Temperature Sensing in Agarose/Silk Fibroin Translucent Hydrogels: Preparation of an Environment for Long-Term Observation
Abstract
1. Introduction
2. Materials and Methods
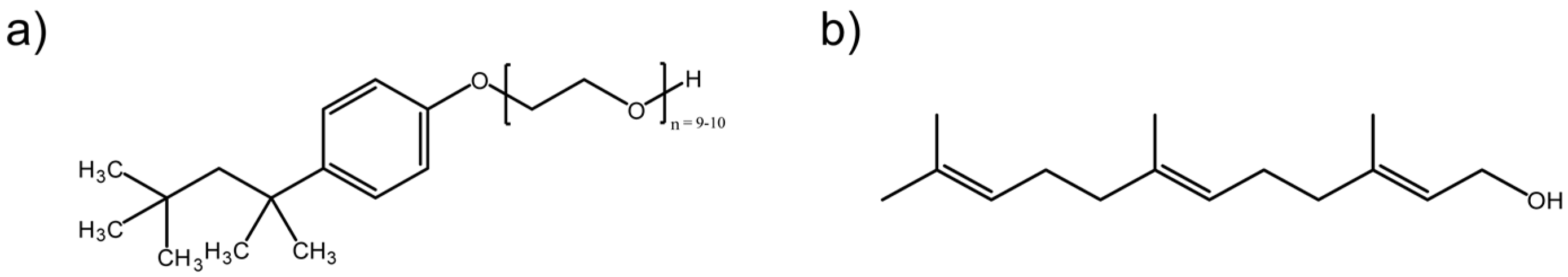
2.1. Materials
2.2. Micelle Preparation
2.3. Hydrogel Preparation
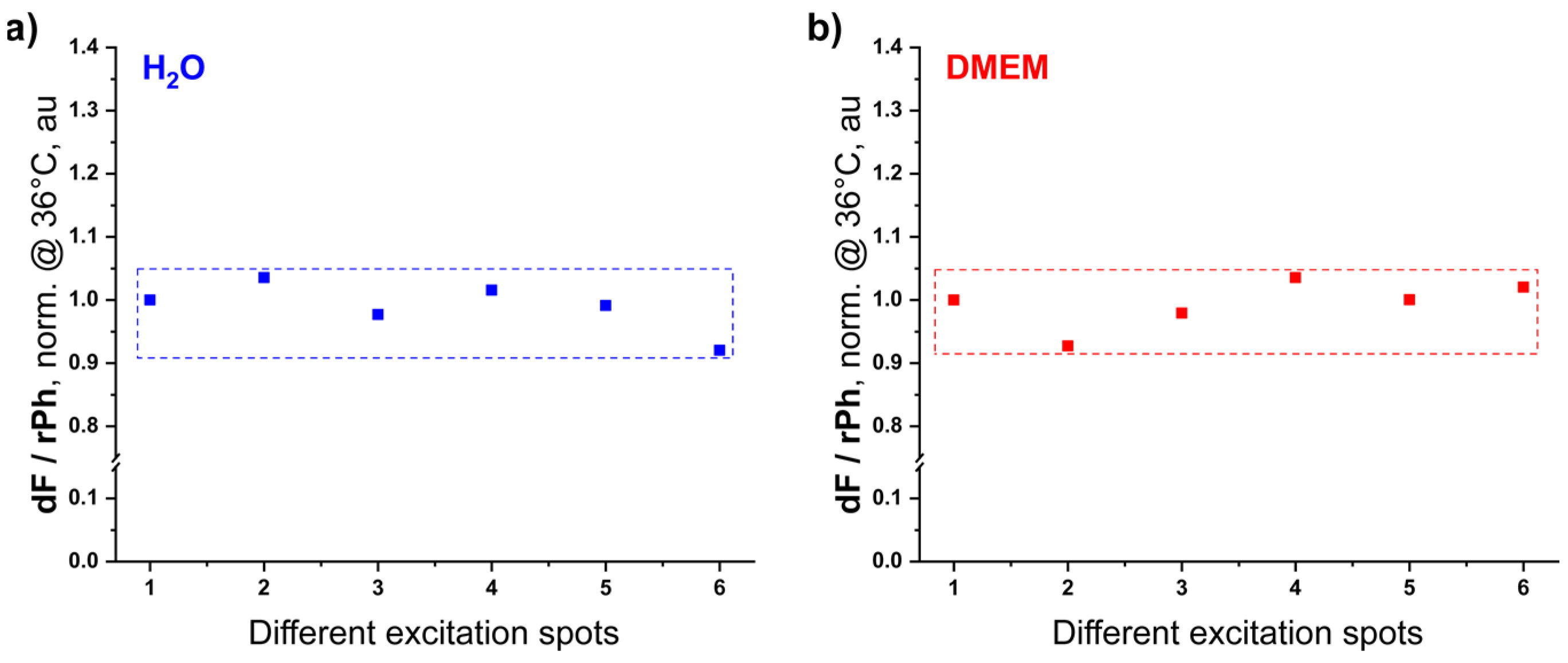
2.4. Spectroscopic Measurements
3. Results and Discussion
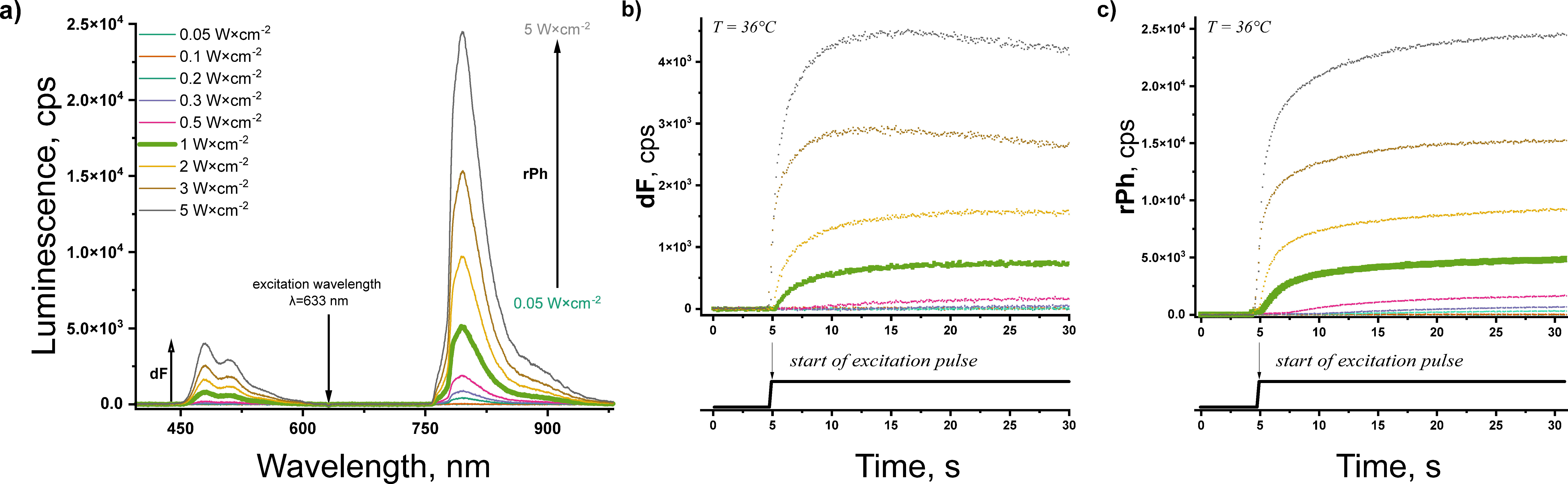
3.1. Micellar Systems
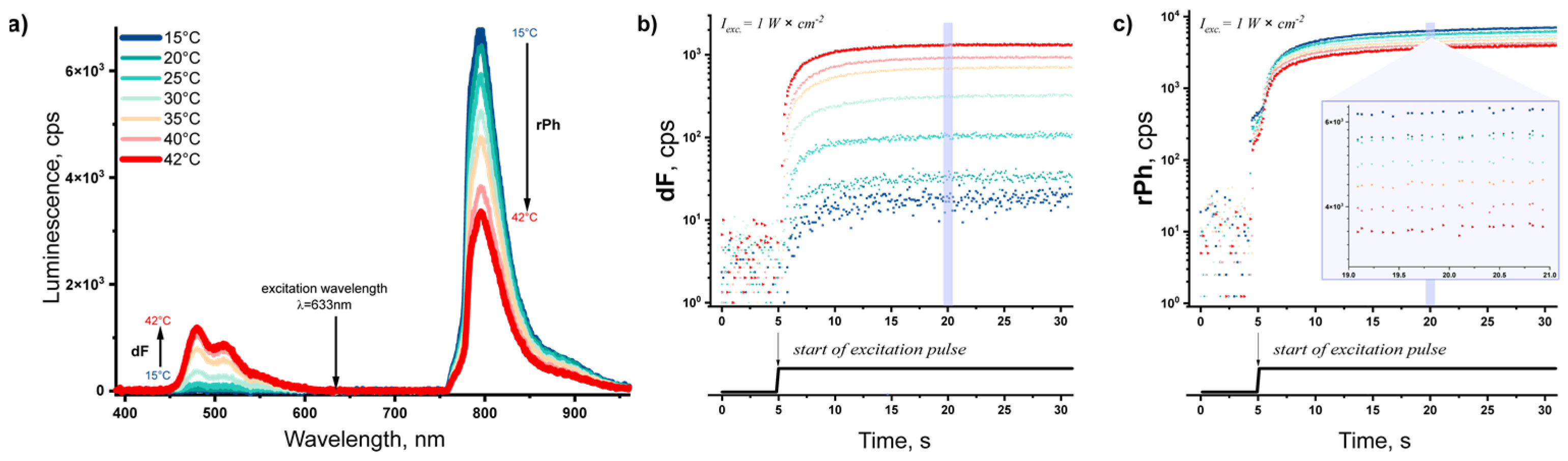
3.2. TTA–UC in Hydrogel
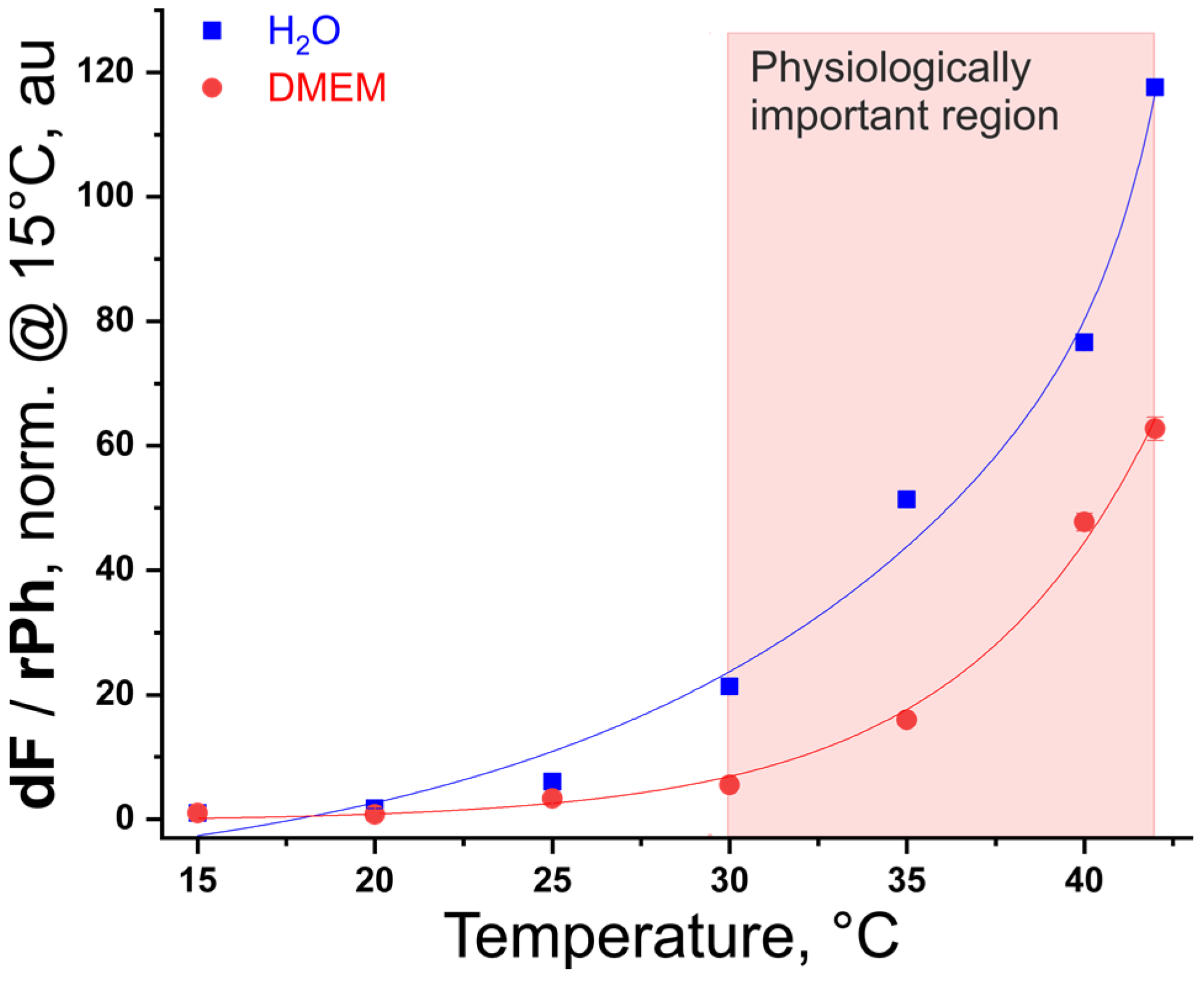
3.3. Creating a Calibration Curve
3.4. TTA-UC in Dulbecco’s Modified Eagle’s Medium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suzuki, M.; Tseeb, V.; Oyama, K.; Ishiwata, S. Microscopic detection of thermogenesis in a single HeLa cell. Biophys. J. 2007, 92, L46–L48. [Google Scholar] [CrossRef]
- Sotoma, S.; Okita, H.; Chuma, S.; Harada, Y. Quantum nanodiamonds for sensing of biological quantities: Angle, temperature, and thermal conductivity. Biophys. Physicobiol. 2022, 19, e190034. [Google Scholar] [CrossRef]
- Yang, F.; Yang, N.; Huo, X.; Xu, S. Thermal sensing in fluid at the micro-nano-scales. Biomicrofluidics 2018, 12, 041501. [Google Scholar] [CrossRef]
- Oliveira, M.; Conceição, P.; Kant, K.; Ainla, A.; Diéguez, L. Electrochemical sensing in 3d cell culture models: New tools for developing better cancer diagnostics and treatments. Cancers 2021, 13, 1381. [Google Scholar] [CrossRef]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Peng, H.S.; Chang, Z.; Hou, L.L.; You, F.T.; Teng, F.; Song, H.W.; Dong, B. Synthesis of ratiometric fluorescent nanoparticles for sensing oxygen. Microchim. Acta 2012, 178, 147–152. [Google Scholar] [CrossRef]
