Dry Reforming of Methane Using Gd-promoted Ni/SBA-16 Catalyst: Structure, Activity and Process Optimization with Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Catalyst Characterization Description
2.4. Catalyst Evaluation
2.5. Experimental Design and Modelling Approach
2.5.1. Central Composite Design (CCD)
2.5.2. Quadratic Polynomial Regression Model
2.5.3. Model Validation and Analysis
3. Results and Discussion
3.1. Catalyst Characterization Results
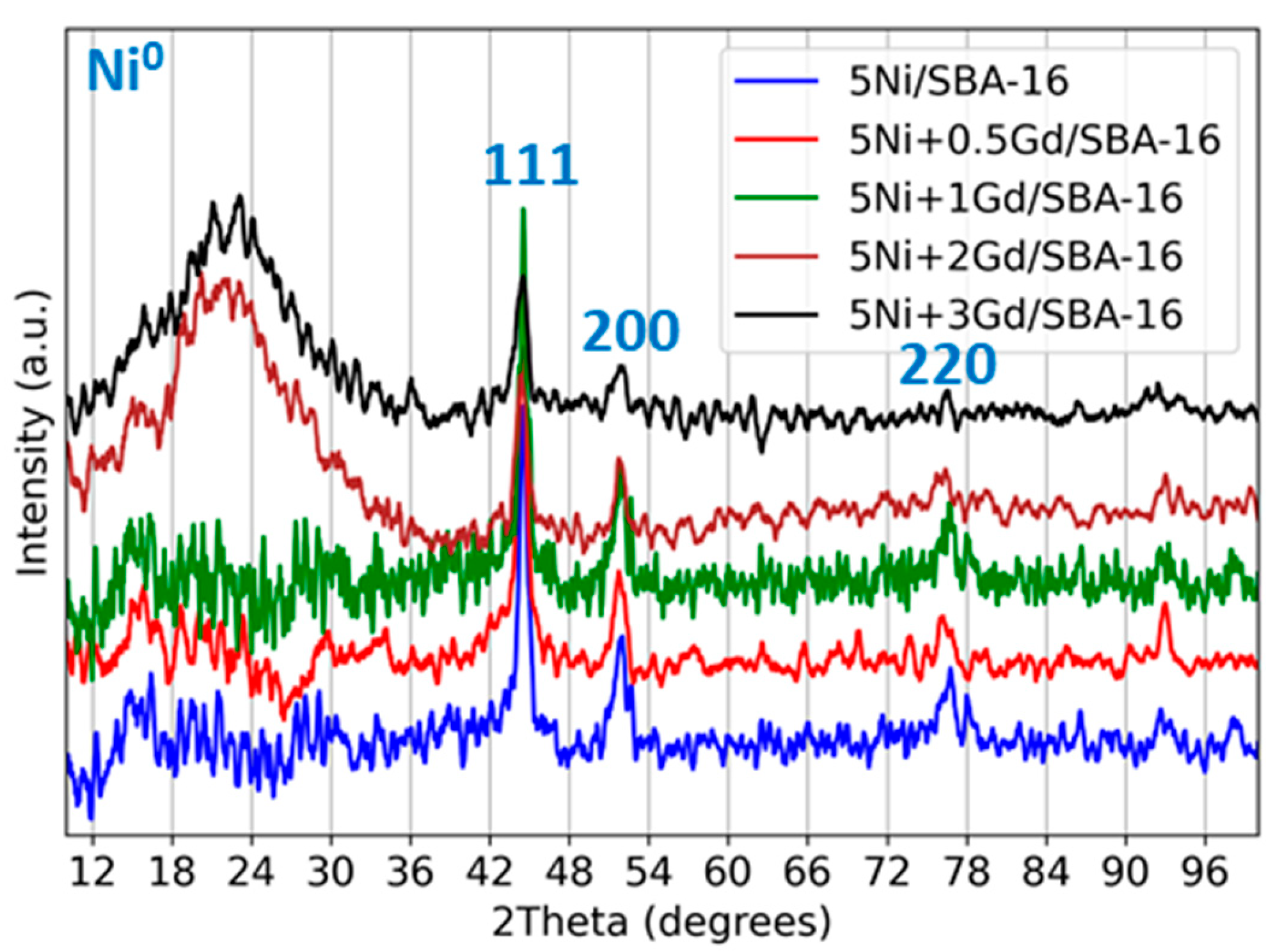
3.1.1. X-Ray Diffraction (XRD) Analysis
3.1.2. N2 Adsorption–Desorption Isotherms
3.1.3. FTIR Analysis
