Strategies for Enhancing BiVO4 Photoanodes for PEC Water Splitting: A State-of-the-Art Review
Abstract
1. Introduction
2. Principle of Operation for Photoanode in PEC Water Splitting

3. Synthesis Methods of BiVO4 Photoelectrodes
3.1. Preparation of Nanoporous BiVO4 Films
3.2. Spin Coating, Drop-Casting, Spray Pyrolysis, and Electrospray Deposition
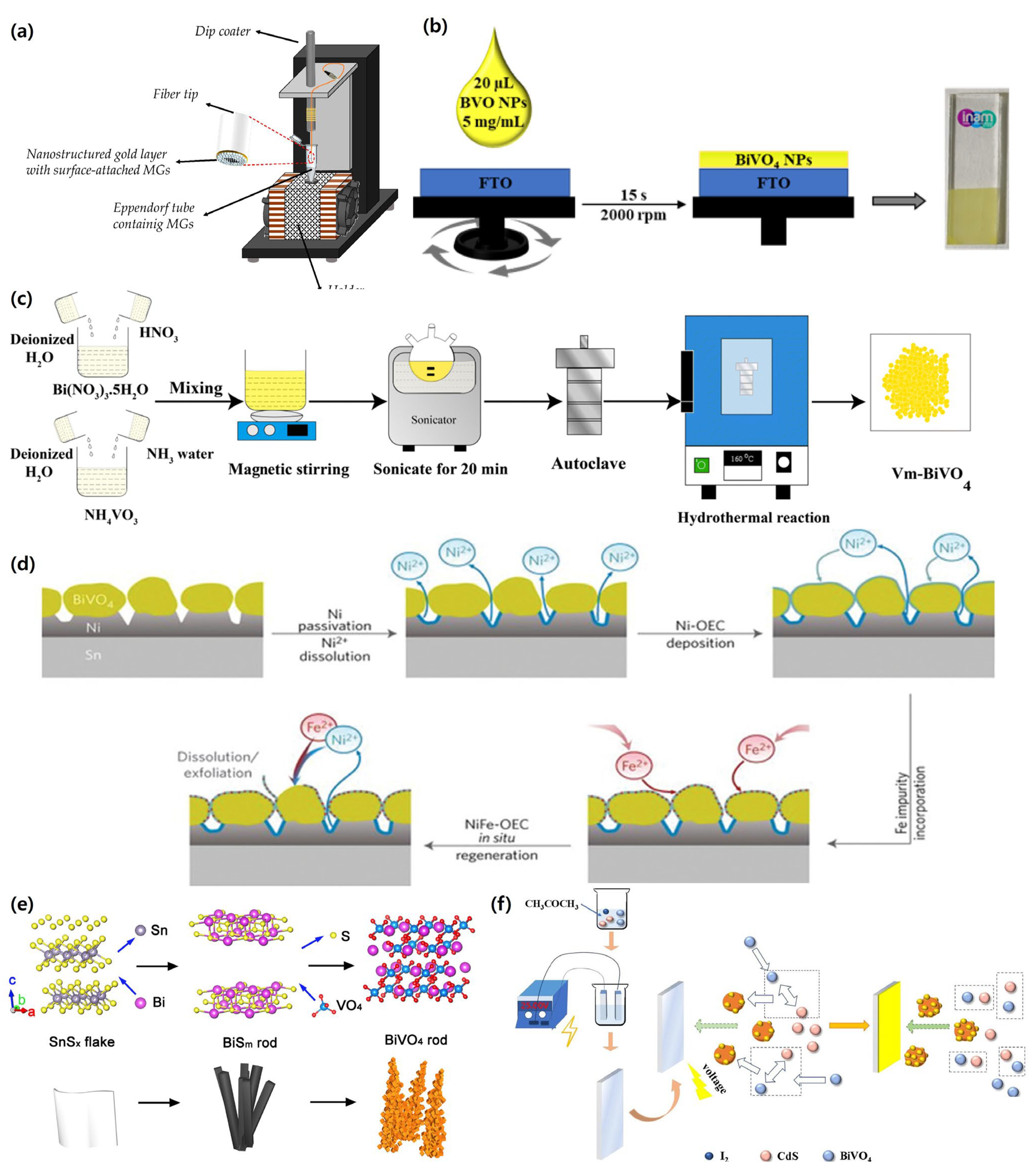
3.3. Sol–Gel Method, Wet-Chemical Process, Hydrothermal, Solvothermal, SILAR
3.4. Chemical Vapor Deposition (CVD), Reactive Co-Sputtering, and Atomic Layer Deposition (ALD)
3.5. In Situ Coordination, Ion Exchange Technique, and Grafting
4. Key Factors Influencing the PEC Performance of the BIVO4 Photoanode

4.1. Introducing Extrinsic/Intrinsic Defects Through Doping
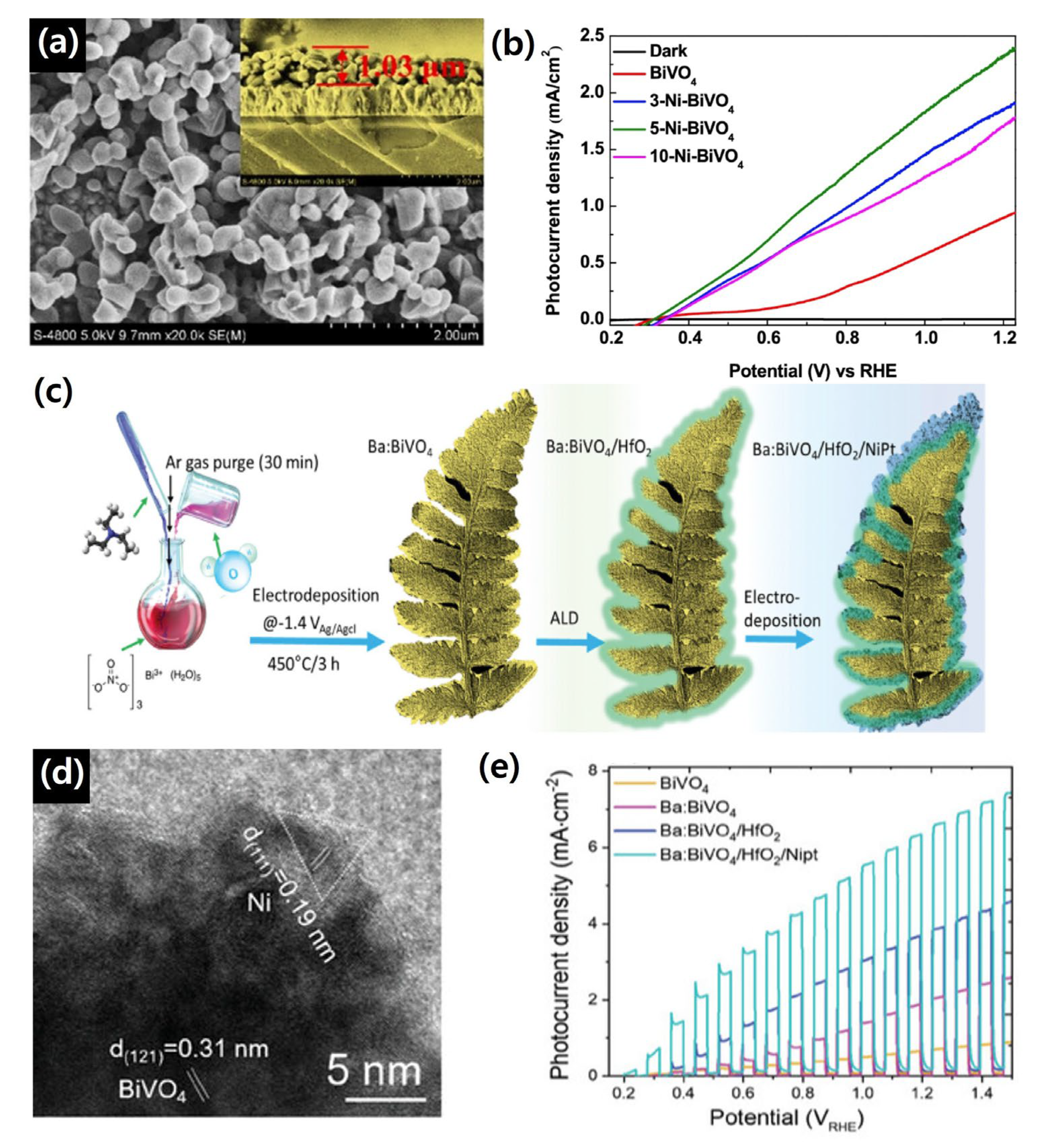
4.2. Heterojunction with ETL (Electron Transport Layer)

4.3. Hole-Transport Layers (HTLs) for BiVO4 Photoanodes
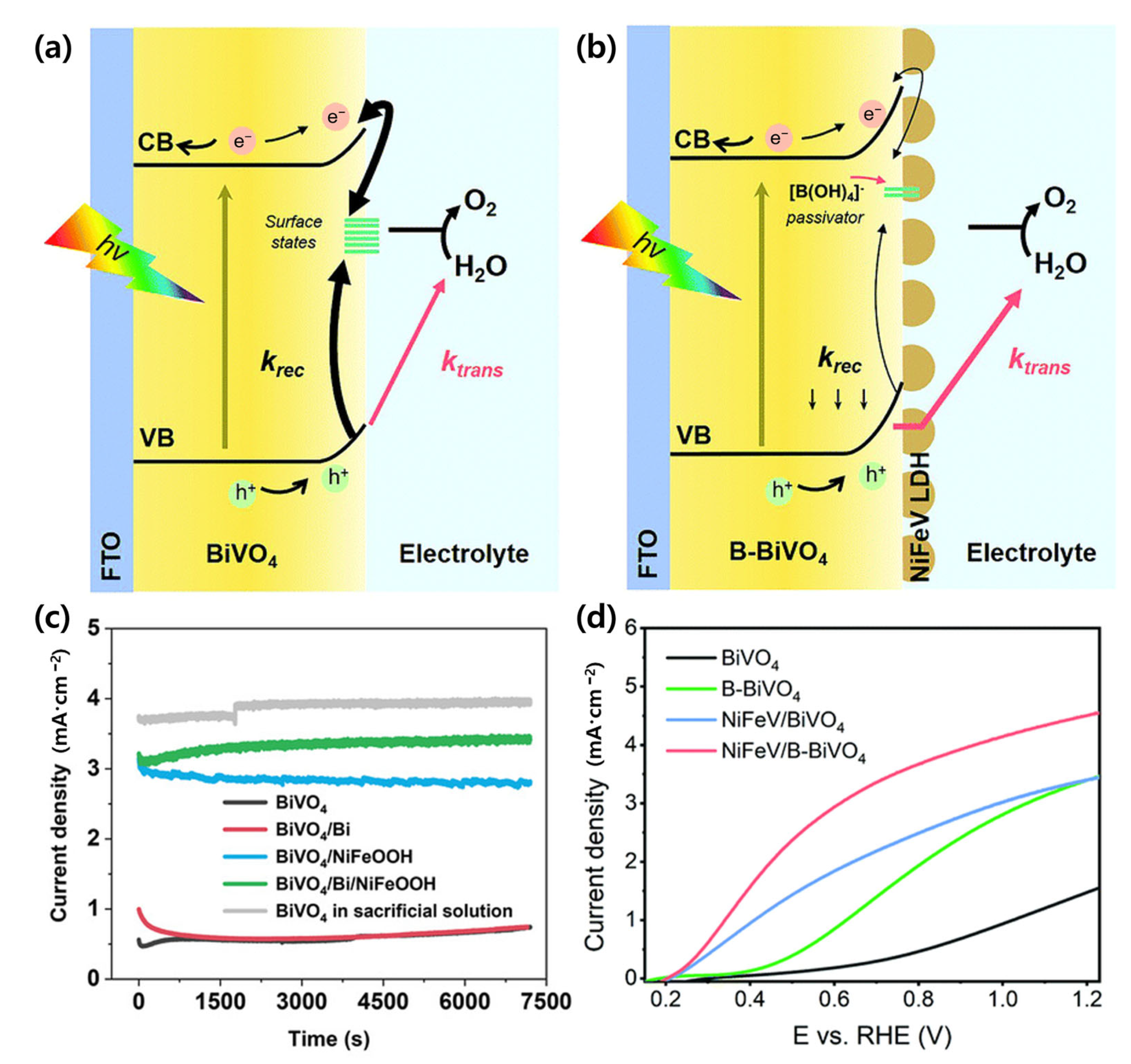
4.4. Cocatalyst and Surface Modification Strategies for BiVO4 Photoanodes
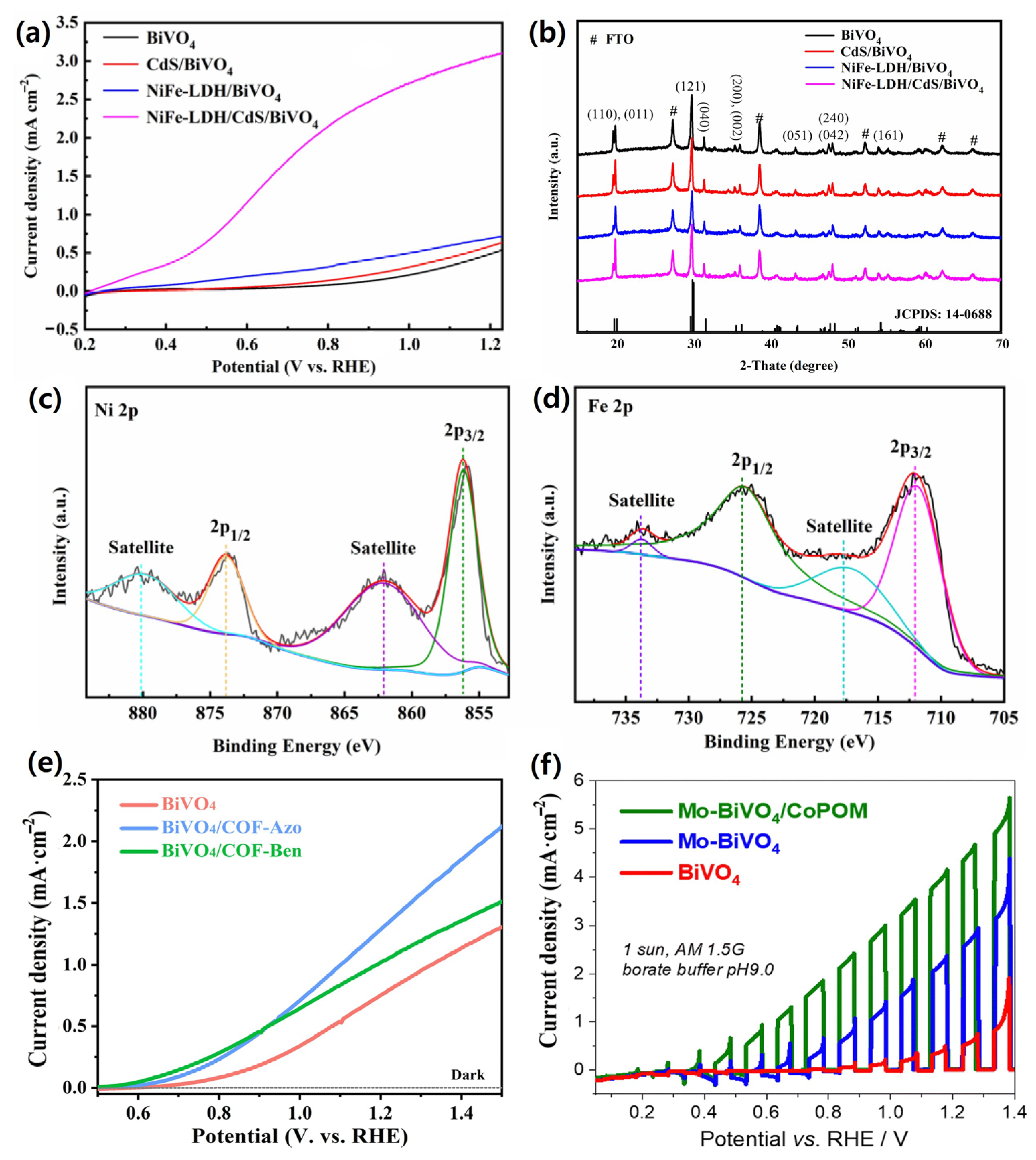
4.5. Light Absorption Efficiency
4.5.1. Band Edge Engineering
4.5.2. Constructing Heterojunction Structure
4.5.3. Optics-Based Elements
4.6. Photogenerated Charge Separation Efficiency (ηsep)
4.6.1. Utilizing in Internal Electric Field
4.6.2. Enhancing Electrical Conductivity and Carrier Concentration
4.7. Charge Transfer Efficiency (ηtrans)
Co-Catalysts Based on Metal (Oxy) Hydroxides
4.8. Stability
4.8.1. Protection Layer Incorporation
4.8.2. Dual-Protection Strategies for Durable BiVO4 Photoanodes
4.8.3. Synergistic Surface–Electrolyte Protection Strategies
5. Emerging Applications of BiVO4 Photoelectrodes in Solar Water Splitting and Beyond
5.1. BiVO4-Based Tandem System for Comprehensive Water Splitting
5.2. PEC Cells for the Generation of Value-Added Chemicals
6. Challenges and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abid, M.Z.; Rafiq, K.; Rauf, A.; Althomali, R.H.; Jin, R.; Hussain, E. Interface engineering of BiVO4/Zn3V2O8 heterocatalysts for escalating the synergism: Impact of Cu electron mediator for overall water splitting. Renew. Energy 2024, 225, 120223. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.-W.; Lee, J.S. Toward practical solar hydrogen production—An artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic water splitting: Quantitative approaches toward photocatalysis by design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Liu, M.; Liu, X.; Zhang, H.; Jiang, W.; Wang, P.; Zheng, Z.; Liu, Y.; Cheng, H.; et al. Boosting H2 Production from a BiVO4 Photoelectrochemical Biomass Fuel Cell by the Construction of a Bridge for Charge and Energy Transfer. Adv. Mater. 2022, 34, e2201594. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2016, 67, 597–611. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Ardo, S.; Fernandez Rivas, D.; Modestino, M.A.; Schulze Greiving, V.; Abdi, F.F.; Alarcon Llado, E.; Artero, V.; Ayers, K.; Battaglia, C.; Becker, J.-P.; et al. Pathways to electrochemical solar-hydrogen technologies. Energy Environ. Sci. 2018, 11, 2768–2783. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Li, C.; Fan, W.; Chen, S.; Zhang, F. Effective charge carrier utilization of BiVO4 for solar overall water splitting. Chemistry 2022, 28, e202201812. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Tan, H.L.; Amal, R.; Ng, Y.H. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: A review. J. Mater. Chem. A 2017, 5, 16498–16521. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation over BiVO4 in an aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Cooper, J.K.; Gul, S.; Toma, F.M. Electronic structure of monoclinic BiVO4. Chem. Mater. 2014, 26, 5365–5373. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting—Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Cooper, J.K.; Scott, S.B.; Ling, Y.; Yang, J.; Hao, S.; Li, Y.; Toma, F.M.; Stutzmann, M.; Lakshmi, K.V.; Sharp, I.D. Role of Hydrogen in Defining the N-Type Character of BiVO4 Photoanodes. Chem. Mater. 2016, 28, 5761–5771. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhou, Y.; Zheng, J. Unbiased photoelectrochemical tandem configuration for water splitting. J. Am. Chem. Soc. 2013, 135, 18661–18664. [Google Scholar] [CrossRef]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A front-illuminated nanostructured transparent BiVO4 photoanode for >2% efficient water splitting. Adv. Energy Mater. 2016, 6, 1501645. [Google Scholar] [CrossRef]
- Kho, Y.K.; Teoh, W.Y.; Iwase, A.; Mädler, L.; Kudo, A.; Amal, R. Flame preparation of visible-light responsive BiVO4 photocatalysts. J. Phys. Chem. C 2011, 115, 8591–8596. [Google Scholar]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode. J. Am. Chem. Soc. 2011, 133, 19306–19309. [Google Scholar]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef]
- Abdi, F.F.; Savenije, T.J.; May, M.M.; Dam, B.; Van De Krol, R. The origin of slow carrier transport in BiVO4 thin film photoanodes. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Zachäus, C.; Abdi, F.F.; Peter, L.M.; van de Krol, R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 2017, 8, 3712–3719. [Google Scholar] [CrossRef]
- Nellist, M.R.; Qiu, J.; Laskowski, F.A.; Toma, F.M.; Boettcher, S.W. Potential-sensing electrochemical AFM shows CoPi as a hole collector and Oxygen Evolution Catalyst on BiVO4 Water-Splitting photoanodes. ACS Energy Lett. 2018, 3, 2286–2291. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 2019, 31, e1806938. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Bai, Y.; Yun, J.-H.; Liu, G.; Wang, L. New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting. Adv. Mater. 2018, 30, 1800486. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, W.; Chen, W.; Chen, W.; Zhou, G.; Hsu, P.-C.; Zhang, R.; Liang, Z.; Fan, S.; Zhang, Y.; et al. Efficient solar-driven water splitting by nanocone BiVO4–perovskite tandem cells. Sci. Adv. 2016, 2, e1501764. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jo, Y.; Kim, J.H.; Jang, J.W.; Kang, H.J.; Lee, Y.H.; Kim, D.S.; Jun, Y.; Lee, J.S. Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: A dual absorber artificial leaf. ACS Nano 2015, 9, 11820–11829. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Z.; Zhang, M.; Feng, J.; Liu, J.; Zhao, Z.; Wang, S.; Yu, T.; Zou, Z. Quantitative analysis and visualized evidence for high charge separation efficiency in a solid/liquid bulk heterojunction. Adv. Energy Mater. 2014, 4, 1301785. [Google Scholar]
- Thomas, N.; Singh, V.; Ahmed, N.; Trinh, D.; Kuss, S.; Halasyamani, P.S.; Parkinson, B.A. Factors in the Metal Doping of BiVO4 for Improved Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2011, 115, 1–9. [Google Scholar]
