Nanoparticles Enhance In Vitro Micropropagation and Secondary Metabolite Accumulation in Origanum petraeum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Source and In Vitro Establishment
2.2. Nanoparticles Applications
2.3. Transmission Electron Microscopy (TEM) Imaging of Roots and Leaves for Nanoparticle Tracking
2.4. Phytochemical Analysis
2.4.1. Essential Oil Extraction Using Hydrodistillation
2.4.2. Analysis of Essential Oils Using Gas Chromatography with Flame Ionization Detection (GC-FID)
2.4.3. Analysis of Essential Oils Using GC-MS
2.5. Effect of Nanoparticles on Nutrients Uptake in Microshoots
2.6. Experiments Designations and Analysis of Data
3. Results and Discussion
3.1. Effect of the NPs Application on O. petraeum Microshoots Growth
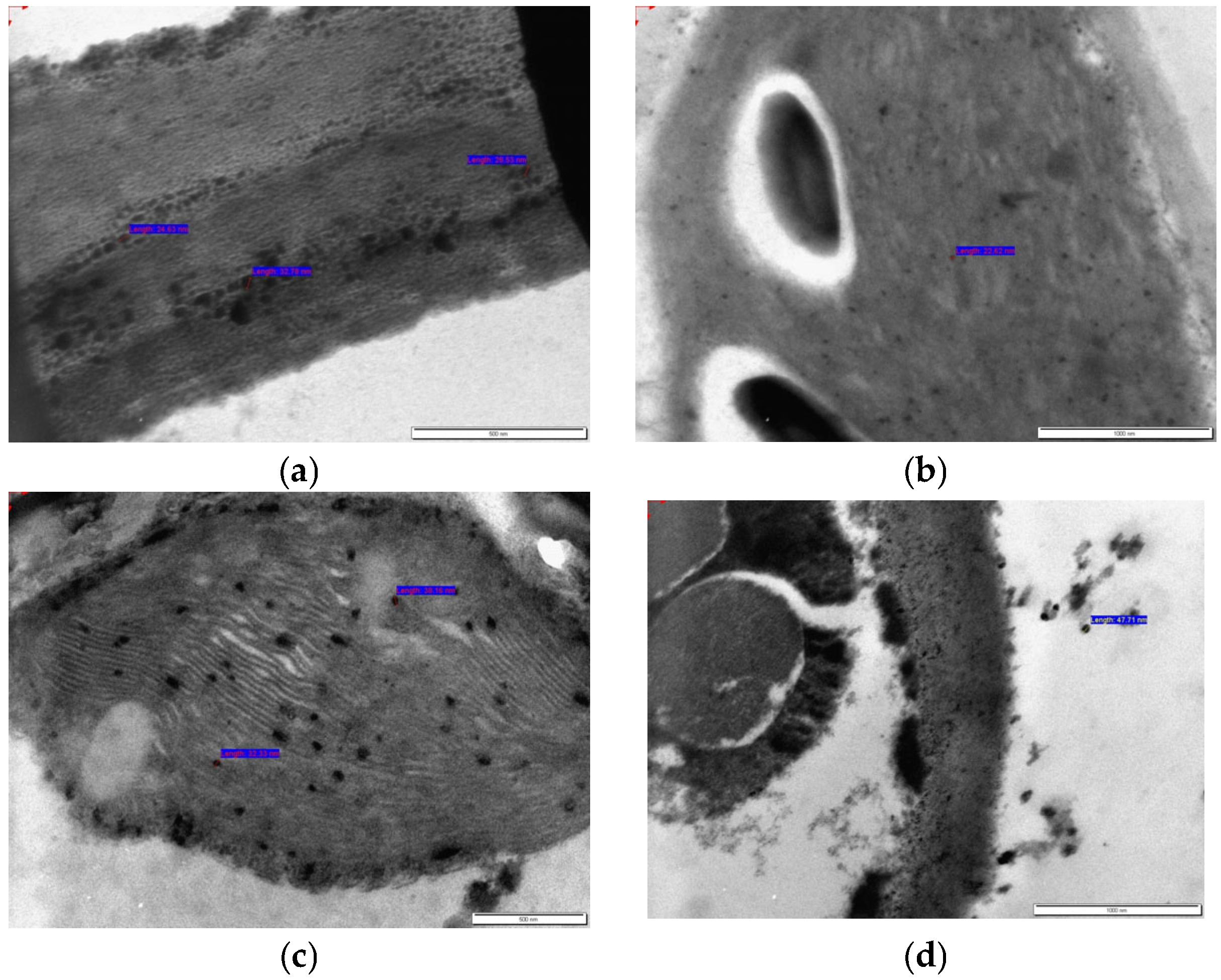
3.2. Transmission Electron Microscopy (TEM) Imaging of Roots and Leaves for Nanoparticle Tracking in O. petraeum Plant Samples
3.3. Effect of Nanoparticles on the Phytochemical Composition of In Vitro O. petraeum
3.4. Effect of Nanoparticles on Nutrient Uptake in In Vitro O. petraeum Microshoots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Taifour, H.; El-Oqlah, A. Jordan Plant Red List, 1st ed.; Royal Botanic Garden: Amman, Jordan, 2014; pp. 78, 572. [Google Scholar]
- Oran, S.A.; Al-Eisawi, D.M. Medicinal plants in the high mountains of northern Jordan. Int. J. Biodivers. Conserv. 2014, 6, 436–443. [Google Scholar] [CrossRef][Green Version]
- Mechergui, K.; Jaouadi, W.; Coelho, J.A.; Serra, M.C.; Khouja, M.L. Biological activities and oil properties of Origanum glandulosum Desf: A review. Phytothérapie 2016, 14, 102–108. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Danin, A. Two new species of Origanum (Labiatae) from Jordan. Willdenowia 1990, 19, 401–404. [Google Scholar]
- Debnath, M.; Malik, C.P. Micropropagation: A tool for production of high-quality plant-based medicines. Curr. Pharm. Biotechnol. 2006, 7, 33–49. [Google Scholar] [CrossRef]
- Al-Qudah, T.S.; Shibli, R.A.; Alali, F.Q. In vitro propagation and secondary metabolites production in wild germander (Teucrium polium L.). In Vitro Cell. Dev. Biol. Plant 2011, 47, 496–505. [Google Scholar] [CrossRef]
- Mherat, M.; Shatnawi, M.; Shibli, R.; Qudah, T.; Malloh, S.A.; Tamadour, S.Q. Clonal propagation of Tetragonolobus palaestinus Bioss: A Jordanian medicinal plant. Acta Agric. Slovenica 2022, 118, 1–9. [Google Scholar] [CrossRef]
- Al-Qudah, T.; Mahmood, S.H.; Abu-Zurayk, R.; Shibli, R.; Khalaf, A.; Lambat, T.L.; Chaudhary, R.G. Nanotechnology applications in plant tissue culture and molecular genetics: A holistic approach. Curr. Nanosci. 2022, 18, 442–464. [Google Scholar] [CrossRef]
- Shibli, R.; Mohusaien, R.; Abu-Zurayk, R.; Qudah, T.; Tahtamouni, R. Silver nanoparticles (Ag NPs) boost mitigation powers of Chenopodium quinoa (Q6 line) grown under in vitro salt-stressing conditions. Water 2022, 14, 3099. [Google Scholar] [CrossRef]