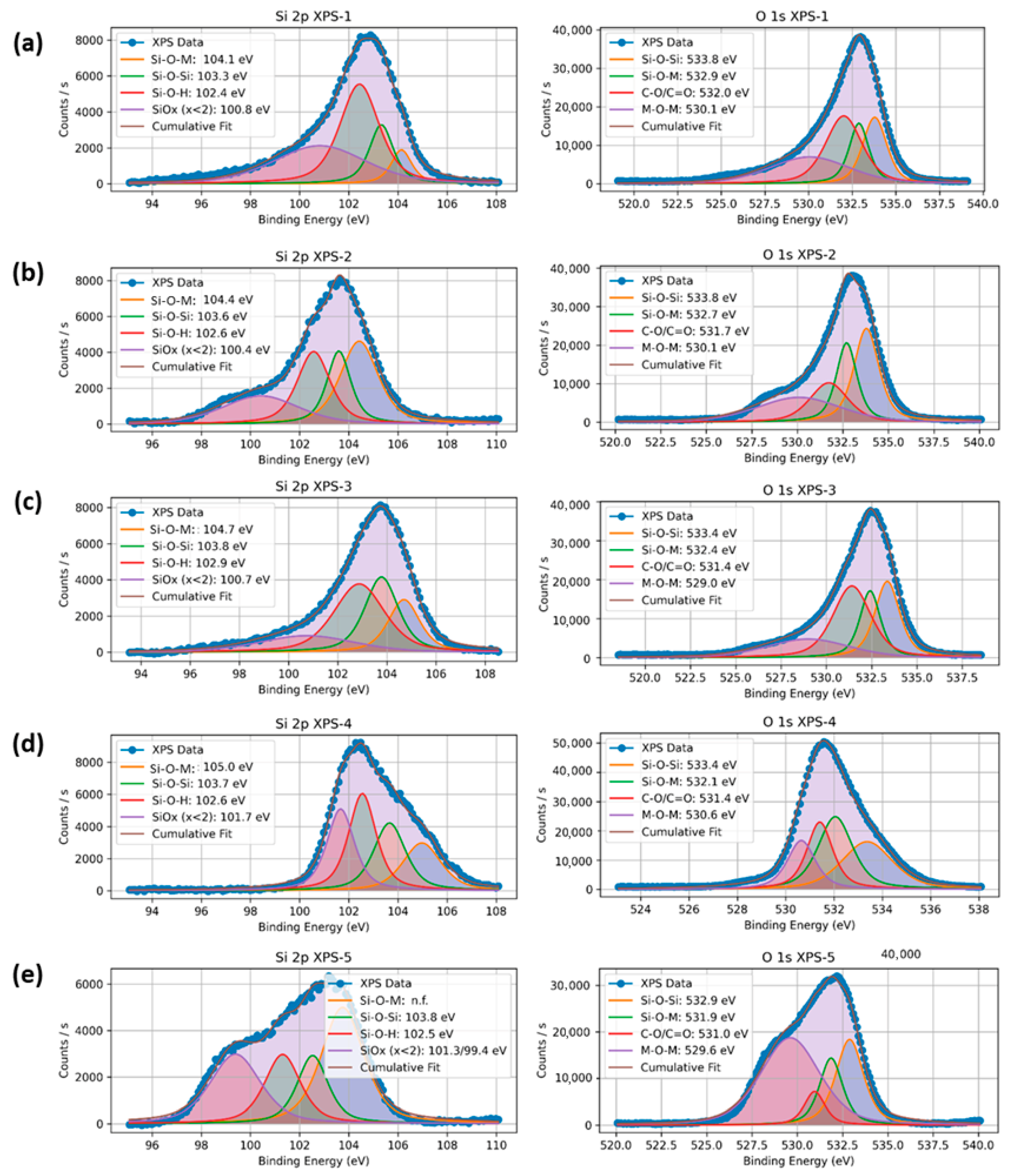
3.1.4. X-Ray Photoelectron Spectroscopy (XPS) Analysis
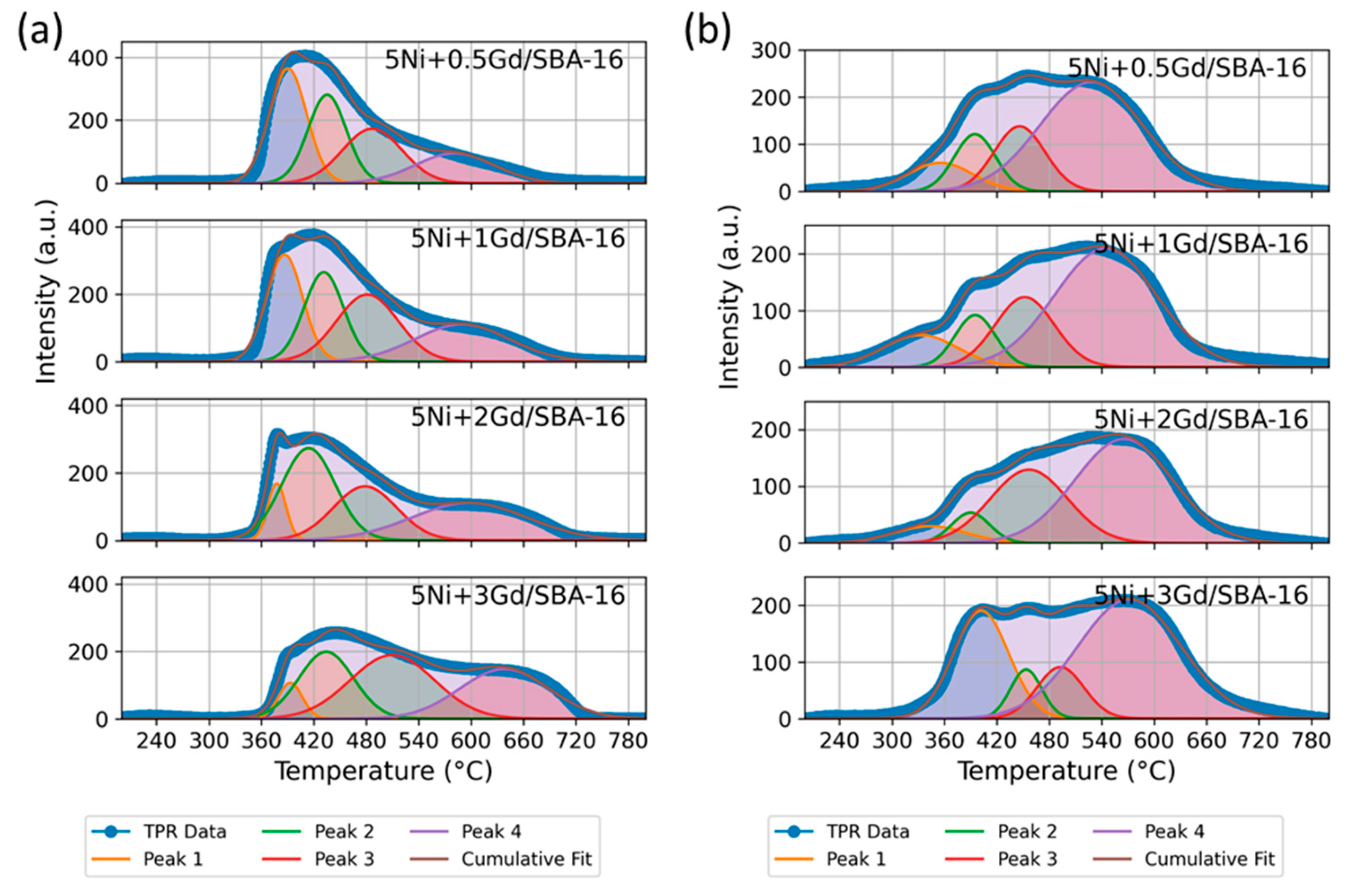
3.1.5. H2-TPR and Post-CO2-TPD Analysis
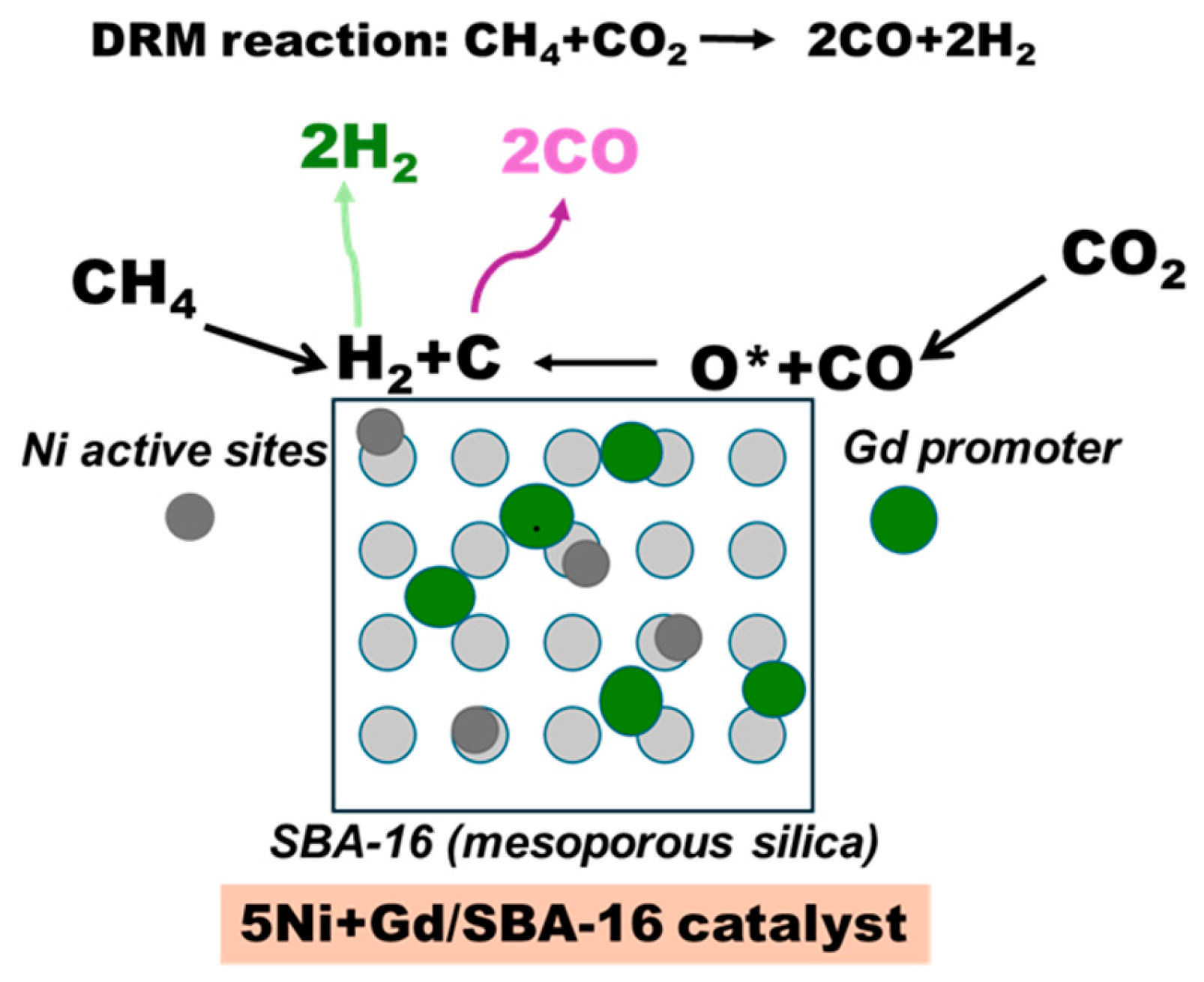
3.2. Catalytic Activity
3.3. Design of Experiments and Model Validation