- Wu, C.; Bull, B.; Christensen, K.; McNeill, J. Ratiometric Single-Nanoparticle Oxygen Sensors for Biological Imaging. Angew. Chem. 2009, 121, 2779–2783. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Nazarova, N.V.; Avlasevich, Y.S.; Landfester, K.; Baluschev, S. Stimuli-responsive protection of optically excited triplet ensembles against deactivation by molecular oxygen. Dalt. Trans. 2018, 47, 8605–8610. [Google Scholar] [CrossRef]
- Vinogradov, S.A.; Lo, L.W.; Jenkins, W.T.; Evans, S.M.; Koch, C.; Wilson, D.F. Noninvasive imaging of the distribution in oxygen in tissue in vivo using near-infrared phosphors. Biophys. J. 1996, 70, 1609–1617. [Google Scholar] [CrossRef]
- Briñas, R.P.; Troxler, T.; Hochstrasser, R.M.; Vinogradov, S.A. Phosphorescent oxygen sensor with dendritic protection and two-photon absorbing antenna. J. Am. Chem. Soc. 2005, 127, 11851–11862. [Google Scholar] [CrossRef]
- Achatz, D.E.; Meier, R.J.; Fischer, L.H.; Wolfbeis, O.S. Luminescent sensing of oxygen using a quenchable probe and upconverting nanoparticles. Angew. Chem.—Int. Ed. 2011, 50, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gorris, H.H.; Stolwijk, J.A.; Meier, R.J.; Groegel, D.B.; Wegener, J.; Wolfbeis, O.S. Self-referenced RGB colour imaging of intracellular oxygen. Chem. Sci. 2011, 2, 901–906. [Google Scholar] [CrossRef]
- Schulze, T.F.; Czolk, J.; Cheng, Y.-Y.; Fückel, B.; MacQueen, R.W.; Khoury, T.; Crossley, M.J.; Stannowski, B.; Lips, K.; Lemmer, U.; et al. Efficiency enhancement of organic and thin-film silicon solar cells with photochemical upconversion. J. Phys. Chem. C 2012, 116, 22794–22801. [Google Scholar] [CrossRef]
- Zou, W.; Visser, C.; Maduro, J.A.; Pshenichnikov, M.S.; Hummelen, J.C. Broadband dye-sensitized upconversion of near-infrared light. Nat. Photonics 2012, 6, 560–564. [Google Scholar] [CrossRef]