- Singh, R.; Ahamad, S. Integration of nanotechnology in plant tissue culture. Curr. Nanosci. 2022, 18, 604–610. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Dubchak, S.; Ogar, A.; Mietelski, J.W.; Turnau, K. Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radiocaesium in Helianthus annuus. Span. J. Agric. Res. 2010, 8, 103–108. [Google Scholar] [CrossRef]
- Selvakesavan, R.K.; Kruszka, D.; Shakya, P.; Mondal, D.; Franklin, G. Impact of Nanomaterials on Plant Secondary Metabolism. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 133–170. [Google Scholar]
- Khalid, M.F.; Khan, R.I.; Jawaid, M.Z.; Shafqat, W.; Hussain, S.; Ahmed, T.; Rizwan, M.; Ercisli, S.; Pop, O.L.; Marc, R.A. Nanoparticles: The plant saviour under abiotic stresses. Nanomaterials 2022, 12, 3915. [Google Scholar] [CrossRef] [PubMed]
- Al-Oubaidi, H.K.M.; Mohammed-Ameen, A.S. The effect of (AgNO3) NPs on increasing of secondary metabolites of Calendula officinalis L. in vitro. Int. J. Pharm. Pract. 2014, 5, 267–272. [Google Scholar]
- Lala, S. Enhancement of secondary metabolites in Bacopa monnieri (L.) Pennell plants treated with copper-based nanoparticles in vivo. IET Nanobiotechnol. 2020, 14, 78–85. [Google Scholar] [CrossRef]
- Kruszka, D.; Selvakesavan, R.K.; Kachlicki, P.; Franklin, G. Untargeted metabolomics analysis reveals the elicitation of important secondary metabolites upon treatment with various metal and metal oxide nanoparticles in Hypericum perforatum L. cell suspension cultures. Ind. Crops Prod. 2022, 178, 114561. [Google Scholar] [CrossRef]
- Pathak, A.; Haq, S.; Meena, N.; Dwivedi, P.; Kothari, S.L.; Kachhwaha, S. Multifaceted role of nanomaterials in modulating in vitro seed germination, plant morphogenesis, metabolism and genetic engineering. Plants 2023, 12, 3126. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Kartsonas, E.; Karras, S.; Mavrommati, E.; Petropoulos, S.A.; Papafotiou, M. In vitro Propagation of Origanum scabrum (Boiss. & Heldr.): An Endemic Medicinal Plant of Greece. Plants 2023, 12, 2118. [Google Scholar] [CrossRef]
- Fokina, A.V.; Satarova, T.M.; Smetanin, V.T.; Kucenko, N.I. Optimization of microclonal propagation in vitro of oregano (Origanum vulgare). Biosyst. Divers. 2018, 26, 98–102. [Google Scholar] [CrossRef]
- Du, W.; Tan, W.; Yin, Y.; Ji, R.; Peralta-Videa, J.R.; Guo, H.; Gardea-Torresdey, J.L. Differential effects of copper nanoparticles/microparticles in agronomic and physiological parameters of oregano (Origanum vulgare). Sci. Total Environ. 2018, 618, 306–312. [Google Scholar] [CrossRef]
- Shaik, M.R.; Khan, M.; Kuniyil, M.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Adil, S.F. Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities. Sustainability 2018, 10, 913. [Google Scholar] [CrossRef]
