Silicon Nanostructures for Hydrogen Generation and Storage
Abstract
1. Introduction
- Thermochemical methods: hydrogen can be extracted from fossil fuels like coal, natural gas, and gasoline through thermochemical methods, with steam reforming being the most commonly employed [12,13]. In this process, fossil fuels react with steam using a nickel-based catalyst, releasing hydrogen. Additionally, hydrogen is derived from bio-oil obtained from biomass through the pyrolysis method, which also involves a reaction with steam.

2. Hydrogen Generation from Silicon Nanostructures
2.1. Hydrogen Generation from a-Si:H Nanostructures
2.2. Hydrogen Generation from Si Nanoparticles
2.2.1. Reactions of Si Nanoparticles with Water
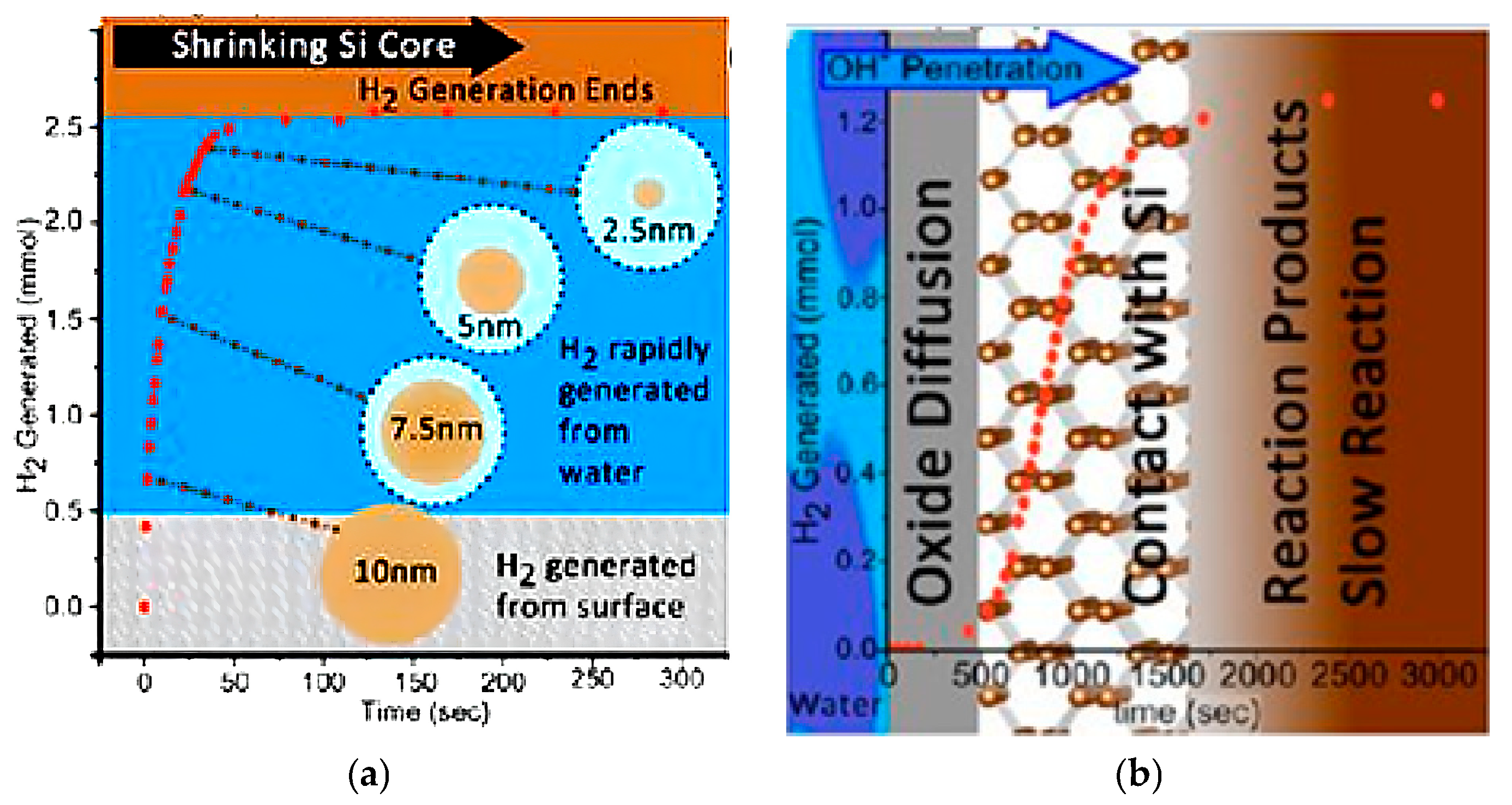
2.2.2. Size Dependance
2.2.3. pH Dependance
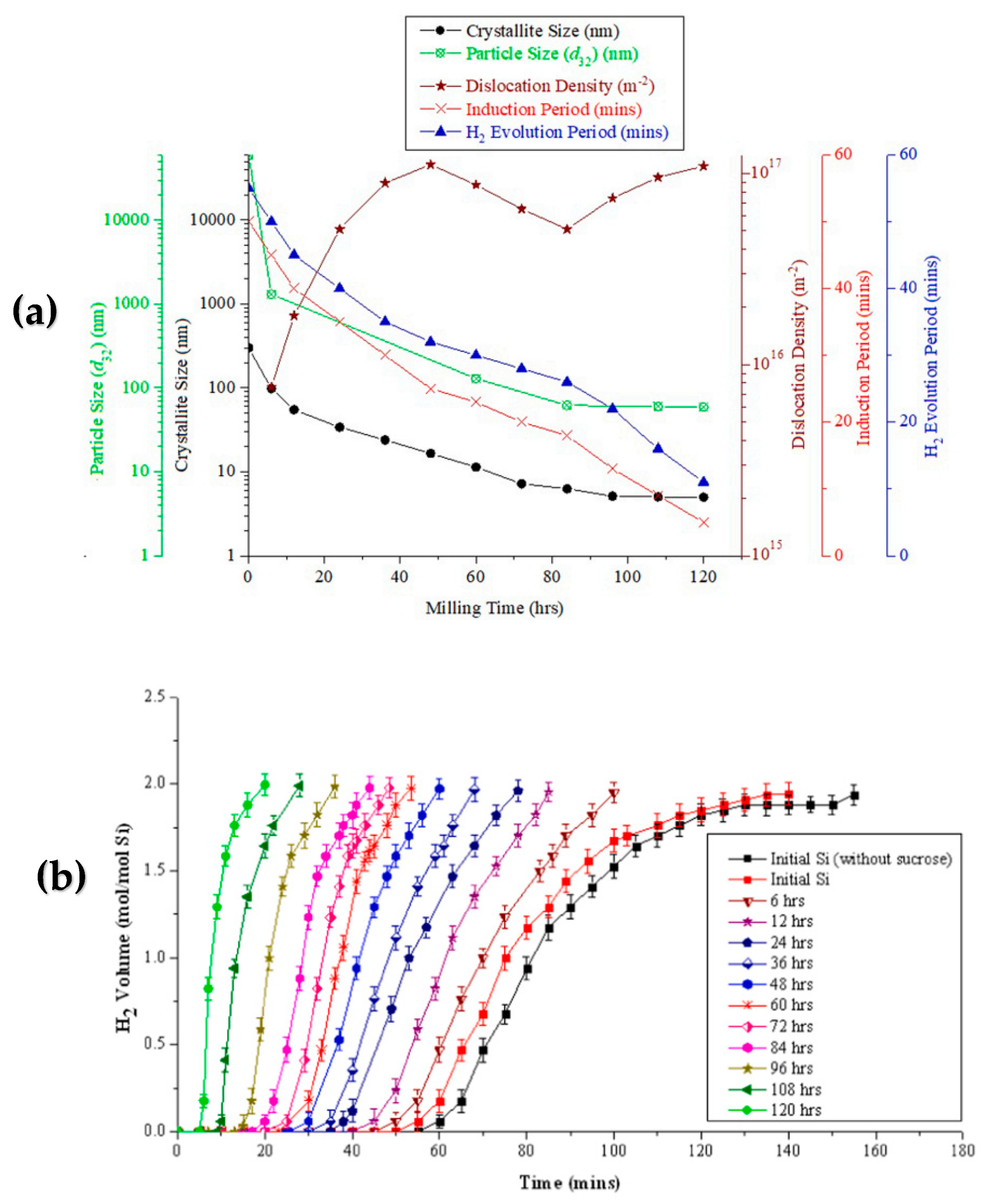