- Berglund, S.P.; Rettie, A.J.E.; Hoang, S.; Mullins, C.B. Incorporation of Mo and W into nanostructured BiVO4 films for efficient photoelectrochemical water oxidation. Phys. Chem. Chem. Phys. 2012, 14, 7065–7075. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, Y.; Liu, J.; Wang, Y.; Li, J.; Li, Y.; Wang, X. BiVO4–graphene catalyst and its high photocatalytic performance under visible light irradiation. Mater. Chem. Phys. 2012, 132, 614–619. [Google Scholar]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by “Co-Pi” Catalyst-Modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Asai, T.; Hisatomi, T.; Uemura, J.; Tosa, M.; Shimamura, K.; Kubota, J.; Domen, K.; et al. Nanostructured WO3/BiVO4 photoanodes for efficient photoelectrochemical water splitting. Small 2014, 10, 3692–3699. [Google Scholar] [CrossRef]
- Trześniewski, B.J.; Digdaya, I.A.; Nagaki, T.; Ravishankar, S.; Herraiz-Cardona, I.; Vermaas, D.A.; Longo, A.; Gimenez, S.; Smith, W.A. Near-complete suppression of surface losses and total internal quantum efficiency in BiVO4 photoanodes. Energy Environ. Sci. 2017, 10, 1517–1529. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, B.J.; Han, G.S.; Hwang, S.W.; Kim, D.R.; Cho, I.S.; Jung, H.S. BiVO4/WO3/SnO2 Double-Heterojunction Photoanode with Enhanced Charge Separation and Visible Transparency for Bias-Free Solar Water Splitting with a Perovskite Solar Cell. ACS Appl. Mater. Interfaces 2017, 9, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, S.K.; Rodene, D.D.; Gupta, R.B. Recent Advancements in Semiconductor Materials for Photoelectrochemical Water Splitting for Hydrogen Production Using Visible Light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sharma, C.; Pooja, D.; Thakur, A.; Negi, Y.S. Review—Combining Experimental and Engineering Aspects of Catalyst Design for Photoelectrochemical Water Splitting. ECS Adv. 2022, 1, 030501. [Google Scholar] [CrossRef]
- Salih, A.K.; Khan, A.Z.; Drmosh, Q.; Kandiel, T.; Qamar, M.; Jahangir, T.; Ton-That, C.; Yamani, Z. Nanostructured BiVO4 Photoanodes Fabricated by Vanadium-Infused Interaction for Efficient Solar Water Splitting. ACS Appl. Nano Mater. 2024, 7, 14115–14122. [Google Scholar] [CrossRef]
- Scherino, L.; Giaquinto, M.; Micco, A.; Aliberti, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Ricciardi, A.; Cusano, A. A Time-Efficient Dip Coating Technique for the Deposition of Microgels onto the Optical Fiber Tip. Fibers 2018, 6, 72. [Google Scholar] [CrossRef]
- Dong, G.; Chen, T.; Kou, F.; Xie, F.; Xiao, C.; Liang, J.; Lou, C.; Zhuang, J.; Du, S. Promoting the Photoelectrochemical Properties of BiVO4 Photoanode via Dual Modification with CdS Nanoparticles and NiFe-LDH Nanosheets. Nanomaterials 2024, 14, 1100. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Wang, H. Fabrication of Cocatalyst NiO-Modified BiVO4 Composites for Enhanced Photoelectrochemical Performances. Front. Chem. 2022, 10, 864143. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Jia, P.; Zou, Y.; Jiang, J. Facile Construction of a BiVO4/CoV-LDHs/Ag Photoanode for Enhanced Photo-Electrocatalytic Glycerol Oxidation and Hydrogen Evolution. Energy Adv. 2023, 2, 1366–1374. [Google Scholar] [CrossRef]
- Ho-Kimura, S.; Soontornchaiyakul, W.; Yamaguchi, Y.; Kudo, A. Preparation of Nanoparticle Porous-Structured BiVO4 Photoanodes by a New Two-Step Electrochemical Deposition Method for Water Splitting. Catalysts 2021, 11, 136. [Google Scholar] [CrossRef]
- Sitaaraman, S.R.; Nirmala Grace, A.; Sellappan, R. Photoelectrochemical Performance of a Spin Coated TiO2 Protected BiVO4-Cu2O Thin Film Tandem Cell for Unassisted Solar Water Splitting. RSC Adv. 2022, 12, 31380–31391. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Ha, S.-B.; Song, I.-C.; Kim, J.-Y. PbS Quantum Dots-Decorated BiVO4 Photoanodes for Highly Efficient Photoelectrochemical Hydrogen Production. Nanomaterials 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Kwan, T.H.; Wu, X.; Yao, Q. Parameter Sizing and Stability Analysis of a Highway Fuel Cell Electric Bus Power System Using a Multi-Objective Optimization Approach. Int. J. Hydrogen Energy 2018, 43, 20976–20992. [Google Scholar] [CrossRef]
- Xia, T.; Chen, M.; Xiao, L.; Fan, W.; Mao, B.; Xu, D.; Guan, P.; Zhu, J.; Shi, W. Phosphorus doped BiVO4 photoanodes prepared by dip coating for improved photoelectrochemical water splitting. J. Taiwan Inst. Chem. Eng. 2018, 82, 582–589. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, R.; Fan, J.; Liu, Y. Direct-Current Piezoelectric Nanogenerator Based on Two-Layer Zinc Oxide Nanorod Arrays with Equal c-Axis Orientation for Energy Harvesting. Chem. Eng. J. 2021, 426, 131262. [Google Scholar] [CrossRef]
- Rohloff, M.; Anke, B.; Wiedemann, D.; Ulpe, A.C.; Kasian, O.; Zhang, S.; Scheu, C.; Bredow, T.; Lerch, M.; Fischer, A. Synthesis and Doping Strategies to Improve the Photoelectrochemical Water Oxidation Activity of BiVO4 Photoanodes. Z. Phys. Chem. 2020, 234, 655–682. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-Light Driven Heterojunction Photocatalysts for Water Splitting—A Critical Review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Liu, R.; Qiang, L.-S.; Yang, W.-D.; Liu, H.-Y. The Effect of Calcination Conditions on the Morphology, the Architecture and the Photo-Electrical Properties of TiO2 Nanotube Arrays. Mater. Res. Bull. 2013, 48, 1458–1467. [Google Scholar] [CrossRef]
- Lai, W.; Di, L.; Zhao, C.; Tian, Y.; Duan, Y.; Pan, Y.; Ye, D.; Jiang, L.; Guo, Y.; He, G.; et al. Electrospray Deposition for Electronic Thin Films on 3D Freeform Surfaces: From Mechanisms to Applications. Adv Mater. Technol. 2024, 9, 2400192. [Google Scholar] [CrossRef]
- Ilyas, A.; Rafiq, K.; Abid, M.Z.; Rauf, A.; Hussain, E. Growth of Villi-Microstructured Bismuth Vanadate (Vm-BiVO4) for Photocatalytic Degradation of Crystal Violet Dye. RSC Adv. 2023, 13, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Montañés, L.; Mesa, C.A.; Gutiérrez-Blanco, A.; Robles, C.; Julián-López, B.; Giménez, S. Facile Surfactant-Assisted Synthesis of BiVO4 Nanoparticulate Films for Solar Water Splitting. Catalysts 2021, 11, 1244. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Bai, L.; Lv, C.; Huang, Z.; Hu, X.; Liu, L. Strategies for Semiconductor/Electrocatalyst Coupling toward Solar-Driven Water Splitting. Adv. Sci. 2020, 7, 1902102. [Google Scholar] [CrossRef]
- Liu, C.; Su, J.; Zhou, J.; Guo, L. A Multistep Ion Exchange Approach for Fabrication of Porous BiVO4 Nanorod Arrays on Transparent Conductive Substrate. ACS Sustain. Chem. Eng. 2016, 4, 4492–4497. [Google Scholar] [CrossRef]
- Shan, L.; Yuan, M.; Ma, C.; Lu, C.; Fang, Z.; Lin, S.; Li, D.; Wu, Z.; Dong, L.; Qian, G.; et al. Z-Scheme CdS/BiVO4 Photocatalyst for Highly Efficient Photodegradation of Organic Pollutants and DFT Calculation. Available online: https://www.researchgate.net/publication/350549778_Z-scheme_CdSBiVO4_Photocatalyst_for_Highly_Efficient_Photodegradation_of_Organic_Pollutants_and_DFT_Calculation (accessed on 1 April 2021).
