Mesoporous Silica Xerogels Prepared by p-toluenesulfonic Acid-Assisted Synthesis: Piperazine-Modification and CO2 Adsorption
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Mesoporous Silica
2.3. Post-Synthesis Surface Modification
2.4. CO2 Adsorption Experiments
2.4.1. CO2 Adsorption Measurements in Dynamic Conditions
2.4.2. CO2 Adsorption Measurements in Static Conditions
2.5. Characterization Techniques
3. Results and Discussion
3.1. Synthesis of Mesoporous Silica Xerogels at Different Synthesis Conditions and Composition
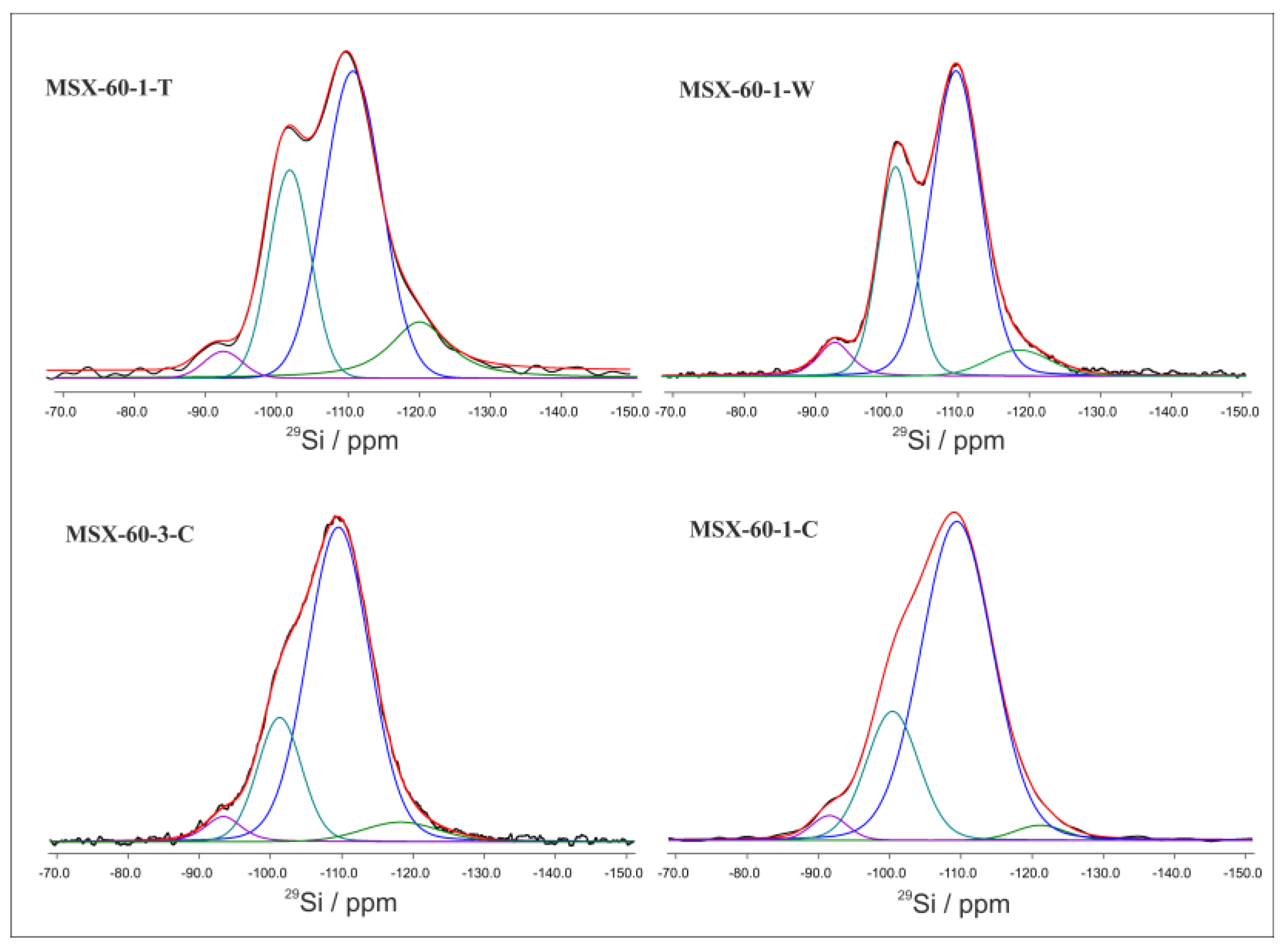
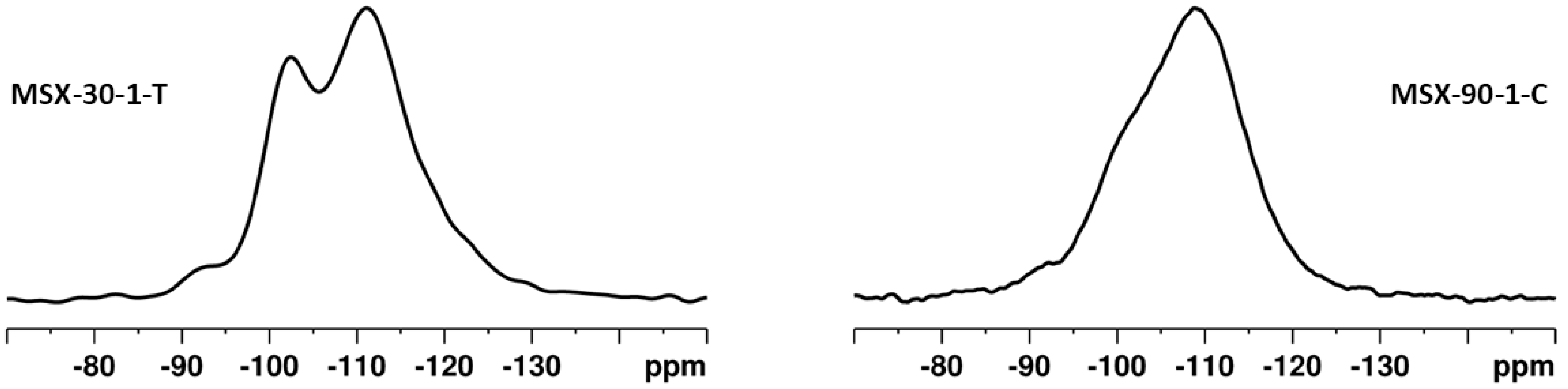
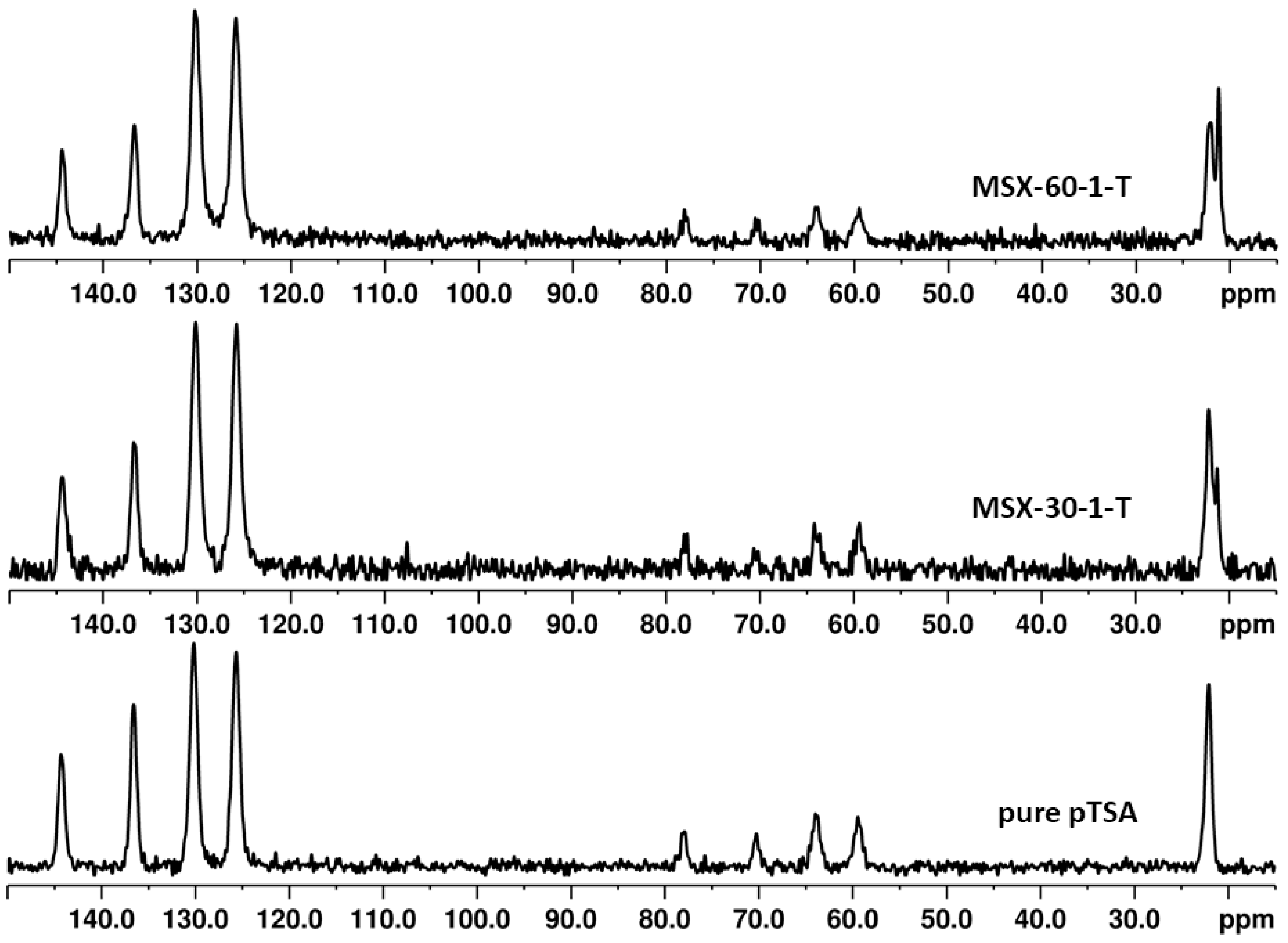
3.2. Surface Modification of the Porous Silica
3.3. Adsorption Capacity and Selectivity for CO2 Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2013, 7, 130–189. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.C.; Loos, M.; Meyer, L.A. Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Reichle, D.; Houghton, J.; Kane, B.; Ekmann, J.; Benson, S.; Clarke, J.; Dahlman, R.; Hendrey, G.; Herzog, H.; Hunter-Cevera, G.; et al. Carbon Sequestration Research and Development; Oak Ridge National Lab.