Magnetic Iron Oxide Nanoparticles: Advances in Synthesis, Mechanistic Understanding, and Magnetic Property Optimization for Improved Biomedical Performance
Abstract
1. Introduction
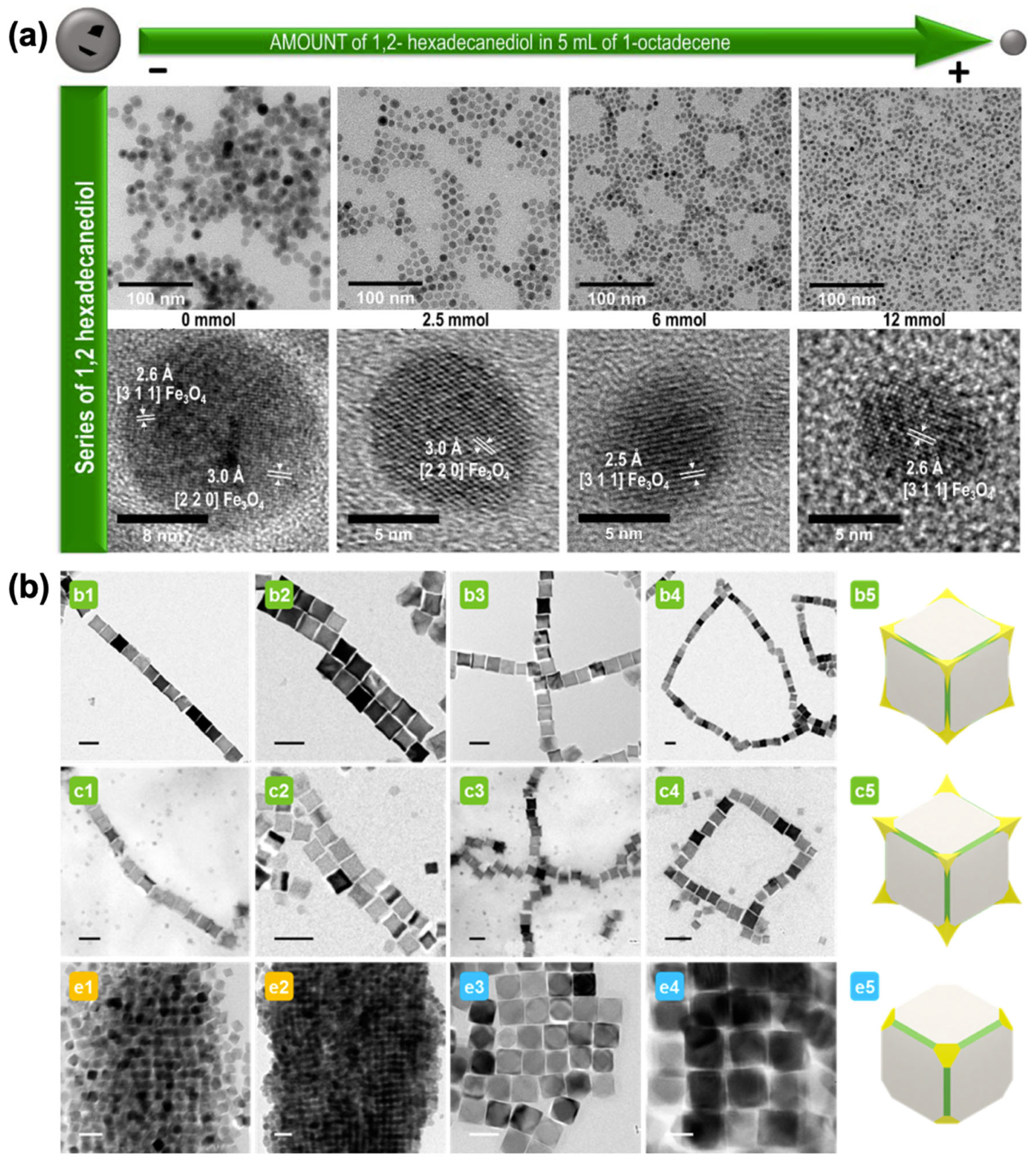
2. Synthesis of Magnetic Iron Oxide Nanoparticles
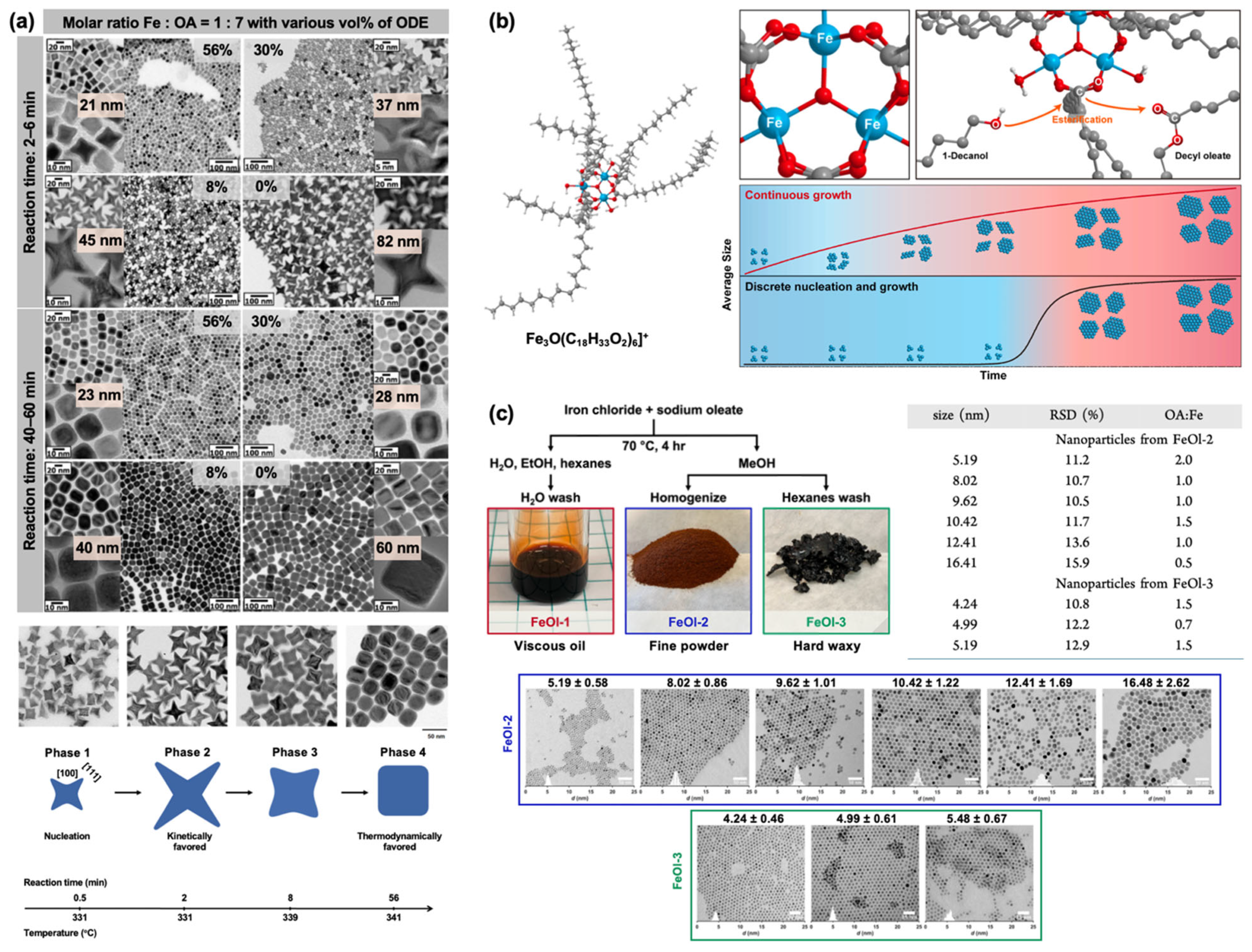
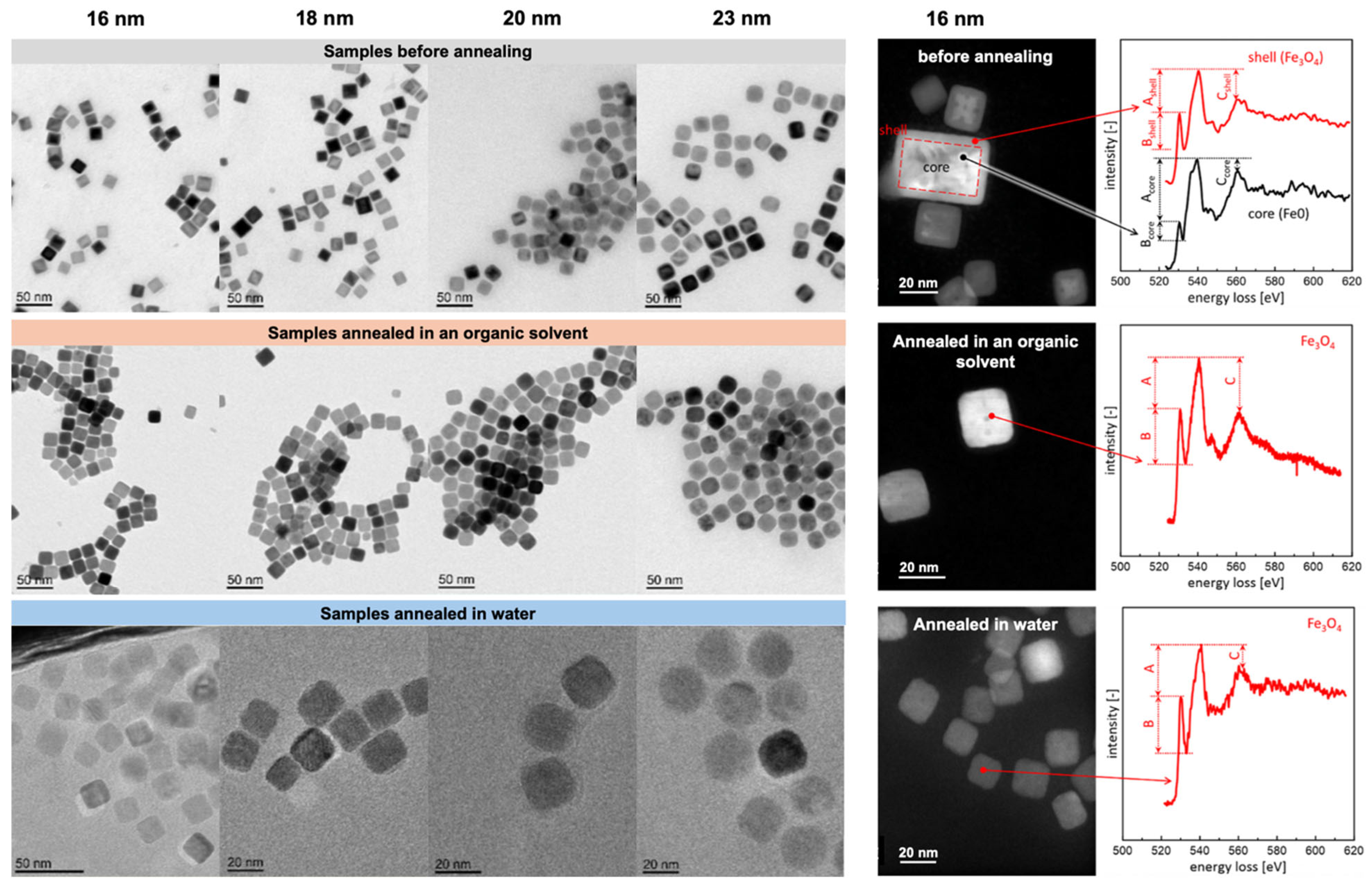
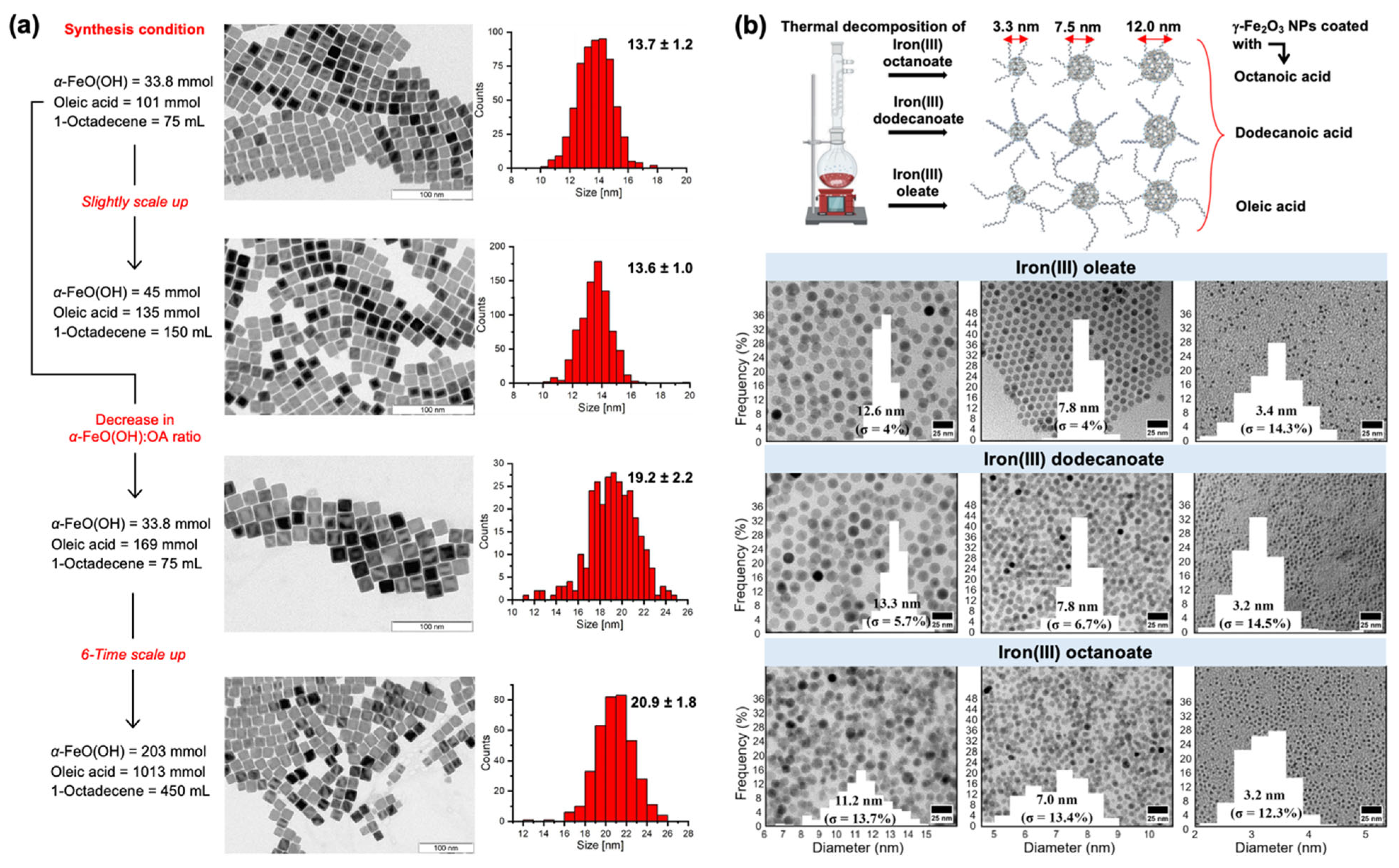
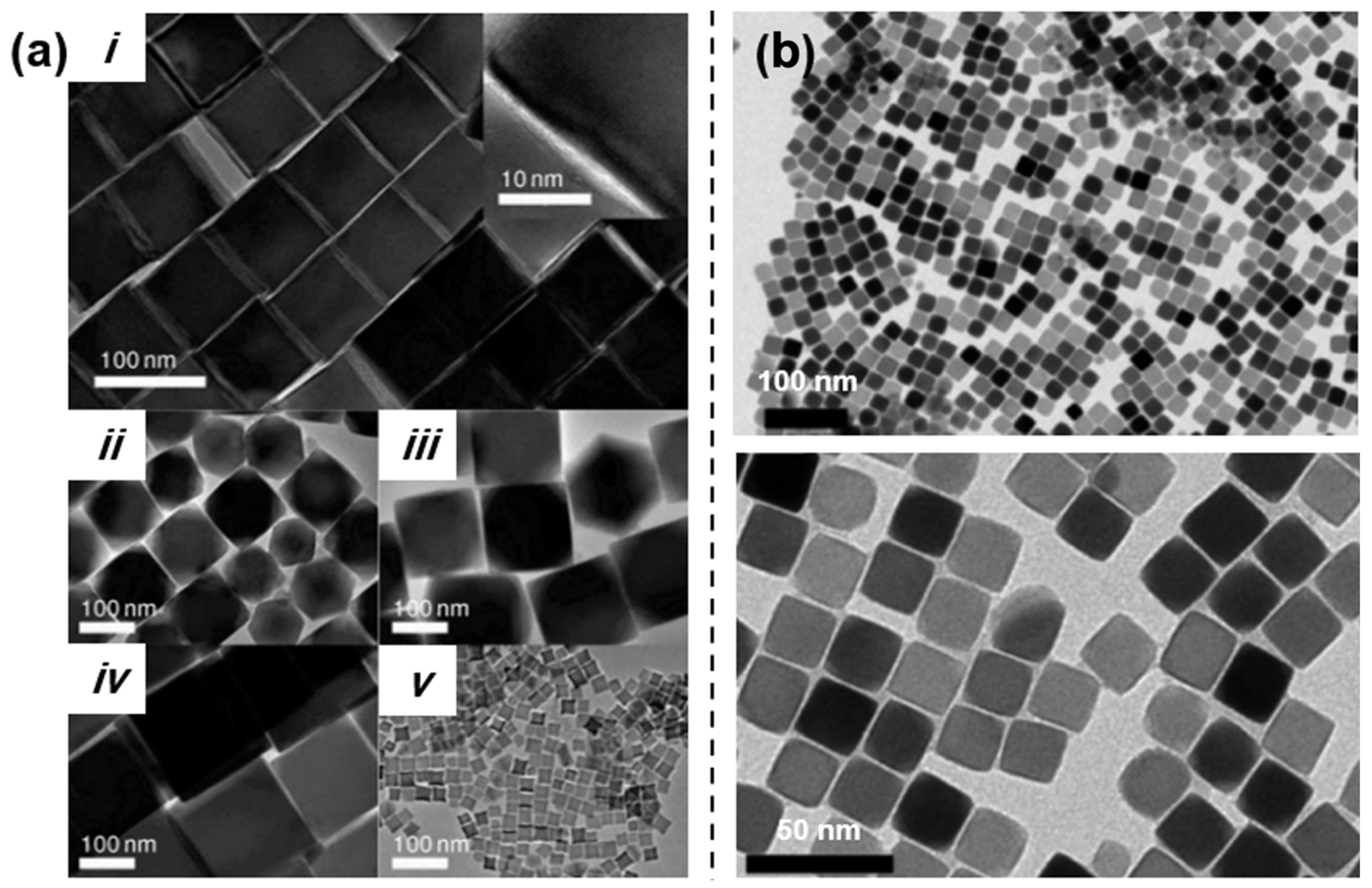
2.1. Iron Oleate, Iron Stearate, and Related Precursors
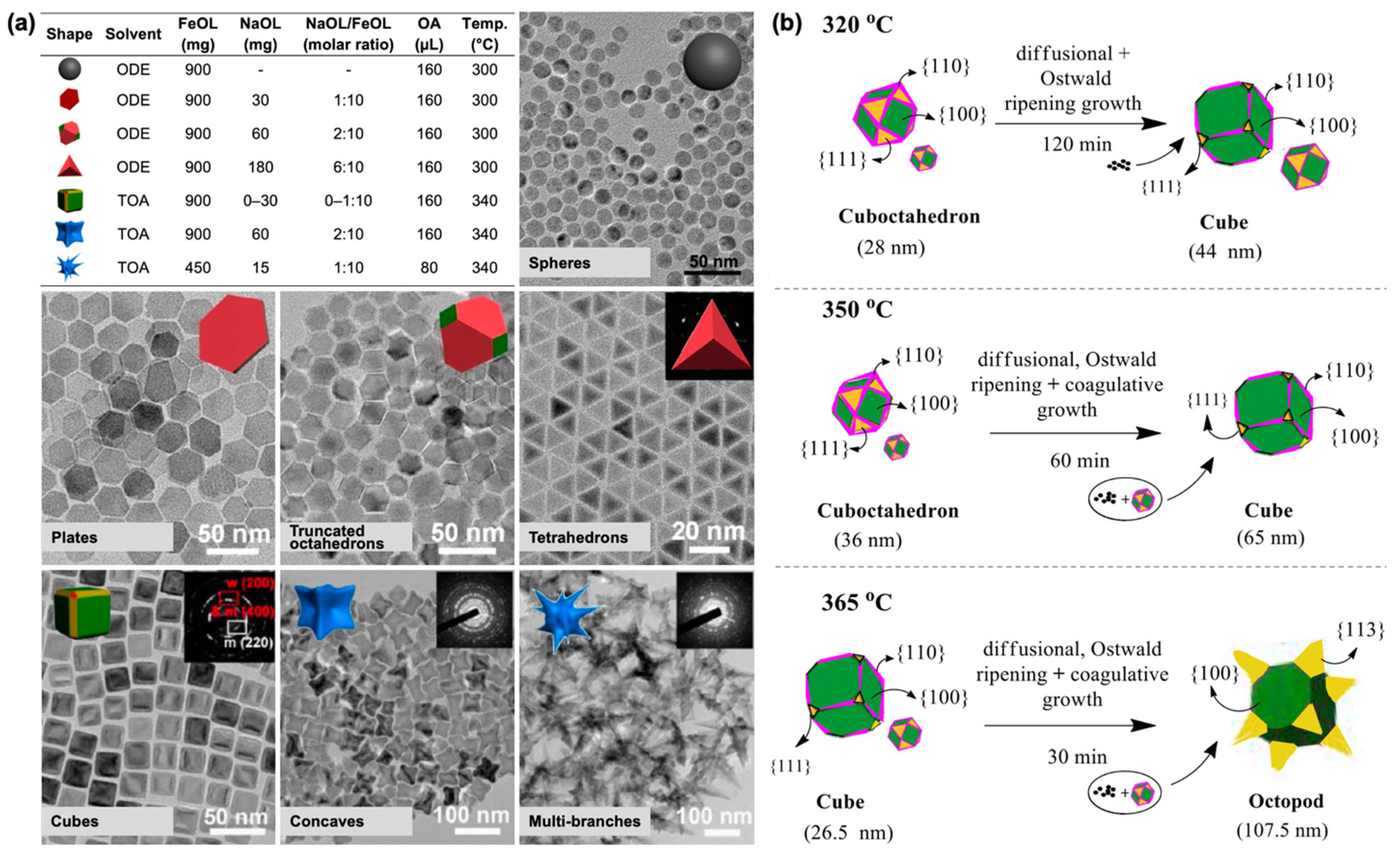
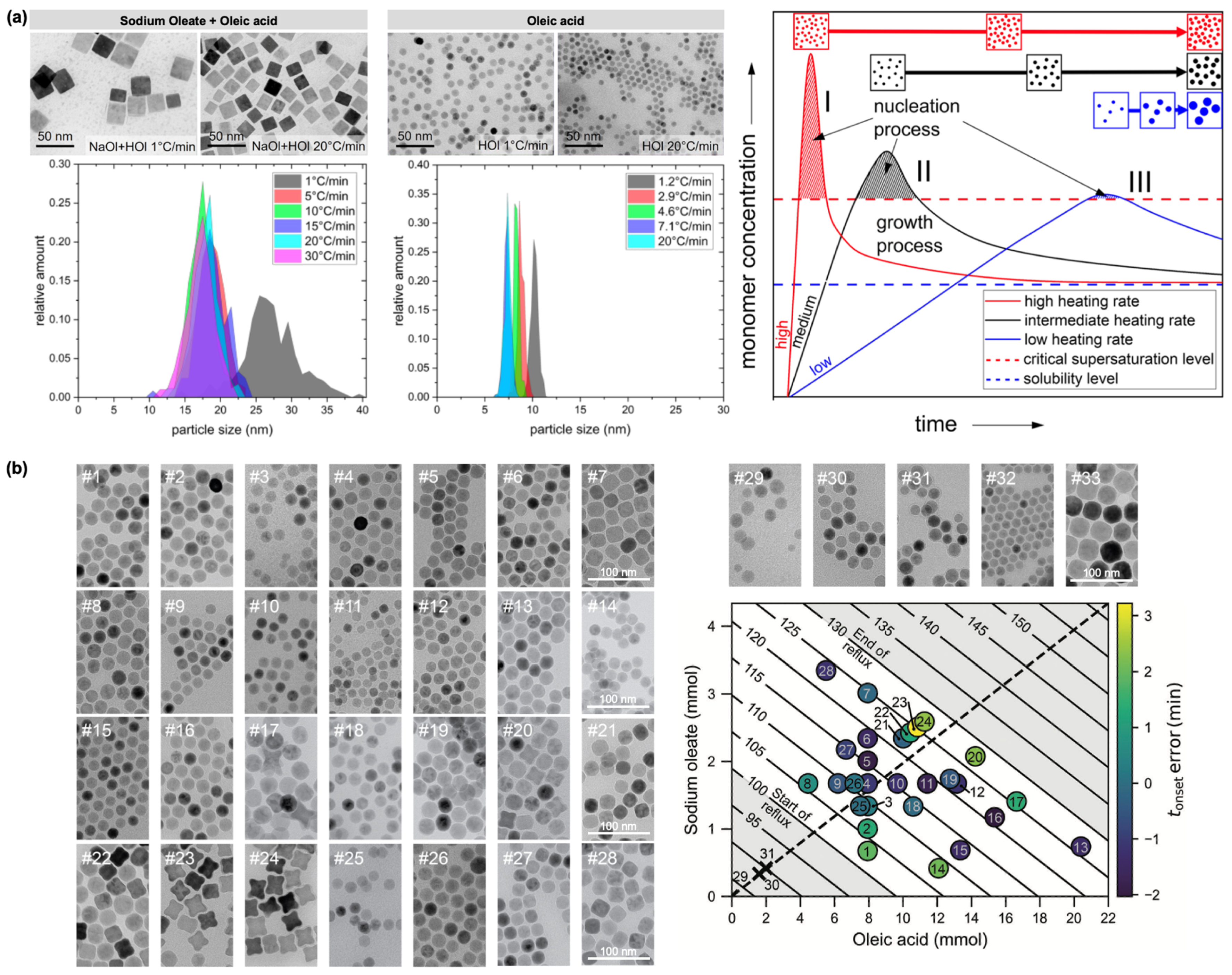
2.2. Iron(III) Acetylacetonate Precursor
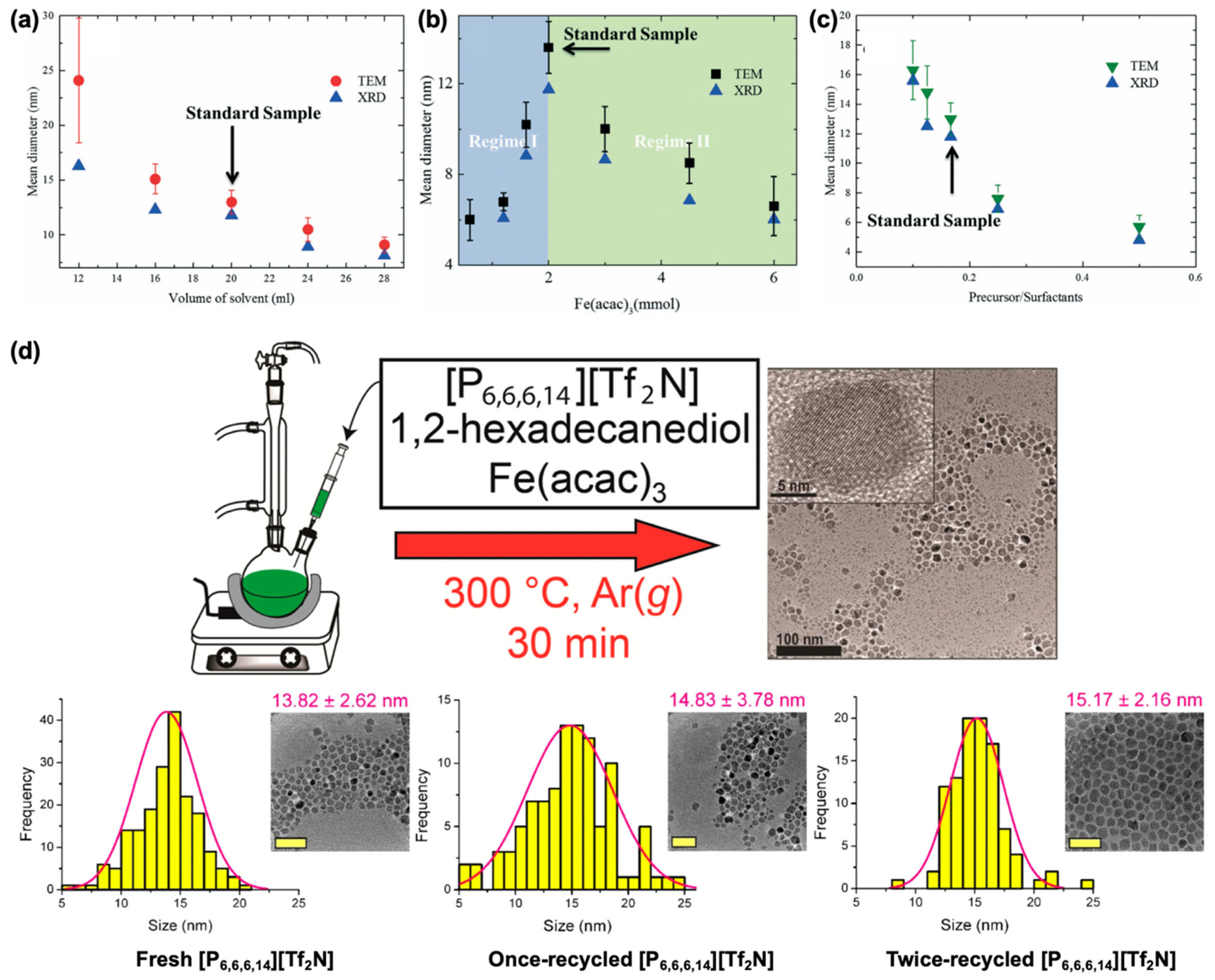
2.3. Iron Pentacarbonyl Precursor

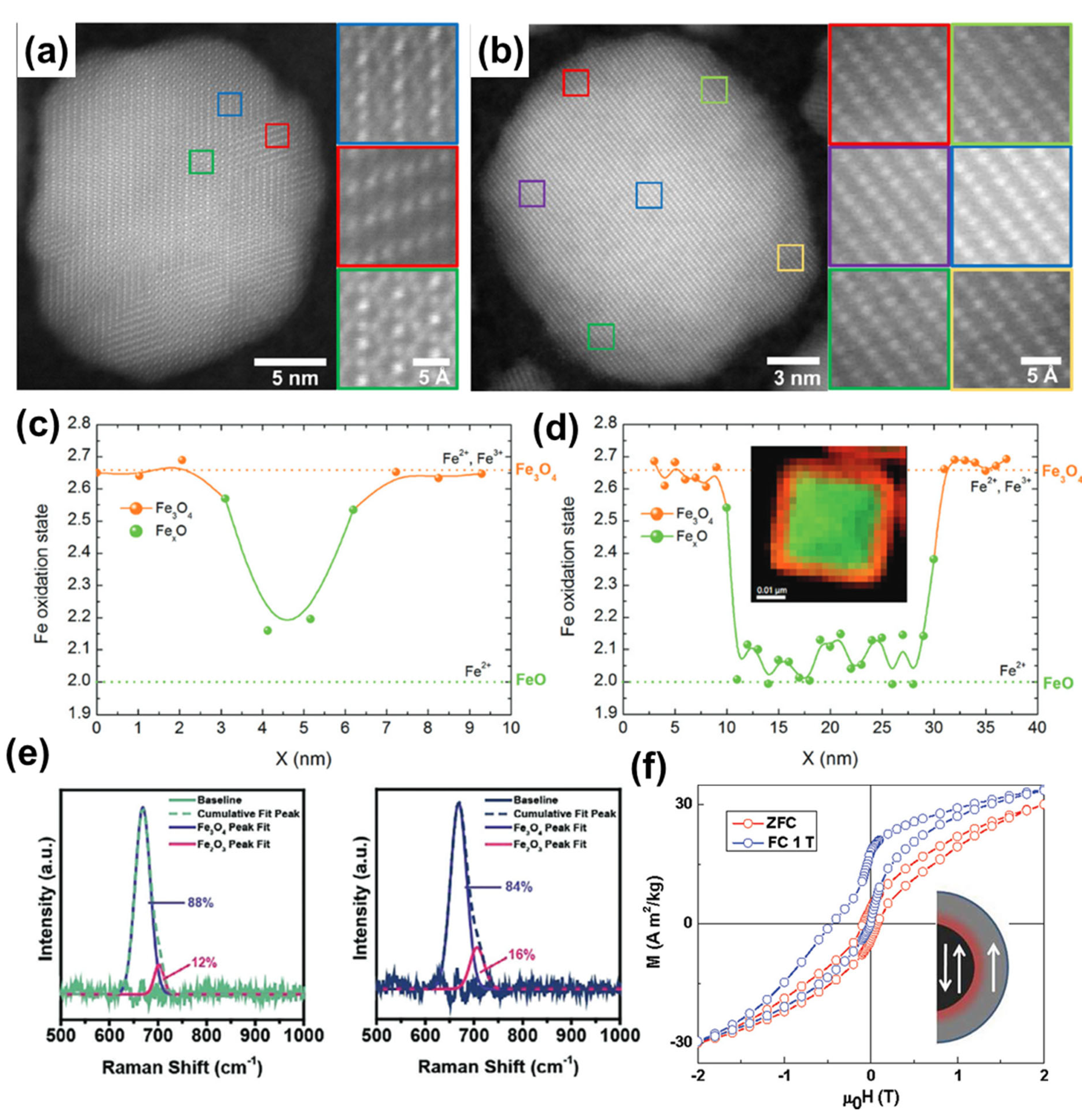
3. Nanoparticle Characterization
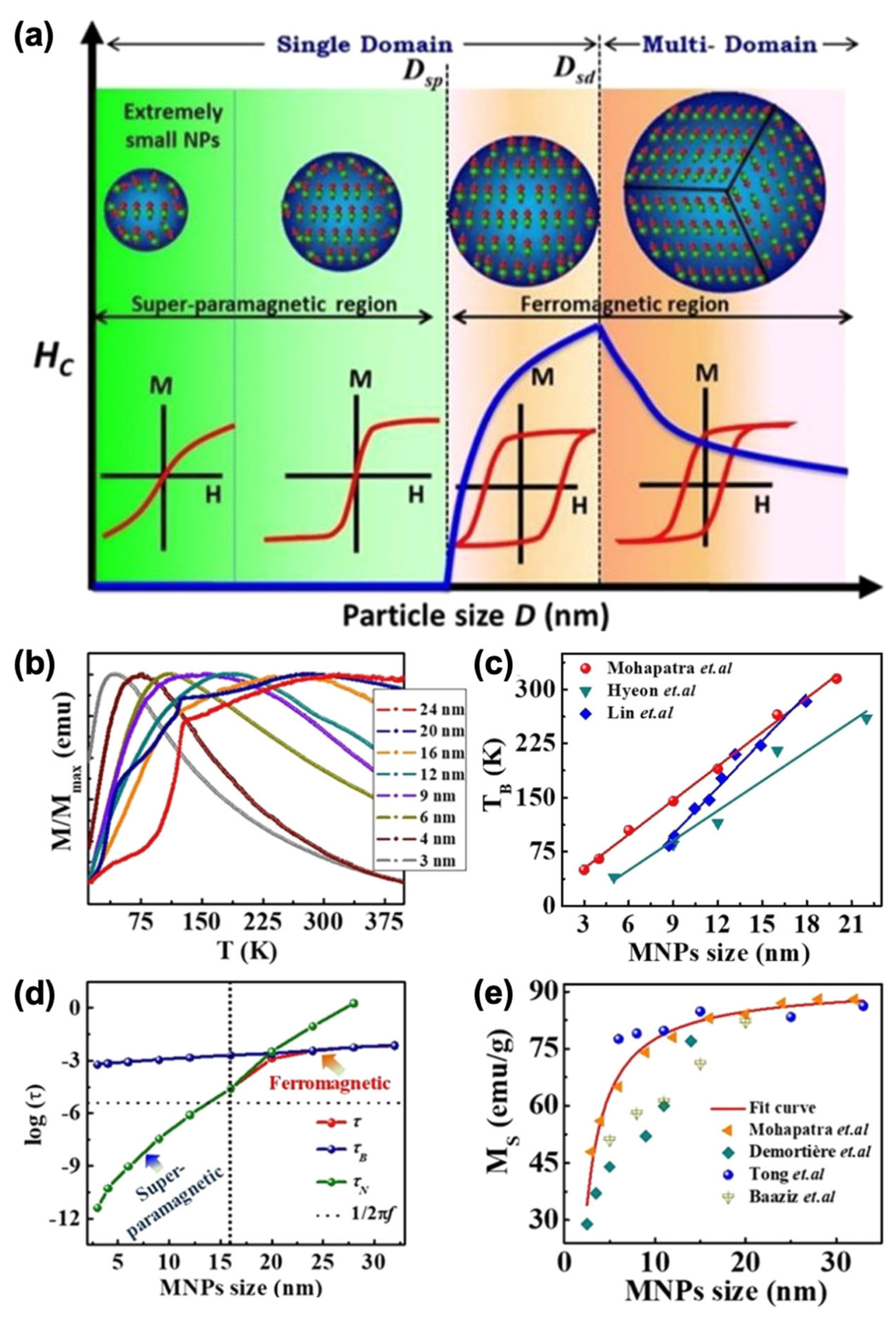
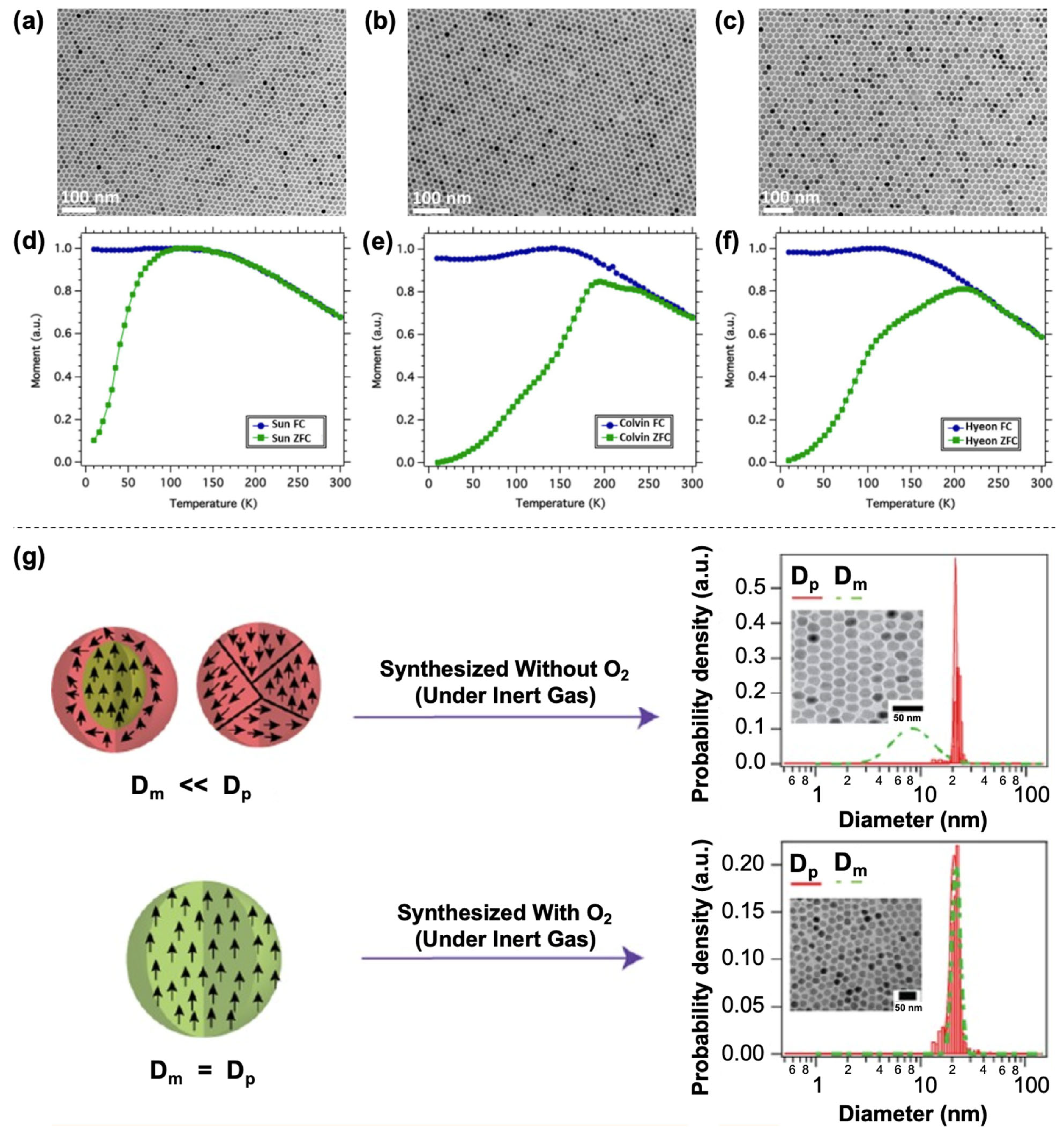
4. Magnetic Properties
5. Aqueous Phase Transfer of Hydrophobic Iron Oxide Nanoparticles
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Delivery Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Yang, C.; Wu, J.; Hou, Y. Fe3O4 Nanostructures: Synthesis, Growth Mechanism, Properties and Applications. Chem. Commun. 2011, 47, 5130–5141. [Google Scholar] [CrossRef]
- Freis, B.; Kiefer, C.; Ramirez, M.L.A.; Harlepp, S.; Mertz, D.; Pichon, B.; Iacovita, C.; Laurent, S.; Begin, S. Defects or No Defects? Or How to Design 20–25 Nm Spherical Iron Oxide Nanoparticles to Harness Both Magnetic Hyperthermia and Photothermia. Nanoscale 2024, 16, 20542–20555. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Hoijang, S.; Fuller, M.; Deng, L.; Chinwangso, P.; DeTellem, D.; Robles Hernandez, F.C.; Chu, C.-W.; Hadjiev, V.G.; Phan, M.-H.; et al. Fine-Tuning the Superparamagnetic Properties of FeO@Fe3O4 Core/Shell Nanoparticles and Superclusters by Controlling Size and Shape. ACS Appl. Mater. Interfaces 2025, 17, 28597–28608. [Google Scholar] [CrossRef]
- Wetterskog, E.; Tai, C.-W.; Grins, J.; Bergström, L.; Salazar-Alvarez, G. Anomalous Magnetic Properties of Nanoparticles Arising from Defect Structures: Topotaxial Oxidation of Fe1–xO|Fe3−δO4 Core|Shell Nanocubes to Single-Phase Particles. ACS Nano 2013, 7, 7132–7144. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Frey Huls, N.; Sigdel, A.; Sun, S. Tuning Exchange Bias in Core/Shell FeO/Fe3O4 Nanoparticles. Nano Lett. 2012, 12, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Sojková, T.; Rizzo, G.M.R.; Di Girolamo, A.; Avugadda, S.K.; Soni, N.; Milbrandt, N.B.; Tsai, Y.H.; Kuběna, I.; Sojka, M.; Silvestri, N.; et al. From Core–Shell FeO/Fe3O4 to Magnetite Nanocubes: Enhancing Magnetic Hyperthermia and Imaging Performance by Thermal Annealing. Chem. Mater. 2023, 35, 6201–6219. [Google Scholar] [CrossRef]
- Mamiya, H.; Fukumoto, H.; Cuya Huaman, J.L.; Suzuki, K.; Miyamura, H.; Balachandran, J. Estimation of Magnetic Anisotropy of Individual Magnetite Nanoparticles for Magnetic Hyperthermia. ACS Nano 2020, 14, 8421–8432. [Google Scholar] [CrossRef]
- García-Acevedo, P.; González-Gómez, M.A.; Arnosa-Prieto, Á.; de Castro-Alves, L.; Piñeiro, Y.; Rivas, J. Role of Dipolar Interactions on the Determination of the Effective Magnetic Anisotropy in Iron Oxide Nanoparticles. Adv. Sci. 2023, 10, 2203397. [Google Scholar] [CrossRef]
- Nedelkoski, Z.; Kepaptsoglou, D.; Lari, L.; Wen, T.; Booth, R.A.; Oberdick, S.D.; Galindo, P.L.; Ramasse, Q.M.; Evans, R.F.L.; Majetich, S.; et al. Origin of Reduced Magnetization and Domain Formation in Small Magnetite Nanoparticles. Sci. Rep. 2017, 7, 45997. [Google Scholar] [CrossRef]
- Mishra, S.; Yadav, M.D. Magnetic Nanoparticles: A Comprehensive Review from Synthesis to Biomedical Frontiers. Langmuir 2024, 40, 17239–17269. [Google Scholar] [CrossRef]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem. Rev. 2023, 123, 3904–3943. [Google Scholar] [CrossRef]
- Ling, D.; Hyeon, T. Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed Synthesis of Uniformly Sized Iron Oxide Nanoparticles for Efficient Magnetic Resonance Imaging Contrast Agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Phan, M.-H.; Alonso, J.; Khurshid, H.; Lampen-Kelley, P.; Chandra, S.; Stojak Repa, K.; Nemati, Z.; Das, R.; Iglesias, Ó.; Srikanth, H. Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems. Nanomaterials 2016, 6, 221. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic Nanoparticles and Clusters for Magnetic Hyperthermia: Optimizing Their Heat Performance and Developing Combinatorial Therapies to Tackle Cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Qiao, R.; Fu, C.; Forgham, H.; Javed, I.; Huang, X.; Zhu, J.; Whittaker, A.K.; Davis, T.P. Magnetic Iron Oxide Nanoparticles for Brain Imaging and Drug Delivery. Adv. Drug Delivery Rev. 2023, 197, 114822. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Patri, S.; Thanh, N.T.K.; Kamaly, N. Magnetic Iron Oxide Nanogels for Combined Hyperthermia and Drug Delivery for Cancer Treatment. Nanoscale 2024, 16, 15446–15464. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Chen, Y.; Peng, X. Size- and Shape-Controlled Magnetic (Cr, Mn, Fe, Co, Ni) Oxide Nanocrystals via a Simple and General Approach. Chem. Mater. 2004, 16, 3931–3935. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of Monodisperse Iron Oxide Nanocrystal Formation by “Heating-Up” Process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Narnaware, P.K.; Ravikumar, C. Mechanistic Insights into the Formation and Growth of Anisotropic-Shaped Wüstite–Spinel Core–Shell Iron Oxide Nanoparticles in a Coordinating Solvent. J. Phys. Chem. C 2020, 124, 25010–25027. [Google Scholar] [CrossRef]
