Green-Synthesized Nanomaterials for Water Disinfection: Mechanisms, Efficacy, and Environmental Safety
Abstract
1. Introduction
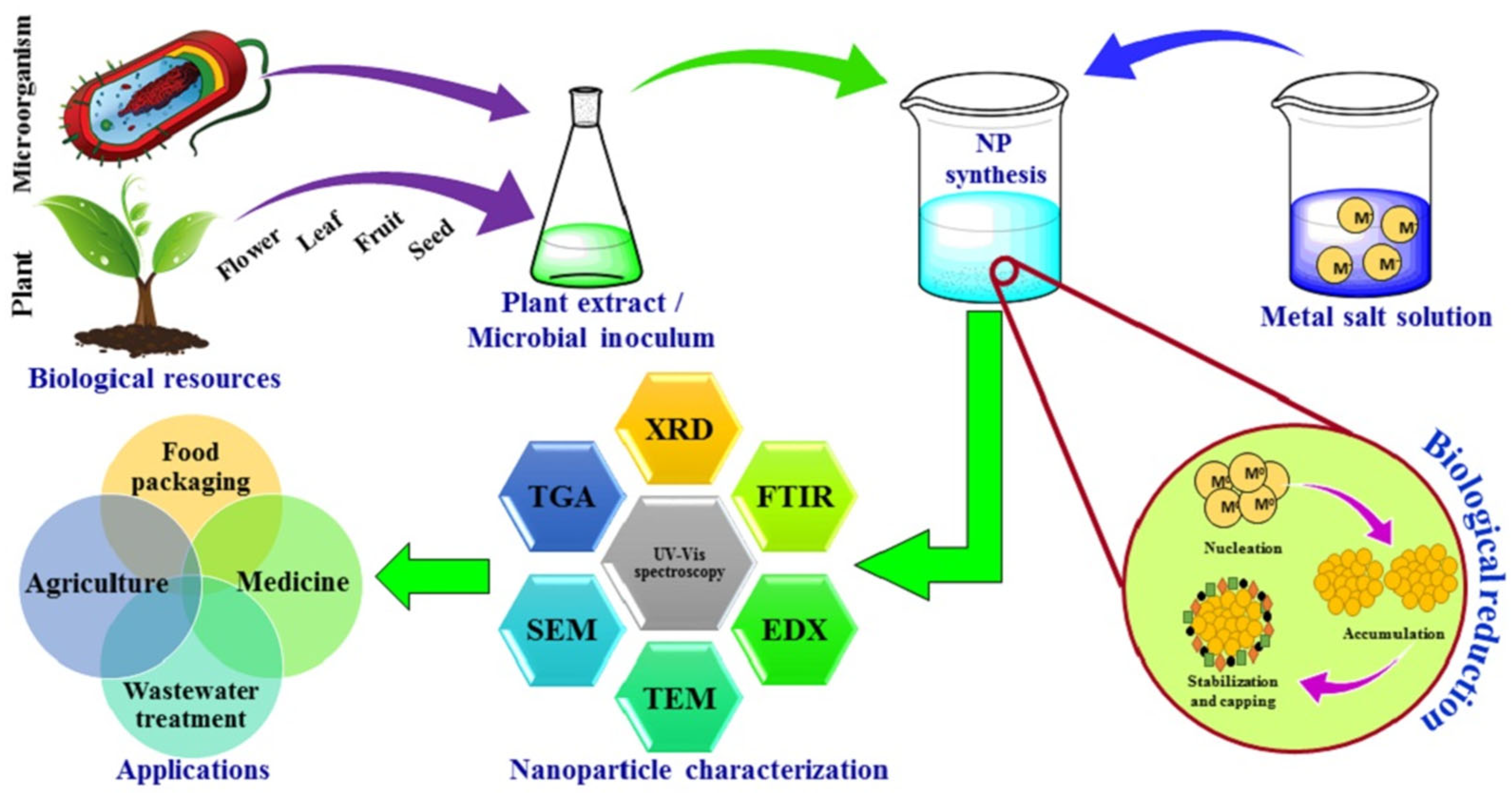
2. Green Synthesis Approaches for Nanomaterials
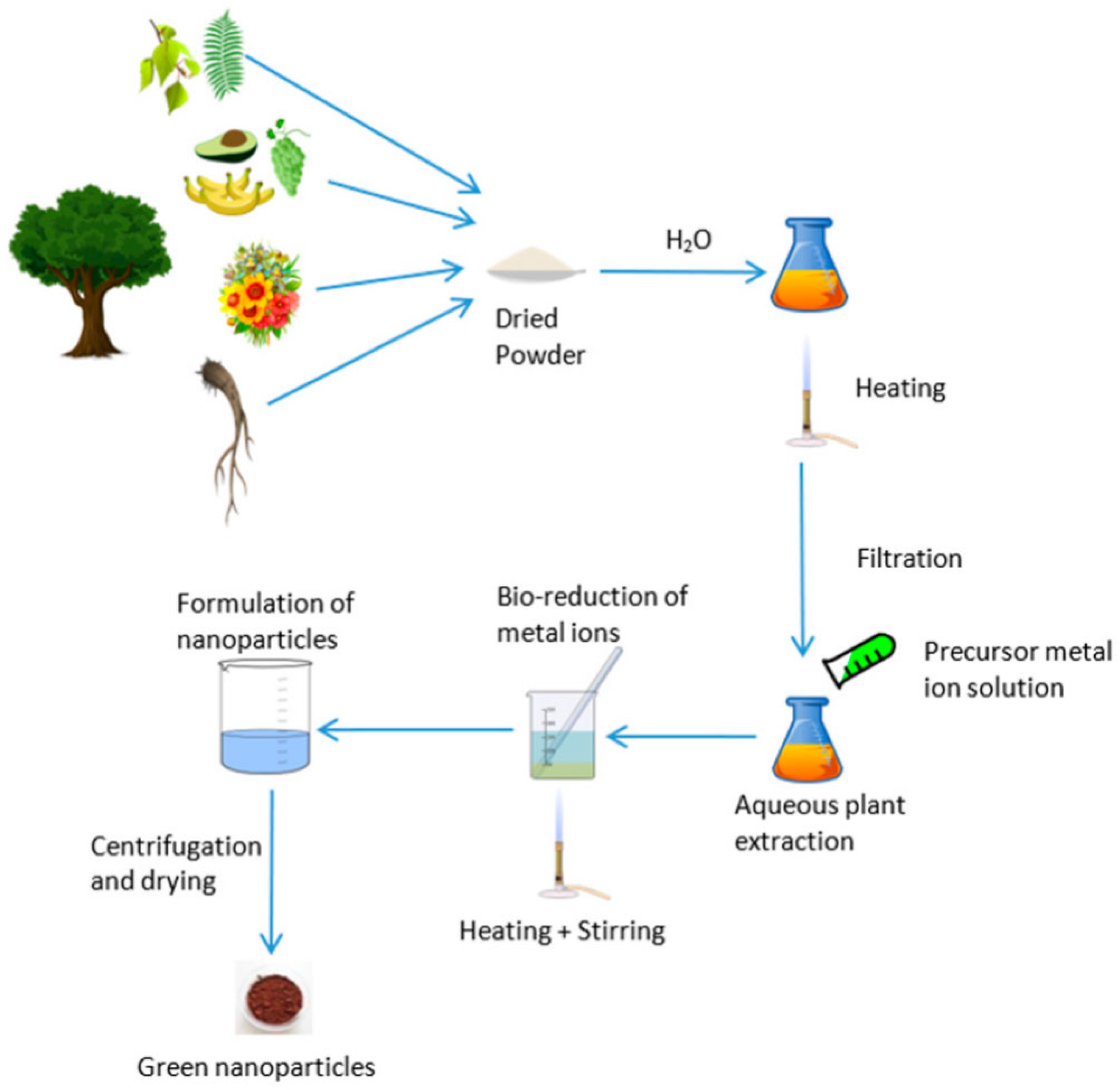
2.1. Plant-Mediated Synthesis
2.2. Microbial-Mediated Synthesis (Bacteria and Fungi)
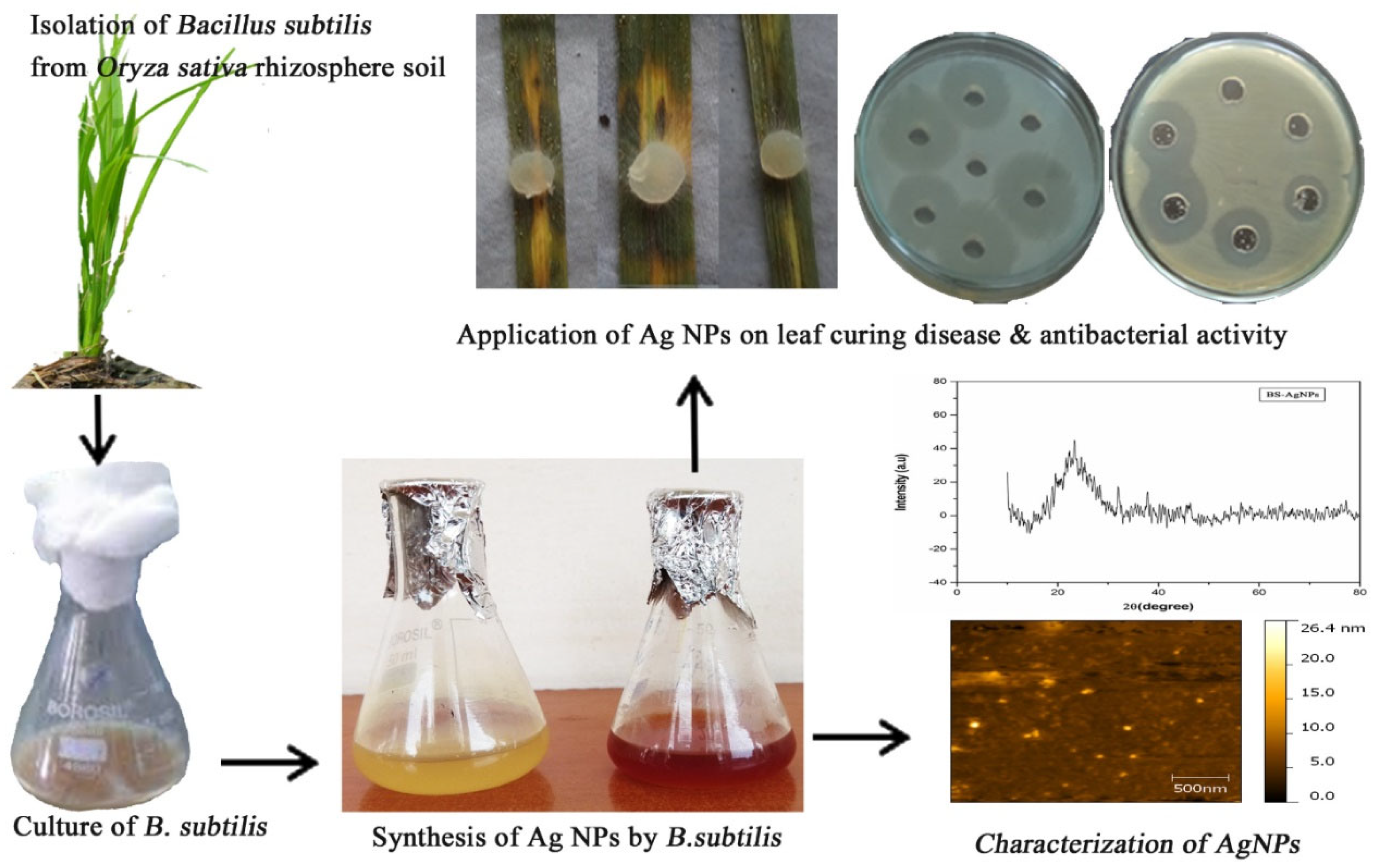
2.2.1. Bacterial-Mediated Nanoparticle Synthesis
2.2.2. Fungal-Mediated Nanoparticle Synthesis
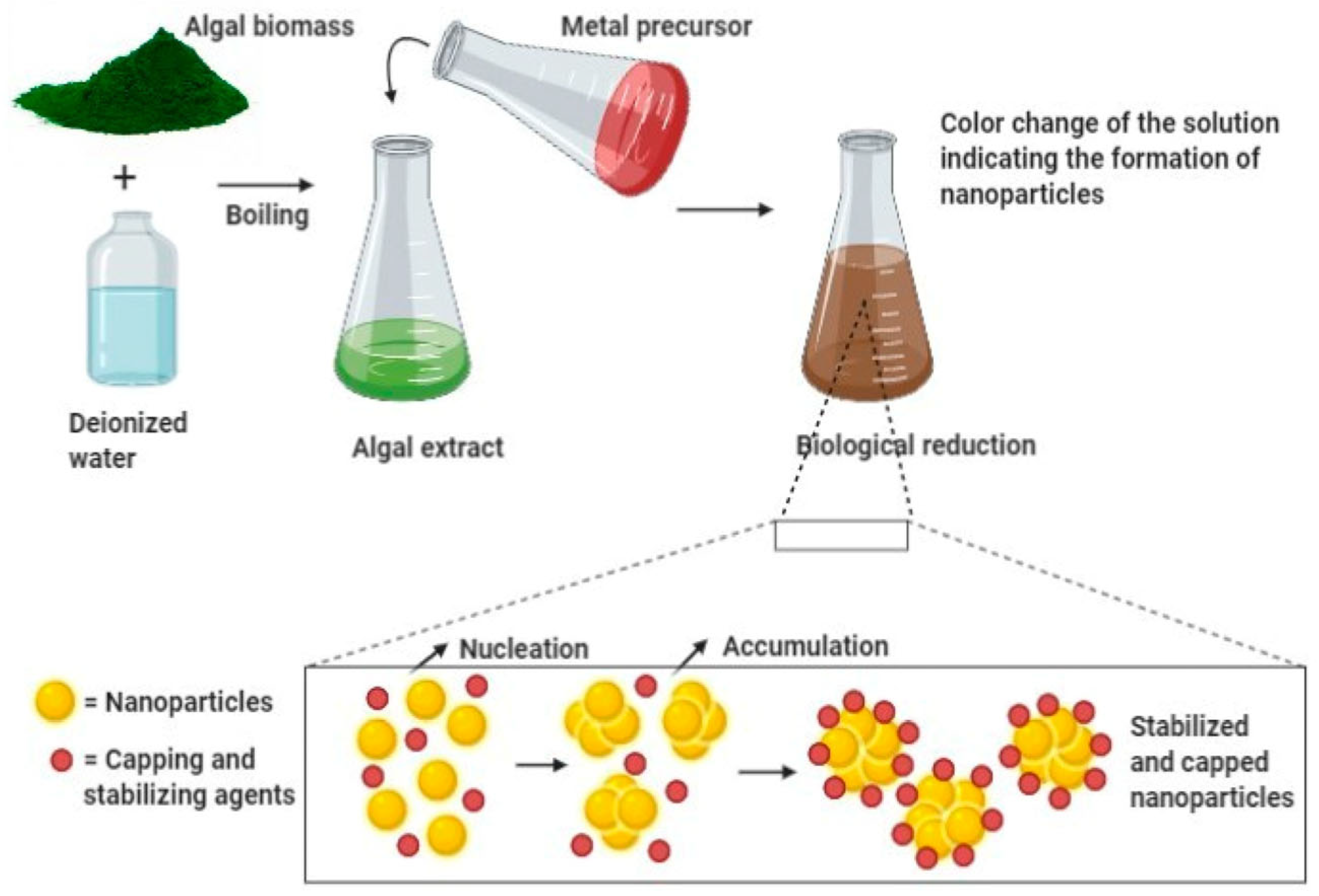
2.3. Algae-Mediated Synthesis
2.4. Biopolymer and Natural Molecule-Mediated Synthesis
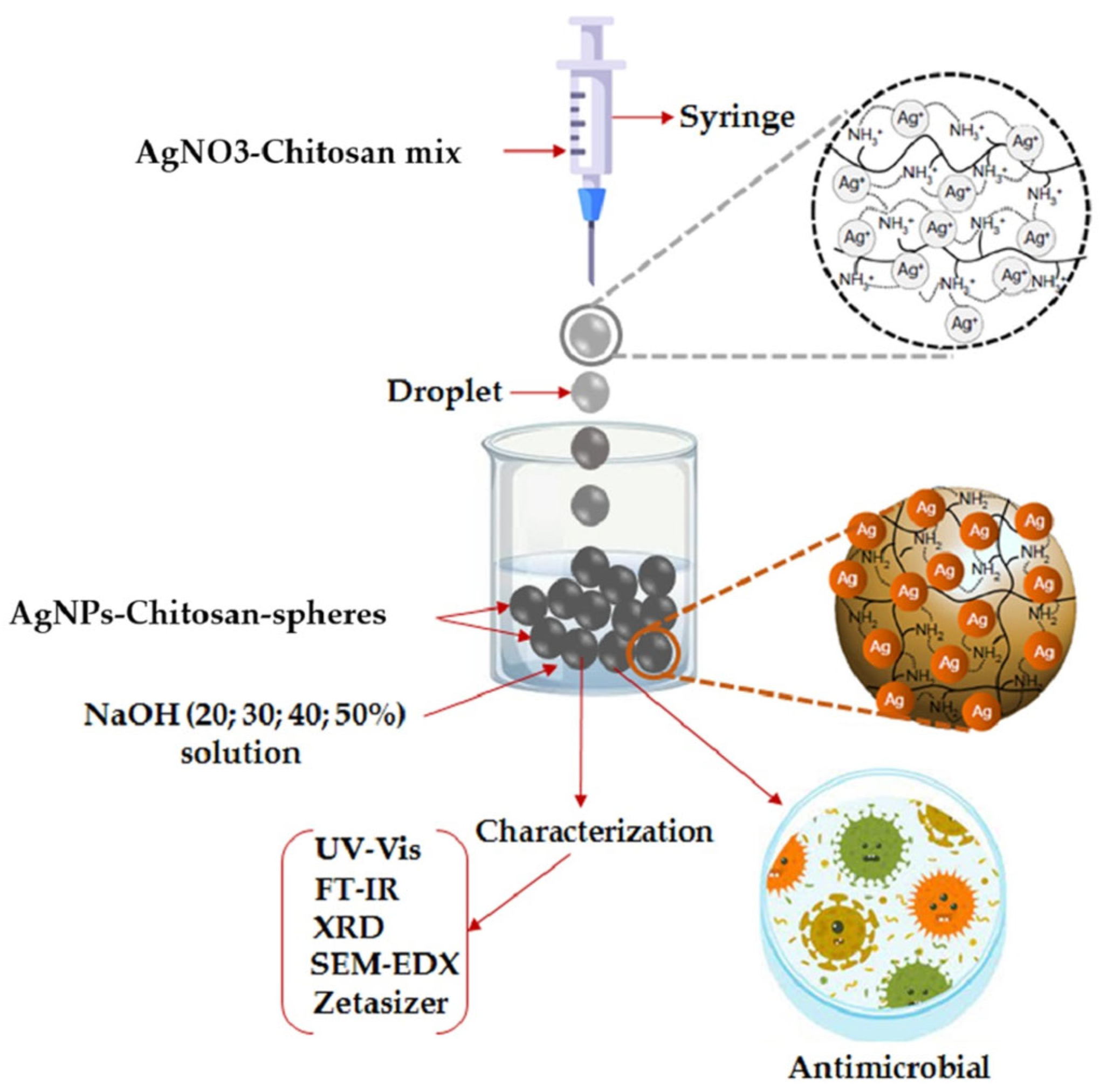
2.4.1. Chitosan-Mediated Nanoparticles
2.4.2. Starch and Gelatin-Mediated Nanoparticles
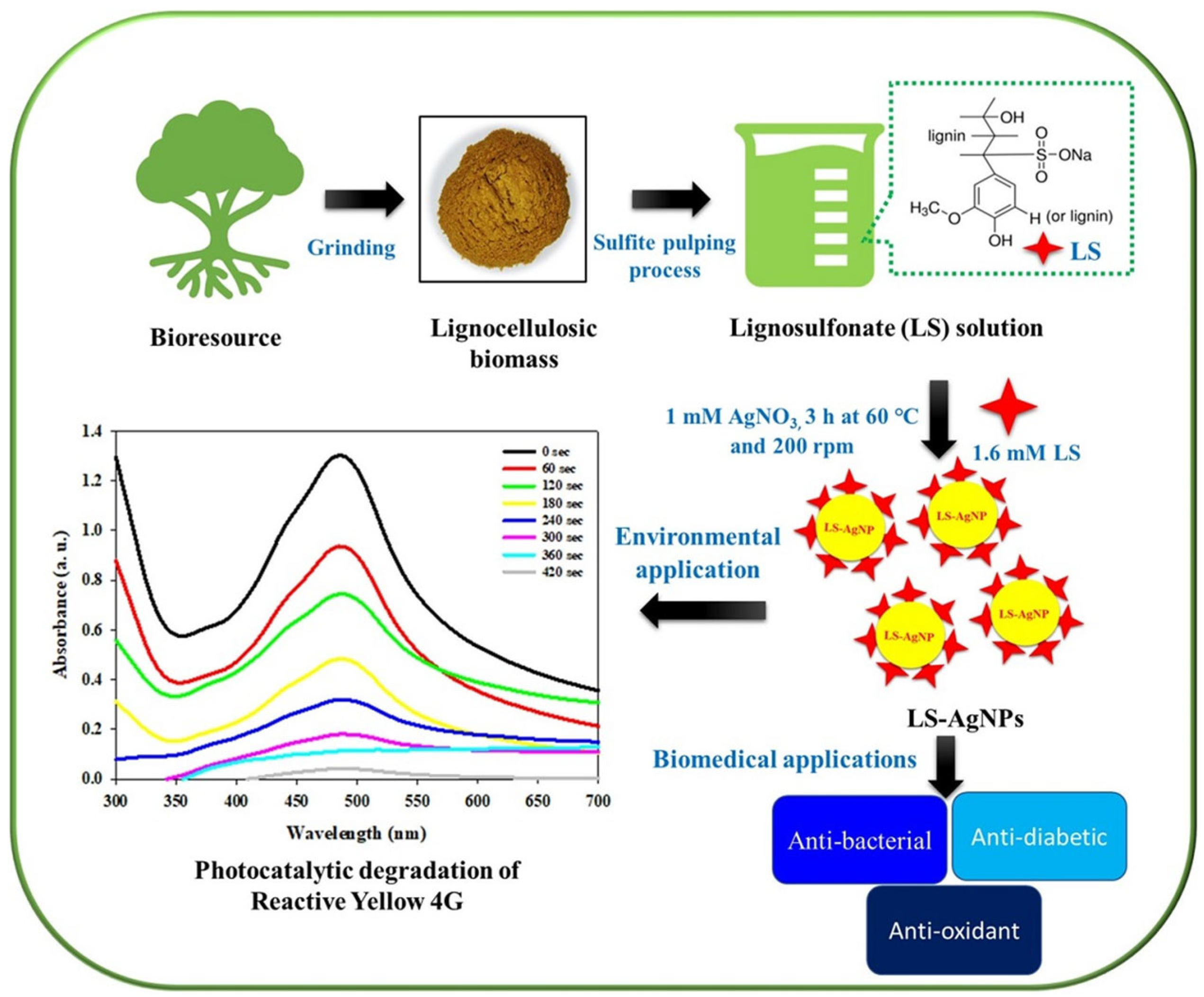
2.4.3. Lignin and Other Natural Molecules
3. Mechanisms of Antimicrobial Action
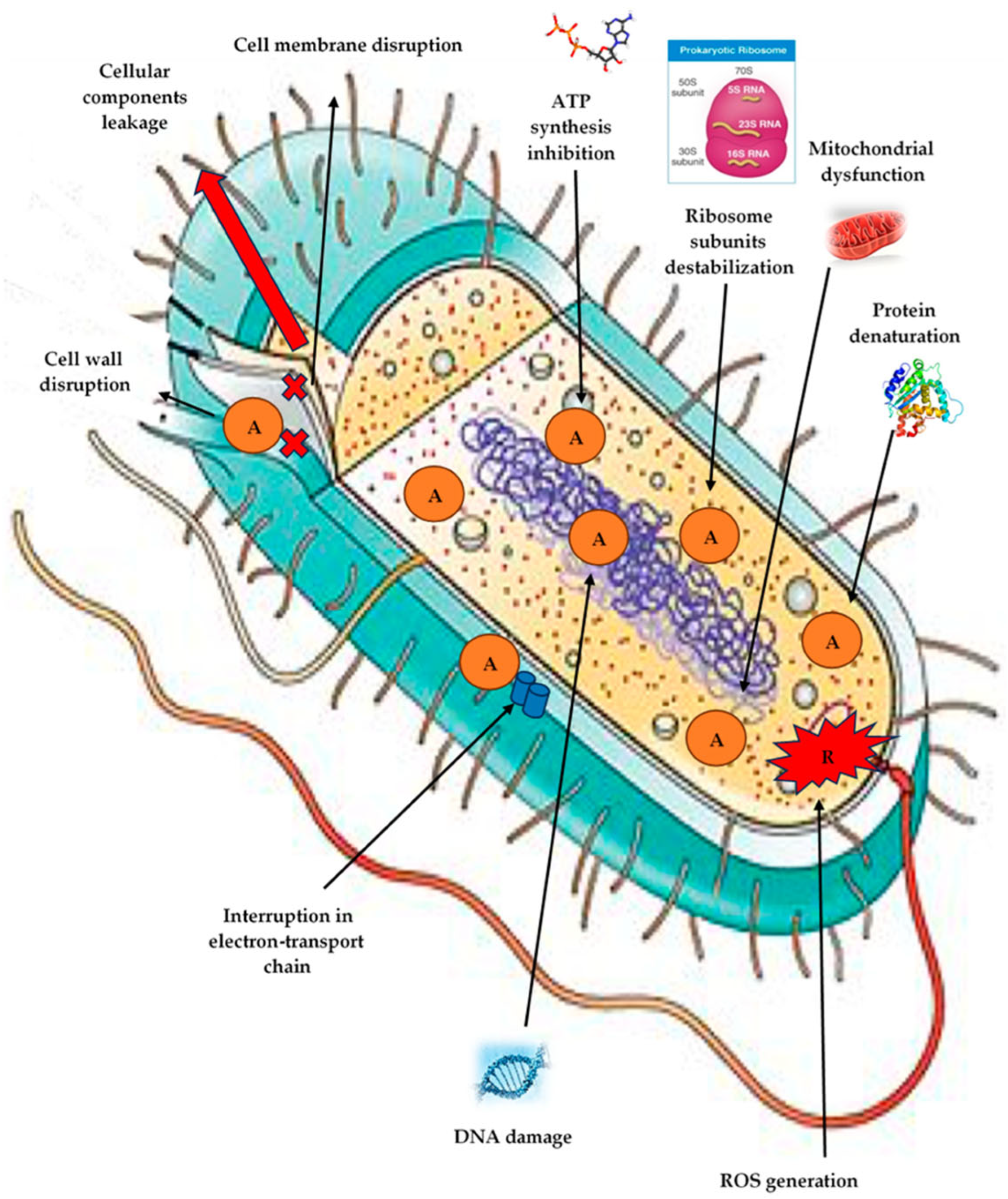
3.1. Disruption of Cell Membrane Integrity
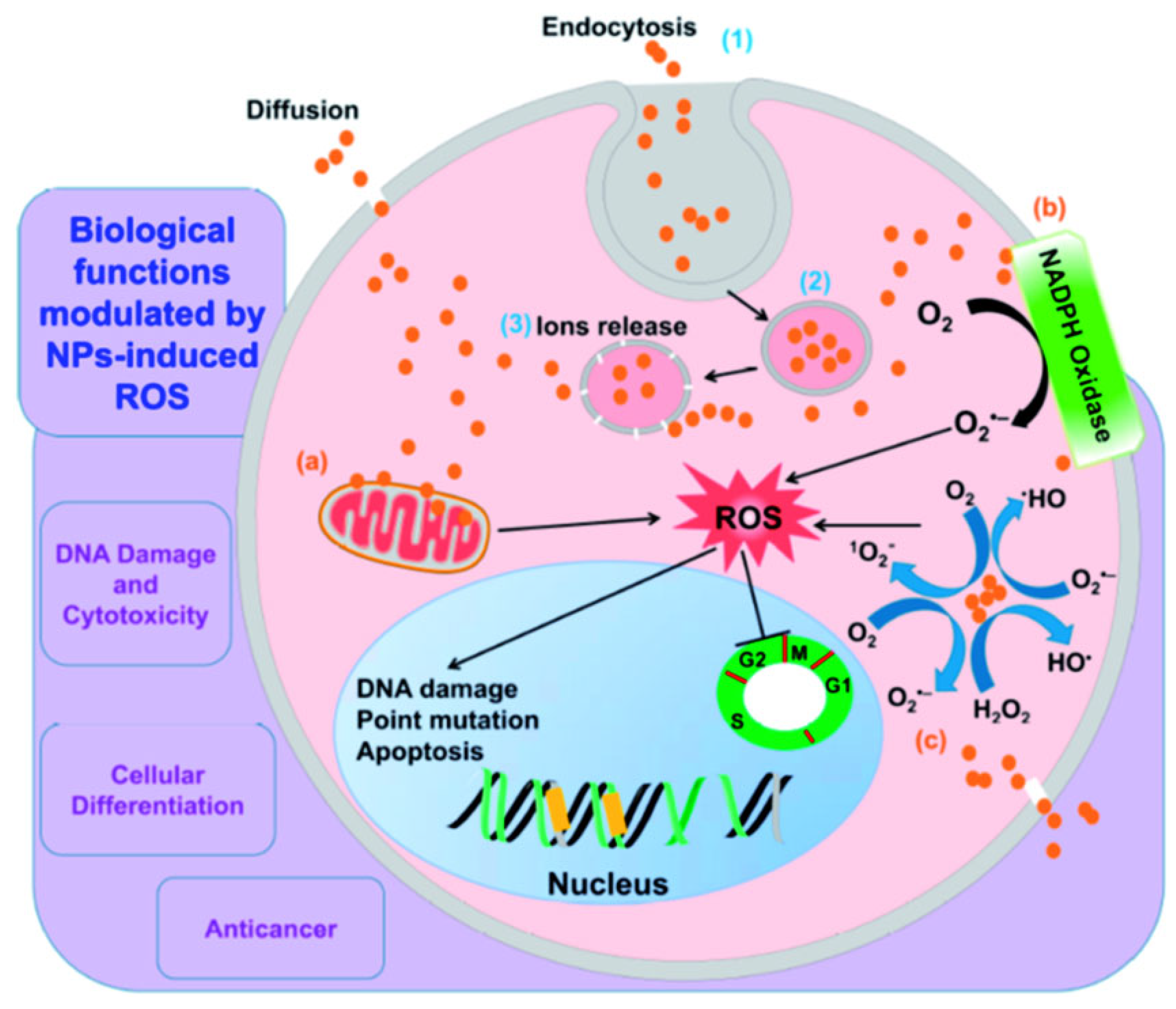
3.2. Generation of Reactive Oxygen Species (ROS)
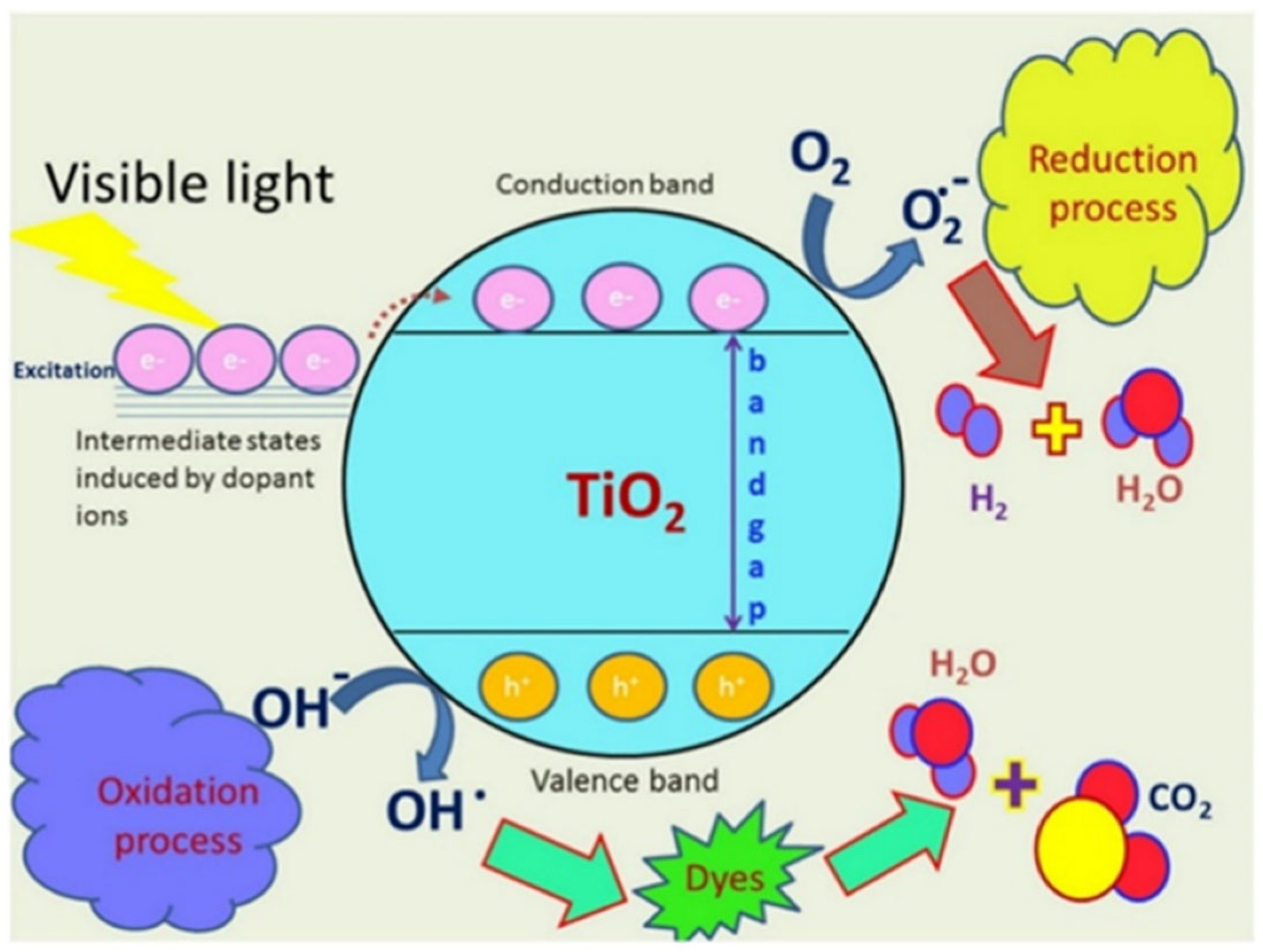
3.2.1. ROS Generation Pathway
3.2.2. Photocatalytic ROS Generation (Semiconductors like ZnO and TiO2)
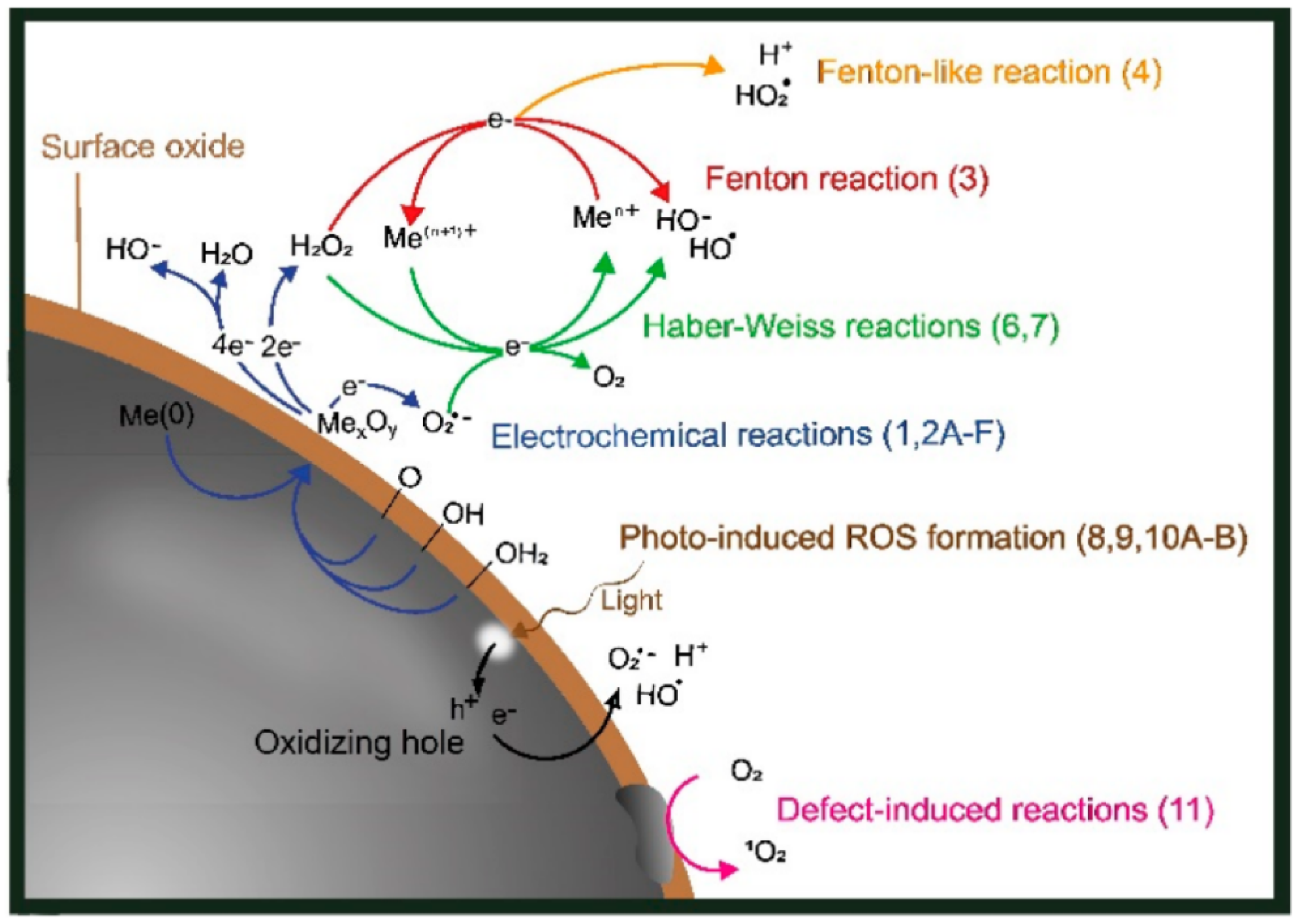
3.2.3. Fenton-like ROS GENERATION (Metal-Based Nanoparticles like Fe3O4, CuO, AgNPs)
