A Cu(I)-Based MOF with Nonlinear Optical Properties and a Favorable Optical Limit Threshold
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synthesis of Cu-Bpy
2.2. The Z-Scan Technique
3. Results
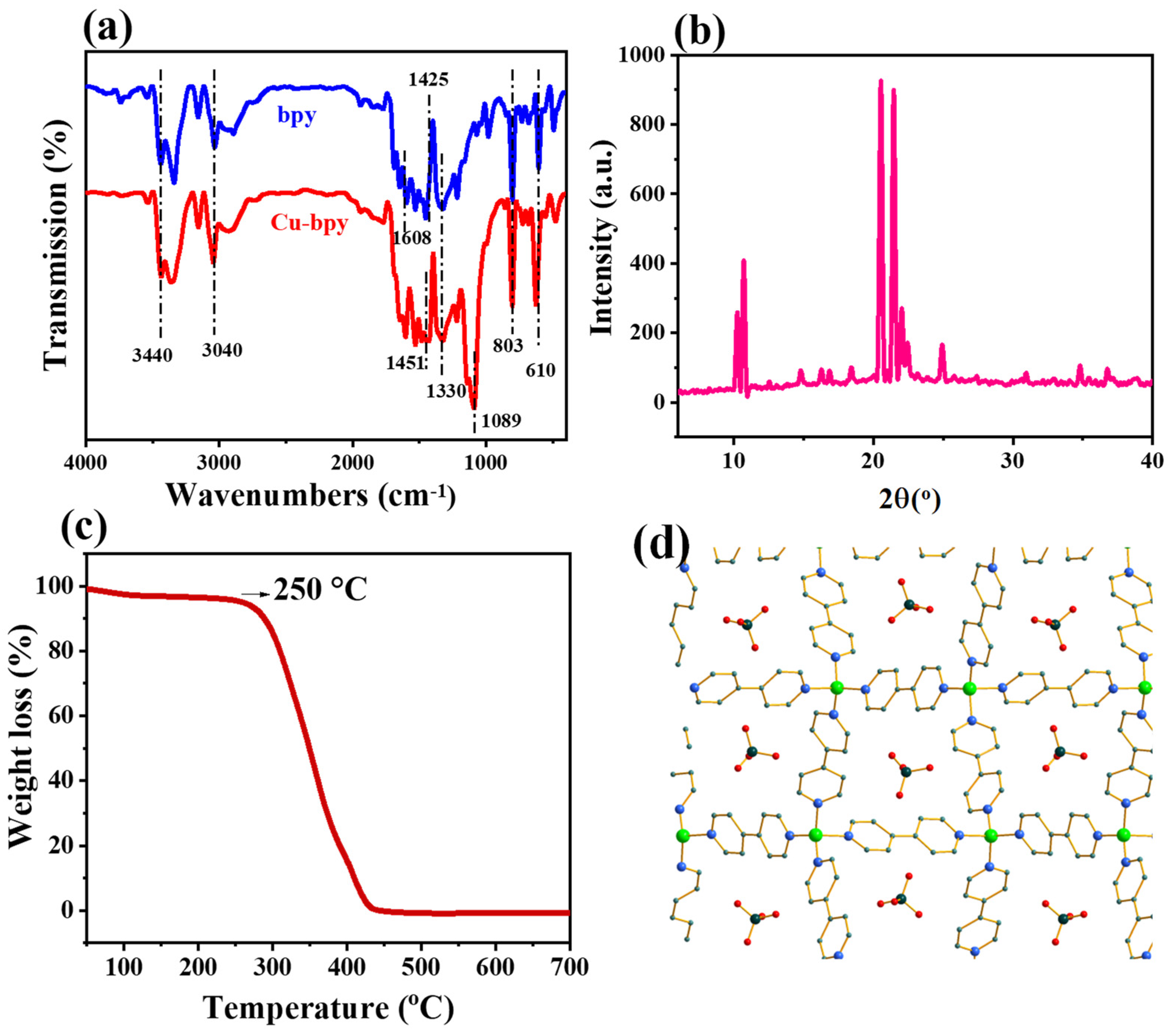
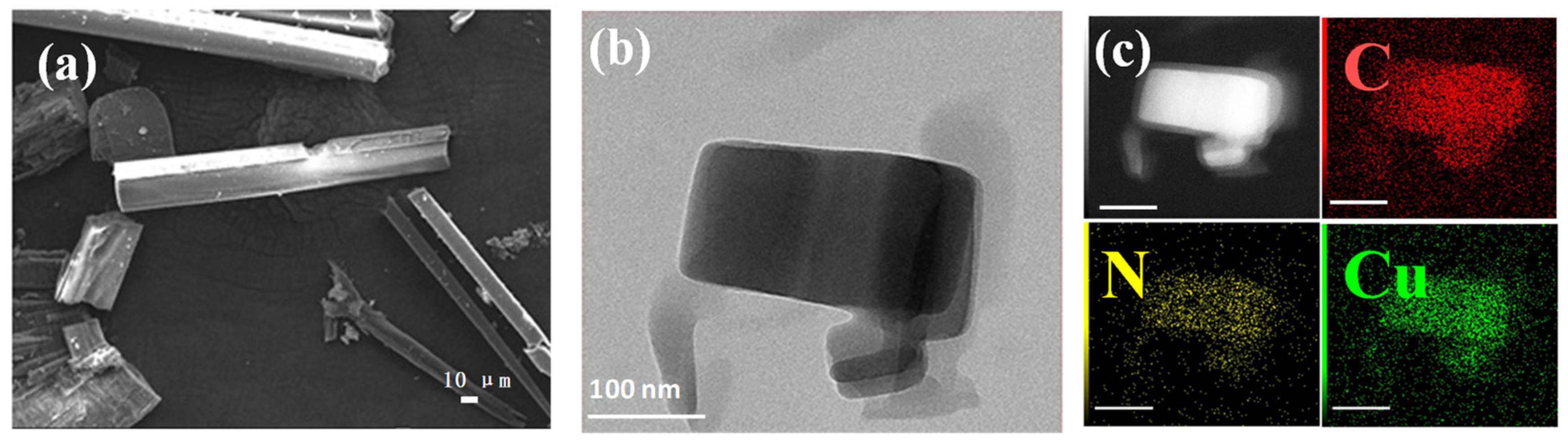
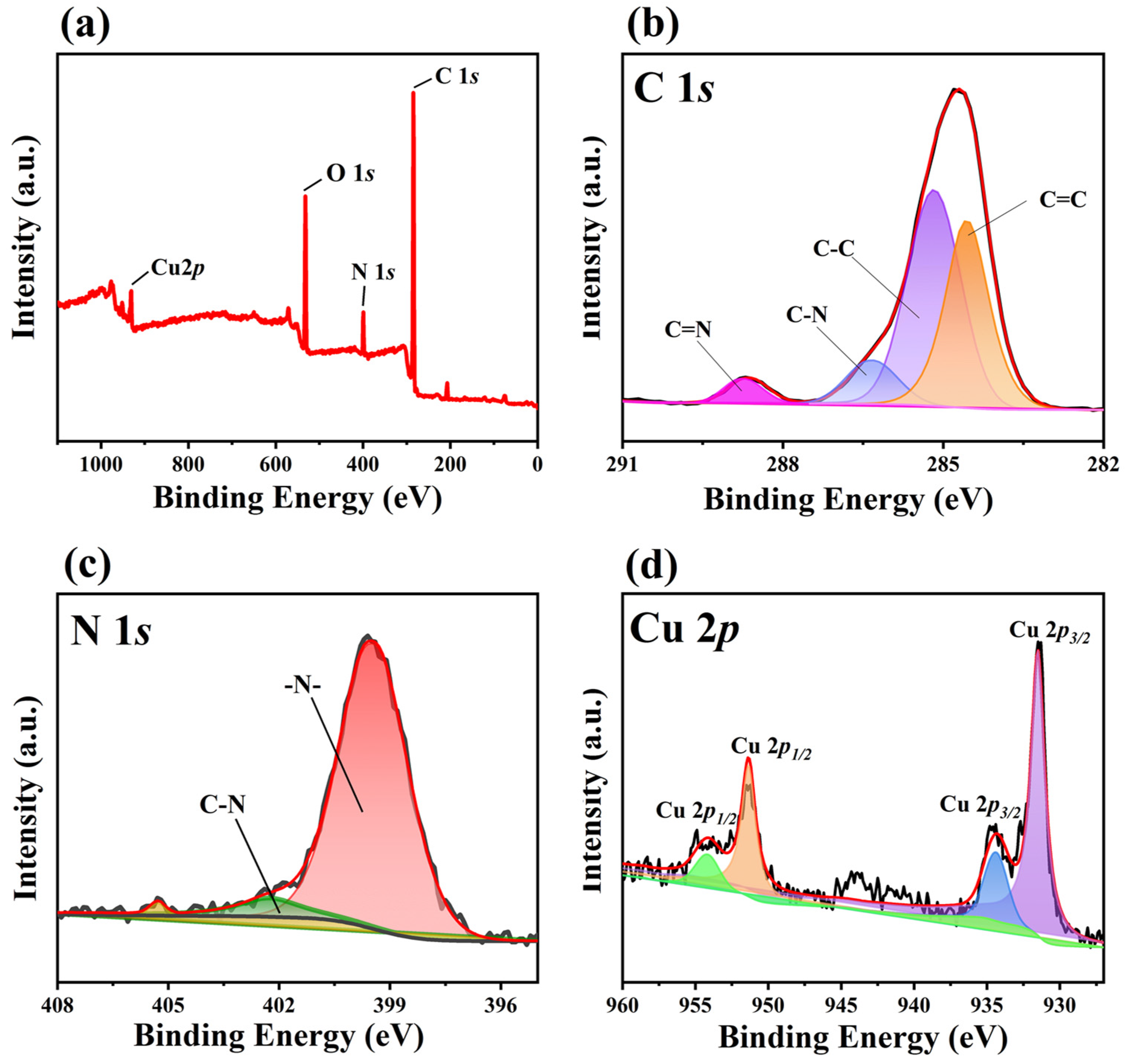
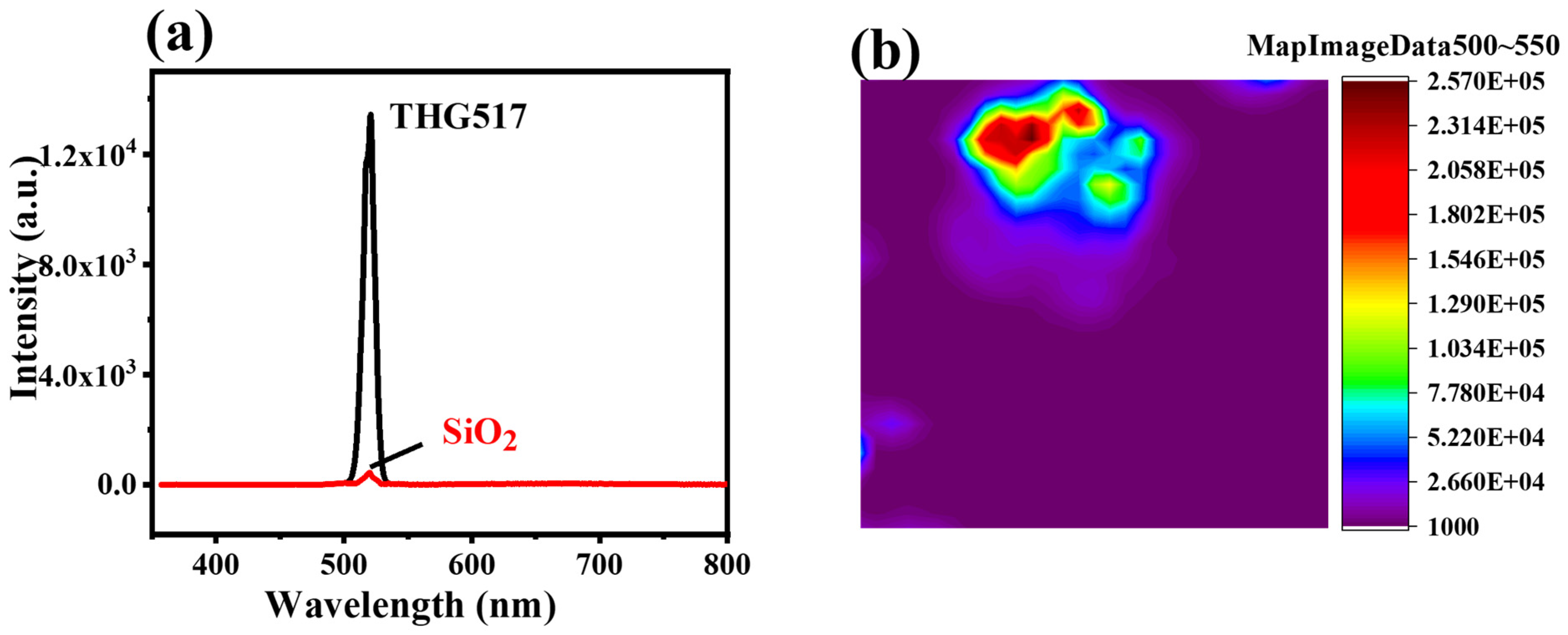
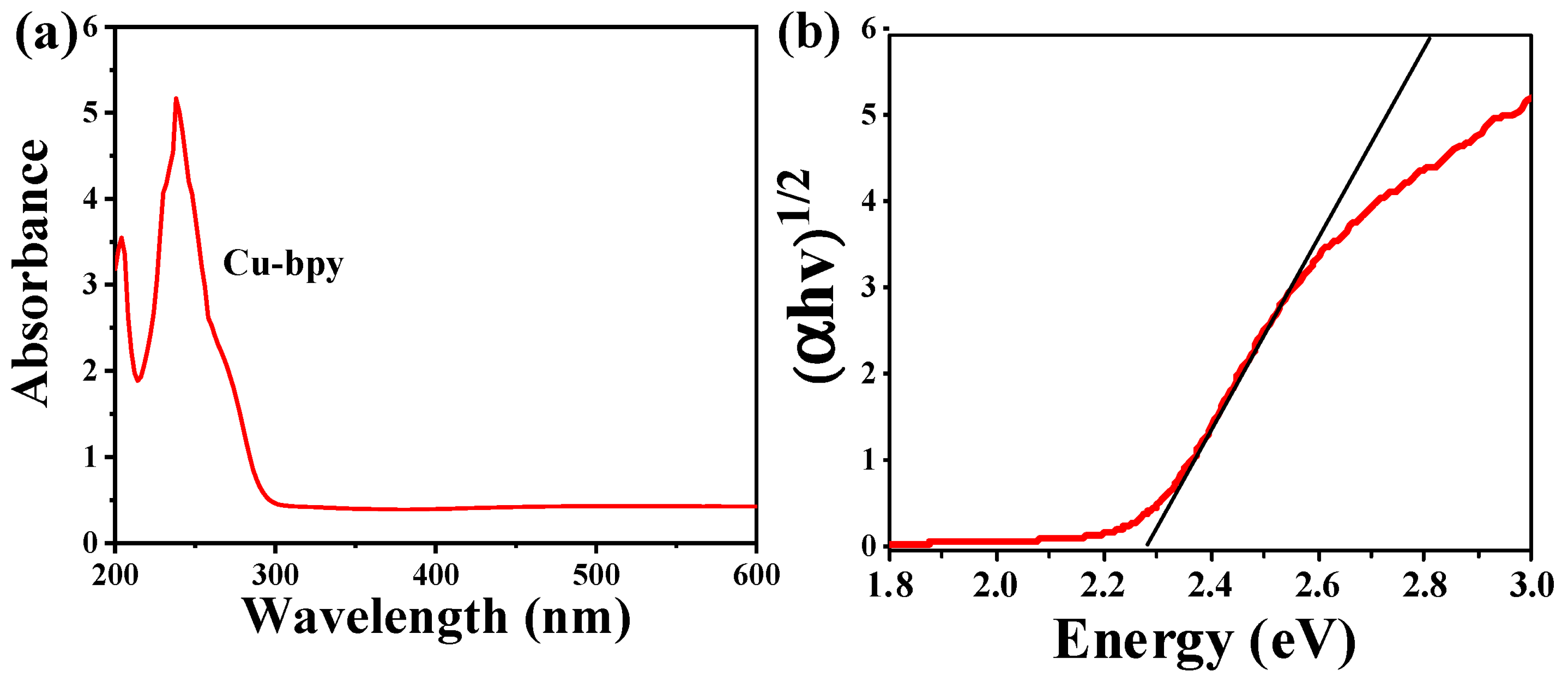
3.1. The Morphology and Structure of Cu-Bpy
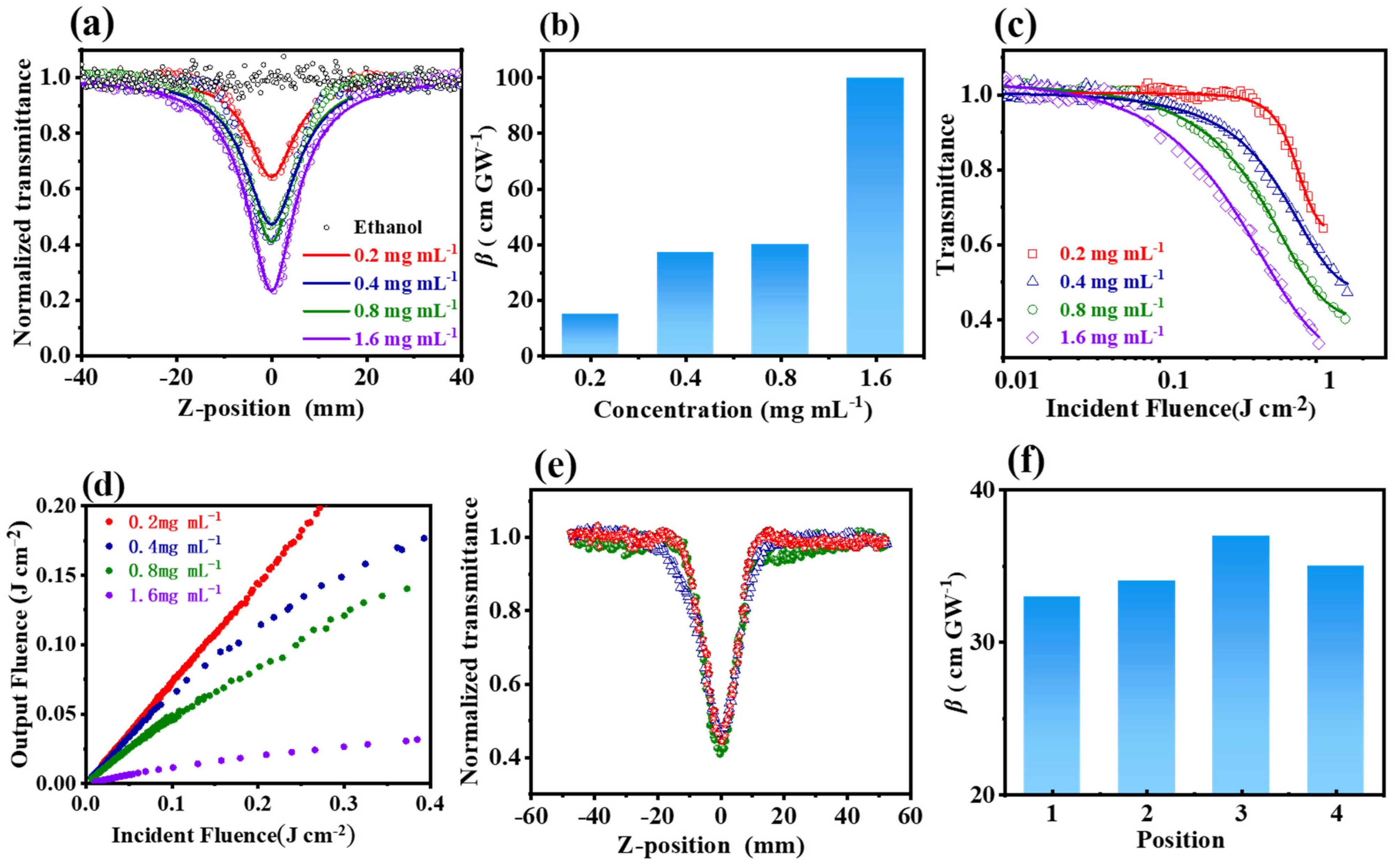
3.2. The Third Nonlinear Optical Properties of Cu-Bpy
| Materials | Timescale | Repetition Rate (Hz) | β (cm GW−1) | Fth (J cm−2) | Ref. |
|---|---|---|---|---|---|
| CDGO | nanosecond | 10 | 70 | - | [34] |
| Mn(dnpi)2 | nanosecond | 10 | 2.3 | [35] | |
| ZnCu-MOF | nanosecond | 10 | 44.7 | - | [36] |
| CH3NH3PbBr3 | nanosecond | 400 | 8.6 | - | [37] |
| MoS2-CuMOF | nanosecond | 10 | 60 | [38] | |
| ZnTPyP(Cu) | nanosecond | 5 | 5.7 × 105 | 7.8 | [39] |
| Zn2(TPyP)(AC)2 | nanosecond | 5 | 3.61 × 106 | 0.32 | [33] |
| Cu-bpy | nanosecond | 10 | 100 | 0.75 a | This work |
3.3. The Mechanism of the NLO Properties of Cu-Bpy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Zhang, C.; Xiao, S.; Wang, Y.; Fan, Y.; Liu, Y.; Zhang, N.; Qu, G.; Ji, H.; Han, J.; et al. Ultrafast control of vortex microlasers. Science 2020, 367, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tikhonov, E.; Tudi, A.; Kruglov, I.; Hou, X.; Xie, C.; Pan, S.; Yang, Z. Target-Driven Design of Deep-UV Nonlinear Optical Materials via Interpretable Machine Learning. Adv. Mater. 2023, 35, 2300848. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xiao, X.; Ye, X.; Li, W.; Zheng, C. Nonlinear optical and optical limiting properties of ultra-long gold nanowires. Mater. Lett. 2016, 166, 51–54. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Li, Y.; Zhang, J.; Xu, F.; Zheng, C.; Li, F. Cs3Sb2Br9: A lead-free perovskite quantum dots as optical limiting material with favourable optical limiting threshold and nonlinear optical properties. Opt. Mater. 2024, 149, 115008. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Wu, J.; Hou, H. Heterogeneous Nanosized Metal (Metallic Compound)@Metal-Organic Framework Composites: Recent Advances in the Preparation and Applications. Adv. Funct. Mater. 2023, 33, 2302573. [Google Scholar] [CrossRef]
- Shao, Z.; Chen, J.; Xie, Q.; Mi, L. Functional metal/covalent organic framework materials for triboelectric nanogenerator. Coord. Chem. Rev. 2023, 486, 215118. [Google Scholar] [CrossRef]
- Gao, K.; Chen, J.; Zhao, M.; Hu, R.; Chen, S.; Xue, X.; Shao, Z.; Hou, H. 3D nanocrystalline metal–organic framework materials for the improved output performance of triboelectric nanogenerators. Dalton Trans. 2023, 52, 444–451. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Díaz, U.; Cohen, B.; Douhal, A. Synthesis, characterization & catalysis of ITQ 2D metal–organic frameworks and spectroscopic & photodynamic properties of their composites with organic dyes. J. Mater. Chem. C 2023, 11, 14043–14069. [Google Scholar] [CrossRef]