(ORNL): Oak Ridge, TN, USA; National Energy Technology Lab.: Pittsburgh, PA, USA; National Energy Technology Lab.: Morgantown, WV, USA, 1999. [Google Scholar]
- Oyenekan, B.A.; Rochelle, G.T. Alternative stripper configurations for CO2 capture by aqueous amines. AIChE J. 2007, 53, 3144–3154. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Gray, M.L.; Champagne, K.J.; Fauth, D.; Baltrus, J.P.; Pennline, H. Performance of immobilized tertiary amine solid sorbents for the capture of carbon dioxide. Int. J. Greenh. Gas Control 2008, 2, 3–8. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- He, Y.; Seaton, N.A. Experimental and Computer Simulation Studies of the Adsorption of Ethane, Carbon Dioxide, and Their Binary Mixtures in MCM-41. Langmuir 2003, 19, 10132–10138. [Google Scholar] [CrossRef]
- Firouzi, A.; Atef, F.; Oertli, A.G.; Stucky, G.D.; Chmelka, B.F. Alkaline Lyotropic Silicate−Surfactant Liquid Crystals. J. Am. Chem. Soc. 1997, 119, 3596–3610. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Pang, J.B.; Qiu, K.Y.; Wei, Y. Preparation of mesoporous silica materials with non-surfactant hydroxy-carboxylic acid compounds as templates via sol–gel process. J. Non. Cryst. Solids 2001, 283, 101–108. [Google Scholar] [CrossRef]
- Lee, D.-W.; Ihm, S.-K.; Lee, K.-H. Mesoporous silica framed by sphere-shaped silica nanoparticles. Microporous Mesoporous Mater. 2005, 83, 262–268. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Kang, S.; Zhang, C.; Li, X. Low-cost route for synthesis of mesoporous silica materials with high silanol groups and their application for Cu(II) removal. Mater. Chem. Phys. 2012, 132, 1053–1059. [Google Scholar] [CrossRef]
- Coltrain, B.K.; Kelts, L.W. The Chemistry of Hydrolysis and Condensation of Silica Sol—Gel Precursors. The Colloid Chemistry of Silica; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1994; Volume 234, pp. 19–403. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Satyapal, S.; Filburn, T.; Trela, J.; Strange, J. Performance and Properties of a Solid Amine Sorbent for Carbon Dioxide Removal in Space Life Support Applications. Energy Fuels 2001, 15, 250–255. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. J. Am. Chem. Soc. 2010, 132, 6312–6314. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Hack, J.; Maeda, N.; Meier, D.M. Review on CO2 Capture Using Amine-Functionalized Materials. ACS Omega 2022, 7, 39520–39530. [Google Scholar] [CrossRef]
- Bae, J.Y.; Jang, S.G.; Cho, J.; Kang, M. Amine-Functionalized Mesoporous Silica for Efficient CO2 Capture: Stability, Performance, and Industrial Feasibility. Int. J. Mol. Sci. 2025, 26, 4313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, G.; Sun, Y.; Xu, Y. CO2 Adsorption Behavior and Kinetics on Amine-Functionalized Composites Silica with Trimodal Nanoporous Structure. Energy Fuels 2017, 31, 12508–12520. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem. Eng. J. 2010, 161, 173–181. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Kawakita, M.; Ogura, K. High Surface-Area Silica with Controlled Pore Size Prepared from Nanocomposite of Silica and Citric Acid. J. Phys. Chem. B 2000, 104, 12184–12191. [Google Scholar] [CrossRef]
- Kleitz, F.; Schmidt, W.; Schüth, F. Calcination behavior of different surfactant-templated mesostructured silica materials. Microporous Mesoporous Mater. 2003, 65, 1–29. [Google Scholar] [CrossRef]
- Ravutsov, M.; Mitrev, Y.; Shestakova, P.; Lazarova, H.; Simeonov, S.; Popova, M. CO2 Adsorption on Modified Mesoporous Silicas: The Role of the Adsorption Sites. Nanomaterials 2021, 11, 2811. [Google Scholar] [CrossRef] [PubMed]
- Tumurbaatar, O.; Popova, M.; Mitova, V.; Shestakova, P.; Koseva, N. Engineering of Silica Mesoporous Materials for CO2 Adsorption. Materials 2023, 16, 1479. [Google Scholar] [CrossRef]
- Son, W.-J.; Choi, J.-S.; Ahn, W.-S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater. 2008, 113, 31–40. [Google Scholar] [CrossRef]
- Jiao, J.; Cao, J.; Xia, Y.; Zhao, L. Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure. Chem. Eng. J. 2016, 306, 9–16. [Google Scholar] [CrossRef]
- Watabe, T.; Yogo, K. Isotherms and isosteric heats of adsorption for CO2 in amine-functionalized mesoporous silicas. Sep. Purif. Technol. 2013, 120, 20–23. [Google Scholar] [CrossRef]