- De Laat, J.; Le, T.G. Effects of Chloride Ions on the Iron(III)-Catalyzed Decomposition of Hydrogen Peroxide and on the Efficiency of the Fenton-like Oxidation Process. Appl. Catal. B Environ. 2006, 66, 137–146. [Google Scholar]
- Laleh, M.; Kargar, F. Suppression of Chromium Depletion and Sensitization in Austenitic Stainless Steel by Surface Mechanical Attrition Treatment. Mater. Lett. 2011, 65, 1935–1937. [Google Scholar] [CrossRef]
- Na, S.; Seo, S.; Lee, H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts 2020, 10, 679. [Google Scholar]
- Haji Yassin, S.N.; Sim, A.S.L.; Jennings, J.R. Photoelectrochemical Evaluation of SILAR-Deposited Nanoporous BiVO4 Photoanodes for Solar-Driven Water Splitting. Nano Mater. Sci. 2020, 2, 227–234. [Google Scholar] [CrossRef]
- Huang, X.H.; Xia, X.H.; Yuan, Y.F.; Zhou, F. Porous ZnO Nanosheets Grown on Copper Substrates as Anodes for Lithium Ion Batteries. Electrochim. Acta 2011, 56, 4960–4965. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z. The Mechanism of the High Resistance to Sulfur Poisoning of the Rhenium Doped Nickel/Yttria-Stabilized Zirconia. Appl. Surf. Sci. 2018, 447, 561–568. [Google Scholar] [CrossRef]
- Liang, Y.; Messinger, J. Improving BiVO4 photoanodes for solar water splitting through surface passivation. Phys. Chem. Chem. Phys 2014, 16, 12014–12020. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Wang, Z.; Liu, R.; Pan, H.; Zhao, Y.; Kong, L.; Li, Y.; Zhu, S.; Wang, L. Organic ligand nanoarchitectonics for BiVO4 photoanodes surface passivation and cocatalyst grafting. Nano Res 2024, 17, 3667–3674. [Google Scholar] [CrossRef]
- Qayum, A.; Guo, M.; Wei, J.; Dong, S.; Jiao, X.; Chen, D.; Wang, T. An in situ combustion method for scale-up fabrication of BiVO4 photoanodes with enhanced long-term photostability for unassisted solar water splitting. J. Mater. Chem. A 2020, 8, 10989–10997. [Google Scholar] [CrossRef]
- Song, J.; Cha, J.; Lee, M.G.; Jeong, H.W.; Seo, S.; Yoo, J.A.; Kim, T.L.; Lee, J.; No, H.; Kim, D.H.; et al. Template-engineered epitaxial BiVO4 photoanodes for efficient solar water splitting. J. Mater. Chem. A 2017, 5, 18831–18838. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.; Li, C. Surface passivation engineering for photoelectrochemical water splitting. Catalysts 2023, 13, 217. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Chang, T.-F.M.; Sone, M.; Shibata, A.; Ishiyama, C.; Higo, Y. Bright Nickel Film Deposited by Supercritical Carbon Dioxide Emulsion Using Additive-Free Watts Bath. Electrochim. Acta 2010, 55, 6469–6475. [Google Scholar] [CrossRef]
- Ojo, A.A.; Dharmadasa, I.M. Electroplating of semiconductor materials for applications in large area electronics: A review. Coatings 2018, 8, 262. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Murugan, C.; Mary, A.S.; Velmurugan, R.; Subramanian, B.; Murugan, P.; Pandikumar, A. Investigating the interfacial charge transfer between electrodeposited BiVO4 and pulsed laser-deposited Co3O4 p-n junction photoanode in photoelectrocatalytic water splitting. Chem. Eng. J. 2024, 483, 149104. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Nguyen, T.N.; Nguyen, H.N.; Nguyen, S.N.; Nguyen, M.T. Photoelectrochemical water splitting with BiVO4 photoanodes synthesized by different methods. Reac. Kinet. Mech. Catal. 2025, 138, 2479–2493. [Google Scholar] [CrossRef]
- Obeso, J.L.; Martínez-Ahumada, E.; López-Olvera, A.; Ortiz-Landeros, J.; Lara-García, H.A.; Balmaseda, J.; López-Morales, S.; Sánchez-González, E.; Solis-Ibarra, D.; Leyva, C.; et al. MOF-303 as an effective adsorbent to clean CH4: SO2 capture and detection. ACS Appl. Energy Mater. 2022, 6, 9084–9091. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Qiao, S.; Lei, D.; Wang, Q.; Shi, X.; Huang, C.; Lu, W.; Yang, S.; Tian, Y.; et al. Synthesis of the Ni2P–Co Mott–Schottky junction as an electrocatalyst to boost sulfur conversion kinetics and application in separator modification in Li–S batteries. ACS Appl. Mater. Interfaces 2023, 15, 5253–5264. [Google Scholar] [CrossRef]
- Tayebi, M.; Lee, B. The effects of W/Mo-co-doped BiVO4 photoanodes for improving photoelectrochemical water splitting performance. Catal. Today 2021, 361, 183–190. [Google Scholar] [CrossRef]
- Huang, M.; Bian, J.; Xiong, W.; Huang, C.; Zhang, R. Low-dimensional Mo:BiVO4 photoanodes for enhanced photoelectrochemical activity. J. Mater. Chem. A 2018, 6, 3602–3609. [Google Scholar] [CrossRef]
- Pham, T.-D.; Lee, B.-K.; Nguyen, V.N.; Dao, V.-D.; Nguyen, T.D.C.; Mai, H.T.; Dao, V.-D.; Nguyen, M.P.; Nguyen, V.N. Fabrication of titanium-doped BiVO4 as a novel visible light-driven photocatalyst for degradation of residual tetracycline pollutant. Ceram. Int. 2021, 47, 34253–34259. [Google Scholar]
- Li, X.; Tang, Q.; Wang, S.; Liu, Y.; Chen, G.; Zhao, W.; Han, M.; Liu, G. Fe-N-C nanoparticles as a highly efficient electrocatalyst for oxygen reduction reaction in alkaline electrolyte. Int. J. Hydrogen Energy 2020, 45, 1686–1695. [Google Scholar]
- Mohd Raub, A.A.; Bahru, R.; Mohd Nashruddin, S.N.A.; Yunas, J. Advances of Nanostructured Metal Oxide as Photoanode in Photoelectrochemical (PEC) Water Splitting Application. Heliyon 2024, 10, e39079. [Google Scholar]
- Fang, G.; Liu, Z.; Han, C. Enhancing the PEC Water Splitting Performance of BiVO4 Co-Modifying with NiFeOOH and Co-Pi Double Layer Cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar]
- Polo, A.; Nomellini, C.; Marra, G.; Selli, E.; Dozzi, M.V. WO3/BiVO4 heterojunction photoanodes: Optimized photoelectrochemical performance in relation to both oxides layer thickness. Catal. Today 2025, 446, 115137. [Google Scholar] [CrossRef]
