Magnetic Cationic Liposomes-Based Delivery System Reduces Drug-Induced Cytotoxicity in an In Vitro Model of Hearing Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Method of Magnetic Cationic Liposomes Coated with Carboxymethyl Chitosan
2.3. Characterization of CMCS-Coated Magnetic Liposomes
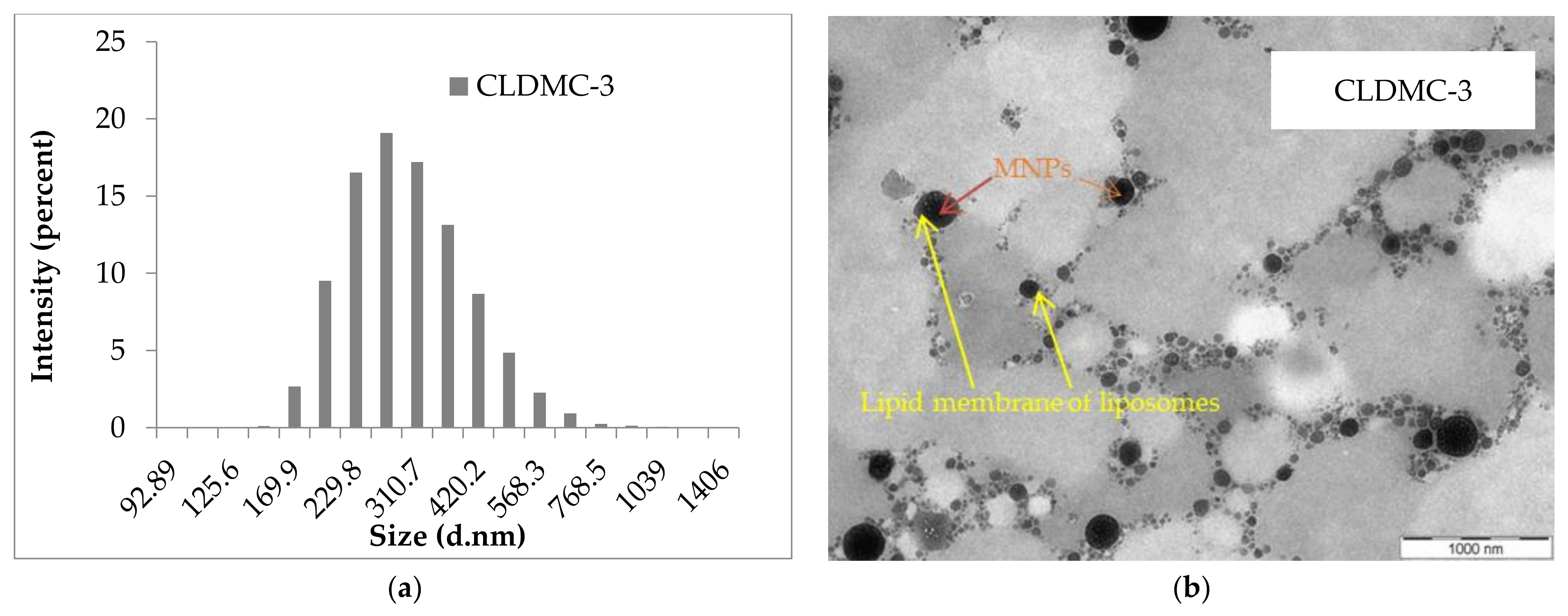
2.3.1. Morphology of Liposomes
2.3.2. FT-IR Spectroscopy
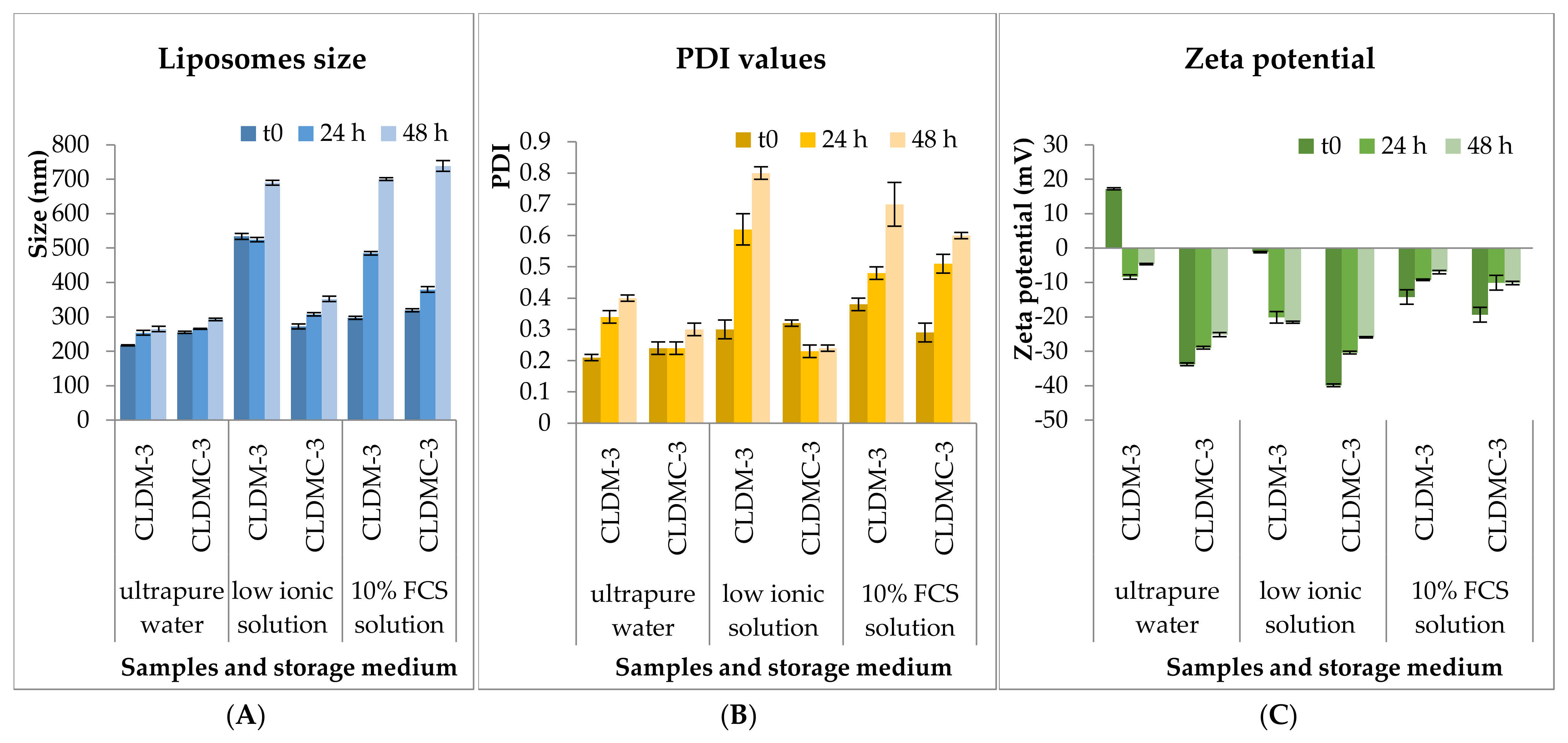
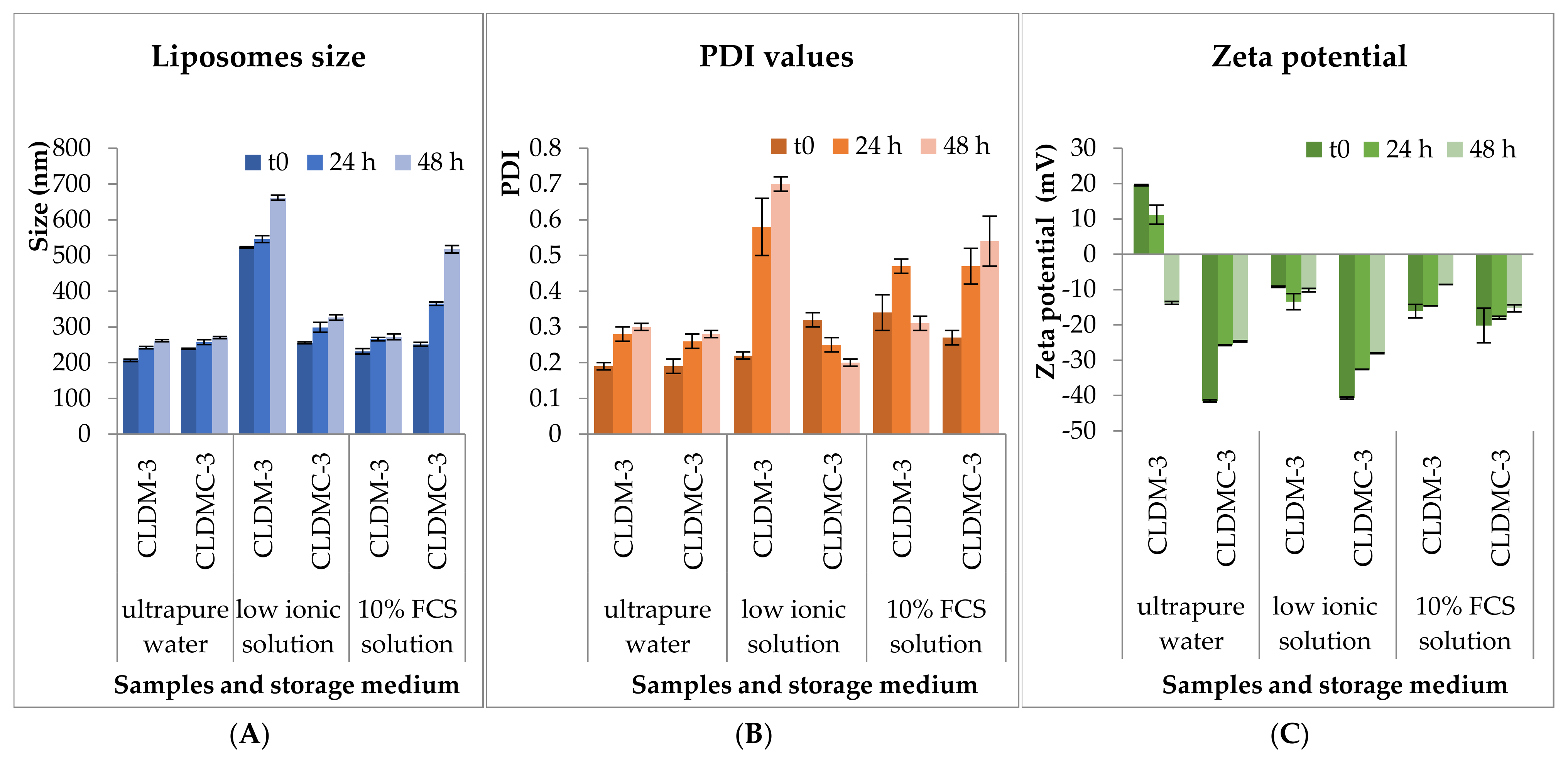
2.3.3. Stability Tests
2.3.4. Drug Encapsulation and Release Efficiency
2.3.5. Magnetic Properties
2.4. In Vitro Studies of HEI-OC1 Cell Cultures
2.4.1. Cells
2.4.2. Liposomes’ Biological Characterization
Cell Viability Tests
Gentamicin and Cisplatin Toxicity
HEI-OC1 Recovery After Gentamicin/Cisplatin Exposure and Treatment with Liposomal Formulations