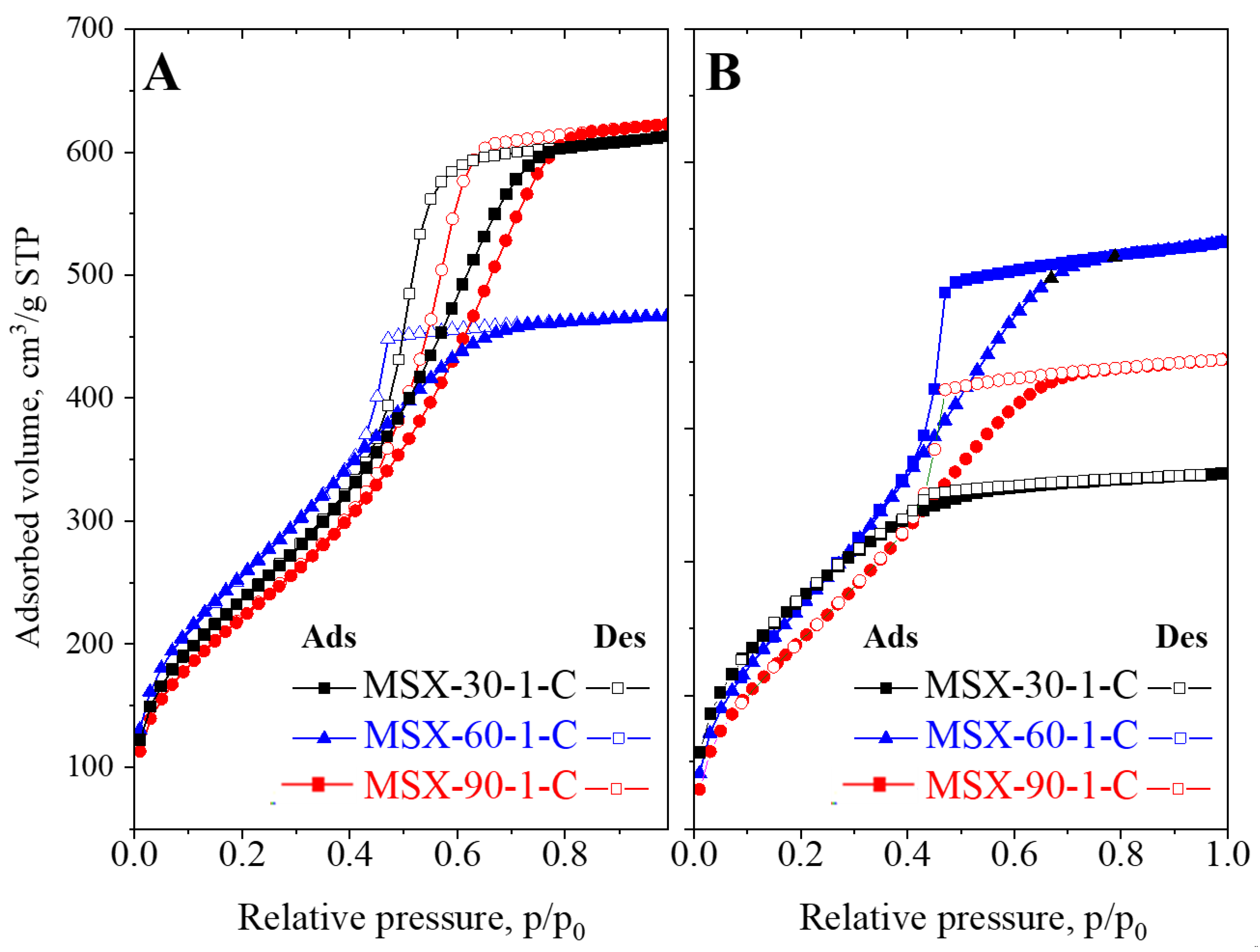

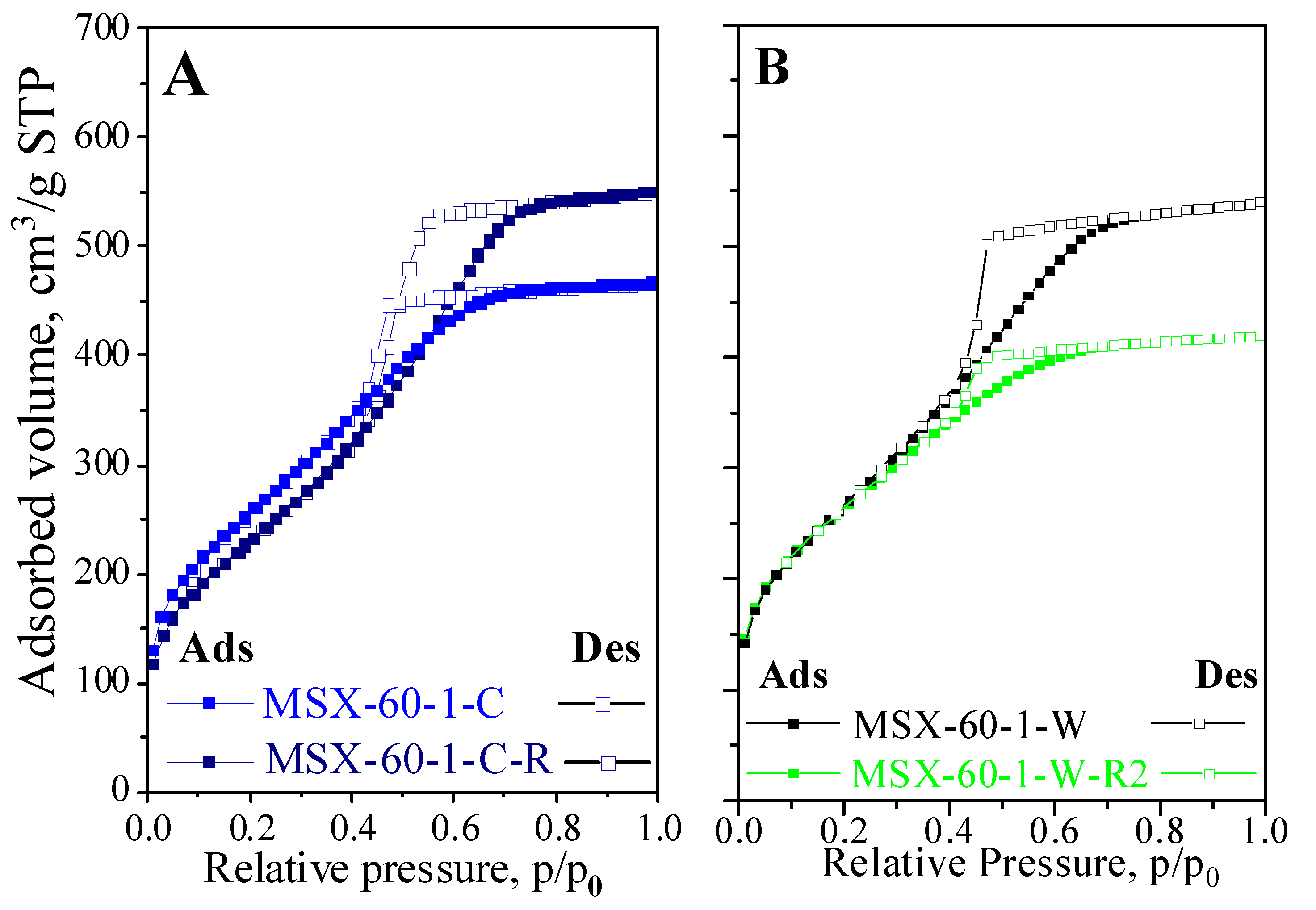

| Samples | Specific Surface Area m2/g | Total Pore Volume cm3/g |
|---|---|---|
| MSX-60-1-C | 885 | 0.91 |
| MSX-60-3-C | 796 | 0.94 |
| MSX-30-1-C | 868 | 0.95 |
| MSX-90-1-C | 817 | 0.97 |
| MSX-60-1-W | 980 | 0.84 |
| MSX-30-1-W | 990 | 0.57 |
| MSX-90-1-W | 886 | 0.70 |
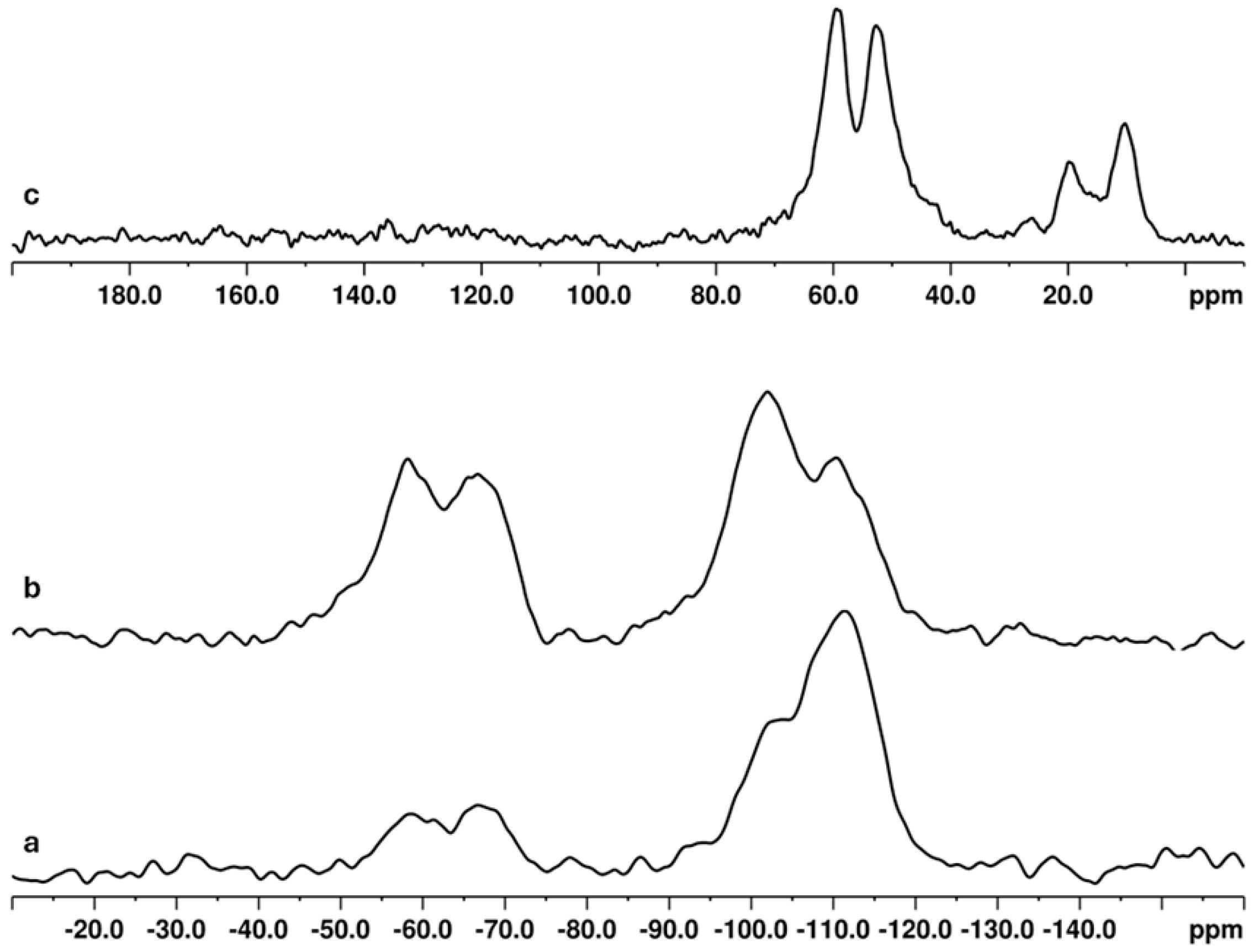
| Samples | Q1/Si(3OH)% | Q2/Si(2OH)% | Q3/Si(1OH)% | Q4/Si(0OH) a % | Q4/Si(0OH) b % |
|---|---|---|---|---|---|
| MSX-30-1-T | 1 | 5 | 24 | 54 | 16 |
| MSX-60-1-T | - | 3 | 27 | 56 | 14 |
| MSX-60-1-C | - | 3 | 22 | 73 | 2 |
| MSX-60-3-C | - | 4 | 20 | 71 | 5 |
| MSX-90-1-C | - | 3 | 20 | 72 | 5 |
| MSX-60-1-W | - | 5 | 29 | 60 | 6 |
| Samples | Specific Surface Area, m2/g | Total Pore Volume, cm3/g |
|---|---|---|
| MSX-60-1-C | 885 | 0.91 |
| MSX-60-1-C-R | 860 ± 18 | 0.85 ± 0.14 |
| MSX-60-1-W | 980 | 0.84 |
| MSX-60-1-W-R | 970 ± 15 | 0.65 ± 0.10 |
| Samples | CO2 Adsorption from CO2/N2 1, mmol/g | Selectivity of CO2 Over N2, Based on IAST Theory | Adsorption of CO2 from CO2/H2O/N2 2, mmol/g |