- Feld, A.; Weimer, A.; Kornowski, A.; Winckelmans, N.; Merkl, J.-P.; Kloust, H.; Zierold, R.; Schmidtke, C.; Schotten, T.; Riedner, M.; et al. Chemistry of Shape-Controlled Iron Oxide Nanocrystal Formation. ACS Nano 2019, 13, 152–162. [Google Scholar] [CrossRef]
- Situ-Loewenstein, S.F.; Wickramasinghe, S.; Abenojar, E.C.; Erokwu, B.O.; Flask, C.A.; Lee, Z.; Samia, A.C.S. A Novel Synthetic Route for High-Index Faceted Iron Oxide Concave Nanocubes with High T2 Relaxivity for in Vivo MRI Applications. J. Mater. Sci. Mater. Med. 2018, 29, 58. [Google Scholar] [CrossRef]
- Matsuo, S.; Kobayashi, S.; Ono, K. Magnetization Process of Concave Fe3O4 Nanoparticles. J. Phys. Chem. Solids 2026, 208, 113020. [Google Scholar] [CrossRef]
- Solodov, A.N.; Shayimova, J.R.; Burilova, E.A.; Shurtakova, D.V.; Zhuravleva, Y.I.; Cherosov, M.A.; Tian, Y.; Kiiamov, A.G.; Amirov, R.R. Understanding the Nucleation and Growth of Iron Oxide Nanoparticle Formation by a “Heating-Up” Process: An NMR Relaxation Study. J. Phys. Chem. C 2021, 125, 20980–20992. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod Iron Oxide Nanoparticles as High-Performance T2 Contrast Agents for Magnetic Resonance Imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Rahman, M.M.; Azhar, A.; Kühn, M.; Albrecht, M. Synthesis of Iron Oxide Nanoparticles by Decomposition of Iron-Oleate: Influence of the Heating Rate on the Particle Size. J. Nanopart. Res. 2022, 24, 183. [Google Scholar] [CrossRef]
- Lak, A.; Ludwig, F.; Scholtyssek, J.M.; Dieckhoff, J.; Fiege, K.; Schilling, M. Size Distribution and Magnetization Optimization of Single-Core Iron Oxide Nanoparticles by Exploiting Design of Experiment Methodology. IEEE Trans. Magn. 2013, 49, 201–207. [Google Scholar] [CrossRef]
- Llacer-Wintle, J.; Hertle, L.; Ziegler, S.; Pellicer, E.; Roca, A.G.; Nogués, J.; Puigmartí-Luis, J.; Nelson, B.J.; Chen, X.-Z.; Pané, S. A Simple In Situ Marker Guiding Shape-Controlled Synthesis of Iron Oxide Nanoparticles. Adv. Funct. Mate 2024, 34, 2404113. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Huang, X.; Retrum, J.; Schmucker, A.; Pink, M.; Stein, B.D.; Dragnea, B. Influence of Iron Oleate Complex Structure on Iron Oxide Nanoparticle Formation. Chem. Mater. 2007, 19, 3624–3632. [Google Scholar] [CrossRef]
- Kim, B.H.; Shin, K.; Kwon, S.G.; Jang, Y.; Lee, H.-S.; Lee, H.; Jun, S.W.; Lee, J.; Han, S.Y.; Yim, Y.-H.; et al. Sizing by Weighing: Characterizing Sizes of Ultrasmall-Sized Iron Oxide Nanocrystals Using MALDI-TOF Mass Spectrometry. J. Am. Chem. Soc. 2013, 135, 2407–2410. [Google Scholar] [CrossRef]
- Vreeland, E.C.; Watt, J.; Schober, G.B.; Hance, B.G.; Austin, M.J.; Price, A.D.; Fellows, B.D.; Monson, T.C.; Hudak, N.S.; Maldonado-Camargo, L.; et al. Enhanced Nanoparticle Size Control by Extending LaMer’s Mechanism. Chem. Mater. 2015, 27, 6059–6066. [Google Scholar] [CrossRef]
- Höfgen, E.G.; Bandyopadhyay, S. Insights into Semi-Continuous Synthesis of Iron Oxide Nanoparticles (IONPs) via Thermal Decomposition of Iron Oleate. Discov. Nano 2025, 20, 5. [Google Scholar] [CrossRef]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous Synthesis of Iron Oxide (Fe3O4) Nanoparticles via Thermal Decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Balakrishnan, T.; Lee, M.-J.; Dey, J.; Choi, S.-M. Sub-Nanometer Scale Size-Control of Iron Oxide Nanoparticles with Drying Time of Iron Oleate. CrystEngComm 2019, 21, 4063–4071. [Google Scholar] [CrossRef]
- Nader, K.; Castellanos-Rubio, I.; Orue, I.; Iglesias-Rojas, D.; Barón, A.; Gil de Muro, I.; Lezama, L.; Insausti, M. Getting Insight into How Iron(III) Oleate Precursors Affect the Features of Magnetite Nanoparticles. J. Solid State Chem. 2022, 316, 123619. [Google Scholar] [CrossRef]
- Chang, H.; Kim, B.H.; Jeong, H.Y.; Moon, J.H.; Park, M.; Shin, K.; Chae, S.I.; Lee, J.; Kang, T.; Choi, B.K.; et al. Molecular-Level Understanding of Continuous Growth from Iron-Oxo Clusters to Iron Oxide Nanoparticles. J. Am. Chem. Soc. 2019, 141, 7037–7045. [Google Scholar] [CrossRef]
- Kirkpatrick, K.M.; Zhou, B.H.; Bunting, P.C.; Rinehart, J.D. Size-Tunable Magnetite Nanoparticles from Well-Defined Iron Oleate Precursors. Chem. Mater. 2022, 34, 8043–8053. [Google Scholar] [CrossRef]