Mitochondrial Membrane Potential—JC-1
Quantitative Assessment of Iron Nanoparticles’ Uptake Using Ferrozine Assay
Beta-Galactosidase Activity (B-Gal)
Statistical Analysis
3. Results
3.1. Characterization of CMCS-Coated Magnetic Cationic Liposomes
3.1.1. Morphology of Liposomes
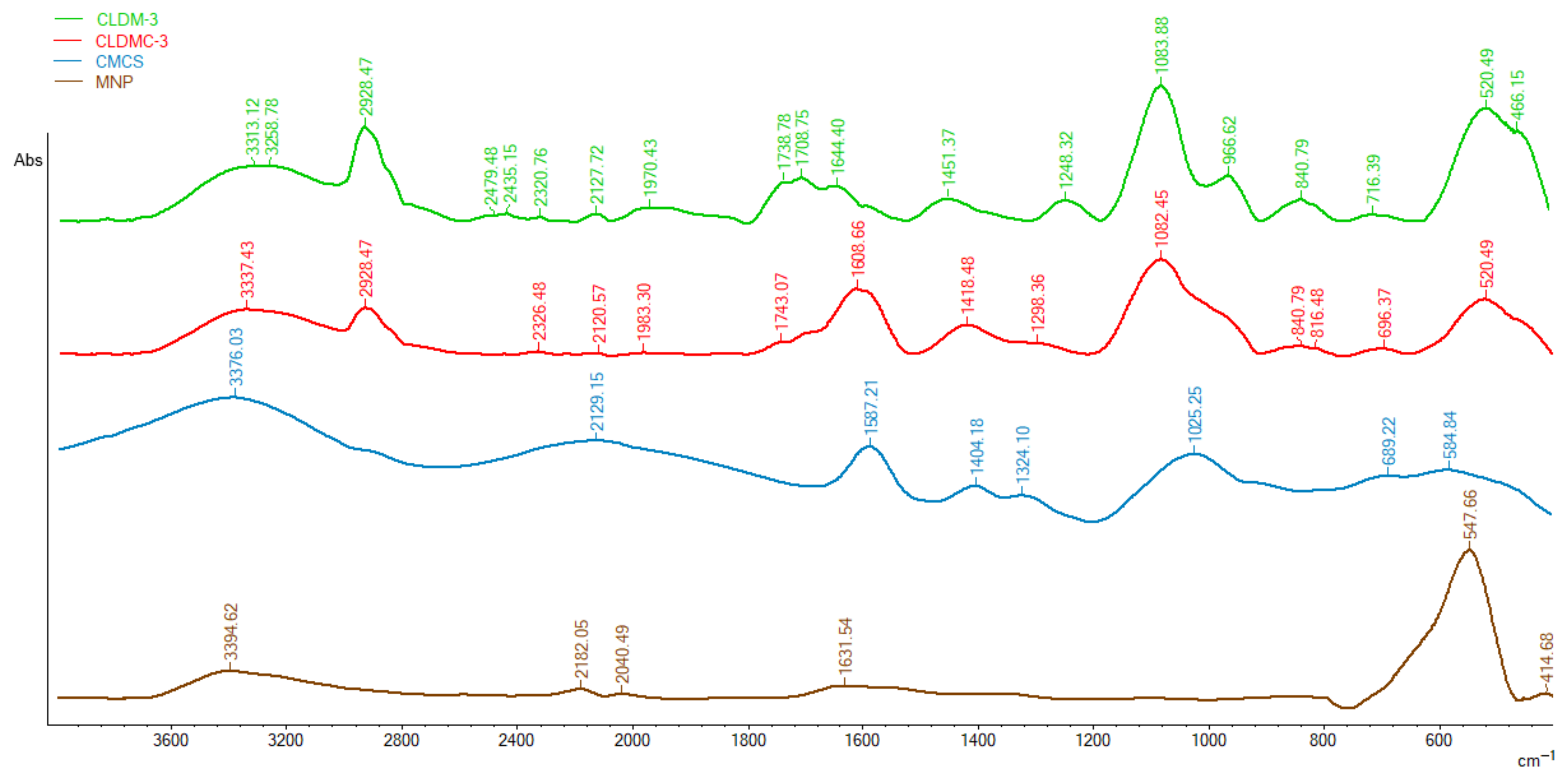
3.1.2. FT-IR Spectroscopy
- (i)
- Appearance in the spectrum of CLDMC-3 sample of peaks at 2928 cm−1, 1082 cm−1, and 840 cm−1 characteristic of the uncoated magnetic liposomes sample;
- (ii)
- The shift of the peaks from 3376 cm−1 to 3337 cm−1, from 1587 cm−1 to 1608 cm−1, and from 1404 cm−1 to 1418 cm−1 in the spectrum of CMCS to spectrum of CLDMC-3;
- (iii)
- The presence of the characteristic CMCS peak at 1025 cm−1 in the broad peak of the coated magnetic liposomes.