3.3. Metal Ion Release
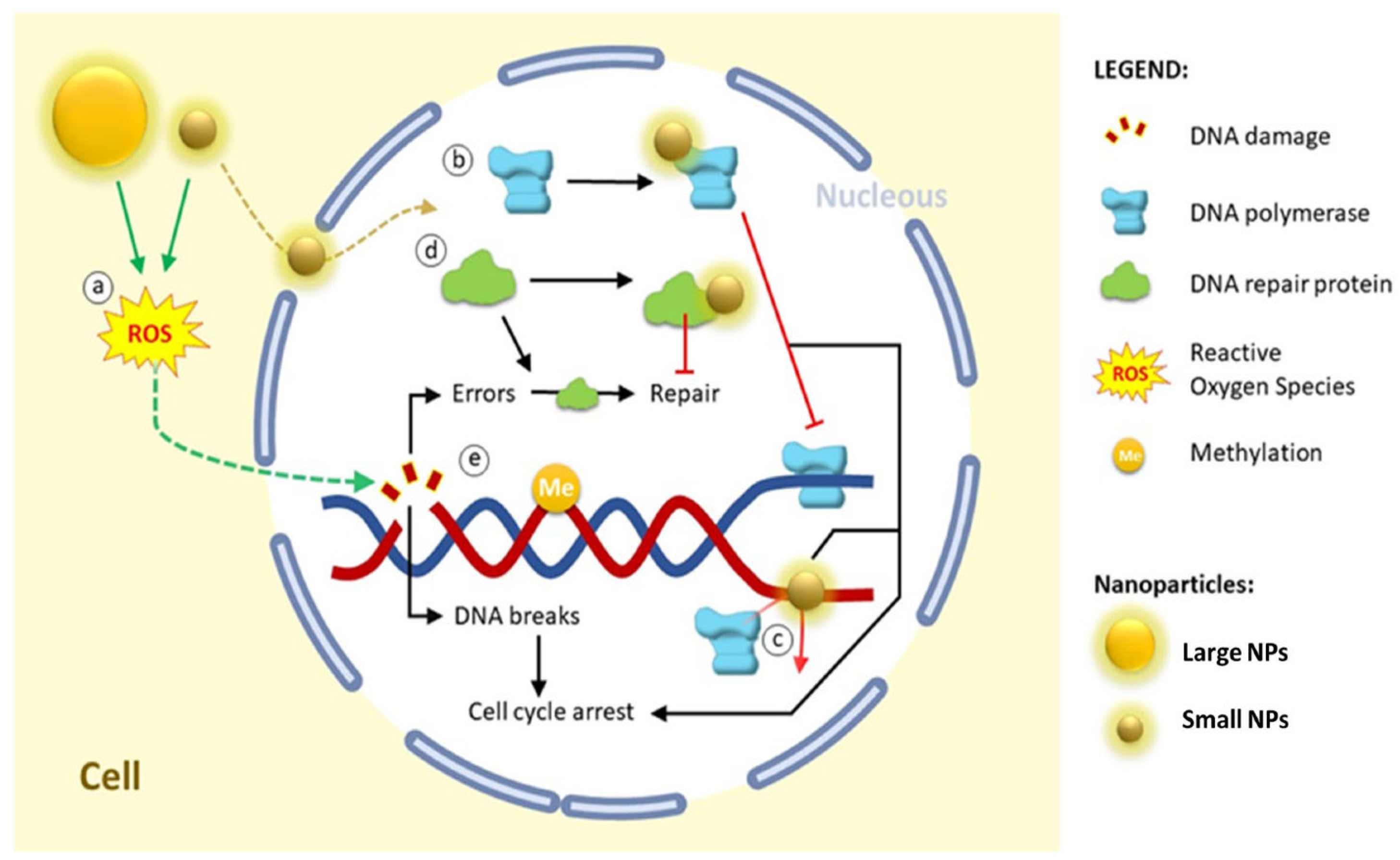
3.4. Intracellular Penetration and DNA Damage
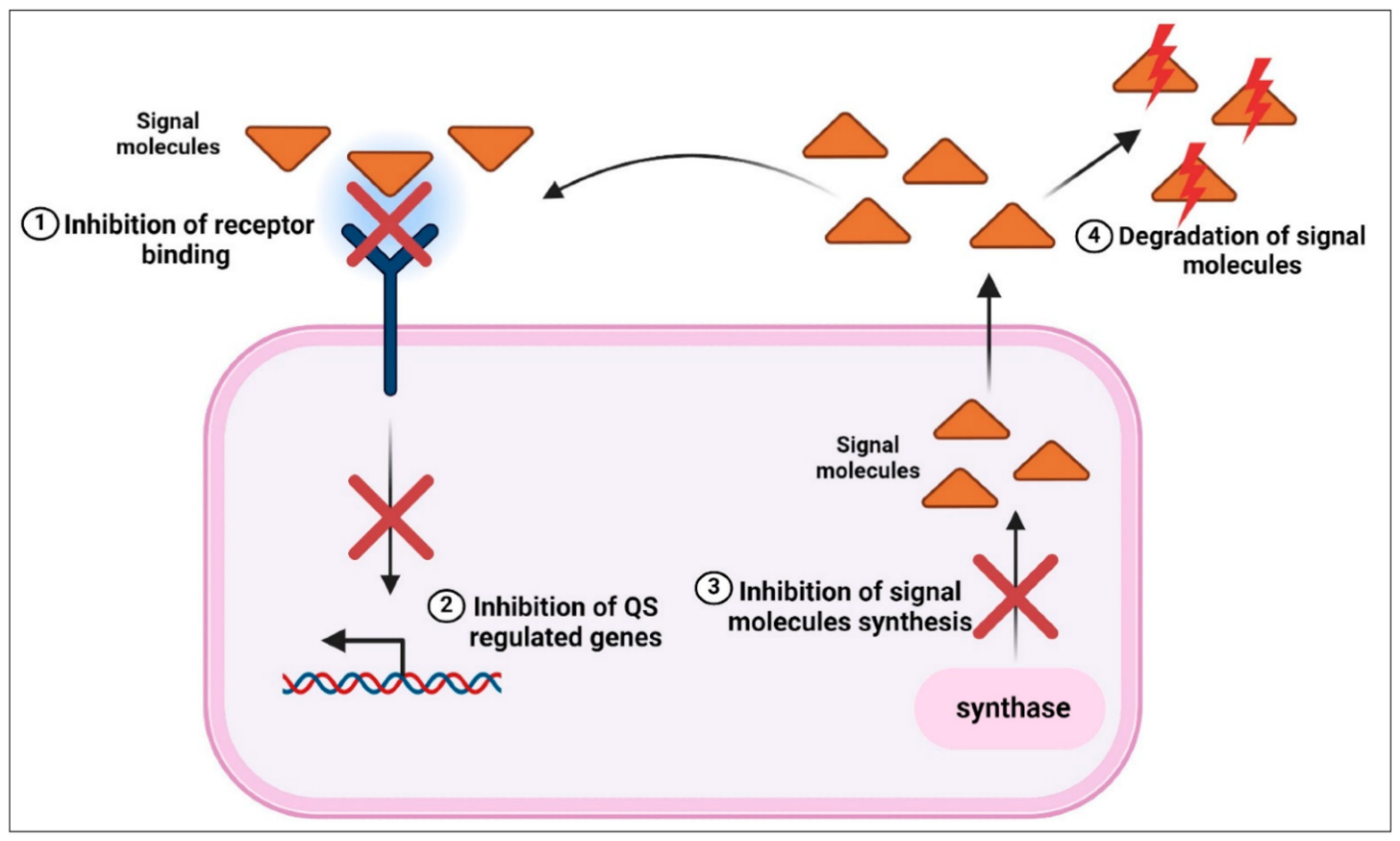
3.5. Biofilm Inhibition and Quorum-Sensing Disruption
3.6. Synergistic Effects with Phytochemicals
3.6.1. Phytochemical-Induced Amplification of ROS Generation
3.6.2. Metal Ion Chelation and Controlled Release by Phytochemical Ligands
3.6.3. Membrane Disruption via Phytochemical–Lipid Interactions
3.6.4. Phytochemical Inhibition of Resistance Pathways
3.6.5. Targeted Antimicrobial Binding Through Phytochemical Functional Groups
3.6.6. Stabilization and Biocompatibility Enhanced by Phytochemicals
4. Performance and Efficacy in Water Disinfection
4.1. Antibacterial and Antiviral Efficacy
4.2. Minimum Inhibitory Concentration (MIC) and Dose-Response
4.3. Biofilm Disruption Performance
4.4. Efficacy in Real and Variable Water Conditions
4.5. Stability and Longevity of Efficacy
5. Applications in Water Disinfection
5.1. Nanomaterial-Embedded Filters and Membranes
5.2. Point-of-Use and Household Water Treatment Devices
5.3. Photocatalytic Water Disinfection Systems
5.4. Hybrid and Smart Water Treatment Systems
6. Ecotoxicity and Environmental Safety
6.1. Cytotoxicity Concerns
6.1.1. In Vitro Evidence
6.1.2. In Vivo Evidence
6.1.3. Non-Target Effects
