Green Synthesis and Characterization of Rosa roxburghii Tratt.-Mediated Gold Nanoparticles for Visual Colorimetric Assay of Tiopronin
Abstract
1. Introduction
2. Experimental Sections
2.1. Instruments and Materials
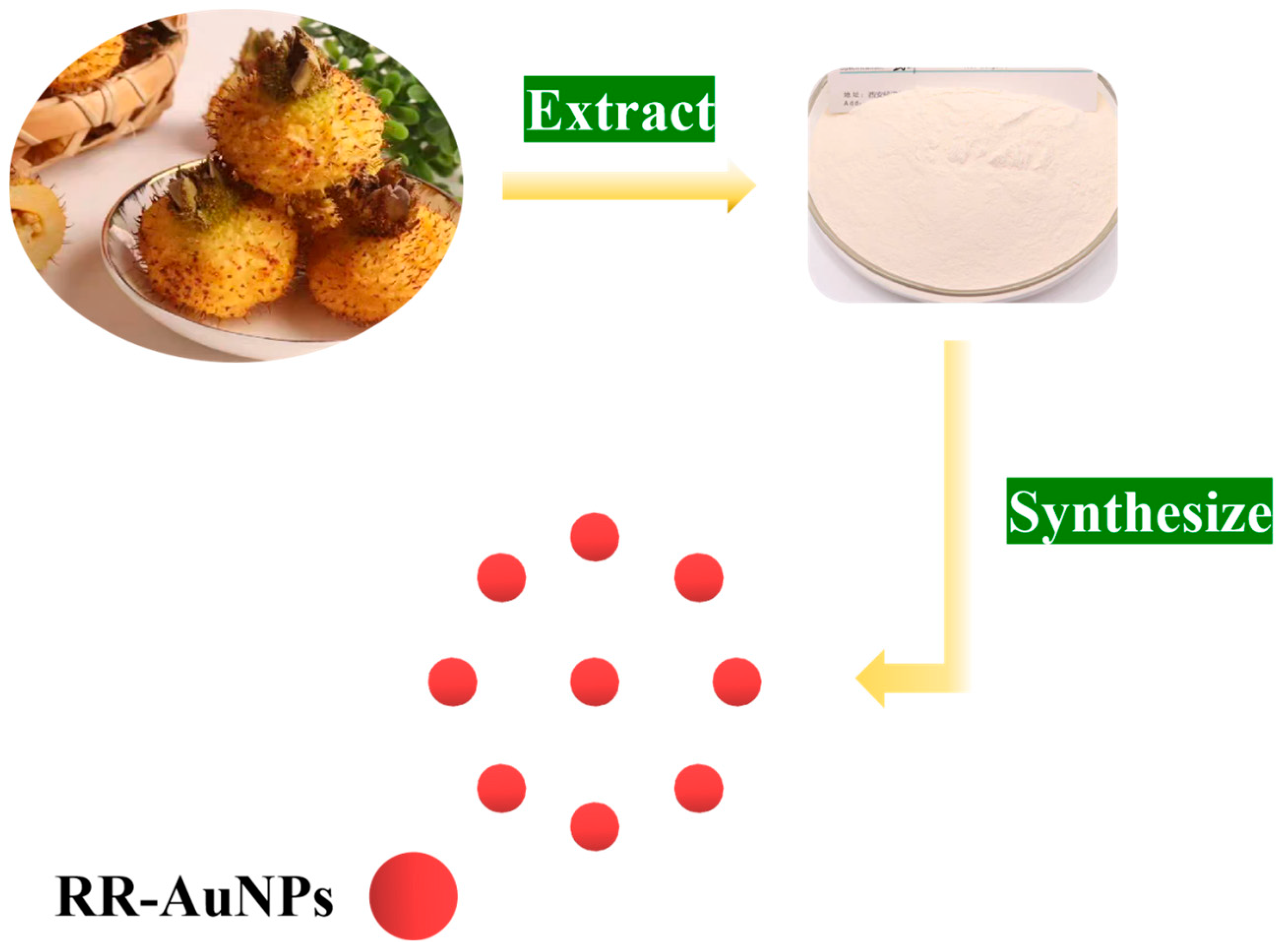
2.2. Green Synthesis of RR-AuNPs
2.3. Optimization of Synthesis Conditions
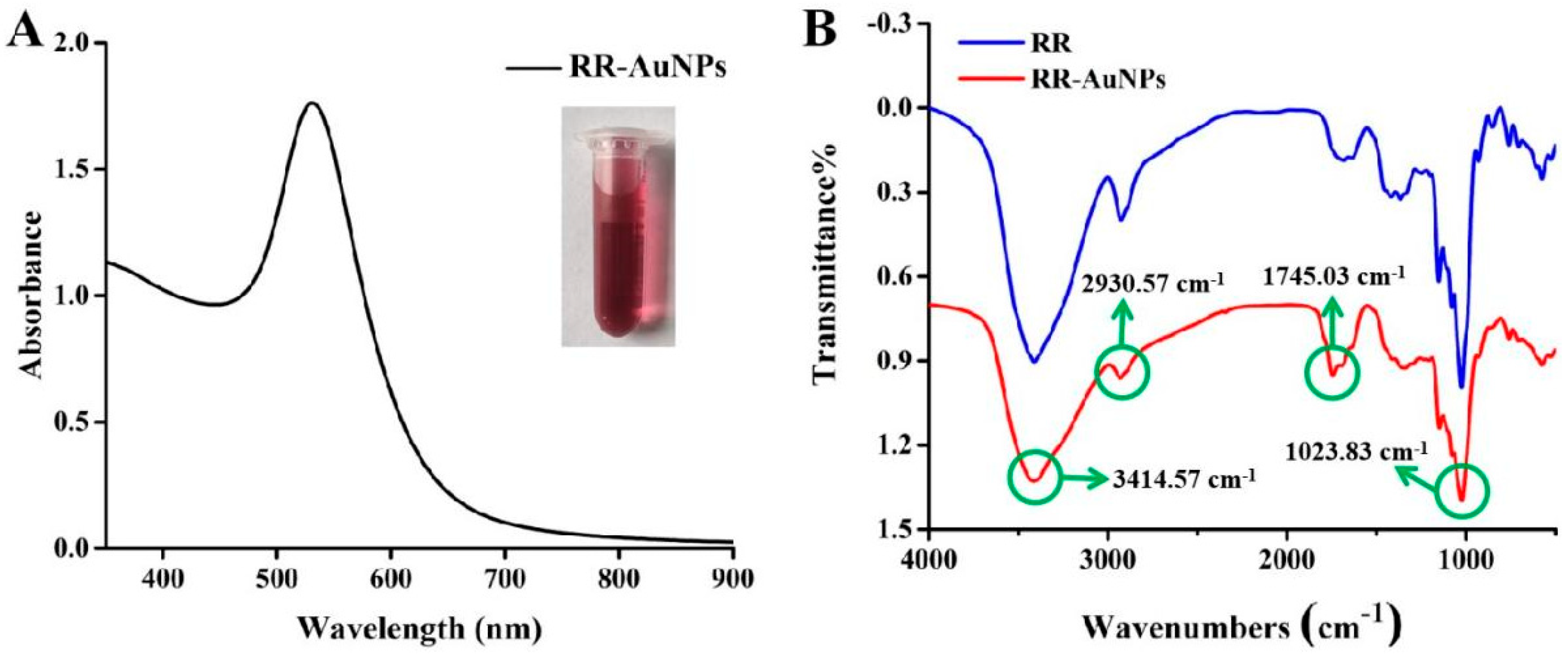
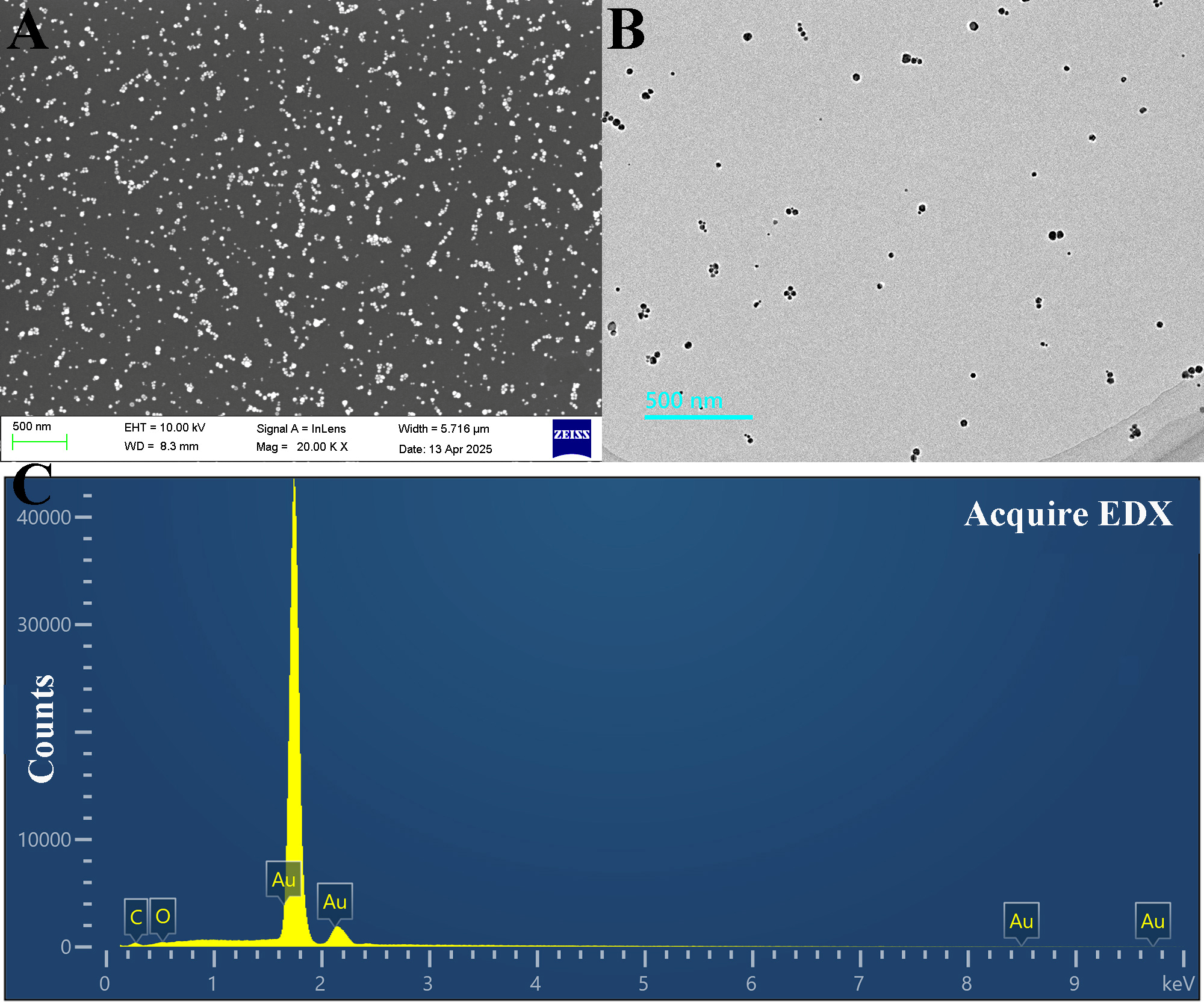
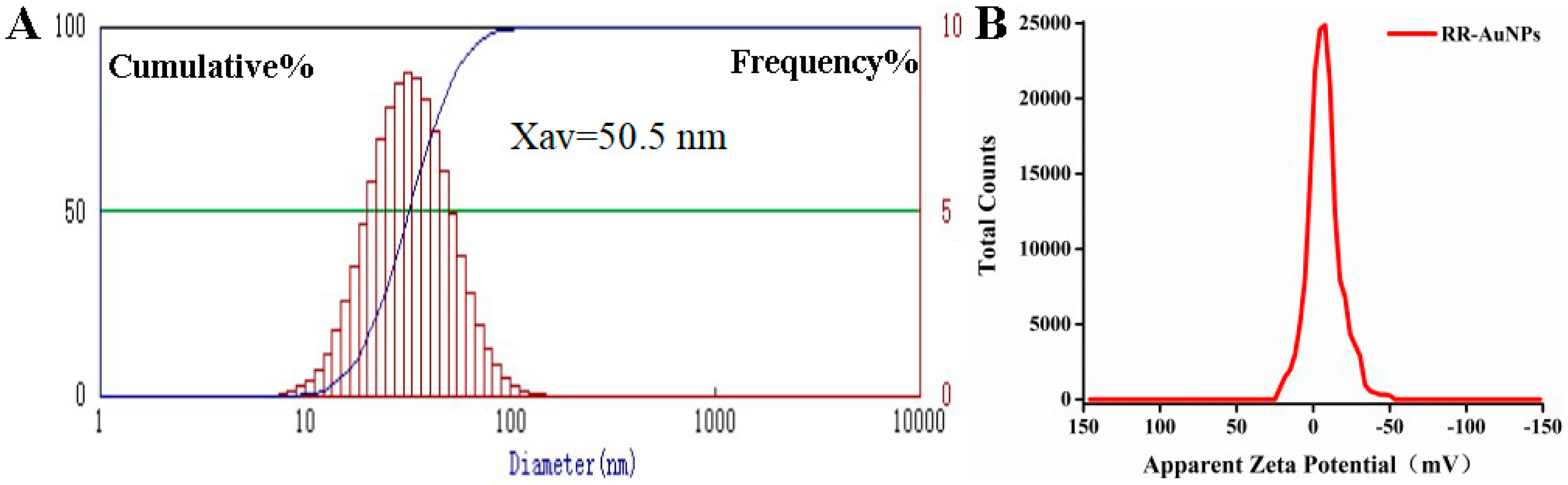
2.4. Characterization of RR-AuNPs
2.5. Investigation of RR-AuNPs’ Stability
2.6. Visual Colorimetric Detection of Tiopronin
2.7. Selectivity Investigation
2.8. Real Sample Detection
3. Results and Discussion
3.1. Green Synthesis of RR-AuNPs
3.2. Synthesis Conditions of RR-AuNPs
3.3. Stability of RR-AuNPs
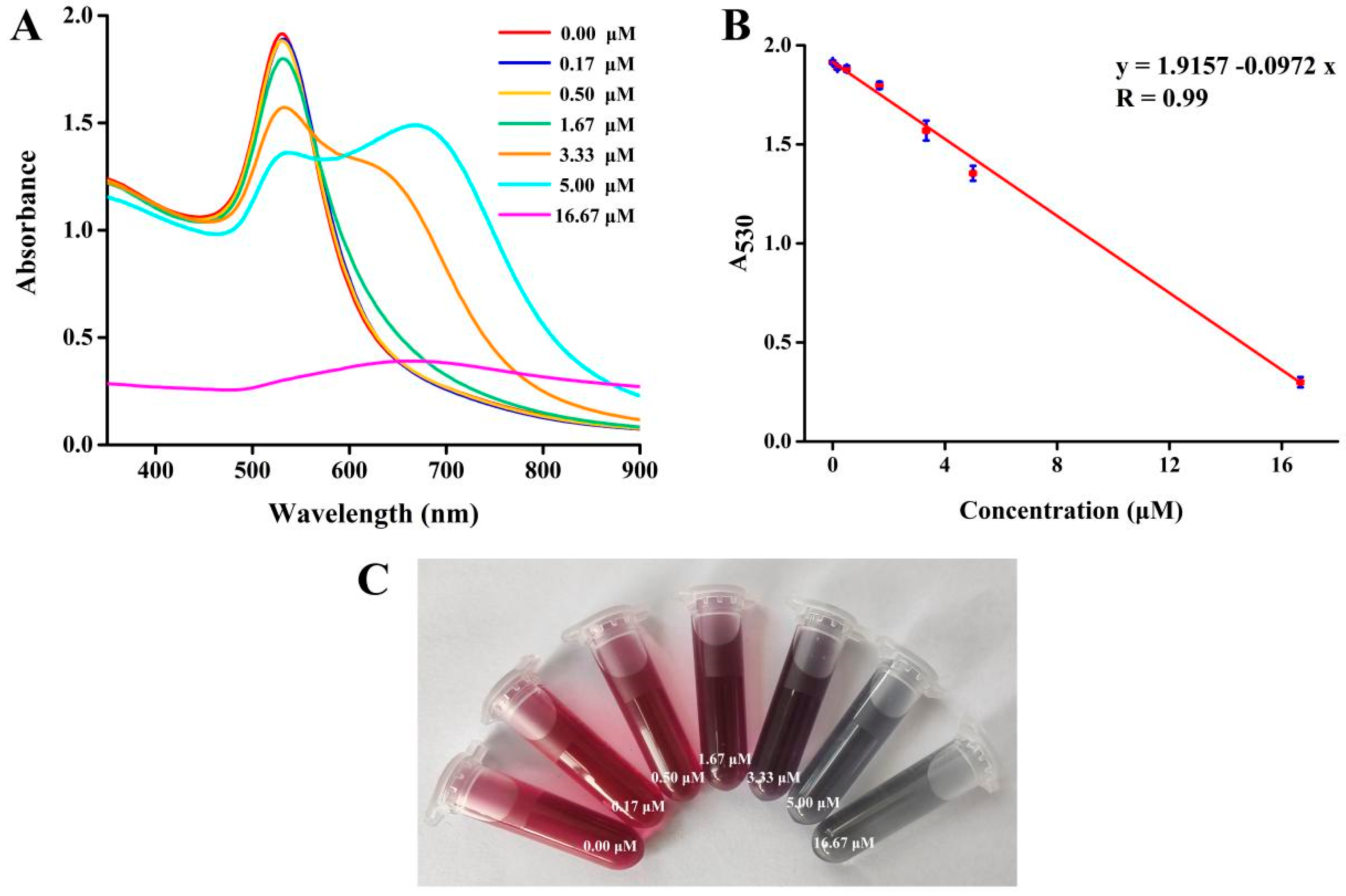
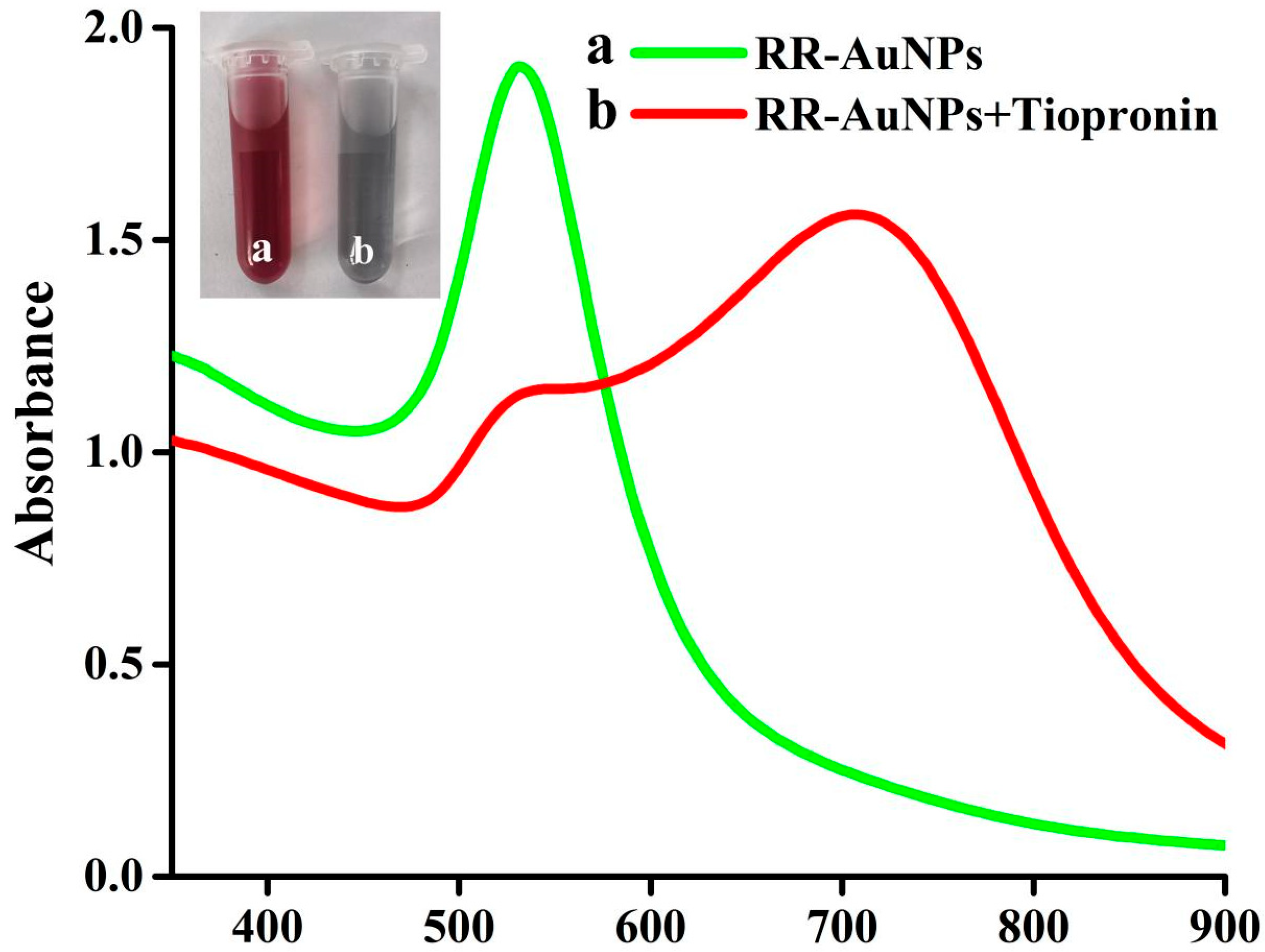
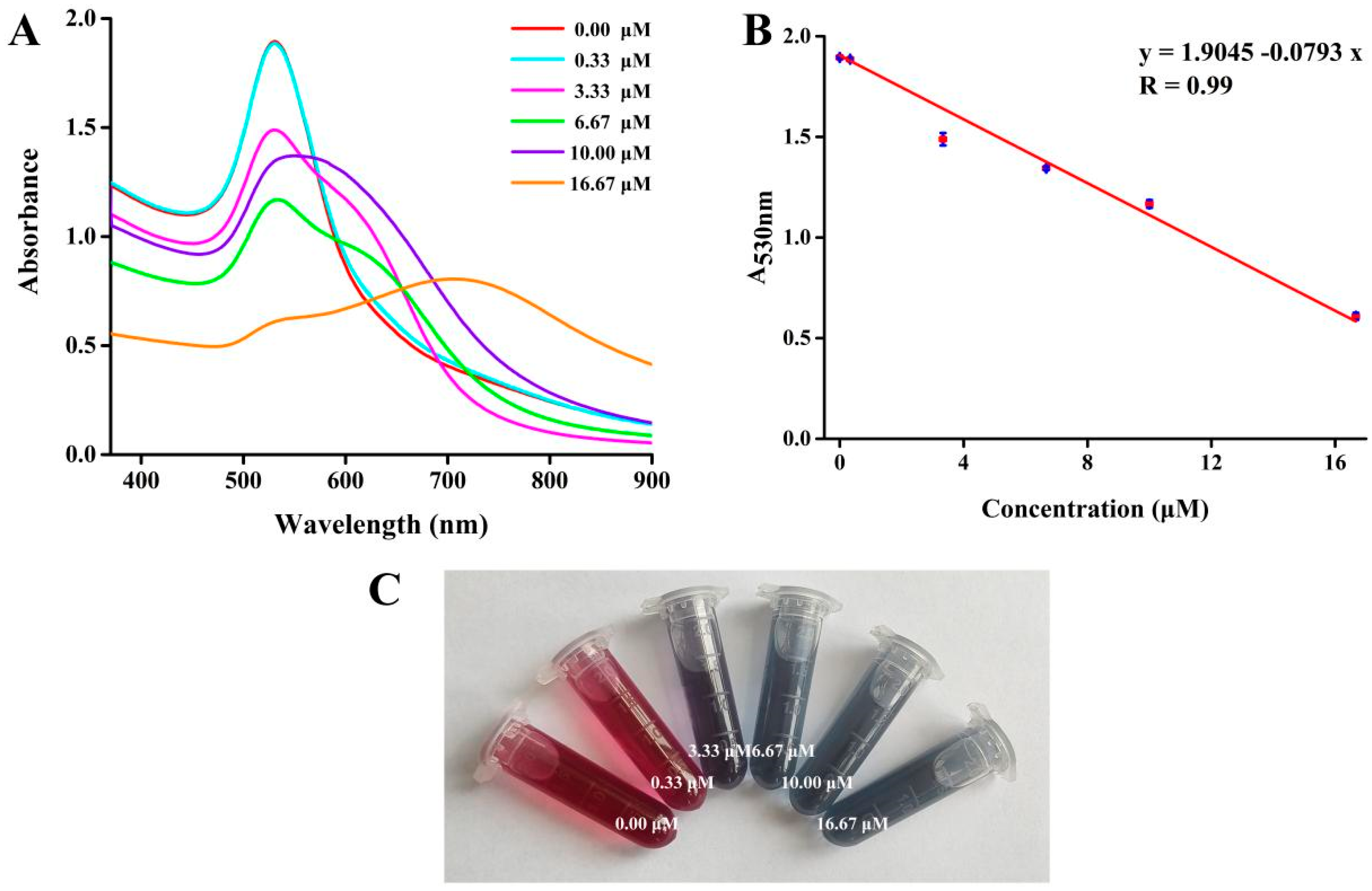
3.4. Visual Colorimetric Detection of Tiopronin
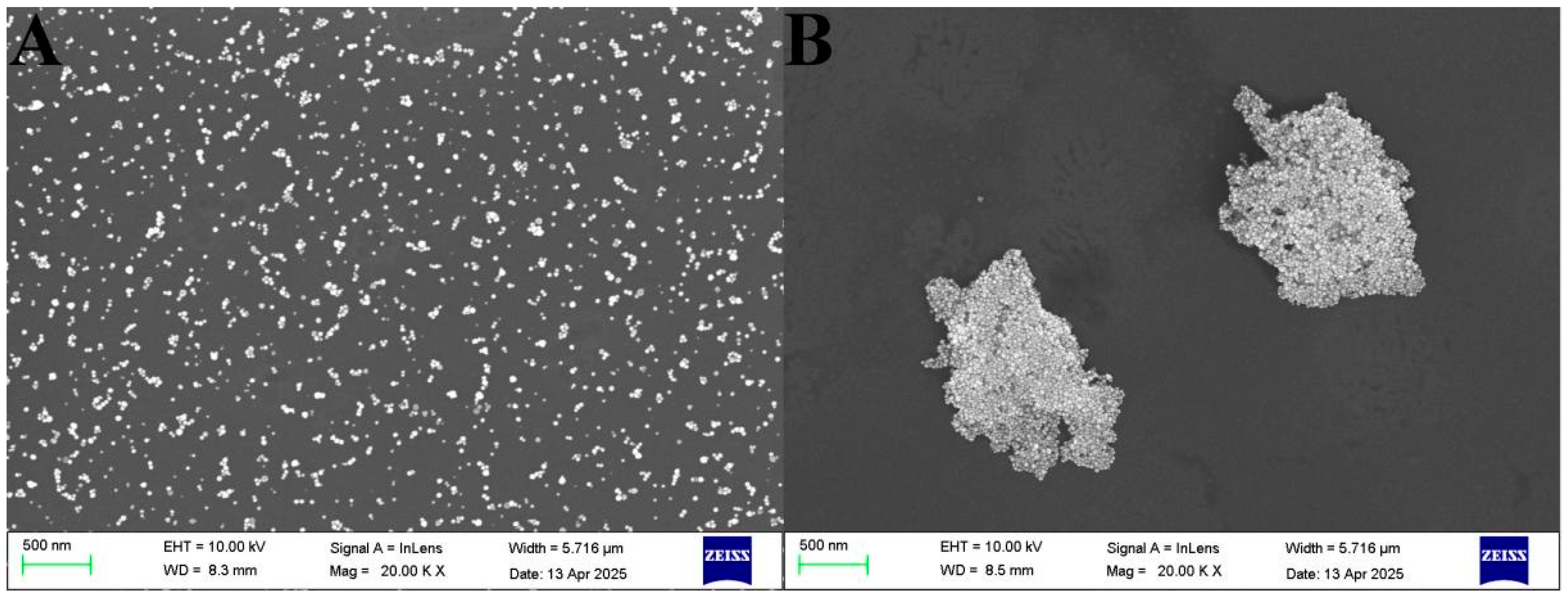
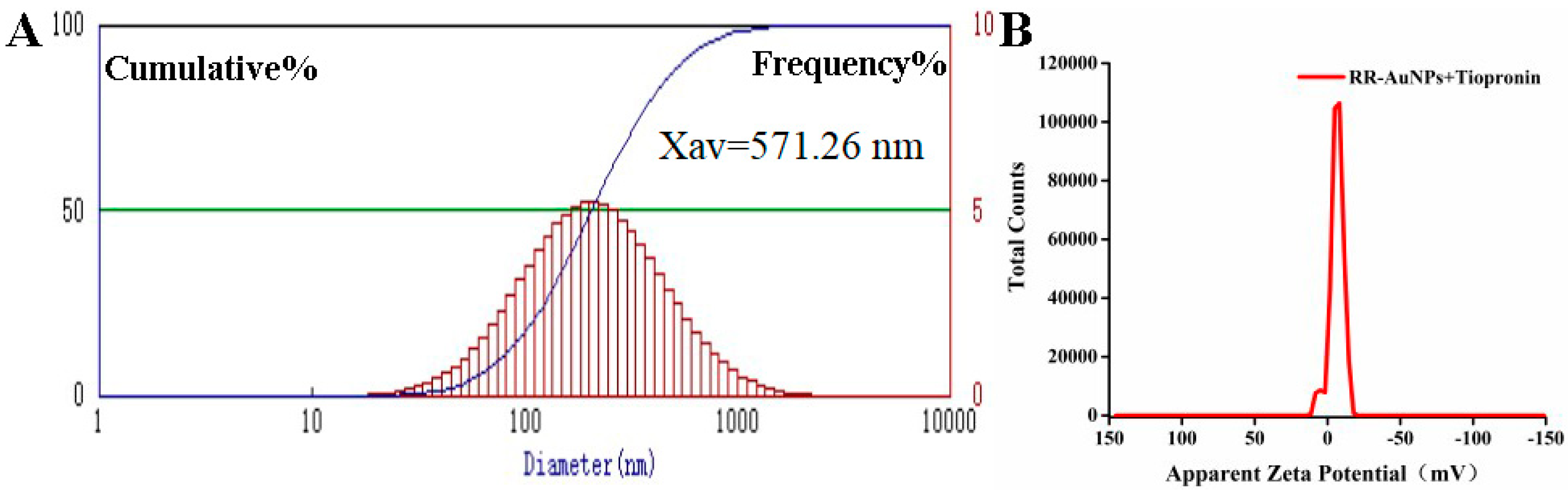
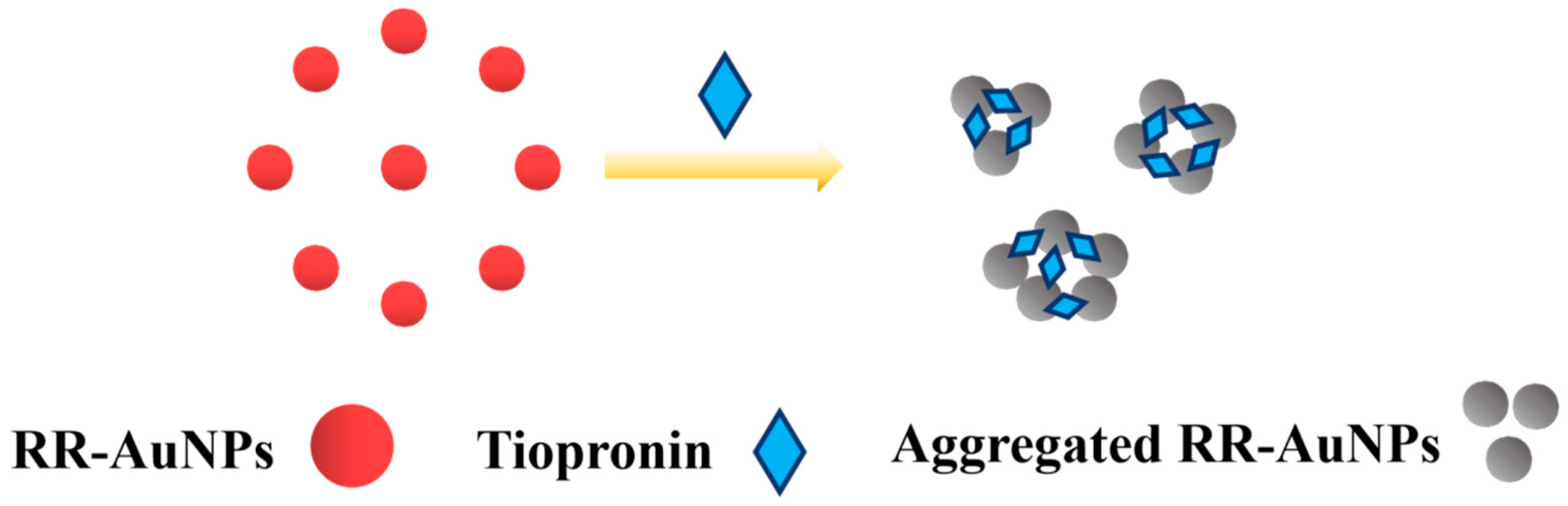
3.5. Colorimetric Detection Mechanism of RR-AuNPs
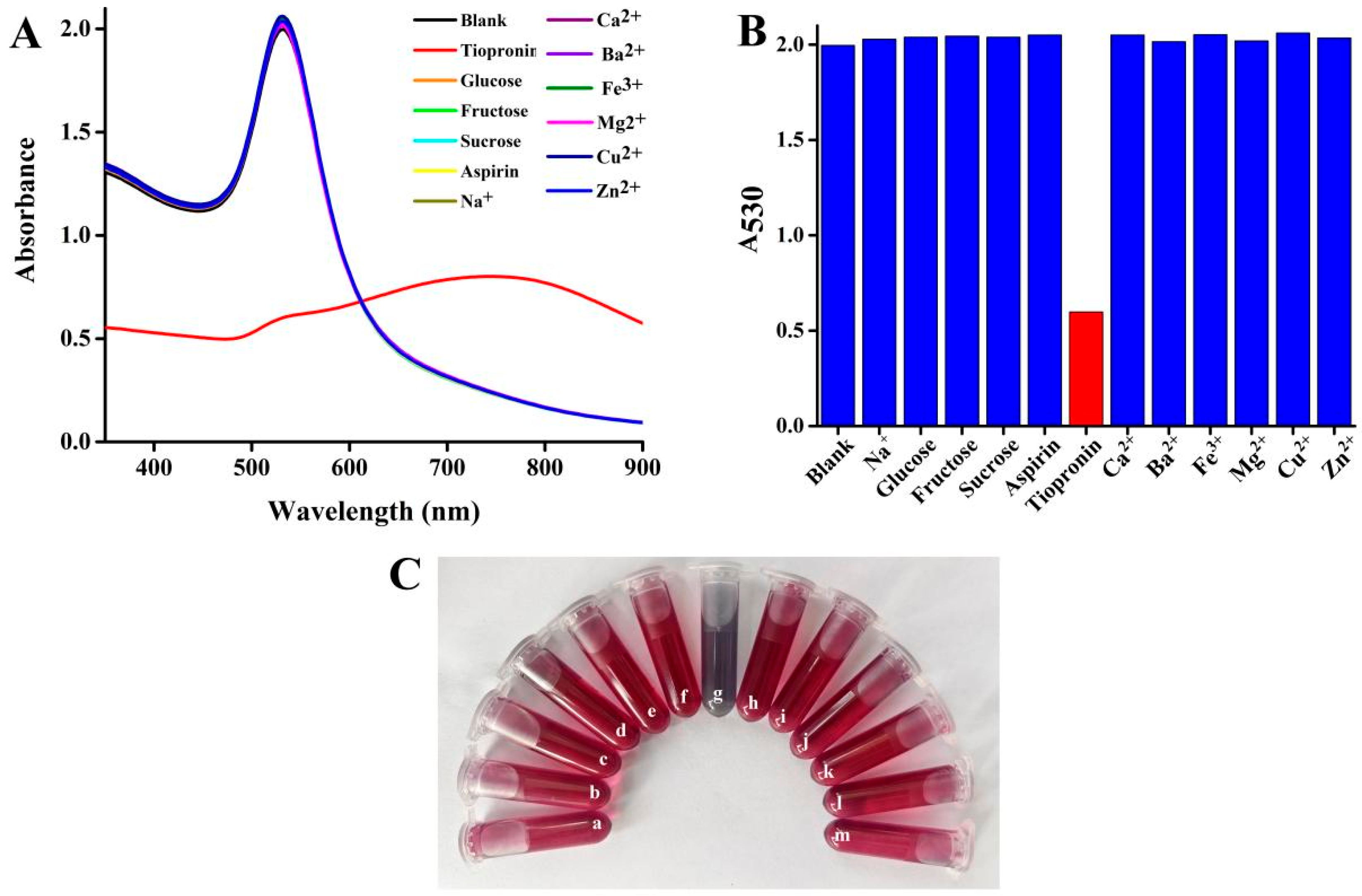
3.6. Selectivity Investigation