3.1.3. Stability Tests
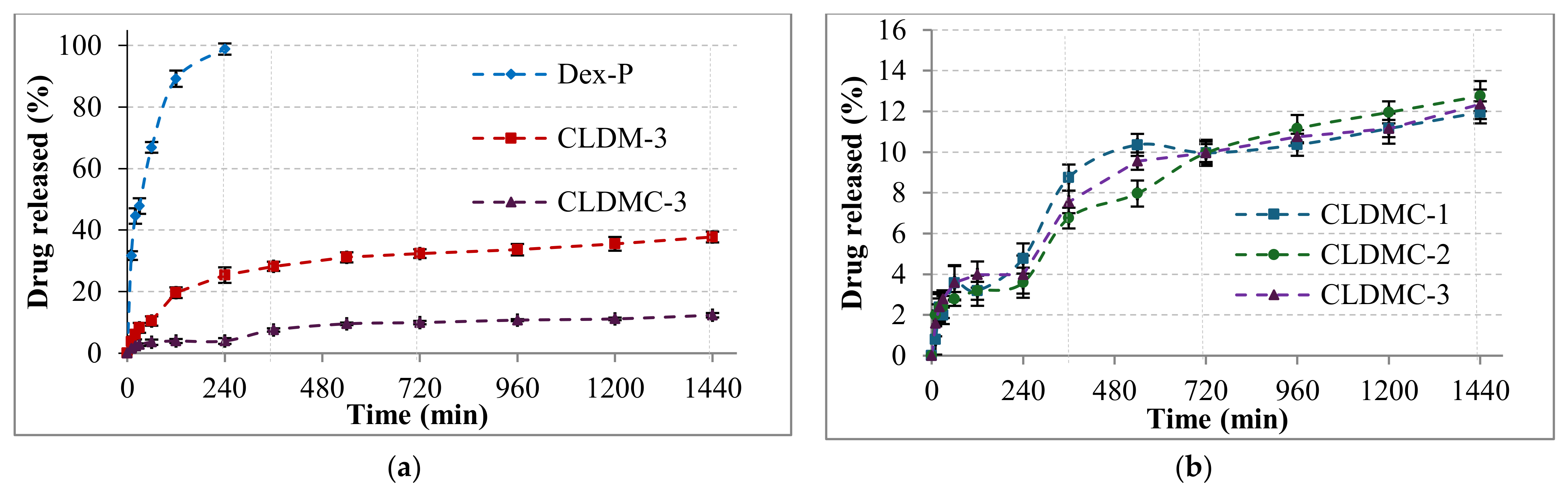
3.1.4. Drug Encapsulation and Release Efficiency
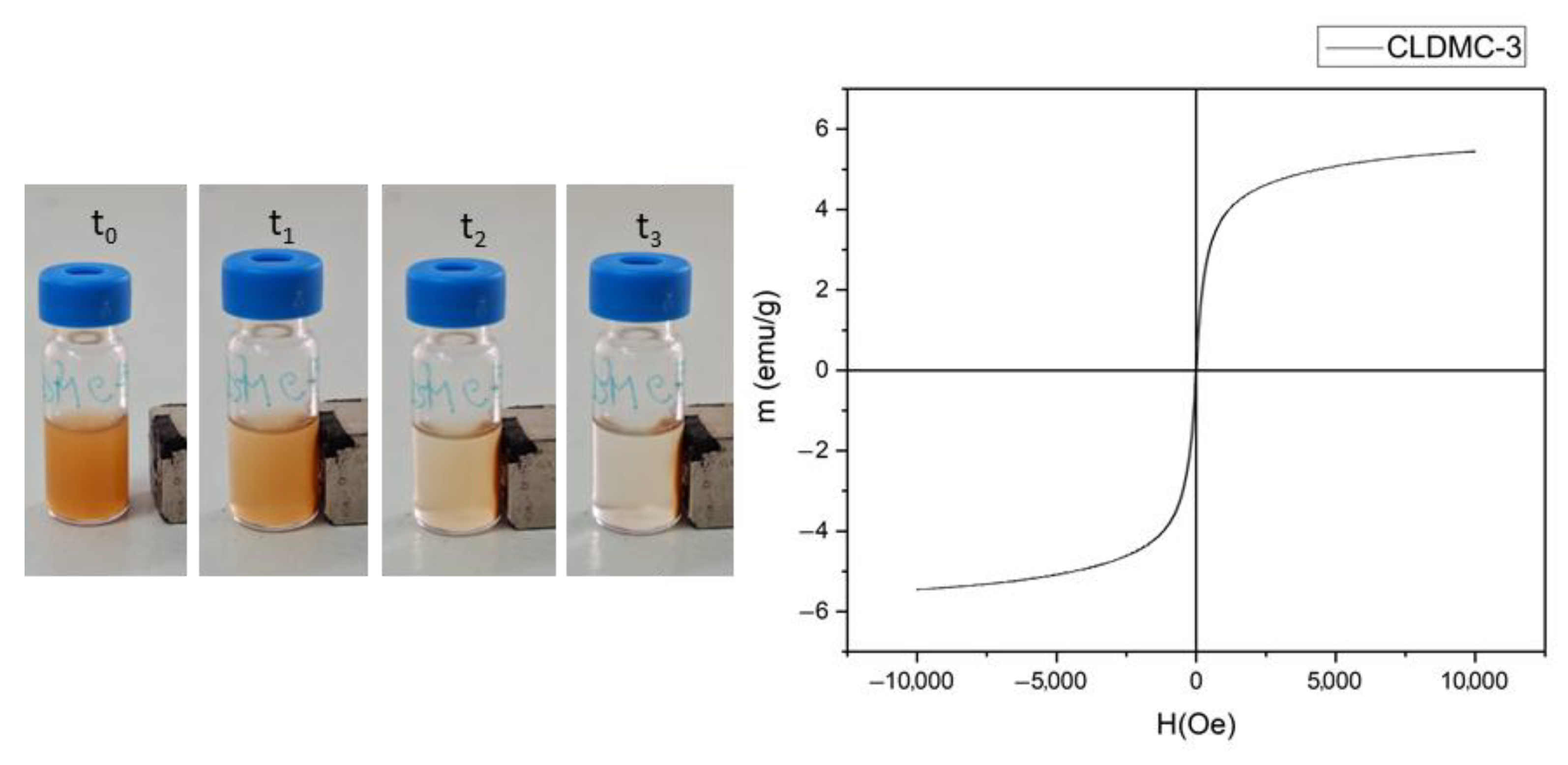
3.1.5. Magnetic Properties
3.2. Liposomes’ Biological Characterization
3.2.1. HEI-OC1 Viability
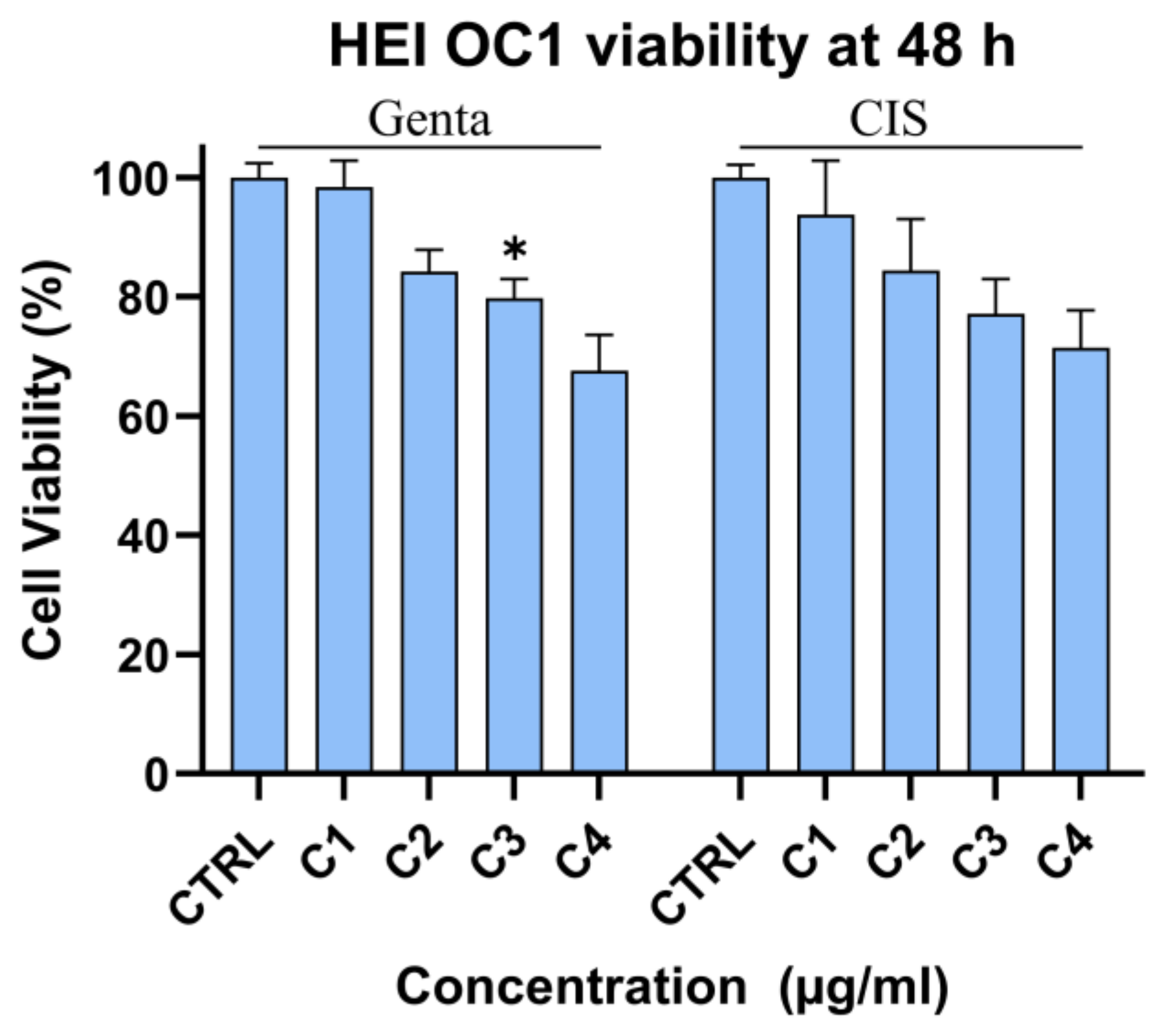
Cytotoxic Effect of Gentamicin and Cisplatin on HEI-OC1 Cells