6.2. Environmental Safety Concerns
6.2.1. Bioaccumulation
6.2.2. Environmental Persistence
6.2.3. Regulatory Gaps
6.3. Essential Studies Before Large-Scale Deployment
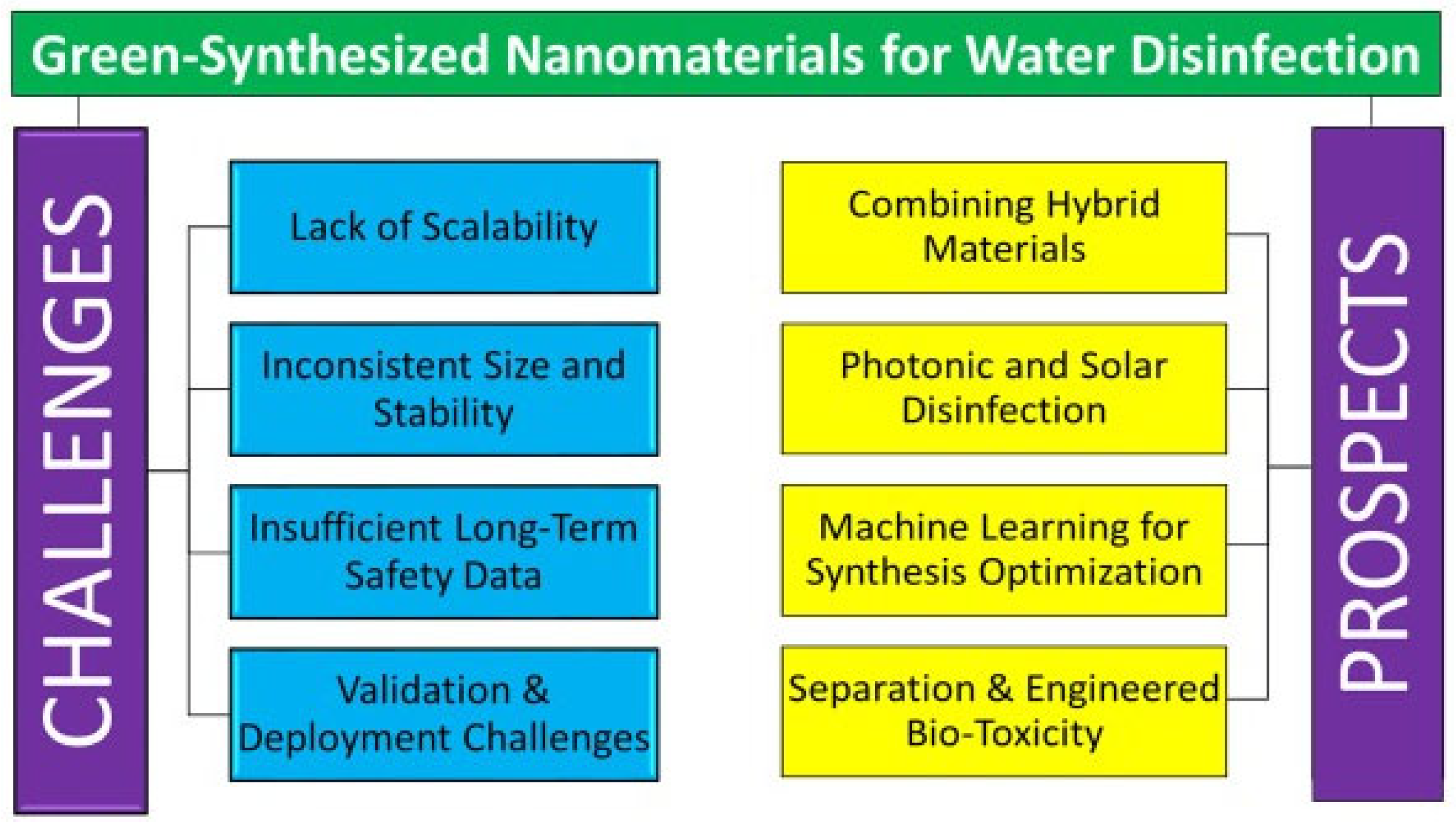
7. Challenges and Future Prospects
7.1. Challenges
7.1.1. Lack of Scalability in Green Synthesis
7.1.2. Inconsistent Nanoparticle Size and Stability
7.1.3. Insufficient Long-Term Safety Data
7.2. Future Directions
7.2.1. Hybrid Materials Combining Multiple Nanoparticles
7.2.2. Light-Activated and Solar-Powered Disinfection Systems
7.2.3. Machine Learning for Synthesis Optimization
7.2.4. Development of GSNM-Integrated Portable Filters
7.2.5. Separation, Toxicity Studies, and Genetically Engineered Biosystems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Ma, S.; Xu, M.; Yuan, M.; Li, J.; Xue, J.; Wang, M. Photo-Fenton Superwettable NiFe2O4/TA/PVDF Composite Membrane for Organic Pollutant Degradation with Successively Oil-in-Water Separation. Chemosphere 2022, 286, 131705. [Google Scholar] [CrossRef]
- UNESCO World Water Assessment Programme; Koncagül, E.; Tran, M.; Connor, R. UN World Water Development Report 2020: Water and Climate Change—Facts and Figures. 2020. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000372876.locale=en (accessed on 30 June 2025).