- Erenler, R.; Dag, B. Biosynthesis of silver nanoparticles using Origanum majorana L. and evaluation of their antioxidant activity. Inorg. Nano-Met. Chem. 2022, 52, 485–492. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas–Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Bonsucesso Santana, J.; Santos do Nascimento, A.; da Silva Conceição, A.L.; Lopes de Carvalho, C.A.; de Souza Dias, F. Efficiency of two digestion methods in determining the presence of metals (Cd, Cu, Cr, Pb and Zn) in geopropolis produced by Melipona scutellaris. Rev. Colomb. Química 2021, 50, 24–29. [Google Scholar] [CrossRef]
- Pequerul, A.; Pérez, C.; Madero, P.; Val, J.; Monge, E. A rapid wet digestion method for plant analysis. In Proceedings of the Optimization of Plant Nutrition: Refereed Papers from the Eighth International Colloquium for the Optimization of Plant Nutrition, Lisbon, Portugal, 31 August–8 September 1992; Springer: Dordrecht, The Netherlands, 1993; pp. 3–6. [Google Scholar]
- Elsayh, S.A.; Arafa, R.N.; Ali, G.A.; Abdelaal, W.B.; Sidky, R.A.; Ragab, T.I. Impact of silver nanoparticles on multiplication, rooting of shoots and biochemical analyses of date palm Hayani cv. by in vitro. Biocatal. Agric. Biotechnol. 2022, 43, 102400. [Google Scholar] [CrossRef]
- Manokari, M.; Raj, M.C.; Dey, A.; Faisal, M.; Alatar, A.A.; Joshee, N.; Shekhawat, M.S. Silver nanoparticles improved morphogenesis, biochemical profile and micro-morphology of Gaillardia pulchella Foug cv. ‘Torch Yellow’. Plant Cell Tissue Organ Cult. 2023, 155, 433–445. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A.; Muthukrishnan, S. Role of biogenic silver nanoparticles on hyperhydricity reversion in Dianthus chinensis L. an in vitro model culture. J. Plant Growth Regul. 2022, 41, 23–39. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Kulus, D. Effect of silver nanoparticles on the in vitro regeneration, biochemical, genetic, and phenotype variation in adventitious shoots produced from leaf explants in Chrysanthemum. Int. J. Mol. Sci. 2022, 23, 7406. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Fahmy, A.H.; Ahmed, S.S. Copper nanoparticles elevate regeneration capacity of Ocimum basilicum L. plant via somatic embryogenesis. Plant Cell Tissue Organ Cult. 2019, 136, 41–50. [Google Scholar] [CrossRef]
- Mawale, K.S.; Praveen, A.; Giridhar, P. Efficacy of copper and silver nanoparticles on seedling growth, biochemical and antioxidant potential of Capsicum annuum L., in vitro and ex vitro. S. Afr. J. Bot. 2024, 175, 1–14. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, J.; Xian, J.; Peijnenburg, W.J.; Du, Y.; Tian, D.; Tu, L. Copper accumulation and physiological markers of soybean (Glycine max) grown in agricultural soil amended with copper nanoparticles. Ecotoxicol. Environ. Saf. 2022, 229, 113088. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Anis, M.; Javed, S.B.; Alatar, A.A. Stimulatory effect of copper and zinc sulphate on plant regeneration, glutathione-S-transferase analysis and assessment of antioxidant activities in Mucuna pruriens L. (DC). Plant Cell Tissue Organ Cult. 2020, 141, 155–166. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Ao, Q.; Yang, Y. Engineered ZnO and CuO nanoparticles ameliorate morphological and biochemical response in tissue culture regenerants of candyleaf (Stevia rebaudiana). Molecules 2020, 25, 1356. [Google Scholar] [CrossRef]