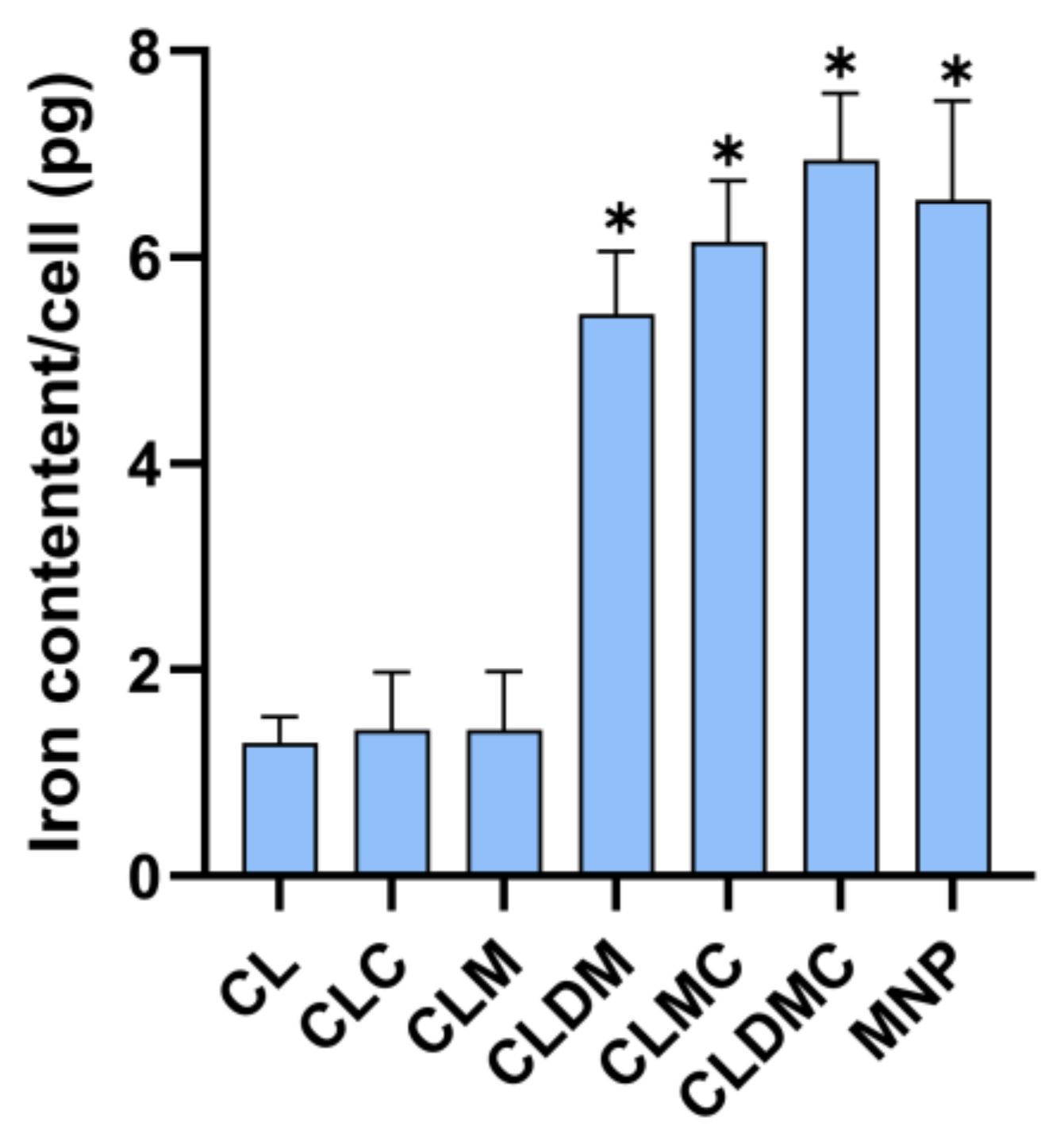
Liposomal Formulation Uptake Based on Iron Content (Ferrozine Assay)
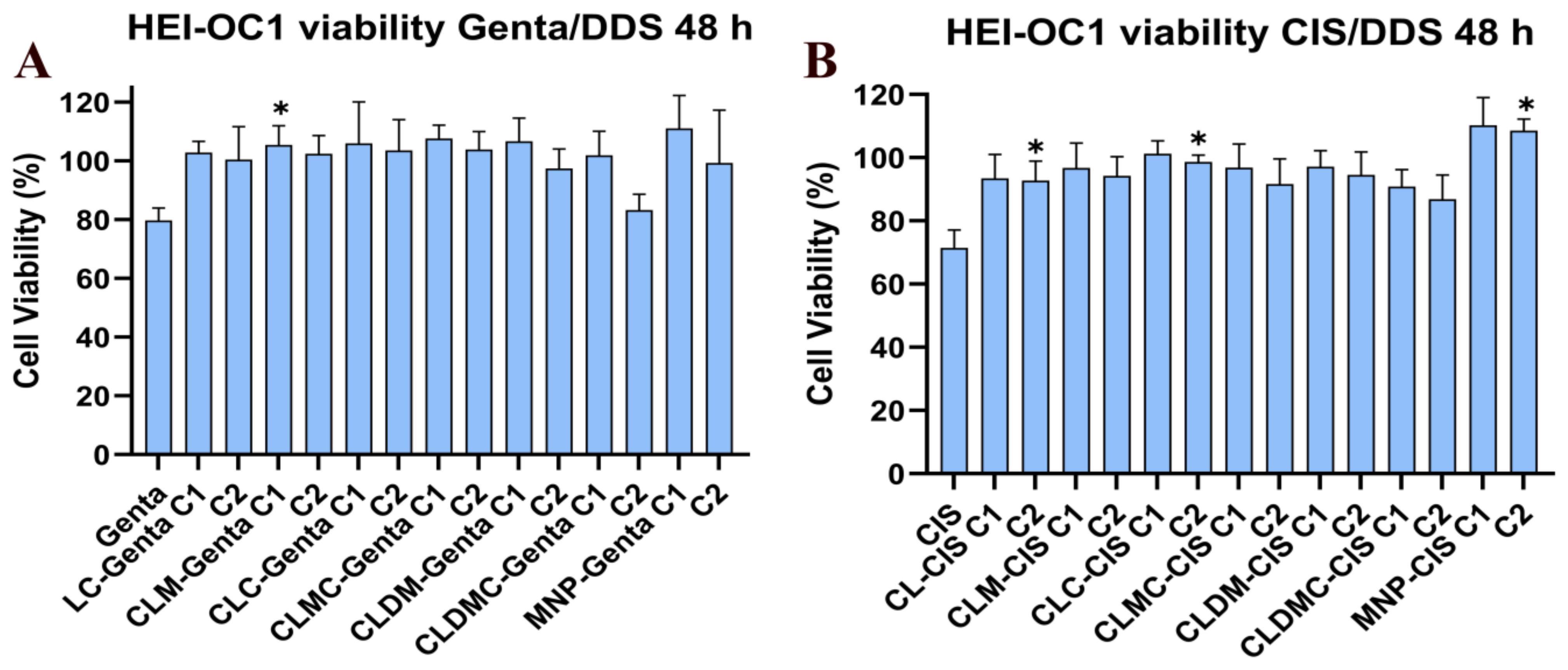
Effect of Liposomal Formulation Treatment on HEI-OC1 Cells Exposed to Drug Toxicity—Cell Viability Using MTT
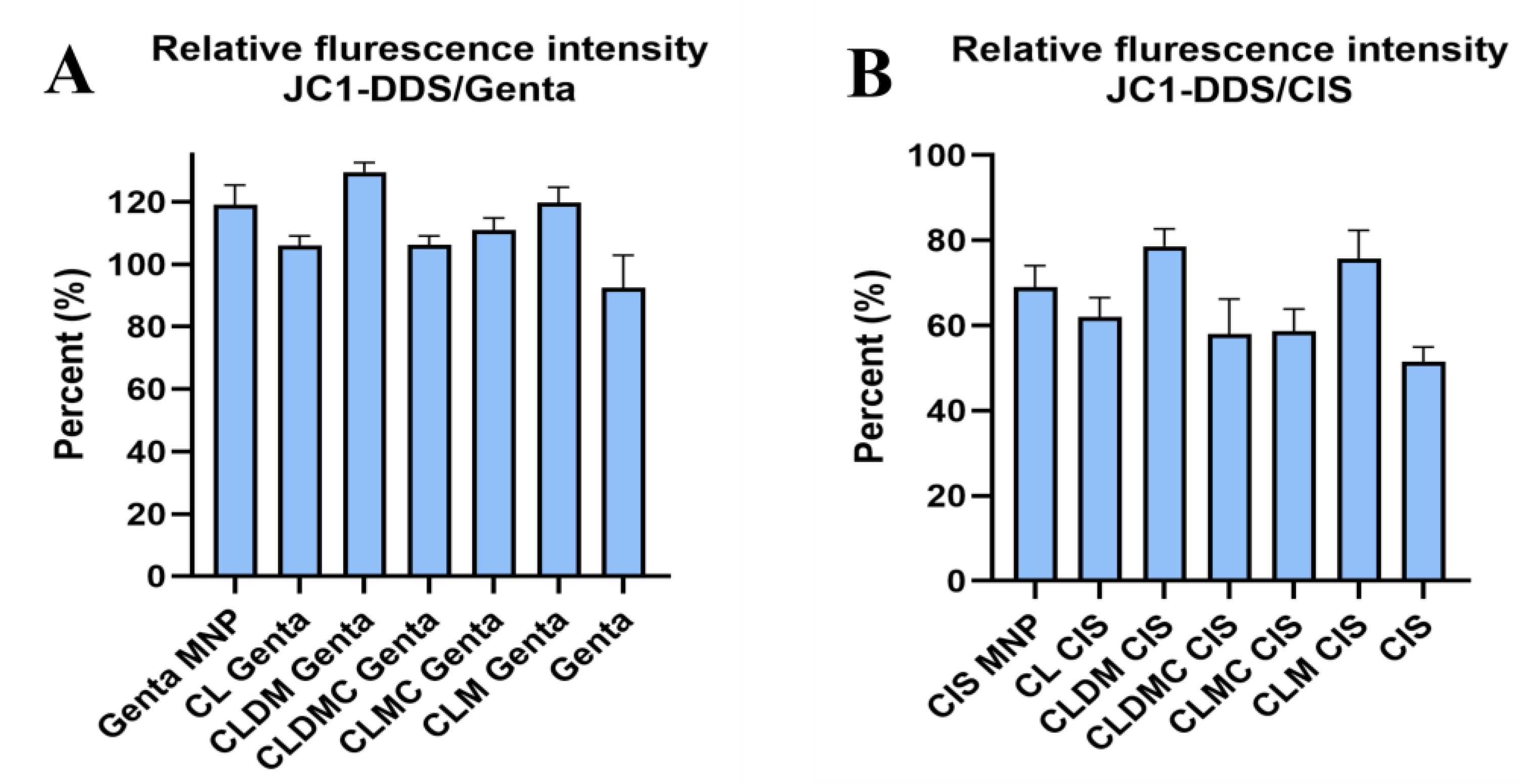