3.7. Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garnier, A.-S.; Laubacher, H.; Briet, M. Drug-induced glomerular diseases. Therapies 2024, 79, 271–281. [Google Scholar] [CrossRef]
- Zheng, Z.; Xue, Y.; Jia, J.; Wei, L.; Shang, W.; Lin, S. Tiopronin-induced membranous nephropathy: A case report. Ren. Fail. 2014, 36, 1455–1460. [Google Scholar] [CrossRef]
- Er, X.; Gu, Q.; Sun, J.; Ding, Z.; Liang, S.; Jin, H. A novel nanoprobe for multimodal instantaneous detection of tiopronin and tetracycline hydrochloride. Anal. Chim. Acta 2025, 1344, 343706. [Google Scholar] [CrossRef]
- Lu, J.; Lau, C.; Yagisawa, S.; Ohta, K.; Kai, M. A simple and sensitive chemiluminescence method for the determination of tiopronin for a pharmaceutical formulation. J. Pharm. Biomed. Anal. 2003, 33, 1033–1038. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Yu, Y.; Zheng, X.; Duan, G. Reverse-phase high performance liquid chromatography for the determination of tiopronin in human plasma after derivatization with p-bromophenacyl bromide. Anal. Chim. Acta 2006, 565, 178–182. [Google Scholar] [CrossRef]