- Ali, N.S.; Kalash, K.R.; Ahmed, A.N.; Albayati, T.M. Performance of a Solar Photocatalysis Reactor as Pretreatment for Wastewater via UV, UV/TiO2, and UV/H2O2 to Control Membrane Fouling. Sci. Rep. 2022, 12, 16782. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Rodríguez, S.; Pardo, F.; Romero, A. Use of Fenton Reagent Combined with Humic Acids for the Removal of PFOA from Contaminated Water. Sci. Total Environ. 2016, 563–564, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.M.H.; Rahman, M.M.; Ferdush, J.; Mohotadi, M.A.A.; Mondal, J.; Uddin, M.N. State-of-the-Art Nanocomposites: Tailoring Material Properties for Next-Generation Applications. Next Res. 2025, 100865. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Umar, K.; Ahmad, A. Modified Graphene Oxide Anode: A Bioinspired Waste Material for Bioremediation of Pb2+ with Energy Generation through Microbial Fuel Cells. Chem. Eng. J. 2021, 417, 128052. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and Environmental Management through Nanotechnology: Eco-Friendly Nanomaterial Synthesis for Soil-Plant Systems, Food Safety, and Sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.-Z.; Dong, Z.-M.; Zhao, T.-L.; Zhou, G.-T.; Fu, S.-Q. Controlled Synthesis of Struvite Nanowires in Synthetic Wastewater. ACS Sustain. Chem. Eng. 2019, 7, 2035–2043. [Google Scholar] [CrossRef]
- Mousa, S.M.; Ammar, N.S.; Ibrahim, H.A. Removal of Lead Ions Using Hydroxyapatite Nano-Material Prepared from Phosphogypsum Waste. J. Saudi Chem. Soc. 2016, 20, 357–365. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-R.; Ahmad, M.S.; Ikram, M. Titanium Dioxide Nanoparticles Elicited Agro-Morphological and Physicochemical Modifications in Wheat Plants to Control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green Synthesis of Metallic Nanoparticles as Effective Alternatives to Treat Antibiotics Resistant Bacterial Infections: A Review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Trimetallic Nanoparticles: Greener Synthesis and Their Applications. Nanomaterials 2020, 10, 1784. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Iram, S.; Ahmad, K.S.; Shaheen, I.; Aljuwayid, A.M.; Ashraf, G.A. Synthesis and Analysis of Euphorbia Cognata Boiss-Assisted Organic–Inorganic Complex: Photosynthesis and Stabilization of CoO Nanoparticles. Appl. Biochem. Biotechnol. 2024, 196, 7994–8008. [Google Scholar] [CrossRef]
- Mubasshira; Rahman, M.M.; Uddin, M.N.; Rhaman, M.; Roy, S.; Sarker, M.S. Next-Generation Smart Carbon–Polymer Nanocomposites: Advances in Sensing and Actuation Technologies. Processes 2025, 13, 2991. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Bahar, M.M.; Megharaj, M.; Rahman, M.M. Recent Developments and Mechanistic Insights on Adsorption Technology for Micro- and Nanoplastics Removal in Aquatic Environments. J. Water Process Eng. 2023, 53, 103777. [Google Scholar] [CrossRef]
- Saini, N.; Sarma, M.P.; Rani, S.; Roy, M. Recent Advances in Phytochemical Based Cost-Effective Metal Oxide Nanoparticles Towards Wastewater Treatment. In Medicinal and Aromatic Plants: Current Research Status, Value-Addition to Their Waste, and Agro-Industrial Potential (Vol II); Kumar, L., Bharadvaja, N., Singh, R., Anand, R., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 173–183. ISBN 978-3-031-64601-0. [Google Scholar]
- Kah, M.; Navarro, D.; Schenkeveld, W.; Kookana, R.S.; Kirby, J.K.; Santra, S.; Ozcan, A. Fate of Copper in Soil: Effect of Agrochemical (Nano)Formulations and Soil Properties. Environ. Sci. Nano 2022, 9, 653–662. [Google Scholar] [CrossRef]
- Tan, K.; Lu, S.-Y.; Tan, K.; Ransangan, J.; Cai, X.; Cheong, K.-L. Bioactivity of Polysaccharides Derived from Bivalves. Int. J. Biol. Macromol. 2023, 250, 126096. [Google Scholar] [CrossRef] [PubMed]
- Narwal, N.; Anwar, Z.; Kakakhel, M.A. Ecotoxicological Effects of Emerging Pollutants (Nanomaterials and Microplastics) on Fish Biology. Anim. Rep. 2025, 1, 60–74. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Ghosh, M.K.; Lal, M.; Shukla, R. A Review on Plant-Mediated Synthesis of Silver Nanoparticles, Their Characterization and Applications. Nano Ex. 2021, 2, 022008. [Google Scholar] [CrossRef]
- Dipta, S.D.; Rahman, M.M.; Ansari, M.J.; Uddin, M.N. A Comprehensive Review of Sustainable and Green Additive Manufacturing: Technologies, Practices, and Future Directions. J. Manuf. Mater. Process. 2025, 9, 269. [Google Scholar] [CrossRef]
- Gunasena, M.D.K.M.; Galpaya, G.D.C.P.; Abeygunawardena, C.J.; Induranga, D.K.A.; Priyadarshana, H.V.V.; Millavithanachchi, S.S.; Bandara, P.K.G.S.S.; Koswattage, K.R. Advancements in Bio-Nanotechnology: Green Synthesis and Emerging Applications of Bio-Nanoparticles. Nanomaterials 2025, 15, 528. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plants and Plant-Based Polymers as Scaffolds for Tissue Engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant Extract Mediated Silver Nanoparticles and Their Applications as Antimicrobials and in Sustainable Food Packaging: A State-of-the-Art Review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Sarwer, Q.; Amjad, M.S.; Mehmood, A.; Binish, Z.; Mustafa, G.; Farooq, A.; Qaseem, M.F.; Abasi, F.; Pérez de la Lastra, J.M. Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine Africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities. Molecules 2022, 27, 7612. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Salem, S.S.; Alharbi, A.A.; Abdelghany, T.M. Ecofriendly Synthesis of Silver Nanoparticles Using Kei-Apple (Dovyalis caffra) Fruit and Their Efficacy against Cancer Cells and Clinical Pathogenic Microorganisms. Arab. J. Chem. 2022, 15, 103927. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Shyam, A.; Chandran, S.S.; George, B.; E., S. Plant Mediated Synthesis of AgNPs and Its Applications: An Overview. Inorg. Nano-Met. Chem. 2021, 51, 1646–1662. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.I.; Aburumman, G.A.; Gholipour, S.; Afrand, M. Nanoparticles in Water Purification: Multifunctional Roles, Challenges, and Sustainable Applications. Environ. Sci. Nano 2025, 12, 3871–3895. [Google Scholar] [CrossRef]
- Uddin, M.N.; Desai, F.; Asmatulu, E. Review of Bioaccumulation, Biomagnification, and Biotransformation of Engineered Nanomaterials. In Nanotoxicology and Nanoecotoxicology Vol. 2; Kumar, V., Guleria, P., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 133–164. ISBN 978-3-030-69492-0. [Google Scholar]
- Kalakonda, P.; Thodeti, M.; Ganneboina, C.; Ankathi, K.; Kathri, S.; Begari, K.; Kante, H.S.; Jupalli, V.; Khaderabad, Y.; Vijaylaxmi, S.; et al. Eco-Friendly Fabrication of Silver Nanoparticles for Sustainable Water Purification and Antibacterial Synergy. Plasmonics 2024, 19, 2857–2869. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Singh, R. Green Biomimetic Synthesis of Ag–TiO2 Nanocomposite Using Origanum Majorana Leaf Extract under Sonication and Their Biological Activities. Bioresour. Bioprocess. 2021, 8, 1. [Google Scholar] [CrossRef]
- Chaudhari, D.S.; Upadhyay, R.P.; Shinde, G.Y.; Gawande, M.B.; Filip, J.; Varma, R.S.; Zbořil, R. A Review on Sustainable Iron Oxide Nanoparticles: Syntheses and Applications in Organic Catalysis and Environmental Remediation. Green Chem. 2024, 26, 7579–7655. [Google Scholar] [CrossRef]
- Nadeem, H.A.; Imran, M.; Saleem, S.; Rafiq, Z.; Batool, A.; Mehmood, K.; Pervaiz, M.; Hussain, S.; Saeed, Z.; Younas, U. GO-Ag-NPs as a Promising Agent for Biomedical, Catalytic, Electrochemical Detection and Water Treatment Technologies; a Comprehensive Review. Z. Phys. Chem. 2023, 237, 1013–1076. [Google Scholar] [CrossRef]
- Singh, D.; Jain, D.; Rajpurohit, D.; Jat, G.; Kushwaha, H.S.; Singh, A.; Mohanty, S.R.; Al-Sadoon, M.K.; Zaman, W.; Upadhyay, S.K. Bacteria Assisted Green Synthesis of Copper Oxide Nanoparticles and Their Potential Applications as Antimicrobial Agents and Plant Growth Stimulants. Front. Chem. 2023, 11, 1154128. [Google Scholar] [CrossRef] [PubMed]
- Kabeerdass, N.; Al Otaibi, A.; Rajendran, M.; Manikandan, A.; Kashmery, H.A.; Rahman, M.M.; Madhu, P.; Khan, A.; Asiri, A.M.; Mathanmohun, M. Bacillus-Mediated Silver Nanoparticle Synthesis and Its Antagonistic Activity against Bacterial and Fungal Pathogens. Antibiotics 2021, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Z.; Cai, A.; Lin, X.; Jiang, X.; Zhou, B.; Wang, J.; Yao, Q.; Chen, R.; Kou, L. Nanoparticle Mediated Codelivery of Nifuratel and Doxorubicin for Synergistic Anticancer Therapy through STAT3 Inhibition. Colloids Surf. B Biointerfaces 2020, 193, 111109. [Google Scholar] [CrossRef]
- Cruz, J.N.; Muzammil, S.; Ashraf, A.; Ijaz, M.U.; Siddique, M.H.; Abbas, R.; Sadia, M.; Saba; Hayat, S.; Lima, R.R. A Review on Mycogenic Metallic Nanoparticles and Their Potential Role as Antioxidant, Antibiofilm and Quorum Quenching Agents. Heliyon 2024, 10, e29500. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Yaseen, T.; Zahra, S.A.; Shahbaz, A.; Shah, S.A.; Uddin, S.; Ma, X.; Raouf, B.; Kanwal, S.; et al. Green Synthesis of Zinc Oxide Nanoparticles Using Elaeagnus Angustifolia L. Leaf Extracts and Their Multiple In Vitro Biological Applications. Sci. Rep. 2021, 11, 20988. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Omara, M.S.; Soliman, A.M. Antifungal Activity of Mycogenic Selenium Nanoparticles Conjugated with Fungal Nano Chitosan Against Three Important Phytopathogenic Fungi. BioNanoScience 2025, 15, 229. [Google Scholar] [CrossRef]
- Malik, S.; Kumar, D. Perspectives of Nanomaterials in Microbial Remediation of Heavy Metals and Their Environmental Consequences: A Review. Biotechnol. Genet. Eng. Rev. 2024, 40, 154–201. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.; Iqbal, D.; Alharbi, R.; Jahan, S.; Darwish, O.; Alshehri, B.; Banawas, S.; Palanisamy, M.; Ismail, A.; Aldosari, S.; et al. Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes. Biology 2023, 12, 661. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- AlNadhari, S.; Al-Enazi, N.M.; Alshehrei, F.; Ameen, F. A Review on Biogenic Synthesis of Metal Nanoparticles Using Marine Algae and Its Applications. Environ. Res. 2021, 194, 110672. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green Synthesis of Silver Nanoparticles Using Marine Algae Caulerpa Racemosa and Their Antibacterial Activity against Some Human Pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Heinemann, M.G.; Rosa, C.H.; Rosa, G.R.; Dias, D. Biogenic Synthesis of Gold and Silver Nanoparticles Used in Environmental Applications: A Review. Trends Environ. Anal. Chem. 2021, 30, e00129. [Google Scholar] [CrossRef]
- Alprol, A.E.; Mansour, A.T.; Abdelwahab, A.M.; Ashour, M. Advances in Green Synthesis of Metal Oxide Nanoparticles by Marine Algae for Wastewater Treatment by Adsorption and Photocatalysis Techniques. Catalysts 2023, 13, 888. [Google Scholar] [CrossRef]
- Rahman, M.M.; Uddin, M.N.; Parvez, M.M.H.; Mohotadi, M.A.A.; Ferdush, J. Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives. Nanomaterials 2025, 15, 933. [Google Scholar] [CrossRef]
- Priyadharshini, R.; Abirami, A.; Rajeshkumar, S. Green and Cost-Effective Nanoparticles Synthesis from Medicinally Important Aquatic Plants and Their Applications. In Secondary Metabolites from Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-321372-7. [Google Scholar]
- Kashid, S.; Joshi, K. Biopolymer-Based Nanomaterials: Synthesis, Characterization, and Applications. In Innovations in Green Nanoscience and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-331915-3. [Google Scholar]
- El-Sharkawy, R.M.; Swelim, M.A.; Hamdy, G.B. Aspergillus Tamarii Mediated Green Synthesis of Magnetic Chitosan Beads for Sustainable Remediation of Wastewater Contaminants. Sci. Rep. 2022, 12, 9742. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Parvez, M.A.K.; Balusamy, S.R.; Rahman, M.M.; Kim, J.H.; Akter, S. Chitosan-Coated Polymeric Silver and Gold Nanoparticles: Biosynthesis, Characterization and Potential Antibacterial Applications: A Review. Polymers 2022, 14, 5302. [Google Scholar] [CrossRef]
- Mirda, E.; Idroes, R.; Khairan, K.; Tallei, T.E.; Ramli, M.; Earlia, N.; Maulana, A.; Idroes, G.M.; Muslem, M.; Jalil, Z. Synthesis of Chitosan-Silver Nanoparticle Composite Spheres and Their Antimicrobial Activities. Polymers 2021, 13, 3990. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, X.; Shi, H.; Ma, T.; Tian, C.; Chen, Y.; Chen, H.; Chen, X.; Luo, K.; Cai, L.; et al. Green Synthesis of an Alginate-Coated Silver Nanoparticle Shows High Antifungal Activity by Enhancing Its Cell Membrane Penetrating Ability. ACS Appl. Bio Mater. 2019, 2, 4087–4096. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Pérez-Marroquín, X.A.; Aguirre-Cruz, G.; Campos-Lozada, G.; Callejas-Quijada, G.; León-López, A.; Campos-Montiel, R.G.; García-Hernández, L.; Méndez-Albores, A.; Vázquez-Durán, A.; Aguirre-Álvarez, G. Green Synthesis of Silver Nanoparticles for Preparation of Gelatin Films with Antimicrobial Activity. Polymers 2022, 14, 3453. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, S.; Mehta, M.; Patel, Y.; Patel, R.; Shah, D.; Patel, D.; Shah, U.; Patel, M.; Patel, S.; et al. A Review on Synthetic Investigation for Quinoline- Recent Green Approaches. Green Chem. Lett. Rev. 2022, 15, 337–372. [Google Scholar] [CrossRef]
- Figueiredo, A.d.O.; de Figueiredo G, A. Sustainable Development and Health: The Regulation of Nanomaterials in Brazil. Ann. Bioeth. Clin. Appl. 2021, 4, 000209. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener Synthesis of Lignin Nanoparticles and Their Applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Magotra, V.K.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Palem, R.R.; et al. Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities. Polymers 2022, 14, 648. [Google Scholar] [CrossRef]
- Andraos, J. Inclusion of Environmental Impact Parameters in Radial Pentagon Material Efficiency Metrics Analysis: Using Benign Indices as a Step Towards a Complete Assessment of “Greenness” for Chemical Reactions and Synthesis Plans. Org. Process Res. Dev. 2012, 16, 1482–1506. [Google Scholar] [CrossRef]