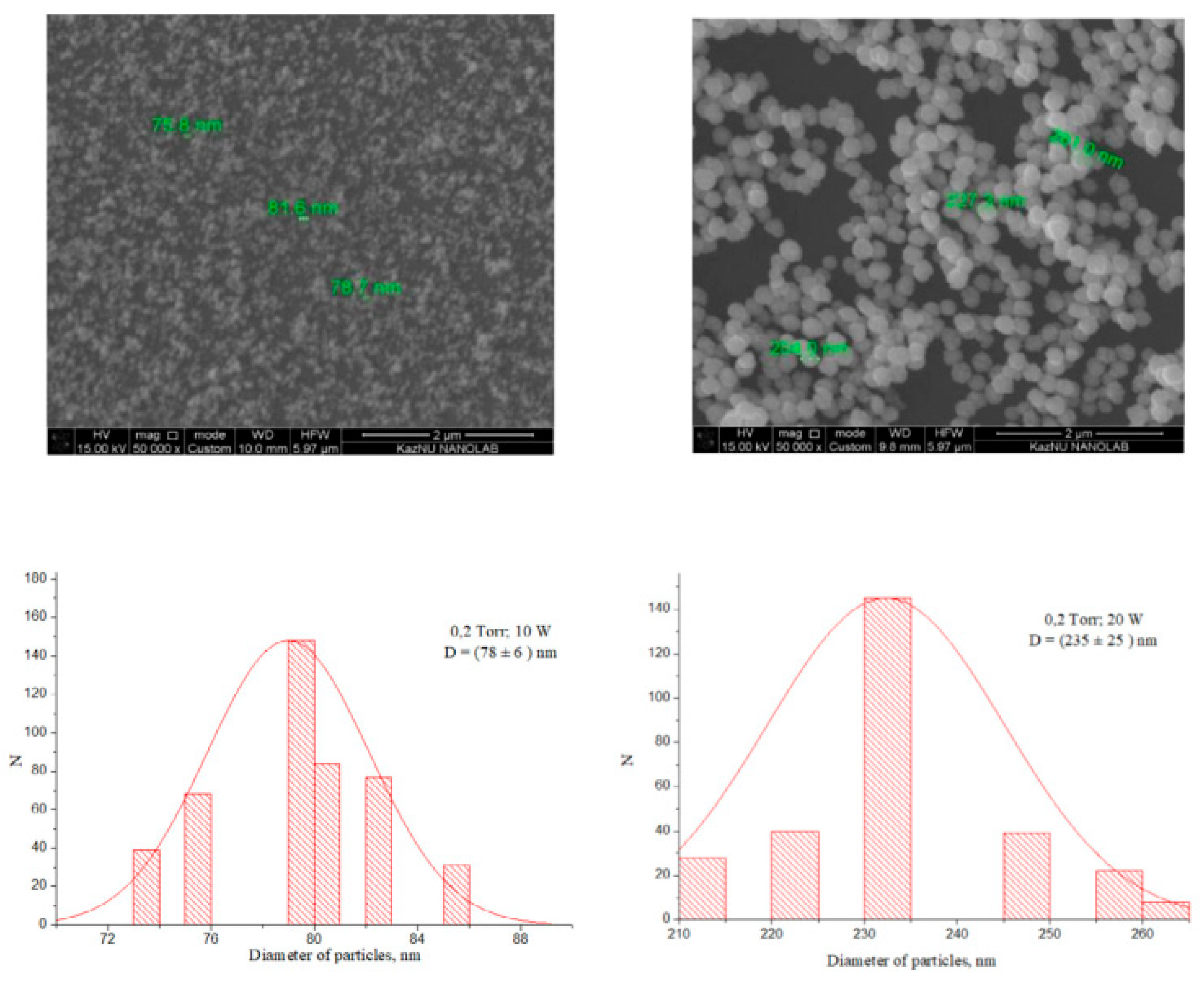
2.2.4. Synthesis of Silicon Nanoparticles for Hydrogen Gas Production
2.3. Hydrogen Generation from Porous Silicon
2.3.1. Porous Silicon
2.3.2. Hydrogen Desorption from Porous Silicon via TPD Technique
2.3.3. Porous Silicon as Photocathode in Hydrogen Production
2.3.4. Mesoporous Silicon Spheres for Photocatalytic Hydrogen Evolution
2.3.5. Stain Etching of Porous Silicon
2.4. Hydrogen Generation from Silicon Nanowires
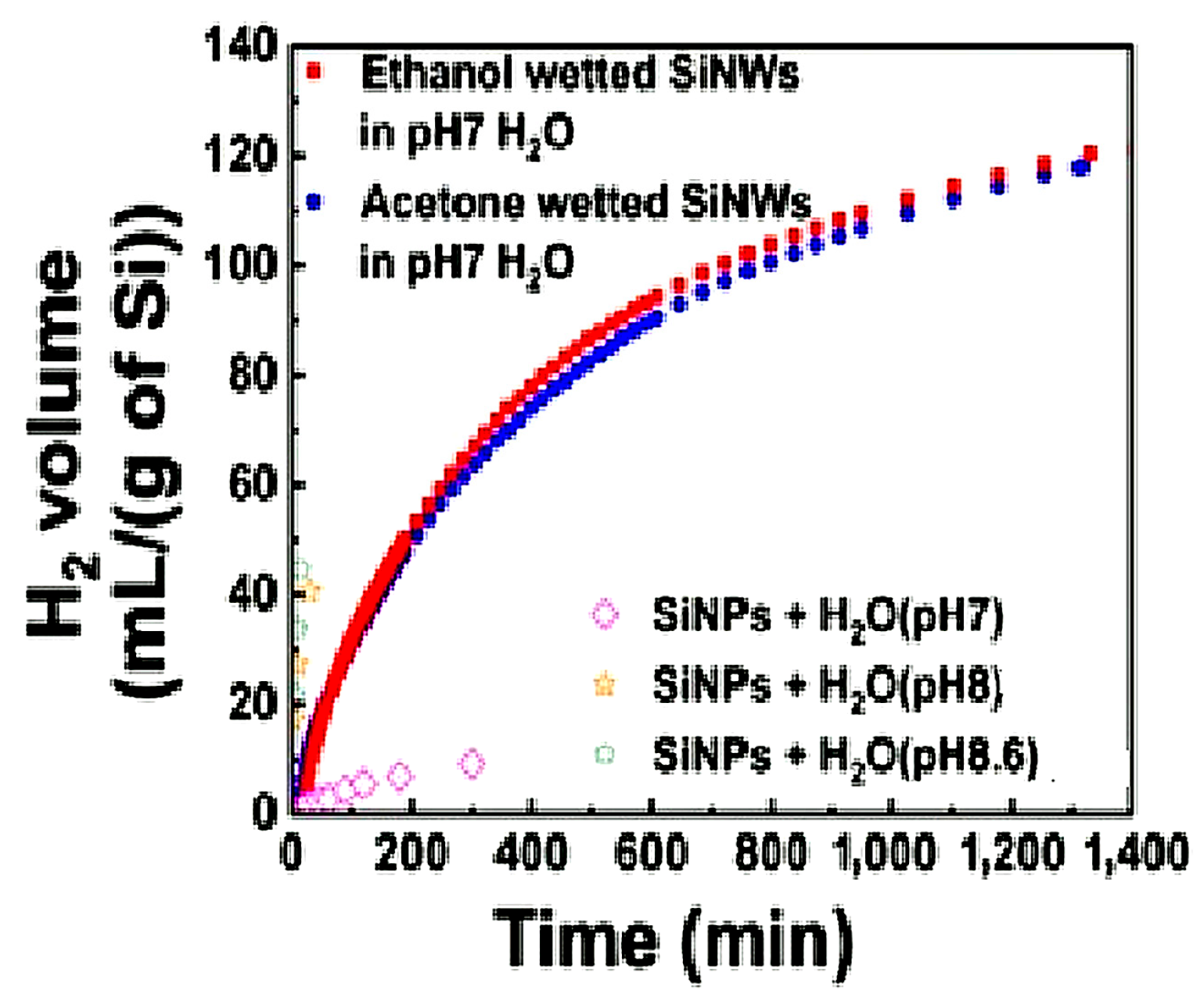
2.4.1. Reaction of Silicon Nanowires with Water
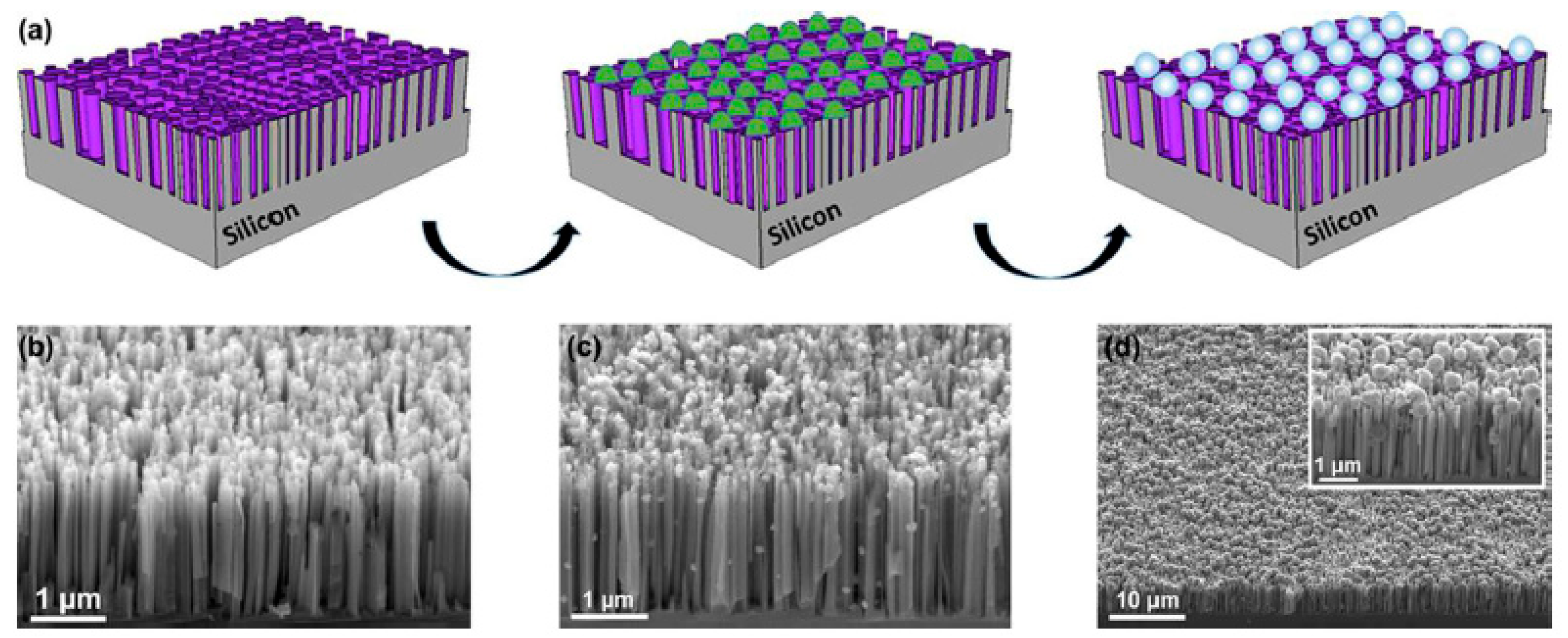
2.4.2. SiNW Arrays with Cobalt Phosphide for Efficient Solar Hydrogen Evolution
2.4.3. SiNWs with AgNPs for Hydrogen Generation
2.5. Silicon Based Nanohybrids for Enhanced Generation of Hydrogen
2.5.1. Nanosilicon with Grafted WS2
2.5.2. Silicon–Carbon Composites Modified with Nickel and/or Platinum
