Synergistic Regulation of Ag Nanoparticles and Reduced Graphene Oxide in Boosting TiO2 Microspheres Photocatalysis for Wastewater Treatment
Abstract
1. Introduction
2. Experimental
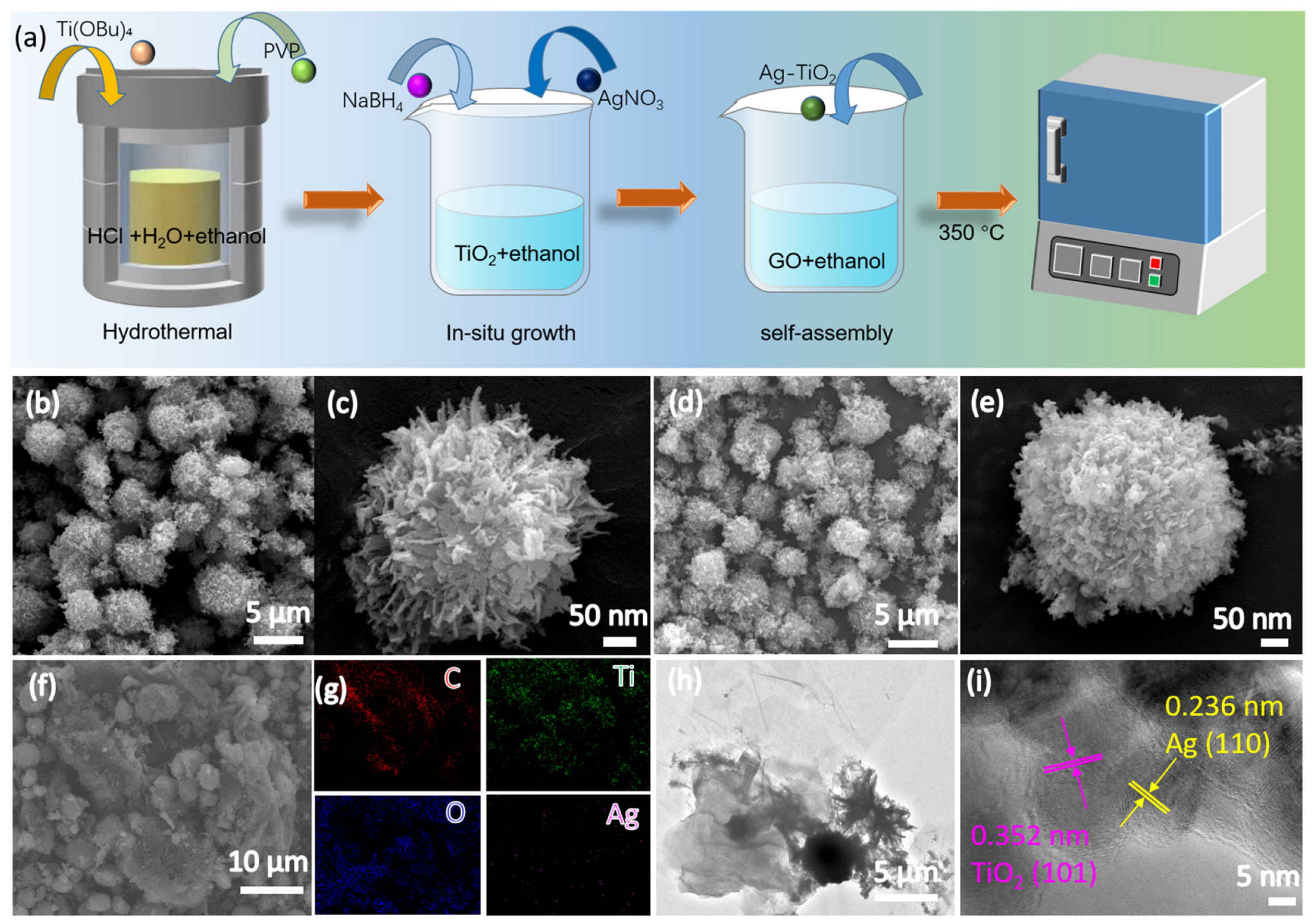
2.1. Preparation of TiO2 and Ag-TiO2-RGO
2.2. Characterization
2.3. Photodegradation Procedures
3. Result and Discussion
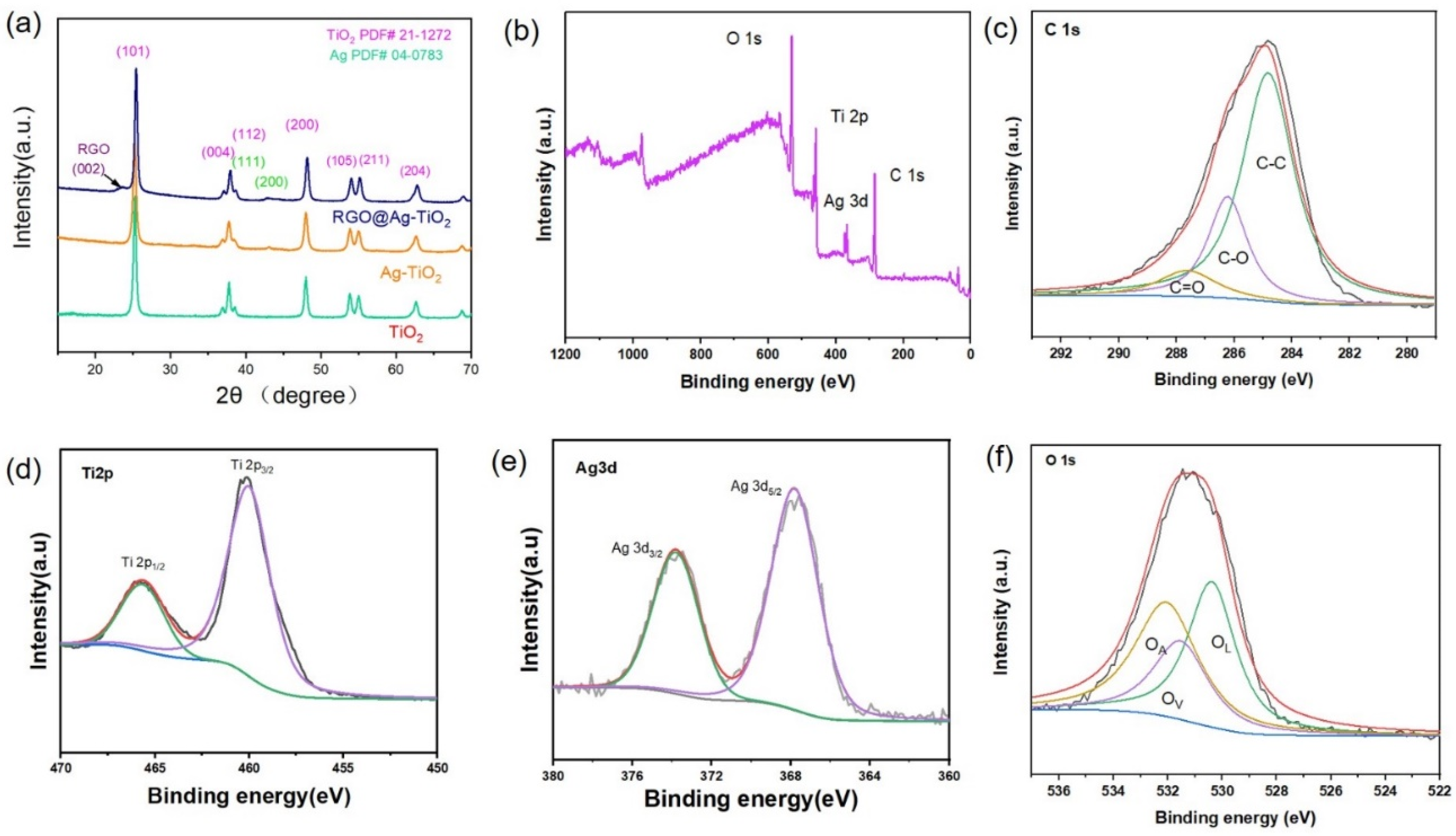
3.1. Morphology and Structural Properties
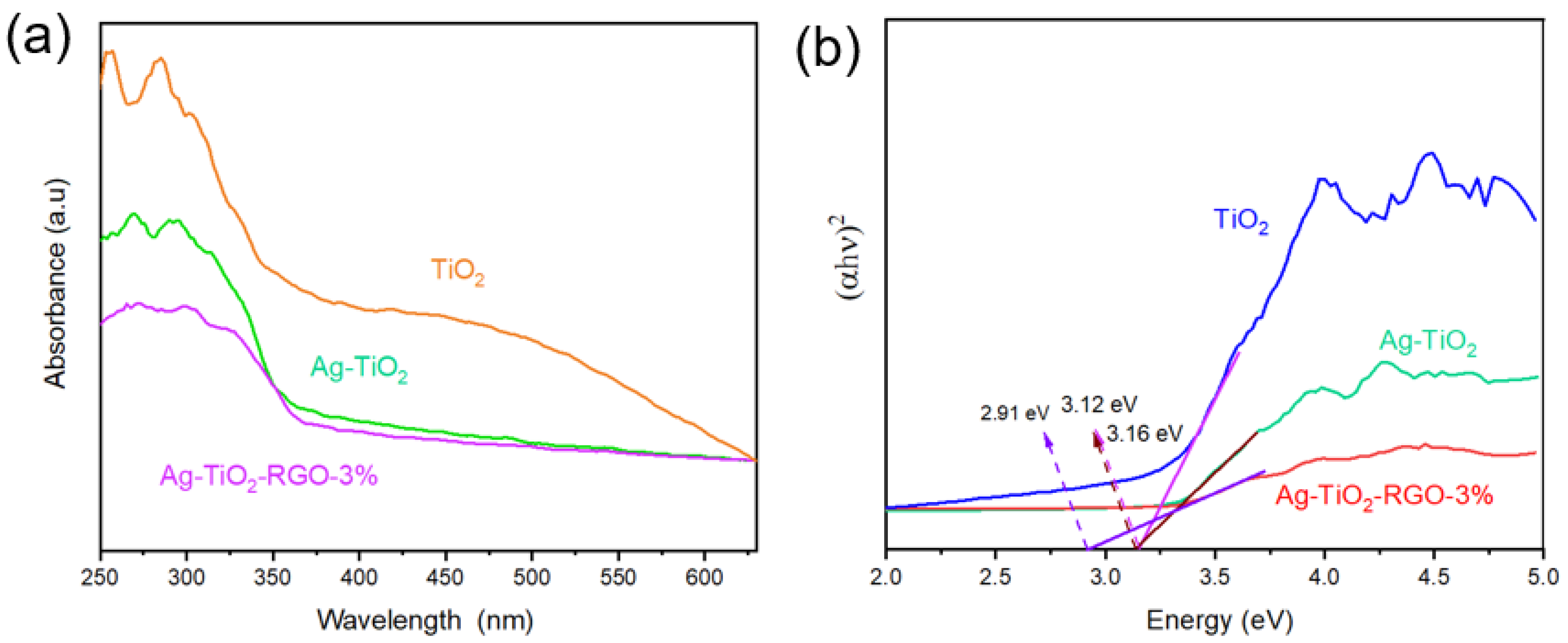
3.2. Optoelectronic Properties
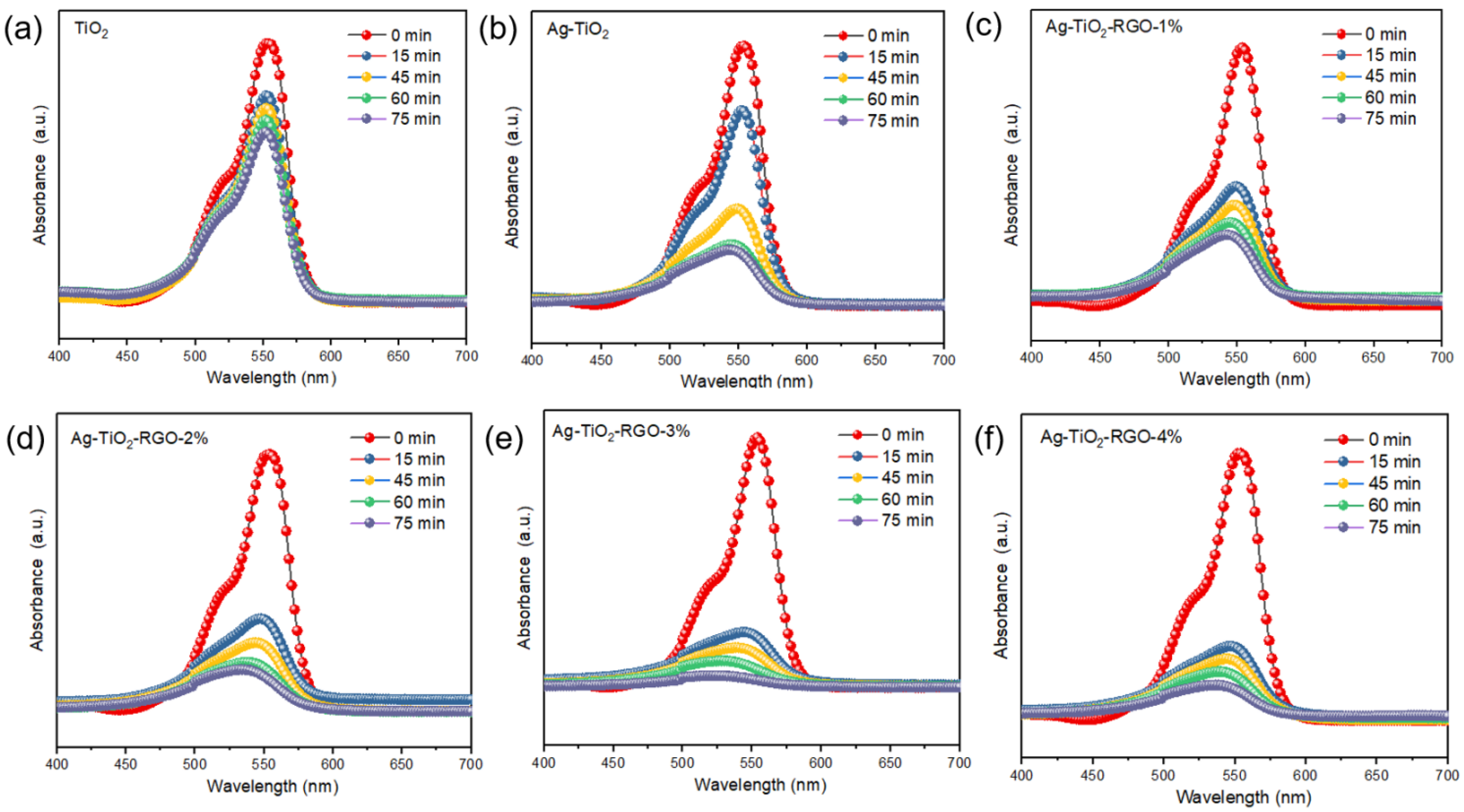
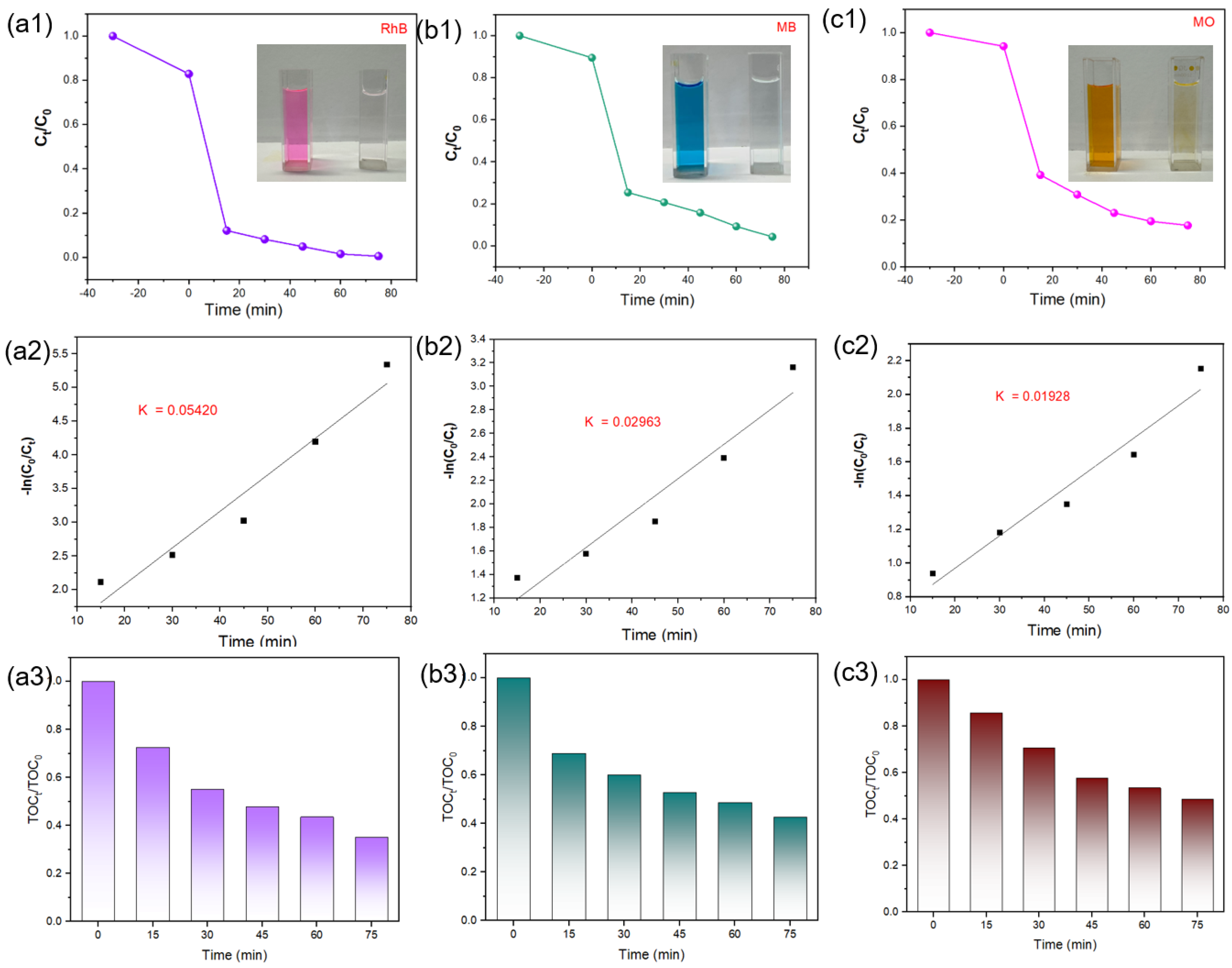
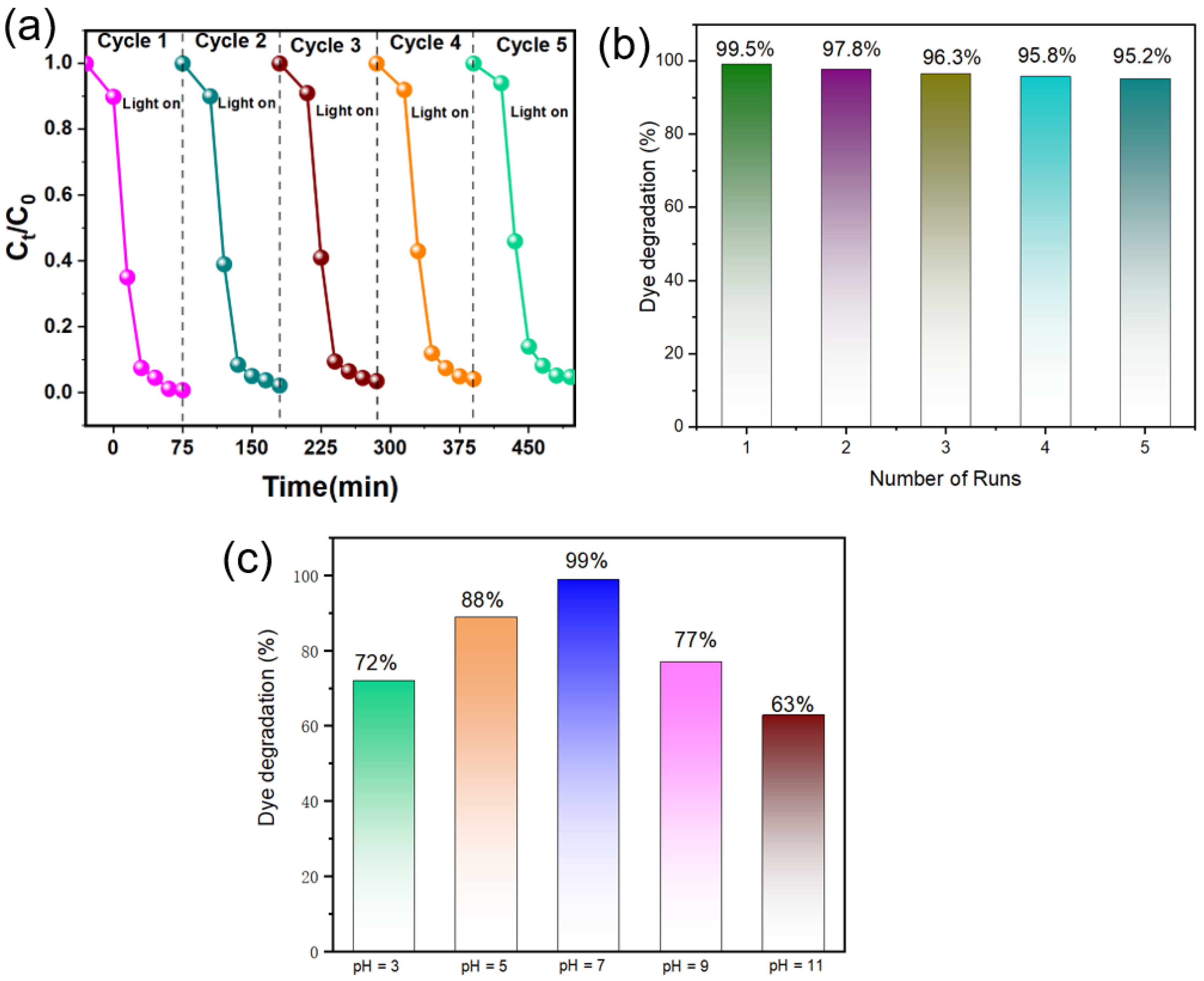
3.3. Photocatalytic Activity
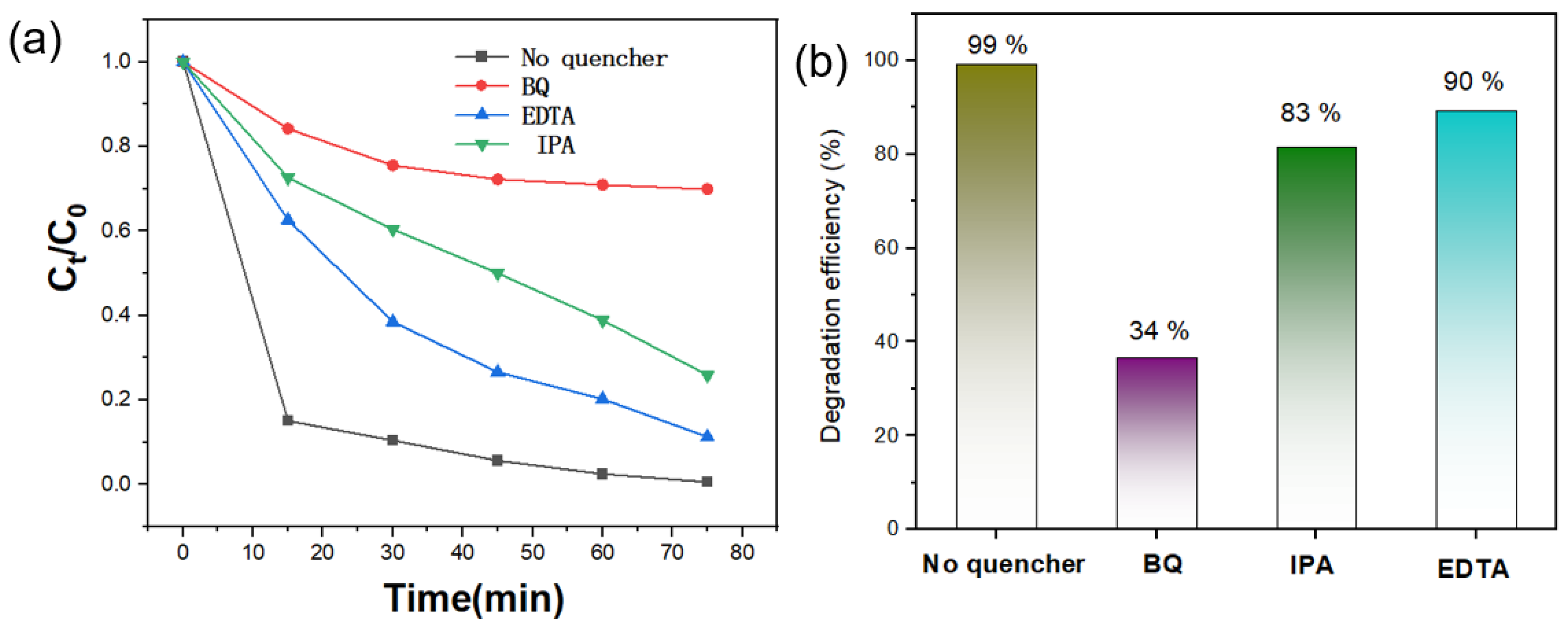
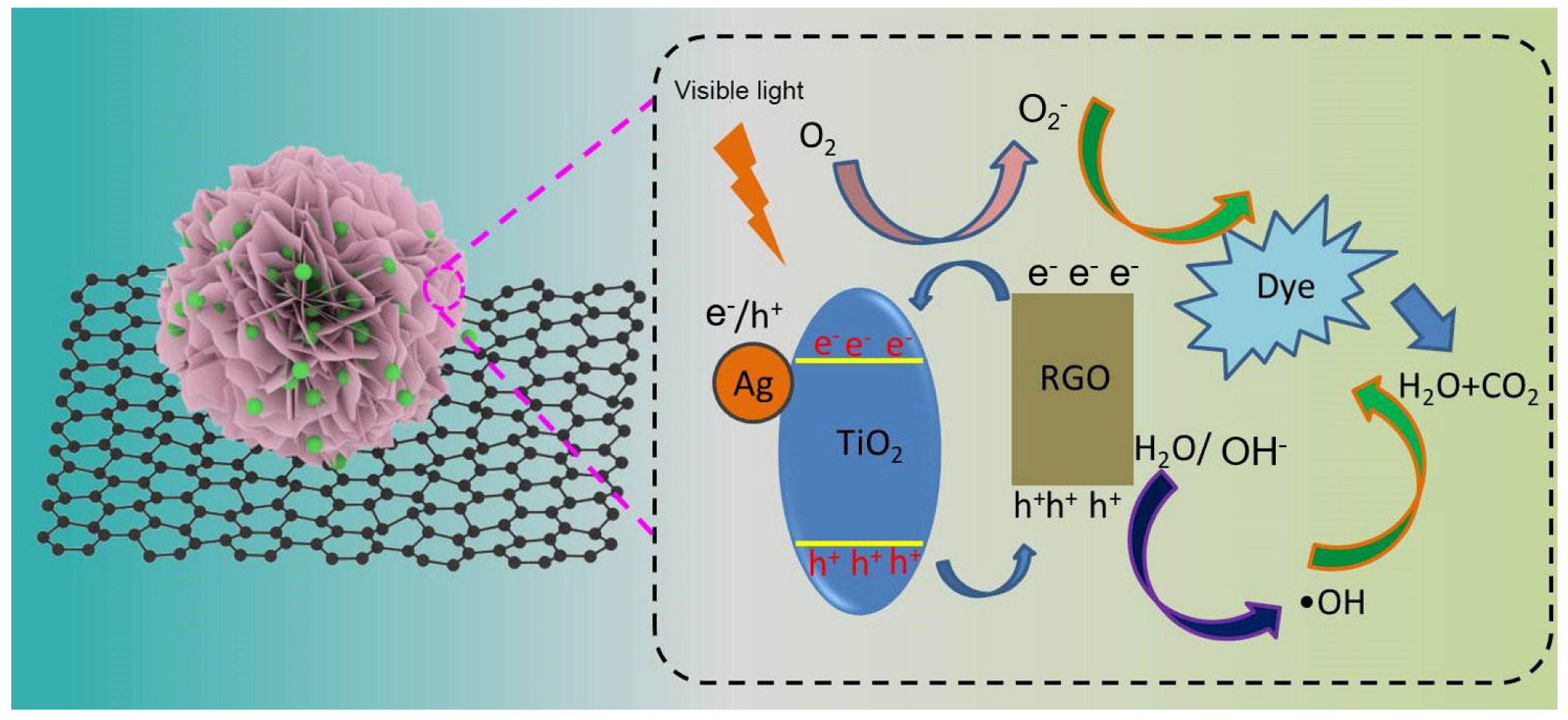
3.4. Photodegradation Mechanism of Dyes for Ag-TiO2-RGO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar] [CrossRef]
- Ran, Y.; Cui, R.; Wang, X.; Wang, H.; Zhang, L.; Xu, L.; Zhu, J.; Huang, Q.; Yuan, W. Advancements in iron-based photocatalytic degradation for antibiotics and dyes. J. Environ. Manag. 2025, 374, 123991. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Sui, J.; Waterhouse, G.I.N.; Zhang, Z.; Yu, L. Kelp-derived Cu-Fe3C@KC nanoclusters for microwave-assisted catalytic dye degradation. Inorg. Chem. Commun. 2025, 178, 114490. [Google Scholar] [CrossRef]
- Vidhya, S.; Subramanian, Y.; Gajendiran, J.; Raj, S.G.; VC, B.S.; Durairajan, A.; Le, M.T.; Mamudu, U.; Kumar, G.R.; Kumar, J.K. Methylene blue dye degradation characteristics of BiFeO3-graphene-LiNbO3 ternary nanocomposites. Sustain. Mater. Technol. 2025, 44, e01331. [Google Scholar] [CrossRef]
- Bi, H.; Zhao, X.; Ma, K.; Wang, Y.; An, L.; Yang, Q.; Liu, J. Role of molecular structure in the electrochemical degradation of coordination structure dye effluents. J. Environ. Chem. Eng. 2025, 13, 117969. [Google Scholar] [CrossRef]
- Gan, M.; Ma, Z.; Zhang, W.; Qie, X.; Liu, S.; He, J.; Wu, Q. BCQDs enhanced Bi-based photocatalysts for dye degradation and visual sewage treatment. J. Water Process Eng. 2025, 70, 107071. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Wang, Y.; Hua, F.; Tong, S. Cationic cellulose nanofibers with efficient anionic dye adsorption: Adsorption mechanism and application in salt-free dyeing of paper. Cellulose 2022, 29, 2047–2061. [Google Scholar] [CrossRef]
- McYotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Singh, V.; Pandit, C.; Roy, A.; Pandit, S.; Rai, A.K.; Rani, A.; Ranjan, N.; Rustagi, S.; Malik, S. Degradation of food dyes via biological methods: A state-of-the-art review. Bioresour. Technol. Rep. 2024, 25, 101780. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, K.; Zhang, H.; Han, L.; He, Q.; Huang, F.; Yu, W.; Guo, F.; Wang, W. Sustainable green conversion of coal gangue waste into cost-effective porous multimetallic silicate adsorbent enables superefficient removal of Cd(II) and dye. Chemosphere 2023, 324, 138287. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.M.S.; Pedroso, P.R.M.; Pereira, J.F.B. Sustainable extraction and utilization of chlorophyll from microalgae for eco-friendly wool dyeing. J. Clean. Prod. 2024, 451, 142009. [Google Scholar] [CrossRef]
- Keshmiri-Naqab, R.; Taghavijeloudar, M. Efficient adsorption of acid orange 7 from wastewater using novel bio-natural granular bentonite-sawdust-corncob (GBSC): Mixture optimization, adsorption kinetic and regeneration. Environ. Res. 2024, 262, 119966. [Google Scholar] [CrossRef]
- Chimupala, Y.; Phromma, C.; Yimklan, S.; Semakul, N.; Ruankham, P. Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv. 2020, 10, 28567–28575. [Google Scholar] [CrossRef]
- Ranjbari, A.; Yu, J.; Kim, J.; Kim, J.; Park, M.; Kim, K.-H.; Heynderickx, P.M. Fundamental kinetic modeling of dye sensitization photocatalysis by oxygen vacancy enriched ZnO for the quantification of degradation by catalyst or dye sensitizer. Appl. Surf. Sci. 2024, 659, 159867. [Google Scholar] [CrossRef]
- Satish Kumar, R.; Min, K.S.; Lee, S.H.; Mergu, N.; Son, Y.-A. Synthesis of novel panchromatic porphyrin-squaraine dye and application towards TiO2 combined photocatalysis. J. Photochem. Photobiol. A Chem. 2020, 397, 112595. [Google Scholar] [CrossRef]
- Deekshitha; Shetty, K.V. Solar light active biogenic titanium dioxide embedded silver oxide (AgO/Ag2O@TiO2) nanocomposite structures for dye degradation by photocatalysis. Mater. Sci. Semicond. Process. 2021, 132, 105923. [Google Scholar] [CrossRef]
