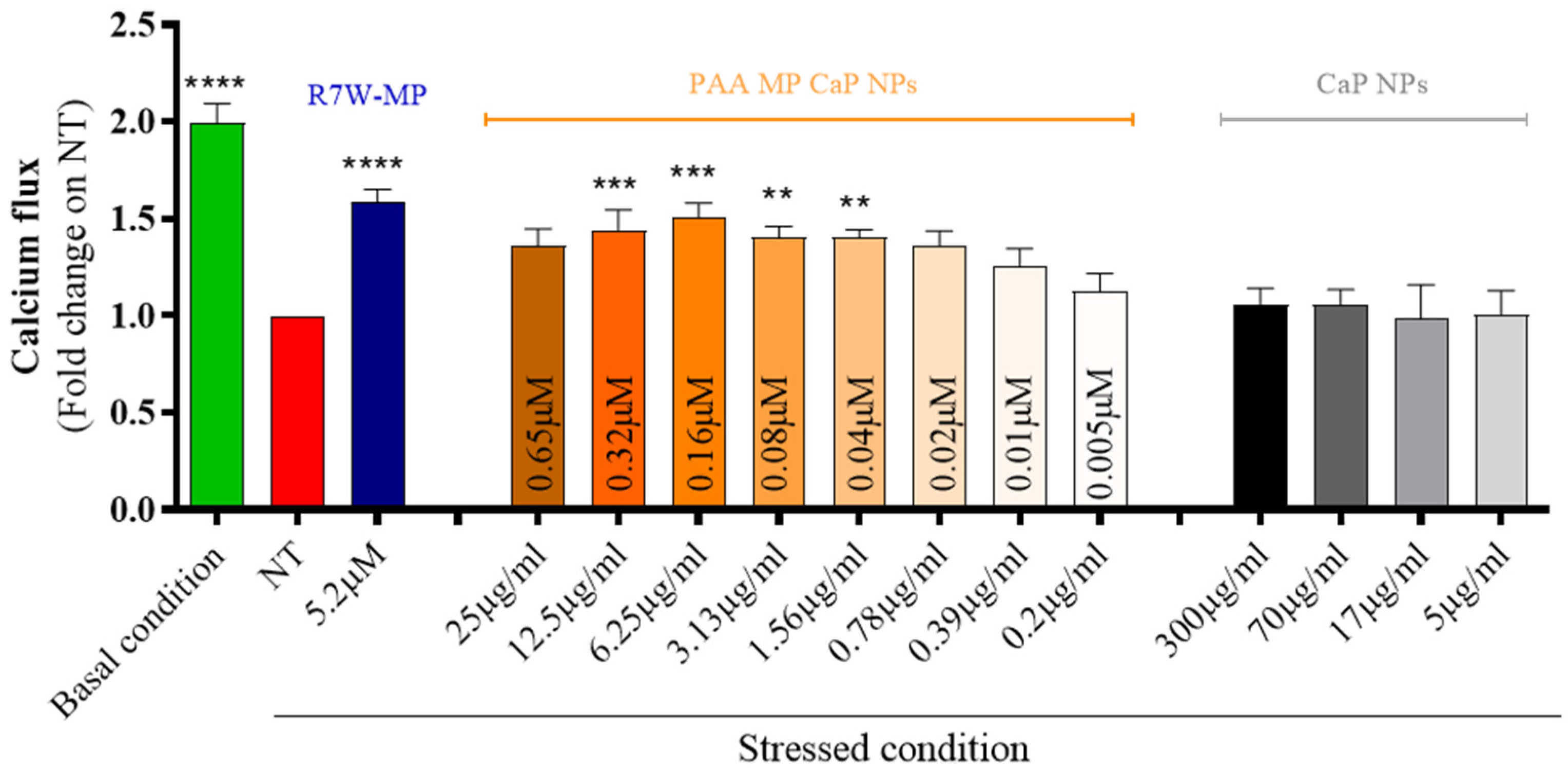
3.2. MP CaP NPs 200 ppm Biological Evaluation
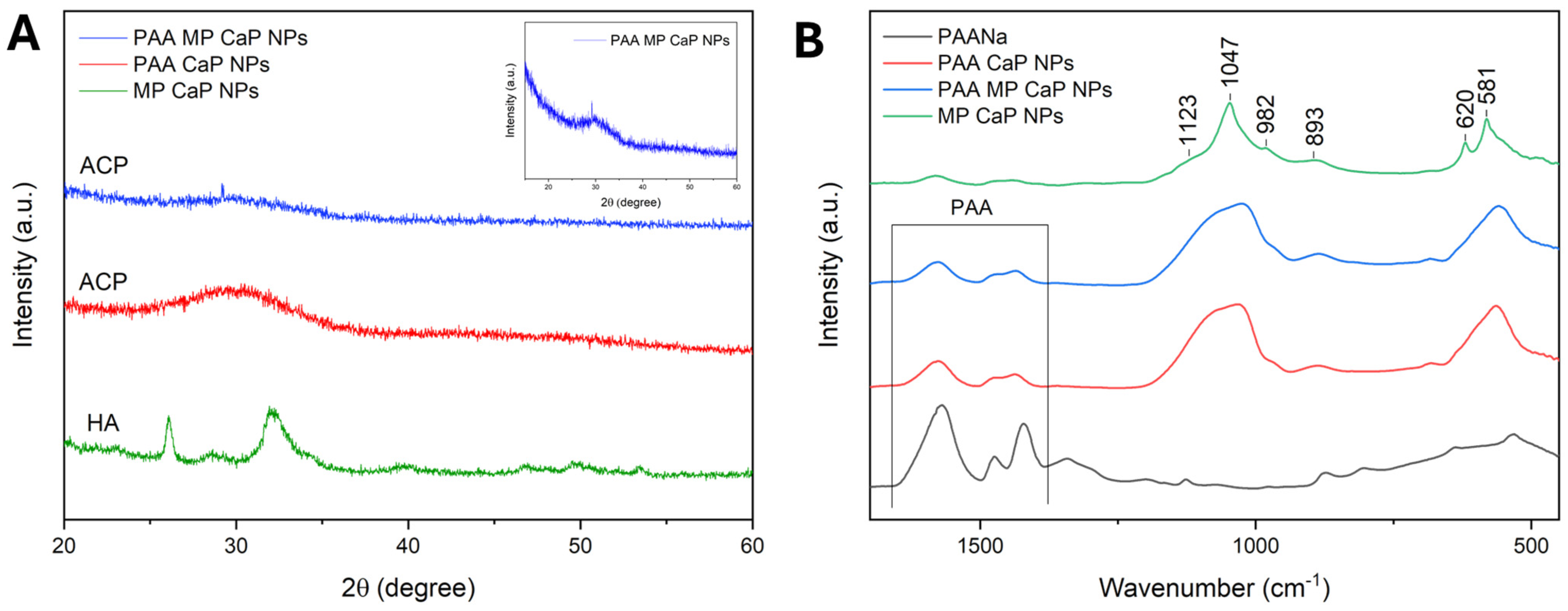
3.3. PAA MP CaP NP Characterization
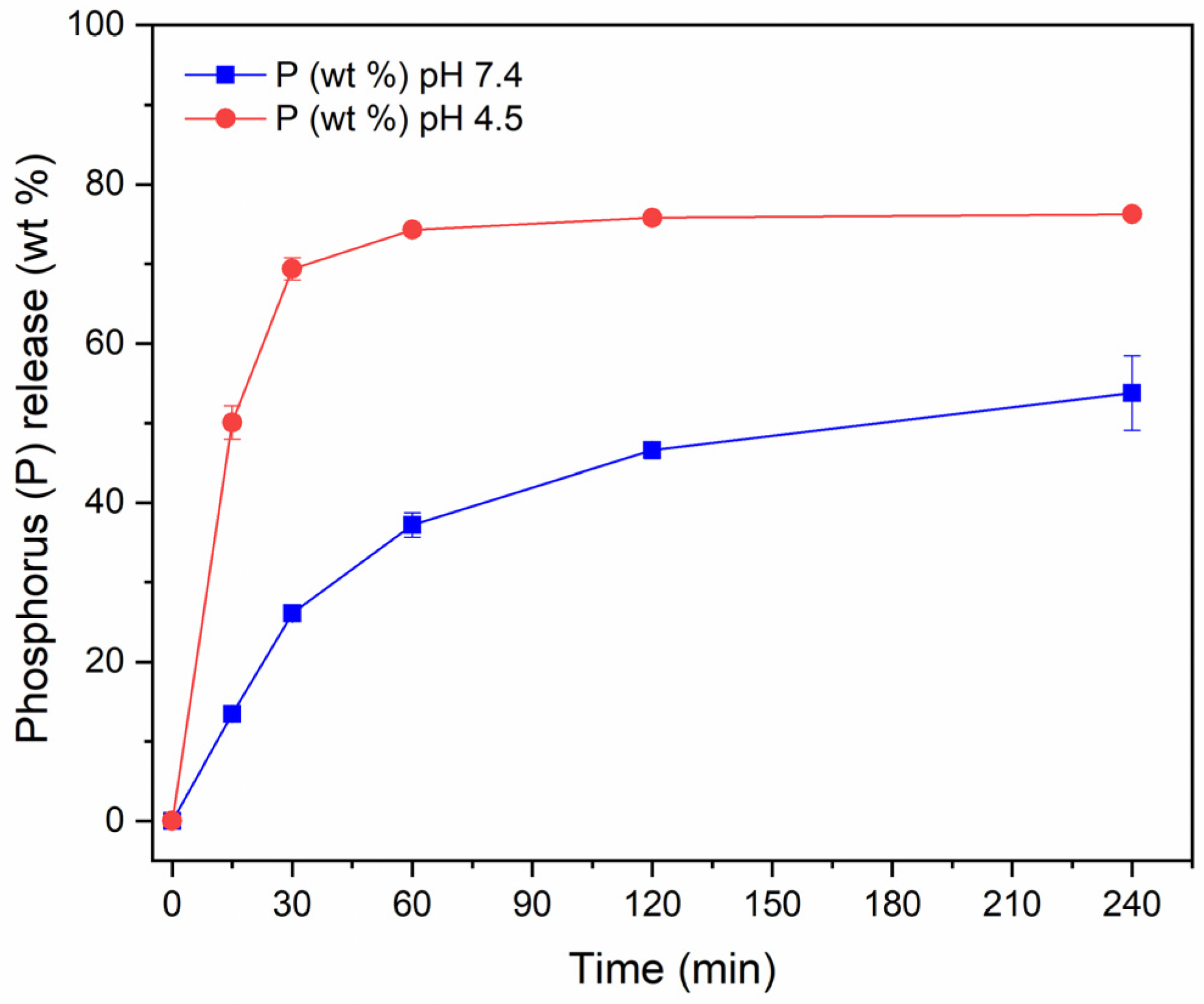
3.4. PAA MP CaP NPs Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diab Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef]
- Greenberg, B.H. Heart failure epidemic. Curr. Cardiol. Rep. 2002, 4, 185. [Google Scholar] [CrossRef]
- Rossin, D.; Vanni, R.; Iacono, M.L.; Cristallini, C.; Giachino, C.; Rastaldo, R. APJ as Promising Therapeutic Target of Peptide Analogues in Myocardial Infarction- and Hypertension-Induced Heart Failure. Pharmaceutics 2023, 15, 1408. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodríguez, G.B.; Patrício, T.; Esposti, L.D.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10, eaan6205. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef]
- Cannone, V.; Cabassi, A.; Volpi, R.; Burnett, J.C. Atrial Natriuretic Peptide: A Molecular Target of Novel Therapeutic Approaches to Cardio-Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 3265. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.J.; Samli, K.N.; Johnston, S.A.; Brown, K.C. In vitro Selection of a Peptide with High Selectivity for Cardiomyocytes In vivo. J. Mol. Biol. 2004, 342, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Sahagun, D.; Zahid, M. Cardiac-Targeting Peptide: From Discovery to Applications. Biomolecules 2023, 13, 1690. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, F.; Ceriotti, P.; Miragoli, M.; Carullo, P.; Salvarani, N.; Rocchetti, M.; Di Pasquale, E.; Rossi, S.; Tessari, M.; Caprari, S.; et al. Peptidomimetic Targeting of Cavβ2 Overcomes Dysregulation of the L-Type Calcium Channel Density and Recovers Cardiac Function. Circulation 2016, 134, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Arina, P.; Sorge, M.; Gallo, A.; Di Mauro, V.; Vitale, N.; Cappello, P.; Brazzi, L.; Barandalla-Sobrados, M.; Cimino, J.; Ranieri, V.M.; et al. Modulation of LTCC Pathways by a Melusin Mimetic Increases Ventricular Contractility During LPS-Induced Cardiomyopathy. Shock 2022, 57, 318–325. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. <Bruno2013.Pdf>. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [PubMed]
- Alogna, A.; Berboth, L.; Faragli, A.; Ötvös, J.; Muzio, F.P.L.; di Mauro, V.; Modica, J.; Quarta, E.; Semmler, L.; Deißler, P.M.; et al. Lung-to-Heart Nano-in-Micro Peptide Promotes Cardiac Recovery in a Pig Model of Chronic Heart Failure. J. Am. Coll. Cardiol. 2024, 83, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Epple, M.; Kovtun, A. Functionalized Calcium Phosphate Nanoparticles for Biomedical Application. Key Eng. Mater. 2010, 441, 299–305. [Google Scholar] [CrossRef]
- Piao, Y.; Bei, H.P.; Tam, A.; Yang, Y.; Zhang, Q.; Yang, M.; Zhao, X. Calcium phosphate nanoparticle-based systems for therapeutic delivery. Theranostic Bionanomaterials 2019, 147–164. [Google Scholar] [CrossRef]
- Amornkitbamrung, U.; In, Y.; Lee, J.H.; Wang, Z.; Oh, S.H.; Shin, H.; Yoon, D.S.; Shin, H. Interface-Controlled Biomimetic Intrafibrillar Mineralization of Collagen: Effect of Ca2+/[PO4]3− Concentration Ratio. Adv. Mater. Interfaces 2023, 10, 2300384. [Google Scholar] [CrossRef]
- Jang, S.; Lee, S.; Kim, H.; Ham, J.; Seo, J.-H.; Mok, Y.; Noh, M.; Lee, Y. Preparation of pH-sensitive CaP nanoparticles coated with a phosphate-based block copolymer for efficient gene delivery. Polymer 2012, 53, 4678–4685. [Google Scholar] [CrossRef]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B.; Gerasimov, K.B.; Prosanov, I.Y.; Kompankov, N.B.; Lapina, O.B.; Papulovskiy, E.S.; Ishchenko, A.V.; Makarova, S.V. Possibilities of Mechanochemical Synthesis of Apatites with Different Ca/P Ratios. Ceramics 2022, 5, 404–422. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. Biomed. Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed]