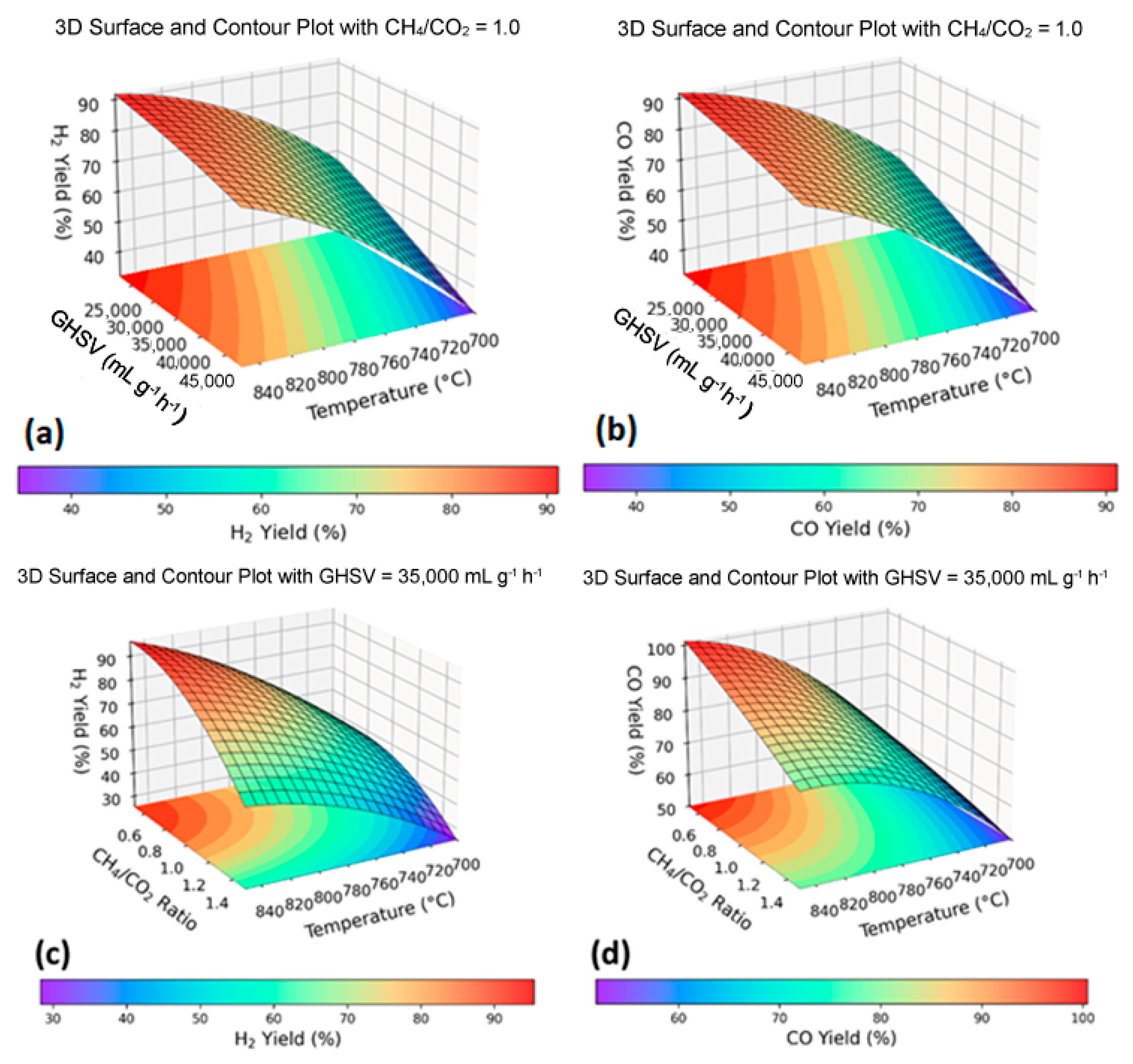
Response Surface Analysis and Optimization of Process Parameters
3.4. TGA–DSC and Raman Characterization of Coke Deposition on Spent Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. The Future of Hydrogen; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 28 July 2025).
