Green Synthesis of Biochar-Supported Nanoscale Zero-Valent Iron Using Tea Polyphenol for Efficient Cadmium Immobilization in Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of nZVI Loading Biochar by Sodium Borohydride
2.3. Preparation of nZVI Loading Biochar by Tea Polyphenols
2.4. Preparation of Soil Sample
2.5. Soil Remediation and Analysis
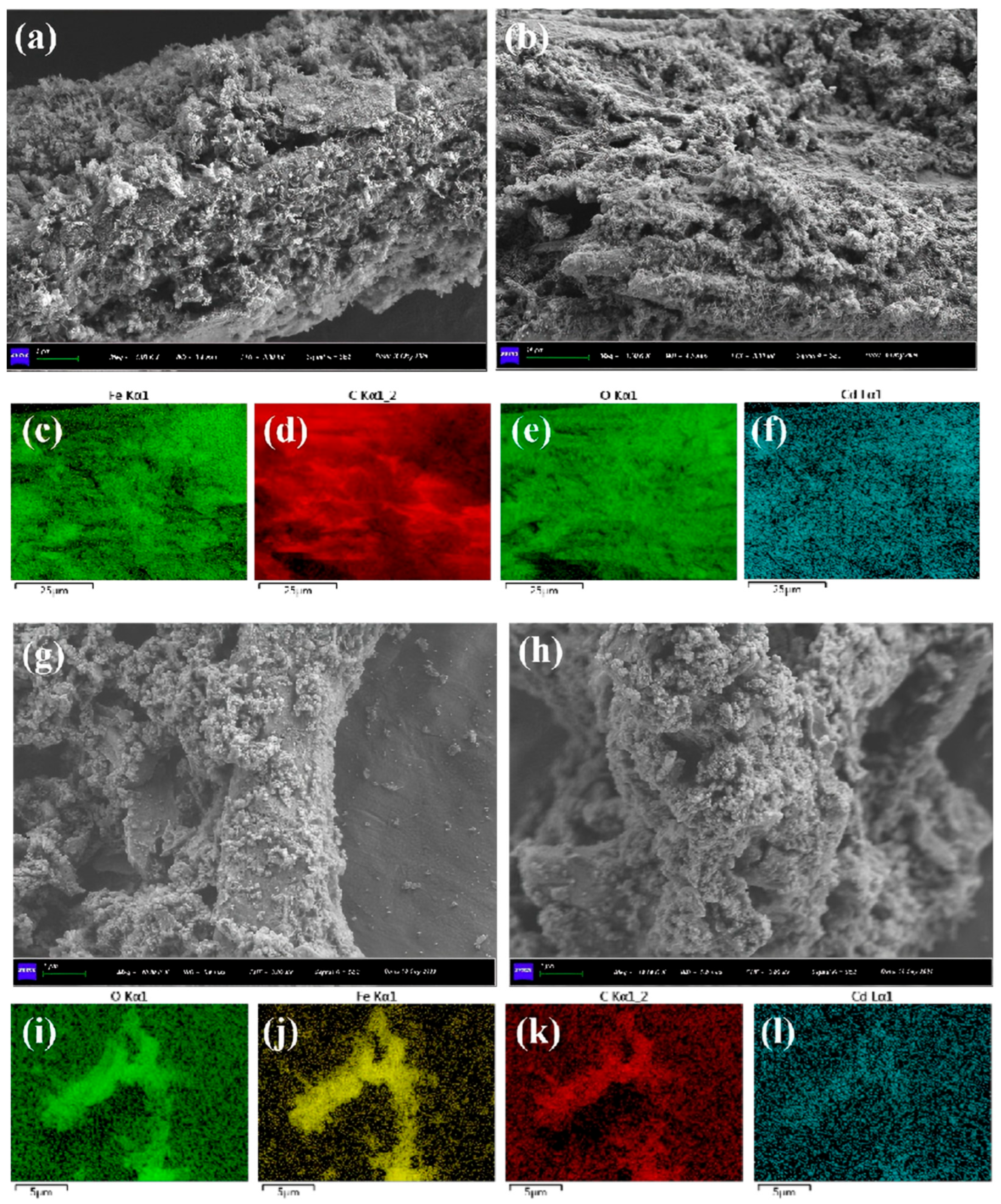
2.6. Characterization and Morphology
3. Results and Discussion
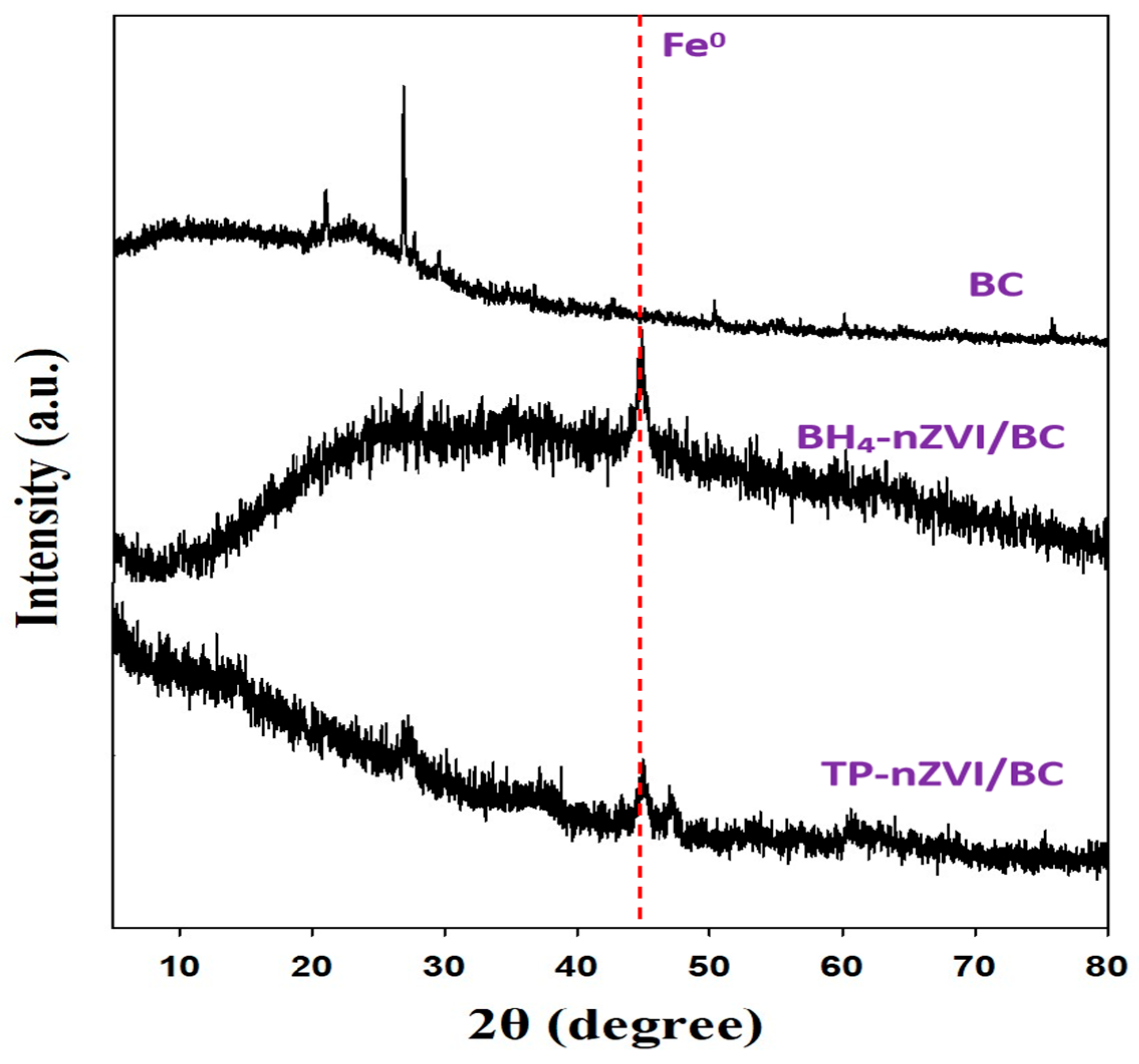
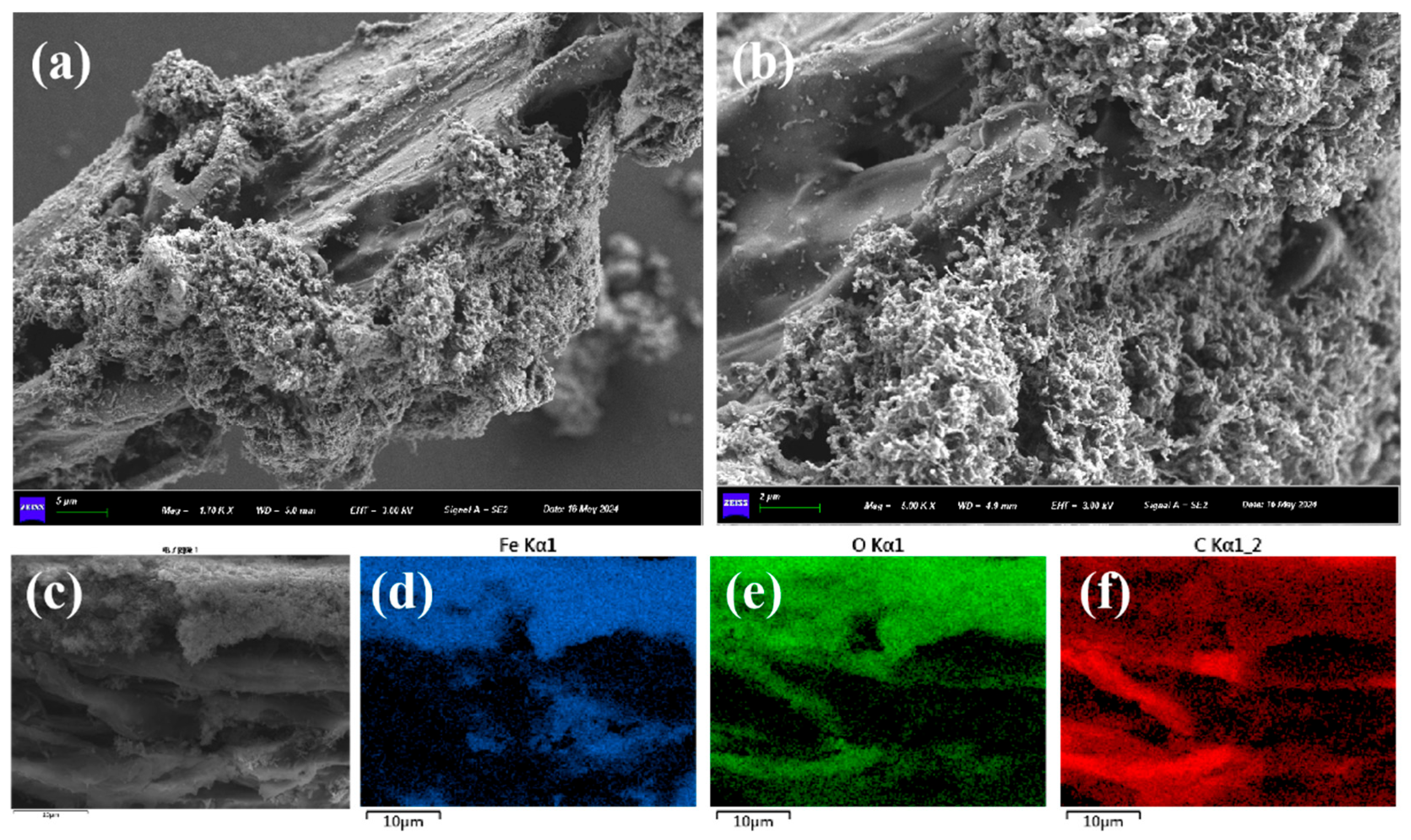
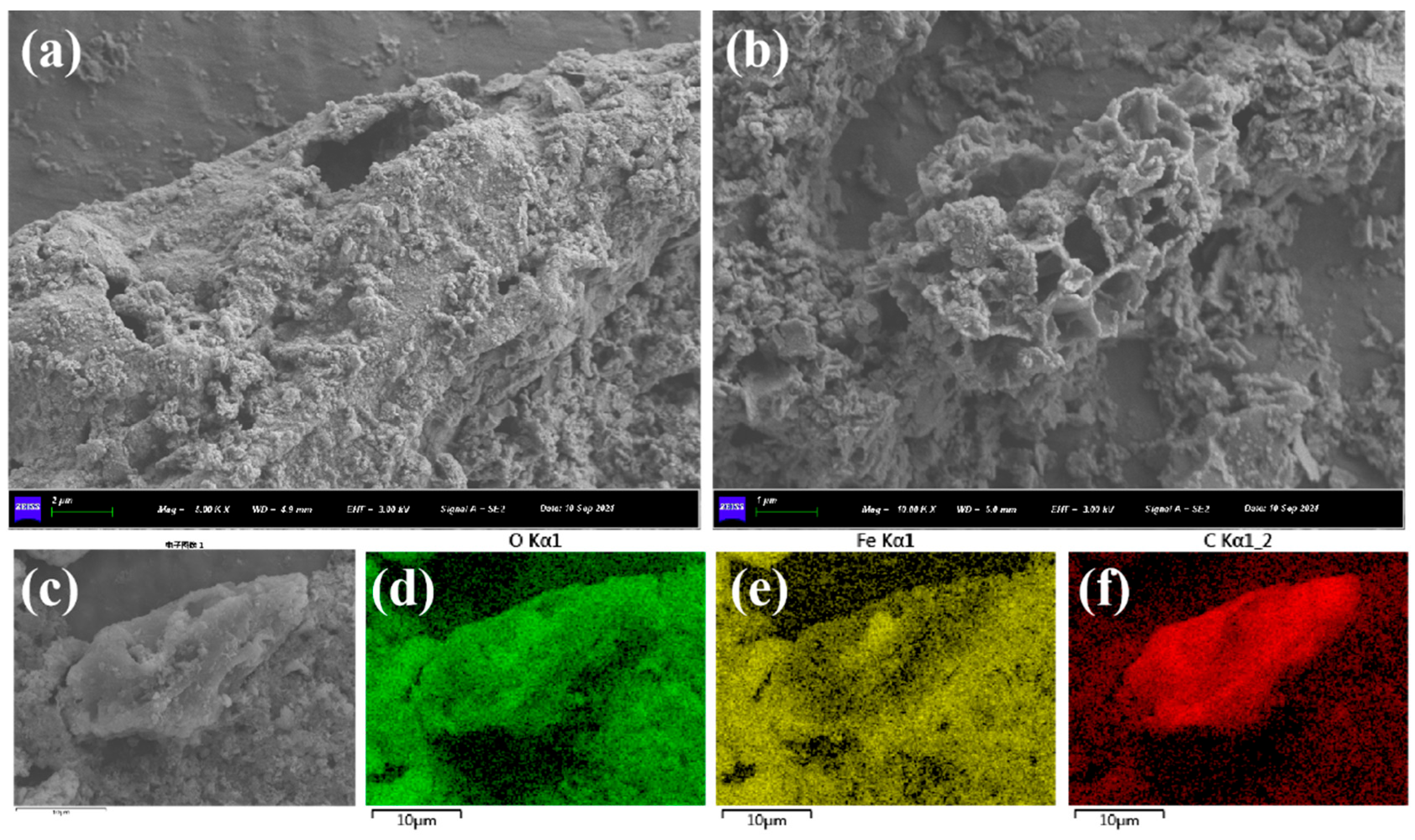
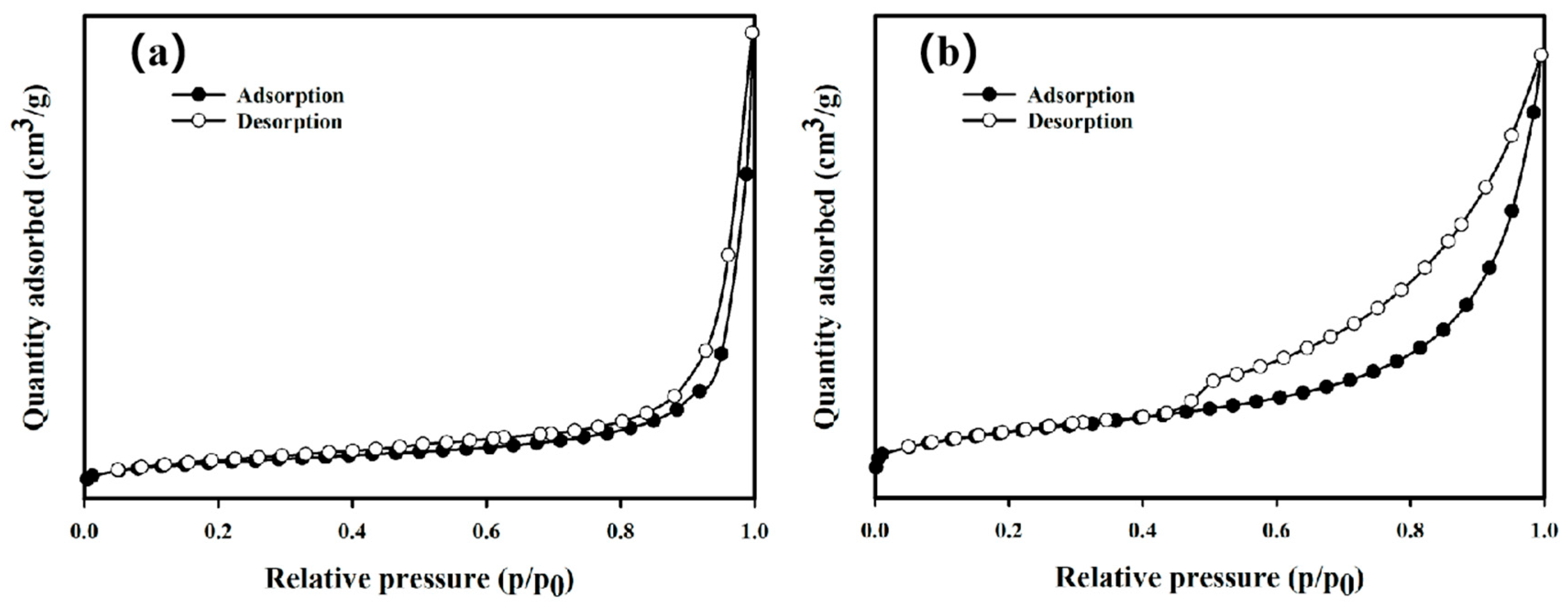
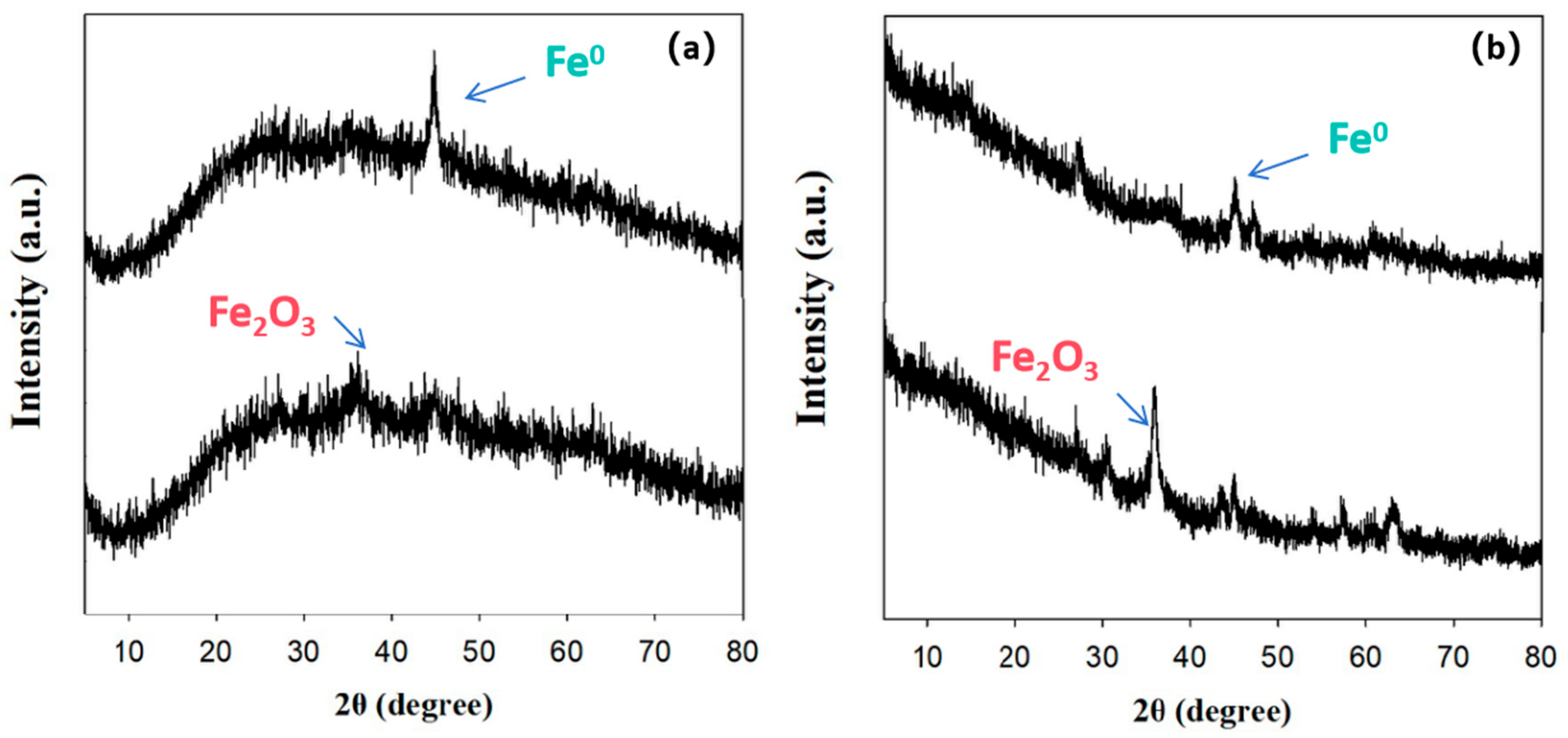
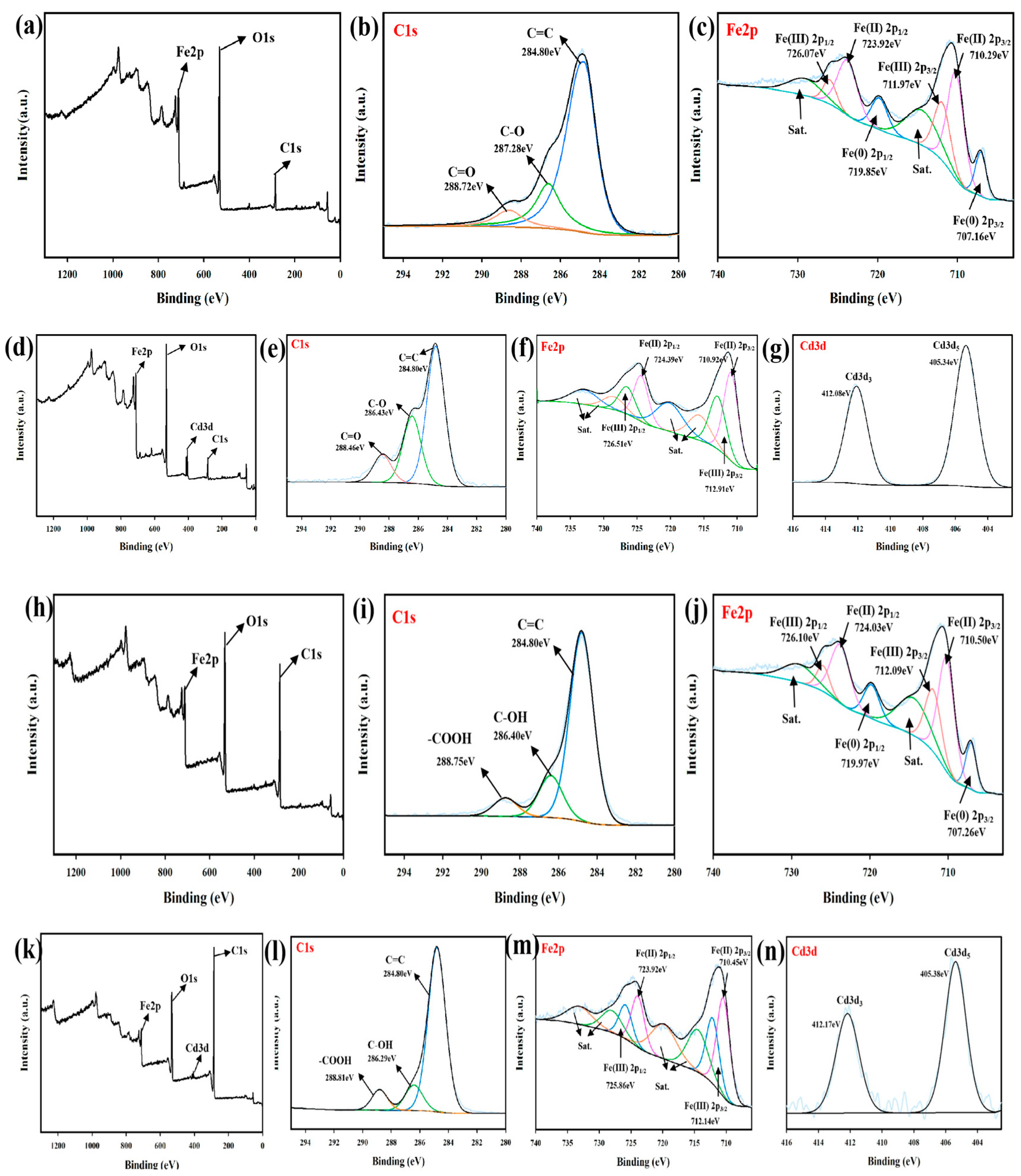
3.1. Characterizations
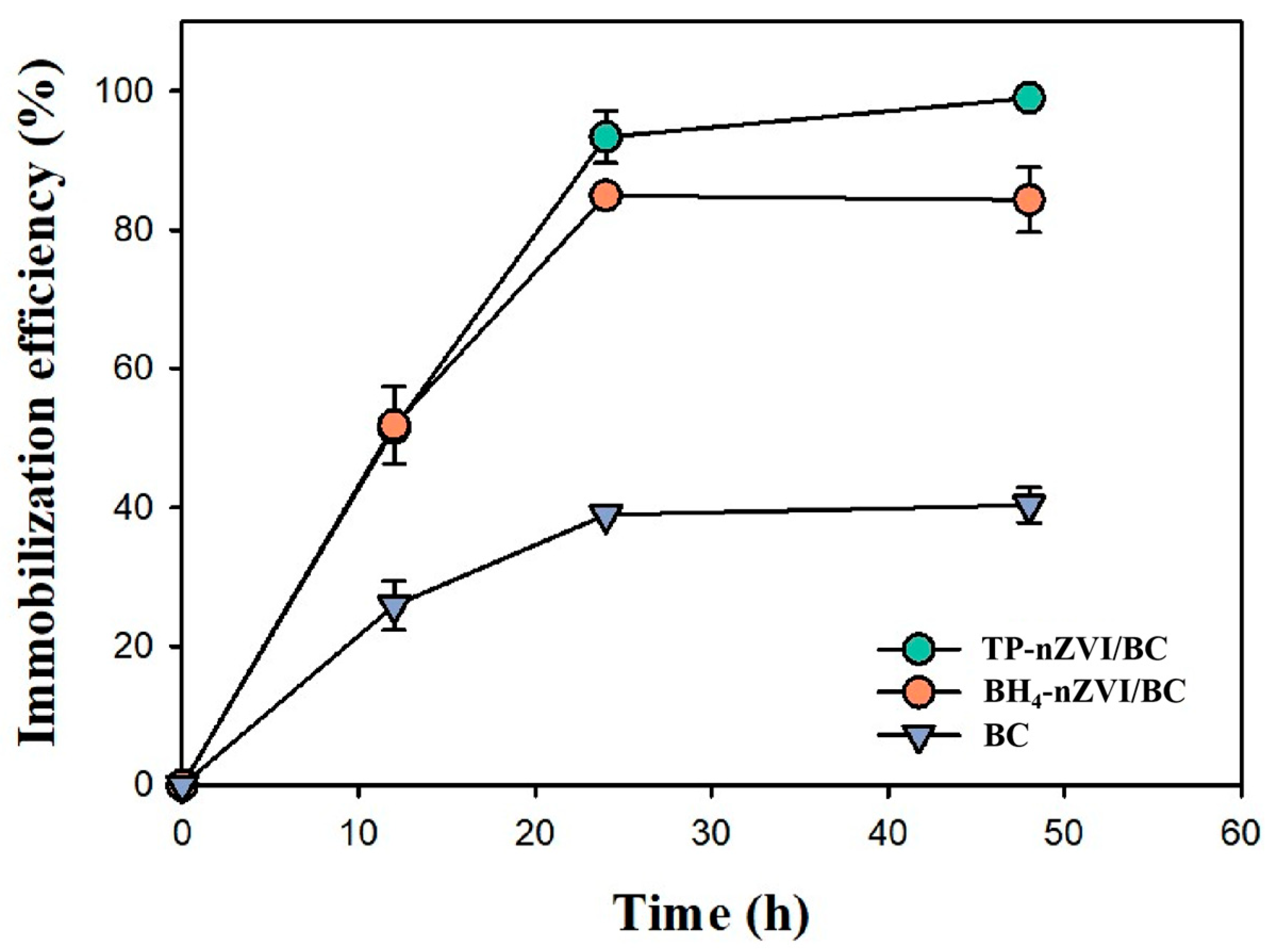
3.2. Immobilization of Cd by BH4-nZVI/BC and TP-nZVI/BC and in the Soil
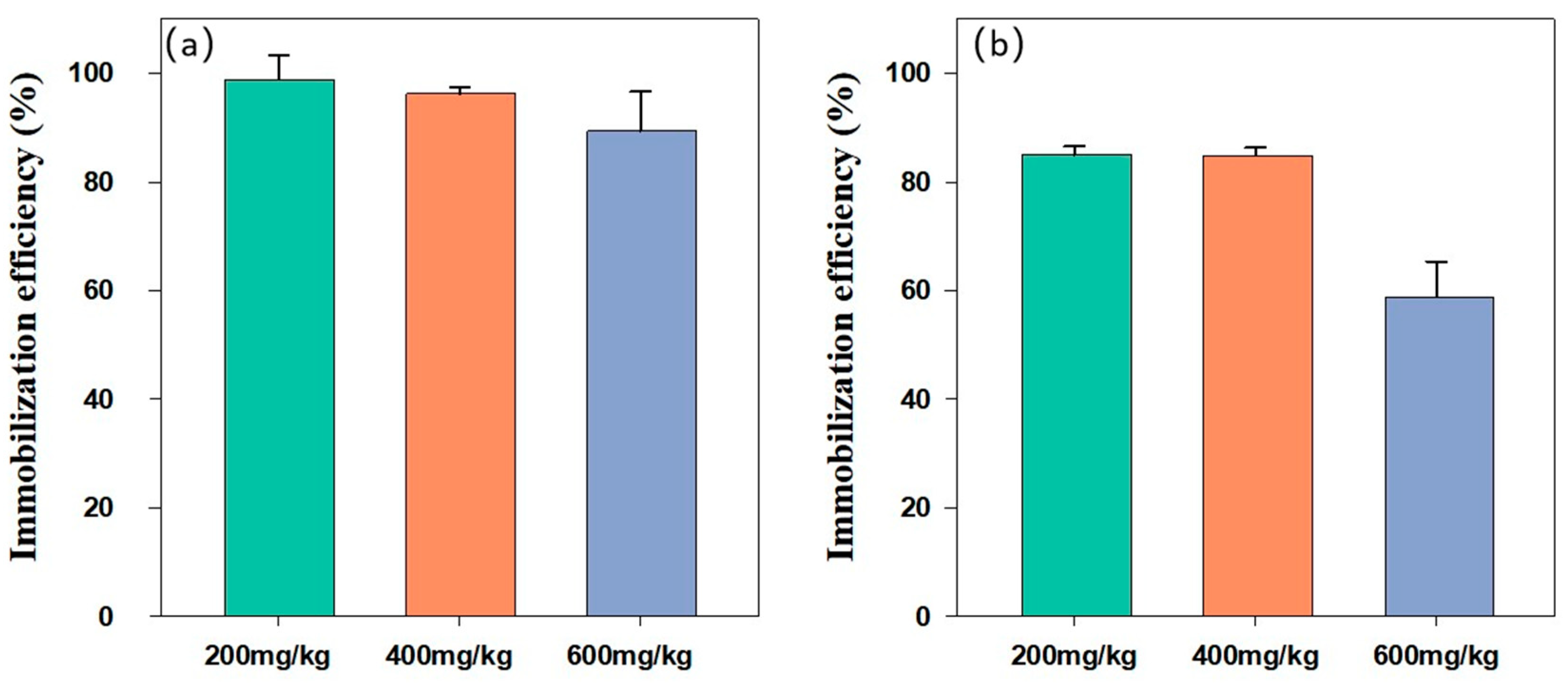
3.3. Effect of the Initial Concentration of Cd in Soil on the Remediation
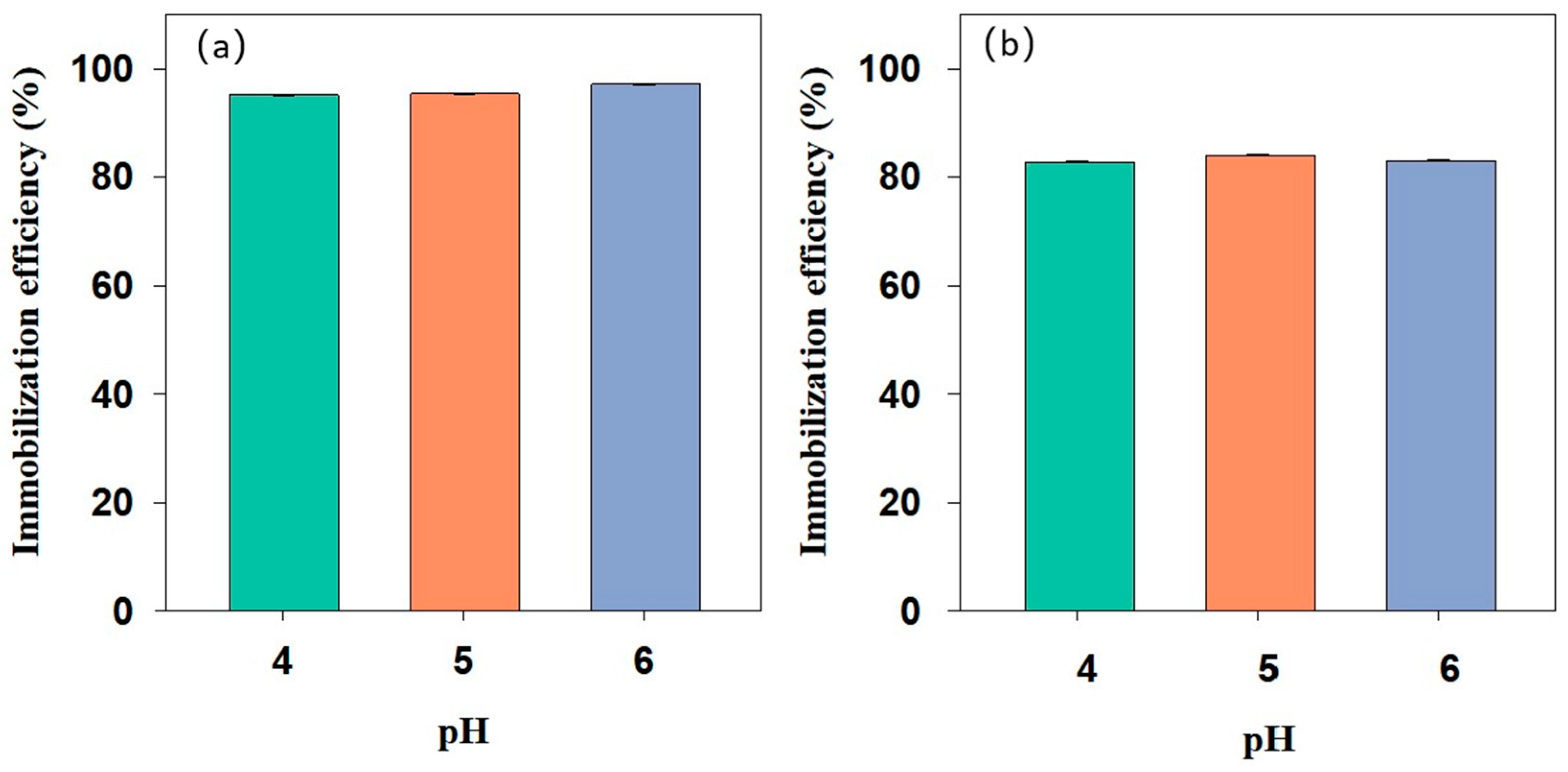
3.4. Effect of the Initial pH of Cd in Soil on the Remediation
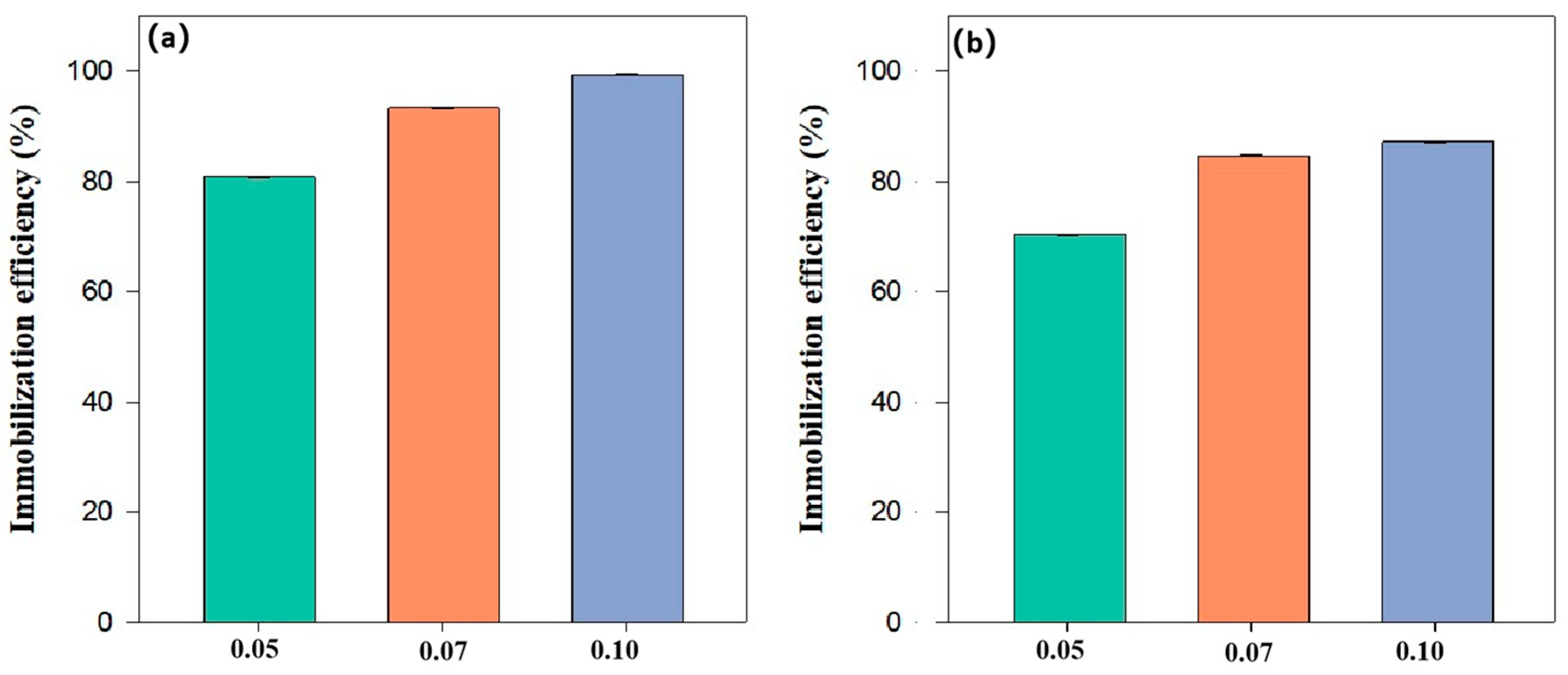
3.5. Effect of Dosage on the Immobilization
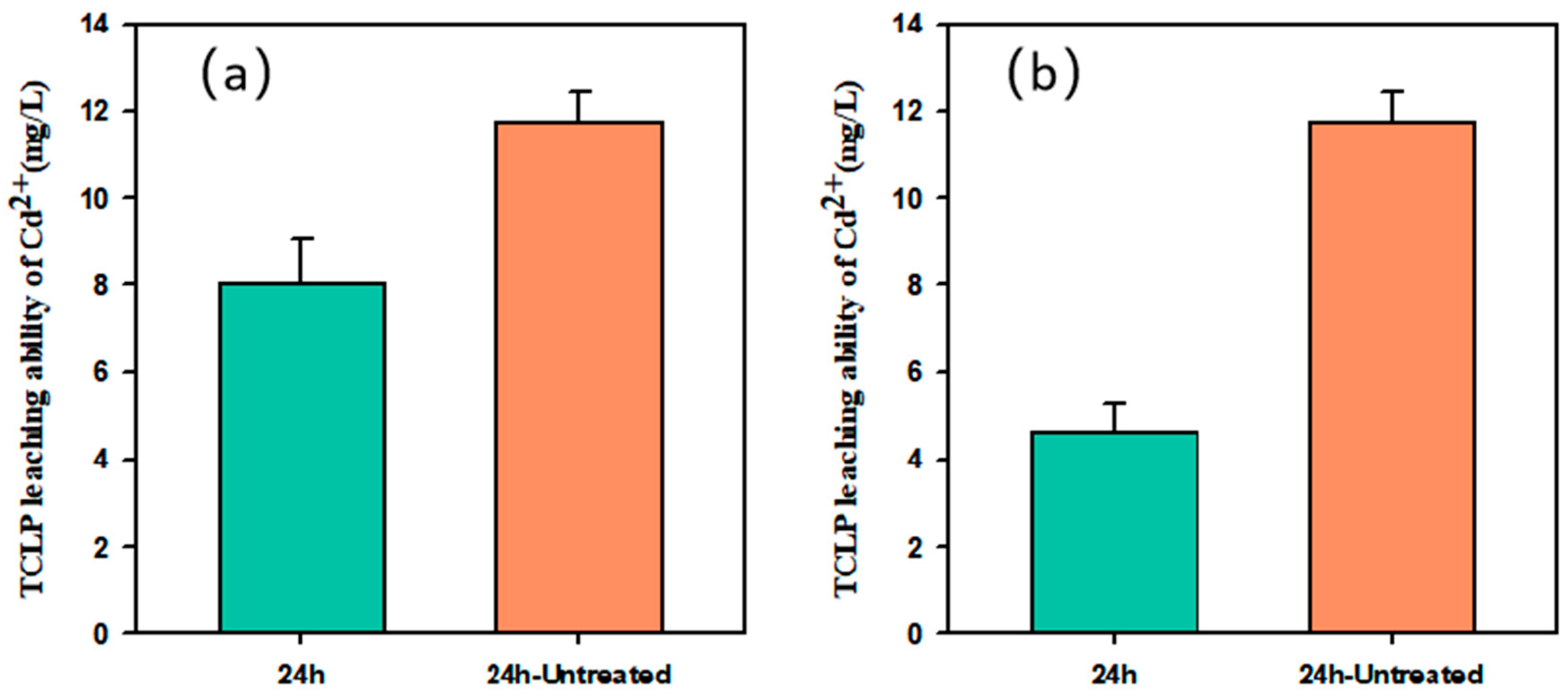
3.6. The Result of TCLP Leaching
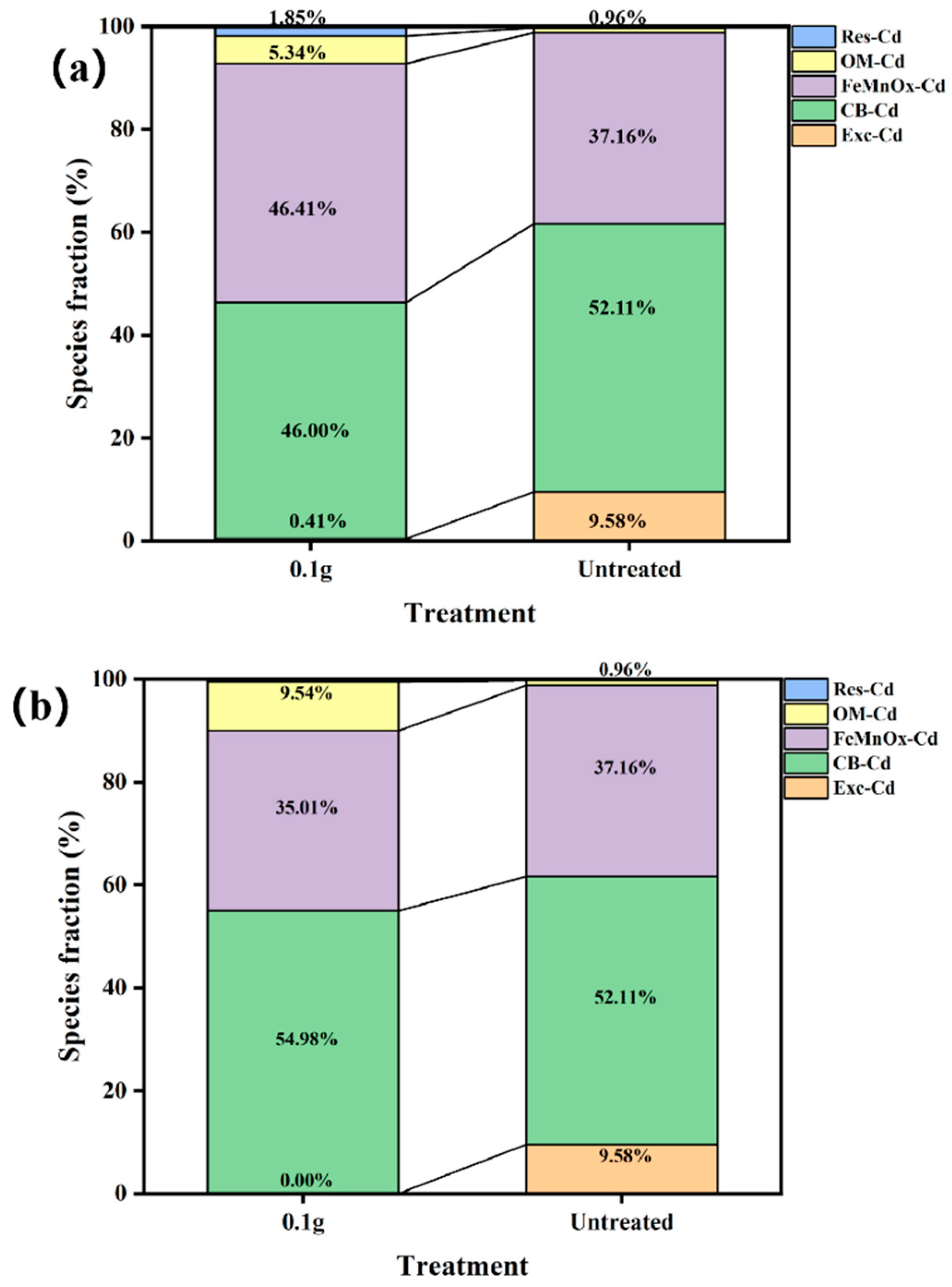
3.7. Heavy Metal Speciation in Soil
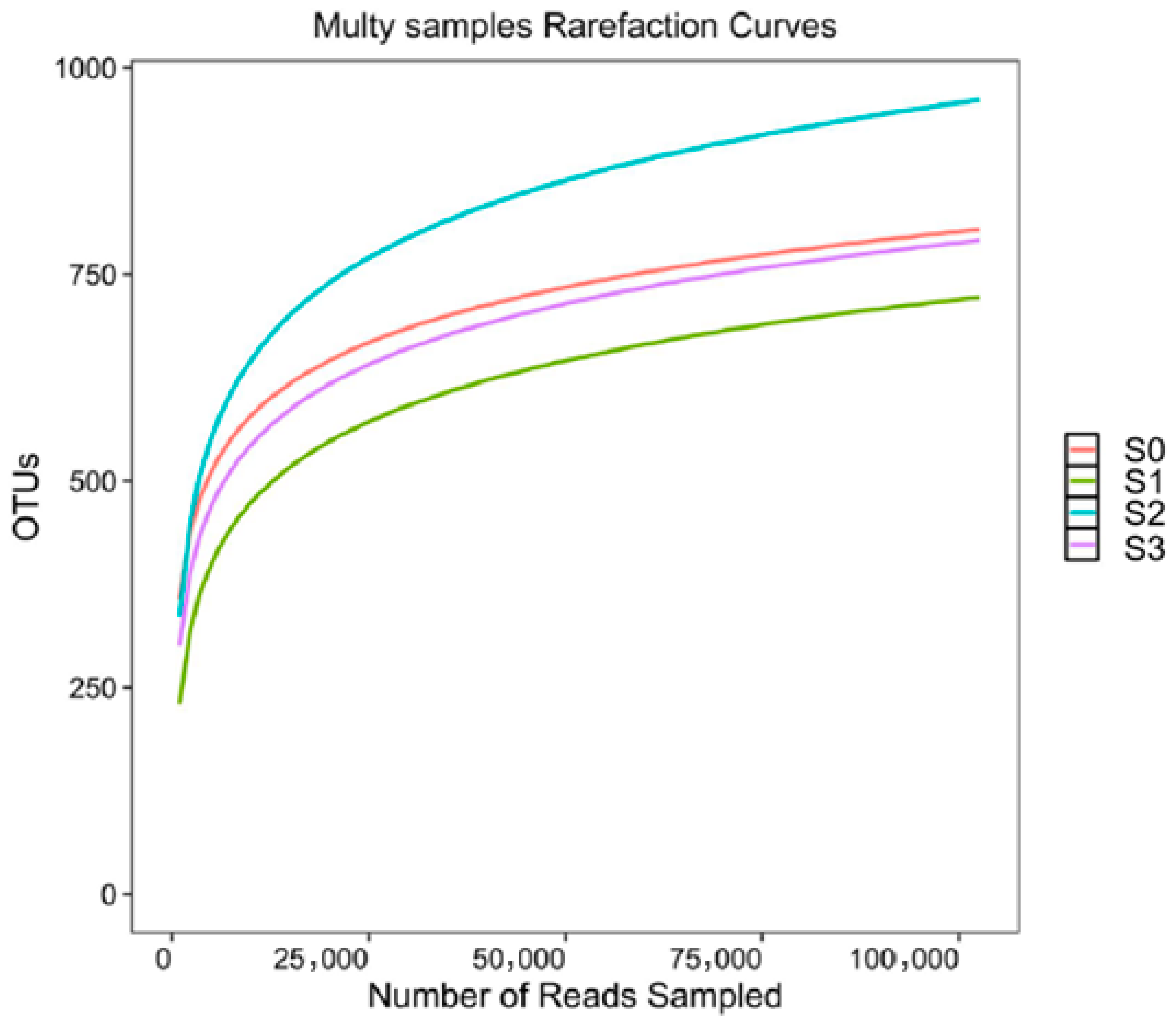
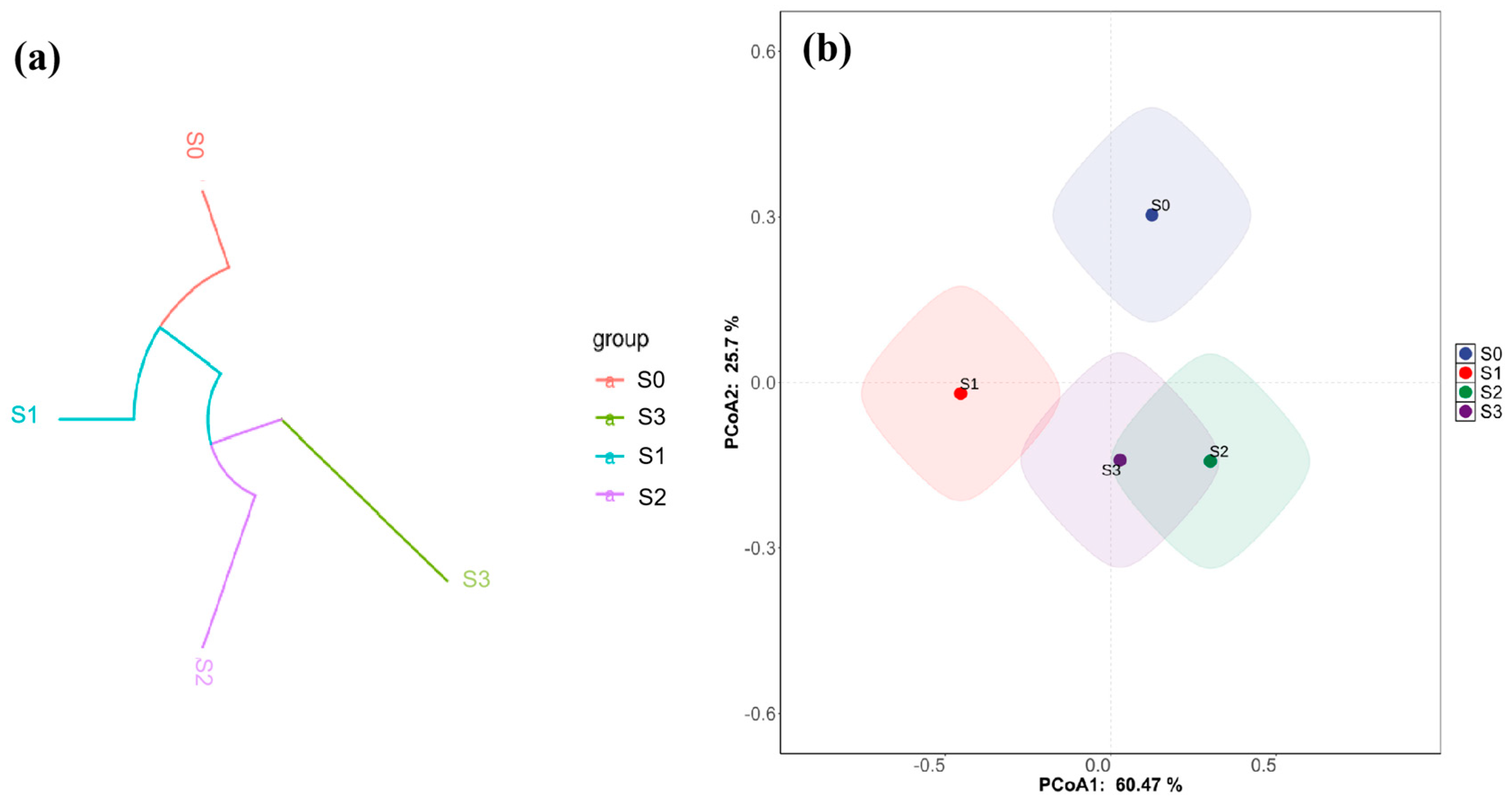
3.8. Soil Microbial Community After Treatment
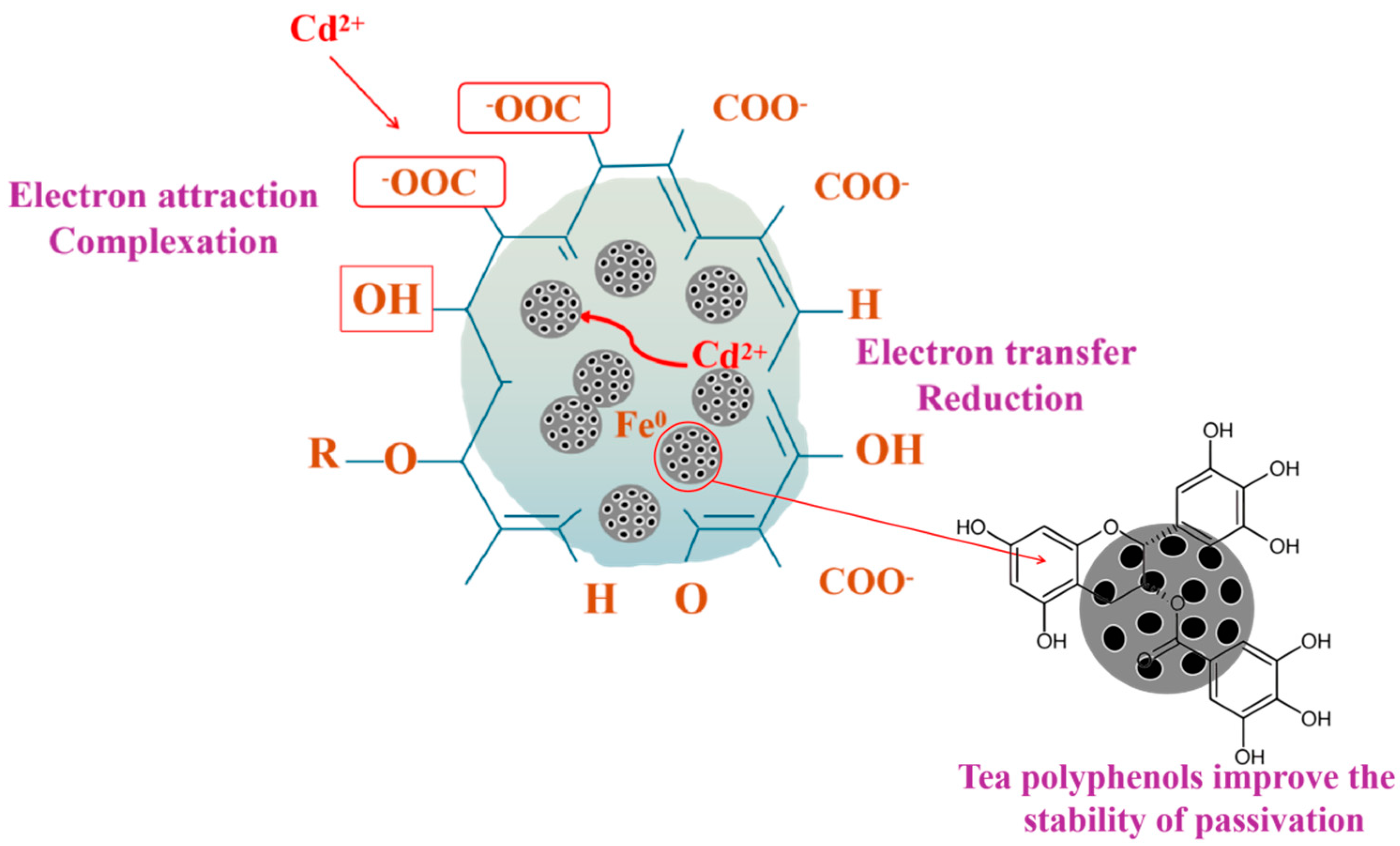
3.9. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, S.; Salehi, F. Comprehensive assessment of heavy metal (HMs) contamination and associated health risks in agricultural soils and groundwater proximal to industrial sites. Sci. Rep. 2025, 15, 7518. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Panchal, D.; Prakash, O.; Bobde, P.; Pal, S. SARS-CoV-2: Sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Res. 2021, 28, 22221–22240. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, R.; Sinha, A.; Lama, Y.; Saha, A.K. A review on synthesis, characterization, and applications of nano zero valent iron (nZVI) for environmental remediation. Crit. Rev. Environ. Sci. Technol. 2016, 46, 443–466. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, Z.; Zhang, M.; Hou, T.; Xiao, X.; Piao, Z.; Lu, G.; Han, Z.; Gao, R.; Nie, L.; et al. A locally solvent-tethered polymer electrolyte for long-life lithium metal batteries. Nat. Commun. 2024, 15, 3914. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, H.; Gu, S.; Qiao, D.; Xia, P.; Zhang, Y.; Dang, W.; Wen, X. Synthesis of (Ti,Me)(C,N)-Co-Ni composite powders produced via polycompound co-reduction and properties of their cermets. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Mohamed, A.; Atta, R.R.; Kotp, A.A.; Abo El-Ela, F.I.; Abd El-Raheem, H.; Farghali, A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mahmoud, R. Green synthesis and characterization of iron oxide nanoparticles for the removal of heavy metals (Cd2+ and Ni2+) from aqueous solutions with antimicrobial investigation. Sci. Rep. 2023, 13, 7227. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhang, W. Nanoscale zero-valent iron (nZVI) for heavy metal wastewater treatment: A perspective. Engineering 2024, 36, 16–20. [Google Scholar] [CrossRef]
- Fajardo, C.; Gil-Díaz, M.; Costa, G.; Alonso, J.; Guerrero, A.M.; Nande, M.; Lobo, M.C.; Martín, M. Residual impact of aged nZVI on heavy metal-polluted soils. Sci. Total Environ. 2015, 535, 79–84. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Bankole, M.T.; Bo, S.; Roos, W.D. Adsorption of Cr(VI), Ni(II), Fe(II) and Cd(II) ions by KIAgNPs decorated MWCNTs in a batch and fixed bed process. Sci. Rep. 2021, 11, 75. [Google Scholar] [CrossRef]
- Bhushan, B.; Negi, P.; Nayak, A.; Goyal, S. Graphene composites for water remediation: An overview of their advanced performance with focus on challenges and future prospects. Adv. Compos. Hybrid Mater. 2025, 8, 55. [Google Scholar] [CrossRef]