- Andraos, J.; Mastronardi, M.L.; Hoch, L.B.; Hent, A. Critical Evaluation of Published Algorithms for Determining Environmental and Hazard Impact Green Metrics of Chemical Reactions and Synthesis Plans. ACS Sustain. Chem. Eng. 2016, 4, 1934–1945. [Google Scholar] [CrossRef]
- Khanam, S.A.; Zaki, M.E.A.; Islam, S.; Saikia, S.; Bania, K.K. Green Chemistry Approaches for Sustainable Synthesis of Inorganic Nanomaterials. In Nanomaterial Green Synthesis; Al-Khayri, J.M., Anju, T.R., Jain, S.M., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 255–284. ISBN 978-3-031-84643-4. [Google Scholar]
- Sangwan, A.; Singh, S.P.; Singh, P.; Gupta, O.P.; Manas, A.; Gupta, S. Role of Molecular Techniques in PMI Estimation: An Update. J. Forensic Leg. Med. 2021, 83, 102251. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Amin, M.; Haider, M.N.; Malik, S.; Malik, H.A.; Alam, M.A.; Xu, J.; Alessa, A.H.; Khan, A.Z.; Boopathy, R. Wastewater-Grown Algal Biomass as Carbon-Neutral, Renewable, and Low Water Footprint Feedstock for Clean Energy and Bioplastics. Curr. Pollut. Rep. 2024, 10, 172–188. [Google Scholar] [CrossRef]
- Nain, R.; Patel, H.; Chahar, M.; Kumar, S.; Rohilla, D.; Pal, M. Biosynthesized Metallic Nanoparticles for Sustainable Environmental Remediation: Mechanisms, Applications, and Future Perspectives. Discov. Chem. 2025, 2, 124. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formations via Cross-Coupling and Atom-Economic Addition Reactions. Achievements and Challenges. Chem. Rev. 2022, 122, 16110–16293. [Google Scholar] [CrossRef]
- Leininger, A.; Chen, J.; Ramaswami, A.; Ren, Z.J. Urban Circular Carbon Economy through Electrochemically Influenced Microbiomes. One Earth 2023, 6, 278–289. [Google Scholar] [CrossRef]
- Dakshinamoorthy, D.; Weinstock, A.K.; Damodaran, K.; Iwig, D.F.; Mathers, R.T. Diglycerol-Based Polyesters: Melt Polymerization with Hydrophobic Anhydrides. ChemSusChem 2014, 7, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Akinhanmi, F.O.; Aworunse, O.S.; Oyewole, O.A.; Adetunji, C.O.; Mathew, J.T.; Igiku, V.; Eniola, K.I.T.; Yerima, M.B. Non-Target Effect, Environmental Impact, and Assessment of Nanobiofertilizer. In Handbook of Agricultural Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 253–270. ISBN 978-1-394-23476-9. [Google Scholar]
- Murínová, S.; Dercová, K. Response Mechanisms of Bacterial Degraders to Environmental Contaminants on the Level of Cell Walls and Cytoplasmic Membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef] [PubMed]
- Lithi, I.J.; Ahmed Nakib, K.I.; Chowdhury, A.M.S.; Sahadat Hossain, M. A Review on the Green Synthesis of Metal (Ag, Cu, and Au) and Metal Oxide (ZnO, MgO, Co3O4, and TiO2) Nanoparticles Using Plant Extracts for Developing Antimicrobial Properties. Nanoscale Adv. 2025, 7, 2446–2473. [Google Scholar] [CrossRef]
- Verma, D.K.; Sharma, A.; Awasthi, L.; Singh, H.; Kumar, P.; Rajput, P.; Sinha, A.; Chaubey, K.K.; Kumar, A.; Rai, N.; et al. Recent Development and Importance of Nanoparticles in Disinfection and Pathogen Control. In Nanomaterials for Environmental and Agricultural Sectors; Bachheti, R.K., Bachheti, A., Husen, A., Eds.; Springer Nature: Singapore, 2023; pp. 83–106. ISBN 978-981-9928-74-3. [Google Scholar]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress Bacterial Cell Death Mediated by Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Koh, T.W.; Bhatnagar, S. Synthesis, Characterization, Antibacterial and Wound Healing Efficacy of Silver Nanoparticles from Azadirachta indica. Front. Microbiol. 2021, 12, 611560. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.M.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Plasma Membrane Is the Target of Rapid Antibacterial Action of Silver Nanoparticles in Escherichia coli and Pseudomonas aeruginosa. Int. J. Nanomed. 2018, 13, 6779–6790. [Google Scholar] [CrossRef] [PubMed]
- Bin Mobarak, M.; Foysal Sikder, M.; Sidratul Muntaha, K.; Islam, S.; Fazle Rabbi, S.M.; Chowdhury, F. Plant Extract-Mediated Green-Synthesized CuO Nanoparticles for Environmental and Microbial Remediation: A Review Covering Basic Understandings to Mechanistic Study. Nanoscale Adv. 2025, 7, 2418–2445. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.S.; Kulkarni, G.R.; Khater, S.S.; Gholap, H.; Patil, R. Study to Elucidate Effect of Titanium Dioxide Nanoparticles on Bacterial Membrane Potential and Membrane Permeability. Mater. Res. Express 2020, 7, 035005. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef]
- Rikta, S.Y. 9—Application of Nanoparticles for Disinfection and Microbial Control of Water and Wastewater. In Nanotechnology in Water and Wastewater Treatment; Ahsan, A., Ismail, A.F., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–176. ISBN 978-0-12-813902-8. [Google Scholar]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Mayegowda, S.B.; Sarma, G.; Gadilingappa, M.N.; Alghamdi, S.; Aslam, A.; Refaat, B.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A.; et al. Green-Synthesized Nanoparticles and Their Therapeutic Applications: A Review. Green Process. Synth. 2023, 12, 20230001. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Waiskopf, N.; Ben-Shahar, Y.; Banin, U. Photocatalytic Hybrid Semiconductor–Metal Nanoparticles; from Synergistic Properties to Emerging Applications. Adv. Mater. 2018, 30, 1706697. [Google Scholar] [CrossRef]
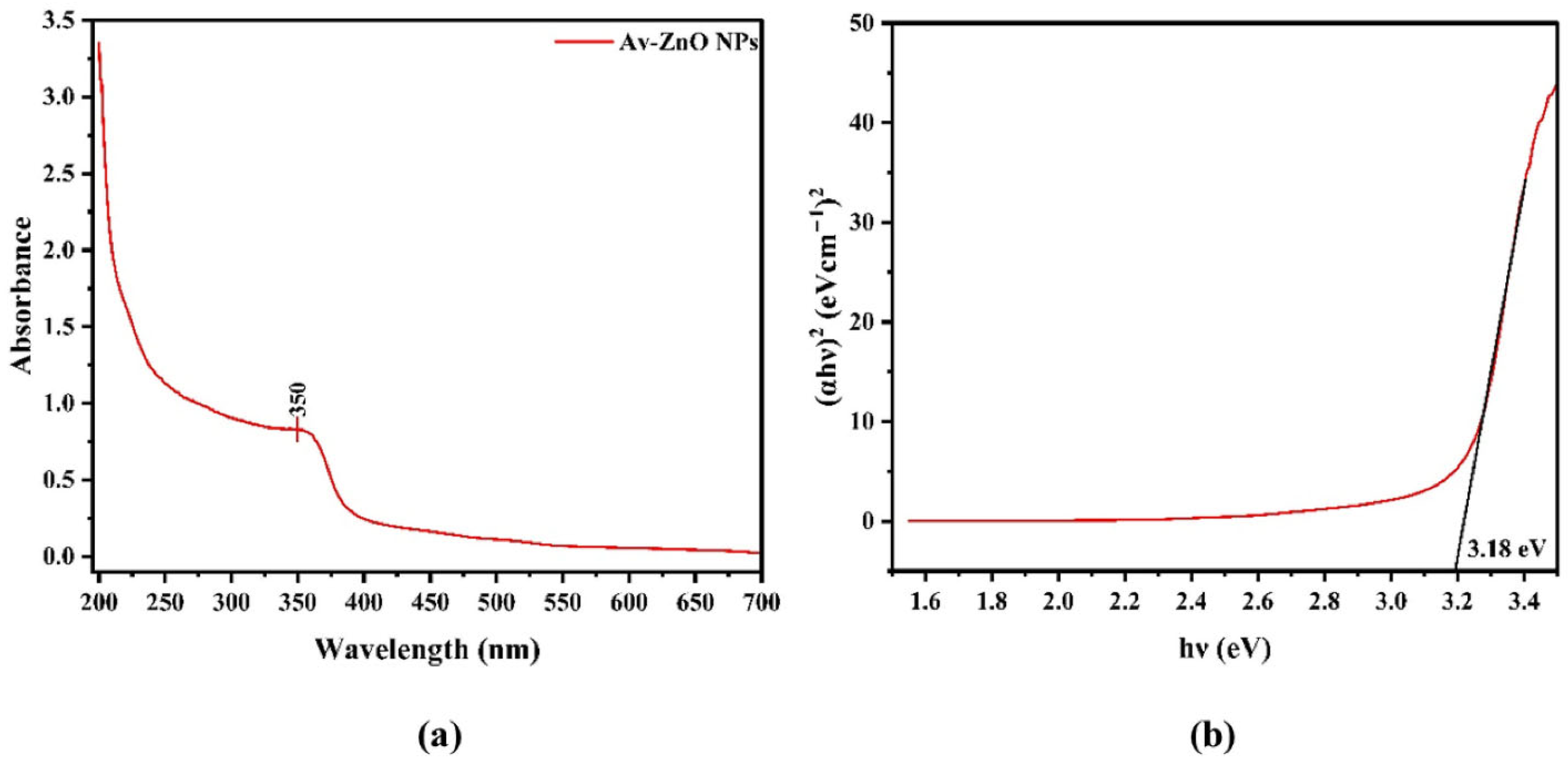
- Rashid, M.H.; Sujoy, S.I.; Rahman, M.S.; Haque, M.J. Aloe Vera Assisted Green Synthesis of Ag and Cu Co-Doped ZnO Nanoparticles and a Comprehensive Analysis of Their Structural, Morphological, Optical, Electrical and Antibacterial Properties. Heliyon 2024, 10, e25438. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Santagostino, S.F.; Assenmacher, C.-A.; Tarrant, J.C.; Adedeji, A.O.; Radaelli, E. Mechanisms of Regulated Cell Death: Current Perspectives. Vet. Pathol. 2021, 58, 596–623. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Manu, B.; Sreenivasa, M.Y.; Manoj, A. Synthesis of Plant-Based Biogenic Jarosite Nanoparticles Using Azadirachta indica and Eucalyptus gunni Leaf Extracts and Its Application in Fenton Degradation of Dicamba. Water Sci. Eng. 2024, 17, 157–165. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Souto, R.S.; Silva, V.C.P.; Prescilio, I.C.; Kauffmann, A.C.; Soares, M.A.; de Souza, J.R.; Bakuzis, A.F.; Fontana, L.C. Biosynthesis of Cube-Shaped Fe3O4 Nanoparticles for Removal of Dyes Using Fenton Process. Water Air Soil. Pollut. 2021, 232, 270. [Google Scholar] [CrossRef]
- Ma, H.; Wallis, L.K.; Diamond, S.; Li, S.; Canas-Carrell, J.; Parra, A. Impact of Solar UV Radiation on Toxicity of ZnO Nanoparticles through Photocatalytic Reactive Oxygen Species (ROS) Generation and Photo-Induced Dissolution. Environ. Pollut. 2014, 193, 165–172. [Google Scholar] [CrossRef]
- Kang, X.; Dong, G.; Dong, T. Oxygen Vacancy Defect Engineering of Heterophase Junction TiO2: Interfacial/Surface Oxygen Vacancies Coadjust the Photocatalytic ROS Production. ACS Appl. Energy Mater. 2023, 6, 1025–1036. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A Review on Metal Ions Modified TiO2 for Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Shrestha, S.; Tiwari, L.; Dhungana, S.; Maharjan, J.; Khadka, D.; Kim, A.A.; Pokhrel, M.R.; Baral, J.; Park, M.; Poudel, B.R. Exploring Photocatalytic, Antimicrobial and Antioxidant Efficacy of Green-Synthesized Zinc Oxide Nanoparticles. Nanomaterials 2025, 15, 858. [Google Scholar] [CrossRef]
- Blosi, M.; Brigliadori, A.; Zanoni, I.; Ortelli, S.; Albonetti, S.; Costa, A.L. Chlorella vulgaris Meets TiO2 NPs: Effective Sorbent/Photocatalytic Hybrid Materials for Water Treatment Application. J. Environ. Manag. 2022, 304, 114187. [Google Scholar] [CrossRef]
- Cao, C.; Wang, X.; Yang, N.; Song, X.; Dong, X. Recent Advances of Cancer Chemodynamic Therapy Based on Fenton/Fenton-like Chemistry. Chem. Sci. 2022, 13, 863–889. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Zúñiga-Miranda, J.; Guerra, J.; Mueller, A.; Mayorga-Ramos, A.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Heredia-Moya, J.; Guamán, L.P. Iron Oxide Nanoparticles: Green Synthesis and Their Antimicrobial Activity. Nanomaterials 2023, 13, 2919. [Google Scholar] [CrossRef] [PubMed]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS Induced Lipid Peroxidation and Their Role in Ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive Oxygen and Nitrogen Species–Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Ghosh, S.K. Apoptosis-Inducing Factor-like Protein-Mediated Stress and Metronidazole-Responsive Programmed Cell Death Pathway in Entamoeba Histolytica. Mol. Microbiol. 2023, 119, 640–658. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Ma, Y.; Zhao, Q.; Jing, B.; Yu, M.; Yang, N.; Yang, A.; Shen, Q.; Wang, Y.; et al. Green Synthesis, Biomedical Effects, and Future Trends of Ag/ZnO Bimetallic Nanoparticles: An Update. Nanotechnol. Rev. 2025, 14, 20250186. [Google Scholar] [CrossRef]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen Sulfide in Physiology and Pathogenesis of Bacteria and Viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Khina, A.G.; Krutyakov, Y.A. Similarities and Differences in the Mechanism of Antibacterial Action of Silver Ions and Nanoparticles. Appl. Biochem. Microbiol. 2021, 57, 683–693. [Google Scholar] [CrossRef]
- Deng, F.; Olvera-Vargas, H.; Zhou, M.; Qiu, S.; Sirés, I.; Brillas, E. Critical Review on the Mechanisms of Fe2+ Regeneration in the Electro-Fenton Process: Fundamentals and Boosting Strategies. Chem. Rev. 2023, 123, 4635–4662. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Jan Iftikhar, F.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Bhattacharjee, B.; Ghose, S.; Tamilanban, T.; Barman, D.; Ahmed, A.B.; Sahu, R.K. Current Status and Future Prospect of Bioremediation Using Green Synthesis of Nanoparticle/Nanomaterials. In Nanomaterials as a Catalyst for Biofuel Production; Dave, V., Kuila, A., Eds.; Springer Nature: Singapore, 2025; pp. 295–327. ISBN 978-981-9617-06-7. [Google Scholar]
- Samejo, S.; Baig, J.A.; Kazi, T.G.; Afridi, H.I.; Hol, A.; Dahshan, A.; Akhtar, K.; Solangi, S.A.; Perveen, S.; Hussain, S. The Green Synthesis of Magnesium Oxide Nanocomposite-Based Solid Phase for the Extraction of Arsenic, Cadmium, and Lead from Drinking Water. Anal. Methods 2023, 15, 3863–3873. [Google Scholar] [CrossRef] [PubMed]
- Tsuneo, I. Anti-Bacterial Mechanism for Metallic Ag+, Cu2+, Zn2+ Ions-Induced Bactertiolysis on Disruptive OM Lpp and PGN Inhibitive Elongations Against S. aureus and E. coli. Mathews J. Cytol. Histol. 2022, 6, 18. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef]
- Casals, E.; Gusta, M.F.; Bastus, N.; Rello, J.; Puntes, V. Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections. Microorganisms 2025, 13, 952. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Roy, S.; Rhim, J.-W. Enhanced Functionality of Green Synthesized Sulfur Nanoparticles Using Kiwifruit (Actinidia deliciosa) Peel Polyphenols as Capping Agents. J. Nanostructure Chem. 2022, 12, 389–399. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Part. Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- Iqbal, J.; Anwar, F.; Afridi, S. Targeted Drug Delivery Systems and Their Therapeutic Applications in Cancer and Immune Pathological Conditions. Infect. Disord.-Drug Targets 2017, 17, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Fatima, S.; Ain, Q.U.; Shafiq, Z.; Ashraf Janjua, M.R.S. A Review on Green Synthesis of Silver Nanoparticles (SNPs) Using Plant Extracts: A Multifaceted Approach in Photocatalysis, Environmental Remediation, and Biomedicine. RSC Adv. 2025, 15, 3858–3903. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zheng, X.; Hu, J.; Ma, S.; Li, K.; Chen, J.; Xu, X.; Lu, X.; Wang, X. Recent Advances of Cell Membrane-Coated Nanoparticles for Therapy of Bacterial Infection. Front. Microbiol. 2023, 14, 1083007. [Google Scholar] [CrossRef]
- Girma, A. Alternative Mechanisms of Action of Metallic Nanoparticles to Mitigate the Global Spread of Antibiotic-Resistant Bacteria. Cell Surf. 2023, 10, 100112. [Google Scholar] [CrossRef]
- Mondal, M. Green Synthesis of Silver Nanoparticles Using Azadirachta indica Leaf Extract for the Application in Cotton Fibres as Antibacterial Coatings. 2021. Available online: http://lib.buet.ac.bd:8080/xmlui/handle/123456789/6649 (accessed on 15 July 2025).
- Suganthy, N.; Sri Ramkumar, V.; Pugazhendhi, A.; Benelli, G.; Archunan, G. Biogenic Synthesis of Gold Nanoparticles from Terminalia Arjuna Bark Extract: Assessment of Safety Aspects and Neuroprotective Potential via Antioxidant, Anticholinesterase, and Antiamyloidogenic Effects. Environ. Sci. Pollut. Res. 2018, 25, 10418–10433. [Google Scholar] [CrossRef]
- Alabresm, A.; Decho, A.W.; Lead, J. A Novel Method to Estimate Cellular Internalization of Nanoparticles into Gram-Negative Bacteria: Non-Lytic Removal of Outer Membrane and Cell Wall. NanoImpact 2021, 21, 100283. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef]
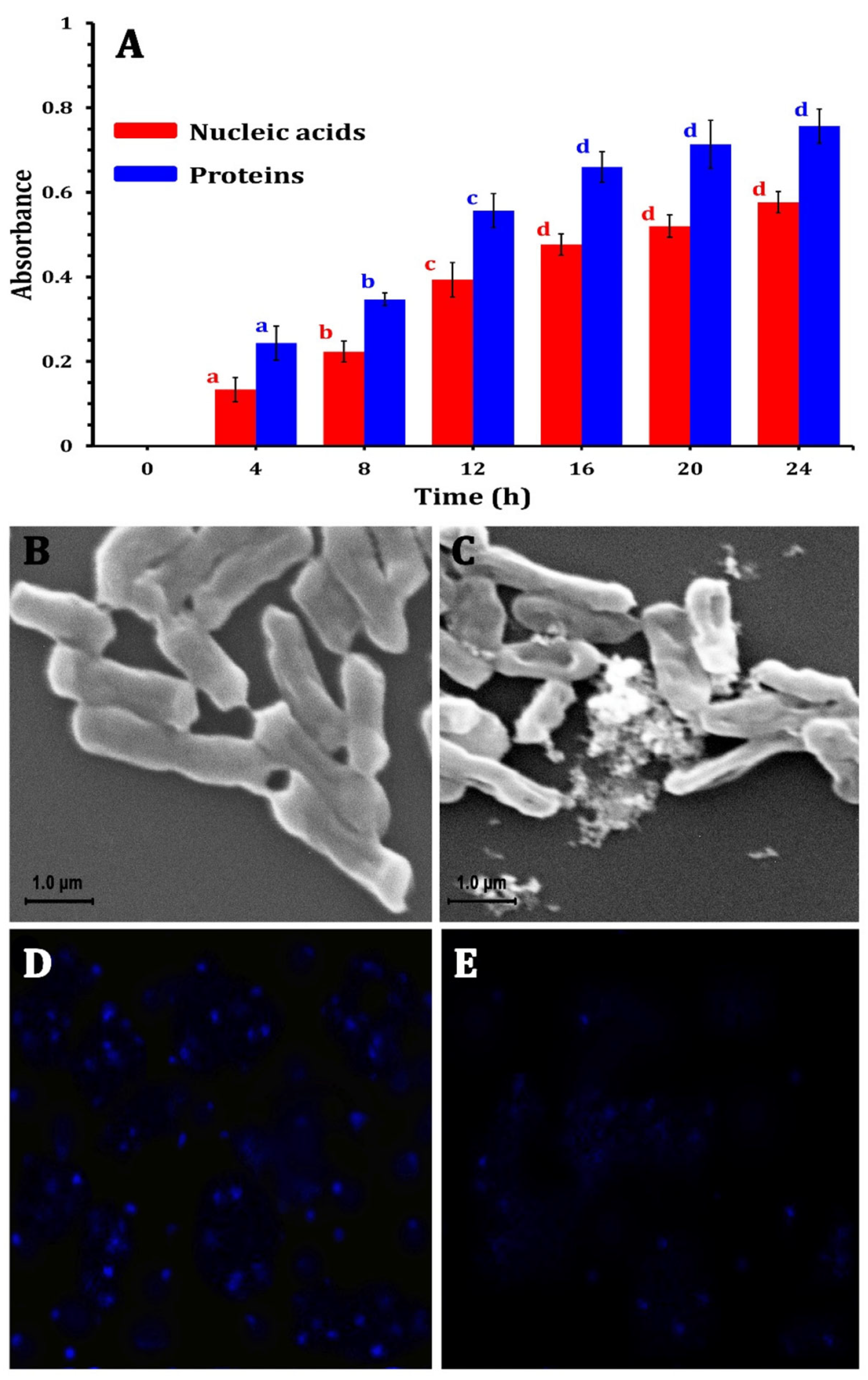
- Encinas-Gimenez, M.; Martin-Duque, P.; Martín-Pardillos, A. Cellular Alterations Due to Direct and Indirect Interaction of Nanomaterials with Nucleic Acids. Int. J. Mol. Sci. 2024, 25, 1983. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I. Green Synthesis of Silver Nanoparticles Using Carum Copticum: Assessment of Its Quorum Sensing and Biofilm Inhibitory Potential against Gram Negative Bacterial Pathogens. Microb. Pathog. 2020, 144, 104172. [Google Scholar] [CrossRef]
- Elumalai, P.; Gao, X.; Cui, J.; Kumar, A.S.; Dhandapani, P.; Parthipan, P.; Karthikeyan, O.P.; Theerthagiri, J.; Kheawhom, S.; Choi, M.Y. Biofilm Formation, Occurrence, Microbial Communication, Impact and Characterization Methods in Natural and Anthropic Systems: A Review. Environ. Chem. Lett. 2024, 22, 1297–1326. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Rashed, Z.I.; Alharbi, A.A.; Alsharef, S.; Alkindy, T.T.; Alkhamali, A.; Albalawi, A.S.; Battah, B.; Donadu, M.G. Quorum Sensing Inhibitors: An Alternative Strategy to Win the Battle against Multidrug-Resistant (MDR) Bacteria. Molecules 2024, 29, 3466. [Google Scholar] [CrossRef]