3. Hydrogen Storage in Silicon Nanostructures
3.1. Modeling Metal-Decorated Porous Silicon for Hydrogen Storage
3.2. Hydrogen Storage in Porous Silicon Nanostructures
3.2.1. Porous Silicon as a Hydrogen Reservoir
3.2.2. Properties of PS Suitable for H2 Storage: Porosity and Surface Area
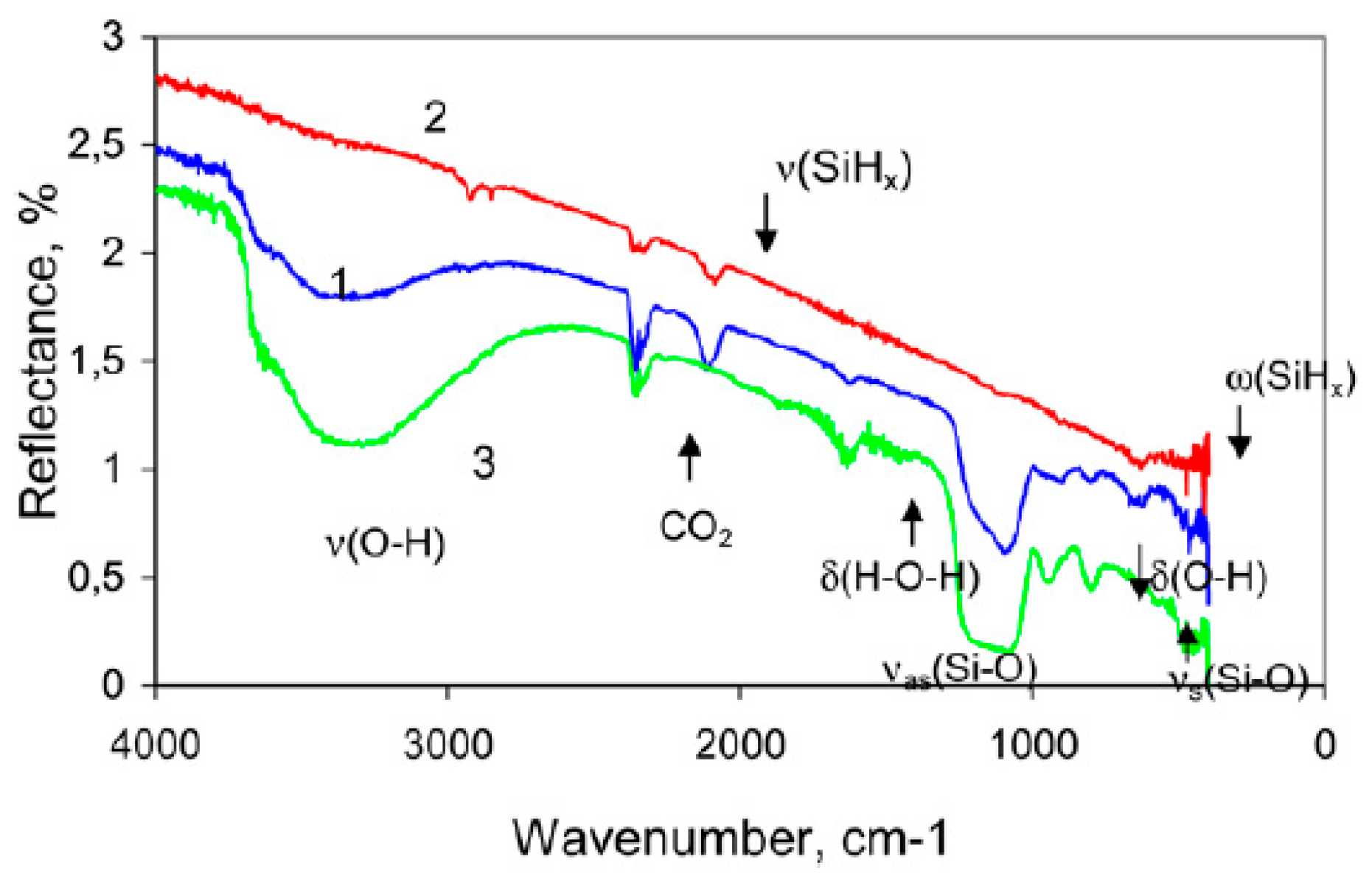
3.2.3. Morphology Dependent SiHX Spectral Features
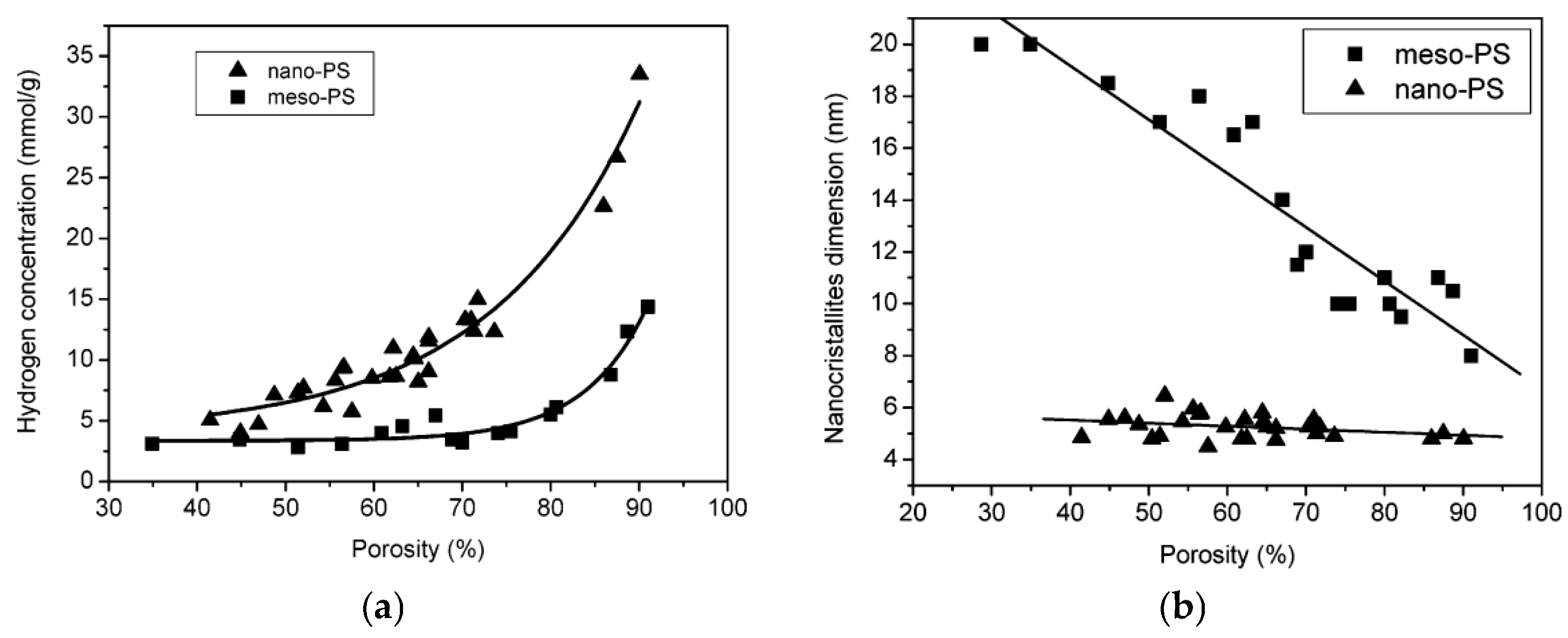
3.2.4. Hydrogen Concentration and Nanocrystallites Dimensions in Porous Silicon
3.3. Hydrogen Storage Effect of Catalyst, Composite Materials, and Porous Silicon as Storage Media
3.3.1. Pd Impregnation in Porous Silicon
3.3.2. Hydrogen Desorption After PS and PS + Pd Treatment in Molecular Hydrogen
3.3.3. Electrochemical Hydrogen Storage in Pd-Coated Porous Silicon/Graphene Oxide