- Chandra, S.; Chowdhuri, A.R.; Mahto, T.K.; Laha, D.; Sahu, S.K. Sulphur and nitrogen doped carbon dots: A facile synthetic strategy for multicolour bioimaging, tiopronin sensing, and Hg2+ ion detection. Nano-Struct. Nano-Objects 2017, 12, 10–18. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Bald, E. Simultaneous determination of tiopronin and d-penicillamine in human urine by liquid chromatography with ultraviolet detection. Anal. Chim. Acta 2007, 590, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Long, H.; Du, M.; Yu, J.; Sun, X.; Wang, Y.; Zheng, J.; Chen, H.; Gao, Y. Fabrication of a nanoplatform based on chitosan and hyaluronic acid containing alkyne-functionalized gold nanoparticles for tumor targeted synergistic phototherapy. Int. J. Biol. Macromol. 2025, 309, 142974. [Google Scholar] [CrossRef]
- Duarah, R.; Gogoi, A.; Baruah, D.J.; Dihingia, K.D.; Dutta, R.; Boruah, P.K.; Saha, S.; Nagamani, S.; Das, M.R. Highly sensitive and rapid colorimetric detection of As3+ in water using label-free silica-coated gold nanoparticles. Microchem. J. 2025, 213, 113596. [Google Scholar] [CrossRef]
- Phosri, C.; Sapyen, W.; Praphairaksit, N.; Imyim, A. Colorimetric detection of arsenic(III) using iron(III) assisted tartrate stabilized gold nanoparticles. Microchem. J. 2025, 212, 113603. [Google Scholar] [CrossRef]