- Majeed, H.; Iftikhar, T.; Nadeem, M.A.; Nazir, M.A. Green Synthesis of Eucalyptus Globulus Zinc Nanoparticles and Its Use in Antimicrobial Insect Repellent Paint Formulation in Bulk Industrial Production. Heliyon 2024, 10, e24467. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Scope of Plant-Based Nanoparticles as Antibacterial and Antibiofilm Agents. In Nanotechnology Horizons in Food Process Engineering; Apple Academic Press: Palm Bay, FL, USA, 2023; ISBN 978-1-00-330537-8. [Google Scholar]
- Marpu, S.B.; Benton, E.N. Shining Light on Chitosan: A Review on the Usage of Chitosan for Photonics and Nanomaterials Research. Int. J. Mol. Sci. 2018, 19, 1795. [Google Scholar] [CrossRef]
- Vetrivel, A.; Vetrivel, P.; Dhandapani, K.; Natchimuthu, S.; Ramasamy, M.; Madheswaran, S.; Murugesan, R. Inhibition of Biofilm Formation, Quorum Sensing and Virulence Factor Production in Pseudomonas aeruginosa PAO1 by Selected LasR Inhibitors. Int. Microbiol. 2023, 26, 851–868. [Google Scholar] [CrossRef]
- Aleksic, I.; Ristivojevic, P.; Pavic, A.; Radojević, I.; Čomić, L.R.; Vasiljevic, B.; Opsenica, D.; Milojković-Opsenica, D.; Senerovic, L. Anti-Quorum Sensing Activity, Toxicity in Zebrafish (Danio rerio) Embryos and Phytochemical Characterization of Trapa natans Leaf Extracts. J. Ethnopharmacol. 2018, 222, 148–158. [Google Scholar] [CrossRef]
- Roy, D.; Nag, M.; Bhattacharya, D.; Lahiri, D. Microbiologically Synthesized Nanoparticles in Inhibition of Quorum Sensing. In Natural Products; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-330055-7. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antibacterial Activity of Green Synthesized Silver Nanomaterials with Colistin Antibiotic against Multidrug-Resistant Bacterial Pathogens. Crystals 2022, 12, 1057. [Google Scholar] [CrossRef]
- Rîmbu, M.C.; Cord, D.; Savin, M.; Grigoroiu, A.; Mihăilă, M.A.; Gălățanu, M.L.; Ordeanu, V.; Panțuroiu, M.; Țucureanu, V.; Mihalache, I.; et al. Harnessing Plant-Based Nanoparticles for Targeted Therapy: A Green Approach to Cancer and Bacterial Infections. Int. J. Mol. Sci. 2025, 26, 7022. [Google Scholar] [CrossRef]
- Elattar, K.M.; Ghoniem, A.A.; Al-Otibi, F.O.; Fakhouri, A.S.; Helmy, Y.A.; Saber, W.I.A.; Hassan, M.A.E.; Elsayed, A. Eco-Friendly Synthesis of Ag/CeO2 and CuO/CeO2 Nanocomposites Using Curcuma Longa Extract and Assessment of Their Antioxidant, Antifungal, and Cytotoxic Activities. RSC Adv. 2025, 15, 12100–12116. [Google Scholar] [CrossRef]
- Murugan, S.; Senthilvelan, T.; Govindasamy, M.; Thangavel, K. A Comprehensive Review on Exploring the Potential of Phytochemicals and Biogenic Nanoparticles for the Treatment of Antimicrobial-Resistant Pathogenic Bacteria. Curr. Microbiol. 2025, 82, 90. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, H.; Batool, I.; Taj, M.B.; Ali, O.M.; Alnajeebi, A.M.; Yahya, R.; Alelwani, W.; Alsabban, M.M.; Kabrah, A.; Alghamdi, S. Phyto-Mechanochemical Synthesis of Iron Oxide Nanoparticles Using Polygonum Paronychioides and Their Pesticidal and Antimicrobial Applications. New J. Chem. 2025, 49, 11705–11728. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Gopinath, N.; Nair, B.G. Ecofriendly Biosynthesis of Copper Nanoparticles from Novel Marine S. Rhizophila Species for Enhanced Antibiofilm, Antimicrobial and Antioxidant Potential. Microb. Pathog. 2024, 194, 106836. [Google Scholar] [CrossRef]
- Singh, N.B.; Susan, M.A.B.H.; Guin, M. Applications of Green Synthesized Nanomaterials in Water Remediation. Curr. Pharm. Biotechnol. 2021, 22, 733–761. [Google Scholar] [CrossRef]
- Şahin, B.; Bülbül, A.; Çelik, İ.; Korkmaz, N.; Karadağ, A. Investigation of Biological Activities of Plant Extract and Green Synthesis Silvernanoparticles Obtained from Gilaburu (Viburnum opulus L.) Fruits. Turk. J. Chem. 2022, 46, 224–235. [Google Scholar] [CrossRef]
- Tahir, S.; Tahir, N.; Hussain, T.; Naseem, Z.; Zahid, M.; Mustafa, G. Non-Photocatalytic and Photocatalytic Inactivation of Viruses Using Antiviral Assays and Antiviral Nanomaterials. In Viral and Antiviral Nanomaterials; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-313664-4. [Google Scholar]
- Thamer, H.; Ibraheem, H.; Alsayed, R.; Makia, R.; Kadhom, M.; Salman, H.; Yousif, E. Synergistic Antimicrobial Potential: Exploring the Efficacy of Poly Methacrylic Acid (PMAA) Polymers, CuO NPs, and Pomegranate Peel Extract against Bacterial Pathogens. Results Chem. 2024, 7, 101365. [Google Scholar] [CrossRef]
- Alherz, F.A.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Haggag, E.M.; Alqahtani, M.J.; Hussein, I.A. Silver Nanoparticles Prepared Using Encephalartos Laurentianus De Wild Leaf Extract Have Inhibitory Activity against Candida Albicans Clinical Isolates. J. Fungi 2022, 8, 1005. [Google Scholar] [CrossRef]
- Elabbasy, M.T.; El Bayomi, R.M.; Abdelkarim, E.A.; Hafez, A.E.-S.E.; Othman, M.S.; Ghoniem, M.E.; Samak, M.A.; Alshammari, M.H.; Almarshadi, F.A.; Elsamahy, T.; et al. Antibacterial and Antibiofilm Activity of Green-Synthesized Zinc Oxide Nanoparticles Against Multidrug-Resistant Escherichia coli Isolated from Retail Fish. Molecules 2025, 30, 768. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative Strategies Toward the Disassembly of the EPS Matrix in Bacterial Biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ghosh, S.; Nayak, B. Phyto-Fabricated Nanoparticles and Their Anti-Biofilm Activity: Progress and Current Status. Front. Nanotechnol. 2021, 3, 739286. [Google Scholar] [CrossRef]
- Husain, F.M.; Qais, F.A.; Ahmad, I.; Hakeem, M.J.; Baig, M.H.; Masood Khan, J.; Al-Shabib, N.A. Biosynthesized Zinc Oxide Nanoparticles Disrupt Established Biofilms of Pathogenic Bacteria. Appl. Sci. 2022, 12, 710. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.S.A.; Altaf, M.; Hossain, I.; El Sayed, M.E.; Kallel, M.; El-Bahy, Z.M.; Rehman, A.U.; Najam, T.; Nazir, M.A. Activated Carbon Derived from Biomass for Wastewater Treatment: Synthesis, Application and Future Challenges. J. Anal. Appl. Pyrolysis 2024, 179, 106480. [Google Scholar] [CrossRef]
- Wang, X.; Liang, D.; Wang, Y.; Peijnenburg, W.J.G.M.; Monikh, F.A.; Zhao, X.; Dong, Z.; Fan, W. A Critical Review on the Biological Impact of Natural Organic Matter on Nanomaterials in the Aquatic Environment. Carbon Res. 2022, 1, 13. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Rahman, A. Integrated Approaches of Arsenic Remediation from Wastewater: A Comprehensive Review of Microbial, Bio-Based, and Advanced Technologies. Toxics 2025, 13, 768. [Google Scholar] [CrossRef]
- Qubtia, M.; Ghumman, S.A.; Noreen, S.; Hameed, H.; Noureen, S.; Kausar, R.; Irfan, A.; Akhtar Shah, P.; Afzal, H.; Hameed, M.; et al. Evaluation of Plant-Based Silver Nanoparticles for Antioxidant Activity and Promising Wound-Healing Applications. ACS Omega 2024, 9, 12146–12157. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Zaghloul, S.; Hashem, M.; El-Shafei, A. A Green Approach to Improve the Antibacterial Properties of Cellulose Based Fabrics Using Moringa Oleifera Extract in Presence of Silver Nanoparticles. Cellulose 2021, 28, 549–564. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, D.; Deb, N.; Swarnakar, S.; Banerjee, S. Application of Green Synthesized ZnO Nanoparticle Coated Ceramic Ultrafiltration Membrane for Remediation of Pharmaceutical Components from Synthetic Water: Reusability Assay of Treated Water on Seed Germination. J. Environ. Chem. Eng. 2020, 8, 103803. [Google Scholar] [CrossRef]
- Abebe, L.S.; Su, Y.-H.; Guerrant, R.L.; Swami, N.S.; Smith, J.A. Point-of-Use Removal of Cryptosporidium Parvum from Water: Independent Effects of Disinfection by Silver Nanoparticles and Silver Ions and by Physical Filtration in Ceramic Porous Media. Environ. Sci. Technol. 2015, 49, 12958–12967. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Negrini, B. Towards Safe and Sustainable Nano-Antimicrobials through a Multidisciplinary Integrated Approach: Application to Two Novel Copper Oxide Nanoparticles-Enabled Case-Studies. Ph.D. Thesis, Università Degli Studi di Milano-Bicocca, Milano, Italy, 2025. [Google Scholar]
- Song, S.H.; Kim, C.-M.; Khirul, M.A.; Ahmad, I.; Jee, H.; Chuah, C.Y.; Park, J.; Chae, K.-J.; Yang, E. Silver Nanoparticle-Decorated Reduced Graphene Oxide/Nanocrystalline Titanium Metal-Organic Frameworks Composite Membranes with Enhanced Nanofiltration Performance and Photocatalytic Ability. Desalination Water Treat. 2024, 320, 100836. [Google Scholar] [CrossRef]
- Băjan, M.; Cursaru, D.L.; Mihai, S. The Impact of Graphene Oxide Nanoparticles Decorated with Silver Nanoparticles (GrO/AgNP) on the Cellulose Acetate (CA) Membrane Matrix Used for Hydrocarbon Removal from Water. Membranes 2025, 15, 158. [Google Scholar] [CrossRef]
- Shahzad, A.; Oh, J.-M.; Azam, M.; Iqbal, J.; Hussain, S.; Miran, W.; Rasool, K. Advances in the Synthesis and Application of Anti-Fouling Membranes Using Two-Dimensional Nanomaterials. Membranes 2021, 11, 605. [Google Scholar] [CrossRef]
- Gloria-Garza, M.A.; Reyna-Martínez, G.R.; Jiménez-Salas, Z.; Campos-Góngora, E.; Kačániová, M.; Aguirre-Cavazos, D.E.; Bautista-Villarreal, M.; Leos-Rivas, C.; Elizondo-Luevano, J.H. Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties. Plants 2025, 14, 1390. [Google Scholar] [CrossRef]
- Khan, H.; Jan, Z.; Ullah, I.; Alwabli, A.; Alharbi, F.; Habib, S.; Islam, M.; Shin, B.-J.; Lee, M.Y.; Koo, J. A Deep Dive into AI Integration and Advanced Nanobiosensor Technologies for Enhanced Bacterial Infection Monitoring. Nanotechnol. Rev. 2024, 13, 20240056. [Google Scholar] [CrossRef]
- Tończyk, A.; Niedziałkowska, K.; Lisowska, K. Ecotoxic Effect of Mycogenic Silver Nanoparticles in Water and Soil Environment. Sci. Rep. 2025, 15, 10815. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles in Therapeutics and Beyond: A Review of Mechanism Insights and Applications. Nanomaterials 2024, 14, 1618. [Google Scholar] [CrossRef]
- Maheswaran, H.; Djearamane, S.; Dhanapal, A.C.T.A.; Wong, L.S. Cytotoxicity of Green Synthesized Zinc Oxide Nanoparticles Using Musa Acuminata on Vero Cells. Heliyon 2024, 10, e31316. [Google Scholar] [CrossRef]
- Al-Asiri, W.Y.; Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Malik, T.; Ahmad, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Cytotoxic and Apoptotic Effects of Green Synthesized Silver Nanoparticles via Reactive Oxygen Species–Mediated Mitochondrial Pathway in Human Breast Cancer Cells. Cell Biochem. Funct. 2024, 42, e4113. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; AL-Abdan, M.; Albasher, G.; Alarifi, S. Effects of Green Silver Nanoparticles on Apoptosis and Oxidative Stress in Normal and Cancerous Human Hepatic Cells In Vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Singh, M. Green Synthesis of Silver Nanoparticles: Methods, Biological Applications, Delivery and Toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Fazel, U.A.; Hasan, A.Y. Inhibitory Effect of the Biosynthesized ZnO Nanoparticles by Musa Acuminata Fruits Peels against Pathogenic Bacteria Isolated from Diabetic Foot Ulcer. Acad. Sci. J. 2024, 2, 31–50. [Google Scholar] [CrossRef]
- Srujana, S.; Bhagat, D. Chemical-Based Synthesis of ZnO Nanoparticles and Their Applications in Agriculture. Nanotechnol. Environ. Eng. 2022, 7, 269–275. [Google Scholar] [CrossRef]
- Ghazy, N.A.; Abd El-Hafez, O.A.; El-Bakery, A.M.; El-Geddawy, D.I.H. Impact of Silver Nanoparticles and Two Biological Treatments to Control Soft Rot Disease in Sugar Beet (Beta vulgaris L). Egypt. J. Biol. Pest Control 2021, 31, 3. [Google Scholar] [CrossRef]
- Chaki, S.H.; Malek, T.J.; Chaudhary, M.D.; Tailor, J.P.; Deshpande, M.P. Magnetite Fe3O4 Nanoparticles Synthesis by Wet Chemical Reduction and Their Characterization. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 035009. [Google Scholar] [CrossRef]
- Di Bona, K.R.; Xu, Y.; Gray, M.; Fair, D.; Hayles, H.; Milad, L.; Montes, A.; Sherwood, J.; Bao, Y.; Rasco, J.F. Short- and Long-Term Effects of Prenatal Exposure to Iron Oxide Nanoparticles: Influence of Surface Charge and Dose on Developmental and Reproductive Toxicity. Int. J. Mol. Sci. 2015, 16, 30251–30268. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Sun, W.; Too, H.Z.; Laserna, A.K.C.; Li, S.F.Y. A Global Metabolomic Insight into the Oxidative Stress and Membrane Damage of Copper Oxide Nanoparticles and Microparticles on Microalga Chlorella vulgaris. Environ. Pollut. 2020, 258, 113647. [Google Scholar] [CrossRef]
- Hanslik, L. Microplastics in Limnic Ecosystems—Investigation of Biological Fate and Effects of Microplastic Particles and Associated Contaminants in Zebrafish (Danio rerio). Available online: https://archiv.ub.uni-heidelberg.de/volltextserver/29130/ (accessed on 22 September 2025).
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological Effects of Carbon Based Nanomaterials in Aquatic Organisms. Sci. Total Environ. 2018, 619–620, 328–337. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Balu, K.S.; Karthik, S.; Prabhu, M.; Rajendran, V.; Aicher, W.K.; Maaza, M. A Sensitive Refining of In Vitro and In Vivo Toxicological Behavior of Green Synthesized ZnO Nanoparticles from the Shells of Jatropha curcas for Multifunctional Biomaterials Development. Ecotoxicol. Environ. Saf. 2019, 184, 109621. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraj, F.; Alotibi, I.; Al-Zahrani, M.; Albiheyri, R.; Alghamdi, M.A.; Nass, N.M.; Abd-Ellatif, S.; Makhlof, R.T.M.; Alsaad, M.A.; Sajer, B.H.; et al. Green Synthesis of Chitosan-Capped Gold Nanoparticles Using Salvia Officinalis Extract: Biochemical Characterization and Antimicrobial and Cytotoxic Activities. Molecules 2023, 28, 7762. [Google Scholar] [CrossRef] [PubMed]
- Bebrevska, L.; Foubert, K.; Hermans, N.; Chatterjee, S.; Van Marck, E.; De Meyer, G.; Vlietinck, A.; Pieters, L.; Apers, S. In Vivo Antioxidative Activity of a Quantified Pueraria lobata Root Extract. J. Ethnopharmacol. 2010, 127, 112–117. [Google Scholar] [CrossRef] [PubMed]