- Sable, H.; Kumar, V.; Singh, V.; Rustagi, S.; Chahal, S.; Chaudhary, V. Strategically engineering advanced nanomaterials for heavy-metal remediation from wastewater. Coord. Chem. Rev. 2024, 518, 216079. [Google Scholar] [CrossRef]
- Twizerimana, P.; Wu, Y. Overview of integrated electrocoagulation-adsorption strategies for the removal of heavy metal pollutants from wastewater. Discov. Chem. Eng. 2024, 4, 14. [Google Scholar] [CrossRef]
- Wang, S.; Hu, H.; Qing, X.; Zhang, Z.; Shao, Z. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69–82. [Google Scholar] [CrossRef]
- Grieger, K.D.; Fjordbøge, A.; Hartmann, N.B.; Eriksson, E.; Bjerg, P.L.; Baun, A. Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: Risk mitigation or trade-off? J. Contam. Hydrol. 2010, 118, 165–183. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef]
- Mahmoudian, F.; Ahmari, A.; Shabani, S.; Sadeghi, B.; Fahimirad, S.; Fattahi, F. Aptamers as an approach to targeted cancer therapy. Cancer Cell Int. 2024, 24, 108. [Google Scholar] [CrossRef]
- Faheem; Du, J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role. Environ. Sci. Pollut. Res. 2020, 27, 37286–37312. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D. Biochar-supported nZVI (BH4-nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Ma, J.; Cui, S.; Quan, G.; Yan, J.; Sui, F.; Wang, H.; Hina, K.; Hussain, Q. Evaluation of Fenton-like reaction for sorption and degradation of kasugamycin in the presence of biochar. Environ. Geochem. Health 2025, 47, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Ma, R.; Li, S.; Song, K.; Zheng, J.; Wang, Y.; Bian, R.; Zhang, X.; Pan, G. Biochar-plant interactions enhance nonbiochar carbon sequestration in a rice paddy soil. Commun. Earth Environ. 2023, 4, 494. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Q.; Zhang, J.; Gu, Q.; Wei, L.; Guo, W.; He, M. Chromium ion removal from raw water by magnetic iron composites and Shewanella oneidensis MR-1. Sci. Rep. 2019, 9, 3687. [Google Scholar] [CrossRef]