- Bai, S.; Cao, S.; Yan, X.; Luo, R.; Wang, X. Two-step electrodeposition to fabricate the p–n heterojunction of a Cu2O/BiVO4 photoanode for the enhancement of photoelectrochemical water splitting. Dalton Trans. 2018, 47, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, L.; Xue, K.; Luo, H.; Zhang, Y. Phase engineering of 1 T-MoS2 on BiVO4 photoanode with p-n junctions establishing high-speed charges transport channels for efficient photoelectrochemical water splitting. Chem. Eng. J. 2023, 470, 144965. [Google Scholar]
- Luo, W.; Tang, T.; Ma, T.; Xu, H.; Cui, Y.; Li, X.; Zhou, H.; Fan, B.; He, Y. Engineering a Tandem S-Scheme TiO2@SrTiO3@BiVO4 Photoanode for Superior PEC Water Oxidation. Electrochim. Acta 2025, 539, 147105. [Google Scholar]
- Dong, L.; Zhang, J.; Wang, J.; Dong, S.; Song, M.; Zhao, C. In situ unravelling surface plasmon resonance subject-object role of BiVO4@Ag in photocatalytic water splitting. J. Colloid Interface Sci. 2025, 701, 138656. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Y.; Liu, C. Sunlight Selective Photodeposition of CoOx(OH)y and NiOx(OH)y on Truncated Bipyramidal BiVO4 for Highly Efficient Photocatalysis. ACS Appl. Mater. Interfaces 2017, 9, 20436–20442. [Google Scholar]
- Sun, Z.J.; Ma, J.; Ma, Y.; Wang, J.G.; Hou, Y.J.; Chen, G.D.; Dissanayake, M.A.W.C.L.N.K.; Hettiarachchi, Y.M.A.G.; Liu, J. Surface Reconstruction and Passivation of BiVO4 Photoanodes Depending on the “Structure Breaker” Cs+. JACS Au 2024, 4, 196–205. [Google Scholar]
- Kim, D.S.; Lee, K.W.; Jung, S.Y.; Choi, J.H.; Lee, H.H.; Yang, W.S.; Cho, Y.S.; Yang, J.W.; Kang, J.; Boettcher, S.W.; et al. Efficient BiVO4/CoFeOxHy photoanodes using controlled annealing and conformal linear-sweep electrocatalyst photodeposition. Appl. Surf. Sci. 2025, 689, 162470. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, S.; Wu, L.; Li, M.; Chong, Y.; Qiu, Y.; Chen, G.; Zhao, Y.; Feng, C.; Ye, D.; et al. Strain-Engineering of Mesoporous Cs3Bi2Br9/BiVO4 S-Scheme Heterojunction for Efficient CO2 Photoreduction. Small 2023, 19, 2302058. [Google Scholar] [CrossRef]
- Arunachalam, M.; Yun, G.; Lee, H.S.; Ahn, K.-S.; Heo, J.; Kang, S.H. Effects of Al2O3 coating on BiVO4 and Mo-doped BiVO4 film for solar water oxidation. J. Electrochem. Sci. Technol 2019, 10, 424–432. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Xiang, Y.; Zhu, Z. Vacancy defect engineered BiVO4 with low-index surfaces for photocatalytic application: A first principles study. RSC Adv. 2022, 12, 31317–31325. [Google Scholar] [CrossRef]
- Ponchio, C.; Nareejun, W. Laser-Assisted FTO/WO3/BiVO4 Photoanode Fabrication for Enhanced Photoelectrocatalytic Performance and Durability for Organic Dye Degradation. J. Appl. Res. Sci. Technol. 2025, 24, 260565. [Google Scholar]
- Park, E.; Yoo, J.; Lee, K. Enhanced Photoelectrochemical Hydrogen Production via Linked BiVO4 Nanoparticles on Anodic WO3 Nanocoral Structures. Sustain. Energy Fuels 2024, 8, 1793. [Google Scholar] [CrossRef]
- Kong, D.; Qi, J.; Liu, D.; Zhang, X.; Pan, L.; Zou, J. Ni-Doped BiVO4 with V4+ Species and Oxygen Vacancies for Efficient Photoelectrochemical Water Splitting. Trans. Tianjin Univ. 2019, 25, 340–347. [Google Scholar] [CrossRef]
- Arunachalam, M.; Lee, K.; Zhu, K.; Kang, S.H. Effective Corrosion-Resistant Single-Atom Alloy Catalyst on HfO2 -Passivated BiVO4 Photoanode for Durable (≈800 h) Solar Water Oxidation. Adv. Energy Mater. 2024, 14, 2402607. [Google Scholar] [CrossRef]
- Yu, S.; Su, C.; Xiao, Z.; Kuang, Y.; Gong, X.; He, X.; Liu, J.; Jin, Q.; Sun, Z. Tuning Surface Hydrophilicity of a BiVO4 Photoanode through Interface Engineering for Efficient PEC Water Splitting. RSC Adv. 2025, 15, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W.; Ji, S.G.; Jeong, C.; Kim, J.; Kwon, H.R.; Lee, T.H.; Lee, S.A.; Cheon, W.S.; Lee, S.; Lee, H.; et al. High-efficiency unbiased water splitting with photoanodes harnessing polycarbazole hole transport layers. Energy Environ. Sci. 2024, 17, 2541–2553. [Google Scholar] [CrossRef]
- Cui, J.; Daboczi, M.; Regue, M.; Chin, Y.; Pagano, K.; Zhang, J.; Isaacs, M.A.; Kerherve, G.; Mornto, A.; West, J.; et al. 2D Bismuthene as a Functional Interlayer between BiVO4 and NiFeOOH for Enhanced Oxygen-Evolution Photoanodes. Adv. Funct. Mater. 2022, 32, 2270250. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Bai, J.; Zeng, Q.; Xia, L.; Zhang, Y.; Chen, S.; Xu, Q.; Zhou, B. Serial Hole Transfer Layers for a BiVO4 Photoanode with Enhanced Photoelectrochemical Water Splitting. Nanoscale 2018, 10, 18378–18386. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, B.; Yang, H.; Liu, C.; Li, Y.; Kravchenko, A.; Sheng, X.; Fan, L.; Li, F.; Sun, L. Remarkable Synergy of Borate and Interfacial Hole Transporter on BiVO4 Photoanodes for Photoelectrochemical Water Oxidation. Mater. Adv. 2021, 2, 4323–4332. [Google Scholar] [CrossRef]
- Yi, Q.; Wang, H.; Lee, J. BiVO4 -Based Photoelectrochemical Water Splitting. ChemElectroChem 2025, 12, e202400600. [Google Scholar] [CrossRef]
- Li, T.-B.; Wang, S.-C.; Li, Y.; Zhao, J.; Su, R.; Wang, J.-G.; Chen, G.; Han, M.-J.; Liu, C.-L.; Liu, J.; et al. Cation Doping to Enable Sub-Bandgap Hydrogen Evolution on BiVO4 Photoanodes. ACS Appl. Mater. Interfaces 2022, 14, 35882–35892. [Google Scholar]
- Li, J.; Xu, D.; Li, T.; Cao, H.; Yu, K.; Li, H.; Chen, G.; Han, M.; Liu, C.; Wang, J.; et al. Band structure and light absorption engineering of Cs-doped BiVO4 photoanodes for efficient photoelectrochemical water splitting. J. Mater. Chem. A 2021, 9, 674–683. [Google Scholar]