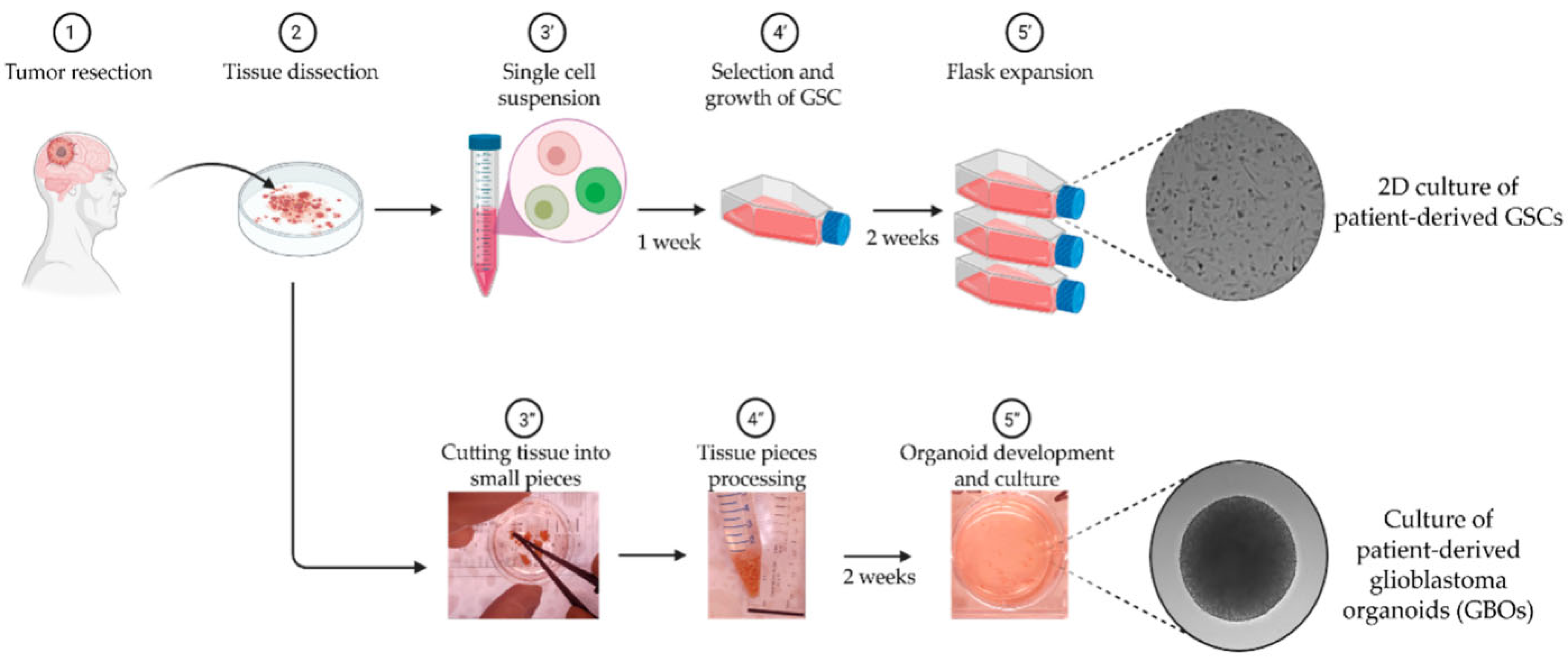
- Lenin, S.; Ponthier, E.; Scheer, K.G.; Yeo, E.C.F.; Tea, M.N.; Ebert, L.M.; Oksdath Mansilla, M.; Poonnoose, S.; Baumgartner, U.; Day, B.W.; et al. A Drug Screening Pipeline Using 2D and 3D Patient-Derived In Vitro Models for Pre-Clinical Analysis of Therapy Response in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 4322. [Google Scholar] [CrossRef]
- Eltwisy, H.O.; Abdel-Fattah, M.; Elsisi, A.M.; Omar, M.M.; Abdelmoteleb, A.A.; El-Mokhtar, M.A. Pathogenesis of Staphylococcus Haemolyticus on Primary Human Skin Fibroblast Cells. Virulence 2020, 11, 1142–1157. [Google Scholar] [CrossRef]
- Sharma, N.K.; Vishwakarma, J.; Rai, S.; Alomar, T.S.; AlMasoud, N.; Bhattarai, A. Green Route Synthesis and Characterization Techniques of Silver Nanoparticles and Their Biological Adeptness. ACS Omega 2022, 7, 27004–27020. [Google Scholar] [CrossRef]
- Heydari, M.; Babaei, A.; Arab-Bafrani, Z.; Zabihi, E. Facile Method to Covalently-Jointed ZnO-NPs with Chitosan by Using GPTMS: A New Strategy to Decrease the Cytotoxicity of ZnO-NPs. Mater. Technol. 2022, 37, 673–683. [Google Scholar] [CrossRef]
- Nagini, S.; Palrasu, M.; Bishayee, A. Limonoids from Neem (Azadirachta indica A. Juss.) Are Potential Anticancer Drug Candidates. Med. Res. Rev. 2024, 44, 457–496. [Google Scholar] [CrossRef]
- Asmaz, A.; Ustun-Alkan, F.; Kalaycilar, I.B.; Kucukdeniz, T.; Özbaş, F.; Karakuş, S. Sonosynthesis and Artificial Intelligence-Enhanced Characterization of PEGylated Camellia Sinensis-Encapsulated Zinc Oxide Nanoparticles: Cytotoxicity Capacity against Cervical Cancer and Fibroblast Cells. J. Taibah Univ. Sci. 2024, 18, 2363579. [Google Scholar] [CrossRef]
- Dutta, G.; Ghosh, D.; Venkatesan, K.; Chakrabarti, G.; Sugumaran, A.; Narayanasamy, D. Biogenic Synthesis of Copper Oxide Nanoparticles: Comprehensive In Vitro Profiling for Cervical Cancer Treatment and Antibacterial Strategies. New J. Chem. 2024, 48, 10697–10716. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green Synthesis of Silver Nanoparticles, Their Characterization, Application and Antibacterial Activity. Int. J. Environ. Res. Public Health 2013, 10, 5221–5238. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, H.; Tu, X.; Zhang, Z. Assessing In Vivo Toxicity of Graphene Materials: Current Methods and Future Outlook. Nanomedicine 2014, 9, 1565–1580. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.F.M.; Sedky, N.K.; Nafie, M.S.; Mahdy, N.K.; Fawzy, I.M.; Fayed, T.W.; Preis, E.; Bakowsky, U.; Fahmy, S.A. Sustainable Nanomedicine: Enhancement of Asplatin’s Cytotoxicity In Vitro and In Vivo Using Green-Synthesized Zinc Oxide Nanoparticles Formed via Microwave-Assisted and Gambogic Acid-Mediated Processes. Molecules 2024, 29, 5327. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, Y.; Zhao, C.; Tian, Q.-W.; Wang, Q.; He, G.-W.; Lun, L.-M.; Xuan, C. Hematological Parameters and Early-Onset Coronary Artery Disease: A Retrospective Case–Control Study Based on 3366 Participants. Ther. Adv. Chronic Dis. 2023, 14, 20406223221142670. [Google Scholar] [CrossRef]
- Alharbi, F.N.; Abaker, Z.M.; Makawi, S.Z.A. Phytochemical Substances—Mediated Synthesis of Zinc Oxide Nanoparticles (ZnO NPS). Inorganics 2023, 11, 328. [Google Scholar] [CrossRef]
- Wang, X.; Shan, M.; Zhang, S.; Chen, X.; Liu, W.; Chen, J.; Liu, X. Stimuli-Responsive Antibacterial Materials: Molecular Structures, Design Principles, and Biomedical Applications. Adv. Sci. 2022, 9, 2104843. [Google Scholar] [CrossRef]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. Int. J. Mol. Sci. 2024, 25, 4057. [Google Scholar] [CrossRef]
- Li, H.; Wen, T.; Wang, T.; Ji, Y.; Shen, Y.; Chen, J.; Xu, H.; Wu, X. In Vivo Metabolic Response upon Exposure to Gold Nanorod Core/Silver Shell Nanostructures: Modulation of Inflammation and Upregulation of Dopamine. Int. J. Mol. Sci. 2020, 21, 384. [Google Scholar] [CrossRef] [PubMed]
- Thejaswini, M.; Lakshmi Ranganatha, V.; Vasanth Patil, H.B.; Pramila, S.; Nagaraju, G.; Mallikarjunaswamy, C. Phyto-Mediated Facile Synthesis of ZnO Nanoparticles: Enhanced Photocatalysis, Biological, and Electrochemical Properties. Ionics 2024, 30, 6611–6629. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Mohammed, H.A.; Khan, R.A.; Singh, V.; Bouazzaoui, A.; Yusuf, M.; Akhtar, N.; Khan, M.; Al-Subaiyel, A.; Mohammed, S.A.A.; et al. Nano-Scale Delivery: A Comprehensive Review of Nano-Structured Devices, Preparative Techniques, Site-Specificity Designs, Biomedical Applications, Commercial Products, and References to Safety, Cellular Uptake, and Organ Toxicity. Nanotechnol. Rev. 2021, 10, 1493–1559. [Google Scholar] [CrossRef]
- Singaravelu, S.; Motsoene, F.; Abrahamse, H.; Dhilip Kumar, S.S. Green-Synthesized Metal Nanoparticles: A Promising Approach for Accelerated Wound Healing. Front. Bioeng. Biotechnol. 2025, 13, 1637589. [Google Scholar] [CrossRef]
- Malik, P.; Rani, R.; Khan, S.; Fernandes, D.; Mukherjee, T.K. Green Essence of Plant Resources Capped Zinc Oxide Nanoparticles: Renewable Distinctions, Size-Shape-Modulated Physicochemical Diversity and Emerging Biomedical-Environmental Usefulness. Part. Part. Syst. Charact. 2025, 42, 2400145. [Google Scholar] [CrossRef]
- Ozbek, O.; Genc, D.E.; Ulgen, K.O. Advances in Physiologically Based Pharmacokinetic (PBPK) Modeling of Nanomaterials. ACS Pharmacol. Transl. Sci. 2024, 7, 2251–2279. [Google Scholar] [CrossRef]
- Johnstone, I. Organisation for Economic Co-Operation and Development (OECD). Yearb. Int. Environ. Law 2023, 34, yvae026. [Google Scholar] [CrossRef]
- Adak, T.; Swain, H.; Munda, S.; Mukherjee, A.K.; Yadav, M.K.; Sundaram, A.; Bag, M.K.; Rath, P.C. Green Silver Nano-Particles: Synthesis Using Rice Leaf Extract, Characterization, Efficacy, and Non-Target Effects. Environ. Sci. Pollut. Res. 2021, 28, 4452–4462. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates With Different Life Strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Huang, Q. Study of the Toxicity of ZnO Nanoparticles to Chlorella sorokiniana under the Influence of Phosphate: Spectroscopic Quantification, Photosynthetic Efficiency and Gene Expression Analysis. Environ. Sci. Nano 2020, 7, 1431–1443. [Google Scholar] [CrossRef]
- Biba, R.; Košpić, K.; Komazec, B.; Markulin, D.; Cvjetko, P.; Pavoković, D.; Peharec Štefanić, P.; Tkalec, M.; Balen, B. Surface Coating-Modulated Phytotoxic Responses of Silver Nanoparticles in Plants and Freshwater Green Algae. Nanomaterials 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.-R.; Raja, N.I. Biogenesis of Silver Nanoparticles to Treat Cancer, Diabetes, and Microbial Infections: A Mechanistic Overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document on Aquatic and Sediment Toxicological Testing of Nanomaterials. Available online: https://www.oecd.org/en/publications/guidance-document-on-aquatic-and-sediment-toxicological-testing-of-nanomaterials_08905c99-en.html (accessed on 11 July 2025).
- Testing of Chemicals. Available online: https://www.oecd.org/en/topics/testing-of-chemicals.html (accessed on 20 September 2025).
- ISO/TS 23302:2021; Nanotechnologies—Requirements and Recommendations for the Identification of Measurands That Characterise Nano-Objects and Materials That Contain Them. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/75190.html (accessed on 20 September 2025).
- ISO/TR 10993-22:2017; Biological Evaluation of Medical Devices. Part 22: Guidance on Nanomaterials. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65918.html (accessed on 20 September 2025).
- Uddin, M.N.; Desai, F.; Asmatulu, E. Engineered Nanomaterials in the Environment: Bioaccumulation, Biomagnification and Biotransformation. Environ. Chem. Lett. 2020, 18, 1073–1083. [Google Scholar] [CrossRef]
- Govahi, M.; Darvishi, E.; Farsani, M.N.; Ghafarifarsani, H. Green Synthesized Zinc Oxide Nanoparticles Using Scrophularia Striata Extract Attenuate Crowding Stress Responses in Common Carp. Sci. Rep. 2025, 15, 19622. [Google Scholar] [CrossRef]
- Mandal, A.H.; Ghosh, S.; Adhurjya, D.; Chatterjee, P.; Samajdar, I.; Mukherjee, D.; Dhara, K.; Saha, N.C.; Piccione, G.; Multisanti, C.R.; et al. Exploring the Impact of Zinc Oxide Nanoparticles on Fish and Fish-Food Organisms: A Review. Aquac. Rep. 2024, 36, 102038. [Google Scholar] [CrossRef]
- Mat Lazim, Z.; Salmiati, S.; Marpongahtun, M.; Arman, N.Z.; Mohd Haniffah, M.R.; Azman, S.; Yong, E.L.; Salim, M.R. Distribution of Silver (Ag) and Silver Nanoparticles (AgNPs) in Aquatic Environment. Water 2023, 15, 1349. [Google Scholar] [CrossRef]
- Liang, D.; Fan, W.; Wu, Y.; Wang, Y. Effect of Organic Matter on the Trophic Transfer of Silver Nanoparticles in an Aquatic Food Chain. J. Hazard. Mater. 2022, 438, 129521. [Google Scholar] [CrossRef]
- Dang, F.; Huang, Y.; Wang, Y.; Zhou, D.; Xing, B. Transfer and Toxicity of Silver Nanoparticles in the Food Chain. Environ. Sci. Nano 2021, 8, 1519–1535. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, Y.; Xu, J.; Qian, J.; Yang, X.; Chen, X.; Zhang, C.; Gao, P. Ecotoxicity and Trophic Transfer of Metallic Nanomaterials in Aquatic Ecosystems. Sci. Total Environ. 2024, 924, 171660. [Google Scholar] [CrossRef]
- Ammar, A.; Evelo, C.; Willighagen, E. FAIR Assessment of Nanosafety Data Reusability with Community Standards. Sci. Data 2024, 11, 503. [Google Scholar] [CrossRef]
- Zheng, Y.; Nowack, B. Meta-Analysis of Bioaccumulation Data for Nondissolvable Engineered Nanomaterials in Freshwater Aquatic Organisms. Environ. Toxicol. Chem. 2022, 41, 1202–1214. [Google Scholar] [CrossRef]
- Kuehr, S.; Kosfeld, V.; Schlechtriem, C. Bioaccumulation Assessment of Nanomaterials Using Freshwater Invertebrate Species. Environ. Sci. Eur. 2021, 33, 9. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Ahmed, W.; Mahmood, M.; Ali, H.M.; Rizwan, M.; Bundschuh, J.; Akmal, M.; Mehmood, S. Study on Application of Green-Synthesized ZnO and Si Nanoparticles in Enhancing Aquaculture Sediment Quality. Chem. Biol. Technol. Agric. 2025, 12, 6. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Wanakai, S.I.; Kiprono, G.K.; Kareru, P.G. Green Synthesis of Copper Oxide Nanoparticles and Its Efficiency in Degradation of Rifampicin Antibiotic. Sci. Rep. 2023, 13, 14030. [Google Scholar] [CrossRef]
- Alsolmi, M.M.; El-Naggar, N.E.-A.; Alqurashi, M.I.; Hamouda, R.A. Biofabrication of Zinc Oxide Nanoparticles Using Moringa Oleifera, Characterization and Statistical Optimization for Their Application in Crystal Violet Adsorption. Sci. Rep. 2025, 15, 3780. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Taha, G.M.; Hagaggi, N.S.A.; Moghazy, M.A. Green Synthesis of Silver Nanoparticles and Its Environmental Sensor Ability to Some Heavy Metals. BMC Chem. 2024, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Doak, S.H.; Andreoli, C.; Burgum, M.J.; Chaudhry, Q.; Bleeker, E.A.J.; Bossa, C.; Domenech, J.; Drobne, D.; Fessard, V.; Jeliazkova, N.; et al. Current Status and Future Challenges of Genotoxicity OECD Test Guidelines for Nanomaterials: A Workshop Report. Mutagenesis 2023, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.H.; Irizarry, N.; Uddin, M.N. Polymer Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]
- OECD. A Tiered Approach for Reliable Bioaccumulation Assessment of Manufactured Nanomaterials in the Environment Whilst Minimising the Use of Vertebrate Testing; OECD Series on the Safety of Manufactured Nanomaterials and other Advanced Materials; OECD: Paris, France, 2024; ISBN 978-92-64-93652-2. [Google Scholar]
- Valdiglesias, V. Cytotoxicity and Genotoxicity of Nanomaterials. Nanomaterials 2022, 12, 634. [Google Scholar] [CrossRef]
- Tareq, M.; Khadrawy, Y.A.; Rageh, M.M.; Mohammed, H.S. Dose-Dependent Biological Toxicity of Green Synthesized Silver Nanoparticles in Rat’s Brain. Sci. Rep. 2022, 12, 22642. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X.; Hong, H.; Chen, J.; Zhao, Q.; Wu, F. Computational Nanotoxicology Models for Environmental Risk Assessment of Engineered Nanomaterials. Nanomaterials 2024, 14, 155. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, M.d.P.; Bustos-Terrones, V.; Díaz-Cárdenas, M.Y.; Vázquez-Vélez, E.; Martínez, H. Life Cycle Assessment of Green Synthesis of TiO2 Nanoparticles vs. Chemical Synthesis. Sustainability 2024, 16, 7751. [Google Scholar] [CrossRef]
- Reijnders, L. Is ‘Green’ Gold and Silver Nanoparticle Synthesis Environmentally Friendly? Nanomaterials 2025, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, D.; Wahid, J.B.A.; Karmegam, N.; Jeevika, R.; Sellapillai, L.; Rajkumar, M.; SenthilKumar, K.J. A Comprehensive Review on the Green Synthesis of Nanoparticles: Advancements in Biomedical and Environmental Applications. Biomed. Mater. Devices 2025, 4, 388–413. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.A.; Kamaraj, C.; Bharathi, A.; Surendra, T.V. Low-Cost and Eco-Friendly Phyto-Synthesis of Silver Nanoparticles Using Cocos nucifera Coir Extract and Its Larvicidal Activity. Ind. Crops Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Jebakumar Immanuel Edison, T.N.; Sethuraman, M.G. Electrocatalytic Reduction of Benzyl Chloride by Green Synthesized Silver Nanoparticles Using Pod Extract of Acacia Nilotica. ACS Sustain. Chem. Eng. 2013, 1, 1326–1332. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green Synthesis of Silver Nanoparticles Using Andean Blackberry Fruit Extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Ma, J.; Fu, M. Facile Green Synthesis of Functional Nanoscale Zero-Valent Iron and Studies of Its Activity toward Ultrasound-Enhanced Decolorization of Cationic Dyes. Chemosphere 2017, 166, 80–88. [Google Scholar] [CrossRef]