- Mehn, D.; Iavicoli, P.; Cabaleiro, N.; Borgos, S.E.; Caputo, F.; Geiss, O.; Calzolai, L.; Rossi, F.; Gilliland, D. Analytical ultracentrifugation for analysis of doxorubicin loaded liposomes. Int. J. Pharm. 2017, 523, 320–326. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Tanaka, Y.; Vegelyte, V.; Nishimoto, Y.; Ishikawa, K.; Kareiva, A. A novel synthetic approach to low-crystallinity calcium deficient hydroxyapatite. Ceram. Int. 2019, 45, 15620–15623. [Google Scholar] [CrossRef]
- Hutchens, S.; Benson, R.; Evans, B.; Oneill, H.; Rawn, C. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef]
- Janairo, J.I.B. Peptide-Mediated Biomineralization; Springer: Berlin, Germany, 20016. [Google Scholar]
- Li, Y.; Huang, Y. Morphology-controlled synthesis of platinum nanocrystals with specific peptides. Adv. Mater. 2010, 22, 1921–1925. [Google Scholar] [CrossRef] [PubMed]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Kim, H.K.; Akaike, T.; Kim, S.C. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Markovic, S.; Ignjatovic, N.; Panseri, S.; Montesi, M.; Adamiano, A.; Fosca, M.; Rau, J.V.; Uskoković, V.; Iafisco, M. Thermal crystallization of amorphous calcium phosphate combined with citrate and fluoride doping: A novel route to produce hydroxyapatite bioceramics. J. Mater. Chem. B 2021, 9, 4832–4845. [Google Scholar] [CrossRef]
- Puvvada, N.; Kumar, P. Effect of temperature on morphology of triethanolamine-assisted synthesized hydroxyapatite nanoparticles. Appl. Nanosci. 2013, 3, 203–209. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A. Calcium phosphate nanoparticles: Second-generation nonviral vectors in gene therapy. Expert. Rev. Mol. Diagn. 2005, 5, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Tang, S.Q.; Wang, Y.J.; Wei, K. Formation of calcium phosphate nanoparticles in reverse microemulsions. Mater. Lett. 2005, 59, 210–214. [Google Scholar] [CrossRef]