- Ali, A.; Mashwani, Z.U.R.; Raja, N.I.; Mohammad, S.; Luna-Arias, J.P.; Ahmad, A.; Kaushik, P. Phytomediated selenium nanoparticles and light regimes elicited in vitro callus cultures for biomass accumulation and secondary metabolite production in Caralluma tuberculata. Front. Plant Sci. 2023, 14, 1253193. [Google Scholar] [CrossRef]
- Alfarraj, N.S.; Tarroum, M.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Salih, A.M.; Perveen, K. Biosynthesis of Silver Nanoparticles and Exploring Their Potential of Reducing the Contamination of the In vitro Culture Media and Inducing the Callus Growth of Rumex nervosus Explants. Molecules 2023, 28, 3666. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Rani, S.; Kumari, N.; Sharma, V. Uptake, translocation, transformation and physiological effects of nanoparticles in plants. Arch. Agron. Soil Sci. 2023, 69, 1579–1599. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Pošćić, F.; Giordano, C.; Musetti, R. In vivo synthesis of nanomaterials in plants: Location of silver nanoparticles and plant metabolism. Nanoscale Res. Lett. 2014, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, L.; He, S.Y.; Ryser, E.T.; Li, H.; Zhang, W. Stomata facilitate foliar sorption of silver nanoparticles by Arabidopsis thaliana. Environ. Pollut. 2022, 292, 118448. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Huang, Y.; Wang, Y.; Zhou, D.; Xing, B. Transfer and toxicity of silver nanoparticles in the food chain. Environ. Sci. Nano 2021, 8, 1519–1535. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Root system architecture, copper uptake and tissue distribution in soybean (Glycine max (L.) Merr.) grown in copper oxide nanoparticle (CuONP)-amended soil and implications for human nutrition. Plants 2020, 9, 1326. [Google Scholar] [CrossRef]
- Shi, J.; Peng, C.; Yang, Y.; Yang, J.; Zhang, H.; Yuan, X.; Hu, T. Phytotoxicity and Accumulation of Copper Oxide Nanoparticles to the Cu-Tolerant Plant Elsholtzia splendens. Nanotoxicology 2014, 8, 179–188. [Google Scholar] [CrossRef]
- Tabatabaee, S.; Iranbakhsh, A.; Shamili, M.; Ardebili, Z.O. Copper nanoparticles mediated physiological changes and transcriptional variations in microRNA159 (miR159) and mevalonate kinase (MVK) in pepper; potential benefits and phytotoxicity assessment. J. Environ. Chem. Eng. 2021, 9, 106151. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Zaman, G.; Khan, T.; Ashraf, H.; Meer, B.; Abbasi, B.H. Copper oxide (CuO) and manganese oxide (MnO) nanoparticles induced biomass accumulation, antioxidants biosynthesis and abiotic elicitation of bioactive compounds in callus cultures of Ocimum basilicum (Thai basil). Artif. Cells Nanomed. Biotechnol. 2021, 49, 625–633. [Google Scholar] [CrossRef]