- Kalikeri, S.; Kodialbail, V.S. Visible light active Bismuth ferrite embedded TiO2 nanocomposite structures for dye mineralization by photocatalysis—A strategy to harness solar energy for remediation of water contaminated with mixture of dyes. Surf. Interfaces 2023, 36, 102492. [Google Scholar] [CrossRef]
- Mahendran, V.; Gogate, P.R. Degradation of Acid Scarlet 3R dye using oxidation strategies involving photocatalysis based on Fe doped TiO2 photocatalyst, ultrasound and hydrogen peroxide. Sep. Purif. Technol. 2021, 274, 119011. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.-L.; Hao, H.; Lang, X. Visible light-induced selective oxidation of alcohols with air by dye-sensitized TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 232, 260–267. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Hasan, J.; Wang, J.; Wang, Z.; Idrees, M.; Batool, S.; Zhang, C.; Qin, C. Enhanced ultraviolet-visible photocatalysis of RGO/equaixial geometry TiO2 composites on degradation of organic dyes in water. Environ. Sci. Pollut. Res. 2022, 29, 12222–12236. [Google Scholar] [CrossRef] [PubMed]
- Chairungsri, W.; Subkomkaew, A.; Kijjanapanich, P.; Chimupala, Y. Direct dye wastewater photocatalysis using immobilized titanium dioxide on fixed substrate. Chemosphere 2022, 286, 131762. [Google Scholar] [CrossRef] [PubMed]
- Bendjabeur, S.; Zouaghi, R.; Zouchoune, B.; Sehili, T. DFT and TD-DFT insights, photolysis and photocatalysis investigation of three dyes with similar structure under UV irradiation with and without TiO2 as a catalyst: Effect of adsorption, pH and light intensity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 494–505. [Google Scholar] [CrossRef]
- Rocha, R.L.P.; Morais, A.Í.S.; Araujo, F.P.; Honório, L.M.C.; Silva, M.P.; Furtini, M.B.; Vieira, E.G.; da Silva-Filho, E.C.; Osajima, J.A. Enhanced Photocatalytic Performance of TiO2@Er-Hydroxyapatite Composite for Cationic Dye and Drug Removal. ACS Omega 2025, 10, 5351–5361. [Google Scholar] [CrossRef]
- Mohd Adnan, M.A.; Muhd Julkapli, N.; Amir, M.N.I.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566. [Google Scholar] [CrossRef]
- Hu, H.; Lin, Y.; Hu, Y.H. Core-shell structured TiO2 as highly efficient visible light photocatalyst for dye degradation. Catal. Today 2020, 341, 90–95. [Google Scholar] [CrossRef]