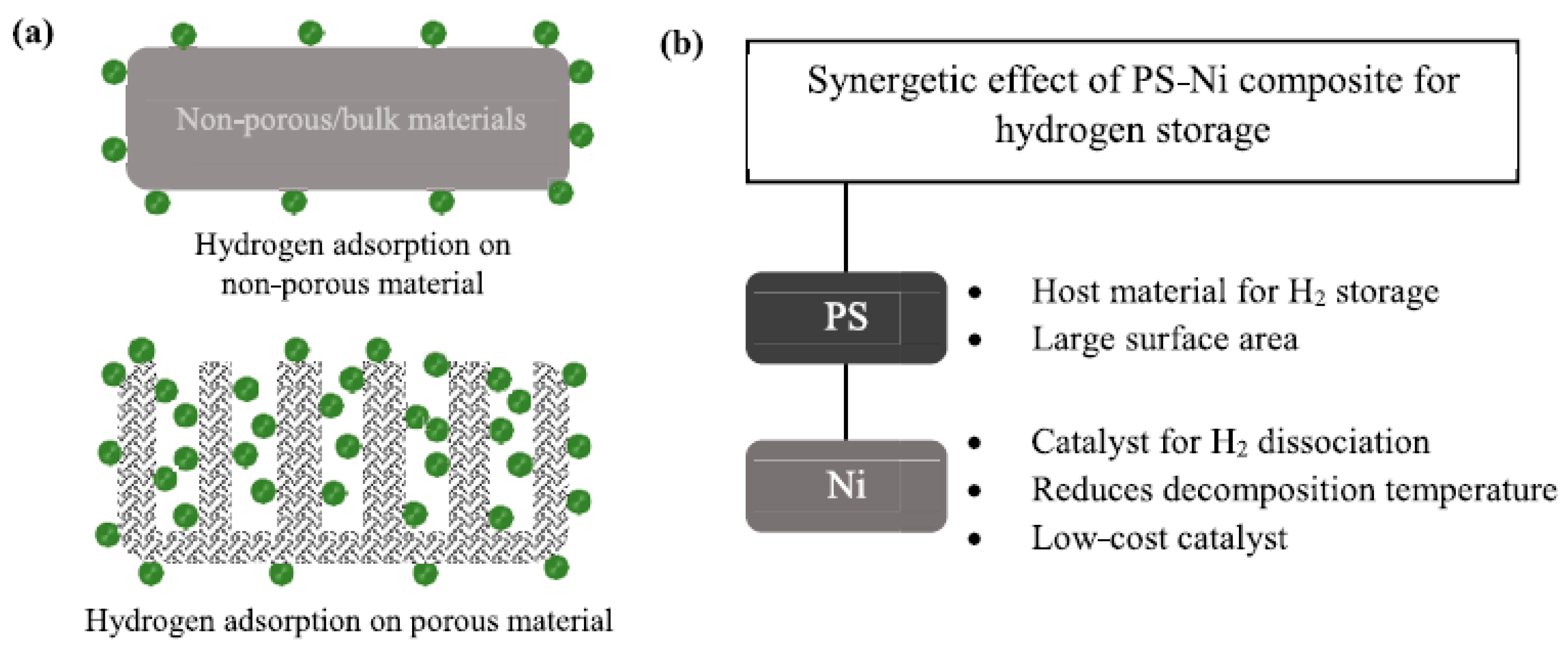
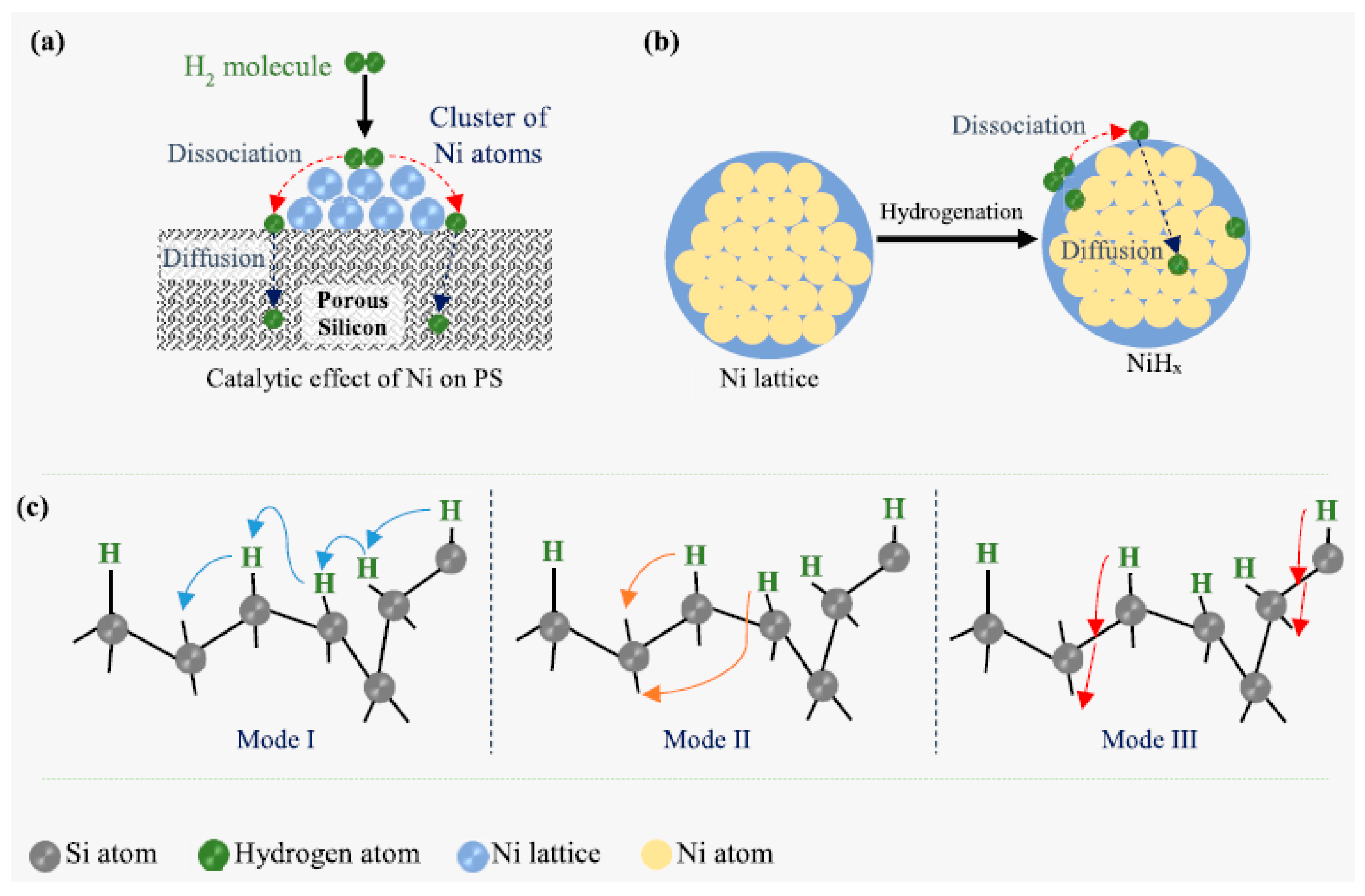
3.3.4. Effect of Nickel Catalyst on Hydrogen Storage and Desorption in Porous Silicon
3.3.5. Enhancing Lithium Hydrides for Hydrogen Storage Using Porous Silicon
4. Applications
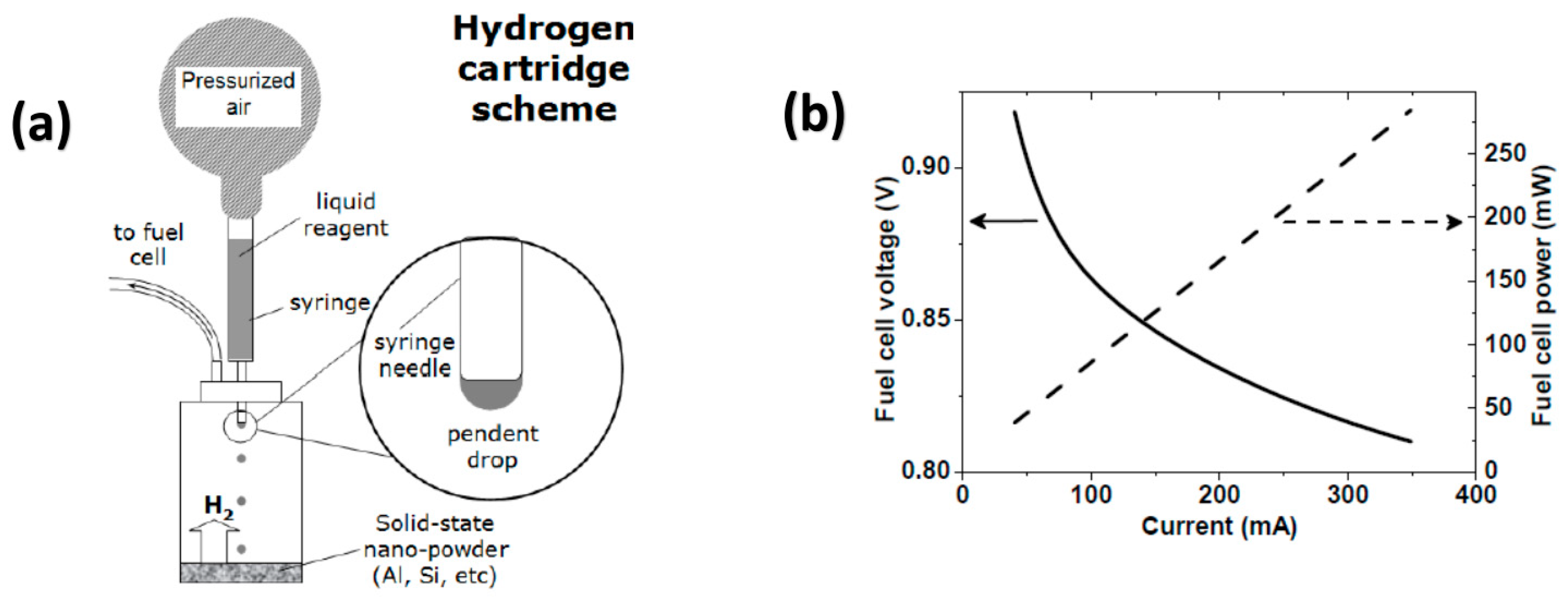
4.1. Self-Regulated System for Application in Portable Fuel Cells
4.2. Water-Emulsified Diesel Fuel in Diesel Engines
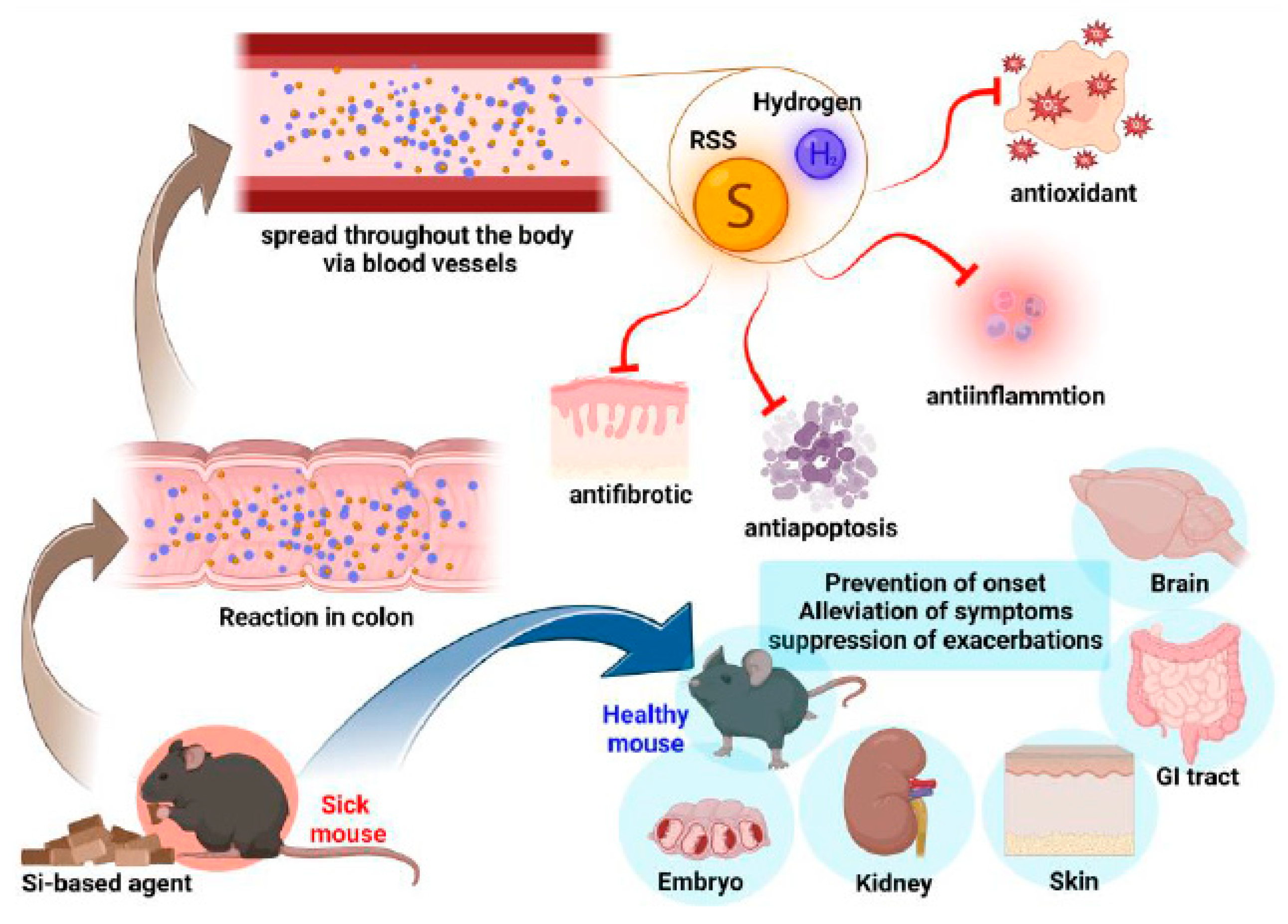
4.3. Si-Based Agent, a Unique New Antioxidant via Hydrogen
4.4. Si-Based Hydrogen Administration for Ischemia–Reperfusion Injury
4.5. Application as an Explosive Material
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Babiker, M.M.; Gurgel, A.C.; Paltsev, S.; Reilly, J.M. Joint Program Report Series Report 161, 18p. Available online: http://globalchange.mit.edu/publication/15538 (accessed on 10 April 2025).