- Phan, T.L.; Le, T.T.; Doan, V.D.; Truong, H.A.V.; Le, V.T. Microwave-assisted synthesis of gold nanoparticle-integrated carbon spheres and graphene oxide: A multifunctional composite for ascorbic acid detection and antibacterial application. Microchem. J. 2025, 214, 114006. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Gubitosa, J.; Rizzi, V.; Cosma, P.; Gaeta, C.; Iannece, P.; Della Sala, P.; Guerrieri, A.; Tesoro, C.; Ciriello, R.; et al. Green synthesis of gold nanoparticles from pomegranate juice ascertained by a combined approach based on MALDI FT-ICR MS and LC-ESI-MS/MS. Food Chem. 2025, 476, 143427. [Google Scholar] [CrossRef]
- Selvarani, J.F.L.; Pushpavalli, K.S.; Kala, S.M.J.; Prakash, K.S. Eco-friendly synthesis of gold nanoparticles using Ruta graveolens Leaf extract and their efficient catalytic reduction of 4-Nitroaniline, Methylene Blue and Antibiotic Nitrofurantoin. J. Organomet. Chem. 2025, 1036, 123706. [Google Scholar] [CrossRef]
- Hameed, R. Synthesis and physical properties of nanocomposite gold nanoparticles attached to graphene quantum dots prepared by laser ablation. Mater. Today Proc. 2021, 42, 1829–1833. [Google Scholar] [CrossRef]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Zhao, P.X.