- Li, J.; Liang, Q.; Yang, Y.; Ya, Y.; Deng, D.; Liu, C. Slide-shaped cotton-based photocatalytic solar evaporator with dual mutually strengthening functions for simultaneous desalination and dynamic fast dye removal. Desalination 2024, 592, 118055. [Google Scholar] [CrossRef]
- Erjeno, D.J.D.; Asequia, D.M.A.; Osorio, C.K.F.; Omisol, C.J.M.; Etom, A.E.; Hisona, R.M.R.; Tilendo, A.C.; Triana, A.P.G.; Dumancas, G.G.; Zoleta, J.B.; et al. Facile Synthesis of Band Gap-Tunable Kappa-Carrageenan-Mediated C,S-Doped TiO2 Nanoparticles for Enhanced Dye Degradation. ACS Omega 2024, 9, 21245–21259. [Google Scholar] [CrossRef]
- Arasu, K.A.S.; Raja, A.G.; Rajaram, R. The encapsulation of noble metal over metal oxide semiconductor TiO2@Ag for the degradation of sulphur dye in effluent water by photocatalysis and electrochemical water splitting. J. Mol. Struct. 2025, 1321, 139986. [Google Scholar] [CrossRef]
- Veziroglu, S.; Obermann, A.-L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leißner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Degradation over TiO2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [Google Scholar] [CrossRef]
- Huo, J.; Yuan, C.; Wang, Y. Nanocomposites of Three-Dimensionally Ordered Porous TiO2 Decorated with Pt and Reduced Graphene Oxide for the Visible-Light Photocatalytic Degradation of Waterborne Pollutants. ACS Appl. Nano Mater. 2019, 2, 2713–2724. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Zhang, Z.; Liao, S.; Deng, Y.; Wang, X.; Ye, Q.; Wang, K. Construction of Sn3O4–Ag–Ag2O Z-scheme heterojunction on TiO2 nanotube arrays: Photocatalytic dye degradation and H2 evolution. Ceram. Int. 2023, 49, 29288–29297. [Google Scholar] [CrossRef]
- Zou, M.; Liu, H.; Feng, L.; Xiong, F.; Thomas, T.; Yang, M. Effect of nitridation on visible light photocatalytic behavior of microporous (Ag, Ag2O) co-loaded TiO2. Microporous Mesoporous Mater. 2017, 240, 137–144. [Google Scholar] [CrossRef]
- Germani, R.; Bini, M.; Fantacci, S.; Simonetti, F.; Tiecco, M.; Vaioli, E.; Del Giacco, T. Influence of surfactants in improving degradation of polluting dyes photocatalyzed by TiO2 in aqueous dispersion. J. Photochem. Photobiol. A Chem. 2021, 418, 113342. [Google Scholar] [CrossRef]
- Khan, S.B.; Hou, M.; Shuang, S.; Zhang, Z. Morphological influence of TiO2 nanostructures (nanozigzag, nanohelics and nanorod) on photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2017, 400, 184–193. [Google Scholar] [CrossRef]