- Di Mauro, V.; Ceriotti, P.; Lodola, F.; Salvarani, N.; Modica, J.; Bang, M.-L.; Mazzanti, A.; Napolitano, C.; Priori, S.G.; Catalucci, D. Peptide-Based Targeting of the L-Type Calcium Channel Corrects the Loss-of-Function Phenotype of Two Novel Mutations of the CACNA1 Gene Associated With Brugada Syndrome. Front. Physiol. 2021, 11, 616819. [Google Scholar] [CrossRef]
- Xu, C.; Yan, Y.; Tan, J.; Yang, D.; Jia, X.; Wang, L.; Xu, Y.; Cao, S.; Sun, S. Biodegradable Nanoparticles of Polyacrylic Acid–Stabilized Amorphous CaCO3 for Tunable pH-Responsive Drug Delivery and Enhanced Tumor Inhibition. Adv. Funct. Mater. 2019, 29, 1808146. [Google Scholar] [CrossRef]
- Wei, X.; Liu, X.; Wang, X.; Bao, Y.; Shi, X.; Sun, L. Synthesis of Calcium Bisphosphonate/Calcium Polyacrylate Spheres for Gene Delivery. ACS Omega 2017, 2, 2017–2025. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Sultana, S.; Alzahrani, N.; Alzahrani, R.; Alshamrani, W.; Aloufi, W.; Ali, A.; Najib, S.; Siddiqui, N.A. Stability issues and approaches to stabilised nanoparticles based drug delivery system. J. Drug Target. 2020, 28, 468–486. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Iafisco, M.; Esposti, L.D.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yang, X.; Hu, Q.; Cai, Z.; Liu, L.M.; Guo, L. Recent Progress of Amorphous Nanomaterials. Chem. Rev. 2023, 123, 8859–8941. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; York, P. Crystallization processes in pharmaceutical technology and drug delivery design. J. Cryst. Growth 2000, 211, 122–136. [Google Scholar] [CrossRef]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]


| Sample | Z-Average (nm) | PDI | ζ-Potential (mV) | CaP NP Concentration (mg/mL) | Ca/P (mol) | MP Loading (wt.%) |
|---|---|---|---|---|---|---|
| CaP NPs | 70 ± 1 | 0.19 | −9.4 ± 0.6 | 3.48 ± 0.08 | 1.48 | - |
| MP CaP NPs 50 ppm | 96 ± 2 | 0.18 | −1.7 ± 0.7 | 3.38 ± 0.01 | 1.45 | 0.4 |
| MP CaP NPs 100 ppm | 85 ± 1 | 0.27 | −2.1 ± 0.6 | 3.29 ± 0.04 | 1.46 | 1.0 |