- Lin, T.; Zhang, Y.; Luo, J.; Yu, K.; Luo, H.; Zhu, H.; Xue, K.; Li, T.; Wang, X.; Zhang, H.; et al. Band-Bending-Driven Enhanced Charge Transfer in a Step-by-Step BiVO4/WO3 Photoanode for Efficient Photoelectrochemical Water Splitting. Adv. Sci. 2023, 10, 2206729. [Google Scholar]
- Zhang, Z.; Liu, H.; Cheng, Z.; Zhou, Y.; Cai, Y.; Xie, L.; Liu, Y.; Cao, H.; Yu, K.; Zhang, H.; et al. BiVO4 photocatalysts co-doped with Cu2+ and Mo6+ via an optimized process for efficient CO2 reduction. Nano Res. 2023, 16, 1943–1953. [Google Scholar]
- Shao, Z.; Zhou, Y.; Liu, H.; Cai, Y.; Zhang, Z.; Lu, Z.; Yu, K.; Cao, H.; Hou, Y.; Liu, J. In-Plane Strain Engineering of Carbon Nitride/BiVO4 S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction. ACS Mater. Lett. 2024, 6, 180–187. [Google Scholar]
- Ren, H.; Zhou, Y.; Liu, H.; Cai, Y.; Zhang, Z.; Lu, Z.; Wang, J.; Yu, K.; Liu, Y.; Cao, H.; et al. Facet engineering of CdS/BiVO4 S-scheme heterojunction for highly efficient photocatalytic CO2 reduction. Nano Energy 2024, 117, 109010. [Google Scholar]
- Han, W.; Li, J.; Yu, K.; Li, H.; Cai, Y.; Zhang, Z.; Zhu, H.; Liu, Y.; Cao, H.; Zhang, H.; et al. Band structure and charge transfer of AgI/Ag/Ag2O/BiVO4 Z-scheme heterojunction photoanodes for efficient photoelectrochemical water splitting. New J. Chem. 2022, 46, 10800–10807. [Google Scholar]
- Wang, L.; Zhang, T.; Su, J.; Guo, L. Room-Temperature Photodeposition of Conformal Transition Metal Based Cocatalysts on BiVO4 for Enhanced Photoelectrochemical Water Splitting. Nano Res. 2020, 13, 231–237. [Google Scholar] [CrossRef]
- LLi, D.; Chen, R.; Wang, P.; Li, Z.; Zhu, J.; Fan, F.; Shi, J.; Li, C. Effect of Facet-Selective Assembly of Cocatalyst on BiVO4 Photoanode for Solar Water Oxidation. ChemCatChem 2019, 11, 3763–3769. [Google Scholar] [CrossRef]
- Guo, A.; Wu, X.; Ali, S.H.; Shen, H.; Chen, L.; Li, Y.; Wang, B. Modified Photoanode by in Situ Growth of Covalent Organic Frameworks on BiVO4 for Oxygen Evolution Reaction. RSC Adv. 2024, 14, 9609–9618. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Mitoraj, D.; Gong, R.; Gao, D.; Elnagar, M.M.; Liu, R.; Beranek, R.; Streb, C. High-Performance BiVO4 Photoanodes: Elucidating the Combined Effects of Mo-Doping and Modification with Cobalt Polyoxometalate. Mater. Adv. 2024, 5, 4932–4944. [Google Scholar] [CrossRef]
- Liang, Y.; Tsubota, T.; Mooij, L.P.A.; Van De Krol, R. Highly Improved Quantum Efficiencies for Thin Film BiVO4 Photoanodes. J. Phys. Chem. Commun. 2011, 115, 17594–17598. [Google Scholar] [CrossRef]
- Abdi, F.F.; Han, L.; Smets, A.H.M.; Zeman, M.; Dam, B.; Van De Krol, R. Efficient Solar Water Splitting by Enhanced Charge Separation in a Bismuth Vanadate-Silicon Tandem Photoelectrode. Nat. Commun. 2013, 4, 2195. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef]
- Zhao, S.; Jia, C.; Shen, X.; Li, R.; Oldham, L.; Moss, B.; Tam, B.; Pike, S.; Harrison, N.; Ahmad, E.; et al. The Aerosol-Assisted Chemical Vapour Deposition of Mo-Doped BiVO4 Photoanodes for Solar Water Splitting: An Experimental and Computational Study. J. Mater. Chem. A 2024, 12, 26645–26666. [Google Scholar] [CrossRef]
- Cheng, S.; Cheng, Y.; Zhou, T.; Li, S.; Xie, D.; Li, X. Enhanced Photoelectrochemical Performance of BiVO4 Photoanodes Co-Modified with Borate and NiFeOx. Micromachines 2025, 16, 866. [Google Scholar] [CrossRef]
- Fu, C.; Xu, B.; Dong, L.; Zhai, J.; Wang, X.; Wang, D.-Y. Highly Efficient BiVO4 Single-Crystal Nanosheets with Dual Modification: Phosphorus Doping and Selective Ag Modification. Nanotechnology 2021, 32, 325701. [Google Scholar] [CrossRef]
- Gil-Rostra, J.; Castillo-Seoane, J.; Guo, Q.; Sobrido, A.J.; González-Elipe, A.R.; Borrás, A. Photoelectrochemical Water Splitting with ITO/WO3/BiVO4/CoPi Multishell Nanotubes Enabled by a Vacuum and Plasma Soft-Template Synthesis. ACS Appl. Mater. Interfaces 2023, 15, 9250–9262. [Google Scholar] [CrossRef]
- Samuel, E.; Joshi, B.; Kim, M.-W.; Swihart, M.T.; Yoon, S.S. Morphology Engineering of Photoelectrodes for Efficient Photoelectrochemical Water Splitting. Nano Energy 2020, 72, 104648. [Google Scholar] [CrossRef]
- Wang, T.; Yu, Q.; Zhang, S.; Kou, X.; Sun, P.; Lu, G. Rational Design of 3D Inverse Opal Heterogeneous Composite Microspheres as Excellent Visible-Light-Induced NO2 Sensors at Room Temperature. Nanoscale 2018, 10, 4841–4851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, C. Three-Dimensional FTO/TiO2/BiVO4 Composite Inverse Opals Photoanode with Excellent Photoelectrochemical Performance. ACS Energy Lett. 2017, 2, 813–821. [Google Scholar] [CrossRef]
- Marinković, D.; Righini, G.C.; Ferrari, M. Synthesis, Optical, and Photocatalytic Properties of the BiVO4 Semiconductor Nanoparticles with Tetragonal Zircon-Type Structure. Photonics 2025, 12, 438. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, X.; Pei, Y.; Han, W. Enhancing Photoelectrocatalytic Efficiency of BiVO4 Photoanodes by Crystal Orientation Control. Nanomaterials 2024, 14, 1870. [Google Scholar] [CrossRef]
- Hu, Z.; Tong, G.; Lin, D.; Nian, Q.; Shao, J.; Hu, Y.; Saeib, M.; Jin, S.; Cheng, G.J. Laser Sintered Graphene Nickel Nanocomposites. J. Mater. Process. Technol. 2016, 231, 143–150. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Y.; Huang, X.; Wang, K.; Gao, F.; Zhao, Y.; Wang, B.; Zhang, L.; Liu, P. One-Pot Construction of 1D/2D Zn1-xCdx S/D-ZnS(en)0.5 Composites with Perfect Heterojunctions and Their Superior Visible-Light-Driven Photocatalytic H2 Evolution. Appl. Catal. B Environ. 2018, 220, 324–336. [Google Scholar] [CrossRef]