- Chang, H.; Kim, B.H.; Lim, S.G.; Baek, H.; Park, J.; Hyeon, T. Role of the Precursor Composition in the Synthesis of Metal Ferrite Nanoparticles. Inorg. Chem. 2021, 60, 4261–4268. [Google Scholar] [CrossRef]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Browning, N.D.; Krishnan, K.M. Synthesis of Phase-Pure and Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef]
- Chen, R.; Christiansen, M.G.; Sourakov, A.; Mohr, A.; Matsumoto, Y.; Okada, S.; Jasanoff, A.; Anikeeva, P. High-Performance Ferrite Nanoparticles through Nonaqueous Redox Phase Tuning. Nano Lett. 2016, 16, 1345–1351. [Google Scholar] [CrossRef]
- Walter, A.; Billotey, C.; Garofalo, A.; Ulhaq-Bouillet, C.; Lefèvre, C.; Taleb, J.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Lartigue, L.; et al. Mastering the Shape and Composition of Dendronized Iron Oxide Nanoparticles to Tailor Magnetic Resonance Imaging and Hyperthermia. Chem. Mater. 2014, 26, 5252–5264. [Google Scholar] [CrossRef]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse Magnetite Nanoparticles with Nearly Ideal Saturation Magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Plummer, L.K.; Hutchison, J.E. Understanding the Effects of Iron Precursor Ligation and Oxidation State Leads to Improved Synthetic Control for Spinel Iron Oxide Nanocrystals. Inorg. Chem. 2020, 59, 15074–15087. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Pichon, B.P.; Grenèche, J.-M.; Begin-Colin, S. Effect of Reaction Environment and In Situ Formation of the Precursor on the Composition and Shape of Iron Oxide Nanoparticles Synthesized by the Thermal Decomposition Method. CrystEngComm 2018, 20, 7206–7220. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Rodríguez-González, B.; Salgueiriño, V. Partial FeO–Fe3O4 Phase Transition Along the <111> Direction of the Cubic Crystalline Structure in Iron Oxide Nanocrystals. Part. Part. Syst. Charact. 2019, 36, 1900283. [Google Scholar]
- Pichon, B.P.; Gerber, O.; Lefevre, C.; Florea, I.; Fleutot, S.; Baaziz, W.; Pauly, M.; Ohlmann, M.; Ulhaq, C.; Ersen, O.; et al. Microstructural and Magnetic Investigations of Wüstite-Spinel Core-Shell Cubic-Shaped Nanoparticles. Chem. Mater. 2011, 23, 2886–2900. [Google Scholar] [CrossRef]
- Cotin, G.; Kiefer, C.; Perton, F.; Boero, M.; Özdamar, B.; Bouzid, A.; Ori, G.; Massobrio, C.; Begin, D.; Pichon, B.; et al. Evaluating the Critical Roles of Precursor Nature and Water Content When Tailoring Magnetic Nanoparticles for Specific Applications. ACS Appl. Nano Mater. 2018, 1, 4306–4316. [Google Scholar] [CrossRef]
- Cotin, G.; Kiefer, C.; Perton, F.; Ihiawakrim, D.; Blanco-Andujar, C.; Moldovan, S.; Lefevre, C.; Ersen, O.; Pichon, B.; Mertz, D.; et al. Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 881. [Google Scholar] [CrossRef]
- Perton, F.; Cotin, G.; Kiefer, C.; Strub, J.-M.; Cianferani, S.; Greneche, J.-M.; Parizel, N.; Heinrich, B.; Pichon, B.; Mertz, D.; et al. Iron Stearate Structures: An Original Tool for Nanoparticles Design. Inorg. Chem. 2021, 60, 12445–12456. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Heinrich, B.; Perton, F.; Kiefer, C.; Francius, G.; Mertz, D.; Freis, B.; Pichon, B.; Strub, J.-M.; Cianférani, S.; et al. A Confinement-Driven Nucleation Mechanism of Metal Oxide Nanoparticles Obtained via Thermal Decomposition in Organic Media. Small 2022, 18, 2200414. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lievanos, K.R.; Tariq, M.; Brennessel, W.W.; Knowles, K.E. Heterometallic Trinuclear Oxo-Centered Clusters as Single-Source Precursors for Synthesis of Stoichiometric Monodisperse Transition Metal Ferrite Nanocrystals. Dalton Trans. 2020, 49, 16348–16358. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, R.; Naka, T.; Kurihara, M.; Togashi, T. Size-Tunable Synthesis of Iron Oxide Nanocrystals by Continuous Seed-Mediated Growth: Role of Alkylamine Species in the Stepwise Thermal Decomposition of Iron(II) Oxalate. Dalton Trans. 2021, 50, 16021–16029. [Google Scholar] [CrossRef]
- Kampferbeck, M.; Klauke, L.R.; Weller, H.; Vossmeyer, T. Little Adjustments Significantly Simplify the Gram-Scale Synthesis of High-Quality Iron Oxide Nanocubes. Langmuir 2021, 37, 9851–9857. [Google Scholar] [CrossRef]
- Meftah, S.; Ngo, A.-T.; Bouteiller, L.; Russier, V.; Hrabovsky, D.; Konaté, A.; Kondo, D.; Bedoui, F.; Lisiecki, I. Synthesis and Magnetic Properties of Spherical Maghemite Nanoparticles with Tunable Size and Surface Chemistry. Langmuir 2024, 40, 22673–22683. [Google Scholar] [CrossRef]
- Corrêa, B.S.; Costa, M.S.; Cabrera-Pasca, G.A.; Sena, C.; Holanda Pinto, R.H.; Silva, A.P.S.; Carvalho Junior, R.N.; Ishida, L.; Ramon, J.G.A.; Freitas, R.S.; et al. High-Saturation Magnetization in Small Nanoparticles of Fe3O4 Coated with Natural Oils. J. Nanoparticle Res. 2020, 22, 68. [Google Scholar] [CrossRef]
- Muro-Cruces, J.; Roca, A.G.; López-Ortega, A.; Fantechi, E.; del-Pozo-Bueno, D.; Estradé, S.; Peiró, F.; Sepúlveda, B.; Pineider, F.; Sangregorio, C.; et al. Precise Size Control of the Growth of Fe3O4 Nanocubes over a Wide Size Range Using a Rationally Designed One-Pot Synthesis. ACS Nano 2019, 13, 7716–7728. [Google Scholar] [CrossRef]