| MP CaP NPs 200 ppm | 71 ± 1 | 0.21 | −6.0 ± 1.0 | 3.78 ± 0.04 | 1.46 | 2.4 |
| Sample | MP Loading (wt.%) | Free MP (%) | Retained MP * (%) | AUC-Corrected MP Loading (wt.%) |
|---|---|---|---|---|
| MP CaP NPs 200 ppm | 2.4 | 30 ± 2 | 70 ± 2 | 1.7 |
| Sample | Z-Average (nm) | PDI | ζ-Potential (mV) | CaP NPs Concentration (mg/mL) | Ca/P (mol) | PAANa (wt.%) | MP Loading (wt.%) |
|---|---|---|---|---|---|---|---|
| PAA MP CaP NPs | 182 ± 4 | 0.14 | −20.0 ± 1.0 | 1.23 ± 0.01 | 1.51 | 13 | 3.2 |
| PAA CaP NPs | 189 ± 2 | 0.12 | −18.5 ± 0.4 | 1.40 ± 0.01 | 1.40 | 13 | - |
| MP CaP NPs | 2505 ± 289 | 0.39 | −4.0 ± 0.6 | 3.11 ± 0.05 | 1.35 | - | 1.3 |
| Sample | MP Loading (wt.%) | Free MP (%) | Retained MP * (%) | AUC-Corrected MP Loading (wt.%) |
|---|---|---|---|---|
| PAA MP CaP NPs | 3.2 | 23 ± 1 | 77 ± 1 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, F.; Degli Esposti, L.; Adamiano, A.; Modica, J.; Catalucci, D.; Mehn, D.; Geiss, O.; Iafisco, M. Calcium Phosphate Nanoparticles Functionalized with a Cardio-Specific Peptide. Nanomaterials 2025, 15, 94. https://doi.org/10.3390/nano15020094
Mancini F, Degli Esposti L, Adamiano A, Modica J, Catalucci D, Mehn D, Geiss O, Iafisco M. Calcium Phosphate Nanoparticles Functionalized with a Cardio-Specific Peptide. Nanomaterials. 2025; 15(2):94. https://doi.org/10.3390/nano15020094
Chicago/Turabian StyleMancini, Federica, Lorenzo Degli Esposti, Alessio Adamiano, Jessica Modica, Daniele Catalucci, Dora Mehn, Otmar Geiss, and Michele Iafisco. 2025. "Calcium Phosphate Nanoparticles Functionalized with a Cardio-Specific Peptide" Nanomaterials 15, no. 2: 94. https://doi.org/10.3390/nano15020094
APA StyleMancini, F., Degli Esposti, L., Adamiano, A., Modica, J., Catalucci, D., Mehn, D., Geiss, O., & Iafisco, M. (2025). Calcium Phosphate Nanoparticles Functionalized with a Cardio-Specific Peptide. Nanomaterials, 15(2), 94. https://doi.org/10.3390/nano15020094