- Namakka, M.; Rahman, M.R.; Said, K.A.B.M.; Muhammad, A. Insights into micro- and nano-zero valent iron materials: Synthesis methods and multifaceted applications. RSC Adv. 2024, 14, 30411–30439. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Castle, A.B.; Murdock, R.C.; Hussain, S.M.; Varma, R.S. In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem. 2010, 12, 114–122. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X. Green synthesis of modified polyethylene packing supported tea polyphenols-NZVI for nitrate removal from wastewater: Characterization and mechanisms. Sci. Total Environ. 2022, 806, 150596. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.; Zhang, Y.; Chang, Q.; Zhang, Y.; Li, D.; Qiu, J.; Hu, L.; Peng, X.; Du, Y.; et al. Congenital heart disease detection by pediatric electrocardiogram based deep learning integrated with human concepts. Nat. Commun. 2024, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cai, X.; Wang, J.; Li, D.; Zhao, S.; Yu, X.; Xu, D.; Zhang, S. Three types of passivators on the stabilization of exogenous lead-contaminated soil with different particle sizes. Sci. Rep. 2021, 11, 22542. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Safonov, A.; Boldyrev, K.; Gundorina, S.; Yushin, N.; Petuhov, O.; Popova, N. Selective metal removal from chromium-containing synthetic effluents using Shewanella xiamenensis biofilm supported on zeolite. Environ. Sci. Pollut. Res. 2020, 27, 10495–10505. [Google Scholar] [CrossRef]
- Habish, A.J.; Lazarević, S.; Janković-Častvan, I.; Jokić, B.; Kovač, J.; Rogan, J.; Janaćković, Đ.; Petrović, R. Nanoscale zerovalent iron (nZVI) supported by natural and acid-activated sepiolites: The effect of the nZVI/support ratio on the composite properties and Cd2+ adsorption. Environ. Sci. Pollut. Res. 2017, 24, 628–643. [Google Scholar] [CrossRef]
- Eljamal, R.; Eljamal, O.; Maamoun, I.; Yilmaz, G.; Sugihara, Y. Enhancing the characteristics and reactivity of nZVI: Polymers effect and mechanisms. J. Mol. Liq. 2020, 315, 113714. [Google Scholar] [CrossRef]