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture Methods, Technologies and Applications; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Elseiver: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Jayabal, R. Hydrogen energy storage in maritime operations: A pathway to decarbonization and sustainability. Int. J. Hydrogen Energy 2025, 109, 1133–1144. [Google Scholar] [CrossRef]
- Yao, Y.; Tan, L.; Chen, F.; Pan, A.; Ma, Q.; Zhao, J.; Kang, R. Hydrogen energy industry in China: The current status, safety problems, and pathways for future safe and healthy development. Saf. Sci. 2025, 186, 106808. [Google Scholar] [CrossRef]
- Yi, X.; Lu, T.; Li Yi Ai, Q.; Hao, R. Collaborative planning of multi-energy systems integrating complete hydrogen energy chain. Renew. Sustain. Energy Rev. 2025, 210, 115147. [Google Scholar] [CrossRef]
- Adaikalam, K.; Vikraman, D.; Karuppasamy, K.; Kim, H. Solar hydrogen production and storage in solid form: Prospects for materials and methods. Nanomaterilas 2024, 14, 1560. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrogen Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Jayachandran, M.; Gatla, R.K.; Flah, A.; Milyani, A.H.; Milyani, H.M.; Blazek, V.; Prokop, L.; Kraiem, H. Challenges and opportunities in green hydrogen adoption for decarbonizing hard-to-abate industries: A comprehensive review. IEEE Access 2024, 12, 23363–23388. [Google Scholar] [CrossRef]
- Muduli, R.C.; Kale, P. Silicon nanostructures for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2023, 48, 1401–1439. [Google Scholar] [CrossRef]
- Larmini, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; John Willey & Sons, Ltd.: West Sussex, UK, 2003. [Google Scholar]
- Ji, G.; Yao, J.G.; Clough, P.T.; Diniz da Costa, J.C.; Anthony, E.J.; Fennell, P.S.; Wanga, W.; Zhao, M. Enhanced hydrogen production from thermochemical processes. Energy Environ. Sci. 2018, 11, 2647–2672. [Google Scholar] [CrossRef]
- Alvarado-Flores, J.J.; Alcaraz-Vera, J.V.; Avalos-Rodriguez, M.L.; Guzman-Mejia, E.; Rutiaga-Quinones, J.G.; Pintor-Ibarra, L.F.; Guevara-Martinez, S.J. Thermochemical production of hydrogen from biomass: Pyrolysis and gasification. Energies 2024, 17, 537. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and future advances in water electrolysis for green hydrogen generation: Critical analysis and perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Belghiti, A.A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Wang, J.; Wen, J.; Wang, J.; Yang, B.; Jiang, L. Water electrolyzer operation scheduling for green hydrogen production: A review. Renew. Sustain. Energy Rev. 2024, 203, 114779. [Google Scholar] [CrossRef]
- Jian, J.; Jiang, G.; van de Krol, R.; Wei, B.; Wang, H. Recent advances in rational engineering of multinary semiconductors for photoelectrochemical hydrogen generation. Nano Energy 2018, 51, 457–480. [Google Scholar] [CrossRef]
- Manohar, A.; Suvarna, T.; Chintagumpala, K.; Ubaidullah, M.; Mameda, N.; Kim, K.H. Exploring progress in binary and ternary nanocomposites for photoelectrochemical water splitting: A comprehensive review. Coord. Chem. Rev. 2025, 522, 216180. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Jalil, A.A.; Ikram, M.; Hussain, I.; Bahari, M.B.; Tran, T.V.; Alhassan, M.; Owgi, A.H.K.; Parashuram, L.; et al. A bibliometric examination and state-of-the-art overview of hydrogen generation from photoelectrochemical water splitting. Int. J. Hydrogen Energy 2024, 52, 358–380. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Jaramillo, A.; Satta, A.; Pinto, F.; Faraloni, C.; Zittelli, G.C.; Benavides, A.M.S.; Torzillo, G.; Schumann, C.; Méndez, J.F.; v Berggren, G.; et al. Outlook on synthetic biology-driven hydrogen production: Lessons from algal photosynthesis applied to cyanobacteria. Energy Fuels 2025, 39, 4987–5006. [Google Scholar] [CrossRef]
- Zhong, S.; Xu, Q. Metal nanoparticle-catalyzed hydrogen generation from liquid chemical hydrides. Bull. Chem. Soc. Jpn. 2018, 91, 1606–1617. [Google Scholar] [CrossRef]
- Sankir, M.; Semiz, L.; Serin, R.B.; Sankir, N.D.; Baker, D. Hydrogen generation from chemical hydrides. In Advanced Catalytic Materials; Tiwari, A., Titinchi, S., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 143–192. [Google Scholar]
- Liu, M.; Yao Zh Gu, J.; Li Ch Huang, X.; Zhang, L.; Huang, Z.; Fan, M. Issues and opportunities facing hydrolytic hydrogen production materials. Chem. Eng. J. 2023, 461, 141918. [Google Scholar] [CrossRef]
- Gor Bolen, M. Hydrogen storage in porous silicon—A review. Silicon 2023, 15, 4663–4673. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioglu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2018, 43, 10810–10821. [Google Scholar] [CrossRef]
- Motalleb, M.; Đukić, A.; Firak, M. Solar hydrogen power system for isolated passive house. Int. J. Hydrogen Energy 2015, 40, 16001–16009. [Google Scholar] [CrossRef]
- Li, Y.; Fu, C. Hydrogen storage-learning from nature: The air clathrate hydrate in polar ice sheets. Sustain. Energy Technol. Assess. 2024, 72, 104007. [Google Scholar] [CrossRef]
- Ozsaban, M.; Midilli, A. A parametric study on exergetic sustainability aspects of high-pressure hydrogen gas compression. Int. J. Hydrogen Energy 2016, 14, 5321–5334. [Google Scholar] [CrossRef]
- Ozcan, H.; Dincer, I. Energy and exergy analyses of a solar based hydrogen production and compression system. Int. J. Hydrogen Energy 2017, 42, 21414–21428. [Google Scholar] [CrossRef]
- Bhouri, M.; Linder, M.; Burger, I. Metal hydride reactor for dual use: Hydrogen storage and cold production. Int. J. Hydrogen Energy 2018, 43, 23357–23371. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Qu, H.; Yu, X.F.; Peng, P. Alkali metal silanides α-MSiH3: A family of complex hydrides for solid-state hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 12405–12413. [Google Scholar] [CrossRef]
- Dincă, M.; Long, J.R. Hydrogen storage in microporous metal–organic frameworks with exposed metal sites. Angew. Chem. Int. Ed. 2008, 47, 6766–6779. [Google Scholar] [CrossRef]
- Mehmood, R.A.; Aslam, A.A.; Iqbal, M.J.; Sajid, A.H.; Hamza, A.; Tahira, H.F.; Islam, I.U.; Yabalak, E. A review on hydrogen production using decorated metal-organic frameworks by electrocatalytic and photocatalytic water splitting. Fuel 2025, 387, 134416. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Tazeen, Z.; Kousar, S.; Li, M.; Zhao, X. Metal–organic framework/graphene-based nanocatalysts for hydrogen evolution reaction: A comprehensive review. Int. J. Hydrogen Energy 2025, 98, 1299–1313. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Dong, H.; Hu, H.; Yu, P.; Peng, L.; Liu, Y. Revealing contribution of pore size to high hydrogen storage capacity. Int. J. Hydrogen Energy 2018, 43, 18077–18082. [Google Scholar] [CrossRef]
- Kohzadi, K.; Mehrabi, M.; Shirkani, H.; Karimr, S. Laser radiation, CDs and Pd-NPs: Three influential factors to enhance the hydrogen storage capacity of porous silicon. Int. J. Hydrogen Energy 2024, 50, 464–474. [Google Scholar] [CrossRef]
- Simanullang, M.; Prost, L. Nanomaterials for on-board solid-state hydrogen storage applications. A review. Int. J. Hydrogen Energy 2022, 47, 29808–29846. [Google Scholar] [CrossRef]
- Nagar, R.; Srivastava, S.; Hudson, S.L.; Amaya, S.L.; Tanna, A.; Sharma, M.; Achayalingam, R.; Sonkaria, S.; Khare, V.; Srinivasan, S.S. Recent developments in state-of-the-art hydrogen energy technologies—Review of hydrogen storage materials. Sol. Compass 2023, 5, 100033. [Google Scholar] [CrossRef]
- Beyazit, N.I. Comparative study of hydrogen storage and metal hydride systems: Future energy storage solutions. Processes 2025, 13, 1506. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Farooqi, A.S.; Fajri, K.; El-Adawy, M.; Hamdy, M.; Farooqi, A.S.; Abdelaziz, O.Y.; Hossain, M.M.; Nemitallah, M.A. Clean hydrogen production via sorption enhanced water gas shift reaction: A comprehensive review. Int. J. Hydrogen Energy 2025, 100, 1483–1512. [Google Scholar] [CrossRef]
- Muduli, R.C.; Kale, P. Sorption properties of nanostructured ball-milled porous silicon for solid-state hydrogen storage up to 80 bar. Int. J. Hydrogen Energy 2024, 140, 920–930. [Google Scholar] [CrossRef]