- Chen, J.; Shao, Z.; Zhao, Y.; Xue, X.; Song, H.; Wu, Z.; Cui, S.; Zhang, L.; Huang, C.; Mi, L.; et al. Metal-Ion Coupling in Metal–Organic Framework Materials Regulating the Output Performance of a Triboelectric Nanogenerator. Inorg. Chem. 2022, 61, 2490–2498. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Meng, X.; Ding, J.; Hou, H. Metal organic framework composites as adsorbents: Synergistic effect for water purification. Coord. Chem. Rev. 2022, 473, 214815. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Z.; Cui, Y.; Geng, K.; Meng, X.; Wu, J.; Hou, H. Guest-Induced Multilevel Charge Transport Strategy for Developing Metal-Organic Frameworks to Boost Photocatalytic CO2 Reduction. Small 2023, 19, 2300398. [Google Scholar] [CrossRef] [PubMed]
- Medishetty, R.; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Huang, G.; Lustig, W.P.; Wang, F.; Wang, H.; Teat, S.J.; Banerjee, D.; Zhang, D.; Li, J. Achieving exceptionally high luminescence quantum efficiency by immobilizing an AIE molecular chromophore into a metal–organic framework. Chem. Commun. 2015, 51, 3045–3048. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincă, M. Grand Challenges and Future Opportunities for Metal–Organic Frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef]
- Hales, J.M.; Cozzuol, M.; Screen, T.E.; Anderson, H.L.; Perry, J.W. Metalloporphyrin polymer with temporally agile, broadband nonlinear absorption for optical limiting in the near infrared. Opt. Express 2009, 17, 18478–18488. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Nyk, M.; Samoć, M. Nonlinear Optical Properties of Emerging Nano-and Microcrystalline Materials. Adv. Opt. Mater. 2021, 9, 2100216. [Google Scholar] [CrossRef]