- Dehghani-Aghchekohal, Z.; Omidi, M.; Azizinezhad, R.; Etminan, A. Stimulation of secondary metabolites and γ-terpinene synthase by silver nanoparticles in callus cultures of Carum carvi. Appl. Biochem. Biotechnol. 2022, 194, 3228–3241. [Google Scholar] [CrossRef]
- El-Naggar, H.M.; Osman, A.R. Enhancing growth and bioactive metabolites characteristics in Mentha pulegium L. via silicon nanoparticles during in vitro drought stress. BMC Plant Biol. 2024, 24, 657. [Google Scholar] [CrossRef] [PubMed]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of callus growth and secondary metabolites in different Thymus species and Zataria multiflora micropropagated under ZnO nanoparticles stress. Biotechnol. Appl. Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic Lamiaceae plants in Greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Özlem Şener, S.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Fragr. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Felisberto, J.S.; Machado, D.B.; Assunção, J.A.; Massau, S.A.; Queiroz, G.A.D.; Guimarães, E.F.; Moreira, D.D.L. Spatio-Temporal Variations of Volatile Metabolites as an Eco-Physiological Response of a Native Species in the Tropical Forest. Plants 2024, 13, 2599. [Google Scholar] [CrossRef]
- Hudaib, M.M.; Abu Hajal, A.F.; Sakkal, M.M. Chemical composition of the volatile oil from aerial parts of Rosmarinus officinalis L. growing in UAE. J. Essent. Oil Bear. Plants 2022, 25, 282–289. [Google Scholar] [CrossRef]
- Darbahani, M.; Ghiyasi, M.R.; Rahaie, M. Nanoparticles as new elicitors for the production of bioactive and phytochemicals in vitro and in vivo plant culture. Phytochem. Rev. 2024, 24, 3179–3203. [Google Scholar] [CrossRef]
- Wen, Y.; Liao, Y.; Tang, Y.; Zhang, H.; Zhang, J.; Liao, Z. Metabolic effects of elicitors on the biosynthesis of tropane alkaloids in medicinal plants. Plants 2023, 12, 3050. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, delivery and advantages in agricultural sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Garg, D.; Sridhar, K.; Inbaraj, B.S.; Chawla, P.; Tripathi, M.; Sharma, M. Nano-biofertilizer formulations for agriculture: A systematic review on recent advances and prospective applications. Bioengineering 2023, 10, 1010. [Google Scholar] [CrossRef]
- Ochatt, S.; Abdollahi, M.R.; Akin, M.; Bello, J.B.; Eimert, K.; Faisal, M.; Nhut, D.T. Application of nanoparticles in plant tissue cultures: Minuscule size but huge effects. Plant Cell Tissue Organ Cult. 2023, 155, 323–326. [Google Scholar] [CrossRef]