- Chen, Z.; Muduli, R.C.; Xu, Z.; Guo, F.; Jain, A.; Isobe, S.; Wang, Y.; Miyaoka, H.; Ichikawa, T.; Kale, P. Exploring the cycling solid-state hydrogen storage performance in lithium Hydride-Porous silicon composite. Chem. Eng. J. 2025, 512, 162492. [Google Scholar] [CrossRef]
- Zhang, H.; Lou, Y.; Tang, F.; Wu, C.; Lin, B. First-principle prediction of one-dimensional silicon allotropes: Promising new candidate for chemical and electrochemical hydrogen storage. Int. J. Hydrogen Energy 2023, 48, 5552–5564. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Seyfollahi, R. Hybrid system for potential room temperature hydrogen storage. Vacuum 2016, 131, 115–119. [Google Scholar] [CrossRef]
- Carraro, P.M.; Blanco, A.G.; Soria, F.A.; Lener, G.; Sapag, K.; Eimer, G.A.; Oliva, M.I. Understanding the role of nickel on the hydrogen storage capacity of Ni/MCM-41 materials. Microporous Mesoporous Mater. 2016, 231, 31–39. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Li, M.; Iqbal, M.Z.; Lu, Y.; Xu, M.; Li, Q. Synthesis, characterization and hydrogen storage characteristics of flower-like SnO2 porous microspheres. Mater. Lett. 2014, 119, 36–38. [Google Scholar] [CrossRef]
- Pei, Z.; Feng, Z.; Yao, Z.; Luo, Y. Emerging molecular sieve regulation strategy for the adsorption and catalysis: Silicon hydroxyl engineering. Mol. Catal. 2025, 570, 114667. [Google Scholar] [CrossRef]
- Ding, F.; Yakobson, B.I. Challenges in hydrogen adsorptions: From physisorption to chemisorption. Front. Phys. 2011, 6, 142–150. [Google Scholar] [CrossRef]
- Efimchenko, V.S.; Meletov, K.P.; Korotkova, M.A.; Masalov, V.M.; Sukhinina, N.S.; Emal’chenko, G.A.; Usmanov, R.I. Hollow silica nanospheres with a high content of sorbed molecular hydrogen. Fuel 2025, 385, 134217. [Google Scholar] [CrossRef]
- Nishad, N.K.; Kale, P. Near-ambient temperature enhancement of hydrogen storage in thermally reduced graphene oxide to 6.53 wt% after porous silicon nanoparticles decoration. J. Energy Storage 2025, 131 Pt B, 117606. [Google Scholar] [CrossRef]
- Saeid, M.F.; Abdulkadir, B.A.; Setiabudi, H.D. Enhancing hydrogen adsorption performance of hollow silica spheres through the addition of Fe: A study on kinetic and thermodynamic. Mater. Sci. Semicond. Process. 2025, 192, 109458. [Google Scholar] [CrossRef]
- Abdulkadir, B.A.; Jalil, A.A.; Cheng, C.K.; Setiabudi, H.D. Progress and advances in porous silica-based scaffolds for enhanced solid-state hydrogen storage: A systematic literature review. Chem. Asian J. 2024, 19, e202300833. [Google Scholar] [CrossRef]
- Ciocarlan, R.G.; Farrando-Perez, J.; Arenas-Esteban, D.; Houlleberghs, M.; Daemen, L.L.; Cheng, Y.; Ramirez-Cuesta, A.J.; Breynaert, E.; Martens, J.; Bals, S.; et al. Tuneable mesoporous silica material for hydrogen storage application via nano-confined clathrate hydrate construction. Nat. Commun. 2024, 15, 8697. [Google Scholar] [CrossRef]
- Merazga, S.; Cheriet, A.; M’hammedi, K.; Mefoued, A.; Gabouze, N. Investigation of porous silicon thin films for electrochemical hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 9994–10002. [Google Scholar] [CrossRef]
- Manilov, A.I.; Skryshevsky, V.A. Hydrogen in porous silicon—A review. Mater. Sci. Eng. B 2013, 178, 942–955. [Google Scholar] [CrossRef]
- Muduli, R.C.; Kale, P. Chemically modified surface of silicon nanostructures to enhance hydrogen uptake capabilities. Int. J. Hydrogen Energy 2023, 48, 37819–37833. [Google Scholar] [CrossRef]
- Curtis, I.S.; Wills, R.J.; Daso, M. Photocatalytic hydrogen generation using mesoporous silicon nanoparticles: Influence of magnesiothermic reduction conditions and nanoparticle aging on the catalytic activity. Nanoscale 2021, 13, 2685–2692. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Filtvedt, W.; Hiorth, M.; Klaveness, J. Silicon nanoparticles for oral administration of molecular hydrogen. Int. J. Pharm. 2022, 629, 122371. [Google Scholar] [CrossRef]
- Koyama, Y.; Kobayashi, Y.; Kobayashi, H.; Shimada, S. Diverse possibilities of Si-based agent, a unique new antioxidant. Antioxidants 2023, 12, 1061. [Google Scholar] [CrossRef]
- Muduli, S.P.; Muduli, R.C.; Kale, P. Decoding hydrogen desorption kinetics in porous silicon: An electrical circuit modeling approach. ACS Appl. Mater. Interfaces 2024, 16, 59663–59673. [Google Scholar] [CrossRef]
- Scryshevsky, V.; Manilov, A.; Ivanov, I. Hydrogen adsorption in porous silicon: Simulation and control method. In Proceedings of the 2023 17th International Conference on the Experience of Designing and Application of CAD Systems (CADSM), Jaroslaw, Poland, 22–25 February 2023. [Google Scholar] [CrossRef]
- Allal, Z.; Noura, H.N.; Salman, O.; Vernier, F.; Chahine, K. A review on machine learning applications in hydrogen energy systems. Int. J. Thermofluids 2025, 26, 101119. [Google Scholar] [CrossRef]
- Pan, B.C.; Biswas, R. Simulation of hydrogen evolution from nano-crystalline silicon. J. Non Cryst. Solids 2004, 33, 44–47. [Google Scholar] [CrossRef]
- Litvinenko, S.; Alekseev, S.; Lysenko, V.; Venturello, A.; Geobaldo, F.; Gulina, L.; Kuznetsov, G.; Tolstoy, V.; Skryshevsky, V.; Garrone, E.; et al. Hydrogen production from nano-porous Si powder formed by stain etching. Int. J. Hydrogen Energy 2010, 35, 6773–6778. [Google Scholar] [CrossRef]
- Goller, B.; Kovalev, D.; Sreseli, O. Nanosilicon in water as a source of hydrogen: Size and pH matter. Nanotechnology 2011, 22, 305402. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Ohta, H.; Uedda, T.; Kashiwagi, T.; Fukuda, T.; Shiobara, T.; Saitow, K. Mechanochemically tailored silicon particles for efficient H2 production: Entropy and enthalpy engineering. ACS Sustain. Chem. Eng. 2023, 32, 11769–11780. [Google Scholar] [CrossRef]
- Kale, P.; Gangal, A.C.; Edla, R.; Sharma, P. Investigation of hydrogen storage behavior of silicon nanoparticles. Int. J. Hydrogen Energy 2012, 37, 3741–3747. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R. Photoactivated reaction of water with silicon nanoparticles. Int. J. Hydrogen Energy 2009, 34, 8504–8510. [Google Scholar] [CrossRef]
- Zhan, C.Y.; Chu, P.K.; Ren, D.; Xin, Y.C.; Huo, K.F.; Zou, Y.; Huang, N.K. Release of hydrogen during transformation from porous silicon to silicon oxide at normal temperature. Int. J. Hydrogen Energy 2011, 36, 4513–4517. [Google Scholar] [CrossRef]
- Bunker, C.E.; Smith, M.J. Nanoparticles for hydrogen generation. J. Mater. Chem. 2011, 21, 12173–12180. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Lin, T.; Tucciarone, P.M.; LaJoie, K.M.; Lai, L.; Patki, G.D.; Prasad, P.N.; Swihart, M.T. On-demand hydrogen generation using nanosilicon: Splitting water without light, heat, or electricity. Nano Lett. 2013, 13, 451–456. [Google Scholar] [CrossRef]
- Bhisikar, A.; Singh, M.N.; Khantwal, N.; Sinha, A.K. Effect of microstructural parameters of ball-milled Si powder on reactivity with water to produce H2: Way forward for on-demand H2 production. Mater. Today Commun. 2022, 33, 104138. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Matsuda, S.; Imamura, K.; Kobayashi, H. Hydrogen generation by reaction of Si nanopowder with neutral water. J. Nanopart. Res. 2017, 19, 176. [Google Scholar] [CrossRef]
- Imamura, K.; Kimura, K.; Fujie, S.; Kobayashi, H. Hydrogen generation from water using Si nanopowder fabricated from swarf. J. Nanopart. Res. 2016, 18, 116. [Google Scholar] [CrossRef]
- Knipping, J.; Wiggers, H.; Rellinghaus, B.; Roth, P.; Konjhodzic, D.; Meier, C. Synthesis of high purity silicon nanoparticles in a low-pressure microwave reactor. J. Nanosci. Nanotechnol. 2004, 4, 1039–1044. [Google Scholar] [CrossRef]
- Abderrafi, K.; García Calzada, R.; Gongalsky, M.B.; Suarez, I.; Abarques, R.; Chirvony, V.S.; Timoshenko VYu Ibanez, R.; Martínez-Pastor, J.P. Silicon nanocrystals produced by nanosecond laser ablation in an organic liquid. J. Phys. Chem. C 2011, 115, 5147–5151. [Google Scholar] [CrossRef]
- Secret, E.; Leonard, C.; Kelly, S.J.; Uhl, A.; Cozzan, C.; Andrew, J.S. Size control of porous silicon-based nanoparticles via pore-wall thinning. Langmuir 2016, 32, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhumadilov, R.; Orazbayev, S.; Zhunisbekov, A.; Ramazanov, T.; Raiymkhanov, Z. Synthesis of silicon nanoparticles for hydrogen gas production. Mater. Today Proc. 2020, 31, 444–448. [Google Scholar] [CrossRef]
- Lysenko, V.; Bidault, F.; Alekseev, S.; Zaitsev, V.; Barbier, D.; Turpin, C.; Geobaldo, F.; Garrone, E. Study of porous silicon nanostructures as hydrogen reservoirs. J. Phys. Chem. B 2005, 109, 19711–19718. [Google Scholar] [CrossRef]