- Remme, U. Global Hydrogen Review 2024; International Energy Agency: Paris, France, 2024; Available online: https://www.iea.org/reports/global-hydrogen-review-2024 (accessed on 31 July 2025).
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Juarez-García, M.; Sánchez-Ramírez, E. Green hydrogen production for sustainable development: A critical examination of barriers and strategic opportunities. RSC Sustain. 2025, 3, 134–157. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Alsaiari, M.A.; Arafat, Y.; Khan, W.U.; Alhamed, Y.A.; Al-Otaibi, R.L. High carbon-resistant nickel supported on yttria–zirconia catalysts for syngas production by dry reforming of methane: The promoting effect of cesium. Alex. Eng. J. 2023, 74, 371–386. [Google Scholar] [CrossRef]
- Chaudhary, K.J.; Rawat, S.; Sharma, A.; Rana, R.; Tiwari, D.; Patel, P.; Yadav, R.; Singh, P.; Yadav, A.K. Enhanced hydrogen production through methane dry reforming: Evaluating the effects of promoter-induced variations in reducibility, basicity, and crystallinity on Ni/ZSM-5 catalyst performance. Energy Convers. Manag. X 2024, 23, 100631. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Al-Zahrani, S.A.; Alharthi, F.A.; Shaikh, H.M.; Albarraq, A.A. Holmium promoted yttria–zirconia supported Ni catalyst for H2 production via dry reforming of methane. Int. J. Hydrogen Energy 2022, 47, 38242–38257. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, H.; Zhang, M.; Liang, C.; Duan, L. Recent advances in promoting dry reforming of methane using nickel-based catalysts. Catal. Sci. Technol. 2024, 14, 1712–1729. [Google Scholar] [CrossRef]
- Alwadai, N.; Khan, W.U.; Osman, A.I.; Alhoshan, M.S.; Al-Zahrani, S.A.; Algarni, T.S. Strontium-promoted Ni/SiO2–ZrO2 catalysts for methane dry reforming: Unraveling the role of basicity, metal–support interaction, and coke resistance. ACS Appl. Energy Mater. 2025, 8, 10423. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Nickel-based nanocatalysts promoted over MgO-modified SBA-16 for dry reforming of methane for syngas production: Impact of support and promoters. J. Energy Inst. 2021, 97, 100–108. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Li, J.; Chen, J.; Zhang, Q.; He, H.; Zhang, H.; Luo, J. Boosting CO2 reforming of methane via the metal–support interaction in mesostructured SBA-16-derived Ni nanoparticles. Appl. Mater. Today 2022, 26, 101354. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Huo, M.; Li, L.; Zhao, X.; Zhang, Y.; Li, J. Synthesis of Ni-based catalysts supported on nitrogen-incorporated SBA-16 and their catalytic performance in the reforming of methane with carbon dioxide. J. Fuel Chem. Technol. 2017, 45, 172–181. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Srivastava, V.K.; Ibrahim, A.A.; Abahussain, A.A.M.; Abu-Dahrieh, J.K.; Alotibi, M.F.; Rawesh, R. Hydrogen production from gadolinium-promoted yttrium–zirconium-supported Ni catalysts through dry methane reforming. ACS Omega 2023, 8, 22108. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Khan, W.U.; Al-Zahrani, S.A. CO2 reforming of CH4: Effect of Gd as promoter for Ni supported over MCM-41 as catalyst. Renew. Energy 2019, 140, 658–667. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, H.; Zhao, L.; Wang, Y.; Li, J. Effect of Gd promoter on the structure and catalytic performance of mesoporous Ni/Al2O3–CeO2 in dry reforming of methane. Ind. Eng. Chem. Res. 2018, 57, 17076–17085. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Ibrahim, A.A.; Khan, W.U.; Al-Zahrani, S.A.; Alsaiari, M.A.; Osman, A.I. Role of promoters over yttria–zirconia supported Ni catalyst for dry reforming of methane. Energy Sci. Eng. 2023, 11, 4366–4380. [Google Scholar] [CrossRef]