- Antunes Filho, S.; Dos Santos, M.S.; Dos Santos, O.A.L.; Backx, B.P.; Soran, M.L.; Opriş, O.; Bououdina, M. Biosynthesis of nanoparticles using plant extracts and essential oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.; Attique, I.; Zahra, M.; Khan, A.K.; Hahim, M.; Hano, C.; Anjum, S. Metal oxide nanoparticles and plant secondary metabolism: Unraveling the game-changer nano-elicitors. Plant Cell Tissue Organ Cult. 2023, 155, 327–344. [Google Scholar] [CrossRef]
- Martinez-Chávez, L.A.; Hernández-Ramírez, M.Y.; Feregrino-Pérez, A.A.; Esquivel Escalante, K. Cutting-edge strategies to enhance bioactive compound production in plants: Potential value of integration of elicitation, metabolic engineering, and green nanotechnology. Agronomy 2024, 14, 2822. [Google Scholar] [CrossRef]
- Singh, R.; Choudhary, P.; Kumar, S.; Daima, H.K. Mechanistic approaches for crosstalk between nanomaterials and plants: Plant immunomodulation, defense mechanisms, stress resilience, toxicity, and perspectives. Environ. Sci. Nano 2024, 11, 2324–2351. [Google Scholar] [CrossRef]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Nanoelicitation: A promising and emerging technology for triggering the sustainable in vitro production of secondary metabolites in medicinal plants. In Plant and Nanoparticles; Springer Nature: Singapore, 2022; pp. 265–280. [Google Scholar]
- Prasad, A.; Sidhic, J.; Sarbadhikary, P.; Narayanankutty, A.; George, S.; George, B.P.; Abrahamse, H. Role of metal nanoparticles in organogenesis, secondary metabolite production and genetic transformation of plants under in vitro condition: A comprehensive review. Plant Cell Tissue Organ Cult. 2024, 158, 33. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Glew, R.S.; Vanderjagt, D.J.; Chuang, L.T.; Huang, Y.S.; Millson, M.; Glew, R.H. Nutrient content of four edible wild plants from West Africa. Plant Foods Hum. Nutr. 2005, 60, 187–193. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Guzmán-Báez, G.A.; Trejo-Téllez, L.I.; Ramírez-Olvera, S.M.; Salinas-Ruíz, J.; Bello-Bello, J.J.; Alcántar-González, G.; Gómez-Merino, F.C. Silver Nanoparticles Increase Nitrogen, Phosphorus, and Potassium Concentrations in Leaves and Stimulate Root Length and Number of Roots in Tomato Seedlings in a Hormetic Manner. Dose-Response 2021, 19, 15593258211044576. [Google Scholar] [CrossRef]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]

| Ag NPs Concentration (mg/L) | Microshoot Number/Explant | Shoot Height (cm) | Root Numbers | Callus Diameter (cm) |
|---|---|---|---|---|
| 0 x | 2.80 ± 0.37 c * | 5.24 ± 0.12 c | 2.60 ± 0.51 c | 0.86 ± 0.50 d |
| 25 | 3.80 ± 0.37 c | 4.54 ± 0.05 d | 3.80 ± 0.37 bc | 2.00 ± 0.71 b |
| 50 | 6.80 ± 0.37 b | 5.94 ± 0.05 b | 4.80 ± 0.37 b | 1.54 ± 0.51 c |
| 100 | 11.60 ± 0.50 a | 9.22 ± 0.11 a | 8.20 ± 0.40 a | 1.84 ± 0.50 b |
| 150 | 7.60 ± 0.51 b | 6.18 ± 0.08 b | 5.2 ± 0.37 b | 2.38 ± 0.86 a |
| Cu NPs Concentration (mg/L) | Microshoot Number/Explant | Shoot Height (cm) | Root Numbers | Callus Diameter (cm) |
|---|---|---|---|---|
| 0 x | 2.80 ± 0.37 cd * | 5.26 ± 0.06 b | 3.20 ± 037 c | 1.03 ± 0.37 c |