- Shabil Sha, M.; Anwar, H.; Musthafa, F.N.; Al-Lohedan, H.; Alfarwati, S.; Rajabathar, J.R.; Khalid Alahmad, J.; Cabibihan, J.-J.; Karnan, M.; Kumar Sadasivuni, K. Photocatalytic degradation of organic dyes using reduced graphene oxide (rGO). Sci. Rep. 2024, 14, 3608. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yang, Y.; Wu, W.; Dai, X.; Zhong, J.; Jian, Y.; Li, R.; Wang, T.; Yu, H.; Xia, X. Synthesis of a novel TiO2/HA/RGO composite material with photocatalytic activity for dye degradation. Mater. Chem. Phys. 2023, 304, 127847. [Google Scholar] [CrossRef]
- Garg, D.; Matai, I.; Garg, A.; Sachdev, A. Tragacanth Hydrogel Integrated CeO2@rGO Nanocomposite as Reusable Photocatalysts for Organic Dye Degradation. ChemistrySelect 2020, 5, 10663–10672. [Google Scholar] [CrossRef]
- Lu, Z.; Bai, Y.; Zhang, S.; Li, Y.; Liu, M.; Fang, L.; Liu, L.; Du, K.; Liu, G.; Xu, L.; et al. Oxygen doping and interface engineering in O-MoS2&rGO heterostructure for efficient piezocatalytic dye degradation. J. Mater. Res. 2024, 39, 1999–2008. [Google Scholar] [CrossRef]
- Sun, K.; Wang, L.; Wu, C.; Deng, J.; Pan, K. Fabrication of α-Fe2O3@rGO/PAN Nanofiber Composite Membrane for Photocatalytic Degradation of Organic Dyes. Adv. Mater. Interfaces 2017, 4, 1700845. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Balaji, V.; Yuvakkumar, R.; Velauthapillai, D.; Ravi, G. Facile synthesis of a heterostructured lanthanum-doped SnO2 anchored with rGO for asymmetric supercapacitors and photocatalytic dye degradation. New J. Chem. 2021, 45, 22497–22513. [Google Scholar] [CrossRef]
- Sowndharya, S.; Nikitha, M.; Meenakshi, S. Designing an in-situ embedment of citric acid@HAp/ZnO as a Z-scheme heterojunction for substantial enhancement in photocatalytic degradation of malachite green under visible light. J. Clean. Prod. 2025, 490, 144723. [Google Scholar] [CrossRef]
- Ge, Y.; Luo, H.; Huang, J.; Zhang, Z. Visible-light-active TiO2 photocatalyst for efficient photodegradation of organic dyes. Opt. Mater. 2021, 115, 111058. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Y.; Wang, L.; Zhang, S.; Ma, J.; Dong, W.; Zeng, Y.; Yuan, J.; Liu, C.; Luo, S. “Dark Deposition” of Ag Nanoparticles on TiO2: Improvement of Electron Storage Capacity To Boost “Memory Catalysis” Activity. ACS Appl. Mater. Interfaces 2018, 10, 25350–25359. [Google Scholar] [CrossRef]