- Kim, D.; Lee, N.; Park, M.; Kim, B.H.; An, K.; Hyeon, T. Synthesis of Uniform Ferrimagnetic Magnetite Nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar] [CrossRef]
- Qiao, L.; Fu, Z.; Li, J.; Ghosen, J.; Zeng, M.; Stebbins, J.; Prasad, P.N.; Swihart, M.T. Standardizing Size- and Shape-Controlled Synthesis of Monodisperse Magnetite (Fe3O4) Nanocrystals by Identifying and Exploiting Effects of Organic Impurities. ACS Nano 2017, 11, 6370–6381. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Ho, C.-H.; Tsai, C.-P.; Chung, C.-C.; Tsai, C.-Y.; Chen, F.-R.; Lin, H.-J.; Lai, C.-H. Shape-Controlled Growth and Shape-Dependent Cation Site Occupancy of Monodisperse Fe3O4 Nanoparticles. Chem. Mater. 2011, 23, 1753–1760. [Google Scholar] [CrossRef]
- Escoda-Torroella, M.; Moya, C.; Rodríguez, A.F.; Batlle, X.; Labarta, A. Selective Control over the Morphology and the Oxidation State of Iron Oxide Nanoparticles. Langmuir 2021, 37, 35–45. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Bao, H.; Gao, M. One-Pot Reaction to Synthesize Water-Soluble Magnetite Nanocrystals. Chem. Mater. 2004, 16, 1391–1393. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vargas, J.M.; Pirota, K.R.; Kumar, S.; Lee, C.G.; Knobel, M. Synthesis and Ageing Effect in FeO Nanoparticles: Transformation to Core–Shell FeO/Fe3O4 and Their Magnetic Characterization. J. Alloys Compd. 2011, 509, 6414–6417. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and Magnetic Properties of Uniform Magnetite Nanoparticles Prepared by High Temperature Decomposition of Organic Precursors. Nanotechnology 2006, 17, 2783. [Google Scholar] [CrossRef]
- Effenberger, F.B.; Couto, R.A.; Kiyohara, P.K.; Machado, G.; Masunaga, S.H.; Jardim, R.F.; Rossi, L.M. Economically Attractive Route for the Preparation of High Quality Magnetic Nanoparticles by the Thermal Decomposition of Iron(III) Acetylacetonate. Nanotechnology 2017, 28, 115603. [Google Scholar] [CrossRef]
- Dehsari, H.S.; Ribeiro, A.H.; Ersöz, B.; Tremel, W.; Jakob, G.; Asadi, K. Effect of Precursor Concentration on Size Evolution of Iron Oxide Nanoparticles. CrystEngComm 2017, 19, 6694–6702. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Toyos-Rodríguez, C.; Calleja-García, J.; Torres-Sánchez, L.; López, A.; Abu-Dief, A.M.; Costa, A.; Elbaile, L.; Crespo, R.D.; Garitaonandia, J.S.; Lastra, E.; et al. A Simple and Reliable Synthesis of Superparamagnetic Magnetite Nanoparticles by Thermal Decomposition of Fe(Acac)3. J. Nanomater. 2019, 2019, 2464010. [Google Scholar] [CrossRef]
- Theppaleak, T.; Tumcharern, G.; Wichai, U.; Rutnakornpituk, M. Synthesis of Water Dispersible Magnetite Nanoparticles in the Presence of Hydrophilic Polymers. Polym. Bull. 2009, 63, 79–90. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Xu, F.; Wei, X. One-Pot Reaction to Synthesize Superparamagnetic Iron Oxide Nanoparticles by Adding Phenol as Reducing Agent and Stabilizer. J. Nanoparticle Res. 2012, 14, 755. [Google Scholar] [CrossRef]
- Pinna, N.; Grancharov, S.; Beato, P.; Bonville, P.; Antonietti, M.; Niederberger, M. Magnetite Nanocrystals: Nonaqueous Synthesis, Characterization, and Solubility. Chem. Mater. 2005, 17, 3044–3049. [Google Scholar] [CrossRef]
- Moya, C.; Batlle, X.; Labarta, A. The Effect of Oleic Acid on the Synthesis of Fe3−xO4 Nanoparticles over a Wide Size Range. Phys. Chem. Chem. Phys. 2015, 17, 27373–27379. [Google Scholar] [CrossRef]
- Shi, R.; Gao, G.; Yi, R.; Zhou, K.; Qiu, G.; Liu, X. Controlled Synthesis and Characterization of Monodisperse Fe3O4 Nanoparticles. Chin. J. Chem. 2009, 27, 739–744. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Gawali, S.L.; Barick, B.K.; Barick, K.C.; Babu, P.D.; Pandey, B.N.; Priyadarsini, K.I.; Hassan, P.A. PEG Mediated Shape-Selective Synthesis of Cubic Fe3O4 Nanoparticles for Cancer Therapeutics. J. Alloys Compd. 2018, 737, 347–355. [Google Scholar] [CrossRef]
- Jović Orsini, N.; Babić-Stojić, B.; Spasojević, V.; Calatayud, M.P.; Cvjetićanin, N.; Goya, G.F. Magnetic and Power Absorption Measurements on Iron Oxide Nanoparticles Synthesized by Thermal Decomposition of Fe(Acac)3. J. Magn. Magn. Mater. 2018, 449, 286–296. [Google Scholar] [CrossRef]
- Oliveira, F.C.C.; Effenberger, F.B.; Sousa, M.H.; Jardim, R.F.; Kiyohara, P.K.; Dupont, J.; Rubim, J.C.; Rossi, L.M. Ionic Liquids as Recycling Solvents for the Synthesis of Magnetic Nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 13558–13564. [Google Scholar] [CrossRef]