- Duan, P.; Yanai, N.; Nagatomi, H.; Kimizuka, N. Photon upconversion in supramolecular gel matrixes: Spontaneous accumulation of light-harvesting donor-acceptor arrays in nanofibers and acquired air stability. J. Am. Chem. Soc. 2015, 137, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.F.; Schmidt, T.W. Photochemical upconversion: Present status and prospects for its application to solar energy conversion. Energy Environ. Sci. 2015, 8, 103–125. [Google Scholar] [CrossRef]
- Keivanidis, P.E.; Baluschev, S.; Lieser, G.; Wegner, G. Inherent photon energy recycling effects in the upconverted delayed luminescence dynamics of poly(fluorene)-PtIIoctaethyl porphyrin blends. ChemPhysChem 2009, 10, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Wohnhaas, C.; Friedemann, K.; Busko, D.; Landfester, K.; Baluschev, S.; Crespy, D.; Turshatov, A. All organic nanofibers as ultralight versatile support for triplet-triplet annihilation upconversion. ACS Macro Lett. 2013, 2, 446–450. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet-triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Lin, T.A.; Perkinson, C.F.; Baldo, M.A. Strategies for High-Performance Solid-State Triplet–Triplet-Annihilation-Based Photon Upconversion. Adv. Mater. 2020, 32, e1908175. [Google Scholar] [CrossRef]
- Joarder, B.; Yanai, N.; Kimizuka, N. Solid-State Photon Upconversion Materials: Structural Integrity and Triplet-Singlet Dual Energy Migration. J. Phys. Chem. Lett. 2018, 9, 4613–4624. [Google Scholar] [CrossRef]
- Iyisan, B.; Thiramanas, R.; Nazarova, N.; Avlasevich, Y.; Mailänder, V.; Baluschev, S.; Landfester, K. Temperature Sensing in Cells Using Polymeric Upconversion Nanocapsules. Biomacromolecules 2020, 21, 4469–4478. [Google Scholar] [CrossRef]