- Gao, J.-F.; Li, H.-Y.; Pan, K.-L.; Si, C.-Y. Green Synthesis of Nanoscale Zero-Valent Iron Using a Grape Seed Extract as a Stabilizing Agent and the Application for Quick Decolorization of Azo and Anthraquinone Dyes. RSC Adv. 2016, 6, 22526–22537. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Fattahi, A. Nigella arvensis Leaf Extract Mediated Green Synthesis of Silver Nanoparticles: Their Characteristic Properties and Biological Efficacy. Adv. Powder Technol. 2018, 29, 202–210. [Google Scholar] [CrossRef]
- Leili, M.; Fazlzadeh, M.; Bhatnagar, A. Green Synthesis of Nano-Zero-Valent Iron from Nettle and Thyme Leaf Extracts and Their Application for the Removal of Cephalexin Antibiotic from Aqueous Solutions. Environ. Technol. 2018, 39, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Kumar Borah, R.; Jyoti Saikia, H.; Mahanta, A.; Kumar Das, V.; Bora, U.; Jyoti Thakur, A. Biosynthesis of Poly(Ethylene Glycol)-Supported Palladium Nanoparticles Using Colocasia Esculenta Leaf Extract and Their Catalytic Activity for Suzuki–Miyaura Cross-Coupling Reactions. RSC Adv. 2015, 5, 72453–72457. [Google Scholar] [CrossRef]
- Ahmad, T.; Irfan, M.; Bhattacharjee, S. Parametric Study on Gold Nanoparticle Synthesis Using Aqueous Elaise guineensis (Oil Palm) Leaf Extract: Effect of Precursor Concentration. Procedia Eng. 2016, 148, 1396–1401. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green Biosynthesis of Gold Nanoparticles Using Galaxaura elongata and Characterization of Their Antibacterial Activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Parab, A.R.; Ramlal, A.; Gopinath, S.C.B.; Subramaniam, S. Forging the Future of Nanotechnology: Embracing Greener Practices for a Resilient Today and a Sustainable Tomorrow. Front. Nanotechnol. 2025, 6, 1506665. [Google Scholar] [CrossRef]
- Yayınları, Y.A.Z. Biyoloji Çalışmaları; Zenodo: Geneva, Switzerland, 2024. [Google Scholar]
- Xuan, L.; Zhao, J.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-Year Chronic Toxicity Evaluation of Single Dose Intravenously Administered Silica Nanoparticles in Mice and Their Ex Vivo Human Hemocompatibility. J. Control Release 2020, 324, 471–481. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-Term Exposure to High-Concentration Silver Nanoparticles Induced Toxicity, Fatality, Bioaccumulation, and Histological Alteration in Fish (Cyprinus carpio). Environ. Sci. Eur. 2021, 33, 14. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chu, H.-Y.; Huang, H.-M.; Li, Z.-L.; Chen, C.-Y.; Shih, Y.-J.; Whang-Peng, J.; Cheng, R.H.; Mo, J.-K.; Lin, H.-Y.; et al. Toxicologic Concerns with Current Medical Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7597. [Google Scholar] [CrossRef]
- Benelli, G. Green Synthesis of Nanomaterials. Nanomaterials 2019, 9, 1275. [Google Scholar] [CrossRef]
- Larrañaga-Tapia, M.; Betancourt-Tovar, B.; Videa, M.; Antunes-Ricardo, M.; Cholula-Díaz, J.L. Green Synthesis Trends and Potential Applications of Bimetallic Nanoparticles towards the Sustainable Development Goals 2030. Nanoscale Adv. 2024, 6, 51–71. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef] [PubMed]
- Gungure, A.S.; Jule, L.T.; Ramaswamy, K.; Nagaprasad, N.; Ramaswamy, S. Photo and Electrochemical Applications of Green Synthesized ZnO/Ag2O Nanocomposites Materials under Visible Light Using P. macrosolen L. Leaf. Sci. Rep. 2025, 15, 7234. [Google Scholar] [CrossRef] [PubMed]
- Abduh, N.A.Y.; Al-Odayni, A.-B. Green Synthesis of ZnO/SnO2 Hybrid Nanocomposite for Degradation of Cationic and Anionic Dyes under Sunlight Radiation. Materials 2023, 16, 7398. [Google Scholar] [CrossRef] [PubMed]
- Degefa, M.; Muleta, G.; Teshome, K. Synthesis of Cu-Doped ZnO Nanoparticles Using Aloe Vera Leaf Extract for Antibacterial and Photocatalytic Activities Evaluation. Curr. Nanomater. 2025, 10, 64–77. [Google Scholar] [CrossRef]
- Benkhira, L.; Ferhat, M.F.; Khaled, M.T.O.; Messai, R.; Bounedjar, N.; Tedjani, M.L.; Zoukel, A.; Humayun, M.; Bououdina, M. Multifunctional Assessment of Copper-Doped ZnO Nanoparticles Synthesized via Gliding Arc Discharge Plasma Technique: Antioxidant, Antibacterial, and Photocatalytic Performance. Environ. Sci. Pollut. Res. 2024, 31, 43743–43756. [Google Scholar] [CrossRef]
- Almansour, K.; Alqahtani, A.S. Utilization of Machine Learning Approach for Production of Optimized PLGA Nanoparticles for Drug Delivery Applications. Sci. Rep. 2025, 15, 8840. [Google Scholar] [CrossRef]
- Diao, S.; Wu, Q.; Li, S.; Xu, G.; Ren, X.; Tan, L.; Jiang, G.; Song, P.; Meng, X. From Synthesis to Properties: Expanding the Horizons of Machine Learning in Nanomaterials Research. Mater. Horiz. 2025, 12, 4133–4164. [Google Scholar] [CrossRef]
- Raheem, S.A.; Alfatlawi, A.H. Surface-Water Purification Using Cellulose Paper Impregnated with Silver Nanoparticles. Drink. Water Eng. Sci. 2021, 14, 95–102. [Google Scholar] [CrossRef]
- Bashir, N.; Afzaal, M.; Khan, A.L.; Nawaz, R.; Irfan, A.; Almaary, K.S.; Dabiellil, F.; Bourhia, M.; Ahmed, Z. Green-Synthesized Silver Nanoparticle-Enhanced Nanofiltration Mixed Matrix Membranes for High-Performance Water Purification. Sci. Rep. 2025, 15, 1001. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P.; Agrawal, S.B.; Bhatia, S. Green Synthesis of Polymeric Nanoparticles: Agricultural Applications and Toxicological Implications. Discov. Appl. Sci. 2025, 7, 505. [Google Scholar] [CrossRef]
- Younas, W.; Khan, F.U.; Zaman, M.; Lin, D.; Zuberi, A.; Wang, Y. Toxicity of Synthesized Silver Nanoparticles in a Widespread Fish: A Comparison between Green and Chemical. Sci. Total Environ. 2022, 845, 157366. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Su, W.; Lu, T.; Li, A.; Pang, X. Effects of Dual Deletion of glnR and mtrA on Expression of Nitrogen Metabolism Genes in Streptomyces venezuelae. Microb. Biotechnol. 2022, 15, 1795–1810. [Google Scholar] [CrossRef]






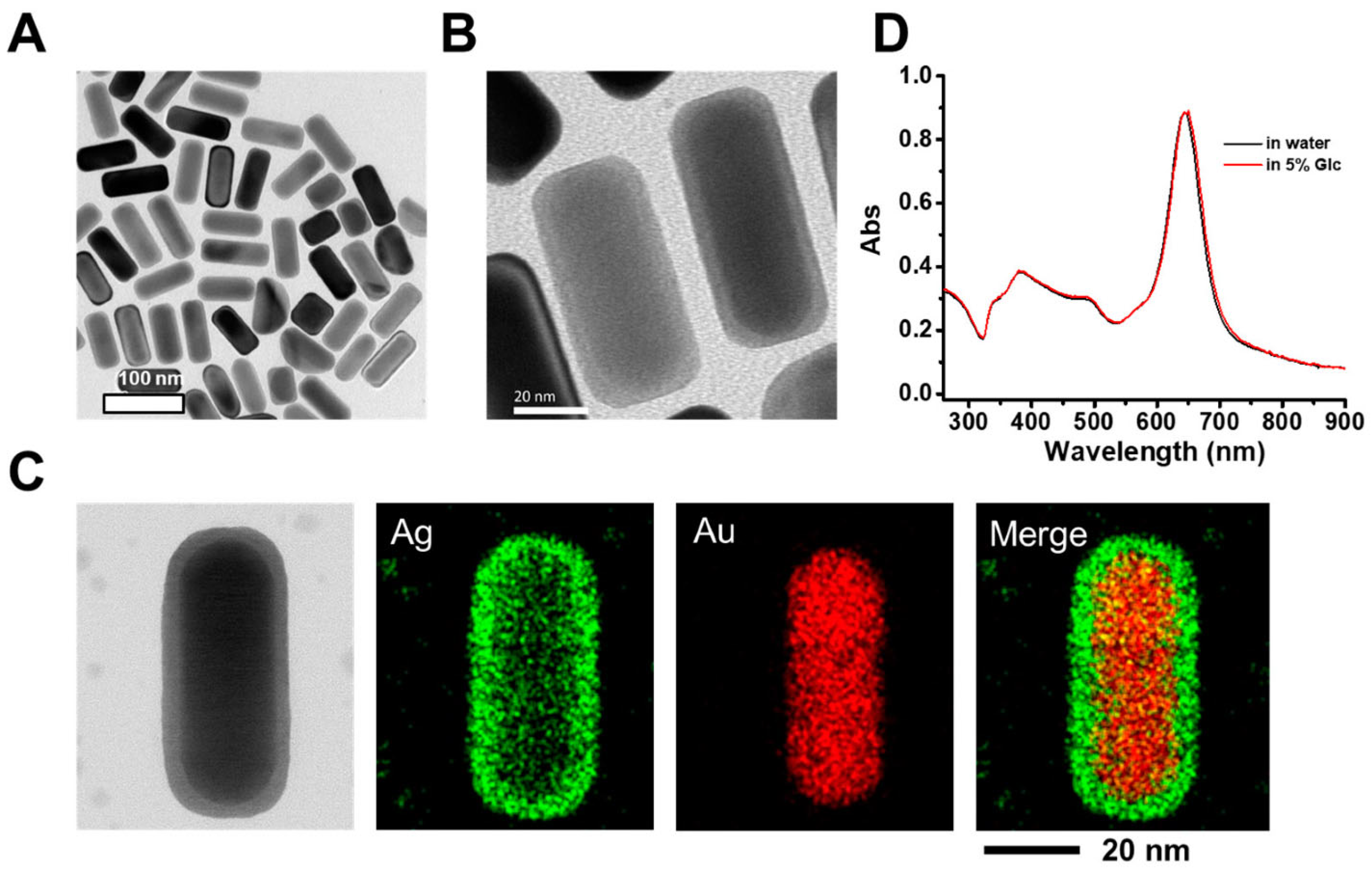
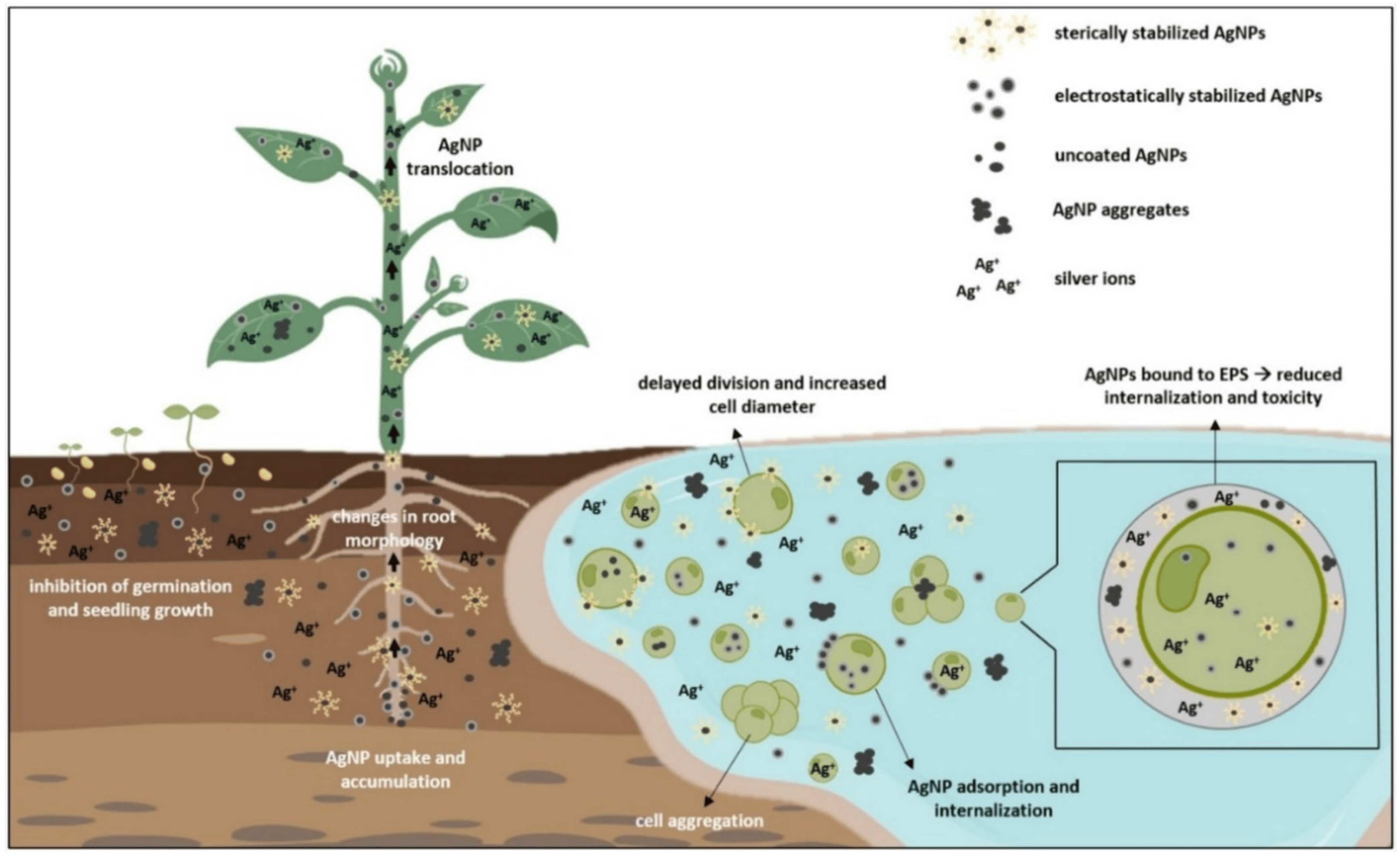
| Metric | Plant-Mediated | Microbial-Mediated (Bacteria and Fungi) | Algae-Mediated | Biopolymer/Natural Molecule-Mediated | References |
|---|---|---|---|---|---|
| Atom economy (AE) | Moderate–high (extracts act as both reducing and capping agents, minimizing extra reagents) | Moderate (nutrient media adds to inputs, lowering AE) | High (algal metabolites directly reduce/cap NPs) | Moderate–high (efficient use of natural molecules, but polymer residues may persist) | [71,72,73] |
| E-Factor (Waste/product ratio) | Medium (biomass waste and extract residues) | High (culture media, biomass disposal) | Low–medium (minimal waste, scalable with photobioreactors) | Medium–high (non-biodegradable polymer residues possible) | [74] |
| PMI (process mass intensity) | Moderate (large extract volumes needed) | High (nutrient-rich media per product yield) | Low (aqueous systems, minimal reagents) | Medium (depends on polymer/natural molecule load) | [69] |
| Energy consumption | Moderate (room-temp synthesis, but extraction may require heating/solvents) | High (long culture times, controlled growth conditions) | Low–moderate (uses sunlight/photosynthesis, but harvesting biomass may be energy-intensive) | Low–moderate (simple mixing, but some polymer processing may require energy) | [30] |
| Toxicity/hazard profile | Low (plant metabolites are generally safe and biodegradable) | Low–Moderate (safe metabolites, but endotoxins/fungal toxins possible) | Very low (nontoxic algal biomolecules) | Moderate (biopolymers safe, but chemical crosslinkers or modified natural molecules may introduce toxicity) | [75] |
| Overall greenness | Good (widely scalable, eco-friendly but extract waste handling needed) | Moderate (effective but resource-intensive) | Excellent (renewable, low waste, safe byproducts) | Good (sustainable, but residues and chemical modifications reduce greenness) | [30] |
| Effect | Description | References |
|---|---|---|
| Lipid peroxidation | Damages microbial membrane integrity by attacking phospholipid bilayers; causes membrane leakage | [106] |
| Protein oxidation | Inactivates key enzymes and structural proteins; disrupts metabolism and cell signaling | [107] |
| DNA/RNA damage | Causes base modifications, strand breaks, and replication failure, leading to mutations or cell death | [108] |
| ROS-induced apoptosis-like death | Triggers programmed like cell death, especially in fungi and protozoa; involves oxidative stress pathways | [109] |
| Capping Agent | Effect on ROS Generation | Effect on Metal Ion Release | Representative Quantitative Examples | Implications for Water Disinfection and Safety | References |
|---|---|---|---|---|---|
| Polyphenols (e.g., flavonoids, tannins, phenolic acids) | Can enhance interfacial ROS via redox cycling at the NPs surface; act as bulk antioxidants, reducing detectable ROS in solution. | May stabilize NPs surface (slower release) or promote dissolution via soluble polyphenolmetal complexes; outcome depends on ligand chemistry and environment. |
| Strong contact-mediated antimicrobial action; but variable ion release may affect long-term safety in aquatic environments. | [121] |
| Proteins (e.g., albumin, microbial proteins, enzymes) | Generally, reduce both interfacial and bulk ROS due to steric shielding and amino acid scavenging. | Strong binding (cysteine, histidine) typically suppresses acute ion release; release may increase if corona degrades in natural waters. |
| Offers lower immediate ROS/ion toxicity and enhanced colloidal stability, but corona degradation can lead to delayed ion release. | [122] |
| Polysaccharides (e.g., starch, chitosan, alginate) | Typically, moderate ROS generation; chitosan can impart intrinsic antimicrobial effects (membrane disruption). | Often reduce ion release by forming dense coatings, though porous polysaccharide shells (e.g., alginate) may allow gradual release. |
| Provide sustained ion release and improve biocompatibility; chitosan adds dual-function antimicrobial action. | [117] |
| Lipids/Terpenoids (e.g., plant essential oils, saponins) | Can act as ROS quenchers (antioxidant terpenoids) or enhance ROS under light via photosensitization. | Lipid layers typically slow ion release, but terpene-metal complexes may promote dissolution under certain pH conditions. |
| Add synergistic antimicrobial properties (membrane disruption), but ROS suppression may limit oxidative disinfection pathways. | [123] |
| Category | Reported Practices |
|---|---|
| Synthesis |
|
| Characterization |
|
| Antimicrobial Testing |
|
| Water Type | Key Characteristics | Waste Removal Efficiency | References |
|---|---|---|---|
| River/surface water | Moderate turbidity (≤50 NTU), NOM present (humic/fulvic acids) | >90% bacterial inactivation by green AgNPs up to 50 NTU; efficacy decreases with high NOM fouling | [160,161] |
| Groundwater | Low turbidity, low NOM, stable pH | Very high removal at MIC doses; stable efficacy with AgNPs, ZnO, TiO2 | [163,164] |
| Wastewater | High NOM, high turbidity, organic/inorganic load | Reduced direct NP efficacy; requires pre-treatment or photocatalytic activation for >80% removal | [100,165] |
| Nanoparticle Type | Synthesis Route | Antimicrobial Efficacy | Cytotoxicity in Mammalian Cells | ROS Generation and Modulation | Environmental Fate and Long-Term Risk | References |
|---|---|---|---|---|---|---|
| ZnO NPs | GSNP (e.g., Musa acuminata extract) | Strong antibacterial, enhanced under UV due to photocatalysis | Moderate, dose and time dependent | Elevated ROS but moderated by bioorganic capping | Reduced ion release (Zn2+); lower sediment toxicity | [180] |
| ZnO NPs | CSNP (precipitation/sol–gel) | Comparable antimicrobial activity | High; apoptosis at lower doses | High ROS without moderation | Significant Zn2+ leaching; higher long-term ecotoxicity | [181] |
| Ag NPs | GSNP (e.g., Euphorbia retusa, Beta vulgaris) | Potent antimicrobial, effective vs. MDR strains | Moderate; apoptosis at higher concentrations | ROS partly mitigated by plant polyphenols | Lower Ag+ release; reduced bioaccumulation risk | [182] |
| Ag NPs | CSNP (NaBH4, citrate reduction) | Very high antimicrobial activity | High; mitochondrial dysfunction and DNA damage | Excess ROS; no antioxidant regulation | High Ag+ release; higher bioaccumulation risk | [120] |
| CuO NPs | GSNP (microbial/algal synthesis) | Effective antibacterial, Fenton-like ROS production | Lower cytotoxicity than chemical CuO | ROS moderated by organic capping | Partial dissolution; sediment accumulation potential | [11] |
| CuO NPs | CSNP (Thermal decomposition) | High antimicrobial activity | Pronounced cytotoxicity; oxidative stress | Persistent high ROS from surface defects | High dissolution rate; elevated aquatic toxicity | [114] |
| Fe3O4 NPs | GSNP (microbial/algal synthesis) | Good antibacterial; magnetically retrievable | Biocompatible at moderate doses | ROS less toxic due to capping | Partially retrievable; sediment accumulation possible | [95] |
| Fe3O4 NPs | CSNP (co-precipitation) | Effective antibacterial | Higher cytotoxicity; stress from surface residues | ROS enhanced by surface defects | Sediment persistence; stronger ecotoxic impact | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdush, J.; Rahman, M.M.; Parvez, M.M.H.; Mohotadi, M.A.A.; Uddin, M.N. Green-Synthesized Nanomaterials for Water Disinfection: Mechanisms, Efficacy, and Environmental Safety. Nanomaterials 2025, 15, 1507. https://doi.org/10.3390/nano15191507
Ferdush J, Rahman MM, Parvez MMH, Mohotadi MAA, Uddin MN. Green-Synthesized Nanomaterials for Water Disinfection: Mechanisms, Efficacy, and Environmental Safety. Nanomaterials. 2025; 15(19):1507. https://doi.org/10.3390/nano15191507
Chicago/Turabian StyleFerdush, Jannatul, Md. Mahbubur Rahman, Md Mahadi Hassan Parvez, Md. Abdullah Al Mohotadi, and Md. Nizam Uddin. 2025. "Green-Synthesized Nanomaterials for Water Disinfection: Mechanisms, Efficacy, and Environmental Safety" Nanomaterials 15, no. 19: 1507. https://doi.org/10.3390/nano15191507
APA StyleFerdush, J., Rahman, M. M., Parvez, M. M. H., Mohotadi, M. A. A., & Uddin, M. N. (2025). Green-Synthesized Nanomaterials for Water Disinfection: Mechanisms, Efficacy, and Environmental Safety. Nanomaterials, 15(19), 1507. https://doi.org/10.3390/nano15191507