| 25 | 4.80± 0.30 b | 3.84 ± 0.09 c | 5.60 ± 0.50 b | 1.08 ± 0.58 c |
| 50 | 6.80 ± 0.37 a | 7.00 ± 0.31 a | 13.80 ± 0.58 a | 1.28 ± 0.37 c |
| 100 | 3.20 ± 0.30 c | 5.20 ± 0.37 b | 6.00 ± 0.44 b | 2.48 ± 0.86 a |
| 150 | 1.60 ± 0.24 d | 4.20 ± 0.30 bc | 4.80 ± 0.58 bc | 2.16 ± 0.92 b |
| SN * | RT ** | RIexp x | RIlit y | Compound a | %Content b | Classification Group z |
|---|---|---|---|---|---|---|
| 1 | 9.831 | 1028 | 1024 | Limonene | 3.60 | Monoterpene Hydrocarbon |
| 2 | 15.100 | 1153 | 1155 | iso-Isopulegol | 4.94 | Monoterpenoid Alcohol |
| 3 | 19.057 | 1242 | 1235 | Neral | 4.64 | Monoterpenoid Aldehyde |
| 4 | 20.421 | 1273 | 1264 | Geraniol | 7.18 | Monoterpenoid Alcohol |
| 5 | 23.620 | 1346 | 1346 | α-Terpinyl Acetate | 29.23 | Monoterpenoid Ester |
| 6 | 24.930 | 1376 | 1379 | Geranyl Acetate | 12.76 | Monoterpenoid Ester |
| 7 | 26.517 | 1413 | 1417 | β-Caryophyllene | 9.13 | Sesquiterpene Hydrocarbon |
| 8 | 28.052 | 1450 | 1452 | α-Humulene | 4.91 | Sesquiterpene Hydrocarbon |
| 9 | 29.129 | 1476 | 1478 | γ-Muurolene | 7.16 | Sesquiterpene Hydrocarbon |
| 10 | 33.384 | 1581 | 1582 | Caryophyllene oxide | 1.73 | Sesquiterpenoid Oxide |
| 11 | 38.217 | 1709 | 1713 | Cedroxyde | 1.74 | Sesquiterpenoid Oxide |
| 12 | 42.543 | 1834 | 1832 | Cyclopentadecanolide | 3.81 | Lactone |
| 13 | 44.930 | 1908 | 1913 | (5E,9E)-Farnesyl acetone | 2.06 | Sesquiterpenoid Ketone |
| 14 | 51.161 | 2100 | 2087 | Abietadiene | 7.09 | Diterpene Hydrocarbon |
| SN * | RT ** | RIexp x | RIlit y | Compound a | %Content b | Classification Group z |
|---|---|---|---|---|---|---|
| 1 | 6.475 | 930 | 932 | α-Pinene | 2.58 | Monoterpene Hydrocarbon |
| 2 | 7.901 | 975 | 974 | β-Pinene | 1.83 | Monoterpene Hydrocarbon |
| 3 | 19.063 | 1242 | 1235 | Neral (z- citral) | 1.98 | Monoterpenoid Aldehyde |
| 4 | 20.413 | 1273 | 1265 | Geranial (E-citral) | 2.94 | Monoterpenoid Aldehyde |
| 5 | 20.746 | 1280 | 1283 | Isobornyl acetat | 1.00 | Monoterpenoid Ester |
| 6 | 23.574 | 1345 | 1346 | α-Terpinyl Acetat | 1.46 | Monoterpenoid Ester |
| 7 | 26.516 | 1413 | 1417 | β-Caryophyllene | 21.94 | Sesquiterpene Hydrocarbon |
| 8 | 28.057 | 1450 | 1452 | α-Humulene | 14.70 | Sesquiterpene Hydrocarbon |
| 9 | 29.125 | 1476 | 1478 | g-Muurolene | 4.76 | Sesquiterpene Hydrocarbon |
| 10 | 29.760 | 1491 | 1500 | bicyclogermacrene | 1.46 | Sesquiterpene Hydrocarbon |
| 11 | 32.060 | 1548 | 1547 | Italicene epoxide | 2.93 | Sesquiterpenoid Oxide |
| 12 | 33.369 | 1580 | 1582 | Caryophyllene oxide | 28.45 | Sesquiterpenoid Oxide |
| 13 | 34.528 | 1610 | 1608 | Humulene epoxide II | 11.40 | Sesquiterpenoid Oxide |
| 14 | 42.596 | 1836 | 1832 | Cyclopentadecanone | 2.57 | Lactone |
| SN * | RT ** | RIexp x | RIlit y | Compound a | %Content b | Classification Group z |
|---|---|---|---|---|---|---|
| 1 | 12.940 | 1103 | 1100 | n-Nonanal | 8.07 | Aldehyde |
| 2 | 17.403 | 1205 | 1201 | n-Decanal | 10.97 | Aldehyde |