- Baluschev, S. Protective Strategies Toward Long-Term Operation of Annihilation Photon Energy Upconversion BT. In Emerging Strategies to Reduce Transmission and Thermalization Losses in Solar Cells; Lissau, J.S., Madsen, M., Eds.; Redefining the Limits of Solar Power Conversion Efficiency; Springer: Cham, Switzerland, 2022; pp. 149–167. [Google Scholar] [CrossRef]
- Busko, D. Noncoherent Upconversion in Multimolecular Organic Systems. Ph.D. Thesis, Johannes Gutenberg University Mainz, Mainz, Germany, 2013. [Google Scholar]
- Nazarova, N.; Avlasevich, Y.; Landfester, K.; Baluschev, S. All-Optical Temperature Sensing in Organogel Matrices via Annihilation Upconversion. ChemPhotoChem 2019, 3, 1020–1026. [Google Scholar] [CrossRef]
- Landfester, K.; Avlasesvich, Y.; Busko, D.; Wurm, F.; Balouchev, S. Single All-Optical Nano-Sensor Device Probing Simultaneously the Local Temperature and Local Oxygen Concentration in Soft-Matter in Non-Invasive Manner. WO2016150677А1, 29 September 2016. [Google Scholar]
- Micheva, M.; Baluschev, S.; Landfester, K. Thermally activated delayed fluorescence in an optically accessed soft matter environment. J. Mater. Chem. C 2022, 10, 4533–4545. [Google Scholar] [CrossRef]
- Penconi, M.; Gentili, P.L.; Massaro, G.; Elisei, F.; Ortica, F. A triplet-triplet annihilation based up-conversion process investigated in homogeneous solutions and oil-in-water microemulsions of a surfactant. Photochem. Photobiol. Sci. 2014, 13, 48–61. [Google Scholar] [CrossRef]
- Lee, H.-L.; Park, J.H.; Choe, H.-S.; Lee, M.-S.; Park, J.-M.; Harada, N.; Sasaki, Y.; Yanai, N.; Kimizuka, N.; Zhu, J.; et al. Upconverting Oil-Laden Hollow Mesoporous Silica Microcapsules for Anti-Stokes-Based Biophotonic Applications. ACS Appl. Mater. Interfaces 2019, 11, 26571–26580. [Google Scholar] [CrossRef]