- Mageed, A.K.; Ayodele, B.V.; Mustapa, S.I. Response surface optimization of hydrogen-rich syngas production by greenhouse gases reforming. Chem. Eng. Technol. 2020, 43, 742–751. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ayodele, B.V.; Cheng, C.K.; Khan, M.R. Optimization of renewable hydrogen-rich syngas production from catalytic reforming of greenhouse gases (CH4 and CO2) over calcium iron oxide supported nickel catalyst. J. Energy Inst. 2019, 92, 177–194. [Google Scholar] [CrossRef]
- Sarrai, A.; Belkacem, M.; Trari, M.; Abdelmalek, F.; Fourcade, F.; Amrane, A. Using central composite experimental design to optimize the degradation of tylosin from aqueous solution by photo-Fenton reaction. Materials 2016, 9, 428. [Google Scholar] [CrossRef]
- Ahmadi Azqhandi, M.H.; Ghaedi, M.; Yousefi, F.; Jamshidi, M. Application of random forest, radial basis function neural networks and central composite design for modeling and/or optimization of the ultrasonic assisted adsorption of brilliant green on ZnS-NP-AC. J. Colloid Interface Sci. 2017, 505, 278–292. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Jadach, B.; Piotrowska, H.; Murias, M.; Lulek, J.; Nowak, I. Synthesis and characterization of SBA-16 type mesoporous materials containing amine groups. Microporous Mesoporous Mater. 2016, 220, 231–238. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Wu, X.; Si, Z.; Weng, D. Dry reforming of methane over Ni catalysts supported on micro- and mesoporous silica. J. CO2 Util. 2023, 68, 102387. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Ide, M.; El Fallah, J.; Galarneau, A.; Di Renzo, F.; Fajula, F. Quantification of silanol sites for the most common mesoporous ordered silicas and organosilicas: Total versus accessible silanols. Phys. Chem. Chem. Phys. 2013, 15, 642–650. [Google Scholar] [CrossRef]
- Gupta, R.; Layek, S.; Pathak, D.D. Synthesis and characterization of guanine-functionalized mesoporous silica (SBA-16-G): A metal-free and recyclable heterogeneous solid base catalyst for synthesis of pyran-annulated heterocyclic compounds. Res. Chem. Intermed. 2019, 45, 1619–1637. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sønderby, C.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079–2088. [Google Scholar] [CrossRef]
- Weidler, N.; Pfaff, C.; Pankrath, T.; Gärtner, F.; Pichler, T.; Fellinger, T.P. X-ray photoelectron spectroscopic investigation of plasma-enhanced chemical vapor deposited NiOx, NiOx(OH)γ, and CoNiOx(OH)γ: Influence of the chemical composition on the catalytic activity for the oxygen evolution reaction. J. Phys. Chem. C 2017, 121, 6455–6463. [Google Scholar] [CrossRef]
- Senna, M.; Li, Y.; Wei, Y.; Yuan, Y.; Xu, S.; Zhang, X.; Wang, Y.; Xu, B. Solid-state reduction of silica nanoparticles via oxygen abstraction from SiO4 units by polyolefins under mechanical stressing. RSC Adv. 2018, 8, 36338–36344. [Google Scholar] [CrossRef]
- Prieto, M.J.; Wehling, T.; Kuhlenbeck, H.; Freund, H.J.; Wlodarczyk, R.; Sierka, M.; Sauer, J. Plasma functionalization of silica bilayer polymorphs. ACS Appl. Mater. Interfaces 2022, 14, 48609–48618. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Chen, W.; Liu, F.; Sun, Z. Energy efficient catalytic CO2 desorption: Mechanism, technological progress and perspective. Carbon Capture Sci. Technol. 2023, 6, 100099. [Google Scholar] [CrossRef]