- Grosman, A.; Ortega, C. Optoelectronic properties of porous silicon. In Properties of Porous Silicon; Canham, L.T., Ed.; INSPEC: London, UK, 1997; p. 145. [Google Scholar]
- Buttard, D.; Dolino, G.; Faivre, C.; Halimaoui, A.; Comin, F.; Formoso, V.; Ortega, L. Porous silicon strain during in situ ultrahigh vacuum thermal annealing. J. Appl. Phys. 1999, 85, 7105–7111. [Google Scholar] [CrossRef]
- Vazques, E.; Taguena-Martinez, J.; Sansores, L.E.; Wang, C. Surface relaxation effects on the properties of porous silicon. J. Appl. Phys. 2002, 91, 3085–3089. [Google Scholar] [CrossRef]
- Lysenko, V.; Vitiello, J.; Remaki, B.; Barbier, D. Gas permeability of porous silicon nanostructures. Phys. Rev. E 2004, 70, 017301. [Google Scholar] [CrossRef]
- Lysenko, V.; Vitiello, J.; Remaki, B.; Barbier, D.; Skryshevsky, V. Nanoscale morphology dependent hydrogen coverage of meso-porous silicon. Appl. Surf. Sci. 2004, 230, 425–430. [Google Scholar] [CrossRef]
- Mussabek, G.; Alekseev, S.A.; Manilov, I.A.; Tutashkonko, S.; Nychyporuk, T.; Shabdan Ye Amirkhanova, G.; Litvinenko, S.V.; Skryshevsky, V.A.; Lysenko, V. Kinetics of hydrogen generation from oxidation of hydrogenated silicon nanocrystals in aqueous solutions. Nanomaterials 2020, 10, 1413. [Google Scholar] [CrossRef]
- Canham, L.T. Silicon quantum wire array fabricaiton by electrochemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Ogata, H.; Kato, F.; Tsuboi, T.; Sakka, T. Changes in the environment of hydrogen in porous silicon with thermal annealing. J. Electrochem. Soc. 1998, 145, 2439. [Google Scholar] [CrossRef]
- Ogata, Y.; Niki, H.; Sakka, T.; Iwasaki, M. Hydrogen in porous silicon: Vibrational analysis of SiHx species. J. Electrochem. Soc. 1995, 142, 195. [Google Scholar] [CrossRef]
- Martin, P.; Fernandez, J.F.; Sanchez, C.R. Hydrogen surface coverage of as-prepared nanocrystalline porous silicon. Mater. Sci. Eng. B 2004, 108, 166–170. [Google Scholar] [CrossRef]
- Rivolo, P.; Geobaldo, F.; Rocchia, M.; Amato, G.; Rossi, A.M.; Garrone, E. Joint FTIR and TPD study of hydrogen desorption from p+-type porous silicon. Phys. Status Solidi A 2003, 197, 217–221. [Google Scholar] [CrossRef]
- Bockris, J.O.M. Hydrogen economy in the future fn2. Int. J. Hydrogen Energy 1999, 24, 1–15. [Google Scholar] [CrossRef]
- Bolton, J.R.; Strickler, S.J.; Connolly, J.S. Limiting and realizable efficiencies of solar photolysis of water. Nature 1985, 316, 495–500. [Google Scholar] [CrossRef]
- Gao, X.; Kocha, S.; Frank, A.J.; Turner, J.A. Photoelectrochemical decomposition of water using modified monolithic tandem cells fn2. Int. J. Hydrogen Energy 1999, 24, 319–325. [Google Scholar] [CrossRef]
- Abruna, H.D.; Bard, A.J. Semiconductor electrodes. 40. Photoassisted hydrogen evolution at poly(benzyl viologen)-coated p-type silicon electrodes. J. Am. Chem. Soc. 1981, 103, 6898–6901. [Google Scholar] [CrossRef]
- Simon, R.A.; Wrighton, M.S. Stabilization of n-type silicon photoanodes against photoanodic decomposition with thin films of polyacetylene. Appl. Phys. Lett. 1984, 44, 930–932. [Google Scholar] [CrossRef]
- Dominey, R.N.; Lewis, N.S.; Bruce, J.A.; Bookbinder, D.C.; Wrighton, M.S. Improvement of photoelectrochemical hydrogen generation by surface modification of p-type silicon semiconductor photocathodes. J. Amer. Chem. Soc. 1982, 104, 467–482. [Google Scholar] [CrossRef]
- Mathews, N.R.; Sebastian, P.J.; Mathew, X.; Agarwal, V. Photoelectrochemical characterization of porous Si. Int. J. Hydrogen Energy 2003, 28, 629–632. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Cheng, H.M. Visible-light-active elemental photocatalysts. Chem. Phys. Chem. 2013, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Kye, J.; Hwang, S. Enhanced photoelectrochemical hydrogen production from silicon nanowire array photocathode. Nano Lett. 2012, 12, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Nann, T.; Voelcker, N.H. Nanostructured silicon photoelectrodes for solar water electrolysis. Nano Energy 2015, 17, 308–322. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Kuang, Q.Q.; Yang, S. Template synthesis of single-crystal-like porous SrTiO3 nanocube assemblies and their enhanced photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2013, 5, 3683–3690. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.C. A sonochemical approach to hierarchical porous titania spheres with enhanced photocatalytic activity. Chem. Commun. 2003, 2078–2079. [Google Scholar] [CrossRef]
- Song, H.; Liu, D.; Yang, J.; Wang, L.; Xu, H.; Xiong, Y. Highly crystalline mesoporous silicon spheres for efficient visible photocatalytic hydrogen evolution. ChemNanoMat 2016, 3, 22–26. [Google Scholar] [CrossRef]
- Ray, U.; Sarkar, S.; Banerjee, D. Silicon nanowires as an efficient material for hydrogen evolution through catalysis: A review. Catal. Today 2023, 423, 113964. [Google Scholar] [CrossRef]
- Ning, R.; Jiang, Y.; Zeng, Y.; Gong, H.; Zhao, J.; Weisse, J.; Shi, X.; Gill, T.M.; Zheng, X. On-demand production of hydrogen by reacting porous silicon nanowires with water. Nano Res. 2020, 13, 1459–1464. [Google Scholar] [CrossRef]
- Weisse, J.M.; Lee, C.H.; Kim, D.R.; Cai, L.L.; Rao, P.M.; Zheng, X.L. Electroassisted transfer of vertical silicon wire arrays using a sacrificial porous silicon layer. Nano Lett. 2013, 13, 4362–4368. [Google Scholar] [CrossRef] [PubMed]
- Weisse, J.M.; Reifenberg, J.P.; Miller, L.M.; Scullin, M.L. Ultralong Silicon Nanostructures, and Methods of Forming and Transferring the Same. U.S. Patent 9691849, 27 June 2017. [Google Scholar]
- Lai, C.Q.; Zheng, W.; Choi, W.K.; Thompson, C.V. Metal assisted anodic etching of silicon. Nanoscale 2015, 7, 11123–11134. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J. Porous Silicon in Practice: Preparation, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Weisse, J.M.; Marconnet, A.M.; Kim, D.R.; Rao, P.M.; Panzer, M.A.; Goodson, K.E.; Zheng, X.L. Thermal conductivity in porous silicon nanowire arrays. Nanoscale Res. Lett. 2012, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.L.; van Duyne, R.P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Hou Yi Zhu, Z.; Xu, Y.; Guo, F.; Zhang, J.; Wang, X. Efficient photoelectrochemical hydrogen production over p-Si nanowire arrays coupled with molybdenum–sulfur clusters. Int. J. Hydrogen Energy 2017, 42, 2832–2838. [Google Scholar] [CrossRef]
- Bao, X.; Cerqueira, M.F.; Alpuima, P.; Liua, L. Silicon nanowire arrays coupled with cobalt phosphide spheres as a low-cost photocathode for efficient solar hydrogen evolution. Chem. Commun. 2015, 51, 10742–10745. [Google Scholar] [CrossRef]
- Ming, T.; Turishchev, S.; Schleusener, A.; Parinova, E.; Koyuda, D.; Chuvenkova, O.; Schulz, M.; Dietzek, B.; Sivakov, V. Silicon suboxides as driving force for efficient light-enhanced hydrogen generation on silicon nanowires. Small 2021, 17, 2007650. [Google Scholar] [CrossRef]
- Lehmann, V.; Gosele, U. Porous silicon formation: A quantum wire effect. Appl. Phys. Lett. 1991, 58, 856. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.T.; Teo, S.H.; Mahmud, H.; Swaraz, A.M.; Rehan, A.I.; Rasee, A.I.; Kubra, K.T.; Hasan, M.M.; Salman, M.S.; et al. Progress in silicon-based materials for emerging solar-powered green hydrogen (H2) production. Adv. Colloid Interface Sci. 2025, 343, 103558. [Google Scholar] [CrossRef] [PubMed]
- Ming, T.; Hu, X.; Wang, Z.; Wu, X.; Zuo, X. The nature of photocatalytic hydrogen generation on silicon nanowires prepared by MAWC. Int. J. Hydrogen Energy 2024, 19, 42–47. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Ahmadi, N.; Rezaei, B.; Abarghoui, M.M. A new electrochemical sensor for the simultaneous determination of acetaminophen and codeine based on porous silicon/palladium nanostructure. Talanta 2015, 134, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Ghadirian, F.; Jafari-Asl, M.; Rezaei, B. WS2 grafted on silicon and nanosilicon particles etched: A high-performance electrocatalyst for hydrogen evolution reaction. J. Iran. Chem. Soc. 2017, 15, 613–620. [Google Scholar] [CrossRef]
- Elsodany, M.N.; Abdel Rahim, M.A.; Shalaby, N.H.; Sultan, M.A. Nickel and/or platinum modified crystalline silicon–carbon composites and their electrochemical behaviour towards the hydrogen evolution reaction. J. Appl. Electrochem. 2023, 54, 531–546. [Google Scholar] [CrossRef]