- Oddo, A.M.; Mani, T.; Kumar, C.V. Micelles Embedded in Multiphasic Protein Hydrogel Enable Efficient and Air-Tolerant Triplet Fusion Upconversion with Heavy-Atom and Spin-Orbit Charge-Transfer Sensitizers. ACS Appl. Mater. Interfaces 2020, 12, 39293–39303. [Google Scholar] [CrossRef]
- Huang, L.; Le, T.; Huang, K.; Han, G. Enzymatic enhancing of triplet–triplet annihilation upconversion by breaking oxygen quenching for background-free biological sensing. Nat. Commun. 2021, 12, 1898. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Kanzaki, T. Swelling of agarose gel and its related changes. Top. Catal. 1987, 1, 317–325. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Li, C.; Jin, H.J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Baluschev, S.; Yakutkin, V.; Wegner, G.; Miteva, T.; Nelles, G.; Yasuda, A.; Chernov, S.; Aleshchenkov, S.; Cheprakov, A. Upconversion with ultrabroad excitation band: Simultaneous use of two sensitizers. Appl. Phys. Lett. 2007, 90, 181103. [Google Scholar] [CrossRef]
- Mattiello, S.; Monguzzi, A.; Pedrini, J.; Sassi, M.; Villa, C.; Torrente, Y.; Marotta, R.; Meinardi, F.; Beverina, L. Self-Assembled Dual Dye-Doped Nanosized Micelles for High-Contrast Up-Conversion Bioimaging. Adv. Funct. Mater. 2016, 26, 8447–8454. [Google Scholar] [CrossRef]
- Healy, B.J.; Zahmatkesh, M.H.; Nitschke, K.N.; Baldock, C. Effect of saccharide additives on response of ferrous-agarose-xylenol orange radiotherapy gel dosimeters. Med. Phys. 2003, 30, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Floren, M.; Migliaresi, C.; Motta, A. Processing Techniques and Applications of Silk Hydrogels in Bioengineering. J. Funct. Biomater. 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, e1800465. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Leisk, G.G.; Lo, T.J.; Yucel, T.; Lu, Q.; Kaplan, D.L. Electrogelation for protein adhesives. Adv. Mater. 2010, 22, 711–715. [Google Scholar] [CrossRef]