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Umamaheswari, K.; Abirami, M. Assessment of antifungal action mechanism of green synthesized gold nanoparticles (AuNPs) using Allium sativum on Candida species. Mater. Lett. 2023, 333, 133616. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Mizwari, Z.M.; Mohammadi-Aghdam, S.; Ahmadi, S.; Ebrahimzadeh, M.A.; Mortazavi-Derazkola, S. Optimization and evaluation of anticancer, antifungal, catalytic, and antibacterial activities: Biosynthesis of spherical-shaped gold nanoparticles using Pistacia vera hull extract (AuNPs@PV). Arab. J. Chem. 2023, 16, 104423. [Google Scholar] [CrossRef]
- Prema, P.; Boobalan, T.; Arun, A.; Rameshkumar, K.; Babu, R.S.; Veeramanikandan, V.; Nguyen, V.-H.; Balaji, P. Green tea extract mediated biogenic synthesis of gold nanoparticles with potent anti-proliferative effect against PC-3 human prostate cancer cells. Mater. Lett. 2022, 306, 130882. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Yao, Q.; Zang, S.; Xie, J. Toward greener synthesis of gold nanomaterials: From biological to biomimetic synthesis. Coord. Chem. Rev. 2021, 426, 213540. [Google Scholar] [CrossRef]