- Niu, H.; Shi, S.; Zhu, S.; Cai, Y.; Cao, D. Biochars-inlaided nano zero-valent iron reactors: A tool for visualized analysis of soil-nanomaterials micro-interfacial interaction in soil remediation process. Sci. Total Environ. 2025, 958, 177829. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Han, Y.; Sun, C.; Zhang, Y.; Zang, S.; Qi, L. Fast and efficient As(III) removal from water by bifunctional nZVI\@NBC. Environ. Geochem. Health 2024, 46, 160. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Chu, G. Comparison of the sorption of Cu(II) and Pb(II) by bleached and activated biochars: Insight into complexation and cation–π interaction. Agronomy 2023, 13, 1282. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; Da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Ma, R.; Liu, X.; Song, S.; Wu, B.; Jiang, Z.; Wang, X. Adsorptive and reductive removal of toxic and radioactive metal ions by nanoscale zero-valent iron–based nanomaterials from wastewater. In Emerging Nanomaterials for Recovery of Toxic and Radioactive Metal Ions from Environmental Media; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–228. [Google Scholar]
- Khurshid, H.; Mustafa, M.R.U.; Isa, M.H. Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: A review of recent trends in the application of AC, BC, nZVI and MXene. Environ. Res. 2022, 212, 113138. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xie, Z.; Xu, J.; Wang, F.; Shi, J.; Zhou, R.; Jin, Z. Immobilization of trace metals by phosphates in contaminated soil near lead/zinc mine tailings evaluated by sequential extraction and TCLP. J. Soils Sediments 2013, 13, 1386–1395. [Google Scholar] [CrossRef]
- Jing, Y.; Chen, R.; Zhang, J.; Hu, L.; Qiu, X. Developed recyclable CaFe-layered double hydroxide for efficient cadmium immobilization in soil: Performance and bioavailability. Minerals 2024, 14, 656. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, S.; Chen, X.; Li, J.; Liu, H.; Gao, Y.; Wen, S.; Guo, J.; Shi, X.; Xue, W. Enhanced immobilization of cadmium in contaminated paddy soil by biochar-supported sulfidized nanoscale zero-valent iron. J. Soils Sediments 2024, 24, 259–274. [Google Scholar] [CrossRef]