|---|---|---|---|
| MSX-60-1-W | 3.4 (3.2) 3 | 272 | 3.3 |
| MSX-60-1-W-HEP | 4.4 (4.1) 3 | 352 | 4.5 |
| Samples | Modification Groups | CO2 Adsorption from CO2/N2, mmol/g | References |
|---|---|---|---|
| MSX-60-1-W-HEP | 1-(2-hydroxyethyl)piperazine | 4.4 | This work |
| SBA-15/DAPTES | DAPTES | 3.9 | [29] |
| MCM-48-P | 1-methylpiperazine | 4.4 | [28] |
| MCM-41-PEI 50 | Polyethyleneimine | 2.5 | [30] |
| SBA-15-PEI 50 | Polyethyleneimine | 2.9 | [30] |
| SBA-16-PEI 50 | Polyethyleneimine | 2.9 | [30] |
| MCM-48-PEI 50 | Polyethyleneimine | 2.7 | [30] |
| KIT-6-PEI 50 | Polyethyleneimine | 3.1 | [30] |
| 25MSU-J-TEPA-20 | tetraethylenepentamine | 3.2 | [31] |
| 45MSU-J-TEPA-50 | tetraethylenepentamine | 3.1 | [31] |
| 45MSU-J-TEPA-70 | tetraethylenepentamine | 3.5 | [31] |
| 65MSU-J-TEPA-50 | tetraethylenepentamine | 3.7 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grozdanova, S.; Trendafilova, I.; Szegedi, A.; Shestakova, P.; Mitrev, Y.; Slavchev, I.; Simeonov, S.; Popova, M. Mesoporous Silica Xerogels Prepared by p-toluenesulfonic Acid-Assisted Synthesis: Piperazine-Modification and CO2 Adsorption. Nanomaterials 2025, 15, 1459. https://doi.org/10.3390/nano15191459