- Yang, W.-D.; Li, Y.-R.; Lee, Y.-C. Synthesis of r-GO/TiO2 composites via the UV-assisted photocatalytic reduction of graphene oxide. Appl. Surf. Sci. 2016, 380, 249–256. [Google Scholar] [CrossRef]
- Shaik, B.B.; Katari, N.K.; Raghupathi, J.K.; Jonnalagadda, S.B.; Rana, S. Titanium Dioxide/Graphene-Based Nanocomposites as Photocatalyst for Environmental Applications: A Review. ChemistrySelect 2024, 9, e202403521. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Maity, P.; Mohammed, O.F.; Gascon, J. Insight into the role of reduced graphene oxide in enhancing photocatalytic hydrogen evolution in disordered carbon nitride. Phys. Chem. Chem. Phys. 2022, 24, 11213–11221. [Google Scholar] [CrossRef] [PubMed]
- Pesci, F.M.; Wang, G.; Klug, D.R.; Li, Y.; Cowan, A.J. Efficient Suppression of Electron–Hole Recombination in Oxygen-Deficient Hydrogen-Treated TiO2 Nanowires for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2013, 117, 25837–25844. [Google Scholar] [CrossRef] [PubMed]
- Tay, Q.; Chen, Z. Effective charge separation towards enhanced photocatalytic activity via compositing reduced graphene oxide with two-phase anatase/brookite TiO2. Int. J. Hydrogen Energy 2016, 41, 10590–10597. [Google Scholar] [CrossRef]
- Rathi, V.H.; Jeice, A.R. Green fabrication of titanium dioxide nanoparticles and their applications in photocatalytic dye degradation and microbial activities. Chem. Phys. Impact 2023, 6, 100197. [Google Scholar] [CrossRef]
- Potle, V.D.; Shirsath, S.R.; Bhanvase, B.A.; Saharan, V.K. Sonochemical preparation of ternary rGO-ZnO-TiO2 nanocomposite photocatalyst for efficient degradation of crystal violet dye. Optik 2020, 208, 164555. [Google Scholar] [CrossRef]
- Roopan, S.M.; Elango, G.; Priya, D.D.; Asharani, I.V.; Kishore, B.; Vinayprabhakar, S.; Pragatheshwaran, N.; Mohanraj, K.; Harshpriya, R.; Shanavas, S.; et al. Sunlight mediated photocatalytic degradation of organic pollutants by statistical optimization of green synthesized NiO NPs as catalyst. J. Mol. Liq. 2019, 293, 111509. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, F.; Zhou, F.; Yang, X.; Zhang, D.; Chen, Y. Synergistic effect of RGO/TiO2 nanosheets with exposed (0 0 1) facets for boosting visible light photocatalytic activity. Appl. Surf. Sci. 2020, 510, 145451. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Chen, Z. Thermal behavior of large-span reticulated domes covered by ETFE membrane roofs under solar radiation. Thin-Walled Struct. 2017, 115, 1–11. [Google Scholar] [CrossRef]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and Characterization of Ag-Ag2O/TiO2@polypyrrole Heterojunction for Enhanced Photocatalytic Degradation of Methylene Blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Wani, S.I.; Ganie, A.S. Ag2O incorporated ZnO−TiO2 nanocomposite: Ionic conductivity and photocatalytic degradation of an organic dye. Inorg. Chem. Commun. 2021, 128, 108567. [Google Scholar] [CrossRef]
- Jain, R.; Upadhyaya, M.; Singh, A.; Jaiswal, R.P. TiO2 nanoparticles synthesized on graphene nanosheets with varied crystallinity for photocatalytic degradation of MB dye. J. Environ. Chem. Eng. 2024, 12, 113881. [Google Scholar] [CrossRef]
- Saensook, S.; Sirisuk, A. A factorial experimental design approach to obtain defect-rich black TiO2 for photocatalytic dye degradation. J. Water Process Eng. 2022, 45, 102495. [Google Scholar] [CrossRef]
- Pal, V.K.; Kumar, D.; Gupta, A.; Neelratan, P.P.; Purohit, L.P.; Singh, A.; Singh, V.; Lee, S.; Mishra, Y.K.; Kaushik, A.; et al. Nanocarbons decorated TiO2 as advanced nanocomposite fabric for photocatalytic degradation of methylene blue dye and ciprofloxacin. Diam. Relat. Mater. 2024, 148, 111435. [Google Scholar] [CrossRef]
- Sultana, R.; Liba, S.I.; Rahman, M.A.; Yeachin, N.; Syed, I.M.; Bhuiyan, M.A. Enhanced photocatalytic activity in RhB dye degradation by Mn and B co-doped mixed phase TiO2 photocatalyst under visible light irradiation. Surf. Interfaces 2023, 42, 103302. [Google Scholar] [CrossRef]
- Madima, N.; Kefeni, K.K.; Mishra, S.B.; Mishra, A.K. TiO2-modified g-C3N4 nanocomposite for photocatalytic degradation of organic dyes in aqueous solution. Heliyon 2022, 8, e10683. [Google Scholar] [CrossRef]