- Prasad, U.; Young, J.L.; Johnson, J.C.; McGott, D.L.; Gu, H.; Garfunkel, E.; Kannan, A.M. Enhancing Interfacial Charge Transfer in a WO3/BiVO4 Photoanode Heterojunction through Gallium and Tungsten Co-Doping and a Sulfur Modified Bi2O3 Interfacial Layer. J. Mater. Chem. A 2021, 9, 16137–16149. [Google Scholar] [CrossRef]
- Nong, S.; Wang, M.; Wang, X.; Li, Y.; Yu, S.; Tang, C.; Li, G.; Xu, L. A Multifunctional Guanosine-Based Carbon Dots for Dead Microbial Imaging and Synergistic Broad-Spectrum Antimicrobial Therapy. Chem. Eng. J. 2024, 485, 150123. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Y.; Liu, X.; Li, R.; Zhang, Z.; Zhang, F.; Cao, D.; Gao, Z.; Mi, B. Plasma treatment induced oxygen vacancies and N doping in the BiVO4 photoanode toward improved charge transfer and facilitated water oxidation. ACS Appl. Mater. Interfaces 2025, 17, 12064–12073. [Google Scholar] [CrossRef]
- Qian, F.; Tian, J.; Bai, Z.; Liu, L.; Cao, M.; Li, J.; Chen, S. CoNi2S4 Cocatalyst Modified TiO2 Nanorods/Nanoflower Homojunction for Efficient Photoelectrochemical Water Splitting. J. Alloys Compd. 2024, 991, 174567. [Google Scholar] [CrossRef]
- Chen, M.; Chang, X.; Li, C.; Wang, H.; Jia, L. Ni-Doped BiVO4 Photoanode for Efficient Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2023, 640, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Park, I.J.; Lee, S.A.; Lee, M.G.; Lee, T.H.; Park, H.; Kim, C.; Park, J.; Moon, J.; Kim, J.Y.; et al. Near-Complete Charge Separation in Tailored BiVO4-Based Heterostructure Photoanodes toward Artificial Leaf. Appl. Catal. B Environ. 2021, 293, 120217. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Zhang, Y.; Xu, L.; Wang, T.; Xiao, X.; Wang, S.; Wang, L.; Huang, W. A BiVO4 photoanode with a VOx layer bearing oxygen vacancies offers improved charge transfer and oxygen evolution kinetics in photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2023, 62, e202217346. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.M.; Lee, S.A.; Suh, J.M.; Hong, S.-P.; Jang, H.W. Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination. Appl. Sci. 2018, 8, 1765. [Google Scholar] [CrossRef]
- Fan, X.; Chen, Q.; Zhu, F.; Wang, T.; Gao, B.; Song, L.; He, J. Preparation of Surface Dispersed WO3/BiVO4 Heterojunction Arrays and Their Photoelectrochemical Performance for Water Splitting. Molecules 2024, 29, 372. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Wang, B.; Tang, W.; Wu, R.; Li, J.; Ma, M. Surface Engineering of WO3/BiVO4 to Boost Solar Water-Splitting. Catalysts 2020, 10, 556. [Google Scholar] [CrossRef]
- Ziwritsch, M.; Müller, S.; Hempel, H.; Unold, T.; Abdi, F.F.; Van De Krol, R.; Friedrich, D.; Eichberger, R. Direct Time-Resolved Observation of Carrier Trapping and Polaron Conductivity in BiVO4. ACS Energy Lett. 2016, 1, 888–894. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Z.; Liu, H.; Zhang, S.; Wang, P.; Lu, J.; Tong, Y. Heterojunction Architecture of N-Doped WO3 Nanobundles with Ce2S3 Nanodots Hybridized on a Carbon Textile Enables a Highly Efficient Flexible Photocatalyst. Adv Funct Mater. 2019, 29, 1903490. [Google Scholar] [CrossRef]
- Nair, V.; Perkins, C.L.; Lin, Q.; Law, M. Textured Nanoporous Mo:BiVO4 Photoanodes with High Charge Transport and Charge Transfer Quantum Efficiencies for Oxygen Evolution. Energy Environ. Sci. 2016, 9, 1412–1429. [Google Scholar] [CrossRef]
- Rettie, A.J.E.; Lee, H.C.; Marshall, L.G.; Lin, J.-F.; Capan, C.; Lindemuth, J.; McCloy, J.S.; Zhou, J.; Bard, A.J.; Mullins, C.B. Combined Charge Carrier Transport and Photoelectrochemical Characterization of BiVO4 Single Crystals: Intrinsic Behavior of a Complex Metal Oxide. J. Am. Chem. Soc. 2013, 135, 11389–11396. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Oropeza, F.E.; Qi, Z.; Einert, M.; Tian, C.; Maheu, C.; Lv, K.; Hofmann, J.P. Influence of Mo Doping on Interfacial Charge Carrier Dynamics in Photoelectrochemical Water Oxidation on BiVO4. Sustain. Energy Fuels 2023, 7, 2923–2933. [Google Scholar] [CrossRef]
- Chaudhari, S.A.; Patil, V.V.; Jadhav, V.A.; Thorat, P.; Sutar, S.S.; Dongale, T.D.; Parale, V.; Patil, V.; Mhamane, D.S.; Mali, M.G.; et al. Conductivity Boosted BiVO4 for Enhanced OER and Supercapacitive Performance: Stability Insights with Modeling, Predictions, and Forecasting Using Machine Learning Technique. Energy Mater. 2025, 5, 500082. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.-S.; Tong, Y.; Huang, Y. Oxygen Vacancy–Based Metal Oxides Photoanodes in Photoelectrochemical Water Splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Li, R.; Zhan, F.; Wen, G.; Wang, B.; Qi, J.; Liu, Y.; Feng, C.; La, P. Facile Synthesis of a Micro–Nano-Structured FeOOH/BiVO4/WO3 Photoanode with Enhanced Photoelectrochemical Performance. Catalysts 2024, 14, 828. [Google Scholar] [CrossRef]
- Creasey, G.H.; McCallum, T.W.; Ai, G.; Tam, B.; Rodriguez Acosta, J.W.; Mohammad Yousuf, A.; Fearn, S.; Eisner, F.; Kafizas, A.; Hankin, A. Mechanically and Photoelectrochemically Stable WO3|BiVO4|NiFeOOH Photoanodes Synthesised by a Scalable Chemical Vapour Deposition Method. J. Mater. Chem. A 2025, 13, 11585–11604. [Google Scholar] [CrossRef]
- Li, Z.; Xie, Z.; Li, W.; Aziz, H.S.; Abbas, M.; Zheng, Z.; Su, Z.; Fan, P.; Chen, S.; Liang, G. Charge Transport Enhancement in BiVO4 Photoanode for Efficient Solar Water Oxidation. Materials 2023, 16, 3414. [Google Scholar] [CrossRef]