- Shao, Y.; Tian, C.; Yang, Y.; Shao, Y.; Zhang, T.; Shi, X.; Zhang, W.; Zhu, Y. Carbothermal synthesis of sludge biochar supported nanoscale zero-valent iron for the removal of Cd2+ and Cu2+: Preparation, performance, and safety risks. Int. J. Environ. Res. Public Health 2022, 19, 16041. [Google Scholar] [CrossRef]
- Razzaq, S.; Zhou, B.; Zia-ur-Rehman, M.; Maqsood, M.A.; Hussain, S.; Bakhsh, G.; Zhang, Z.; Yang, Q.; Altaf, A.R. Cadmium stabilization and redox transformation mechanism in maize using nanoscale zerovalent-iron-enriched biochar in cadmium-contaminated soil. Plants 2022, 11, 1074. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.; Nakanishi, H.; McBride, M.B.; Williams, M.A.; Yoshihara, T. Chemical speciation, plant uptake, and toxicity of heavy metals in agricultural soils. J. Agric. Food Chem. 2020, 68, 12856–12869. [Google Scholar] [CrossRef]
- Jatoi, W.A.; Kaleri, M.H.; Fatima, N.; Memon, S.; Mengal, A.; Baloch, T.; Jatoi, I.; Samo, R.; Anwar, A.; Khokhar, N. Exploring genetic variation through line × tester analysis of sunflower (Helianthus annuus) genotypes for yield and oil traits under adverse environment. Egypt. J. Agron. 2024, 46, 131–139. [Google Scholar]
- Passos, J.G.D.C.; Silva, R.D.; Rovere, C.A.D.; de Sousa Malafaia, A.M. Improvement of the oxidation resistance of FeMnSiCrNi alloys with a pre-oxidation treatment. Metals 2023, 13, 1928. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, J.; Dong, T.; Gai, X.; Zhang, H.; Li, Y. Prospective study on the association between blood heavy metal levels and pulmonary function in university students from a medical college in Shandong Province, China. Int. J. Gen. Med. 2024, 17, 4257. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Y.; Wu, Y.; Yan, C.; Dang, Z.; Yin, H. CaAl-layered double hydroxides-modified biochar composites mitigate the toxic effects of Cu and Pb in soil on pea seedlings. Mater. 2024, 18, 15. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Yue, J.; Li, T.; Tian, J.; Ge, F.; Li, F.; Liu, Y.; Zhang, D.; Li, J. Penicillium oxalicum-induced phosphate precipitation enhanced cadmium (Cd) immobilization by simultaneously accelerating Cd biosorption and biomineralization. J. Hazard. Mater. 2024, 470, 134306. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yao, J.; Ma, B.; Chen, Z.; Zhao, C.; Zhu, X.; Li, M.; Cao, Y.; Pang, W.; Li, H.; et al. Microbial community profiles in soils adjacent to mining and smelting areas: Contrasting potentially toxic metals and co-occurrence patterns. Chemosphere 2021, 282, 130992. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Huang, C.; Huang, G.; Gu, M.; Shen, F.; Lu, D.; Zhang, M.; Wei, Y.; Li, H.; Shohag, M.J.I. Relative bioavailability of selenium in rice using a rat model and its application to human health risk assessment. Environ. Pollut. 2023, 338, 122675. [Google Scholar] [CrossRef] [PubMed]
- Momina, M.; Qurtulen, Q.; Shahraki, H.S.; Ahmad, A.; Zaheer, Z. Machine learning approaches to predict adsorption performance of sugarcane derived-carbon dot-based composite in the removal of dyes. Sep. Purif. Technol. 2024, 351, 127937. [Google Scholar] [CrossRef]
- Mussttaf, D.J.; Abdul-Hamead, A.A.; Othman, F.M. Green synthesis and characterization of CuO, Fe2O3 and CuO/Fe2O3 compounds investigation. Results Eng. 2024, 22, 102282. [Google Scholar] [CrossRef]
- Xu, W.; Huang, D.; Wang, G.; Li, S.; Du, L.; Zhou, W.; Huang, H. Enhanced sulfamethazine degradation via peroxydisulfate activation by biochar-supported nano zero-valent iron-copper: The key role of Fe(IV) and electron transfer induced by doped Cu. J. Clean. Prod. 2024, 434, 140133. [Google Scholar] [CrossRef]
- Bennici, S. Integration of digestate-derived biochar into the anaerobic digestion process through circular economic and environmental approaches—A review. Mater. 2024, 17, 43527. [Google Scholar]