- Parthiban, A.; Sachithanandam, V.; Sarangapany, S.; Misra, R.; Muthukrishnan, P.; Jeyakumar, T.C.; Purvaja, R.; Ramesh, R. Green synthesis of gold nanoparticles using quercetin biomolecule from mangrove plant, Ceriops tagal: Assessment of antiproliferative properties, cellular uptake and DFT studies. J. Mol. Struct. 2023, 1272, 134167. [Google Scholar] [CrossRef]
- Patil, T.P.; Vibhute, A.A.; Patil, S.L.; Dongale, T.D.; Tiwari, A.P. Green synthesis of gold nanoparticles via Capsicum annum fruit extract: Characterization, antiangiogenic, antioxidant and anti-inflammatory activities. Appl. Surf. Sci. Adv. 2023, 13, 100372. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Bekmurzayeva, A.; Myrkhiyeva, Z.; Assylbekova, N.; Atabaev, T.S.; Tosi, D. Green-synthesized gold nanoparticle-based optical fiber ball resonator biosensor for cancer biomarker detection. Opt. Laser Technol. 2023, 161, 109136. [Google Scholar] [CrossRef]
- Pingping, W.; Yutong, D.; Xianghua, C.; Chun, C.; Kegang, W.; Xiong, F. Effect of bioactive Rosa roxburghii Tratt fruit polysaccharide on the structure and emulsifying property of lactoferrin protein. Int. J. Biol. Macromol. 2025, 298, 140016. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Pensa, E.; Cortés, E.; Corthey, G.; Carro, P.; Vericat, C.; Fonticelli, M.H.; Benítez, G.; Rubert, A.A.; Salvarezza, R.C. The Chemistry of the Sulfur-Gold Interface: In Search of a Unified Model. Acc. Chem. Res. 2012, 45, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Veiros, L.F.; Calhorda, M.J. How bridging ligands and neighbouring groups tune the gold-gold bond strength. J. Organomet. Chem. 1996, 510, 71–81. [Google Scholar] [CrossRef]
- Kokkin, D.L.; Zhang, R.; Steimle, T.C.; Wyse, I.A.; Pearlman, B.W.; Varberg, T.D. Au-S Bonding Revealed from the Characterization of Diatomic Gold Sulfide, AuS. J. Phys. Chem. 2015, 119, 11659–11667. [Google Scholar] [CrossRef] [PubMed]
- Kaim, A.; Szydłowska, J.; Piotrowski, P.; Megiel, E. One-pot synthesis of gold nanoparticles densely coated with nitroxide spins. Polyhedron 2012, 46, 119–123. [Google Scholar] [CrossRef]

| Various Detection Methods | Linear Range | LOD | Ref |
|---|---|---|---|
| Novel nanoprobe | 50–400 μM | 146 μM | [3] |
| Chemiluminescence | 0.5 μM–3000 μM | 0.2 μM | [4] |
| High-performance liquid chromatography | 0.25~24.54 μM | − | [5] |
| Sulphur- and nitrogen-doped carbon dots | 4–18 μM | 3.8 μM | [6] |