- Mandal, S.K.; Dutta, K.; Pal, S.; Mandal, S.; Naskar, A.; Pal, P.K.; Bhattacharya, T.S.; Singha, A.; Saikh, R.; De, S.; et al. Engineering of ZnO/rGO nanocomposite photocatalyst towards rapid degradation of toxic dyes. Mater. Chem. Phys. 2019, 223, 456–465. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Maiti, M.; Sarkar, M.; Maiti, S.; Malik, M.A.; Xu, S. Modification of geopolymer with size controlled TiO2 nanoparticle for enhanced durability and catalytic dye degradation under UV light. J. Clean. Prod. 2020, 255, 120183. [Google Scholar] [CrossRef]


| Catalyst | Light Source | Dye | Irradiation Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|
| TiO2 | UV | MB | 40 | 86 | [50] |
| TiO2 | UV | RhB | 120 | 96 | [51] |
| TiO2 | Visible light | MB | 120 | 93 | [52] |
| RGO/TiO2 | UV | MO | 180 | 94 | [53] |
| Ag/TiO2 | Visible light | RhB | 150 | 79 | [54] |
| Ag-Ag2O/TiO2@PP | UV | MB | 120 | 83 | [55] |
| Ag2O/ZnO–TiO2 | Visible light | RhB | 90 | 30 | [56] |
| Ag-TiO2-RGO-3% | Visible light | RhB | 75 | 99 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; An, Z.; Yang, Y.; Wang, W.; Wang, Y.; Tian, S.; Gao, J.; Gong, X.-Z.; Belfoire, L.A.; Tang, J. Synergistic Regulation of Ag Nanoparticles and Reduced Graphene Oxide in Boosting TiO2 Microspheres Photocatalysis for Wastewater Treatment. Nanomaterials 2025, 15, 1510. https://doi.org/10.3390/nano15191510
Ma G, An Z, Yang Y, Wang W, Wang Y, Tian S, Gao J, Gong X-Z, Belfoire LA, Tang J. Synergistic Regulation of Ag Nanoparticles and Reduced Graphene Oxide in Boosting TiO2 Microspheres Photocatalysis for Wastewater Treatment. Nanomaterials. 2025; 15(19):1510. https://doi.org/10.3390/nano15191510
Chicago/Turabian StyleMa, Guoshuai, Zhijian An, Yinqi Yang, Wei Wang, Yao Wang, Shuting Tian, Jingwen Gao, Xue-Zhong Gong, Laurence A. Belfoire, and Jianguo Tang. 2025. "Synergistic Regulation of Ag Nanoparticles and Reduced Graphene Oxide in Boosting TiO2 Microspheres Photocatalysis for Wastewater Treatment" Nanomaterials 15, no. 19: 1510. https://doi.org/10.3390/nano15191510
APA StyleMa, G., An, Z., Yang, Y., Wang, W., Wang, Y., Tian, S., Gao, J., Gong, X.-Z., Belfoire, L. A., & Tang, J. (2025). Synergistic Regulation of Ag Nanoparticles and Reduced Graphene Oxide in Boosting TiO2 Microspheres Photocatalysis for Wastewater Treatment. Nanomaterials, 15(19), 1510. https://doi.org/10.3390/nano15191510