- Cheng, X.; Yao, J.; Zhang, H.; Wang, X.; Bai, J. The nonlinear optical properties of two-dimensional metal-organic framework. J. Alloys Compd. 2021, 855, 157433. [Google Scholar] [CrossRef]
- Teran, N.B.; He, G.S.; Baev, A.; Shi, Y.R.; Swihart, M.T.; Prasad, P.N.; Marks, T.J.; Reynolds, J.R. Twisted Thiophene-Based Chromophores with Enhanced Intramolecular Charge Transfer for Cooperative Amplification of Third-Order Optical Nonlinearity. J. Am. Chem. Soc. 2016, 138, 6975–6984. [Google Scholar] [CrossRef]
- Ravindra, H.J.; Kiran, A.J.; Chandrasekharan, K.; Shashikala, H.D.; Dharmaprakash, S.M. Third order nonlinear optical properties and optical limiting in donor/acceptor substituted 4′-methoxy chalcone derivatives. Appl. Phys. B 2007, 88, 105–110. [Google Scholar] [CrossRef]
- Sunitha, M.S.; Adhikari, A.V.; Vishnumurthy, K.A.; Safakath, K.; Philip, R. Large Third-Order Nonlinearity of New π-conjugated Donor-Acceptor Polymers with Substituted Thiophene and 1,3,4-Oxadiazole Moieties. Int. J. Polym. Mater. Polym. Biomater. 2012, 61, 483–504. [Google Scholar] [CrossRef]
- Ricci, F.; Carlotti, B.; Keller, B.; Bonaccorso, C.; Fortuna, C.G.; Goodson, T.; Elisei, F.; Spallettit, A. Enhancement of Two-Photon Absorption Parallels Intramolecular Charge-Transfer Efficiency in Quadrupolar versus Dipolar Cationic Chromophores. J. Phys. Chem. C 2017, 121, 3987–4001. [Google Scholar] [CrossRef]