JC-1 Fluctuation in HEI-OC1 Exposed to Drugs and Treated with Liposomal Formulations
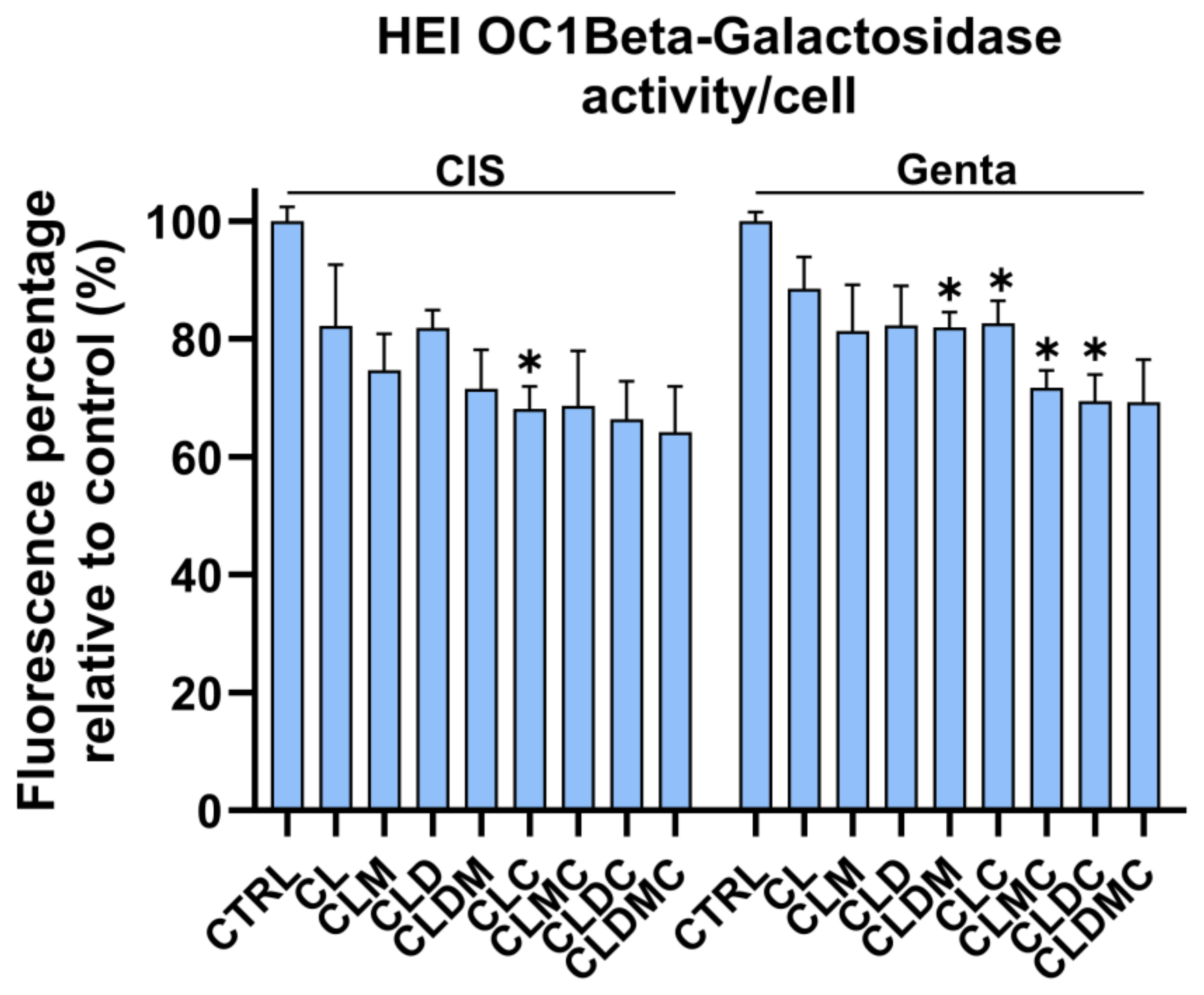
Senescence-Associated Activity in HEI-OC1 Cells Treated with Gentamicin and Cisplatin Liposomal Formulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Deafness and Hearing Loss. Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 26 February 2025).