- Wagle, D.V.; Rondinone, A.J.; Woodward, J.D.; Baker, G.A. Polyol Synthesis of Magnetite Nanocrystals in a Thermostable Ionic Liquid. Cryst. Growth Des. 2017, 17, 1558–1567. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Chen, Y.-T.; Chinwangso, P.; Nekrashevich, I.; Dannangoda, G.C.; Singh, A.; Jamison, A.C.; Zenasni, O.; Rusakova, I.A.; Martirosyan, K.S.; et al. Magnetic Sensing Potential of Fe3O4 Nanocubes Exceeds That of Fe3O4 Nanospheres. ACS Omega 2017, 2, 8010–8019. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Deng, L.; Lee, J.M.; Resendez, K.M.; Fuller, M.; Hoijang, S.; Robles-Hernandez, F.; Chu, C.-W.; Litvinov, D.; Hadjiev, V.G.; et al. Magnetic Tunability via Control of Crystallinity and Size in Polycrystalline Iron Oxide Nanoparticles. Small 2024, 20, 2402940. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Pérez, N.; Labarta, A.; Batlle, X. Controlled Synthesis of Iron Oxide Nanoparticles over a Wide Size Range. Langmuir 2010, 26, 5843–5847. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Pérez-Juste, J.; Labarta, A.; Batlle, X.; Liz-Marzán, L.M. Heating Rate Influence on the Synthesis of Iron Oxide Nanoparticles: The Case of Decanoic Acid. Chem. Commun. 2010, 46, 6108–6110. [Google Scholar] [CrossRef]
- Guardia, P.; Riedinger, A.; Nitti, S.; Pugliese, G.; Marras, S.; Genovese, A.; Materia, M.E.; Lefevre, C.; Manna, L.; Pellegrino, T. One Pot Synthesis of Monodisperse Water Soluble Iron Oxide Nanocrystals with High Values of the Specific Absorption Rate. J. Mater. Chem. B 2014, 2, 4426–4434. [Google Scholar] [CrossRef]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.K.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios Between the Chemicals. ACS Omega 2022, 7, 15996–16012. [Google Scholar] [CrossRef]
- Mekseriwattana, W.; Silvestri, N.; Brescia, R.; Tiryaki, E.; Barman, J.; Mohammadzadeh, F.G.; Jarmouni, N.; Pellegrino, T. Shape-Control in Microwave-Assisted Synthesis: A Fast Route to Size-Tunable Iron Oxide Nanocubes with Benchmark Magnetic Heat Losses. Adv. Funct. Mater. 2025, 35, 2413514. [Google Scholar] [CrossRef]
- Gavilán, H.; Rizzo, G.M.; Silvestri, N.; Mai, B.T.; Pellegrino, T. Scale-Up Approach for the Preparation of Magnetic Ferrite Nanocubes and Other Shapes with Benchmark Performance for Magnetic Hyperthermia Applications. Nat. Protoc. 2023, 18, 783–809. [Google Scholar] [CrossRef]
- Lohr, J.; Vasquez Mansilla, M.; Gerbaldo, M.V.; Moreno, M.S.; Tobia, D.; Goya, G.F.; Winkler, E.L.; Zysler, R.D.; Lima, E. Dependence of the Composition, Morphology and Magnetic Properties with the Water and Air Exposure During the Fe1-yO/Fe3O4 Core–Shell Nanoparticles Synthesis. J. Nanoparticle Res. 2021, 23, 140. [Google Scholar] [CrossRef]
- McDonagh, B.H.; Staudinger, C.; Normile, P.S.; De Toro, J.A.; Bandyopadhyay, S.; Glomm, W.R.; Singh, G. New Insights into Controlling the Twin Structure of Magnetic Iron Oxide Nanoparticles. Appl. Mater. Today 2021, 24, 101084. [Google Scholar] [CrossRef]
- Jiao, M.; Zeng, J.; Jing, L.; Liu, C.; Gao, M. Flow Synthesis of Biocompatible Fe3O4 Nanoparticles: Insight into the Effects of Residence Time, Fluid Velocity, and Tube Reactor Dimension on Particle Size Distribution. Chem. Mater. 2015, 27, 1299–1305. [Google Scholar] [CrossRef]
- Uson, L.; Arruebo, M.; Sebastian, V.; Santamaria, J. Single Phase Microreactor for the Continuous, High-Temperature Synthesis of <4 nm Superparamagnetic Iron Oxide Nanoparticles. Chem. Eng. J. 2018, 340, 66–72. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Famiani, S.; Pucciarelli, M.; Lettieri, P.; Thanh, N.T.K.; Gavriilidis, A. Continuous Production of Iron Oxide Nanoparticles via Fast and Economical High Temperature Synthesis. React. Chem. Eng. 2020, 5, 1474–1483. [Google Scholar] [CrossRef]
- Suslick, K.S.; Goodale, J.W.; Schubert, P.F.; Wang, H.H. Sonochemistry and Sonocatalysis of Metal Carbonyls. J. Am. Chem. Soc. 1983, 105, 5781–5785. [Google Scholar] [CrossRef]
- Lassenberger, A.; Grünewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition: Elucidating Particle Formation by Second-Resolved in Situ Small-Angle X-Ray Scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef]
- Woo, K.; Hong, J.; Choi, S.; Lee, H.-W.; Ahn, J.-P.; Kim, C.S.; Lee, S.W. Easy Synthesis and Magnetic Properties of Iron Oxide Nanoparticles. Chem. Mater. 2004, 16, 2814–2818. [Google Scholar] [CrossRef]
- Martínez-Boubeta, C.; Simeonidis, K.; Angelakeris, M.; Pazos-Pérez, N.; Giersig, M.; Delimitis, A.; Nalbandian, L.; Alexandrakis, V.; Niarchos, D. Critical Radius for Exchange Bias in Naturally Oxidized Fe Nanoparticles. Phys. Rev. B 2006, 74, 054430. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, F.Y.; Chen, K.; Kang, F.; Tang, Z.K. Size-Controlled Synthesis of Monodisperse Superparamagnetic Iron Oxide Nanoparticles. J. Alloys Compd. 2011, 509, 8549–8553. [Google Scholar] [CrossRef]
- Gonzales, M.; Mitsumori, L.M.; Kushleika, J.V.; Rosenfeld, M.E.; Krishnan, K.M. Cytotoxicity of Iron Oxide Nanoparticles Made from the Thermal Decomposition of Organometallics and Aqueous Phase Transfer with Pluronic F127. Contrast Media Mol. Imaging 2010, 5, 286–293. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Moulla, M.; Tsiaoussis, I.; Martinez-Boubeta, C.; Angelakeris, M.; Dendrinou-Samara, C.; Kalogirou, O. Controlled Synthesis and Phase Characterization of Fe-Based Nanoparticles Obtained by Thermal Decomposition. J. Magn. Magn. Mater. 2007, 316, e1–e4. [Google Scholar] [CrossRef]