- Liang, Y.; Hu, W.; Yuan, X.; Zeng, Z.; Zhu, B.; Gu, Y. Switchable Nonlinear Optical Absorption of Metal–Organic Frameworks. Adv. Opt. Mater. 2022, 10, 2200779. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yao, X.J.; Wang, R.L.; Xie, C.Z. Poly[[bis(μ-4,4′-bipyridine-κ2 N:N′)copper(I)] perchlorate 0.24-hydrate]. Acta Crystallogr. E 2012, 68, m660–m661. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, J.; Tian, F.; Yan, W.; Tang, J.; Chen, Z.; Liang, W.; Shi, D.; Chen, D. Cu(I)-4,4′-bipyridine coordination polymer for photocatalytic H2 generation. J. Mol. Struct. 2024, 1301, 137332. [Google Scholar] [CrossRef]
- Crist, B.V. The XPS library website: A resource for the XPS community including—The XPS library of information, XPS spectra-base having >70,000 monochromatic XPS spectra, and spectral data processor (SDP) v8.0 software. J. Electron. Spectrosc. Relat. Phenom. 2021, 248, 147046. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Lun, K.; Wu, B.; He, L.; Wang, M.; Fang, S.; Zhang, Z.; Zhou, L. Sensitive detection of Escherichia coli in diverse foodstuffs by electrochemical aptasensor based on 2D porphyrin-based COF. Microchim. Acta 2023, 190, 421. [Google Scholar] [CrossRef]
- Cui, J.; Kan, L.; Li, Z.; Yang, L.; Wang, M.; He, L.; Lou, Y.; Xue, Y.; Zhang, Z. Porphyrin-based covalent organic framework as bioplatfrom for detection of vascular endothelial growth factor 165 through fluorescence resonance energy transfer. Talanta 2021, 228, 122060. [Google Scholar] [CrossRef]