| 3 | 23.534 | 1344 | 1346 | α-Terpinyl Acetat | 18.98 | Monoterpenoid Ester |
| 4 | 26.483 | 1412 | 1417 | β-Caryophyllene | 10.34 | Sesquiterpene Hydrocarbon |
| 5 | 28.035 | 1449 | 1452 | α-Humulene | 9.54 | Sesquiterpene Hydrocarbon |
| 6 | 33.316 | 1579 | 1582 | Caryophyllene oxide | 25.71 | Sesquiterpenoid Oxide |
| 7 | 38.194 | 1708 | 1713 | Cedroxyde | 16.39 | Sesquiterpenoid Oxide |
| SN * | RT ** | RIexp x | RIlit y | Compound a | %Content b | Classification Group z |
|---|---|---|---|---|---|---|
| 1 | 9.265 | 1014 | 1014 | α-Terpinene | 2.56 | Monoterpene Hydrocarbon |
| 2 | 10.956 | 1055 | 1054 | γ-Terpinene | 4.70 | Monoterpene Hydrocarbon |
| 3 | 16.801 | 1191 | 1174 | Terpinen-4-ol (mix 1174) | 19.55 | Monoterpenoid Alcohol |
| 4 | 21.308 | 1293 | 1299 | Terpinen-4-ol acetate | 4.22 | Monoterpenoid Ester |
| 5 | 26.527 | 1413 | 1417 | β-Caryophyllene | 12.20 | Sesquiterpene Hydrocarbon |
| 6 | 29.088 | 1475 | 1478 | γ-Muurolene | 4.61 | Sesquiterpene Hydrocarbon |
| 7 | 30.163 | 1500 | 1505 | β-Bisabolene | 3.42 | Sesquiterpene Hydrocarbon |
| 8 | 30.751 | 1515 | 1522 | δ-Cadinene | 4.75 | Sesquiterpene Hydrocarbon |
| 9 | 33.351 | 1580 | 1582 | Caryophyllene oxide | 7.16 | Sesquiterpenoid Oxide |
| 10 | 37.827 | 1698 | 1700 | Eudesm-7(11)-en-4-ol | 25.10 | Sesquiterpenoid Alcohol |
| 11 | 42.560 | 1834 | NA m | 6,10,14-trimethyl-2- Pentadecanone (Or Unk HC) | 2.53 | Hydrocarbon |
| 12 | 43.326 | 1859 | 1867 | (E)-β-Santalol acetate | 2.07 | Sesquiterpenoid Ester |
| 13 | 49.669 | 2053 | 2055 | Abietatriene | 7.13 | Diterpene Hydrocarbon |
| Sample | Element (mg/g) | ||
|---|---|---|---|
| P | K | Ca | |
| Control x | 3.15133 ± 0.00491 b * | 36.31067± 0.00348 a | 12.11567 ± 0.00348 b |
| 50 mg/L CuNPs | 3.43533 ± 0.00819 a | 24.97567± 0.00348 b | 3.75133 ± 0.0041 c |
| 50 mg/L AgNPs | 2.95233 ± 0.01178 c | 24.41100± 0.00665 c | 13.310 ± 0.00577 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Qudah, T.S.; Shibli, R.A.; Abu-Zurayk, R.; Hudaib, M. Nanoparticles Enhance In Vitro Micropropagation and Secondary Metabolite Accumulation in Origanum petraeum. Nanomaterials 2025, 15, 1496. https://doi.org/10.3390/nano15191496
Al Qudah TS, Shibli RA, Abu-Zurayk R, Hudaib M. Nanoparticles Enhance In Vitro Micropropagation and Secondary Metabolite Accumulation in Origanum petraeum. Nanomaterials. 2025; 15(19):1496. https://doi.org/10.3390/nano15191496
Chicago/Turabian StyleAl Qudah, Tamara S., Rida A. Shibli, Rund Abu-Zurayk, and Mohammad Hudaib. 2025. "Nanoparticles Enhance In Vitro Micropropagation and Secondary Metabolite Accumulation in Origanum petraeum" Nanomaterials 15, no. 19: 1496. https://doi.org/10.3390/nano15191496
APA StyleAl Qudah, T. S., Shibli, R. A., Abu-Zurayk, R., & Hudaib, M. (2025). Nanoparticles Enhance In Vitro Micropropagation and Secondary Metabolite Accumulation in Origanum petraeum. Nanomaterials, 15(19), 1496. https://doi.org/10.3390/nano15191496