- Shao, H.; Lee, H.; Huang, Y.; Ko, I.; Kim, C. Control of Iron Nanoparticles Size and Shape by Thermal Decomposition Method. IEEE Trans. Magn. 2005, 41, 3388–3390. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lim, N.H.; Seo, J.A.; Yuk, S.H.; Kwak, B.K.; Khang, G.; Lee, H.B.; Cho, S.H. Preparation and Magnetic Resonance Imaging Effect of Polyvinylpyrrolidone-Coated Iron Oxide Nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79B, 142–150. [Google Scholar] [CrossRef]
- Redl, F.X.; Black, C.T.; Papaefthymiou, G.C.; Sandstrom, R.L.; Yin, M.; Zeng, H.; Murray, C.B.; O’Brien, S.P. Magnetic, Electronic, and Structural Characterization of Nonstoichiometric Iron Oxides at the Nanoscale. J. Am. Chem. Soc. 2004, 126, 14583–14599. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Snoeck, E.; Garcia, M.A.; Giannini, C.; Guagliardi, A.; Cervellino, A.; Gozzo, F.; Hernando, A.; Achterhold, K.; Ciobanu, N.; et al. Colloidal Synthesis and Characterization of Tetrapod-Shaped Magnetic Nanocrystals. Nano Lett. 2006, 6, 1966–1972. [Google Scholar] [CrossRef]
- Sun, H.; Chen, B.; Jiao, X.; Jiang, Z.; Qin, Z.; Chen, D. Solvothermal Synthesis of Tunable Electroactive Magnetite Nanorods by Controlling the Side Reaction. J. Phys. Chem. C 2012, 116, 5476–5481. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Nemati Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.-H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Mahin, J.; Torrente-Murciano, L. Continuous Synthesis of Monodisperse Iron@Iron Oxide Core@Shell Nanoparticles. Chem. Eng. J. 2020, 396, 125299. [Google Scholar] [CrossRef]
- Lacroix, L.-M.; Frey Huls, N.; Ho, D.; Sun, X.; Cheng, K.; Sun, S. Stable Single-Crystalline Body Centered Cubic Fe Nanoparticles. Nano Lett. 2011, 11, 1641–1645. [Google Scholar] [CrossRef]
- Mehta, J.P.; Knappett, B.R.; Divitini, G.; Ringe, E.; Midgley, P.A.; Fairen-Jimenez, D.; Wheatley, A.E.H. Advances in the Synthesis and Long-Term Protection of Zero-Valent Iron Nanoparticles. Part. Part. Syst. Charact. 2018, 35, 1800120. [Google Scholar] [CrossRef]
- Koo, C.; Hong, H.; Im, P.W.; Kim, H.; Lee, C.; Jin, X.; Yan, B.; Lee, W.; Im, H.-J.; Paek, S.H.; et al. Magnetic and Near-Infrared Derived Heating Characteristics of Dimercaptosuccinic Acid Coated Uniform Fe@Fe3O4 Core–Shell Nanoparticles. Nano Converg. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, P.A.; Lundgren, C.; van Helvoort, A.T.J.; Mathieu, R.; Wahlström, E.; Glomm, W.R. Tunability in Crystallinity and Magnetic Properties of Core–Shell Fe Nanoparticles. Part. Part. Syst. Charact. 2014, 31, 1054–1059. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Khurshid, H.; Phan, M.H.; Srikanth, H. Core/Shell Iron/Iron Oxide Nanoparticles: Are They Promising for Magnetic Hyperthermia? RSC Adv. 2016, 6, 38697–38702. [Google Scholar] [CrossRef]
- Estrader, M.; López-Ortega, A.; Golosovsky, I.V.; Estradé, S.; Roca, A.G.; Salazar-Alvarez, G.; López-Conesa, L.; Tobia, D.; Winkler, E.; Ardisson, J.D.; et al. Origin of the Large Dispersion of Magnetic Properties in Nanostructured Oxides: FexO/Fe3O4 Nanoparticles as a Case Study. Nanoscale 2015, 7, 3002–3015. [Google Scholar] [CrossRef]
- Xie, L.; Shen, Y.; Franke, D.; Sebastián, V.; Bawendi, M.G.; Jensen, K.F. Characterization of Indium Phosphide Quantum Dot Growth Intermediates Using MALDI-TOF Mass Spectrometry. J. Am. Chem. Soc. 2016, 138, 13469–13472. [Google Scholar] [CrossRef]
- Li, J.; Yang, T.; Chan, W.H.; Choi, M.M.F.; Zhao, D. Synthesis of High-Quality N-Acetyl-l-Cysteine-Capped CdTe Quantum Dots by Hydrothermal Route and the Characterization through MALDI-TOF Mass Spectrometry. J. Phys. Chem. C 2013, 117, 19175–19181. [Google Scholar] [CrossRef]
- Kim, B.H.; Chang, H.; Hackett, M.J.; Park, J.; Seo, P.; Hyeon, T. Size Characterization of Ultrasmall Silver Nanoparticles Using MALDI-TOF Mass Spectrometry. Bull. Korean Chem. Soc. 2014, 35, 961–964. [Google Scholar] [CrossRef]
- Sandler, S.E.; Fellows, B.; Mefford, O.T. Best Practices for Characterization of Magnetic Nanoparticles for Biomedical Applications. Anal. Chem. 2019, 91, 14159–14169. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ingham, B.; Toney, M.F.; Tilley, R.D. Effect of Surfactant Concentration and Aggregation on the Growth Kinetics of Nickel Nanoparticles. J. Phys. Chem. C 2013, 117, 16709–16718. [Google Scholar] [CrossRef]
- Tancredi, P.; Rojas, P.C.R.; Moscoso-Londoño, O.; Wolff, U.; Neu, V.; Damm, C.; Rellinghaus, B.; Knobel, M.; Socolovsky, L.M. Synthesis Process, Size and Composition Effects of Spherical Fe3O4 and FeO@Fe3O4 Core/Shell Nanoparticles. New J. Chem. 2017, 41, 15033–15041. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman Spectroscopy to Unravel the Magnetic Properties of Iron Oxide Nanocrystals for Bio-Related Applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Fock, J.; Bogart, L.K.; González-Alonso, D.; Espeso, J.I.; Hansen, M.F.; Varón, M.; Frandsen, C.; Pankhurst, Q.A. On the ‘Centre of Gravity’ Method for Measuring the Composition of Magnetite/Maghemite Mixtures, or the Stoichiometry of Magnetite-Maghemite Solid Solutions, via 57Fe Mössbauer Spectroscopy. J. Phys. D Appl. Phys. 2017, 50, 265005. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite–Maghemite Nanoparticles in the 5–15 Nm Range: Correlating the Core–Shell Composition and the Surface Structure to the Magnetic Properties. A Total Scattering Study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef]