- Sheffield, A.M.; Smith, R.J.H. The Epidemiology of Deafness. Cold Spring Harb. Perspect. Med. 2019, 9, a033258. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Cisplatin ototoxicity and protection: Clinical and experimental studies. Tohoku J. Exp. Med. 2009, 219, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Pasdelou, M.P.; Byelyayeva, L.; Malmström, S.; Pucheu, S.; Peytavy, M.; Laullier, H.; Hodges, D.B., Jr.; Tzafriri, A.R.; Naert, G. Ototoxicity: A high risk to auditory function that needs to be monitored in drug development. Front. Mol. Neurosci. 2024, 2, 1379743. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J. Recent advances in stem cells and regenerative medicine. QJM 2014, 107, 251–252. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Cumpata, A.J.; Labusca, L.; Radulescu, L.M. Stem Cell-Based Therapies for Auditory Hair Cell Regeneration in the Treatment of Hearing Loss. Tissue Eng. Part B Rev. 2024, 30, 15–28. [Google Scholar] [CrossRef]
- Takeda, H.; Dondzillo, A.; Randall, J.A.; Gubbels, S.P. Challenges in Cell-Based Therapies for the Treatment of Hearing Loss. Trends Neurosci. 2018, 41, 823–837. [Google Scholar] [CrossRef]
- Guo, J.; Chai, R.; Li, H.; Sun, S. Protection of Hair Cells from Ototoxic Drug-Induced Hearing Loss. Adv. Exp. Med. Biol. 2019, 1130, 17–36. [Google Scholar]
- Flaherty, S.M.; Russell, I.J.; Lukashkin, A.N. Drug distribution along the cochlea is strongly enhanced by low-frequency round window micro vibrations. Drug Deliv. 2021, 28, 1312–1320. [Google Scholar] [CrossRef]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2019, 5, 122–131. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Vallecillo, N.; Cediel, R.; Celaya, A.M.; Lassaletta, L.; Varela-Nieto, I.; Contreras, J. A Comparative Study of Drug Delivery Methods Targeted to the Mouse Inner Ear: Bullostomy Versus Transtympanic Injection. J. Vis. Exp. 2017, 8, 54951. [Google Scholar]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, H.; Xu, L.; Pei, X.; Xiong, J.; Wang, Y.; Zhan, X.; Li, S.; He, Y. Liposomes in the cosmetics: Present and outlook. J. Liposome Res. 2024, 34, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef]
- Labusca, L.S.; Herea, D.D.; Radu, E.; Danceanu, C.; Chiriac, H.; Lupu, N. Human Adipose Derived Stem Cells and Osteoblasts Interaction with Fe-Cr-Nb-B Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 5143–5153. [Google Scholar] [CrossRef]
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G.; et al. A Review of Magnetic Particle Imaging and Perspectives on Neuroimaging. AJNR Am. J. Neuroradiol. 2019, 40, 206–212. [Google Scholar] [CrossRef]
- Cumpata, A.J.; Peptanariu, D.; Lungoci, A.L.; Labusca, L.; Pinteala, M.; Radulescu, L. Towards Regenerative Audiology: Immune Modulation of Adipose-Derived Mesenchymal Cells Preconditioned with Citric Acid-Coated Antioxidant-Functionalized Magnetic Nanoparticles. Medicina 2023, 59, 587. [Google Scholar] [CrossRef]
- Cheng, Z.; Huang, H.; Yin, M.; Liu, H. Applications of liposomes and lipid nanoparticles in cancer therapy: Current advances and prospects. Exp. Hematol. Oncol. 2025, 14, 11. [Google Scholar] [CrossRef]
- Vlasova, K.Y.; Piroyan, A.; Le-Deygen, I.M.; Vishwasrao, H.M.; Ramsey, J.D.; Klyachko, N.L.; Golovin, Y.I.; Rudakovskaya, P.G.; Kireev, I.I.; Kabanov, A.V.; et al. Magnetic liposome design for drug release systems responsive to super-low frequency alternating current magnetic field (AC MF). J. Colloid Interface Sci. 2019, 552, 689–700. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Roy, S.; Apolo, A.B.; Shapiro, B.; Depireux, D.A. Magnetic Nanoparticle Mediated Steroid Delivery Mitigates Cisplatin Induced Hearing Loss. Front. Cell. Neurosci. 2017, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Hussain, N.; Pafford, R.; Parham, K. Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngol.-Head. Neck Surg. 2014, 150, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.; Koo, C.; Richter, C.P.; Tan, X. Hearing loss during chemotherapy: Prevalence, mechanisms, and protection. Am. J. Cancer Res. 2024, 14, 4597–4632. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, X.; Chen, X. Polymer-Modified Liposomes for Drug Delivery: From Fundamentals to Applications. Pharmaceutics 2022, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, Y.; Zhao, X.; Wang, Y.; Zhang, Z.; Zhang, X.; Li, J.; Zheng, H.; Zhang, Y.; Shi, H.; et al. Pathologic Tissue Injury and Inflammation in Mice Immunized with Plasmid DNA-Encapsulated DOTAP-Based Lipid Nanoparticles. Bioconjug. Chem. 2024, 35, 2015–2026. [Google Scholar] [CrossRef]
- Filion, M.C.; Phillips, N.C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta 1997, 1329, 345. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Barea, M.J.; Jenkins, M.J.; Gaber, M.H.; Bridson, R.H. Evaluation of liposomes coated with a pH responsive polymer. Int. J. Pharm. 2010, 402, 89–94. [Google Scholar] [CrossRef]