- Movahedi, F.; Nirmal, N.; Wang, P.; Jin, H.; Grøndahl, L.; Li, L. Recent advances in essential oils and their nanoformulations for poultry feed. J. Anim. Sci. Biotechnol. 2024, 15, 110. [Google Scholar] [CrossRef]
- Cao, Y.; Ai, S.; True, D.; Xiong, L. Effects of (−)-epigallocatechin-3-gallate incorporation on the physicochemical and oxidative stability of myofibrillar protein-soybean oil emulsions. Food Chem. 2018, 245, 439–445. [Google Scholar] [CrossRef]

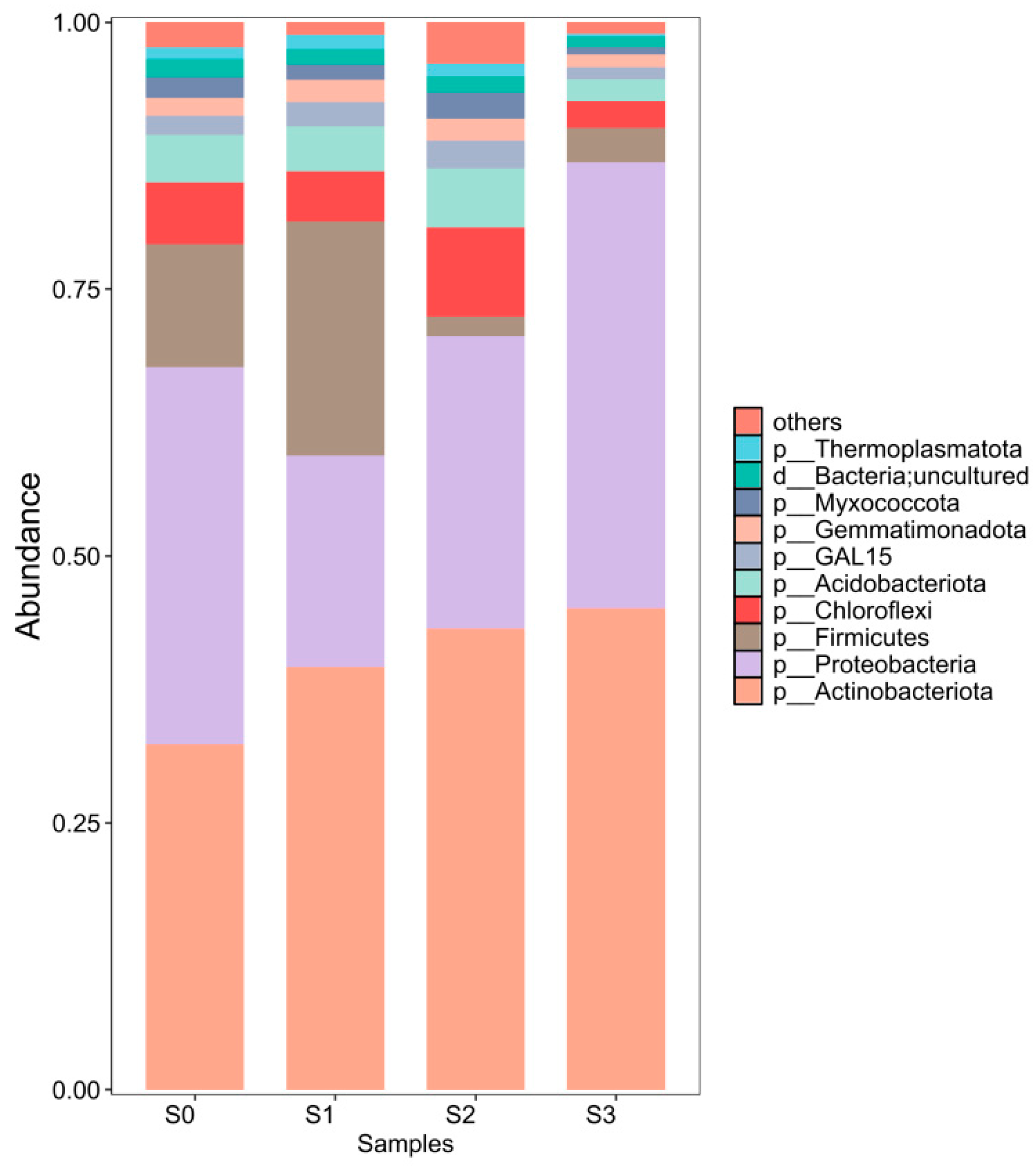

| Sample ID | Richness | Chao1 | ACE | Shannon | Simpson | Good’s Coverage |
|---|---|---|---|---|---|---|
| S0 | 942 | 1011.76 | 1000.06 | 4.88 | 0.96 | 0.9958 |
| S1 | 795 | 891.87 | 849.81 | 5.34 | 0.99 | 0.9963 |
| S2 | 776 | 853.17 | 819.94 | 4.88 | 0.98 | 0.9976 |
| S3 | 706 | 768.34 | 749.52 | 3.32 | 0.87 | 0.9983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Wang, H.; Yuan, S.; Zhang, W.; Zhang, D. Green Synthesis of Biochar-Supported Nanoscale Zero-Valent Iron Using Tea Polyphenol for Efficient Cadmium Immobilization in Soil. Nanomaterials 2025, 15, 1460. https://doi.org/10.3390/nano15191460
Jia Z, Wang H, Yuan S, Zhang W, Zhang D. Green Synthesis of Biochar-Supported Nanoscale Zero-Valent Iron Using Tea Polyphenol for Efficient Cadmium Immobilization in Soil. Nanomaterials. 2025; 15(19):1460. https://doi.org/10.3390/nano15191460
Chicago/Turabian StyleJia, Ziyong, Huizi Wang, Shupei Yuan, Weifeng Zhang, and Daijun Zhang. 2025. "Green Synthesis of Biochar-Supported Nanoscale Zero-Valent Iron Using Tea Polyphenol for Efficient Cadmium Immobilization in Soil" Nanomaterials 15, no. 19: 1460. https://doi.org/10.3390/nano15191460
APA StyleJia, Z., Wang, H., Yuan, S., Zhang, W., & Zhang, D. (2025). Green Synthesis of Biochar-Supported Nanoscale Zero-Valent Iron Using Tea Polyphenol for Efficient Cadmium Immobilization in Soil. Nanomaterials, 15(19), 1460. https://doi.org/10.3390/nano15191460