- Zhou, Q.-Q.; Miao, R.-Q.; Wang, D.-F.; Huang, R.-B. Syntheses, structures and properties of three novel Cu(II) coordination compounds based on 4,4′-oxybisbenzoic acid. J. Mol. Struct. 2020, 1206, 127688. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Z.; Wang, Y.; Wang, J.; Guo, Y.; Hu, H.; He, X.; Wang, C.; Lin, W. Photoactivation of Cu Centers in Metal–Organic Frameworks for Selective CO2 Conversion to Ethanol. J. Am. Chem. Soc. 2020, 142, 75–79. [Google Scholar] [CrossRef]
- Yang, E.-C.; Liu, Z.-Y.; Shi, X.-J.; Liang, Q.-Q.; Zhao, X.-J. Two 3D Triazolate−Tricarboxylate-Bridged CuII/I Frameworks by One-Pot Hydrothermal Synthesis Exhibiting Spin-Canted Antiferromagnetism and Strong Antiferromagnetic Couplings. Inorg. Chem. 2010, 49, 7969–7975. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Stryland, E.W.V. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, H.; Tian, X.; Qiao, T.; Hu, Z.; Chen, Z.; He, X.; Yu, Y.; Qiu, J. MoS2 nanoflowers as high performance saturable absorbers for an all-fiber passively Q-switched erbium-doped fiber laser. Nanoscale 2016, 8, 7704–7710. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Li, Q.-H.; Wang, Z.-R.; Ma, Z.-Z.; Gu, Z.-G.; Zhang, J. Interpenetrated Metal-Porphyrinic Framework for Enhanced Nonlinear Optical Limiting. J. Am. Chem. Soc. 2021, 143, 17162–17169. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Liang, C.; Li, J.; Dong, W.; Cui, X.; Duan, Q.J.O.; Technology, L. Synthesis of heteroatom Doped-Carbon dot graphene oxide nanocomposites and investigation of nonlinear optical properties. Opt. Laser Technol. 2023, 164, 109469. [Google Scholar] [CrossRef]