- Shalaby, T.I.; El-Refaie, W.M. Bioadhesive Chitosan-Coated Cationic Nanoliposomes with Improved Insulin Encapsulation and Prolonged Oral Hypoglycemic Effect in Diabetic Mice. J. Pharm. Sci. 2018, 107, 2136–2143. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-Based Drug Delivery Systems: From Laboratory Research to Industrial Production—Instruments and Challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Iftode, L.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G.; Radulescu, L.; Popa, M.; Gherghel, D. Double Peptide-Functionalized Carboxymethyl Chitosan-Coated Liposomes Loaded with Dexamethasone as a Potential Strategy for Active Targeting Drug Delivery. Int. J. Mol. Sci. 2025, 26, 922. [Google Scholar] [CrossRef] [PubMed]
- Vochita, G.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Popa, M.; Mahdieh, A.; Mihai, C.T.; Stache, A.B.; Moldovan, C.V.; Bacaita, E.S.; et al. Comparative In Vitro Study between Biocompatible Chitosan-Based Magnetic Nanocapsules and Liposome Formulations with Potential Application in Anti-Inflammatory Therapy. Int. J. Mol. Sci. 2024, 25, 8454. [Google Scholar] [CrossRef] [PubMed]
- Dellali, K.Z.; Rata, D.M.; Popa, M.; Djennad, M.; Ouagued, A.; Gherghel, D. Antitumoral Drug: Loaded Hybrid Nanocapsules Based on Chitosan with Potential Effects in Breast Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 5659. [Google Scholar] [CrossRef] [PubMed]
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Vochița, G.; Sande, S.A.; Popa, M. Peptide-functionalized magnetic microcapsules loaded with dexamethasone for dual active targeted treatment of inner ear inflammation. Polymer 2025, 316, 127864. [Google Scholar] [CrossRef]
- Kalinec, F.; Kalinec, G.; Boukhvalova, M.; Kachar, B. Establishment and characterization of conditionally immortalized organ of corti cell lines. Cell Biol. Int. 1999, 23, 175–184. [Google Scholar] [CrossRef]
- Süleymanoglu, E. The use of infrared spectroscopy for following drug-membrane interactions: Probing paclitaxel (taxol)-cell phospholipid surface recognition. Electron. J. Biomed. 2009, 3, 19. [Google Scholar]
- Mohamed, A.L.; Hassabo, A.G. Composite material based on pullulan/silane/ZnO-NPs as pH, thermo-sensitive and antibacterial agent for cellulosic fabrics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045005. [Google Scholar] [CrossRef]
- Zaheer, J.; Lee, H.S.; Kim, S.; Jang, J.; Kim, H.; Choi, J.; Park, M.H.; Kim, J.S. Microplastic polyethylene induced inner ear dysfunction in murine model. J. Hazard. Mater. 2024, 476, 135193. [Google Scholar] [CrossRef]
- Barreto, G.R.; Kawai, C.; Tofanello, A.; Neves, A.A.R.; Araujo-Chaves, J.C.; Belleti, E.; Lanfredi, A.J.C.; Crespilho, F.N.; Nantes-Cardoso, I.L. Magnetoliposomes as model for signal transmission. R. Soc. Open Sci. 2019, 6, 181108. [Google Scholar] [CrossRef]
- Xu, K.; Xu, B.; Gu, J.; Wang, X.; Yu, D.; Chen, Y. Intrinsic mechanism and pharmacologic treatments of noise-induced hearing loss. Theranostics 2023, 13, 3524–3549. [Google Scholar] [CrossRef]
- Fu, X.; Wan, P.; Li, P.; Wang, J.; Guo, S.; Zhang, Y.; An, Y.; Ye, C.; Liu, Z.; Gao, J.; et al. Mechanism and Prevention of Ototoxicity Induced by Aminoglycosides. Front. Cell. Neurosci. 2021, 15, 692762. [Google Scholar] [CrossRef]
- Li, L.; Luo, J.; Lin, X.; Tan, J.; Li, P. Nanomaterials for Inner Ear Diseases: Challenges, Limitations and Opportunities. Materials 2022, 15, 3780. [Google Scholar] [CrossRef]
- Goyal, M.M.; Zhou, N.J.; Vincent, P.F.Y.; Hoffman, E.S.; Goel, S.; Wang, C.; Sun, D.Q. Rationally Designed Magnetic Nanoparticles for Cochlear Drug Delivery: Synthesis, Characterization, and In Vitro Biocompatibility in a Murine Model. Otol. Neurotol. Open 2022, 2, e013. [Google Scholar] [CrossRef]
- Li, L.; Chao, T.; Brant, J.; O’Malley, B., Jr.; Tsourkas, A.; Li, D. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv. Drug Deliv. Rev. 2017, 108, 2–12. [Google Scholar] [CrossRef]
- Lin, Q.; Guo, Q.; Zhu, M.; Zhang, J.; Chen, B.; Wu, T.; Jiang, W.; Tang, W. Application of Nanomedicine in Inner Ear Diseases. Front. Bioeng. Biotechnol. 2022, 9, 809443. [Google Scholar] [CrossRef]
- Kopke, R.D.; Wassel, R.A.; Mondalek, F.; Grady, B.; Chen, K.; Liu, J.; Gibson, D.; Dormer, K.J. Magnetic nanoparticles: Inner ear targeted molecule delivery and middle ear implant. Audiol. Neurootol. 2006, 11, 123–133. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Shu, Y.; Zhang, H. Nanoparticle-based inner ear delivery systems for the treatment of hearing loss. Smart Mater. Med. 2021, 2, 350–353. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Lin, L. Characterization, Stability, andIn VitroRelease Evaluation of Carboxymethyl Chitosan Coated Liposomes Containing Fish Oil. J. Food Sci. 2015, 80, C1460–C1467. [Google Scholar] [CrossRef] [PubMed]
- Kalinec, G.; Thein, P.; Park, C.; Kalinec, F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear. Res. 2016, 335, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, X.; Jin, H.; Mi, Y.; Liu, L.; Dong, M.; Chen, Y.; Zou, Z. Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur. J. Pharm. Biopharm. 2021, 163, 60–71. [Google Scholar] [CrossRef]