| Liquid chromatography with ultraviolet detection | 1~200 μM | 0.5 μM | [7] |
| This work | 0.17 μM~16.67 μM | 0.19 μM | − |
| RR-AuNPs | RR-AuNPs + Tiopronin | |
|---|---|---|
| zeta potential | −7.12 mV | −6.36 mV |
| hydrodynamic diameters | 50.5 nm | 571.26 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Feng, S. Green Synthesis and Characterization of Rosa roxburghii Tratt.-Mediated Gold Nanoparticles for Visual Colorimetric Assay of Tiopronin. Nanomaterials 2025, 15, 1513. https://doi.org/10.3390/nano15191513
Liu D, Feng S. Green Synthesis and Characterization of Rosa roxburghii Tratt.-Mediated Gold Nanoparticles for Visual Colorimetric Assay of Tiopronin. Nanomaterials. 2025; 15(19):1513. https://doi.org/10.3390/nano15191513
Chicago/Turabian StyleLiu, Dan, and Shilan Feng. 2025. "Green Synthesis and Characterization of Rosa roxburghii Tratt.-Mediated Gold Nanoparticles for Visual Colorimetric Assay of Tiopronin" Nanomaterials 15, no. 19: 1513. https://doi.org/10.3390/nano15191513
APA StyleLiu, D., & Feng, S. (2025). Green Synthesis and Characterization of Rosa roxburghii Tratt.-Mediated Gold Nanoparticles for Visual Colorimetric Assay of Tiopronin. Nanomaterials, 15(19), 1513. https://doi.org/10.3390/nano15191513