- Zhang, S.; Ahmet, I.; Kim, S.-H.; Kasian, O.; Mingers, A.M.; Schnell, P.; Kolbach, M.; Lim, J.; Fischer, A.; Mayrhofer, K.J.J.; et al. Different Photostability of BiVO4 in Near-pH-Neutral Electrolytes. ACS Appl. Energy Mater. 2020, 3, 9523–9527. [Google Scholar]
- Wang, L.; Zhang, Y.; Li, W.; Wang, L. Recent Advances in Elaborate Interface Regulation of BiVO4 Photoanode for Photoelectrochemical Water Splitting. Mater. Rep. Energy 2023, 3, 100232. [Google Scholar] [CrossRef]
- Lei, R.; Tang, Y.; Qiu, W.; Yan, S.; Tian, X.; Wang, Q.; Chen, Q.; Wang, Z.; Qian, W.; Xu, Q.; et al. Prompt Hole Extraction Suppresses V5+ Dissolution and Sustains Large-Area BiVO4 Photoanodes for Over 2100 h Water Oxidation. Nano Lett. 2023, 23, 11785–11792. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, D.S.; Jung, S.Y.; Kang, J.; Cho, H.K. Oxygen Network Formation for Efficient Charge Transport and Durable BiVO4 Photoanodes with Ultra-Thin TiO2 Layer in Solar Water Splitting. Appl. Surf. Sci. Adv. 2025, 27, 100737. [Google Scholar] [CrossRef]
- Zhou, G.; Aletsee, C.C.; Lemperle, A.; Rieth, T.; Mengel, L.; Gao, J.; Tschurl, M.; Heiz, U.; Sharp, I.D. Water Oxidation and Degradation Mechanisms of BiVO4 Photoanodes in Bicarbonate Electrolytes. ACS Catal. 2025, 15, 13048–13058. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yu, X.; Chen, X.; Ye, R. Boosting the Photoelectrochemical Performance of BiVO4 by Borate Buffer Activation: The Role of Trace Iron Impurities. Chem. Commun. 2024, 60, 10330–10333. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; An, S.; Qin, W.; Kuang, Y.; Liu, D. Stabilizing BiVO4 Photoanode in Bicarbonate Electrolyte for Efficient Photoelectrocatalytic Alcohol Oxidation. Molecules 2024, 29, 1554. [Google Scholar] [CrossRef]
- Khan, H.; Kim, M.-J.; Balasubramanian, P.; Jung, M.-J.; Kwon, S.-H. Improving the Photoelectrochemical Stability of a Bismuth Vanadate Photoanode for Solar Water Oxidation. Cell Rep. Phys. Sci. 2023, 4, 101652. [Google Scholar] [CrossRef]
- Jung, G.; Moon, C.; Martinho, F.; Jung, Y.; Chu, J.; Park, H.; Hajijafarassar, A.; Nielsen, R.; Schou, J.; Park, J.; et al. Monolithically Integrated BiVO4/Si Tandem Devices Enabling Unbiased Photoelectrochemical Water Splitting. Adv. Energy Mater. 2023, 13, 2301235. [Google Scholar] [CrossRef]
- Sitaaraman, S.R.; Grace, A.N.; Zhu, J.; Sellappan, R. Photoelectrochemical Performance of a Nanostructured BiVO4/NiOOH/FeOOH–Cu2O/CuO/TiO2 Tandem Cell for Unassisted Solar Water Splitting. Nanoscale Adv. 2024, 6, 2407–2418. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Zhang, Y.; Zhu, M.; Zhang, C.; Li, S.; Ma, Y.; Huang, W.; Wang, S. A Standalone Bismuth Vanadate-Silicon Artificial Leaf Achieving 8.4% Efficiency for Hydrogen Production. Nat. Commun. 2025, 16, 2792. [Google Scholar] [CrossRef]
- Shu, X.; Wang, R.; Yang, L.; Liu, S.; Yin, Y.; Liang, X.; Hu, K.; Zhang, M. Bifunctional BiVO4/Cu2O Photoelectrode for Bias-Free Solar Water Splitting Tandem Cells. Sol. Energy Mater. Sol. Cells 2025, 282, 113386. [Google Scholar] [CrossRef]
- Ahmet, I.Y.; Ma, Y.; Jang, J.-W.; Henschel, T.; Stannowski, B.; Lopes, T.; Vilanova, A.; Mendes, A.; Abdi, F.F.; Van De Krol, R. Demonstration of a 50 Cm2 BiVO4 Tandem Photoelectrochemical-Photovoltaic Water Splitting Device. Sustain. Energy Fuels 2019, 3, 2366–2379. [Google Scholar] [CrossRef]
- Wan, S.; Jin, J.; Dong, C.; Lu, Y.; Zhong, Q.; Zhang, K.; Park, J.H. Promoting Water Oxidative Hydrogen Peroxide Production by Dynamic Anion Exchange on BiVO4 Photoanodes. ACS Catal. 2023, 13, 14845–14852. [Google Scholar] [CrossRef]
- Shi, S.; Song, Y.; Jiao, Y.; Jin, D.; Li, Z.; Xie, H.; Gao, L.; Sun, L.; Hou, J. BiVO4-Based Heterojunction Photocathode for High-Performance Photoelectrochemical Hydrogen Peroxide Production. Nano Lett. 2024, 24, 6051–6060. [Google Scholar] [CrossRef]
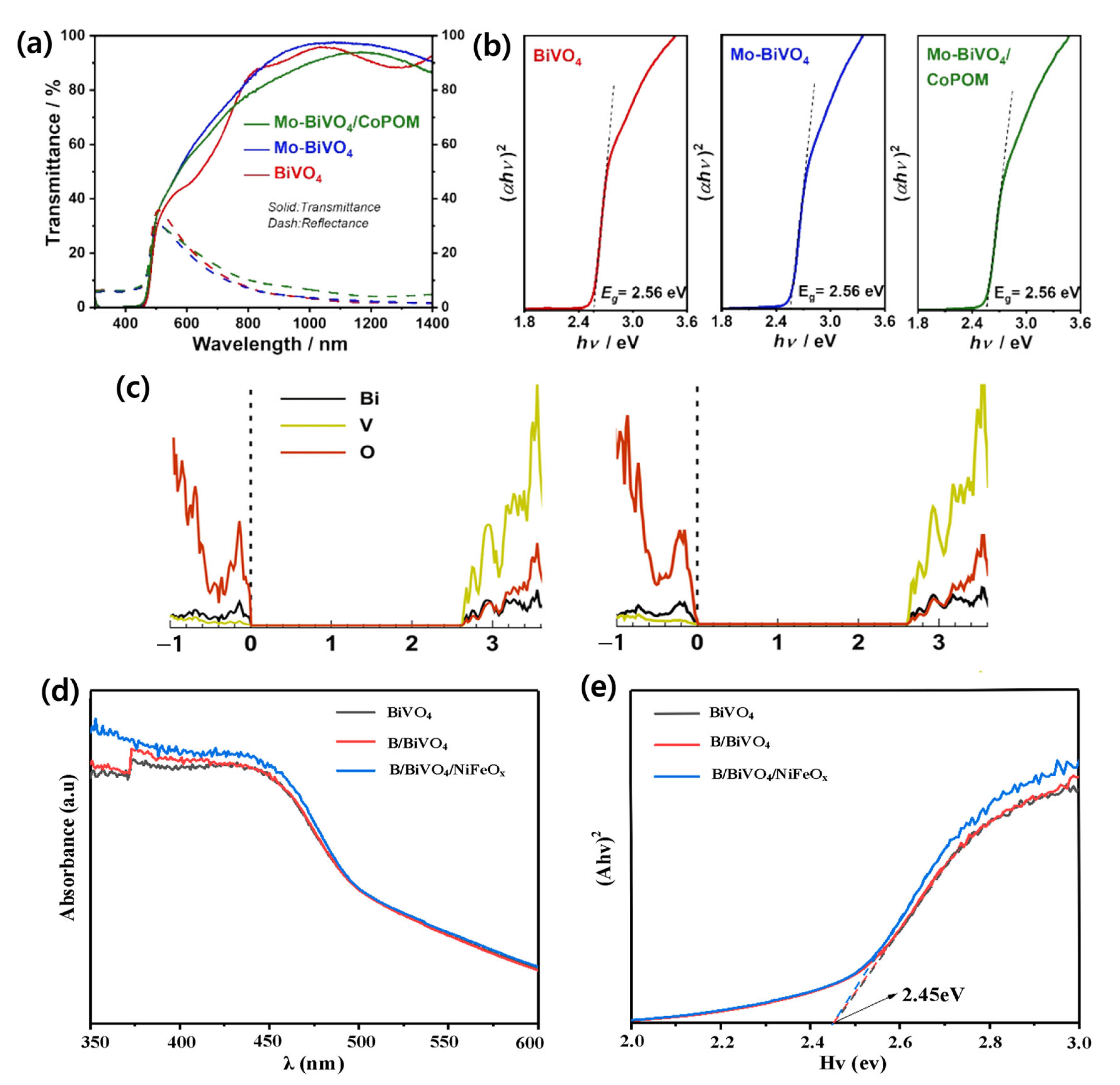
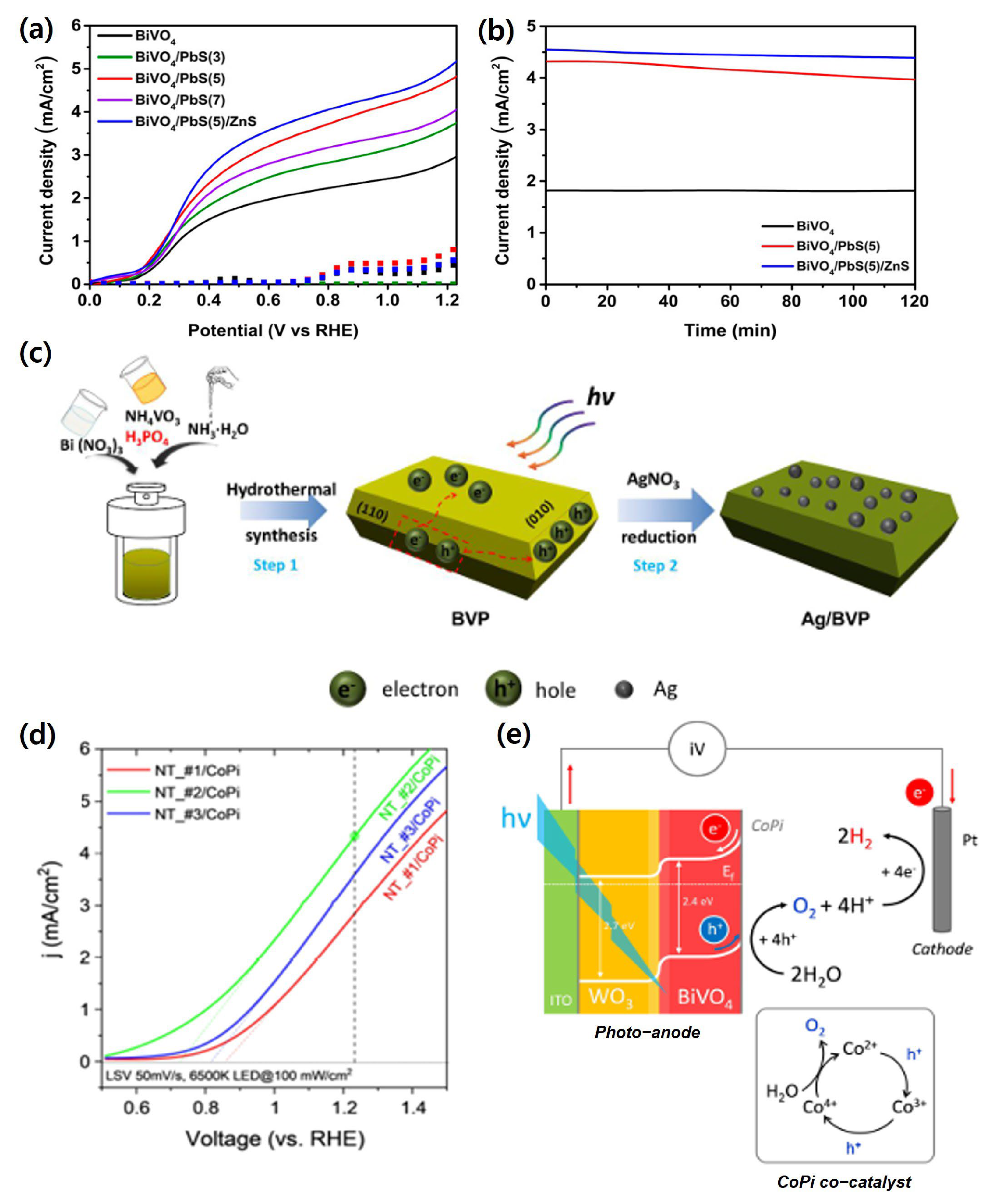
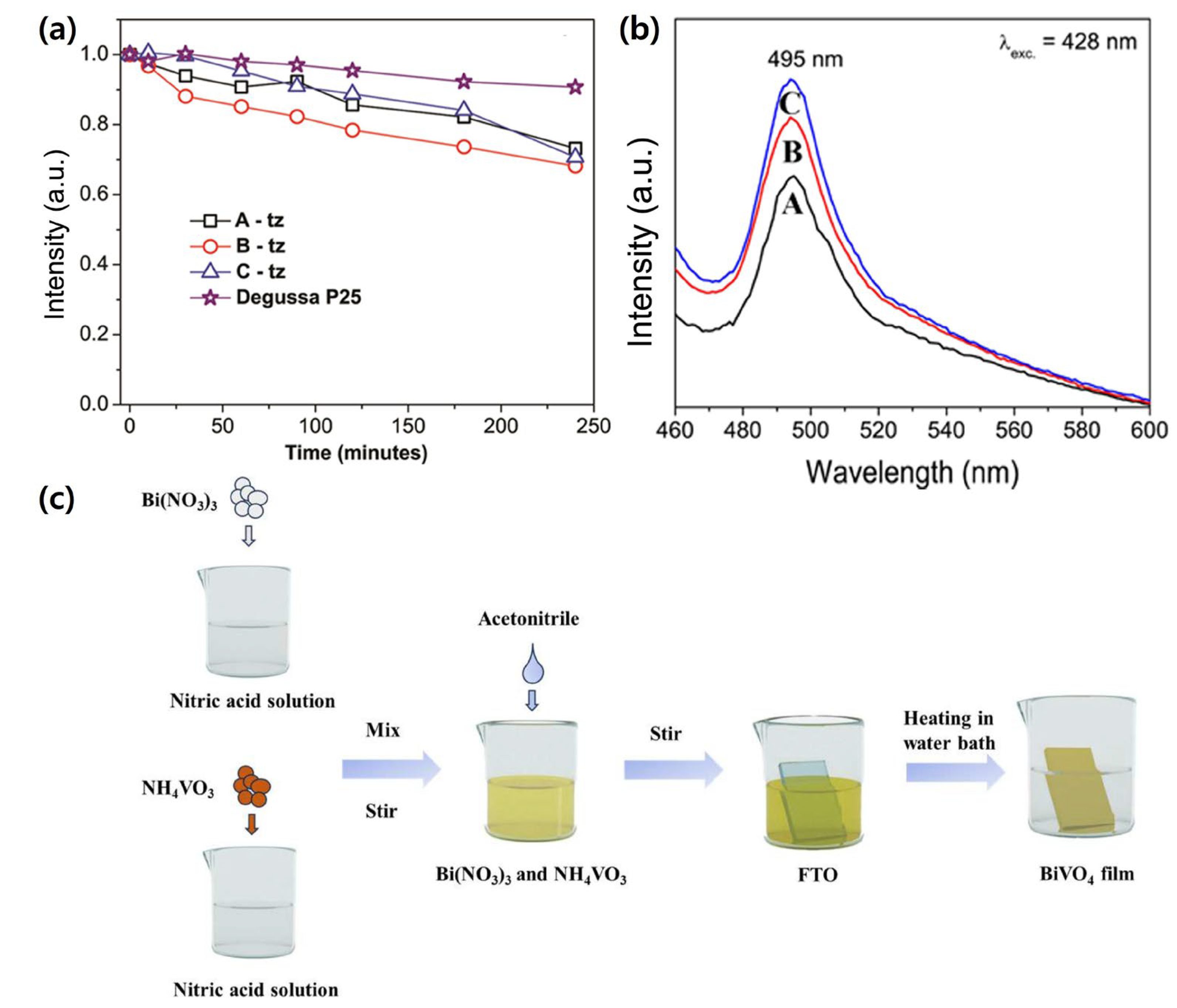
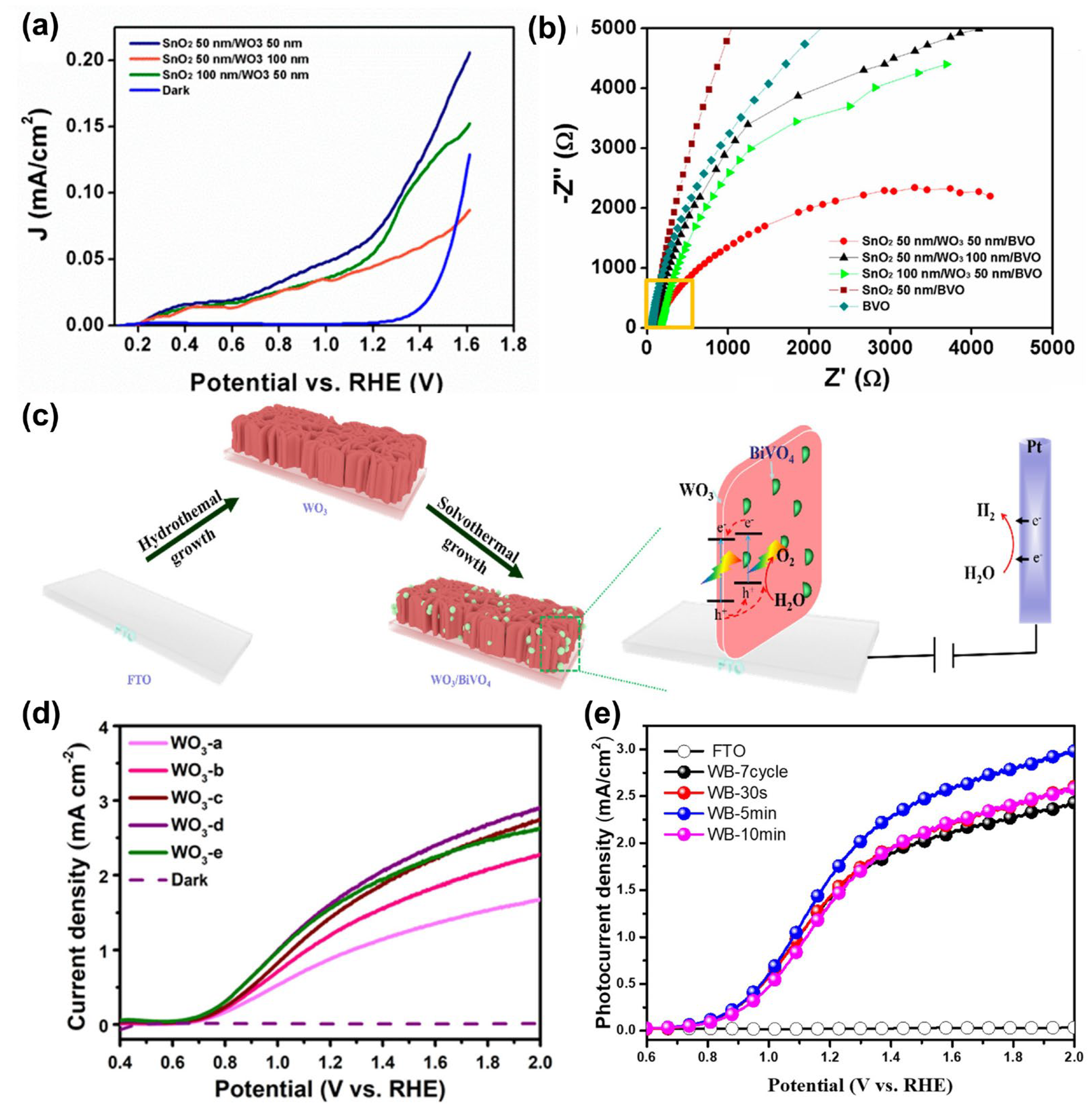
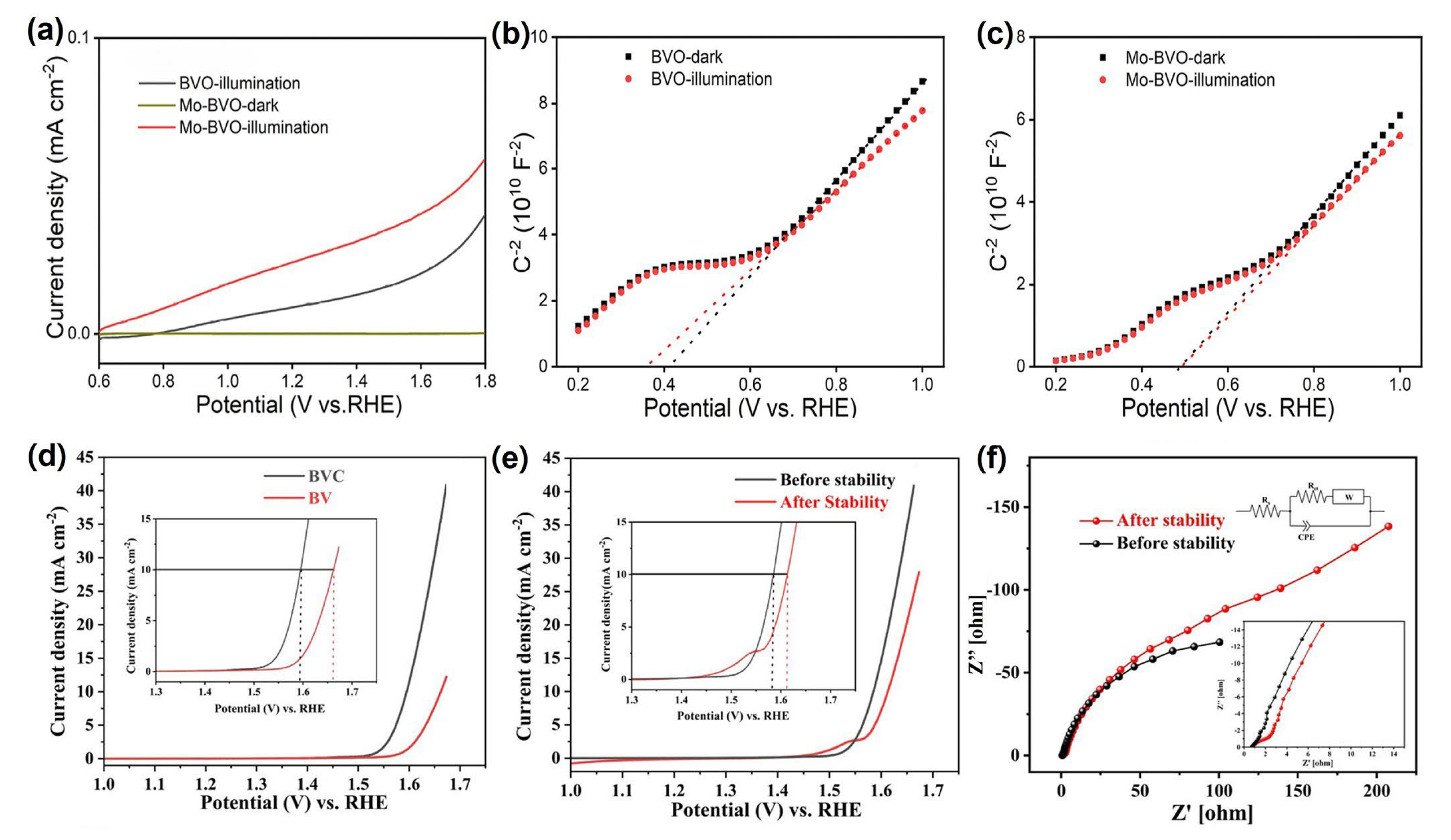
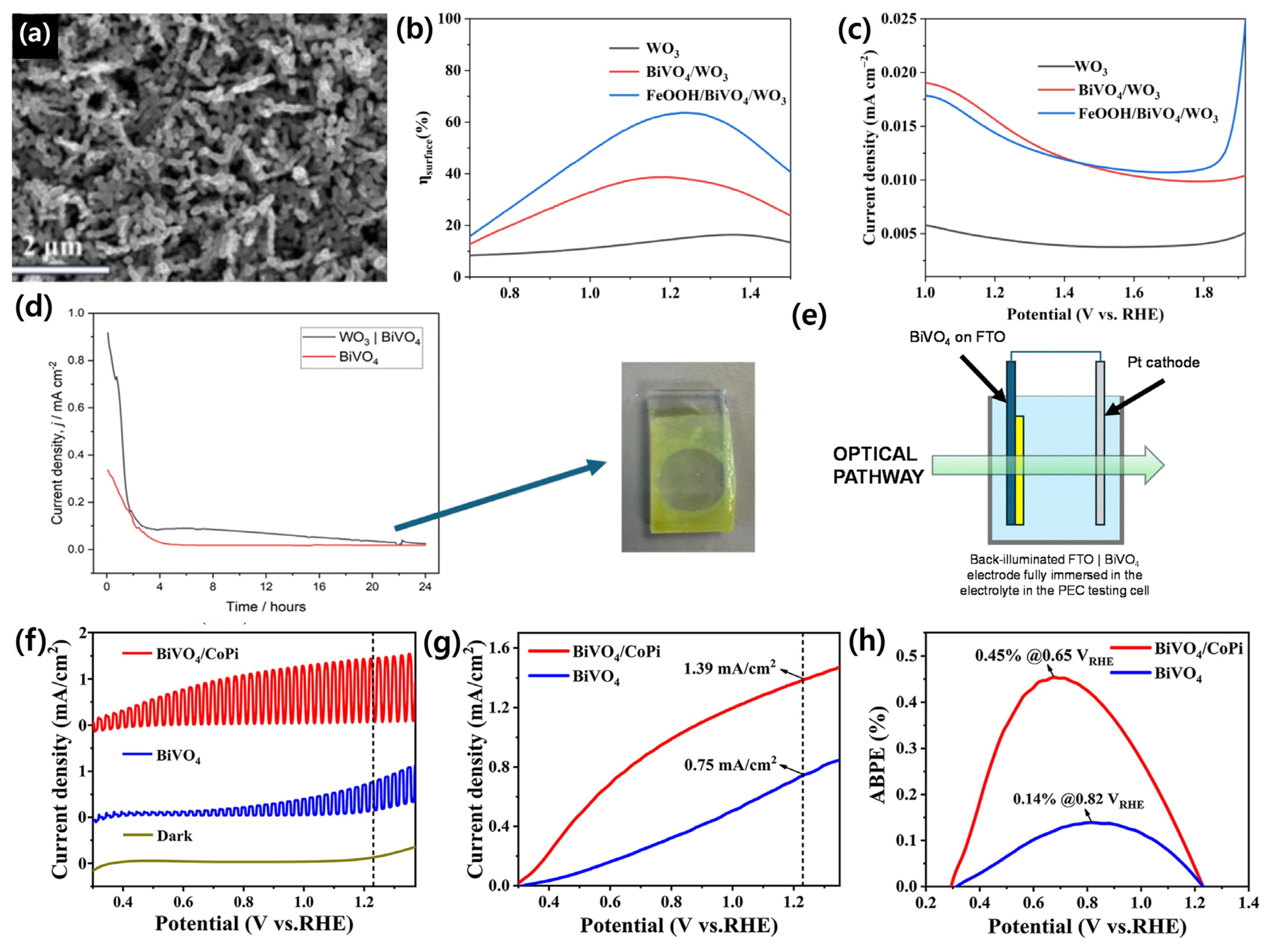

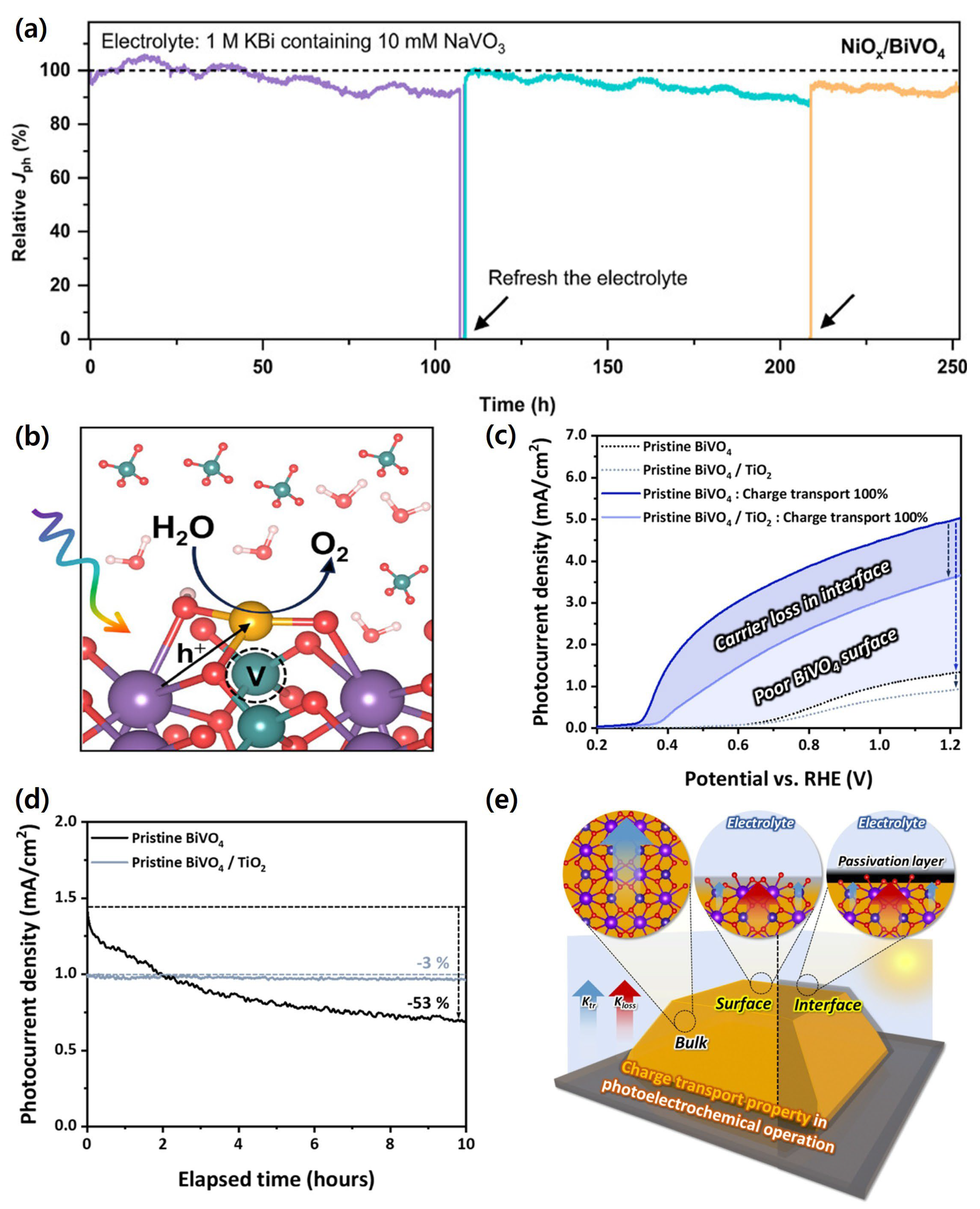
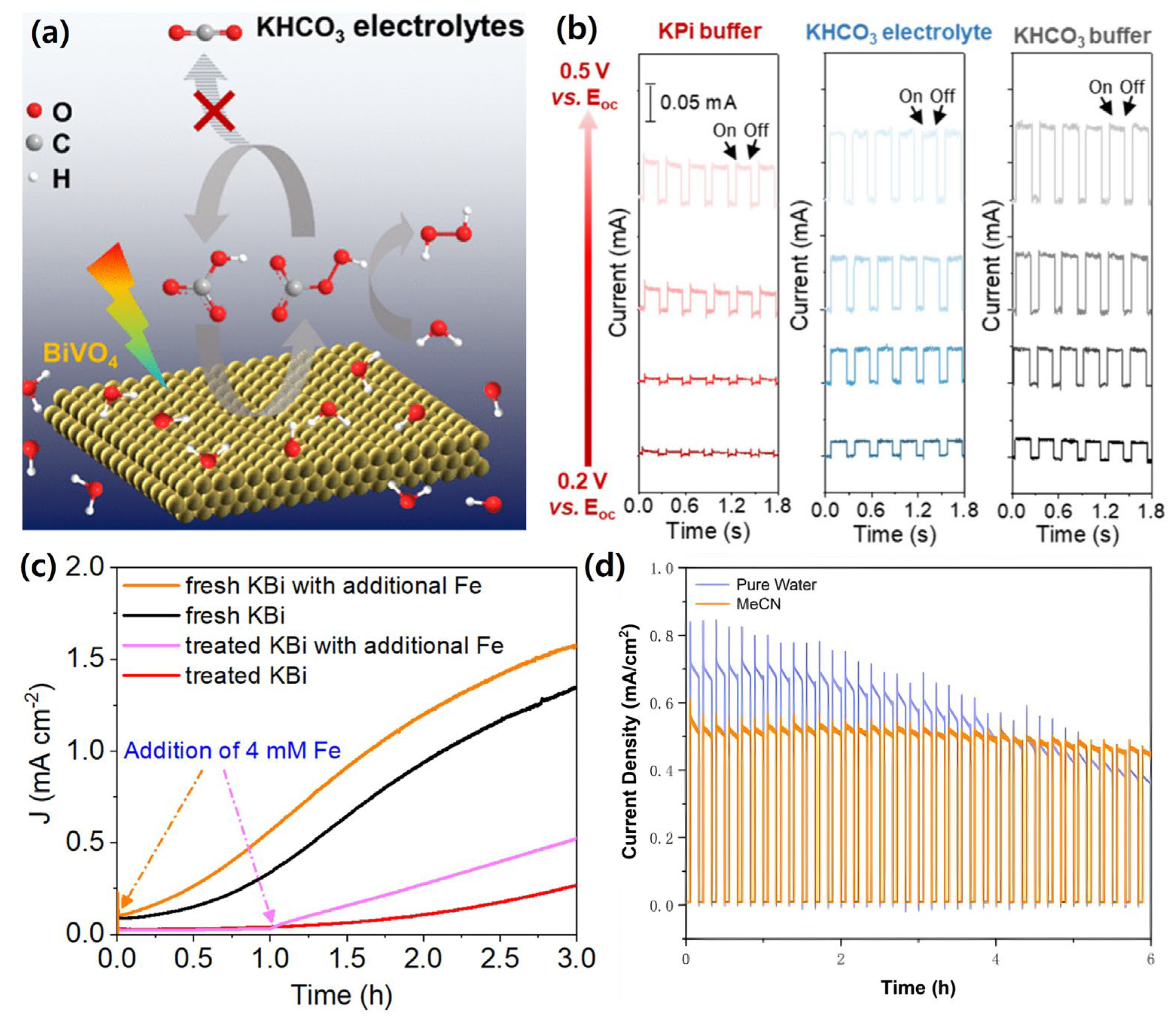
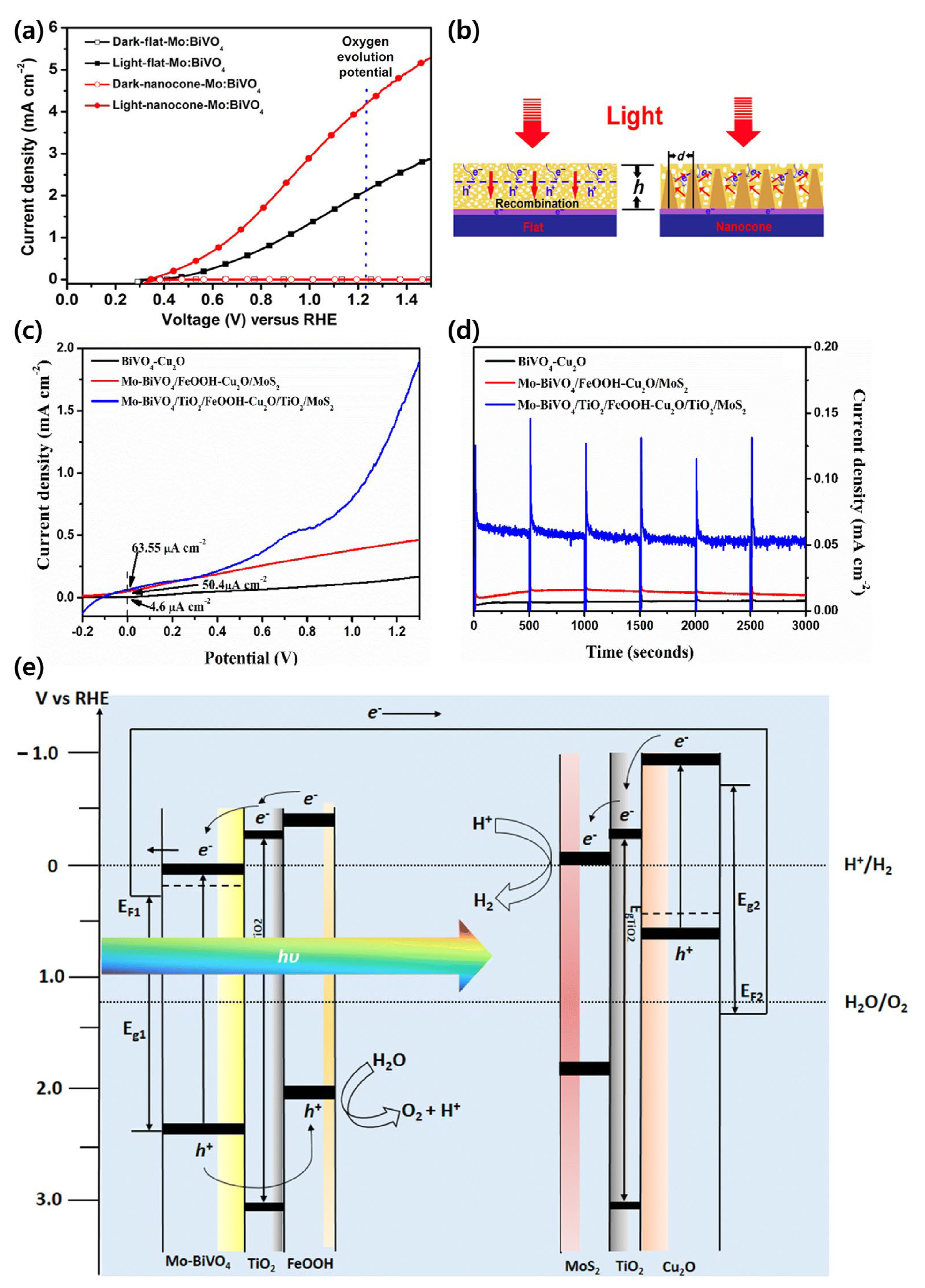
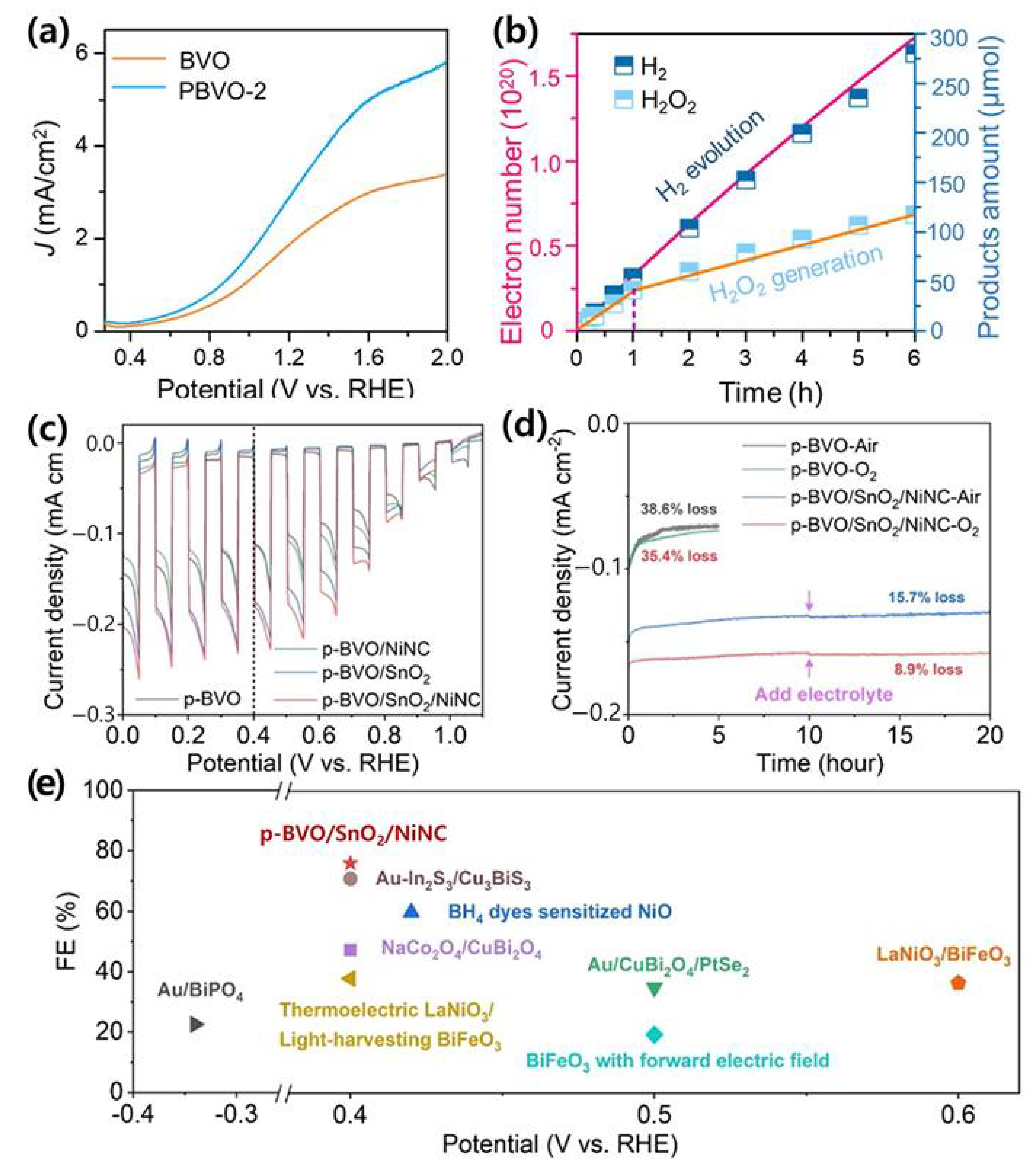
| Materials | Fabrication Method | Annealing Condition (Temp., Time, Atmosphere) | Electrolyte Condition | Photocurrent Density @ 1.23 VRHE (mA/cm2) |
|---|---|---|---|---|
| Bare BiVO4 [47] | Electrodeposition BiOI + VO(acac)2 drop | 450 °C, 2 h, air | 0.1 M Na2SO4 (pH 6) | 0.65 |
| NiOOH/FeOOH/BiVO4 [48] | Electrodeposition BiOI + VO(acac)2 drop + cocatalysts deposition | 450 °C, 2 h, air | 0.1 M Na2SO4 | ~1.3 |
| CdS/NiFe-LDH/BiVO4 [49] | BiOI + VO(acac)2 + CdS hydrothermal + NiFe-LDH deposition | 450 °C, 2 h, air | 0.5 M Na2SO4 | 3.10 |
| BiVO4/NiO composite [50] | BiOI + VO(acac)2 conversion + NiO HT | 450 °C, 2 h, air | 0.5 M Na2SO4 | 1.20 |
| CoV-LDH/Ag/BiVO4 [51] | BiOI + VO(acac)2→CoV-LDH/Ag coating | 450 °C, 2 h, air | 0.5 M Na2SO4 + 0.1 M glycerol | 7.15 |
| Bare BiVO4 [52] | BiOI electrodeposition + V2O5 electrodeposition + calcination | 475 °C, 2 h, air | 0.5 M potassium borate (pH 9.5) | 2.2 |
| Mo-BiVO4/TiO2/FeOOH [53] | BiOI + VO/(Mo dopant) + TiO2 + FeOOH | 450 °C, 2 h, air | 0.1 M Na2SO4 | 0.81 |
| BiVO4/PbS QDs/ZnS [54] | BiOI + VO(acac)2→BiVO4 + PbS/ZnS (SILAR) | 450 °C, 2 h, air | 0.5 M KH2PO4 + 1.0 M Na2SO3 | 5.19 |
| Techniques | Materials/Structures/Methods | Functionality |
|---|---|---|