- Ares, G. Mathematical and Statistical Methods in Food Science and Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Khan, W.U.; Al-Zahrani, S.A.; Osman, A.I.; Alqahtani, A.S.; Al-Kahtani, A.A. Tailoring strontium-promoted alumina–zirconia supported Ni-catalysts for enhanced CO2 utilization via dry reforming of methane: Sr loading effects and process optimization. J. CO2 Util. 2023, 75, 102578. [Google Scholar] [CrossRef]
- Yang, L.; Liu, R.; Zhao, G.; Wang, X.; Zhang, Y. Response surface methodology optimization of ultrasonic-assisted extraction of Acer truncatum leaves for maximal phenolic yield and antioxidant activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Sasaki, T.; Olea, M. Original method to predict and monitor carbon deposition on Ni-based catalysts during dry reforming of methane. Front. Chem. Eng. 2020, 2, 9. [Google Scholar] [CrossRef]
- Puech, P.; Flahaut, E.; Lavin, J.G.; Rols, S.; Monthioux, M. Analyzing the Raman spectra of graphenic carbon materials from kerogens to nanotubes: What type of information can be extracted from defect bands? C 2019, 5, 69. [Google Scholar] [CrossRef]
- Owen, R.E.; Hall, J.; Williams, H.; Jones, J.; Chen, R. Visualising coke-induced degradation of catalysts used for CO2-reforming of methane with X-ray nano-computed tomography. Carbon Capture Sci. Technol. 2022, 5, 100068. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Khatri, J.; Kumar, R.; Kumar Srivastava, V.; Osman, A.I.; AlGarni, T.S.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H.; Rooney, D.W. Role of Ca, Cr, Ga and Gd Promotor over Lanthana-Zirconia–Supported Ni Catalyst towards H2-Rich Syngas Production through Dry Reforming of Methane. Energy Sci. Eng. 2022, 10, 866–880. [Google Scholar] [CrossRef]
- Khatri, J.; Al-Fatesh, A.S.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Kasim, S.O.; Osman, A.I.; Patel, R.; Kumar, R. Ce Promoted Lanthana-Zirconia Supported Ni Catalyst System: A Ternary Redox System for Hydrogen Production. Mol. Catal. 2021, 504, 111498. [Google Scholar] [CrossRef]


| Process Parameters | Levels | Levels |
|---|---|---|
| −1 (Low) | +1(High) | |
| GHSV: Gas hourly space velocity (mL g−1 h−1) | 22,000 | 48,000 |
| T: Temperature (°C) | 700 | 850 |
| R: CH4/CO2 | 0.5 | 1.5 |
| Run | T (Coded) | SV (Coded) | R (Coded) | Temperature (°C) | GHSV (mL g−1 h−1) | CH4/CO2 |
|---|---|---|---|---|---|---|
| 1 | +1.000 | −1.000 | +1.000 | 850 | 22,000 | 1.25 |
| 2 | +1.000 | −1.000 | −1.000 | 850 | 22,000 | 0.75 |
| 3 | −1.000 | −1.000 | +1.000 | 700 | 22,000 | 1.25 |
| 4 | −1.000 | −1.000 | −1.000 | 700 | 22,000 | 0.75 |
| 5 | 0.000 | −1.000 | 0.000 | 775 | 22,000 | 1.00 |
| 6 | +1.000 | 0.000 | 0.000 | 850 | 35,000 | 1.00 |
| 7 | 0.000 | 0.000 | +1.000 | 775 | 35,000 | 1.25 |
| 8 | −1.000 | 0.000 | 0.000 | 700 | 35,000 | 1.00 |
| 9 | 0.000 | 0.000 | 0.000 | 775 | 35,000 | 1.00 |
| 10 | 0.000 | 0.000 | −1.000 | 775 | 35,000 | 0.75 |
| 11 | 0.000 | +1.000 | 0.000 | 775 | 48,000 | 1.00 |
| 12 | +1.000 | +1.000 | +1.000 | 850 | 48,000 | 1.25 |
| 13 | +1.000 | +1.000 | −1.000 | 850 | 48,000 | 0.75 |
| 14 | −1.000 | +1.000 | +1.000 | 700 | 48,000 | 1.25 |
| 15 | −1.000 | +1.000 | −1.000 | 700 | 48,000 | 0.75 |
| 16 | +0.333 | +1.000 | −1.000 | 800 | 48,000 | 0.75 |
| 17 | +0.333 | 0.000 | 0.000 | 800 | 35,000 | 1.00 |
| (a) | ||||||
| Peak Assignment | Parameter | 5Ni/SBA-16 | 5Ni+0.5Gd/SBA-16 | 5Ni+1Gd/SBA-16 | 5Ni+2Gd/SBA-16 | 5Ni+3Gd/SBA-16 |
| Si–O–M | B.E. (eV) | 104.1 | 104.4 | 104.7 | 105.0 | Not observed |
| % area | 8.1 | 33.4 | 18.7 | 19.6 | Not observed | |