Grozdanova S, Trendafilova I, Szegedi A, Shestakova P, Mitrev Y, Slavchev I, Simeonov S, Popova M. Mesoporous Silica Xerogels Prepared by p-toluenesulfonic Acid-Assisted Synthesis: Piperazine-Modification and CO2 Adsorption. Nanomaterials. 2025; 15(19):1459. https://doi.org/10.3390/nano15191459
Chicago/Turabian StyleGrozdanova, Stela, Ivalina Trendafilova, Agnes Szegedi, Pavletta Shestakova, Yavor Mitrev, Ivailo Slavchev, Svilen Simeonov, and Margarita Popova. 2025. "Mesoporous Silica Xerogels Prepared by p-toluenesulfonic Acid-Assisted Synthesis: Piperazine-Modification and CO2 Adsorption" Nanomaterials 15, no. 19: 1459. https://doi.org/10.3390/nano15191459
APA StyleGrozdanova, S., Trendafilova, I., Szegedi, A., Shestakova, P., Mitrev, Y., Slavchev, I., Simeonov, S., & Popova, M. (2025). Mesoporous Silica Xerogels Prepared by p-toluenesulfonic Acid-Assisted Synthesis: Piperazine-Modification and CO2 Adsorption. Nanomaterials, 15(19), 1459. https://doi.org/10.3390/nano15191459