- Tang, G.; Kou, S.; Zhang, Z.; Tang, T.; Culnane, L.F.; Zhang, Y.; Song, Y. Synthesis, characterization and theoretical investigation of the structure, electronic and third-order nonlinear optical (NLO) properties of M(dnpi)2 (M = Mn2+ and Ni2+). Synth. Met. 2013, 182, 60–66. [Google Scholar] [CrossRef]
- Biswal, B.P.; Valligatla, S.; Wang, M.; Banerjee, T.; Saad, N.A.; Mariserla, B.M.K.; Chandrasekhar, N.; Becker, D.; Addicoat, M.; Senkovska, I.; et al. Nonlinear Optical Switching in Regioregular Porphyrin Covalent Organic Frameworks. Angew. Chem. Int. Edit. 2019, 58, 6896–6900. [Google Scholar] [CrossRef]
- Walters, G.; Sutherland, B.R.; Hoogland, S.; Shi, D.; Comin, R.; Sellan, D.P.; Bakr, O.M.; Sargent, E.H. Two-photon absorption in organometallic bromide perovskites. ACS Nano 2015, 9, 9340–9346. [Google Scholar] [CrossRef]
- Shang, L.; Wu, S.; Liu, X.; Duan, Y.; Li, Y.; Duan, Q. Novel structure and excellent nonlinear optical performance nanocomposite derived from flower-like MOF grown on MoS2. Dyes Pigm. 2024, 225, 112089. [Google Scholar] [CrossRef]
- Li, D.-J.; Li, Q.-H.; Gu, Z.-G.; Zhang, J. Oriented Assembly of 2D Metal-Pyridylporphyrinic Framework Films for Giant Nonlinear Optical Limiting. Nano Lett. 2021, 21, 10012–10018. [Google Scholar] [CrossRef]
- Zeki, H.F. Optical switching in organometallic phthalocyanine. J. Opt. A Pure Appl. Opt. 2001, 3, 188. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Q.; Shen, X.; Huang, D.; Yuan, C.; Wang, Y.; Ouyang, Q.; Fang, X.; Chen, X. Nonlinear Optical and Optical Limiting Properties of MAPbBr3/Polymethyl Methacrylate Organic Glasses. J. Phys. Chem. C 2022, 126, 5743–5750. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Utama, M.I.B.; Xing, G.; Sum, T.C.; Xiong, Q. Phonon-Assisted Anti-Stokes Lasing in ZnTe Nanoribbons. Adv. Mater. 2016, 28, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Gao, X.; Yu, Z.; Huang, Z.; Zhang, C. Superb Nonlinear Absorption of Triphenylene-Based Metal–Organic Frameworks Associated with Abundant Metal d Electrons. Adv. Opt. Mater. 2021, 9, 2100622. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Yang, Z.; Zhang, Y.; Fan, Z.; Wang, J.; Qin, X.; Gao, L.; Yang, H.; Liu, S.; Zhou, L.; et al. A Cu(I)-Based MOF with Nonlinear Optical Properties and a Favorable Optical Limit Threshold. Nanomaterials 2025, 15, 145. https://doi.org/10.3390/nano15020145
Cui J, Yang Z, Zhang Y, Fan Z, Wang J, Qin X, Gao L, Yang H, Liu S, Zhou L, et al. A Cu(I)-Based MOF with Nonlinear Optical Properties and a Favorable Optical Limit Threshold. Nanomaterials. 2025; 15(2):145. https://doi.org/10.3390/nano15020145
Chicago/Turabian StyleCui, Jing, Zhaohui Yang, Yu Zhang, Zhaoxuan Fan, Jianquan Wang, Xiaoyun Qin, Lijun Gao, Haoran Yang, Shuangliang Liu, Liming Zhou, and et al. 2025. "A Cu(I)-Based MOF with Nonlinear Optical Properties and a Favorable Optical Limit Threshold" Nanomaterials 15, no. 2: 145. https://doi.org/10.3390/nano15020145
APA StyleCui, J., Yang, Z., Zhang, Y., Fan, Z., Wang, J., Qin, X., Gao, L., Yang, H., Liu, S., Zhou, L., Fang, S., & Zhang, Z. (2025). A Cu(I)-Based MOF with Nonlinear Optical Properties and a Favorable Optical Limit Threshold. Nanomaterials, 15(2), 145. https://doi.org/10.3390/nano15020145