- Silva, S.S.; Motta, A.; Rodrigues, M.T.; Pinheiro, A.F.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules 2008, 9, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Richterová, V.; Pekař, M. Effect of Silk Fibroin on the Mechanical and Transport Properties of Agarose Hydrogels. Gels 2024, 10, 611. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Tramsport Phenomena, 2nd ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Davis, G. Absorbance Spectra of Phenol Red at Different pH Values. Available online: https://cran.r-project.org/web/packages/colorSpec/vignettes/phenolred.html (accessed on 10 December 2024).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheva, M.; Baluschev, S.; Landfester, K. Temperature Sensing in Agarose/Silk Fibroin Translucent Hydrogels: Preparation of an Environment for Long-Term Observation. Nanomaterials 2025, 15, 123. https://doi.org/10.3390/nano15020123
Micheva M, Baluschev S, Landfester K. Temperature Sensing in Agarose/Silk Fibroin Translucent Hydrogels: Preparation of an Environment for Long-Term Observation. Nanomaterials. 2025; 15(2):123. https://doi.org/10.3390/nano15020123
Chicago/Turabian StyleMicheva, Maria, Stanislav Baluschev, and Katharina Landfester. 2025. "Temperature Sensing in Agarose/Silk Fibroin Translucent Hydrogels: Preparation of an Environment for Long-Term Observation" Nanomaterials 15, no. 2: 123. https://doi.org/10.3390/nano15020123
APA StyleMicheva, M., Baluschev, S., & Landfester, K. (2025). Temperature Sensing in Agarose/Silk Fibroin Translucent Hydrogels: Preparation of an Environment for Long-Term Observation. Nanomaterials, 15(2), 123. https://doi.org/10.3390/nano15020123