- Guimarães, A.P. Principles of Nanomagnetism; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mohapatra, J.; Liu, J.P. Chapter 1-Rare-Earth-Free Permanent Magnets: The Past and Future. In Handbook of Magnetic Materials; Brück, E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 1–57. [Google Scholar]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Edi. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Noh, S.; Na, W.; Jang, J.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Mohapatra, J.; Zeng, F.; Elkins, K.; Xing, M.; Ghimire, M.; Yoon, S.; Mishra, S.R.; Liu, J.P. Size-Dependent Magnetic and Inductive Heating Properties of Fe3O4 Nanoparticles: Scaling Laws across the Superparamagnetic Size. Phys. Chem. Chem. Phys. 2018, 20, 12879–12887. [Google Scholar] [CrossRef]
- Lin, C.-R.; Chiang, R.-K.; Wang, J.-S.; Sung, T.-W. Magnetic Properties of Monodisperse Iron Oxide Nanoparticles. J. Appl. Phys. 2006, 99, 08N710. [Google Scholar] [CrossRef]
- Shliomis, M.I.; Stepanov, V.I. Theory of the Dynamic Susceptibility of Magnetic Fluids. In Advances in Chemical Physics; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 1–30. [Google Scholar]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-Dependent Properties of Magnetic Iron Oxide Nanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shah, N.; Huffman, G.P. Size Dependence of Magnetic Parameters and Surface Disorder in Magnetite Nanoparticles. J. Appl. Phys. 2009, 105, 07B501. [Google Scholar] [CrossRef]
- Yu, W.W.; Falkner, J.C.; Yavuz, C.T.; Colvin, V.L. Synthesis of Monodisperse Iron Oxide Nanocrystals by Thermal Decomposition of Iron Carboxylate Salts. Chem. Commun. 2004, 2306–2307. [Google Scholar] [CrossRef]
- Andersson, M.S.; Mathieu, R.; Lee, S.S.; Normile, P.S.; Singh, G.; Nordblad, P.; Toro, J.A.D. Size-Dependent Surface Effects in Maghemite Nanoparticles and Its Impact on Interparticle Interactions in Dense Assemblies. Nanotechnology 2015, 26, 475703. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Miao, Y.Q.; Yu, B.Z.; Ma, P.; Li, L.; Fan, H.M. Large-Scale, Facile Transfer of Oleic Acid-Stabilized Iron Oxide Nanoparticles to the Aqueous Phase for Biological Applications. Langmuir 2017, 33, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.C.; Seabra, A.B.; Pelegrino, M.T.; Araujo, D.R.d.; Bernardes, J.S.; Haddad, P.S. Superparamagnetic Iron Oxide Nanoparticles Dispersed in Pluronic F127 Hydrogel: Potential Uses in Topical Applications. RSC Adv. 2017, 7, 14496–14503. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Garcia-Martin, M.L.; Leal, M.P. Deciphering the Parameters to Produce Highly Reproducible and Scalable Iron Oxide Nanoparticles. React. Chem. Eng. 2023, 8, 1638–1653. [Google Scholar] [CrossRef]
- Wei, H.; Insin, N.; Lee, J.; Han, H.-S.; Cordero, J.M.; Liu, W.; Bawendi, M.G. Compact Zwitterion-Coated Iron Oxide Nanoparticles for Biological Applications. Nano Lett. 2012, 12, 22–25. [Google Scholar] [CrossRef]
- Mondini, S.; Leonzino, M.; Drago, C.; Ferretti, A.M.; Usseglio, S.; Maggioni, D.; Tornese, P.; Chini, B.; Ponti, A. Zwitterion-Coated Iron Oxide Nanoparticles: Surface Chemistry and Intracellular Uptake by Hepatocarcinoma (HepG2) Cells. Langmuir 2015, 31, 7381–7390. [Google Scholar] [CrossRef]
- Panja, P.; Debnath, K.; Jana, N.R.; Jana, N.R. Surface Chemistry- and Intracellular Trafficking-Dependent Autophagy Induction by Iron Oxide Nanoparticles. ACS Appl. Bio Mater. 2020, 3, 5974–5983. [Google Scholar] [CrossRef]
- Mandal, S.; Nguyen, M.D.; Jana, N.R.; Lee, T.R. Nanoparticles with Ampholytic Surfaces for Binding and Disintegration of Amyloid Fibrils. ACS Cent. Sci. 2025, 11, 1218–1229. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Kostiv, U.; Patsula, V.; Šlouf, M.; Pongrac, I.M.; Škokić, S.; Radmilović, M.D.; Pavičić, I.; Vrček, I.V.; Gajović, S.; Horák, D. Physico-Chemical Characteristics, Biocompatibility, and MRI Applicability of Novel Monodisperse PEG-Modified Magnetic Fe3O4&SiO2 Core–Shell Nanoparticles. RSC Adv. 2017, 7, 8786–8797. [Google Scholar]
- Qi, G.; Shi, G.; Wang, S.; Hu, H.; Zhang, Z.; Yin, Q.; Li, Z.; Hao, L. A Novel pH-Responsive Iron Oxide Core-Shell Magnetic Mesoporous Silica Nanoparticle (M-MSN) System Encapsulating Doxorubicin (DOX) and Glucose Oxidase (Gox) for Pancreatic Cancer Treatment. Int. J. Nanomed. 2023, 18, 7133–7147. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Ahn, J.-P.; Han, S.Y.; Lee, N.-S.; Jeong, Y.G.; Kim, D.K. Highly Luminescent and Anti-Photobleaching Core-Shell Structure of Mesoporous Silica and Phosphatidylcholine Modified Superparamagnetic Iron Oxide Nanoparticles. Nanomaterials 2020, 10, 1312. [Google Scholar] [CrossRef]