- González, I.; De Santiago, F.; Arellano, L.G.; Miranda, A.; Trejo, A.; Salazar, F.; Cruz-Irisson, M. Theoretical modelling of porous silicon decorated with metal atoms for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 26321–26333. [Google Scholar] [CrossRef]
- Merazga, S.; Kerrar, H.; Larabi, A.; Gabouze, N. Palladium effect on electrochemical hydrogen storage properties of nanoporous silicon. Int. J. Hydrogen Energy 2024, 70, 53–60. [Google Scholar] [CrossRef]
- Herino, R. Pore size distribution. In Properties of Porous Silicon; Canham, L.T., Ed.; INSPEC, The IEE: London, UK, 1997; pp. 89–97. [Google Scholar]
- Martin, P.; Fernandez, J.F.; Sanchez, C.R. TDS applied to investigate the hydrogen and silane desorption from porous silicon. Phys. Status Solidi A 2000, 182, 255–260. [Google Scholar] [CrossRef]
- Bisi, O.; Ossicini, S.; Pavesi, L. Porous silicon: A quantum sponge structure for silicon based optoelectronics. Surf. Sci. Rep. 2000, 38, 1–126. [Google Scholar] [CrossRef]
- Herino, R.; Bomchil, G.; Barla, K.; Bertrand, C.; Ginoux, J.L. Porosity and pore size distributions of porous silicon layers. J. Electrochem. Soc. 1987, 134, 1994–2000. [Google Scholar] [CrossRef]
- Vazsonyi, E.; Barsony, I.; Lohner, T.; Fried, M.; Erostyak, J.; Racz, M.; Pászti, F. Light emission versus excitation from porous structures in ion-implanted silicon. MRS Online Proc. Libr. 1995, 358, 653–658. [Google Scholar] [CrossRef]
- Dai, F.; Zai, J.; Yi, R.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, D. Bottomup synthesis of high surface area mesoporous crystalline silicon and evaluation of its hydrogen evolution performance. Nat. Commun. 2014, 5, 3605. [Google Scholar] [CrossRef] [PubMed]
- Manilov, A.I.; Alekseev, S.A.; Skryshevsky, V.A.; Litvinenko, S.V.; Kuznetsov, G.V.; Lysenko, V. Influence of palladium particles impregnation on hydrogen behavior in meso-porous silicon. J. Alloys Compd. 2010, 492, 466–472. [Google Scholar] [CrossRef]
- Honarpazhouh, Y.; Astaraei, F.R.; Naderi, H.R.; Tavakoli, O. Electrochemical hydrogen storage in Pd-coated porous silicon/graphene oxide. Int. J. Hydrogen Energy 2016, 41, 2175–12182. [Google Scholar] [CrossRef]
- Muduli, R.C.; Kale, P. Synergetic effect of porous silicon-nickel composite on its solid-state hydrogen energy storage properties. Int. J. Hydrogen Energy 2023, 48, 35185–35196. [Google Scholar] [CrossRef]
- Muduli, R.C.; Chen, Z.; Shinzato, K.; Guo, F.; Ichikawa, T.; Jain, A.; Miyaoka, H.; Kale, P. Thermodynamic improvement of lithium hydrides for hydrogen absorption and desorption by incorporation of porous silicon. Int. J. Hydrogen Energy 2024, 50, 1094–1102. [Google Scholar] [CrossRef]
- Schlapbach, L. Hydrogen as a fuel and its storage for mobility and transport. MRS Bull. 2002, 27, 675–679. [Google Scholar] [CrossRef]
- Pastushenko, A.; Litvinenko, S.; Lysenko, V.; Skryshevsky, V. Self-regulated hydrogen generation with the use of nano-powders: Application for portable fuel cells. Phys. Sci. Technol. 2018, 5, 50–59. [Google Scholar] [CrossRef]
- Tazi, B.; Savadogo, O. Parameters of PEM fuel-cells based on new membranes fabricated from Nafion®, silicotungstic acid and thiophene. Electrochim. Acta 2000, 45, 4329–4339. [Google Scholar] [CrossRef]
- Harbash, J.A.; Agosta, F. Effect of emulsified fuel on combustion in a four-stroke diesel engine. J. Ship Res. 1991, 35, 356–363. [Google Scholar] [CrossRef]
- Lin, C.; Wang, K.H. Effect of a combustion improver on diesel engine performance and emission characteristics when using three-phase emulsions as an alternative. Energy Fuel 2004, 18, 477–484. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil-water emulsion. Fuel Process Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Impact of hydrogen generated by splitting water with nano-silicon and nano-aluminum on diesel engine performance. Int. J. Hydrogen Energy 2014, 38, 8098–8105. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Kawamura, M.; Imamura, R.; Kobayashi, Y.; Taniguchi, A.; Nakazawa, S.; Kato, T.; Namba-Hamano, T.; Abe, T.; Uemura, M.; Kobayashi, H.; et al. Oral administration of Si-based agent attenuates oxidative stress and ischemia-reperfusion injury in a rat model: A Nobel hydrogen administration method. Front. Med. 2020, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Imamura, R.; Koyama, Y.; Kondo, M.; Kobayashi, H.; Nonomura, N.; Shimada, S. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci. Rep. 2020, 10, 5859. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhao, P.; Gong, W.; Ding, W. Fe-porphyrin: A redox-related biosensor of hydrogen molecule. Nano Res. 2023, 16, 2020–2025. [Google Scholar] [CrossRef]
- Imamura, R.; Kawamura, M.; Taniguchi, A.; Kobayashi, Y.; Nakazawa, S.; Kato, T.; Abe, T.; Uemura, M.; Kobayashi, H.; Nonomura, N. Efficacy of a Si-based agent against developing renal failure in a rat remnant kidney model. Biochem. Biophys. Res. Commun. 2020, 533, 698–703. [Google Scholar] [CrossRef]
- Uchiyama, J.; Akiyama, M.; Hase, K.; Kumagai, Y.; Kim, Y.G. Gut microbiota reinforce host antioxidant capacity via the generation of reactive sulfur species. Cell Rep. 2022, 38, 110479. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 2009, 34, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzym. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Kovalev, D.; Timoshenko, V.Y.; Künzner, N.; Gross, E.; Koch, F. Strong explosive interaction of hydrogenated porous silicon with oxygen at cryogenic temperatures. Phys. Rev. Lett. 2001, 87, 068301. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, S.; Zhu, W.; Ren, D.; Xu, Q.; Feng, Y. A review on key technologies and developments of hydrogen fuel cell multi-rotor drones. Energies 2024, 17, 4193. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Jia, Y.; Li, C.; Zhang, A.; Hu, R.; Liu, J.; Wang, C.; Ma, L.; Ouyang, L. Hydrolysis-engineered robust porous micron Silicon anode for high-energy lithium-ion batteries. Nano Micro Lett. 2025, 17, 297. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Y.; Liu, J.; Chen, K.; Zhong, H.; Jiang, L.; Liu, H.; Ouyang, L.; Zhu, M. Activating silicon for high hydrogen conversion and sustainable anode recovery. Nat. Commun. 2025, 16, 7772. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Sun, J.; Zhu, Y.; Chen, K.; Zhong, H.; Ouyang, L.; Liu, H. In situ formation of Li2SiO3-Li-NaCl interface on Si and its effect on hydrogen evolution. ACS Appl. Mater. Interfaces 2023, 15, 20917–20924. [Google Scholar] [CrossRef]










| Sample | Hydrogen Volume, mL/g × 10−3 |
|---|---|
| PS | 1.2–1.3 |
| PS + 1%Pd | 0.90–1.14 |
| PS + 1%Pd with H2 treatment | 1.04–1.27 |
| PS + 10%Pd | 0.52–0.65 |
| PS + 10%Pd with H2 treatment | 0.17–0.65 |
| Materials | Atomic Hydrogen Content, (mmol g−1) | Theoretical Mass Energy Density, (W-h kg−1) | Ref. |
|---|---|---|---|
| meso-PS (90%, 10 nm) | 13 | 429 | [80] |
| nano-PS (90%, 5 nm) | 34 | 1120 | [80] |
| nano-PS powder (>95%, 2–3 nm) | 66 | 2176 | [80] |
| reversible metal hydrides | [136] | ||
| MgH2 →Mg + H2 | 76 | 2505 | |
| LaNi5H6 → LaNi5 + 3H2 | 14 | 461 | |
| hydride hydrolysis | [136] | ||
| (NaBH4 + 2H2O) →NaBO2 + 4H2 | 108 | 3560 | |
| (LiBH4 + 4H2O) → LiOH + H3 BO3 + 4H2 | 85 | 2802 | |
| hydride thermolysis | [136] | ||
| NH4BH4 → BN + 4H2 | 244 | 8043 | |
| NH3BH3 → BN + 3H2 | 195 | 6428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussabek, G.; Yar-Mukhamedova, G.; Orazbayev, S.; Skryshevsky, V.; Lysenko, V. Silicon Nanostructures for Hydrogen Generation and Storage. Nanomaterials 2025, 15, 1531. https://doi.org/10.3390/nano15191531
Mussabek G, Yar-Mukhamedova G, Orazbayev S, Skryshevsky V, Lysenko V. Silicon Nanostructures for Hydrogen Generation and Storage. Nanomaterials. 2025; 15(19):1531. https://doi.org/10.3390/nano15191531
Chicago/Turabian StyleMussabek, Gauhar, Gulmira Yar-Mukhamedova, Sagi Orazbayev, Valeriy Skryshevsky, and Vladimir Lysenko. 2025. "Silicon Nanostructures for Hydrogen Generation and Storage" Nanomaterials 15, no. 19: 1531. https://doi.org/10.3390/nano15191531
APA StyleMussabek, G., Yar-Mukhamedova, G., Orazbayev, S., Skryshevsky, V., & Lysenko, V. (2025). Silicon Nanostructures for Hydrogen Generation and Storage. Nanomaterials, 15(19), 1531. https://doi.org/10.3390/nano15191531