| 1. Synthesis and Film Morphology Engineering | (1) Controls morphology and film thickness. (2) Increases surface area. (3) Improves light absorption and bulk charge transport (ηbulk). (4) Precisely controls composition and film density. (5) Enables conformal coating on complex nanostructures. | |
| 2. Doping | (1) Increases bulk conductivity. (2) Tunes the bandgap. (3) Reduces bulk recombination. | |
| 3. Surface and Cocatalyst Modification | (1) Lowers OER overpotential. (2) Improves surface reaction kinetics (ηsurf). (3) Increases chemical stability. (4) Accelerates oxygen evolution reaction. (5) Enhances charge transfer efficiency at the surface. | |
| 4. Heterojunction Engineering | (1) Increases electron–hole separation efficiency. (2) Improves quantum efficiency. (3) Enhances light absorption. (4) Boosts performance via surface resonance effect. (5) Provides a direct pathway for hole extraction. (6) Expands absorption into the near-infrared and enhances energy conversion efficiency. | |
| 5. Post-treatment and Passivation | (1) Passivates surface defects. (2) Improves crystallinity. (3) Tunes electronic band structure. (4) Increases stability and reduces recombination. (5) Creates a protective layer against photocorrosion. | |
| 6. Other Treatment Methods | (1) Enhances photon absorption. (2) Improves electrical conductivity. (3) Promotes desired defect states for enhanced activity. |
| Structure/Strategy | Cocatalyst or Overlayer | Performance Highlight | Key Effect | Ref. |
|---|---|---|---|---|
| One-step PEC deposition | FeOOH (inner) + Co–Sil (outer) | 6.10 mA/cm2 @1.23 V | Dual-layer cocatalyst boosts charge separation and reduces recombination | [115] |
| Fluoride-assisted in situ passivation | F− ions | Long-term stability >100 h @0.6 V | Surface passivation and cocatalyst reactivation | [116] |
| Porphyrin-based surface ligand | Co–He (Co–O–V linkage) | 5.3 mA/cm2 @1.23 V, Von = 0.07 V | Low overpotential and efficient hole transfer | [117] |
| Organic ligand modification | Co2+ + BTC ligand | 4.82 mA/cm2, onset 0.22 V | Surface passivation + cocatalyst anchoring | [118] |
| Magnetic overlayer | Co-doped Fe3O4 | 1.9× higher OER activity, Faradaic efficiency >85% | Improves surface kinetics and protects BiVO4 | [119] |
| Dual cocatalyst immersion | FeOOH + Co(OH)2 | 2.56 mA/cm2, 71.6% retention (10 h) | Synergistic catalytic enhancement + stability | [120] |
| Bilayer MOF cocatalyst | Fe-MOF/Ni-MOF | 1.80 mA/cm2, V_on dropped from 0.9 V to 0.69 V | Facilitated interfacial charge transfer | [121] |
| Room-temp photodeposition | Co-Pi, Ni-Bi, Mn-Pi/BiVO4 | Hole transfer efficiency up to 94.5 % @1.23 VRHE | Conformal, uniform cocatalyst deposition | [122] |
| Facet-selective cocatalyst loading | Selective facet-modified MnOx | 0.74 mA/cm2 @1.23 VRHE | Maximize catalytic activity by crystal facet control | [123] |
| In situ solvothermal growth | COF–Azo | 1.38 mA/cm2 @1.23 VRHE | Improves carrier separation, lowers impedance, and accelerates OER | [124] |
| Bulk Mo doping + surface molecular catalyst deposition | CoPOM | 4.32 mA/cm2 @1.23 VRHE | Conductivity enhancement + catalytic activation | [125] |
| Photoanodes | JPEC @1.23 VRHE (mA/cm2) | ηsep (%) | ηinj (%) | Modification Method | Ref. |
|---|---|---|---|---|---|
| WO3/S:Bi2O3/(Ga,W):BiVO4/Co-Pi | 5.10 | N/R | N/R | Interface design | [140] |
| Co3O4/BiVO4 | ~2.3 | N/R | N/R | Cocatalyst interface | [141] |
| Plasma-treated N-doped BiVO4 | 1.39 | ~4.6× higher vs. pristine | N/R | N doping + oxygen vacancies | [142] |
| Co:BiVO4/Mo:BiVO4 | 2.09 | 77.8 | 86.5 | Homojunction (doped layers) | [139] |
| Zn:BiVO4/Mo:BiVO4 | 2.70 | 65.0 | 89.0 | Homojunction | [143] |
| Ni-BiVO4/FeOOH | 3.02 | N/R | 73.3 | Homojunction (+OER overlayer) | [144] |
| BiVO4/SnO2 (heterostructure) | 5.61 | 97 | N/R | Heterojunction + cocatalyst | [145] |
| Ov-BiVO4 (VOx engineered) | 6.29 | 94 | 96 | VOx (oxygen vacancy-engineered) | [146] |
| Photoanodes | Bias | Electrolyte | JPEC @1.23 VRHE (mA/cm2) | STH (%) | Ref |
|---|---|---|---|---|---|
| NiOOH/FeOOH/BiVO4/SnO2//TTO//TOPCon-Si | Unbiased | 1.0 M potassium borate, pH 9 | 1.40 | 1.72 | [168] |
| Nanocone/Mo:BiVO4/Fe(Ni)OOH | Unassisted | Phosphate buffer, pH 7 | 5.82 ± 0.36 | 6.2 | [33] |
| BiVO4/NiOOH/FeOOH (top)//Cu2O/CuO/TiO2 (bottom) | Unassisted | 0.1 M Na2SO4, pH 6 | 2.05 | 0.27 | [169] |
| BiVO4/FeOOH (oxygen vacancy gradient; FeOOH OEC) | Unassisted | 1 M borate buffer (pH ≈ 9) | 7.0 | 8.4 | [170] |
| BiVO4/Cu2O/NiFe-LDH | Unassisted | 0.1 M Na2SO4, pH 6 | 5.01 | 1.18 | [171] |
| Mo:BiVO4 + polycarbazole HTL (CPF-TCB) + NiFeCoOx OEC | Unassisted | K–borate buffer (pH ≈ 9–10) | ≈6.6 | ~9 | [110] |
| CoPi/W:BiVO4/Ni | Unassisted | K-phosphate buffer (pH 7) | 1.5 | 2.1–6.3 | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, B.D.; Choi, I.-H.; Kim, J.-Y. Strategies for Enhancing BiVO4 Photoanodes for PEC Water Splitting: A State-of-the-Art Review. Nanomaterials 2025, 15, 1494. https://doi.org/10.3390/nano15191494
Nguyen BD, Choi I-H, Kim J-Y. Strategies for Enhancing BiVO4 Photoanodes for PEC Water Splitting: A State-of-the-Art Review. Nanomaterials. 2025; 15(19):1494. https://doi.org/10.3390/nano15191494
Chicago/Turabian StyleNguyen, Binh Duc, In-Hee Choi, and Jae-Yup Kim. 2025. "Strategies for Enhancing BiVO4 Photoanodes for PEC Water Splitting: A State-of-the-Art Review" Nanomaterials 15, no. 19: 1494. https://doi.org/10.3390/nano15191494
APA StyleNguyen, B. D., Choi, I.-H., & Kim, J.-Y. (2025). Strategies for Enhancing BiVO4 Photoanodes for PEC Water Splitting: A State-of-the-Art Review. Nanomaterials, 15(19), 1494. https://doi.org/10.3390/nano15191494