- Ménard, M.; Ali, L.M.A.; Vardanyan, A.; Charnay, C.; Raehm, L.; Cunin, F.; Bessière, A.; Oliviero, E.; Theodossiou, T.A.; Seisenbaeva, G.A.; et al. Upscale Synthesis of Magnetic Mesoporous Silica Nanoparticles and Application to Metal Ion Separation: Nanosafety Evaluation. Nanomaterials 2023, 13, 3155. [Google Scholar] [CrossRef]
- Shatan, A.B.; Venclíková, K.; Zasońska, B.A.; Patsula, V.; Pop-Georgievski, O.; Petrovský, E.; Horák, D. Antibacterial Silver-Conjugated Magnetic Nanoparticles: Design, Synthesis and Bactericidal Effect. Pharm. Res. 2019, 36, 147. [Google Scholar] [CrossRef]
- Hegazy, M.; Zhou, P.; Wu, G.; Wang, L.; Rahoui, N.; Taloub, N.; Huang, X.; Huang, Y. Construction of Polymer Coated Core–Shell Magnetic Mesoporous Silica Nanoparticles with Triple Responsive Drug Delivery. Polym. Chem. 2017, 8, 5852–5864. [Google Scholar] [CrossRef]
- Zhao, X.; Shang, T.; Zhang, X.; Ye, T.; Wang, D.; Rei, L. Passage of Magnetic Tat-Conjugated Fe3O4@SiO2 Nanoparticles Across In Vitro Blood-Brain Barrier. Nanoscale Res. Lett. 2016, 11, 451. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Medhi, R.; Nekrashevich, I.; Litvinov, D.; Xu, S.; Lee, T.R. Specific Detection of Proteins Using Exceptionally Responsive Magnetic Particles. Anal. Chem. 2018, 90, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Maximenko, A.; Depciuch, J.; Łopuszyńska, N.; Stec, M.; Światkowska-Warkocka, Ż.; Bayev, V.; Zieliński, P.M.; Baran, J.; Fedotova, J.; Węglarz, W.P.; et al. Fe3O4@SiO2@Au Nanoparticles for MRI-Guided Chemo/NIR Photothermal Therapy of Cancer Cells. RSC Adv. 2020, 10, 26508–26520. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.-I.; Crăciun, L.N.; Ficai, D.; Ficai, A.; Andronescu, E. Magnetite-Silica Core/Shell Nanostructures: From Surface Functionalization towards Biomedical Applications—A Review. Appl. Sci. 2021, 11, 11075. [Google Scholar] [CrossRef]
- Asad, S.; Ahl, D.; Suárez-López, Y.d.C.; Erdélyi, M.; Phillipson, M.; Teleki, A. Click Chemistry-Based Bioconjugation of Iron Oxide Nanoparticles. Small 2025, 21, 2407883. [Google Scholar] [CrossRef]
- Mejías, R.; Gutiérrez, L.; Salas, G.; Pérez-Yagüe, S.; Zotes, T.M.; Lázaro, F.J.; Morales, M.P.; Barber, D.F. Long Term Biotransformation and Toxicity of Dimercaptosuccinic Acid-Coated Magnetic Nanoparticles Support Their Use in Biomedical Applications. J. Control. Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- Paulini, F.; Marangon, A.R.M.; Azevedo, C.L.; Brito, J.L.M.; Lemos, M.S.; Sousa, M.H.; Veiga-Souza, F.H.; Souza, P.E.N.; Lucci, C.M.; Azevedo, R.B. In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up. Nanomaterials 2022, 12, 3513. [Google Scholar] [CrossRef]
- Deblock, L.; Goossens, E.; Pokratath, R.; De Buysser, K.; De Roo, J. Mapping out the Aqueous Surface Chemistry of Metal Oxide Nanocrystals: Carboxylate, Phosphonate, and Catecholate Ligands. JACS Au 2022, 2, 711–722. [Google Scholar] [CrossRef]
- Guénin, E.; Lalatonne, Y.; Bolley, J.; Milosevic, I.; Platas-Iglesias, C.; Motte, L. Catechol versus Bisphosphonate Ligand Exchange at the Surface of Iron Oxide Nanoparticles: Towards Multi-Functionalization. J. Nanopart. Res. 2014, 16, 2596. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Chen, O.; Bawendi, M.G. Compact Zwitterion-Coated Iron Oxide Nanoparticles for In Vitro and In Vivo Imaging. Int. Bio. 2013, 5, 108–114. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.D.; Hoijang, S.; Yarinia, R.; Ariza Gonzalez, M.; Mandal, S.; Tran, Q.M.; Chinwangso, P.; Lee, T.R. Magnetic Iron Oxide Nanoparticles: Advances in Synthesis, Mechanistic Understanding, and Magnetic Property Optimization for Improved Biomedical Performance. Nanomaterials 2025, 15, 1500. https://doi.org/10.3390/nano15191500
Nguyen MD, Hoijang S, Yarinia R, Ariza Gonzalez M, Mandal S, Tran QM, Chinwangso P, Lee TR. Magnetic Iron Oxide Nanoparticles: Advances in Synthesis, Mechanistic Understanding, and Magnetic Property Optimization for Improved Biomedical Performance. Nanomaterials. 2025; 15(19):1500. https://doi.org/10.3390/nano15191500
Chicago/Turabian StyleNguyen, Minh Dang, Supawitch Hoijang, Ramtin Yarinia, Melissa Ariza Gonzalez, Suman Mandal, Quoc Minh Tran, Pailinrut Chinwangso, and T. Randall Lee. 2025. "Magnetic Iron Oxide Nanoparticles: Advances in Synthesis, Mechanistic Understanding, and Magnetic Property Optimization for Improved Biomedical Performance" Nanomaterials 15, no. 19: 1500. https://doi.org/10.3390/nano15191500
APA StyleNguyen, M. D., Hoijang, S., Yarinia, R., Ariza Gonzalez, M., Mandal, S., Tran, Q. M., Chinwangso, P., & Lee, T. R. (2025). Magnetic Iron Oxide Nanoparticles: Advances in Synthesis, Mechanistic Understanding, and Magnetic Property Optimization for Improved Biomedical Performance. Nanomaterials, 15(19), 1500. https://doi.org/10.3390/nano15191500