| Si–O–Si | B.E. (eV) | 103.3 | 103.6 | 103.8 | 103.7 | 103.8 |
| % area | 16.1 | 19.7 | 28.5 | 24.1 | 37.9 | |
| Si–O–H | B.E. (eV) | 102.4 | 102.6 | 102.9 | 102.6 | 102.5 |
| % area | 42.1 | 26.1 | 37.0 | 30.5 | 16.8 | |
| SiOx (x < 2) | B.E. (eV) | 100.8 | 100.4 | 100.7 | 101.7 | 101.3 |
| % area | 33.7 | 20.9 | 15.8 | 25.8 | 19.4 | |
| (b) | ||||||
| Peak Assignment | Parameter | 5Ni/ SBA-16 | 5Ni+0.5Gd/SBA-16 | 5Ni+1Gd/SBA-16 | 5Ni+2Gd/SBA-16 | 5Ni+3Gd/SBA-16 |
| Si–O–Si | B.E. (eV) | 533.8 | 533.8 | 533.4 | 533.4 | 532.9 |
| % area | 21.8 | 32.3 | 26.1 | 28.5 | 25.3 | |
| Si–O–M | B.E. (eV) | 532.9 | 532.7 | 532.4 | 532.1 | 531.9 |
| % area | 17.6 | 23.2 | 19.4 | 31.3 | 16.9 | |
| C–O / C=O | B.E. (eV) | 532.0 | 531.7 | 531.4 | 531.4 | 531 |
| % area | 33.8 | 19.7 | 35.8 | 22.6 | 7.6 | |
| M–O–M | B.E. (eV) | 530.1 | 530.1 | 529.0 | 530.6 | 529.6 |
| % area | 26.8 | 24.9 | 18.6 | 17.6 | 50.1 | |
| Catalyst | Peak | Temp. (°C) Fresh | % Area Fresh | Temp. (°C) After CO2-TPD | % Area After CO2-TPD |
|---|---|---|---|---|---|
| 5Ni+0.5Gd/SBA-16 | 1 | 390.4 | 32.3 | 355.0 | 10 |
| 2 | 435.2 | 25.8 | 394.9 | 12.7 | |
| 3 | 486.9 | 24.4 | 446.0 | 17.8 | |
| 4 | 580.9 | 17.6 | 528.1 | 59.5 | |
| 5Ni+1Gd/SBA-16 | 1 | 386.5 | 25.7 | 332.3 | 11.5 |
| 2 | 431.4 | 23.7 | 395.2 | 10.2 | |
| 3 | 481.3 | 27.5 | 451.9 | 20.1 | |
| 4 | 589.6 | 23.1 | 544.4 | 58.2 | |
| 5Ni+2Gd/SBA-16 | 1 | 377.7 | 7.7 | 343.6 | 6.8 |
| 2 | 414.0 | 36.4 | 390.0 | 6.4 | |
| 3 | 478.6 | 25.1 | 456.8 | 31.1 | |
| 4 | 598.0 | 30.8 | 565.3 | 55.7 | |
| 5Ni+3Gd/SBA-16 | 1 | 393.1 | 6.1 | 401.9 | 26.9 |
| 2 | 434.1 | 25.9 | 453.4 | 7.1 | |
| 3 | 509.9 | 37.7 | 492.8 | 10.9 | |
| 4 | 638.0 | 30.3 | 571.6 | 55.1 |
| Variables | Goal Function | Theoretical Optimum | Experimental Validation |
|---|---|---|---|
| Temperature (°C) | 700–850 | 848.9 | 845 |
| GHSV (mL g−1 h−1) | 22,000–48,000 | 31,283 | 31,283 |
| CH4/CO2 ratio | 0.5–15 | 0.61 | 0.61 |
| H2 yield (%) | Maximum | 96.64 | 96.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, S.A.; Alotibi, M.F.; Osman, A.I.; Bhran, A.A.; Alreshidi, M.A.; Al Otaibi, A.; Al-Enazy, H.D.A.; Alsaif, N.O.S.; Al-Fatesh, A.S. Dry Reforming of Methane Using Gd-promoted Ni/SBA-16 Catalyst: Structure, Activity and Process Optimization with Response Surface Methodology. Nanomaterials 2025, 15, 1527. https://doi.org/10.3390/nano15191527
Al-Zahrani SA, Alotibi MF, Osman AI, Bhran AA, Alreshidi MA, Al Otaibi A, Al-Enazy HDA, Alsaif NOS, Al-Fatesh AS. Dry Reforming of Methane Using Gd-promoted Ni/SBA-16 Catalyst: Structure, Activity and Process Optimization with Response Surface Methodology. Nanomaterials. 2025; 15(19):1527. https://doi.org/10.3390/nano15191527
Chicago/Turabian StyleAl-Zahrani, Salma A., Mohammed F. Alotibi, Ahmed I. Osman, Ahmed A. Bhran, Maha Awjan Alreshidi, Ahmed Al Otaibi, Hessah Difallah A. Al-Enazy, Nuha Othman S. Alsaif, and Ahmed S. Al-Fatesh. 2025. "Dry Reforming of Methane Using Gd-promoted Ni/SBA-16 Catalyst: Structure, Activity and Process Optimization with Response Surface Methodology" Nanomaterials 15, no. 19: 1527. https://doi.org/10.3390/nano15191527
APA StyleAl-Zahrani, S. A., Alotibi, M. F., Osman, A. I., Bhran, A. A., Alreshidi, M. A., Al Otaibi, A., Al-Enazy, H. D. A., Alsaif, N. O. S., & Al-Fatesh, A. S. (2025). Dry Reforming of Methane Using Gd-promoted Ni/SBA-16 Catalyst: Structure, Activity and Process Optimization with Response Surface Methodology. Nanomaterials, 15(19), 1527. https://doi.org/10.3390/nano15191527