- Zou, J.; Feng, H.; Sood, R.; Kinnunen, P.K.; Pyykko, I. Biocompatibility of Liposome Nanocarriers in the Rat Inner Ear After Intratympanic Administration. Nanoscale Res. Lett. 2017, 12, 372. [Google Scholar] [CrossRef]
- Shahsavari, S.; Rad, M.B.; Hajiaghajani, A.; Rostami, M.; Hakimian, F.; Jafarzadeh, S.; Hasany, M.; Collingwood, J.F.; Aliakbari, F.; Fouladiha, H.; et al. Magnetoresponsive liposomes applications in nanomedicine: A comprehensive review. Biomed. Pharmacother. 2024, 181, 117665. [Google Scholar] [CrossRef]
- Lee, D.S.; Schrader, A.; Zou, J.; Ang, W.H.; Warchol, M.E.; Sheets, L. Direct targeting of mitochondria by cisplatin leads to cytotoxicity in zebrafish lateral-line hair cells. iScience 2024, 27, 110975. [Google Scholar] [CrossRef]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef]
- Cho, S.I.; Jo, E.R.; Song, H. Urolithin A attenuates auditory cell senescence by activating mitophagy. Sci. Rep. 2022, 12, 7704. [Google Scholar] [CrossRef]


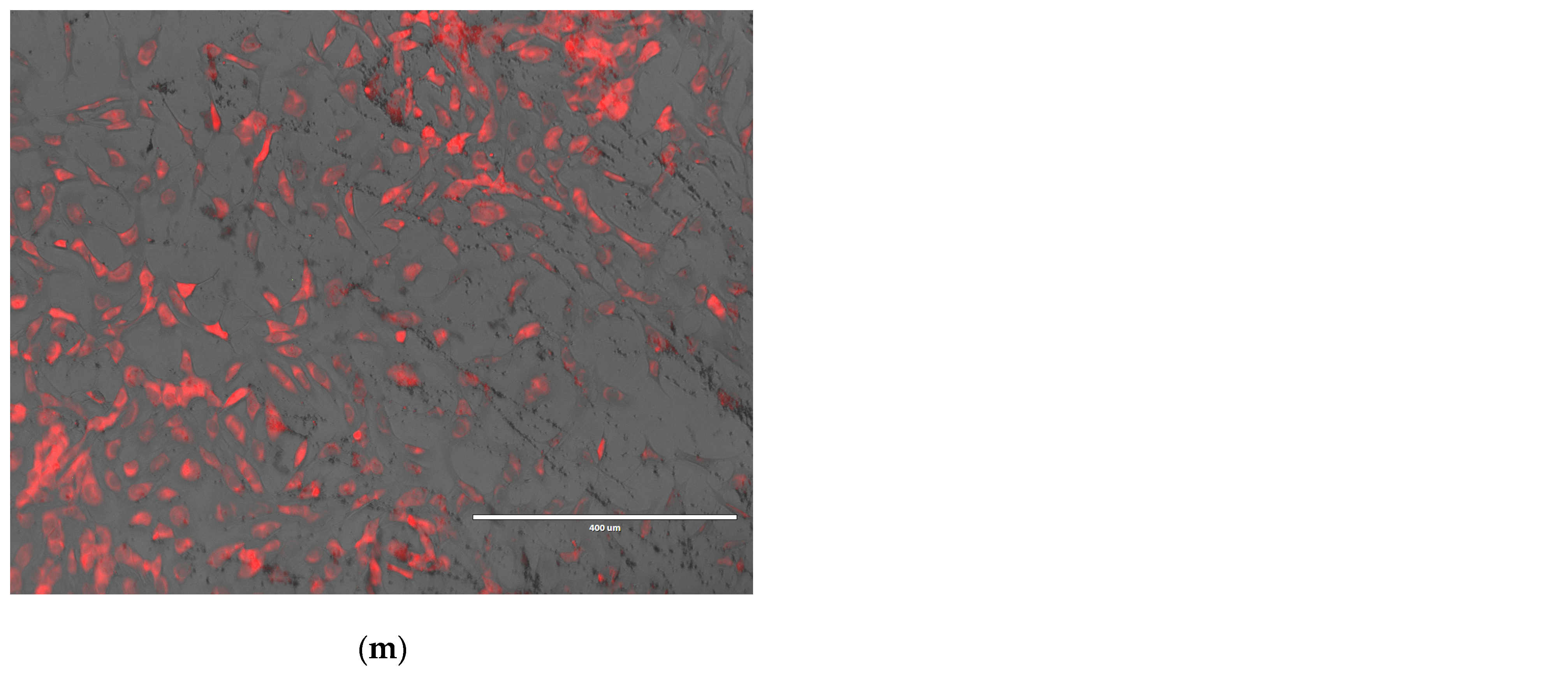
| Sample Code | EPC/Chol/DOTAP (Molar Ratio) | EPC/Chol/DOTAP (mg/mg/mg) | Dexamethasone Phosphate (Dex-P) (mg) | MNPs (mg) | CMCS (1%)/ Liposomes Suspension (v/v) | Sonication Time (min) |
|---|---|---|---|---|---|---|
| CMCS-Coated Magnetic Liposomes | ||||||
| CLDMC-1 | 1.00/0.18/0.05 | 22/2/1 | 25 | 12.5 | 1/1 | 80 |
| CLDMC-2 | 0.95/0.18/0.10 | 21/2/2 | ||||
| CLDMC-3 | 0.86/0.36/0.10 | 19/4/2 | ||||
| Sample Code | Mean Diameter (nm) | PDI | Zeta Potential (mV) | MNPs Loading Efficiency (%) | Drug Loading Efficiency (%) | The Final Composition (mg/mL of Each Component) EPC/Chol/DOTAP/MNPs/Dex-P/CMCS |
|---|---|---|---|---|---|---|
| CLDM-1 | 212.4 ± 7.2 | 0.47 | 14.5 ± 4.1 | 34.4 ± 1.6 | 9.2 ± 1.3 | 4.40/0.40/0.20/0.86/0.46/0.01 |
| CLDM-2 | 210.2 ± 6.0 | 0.35 | 15.7 ± 1.1 | 31.7 ± 3.2 | 8.8 ± 0.8 | 4.20/0.40/0.40/0.79/0.44/0.01 |
| CLDM-3 | 207.6 ± 8.2 | 0.30 | 17.5 ± 1.2 | 29.5 ± 1.2 | 8.4 ± 1.1 | 3.80/0.80/0.40/0.74/0.44/0.01 |
| CLDMC-1 | 306.7 ± 7.6 | 0.28 | −10.3 ± 0.2 | 34.4 ± 1.6 | 7.7 ± 1.9 | 4.40/0.40/0.20/0.86/0.39/0.01 |
| CLDMC-2 | 272.3 ± 9.9 | 0.23 | −11.2 ± 0.9 | 31.7 ± 3.2 | 7.1 ± 0.6 | 4.20/0.40/0.40/0.79/0.36/0.01 |
| CLDMC-3 | 268.3 ± 5.8 | 0.32 | −20.4 ± 1.6 | 29.5 ± 1.2 | 6.5 ± 0.4 | 3.80/0.80/0.40/0.74/0.33/0.01 |
| Formulation | Cell Viability ↑ (%) vs. Drug Only | JC-1 Mitochondrial Potential (% of Control) | β-Gal Activity ↓ (Senescence) |
|---|---|---|---|
| MNPs | Significant ↑ (both cis and genta) | ↑ non-significant | Non-significant ↓ |
| Cationic Liposomes (CL) | Non-significant ↑ | ↑ non-significant | Non-significant ↓ |
| Magnetic Cationic Liposomes (CLM) | Significant ↑ (best for cis) | Cis: ~75.7% ↑ significant; Genta: ~119.6% ↑ significant | Non-significant ↓ |
| CMCS-Coated Liposomes (CLC) | Non-significant ↑ | ↑ non-significant | Non-significant ↓ |
| CMCS-Coated Magnetic Liposomes (CLMC) | Significant ↑ (dose-dependent, better at low conc.) | ↑, but less than full restoration at high conc. | Non-significant ↓ |
| Cationic Liposomes + Dex (CLD) | Non-significant ↑ | ↑ non-significant | Non-significant ↓ |
| Magnetic Liposomes + Dex (CLDM) | Significant ↑ (best for genta) | Cis: ~78.6% ↑ significant; Genta: ~129.6% ↑ significant | Non-significant ↓ |
| CMCS-Coated Liposomes + Dex (CLDC) | Non-significant ↑ | ↑ non-significant | Non-significant ↓ |
| CMCS-Coated Magnetic Liposomes + Dex (CLDMC) | Significant ↑ (esp. β-gal reduction) | ↑ moderate; protective vs. both cis and genta | Significant ↓ (both cis and genta) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftode, L.; Zara Danceanu, C.M.; Cadinoiu, A.N.; Raţă, D.M.; Popa, M.; Labusca, L.; Radulescu, L. Magnetic Cationic Liposomes-Based Delivery System Reduces Drug-Induced Cytotoxicity in an In Vitro Model of Hearing Loss. Nanomaterials 2025, 15, 1529. https://doi.org/10.3390/nano15191529
Iftode L, Zara Danceanu CM, Cadinoiu AN, Raţă DM, Popa M, Labusca L, Radulescu L. Magnetic Cationic Liposomes-Based Delivery System Reduces Drug-Induced Cytotoxicity in an In Vitro Model of Hearing Loss. Nanomaterials. 2025; 15(19):1529. https://doi.org/10.3390/nano15191529
Chicago/Turabian StyleIftode, Loredana, Camelia Mihaela Zara Danceanu, Anca Niculina Cadinoiu, Delia Mihaela Raţă, Marcel Popa, Luminița Labusca, and Luminita Radulescu. 2025. "Magnetic Cationic Liposomes-Based Delivery System Reduces Drug-Induced Cytotoxicity in an In Vitro Model of Hearing Loss" Nanomaterials 15, no. 19: 1529. https://doi.org/10.3390/nano15191529
APA StyleIftode, L., Zara Danceanu, C. M., Cadinoiu, A. N., Raţă, D. M., Popa, M., Labusca, L., & Radulescu, L. (2025). Magnetic Cationic Liposomes-Based Delivery System Reduces Drug-Induced Cytotoxicity in an In Vitro Model of Hearing Loss. Nanomaterials, 15(19), 1529. https://doi.org/10.3390/nano15191529









