Comparative Efficiencies of TiO2 Photocatalysts on β-Blocker Metoprolol Degradation by Solar Heterogeneous Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
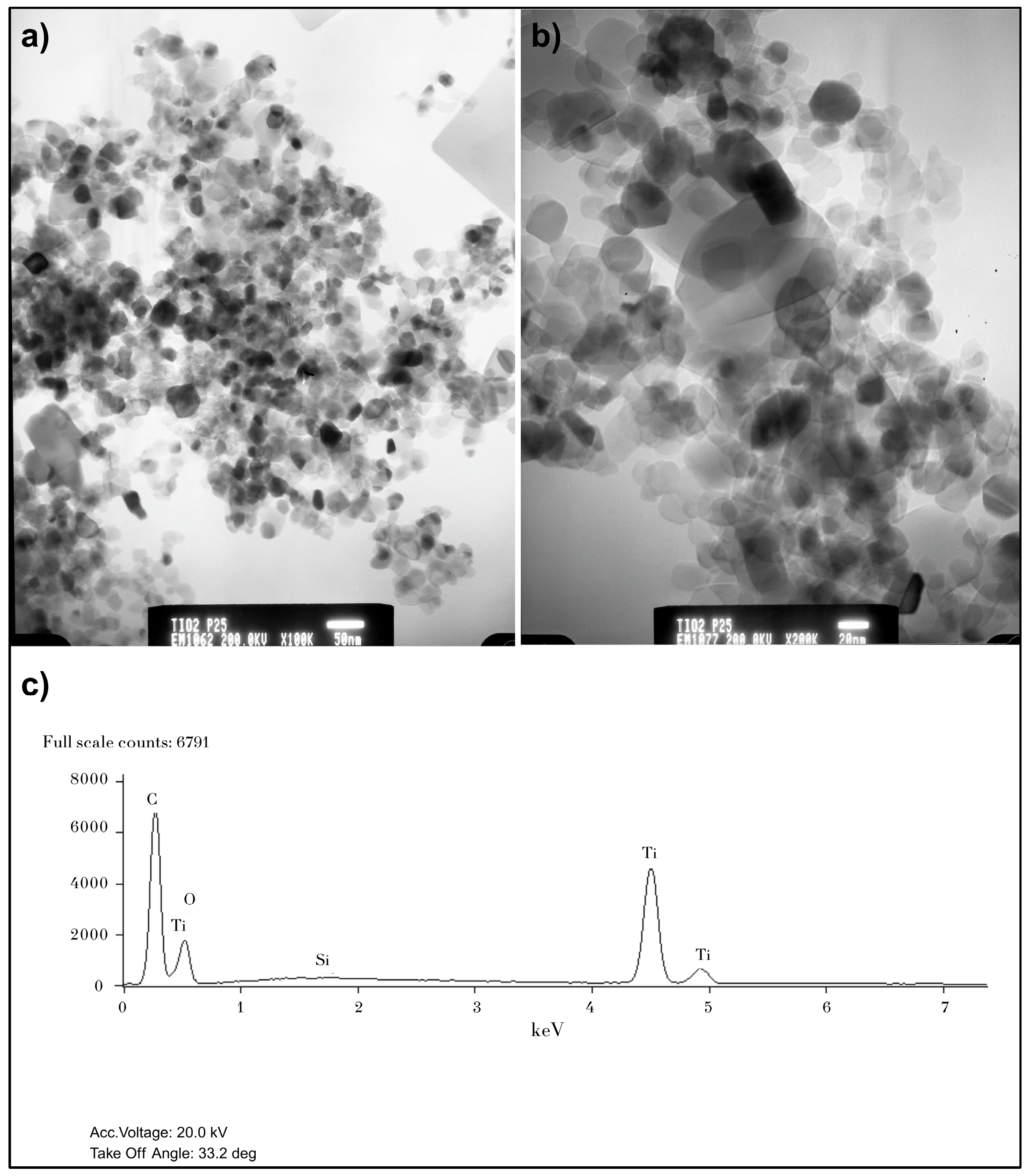
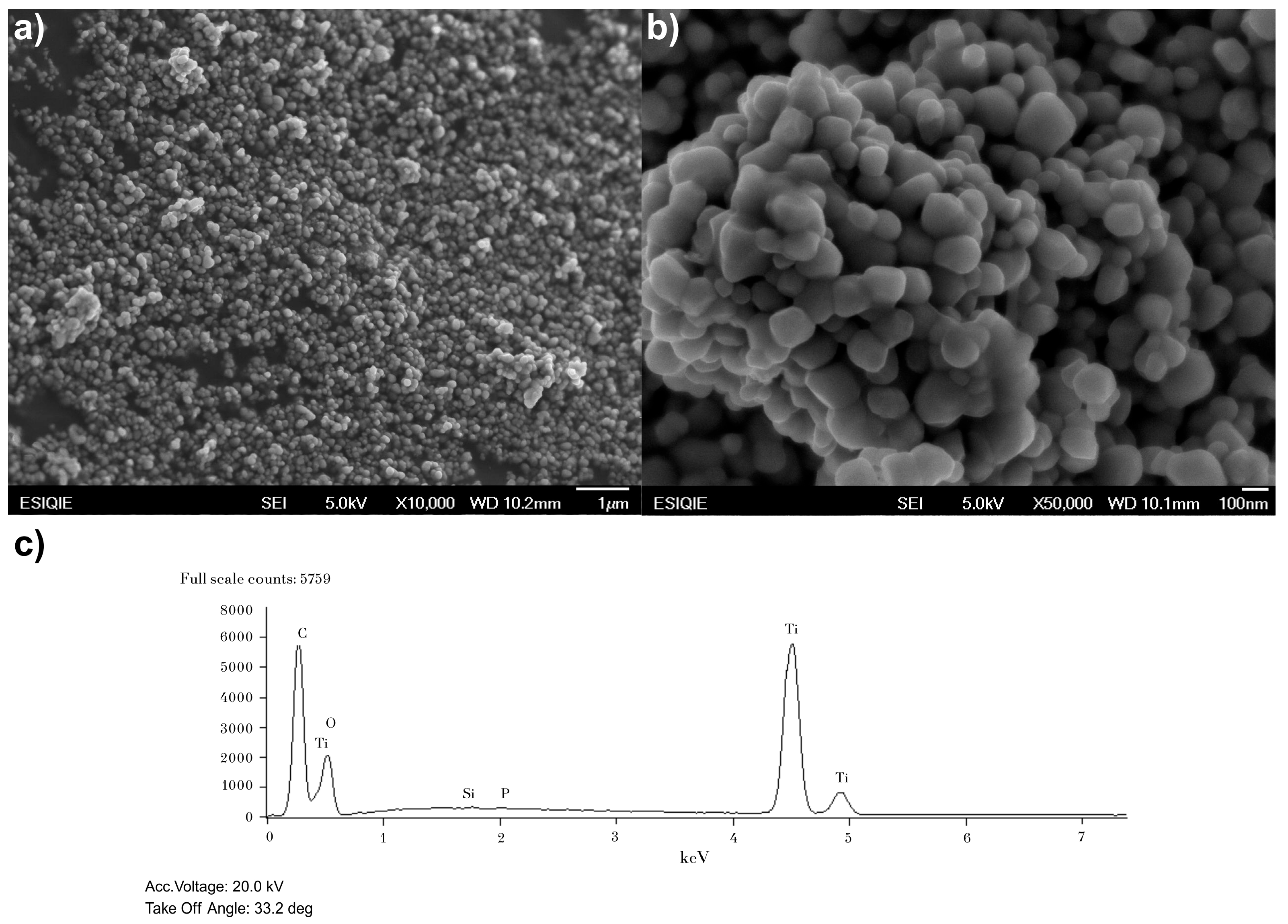
2.2. Characterization of Catalysts
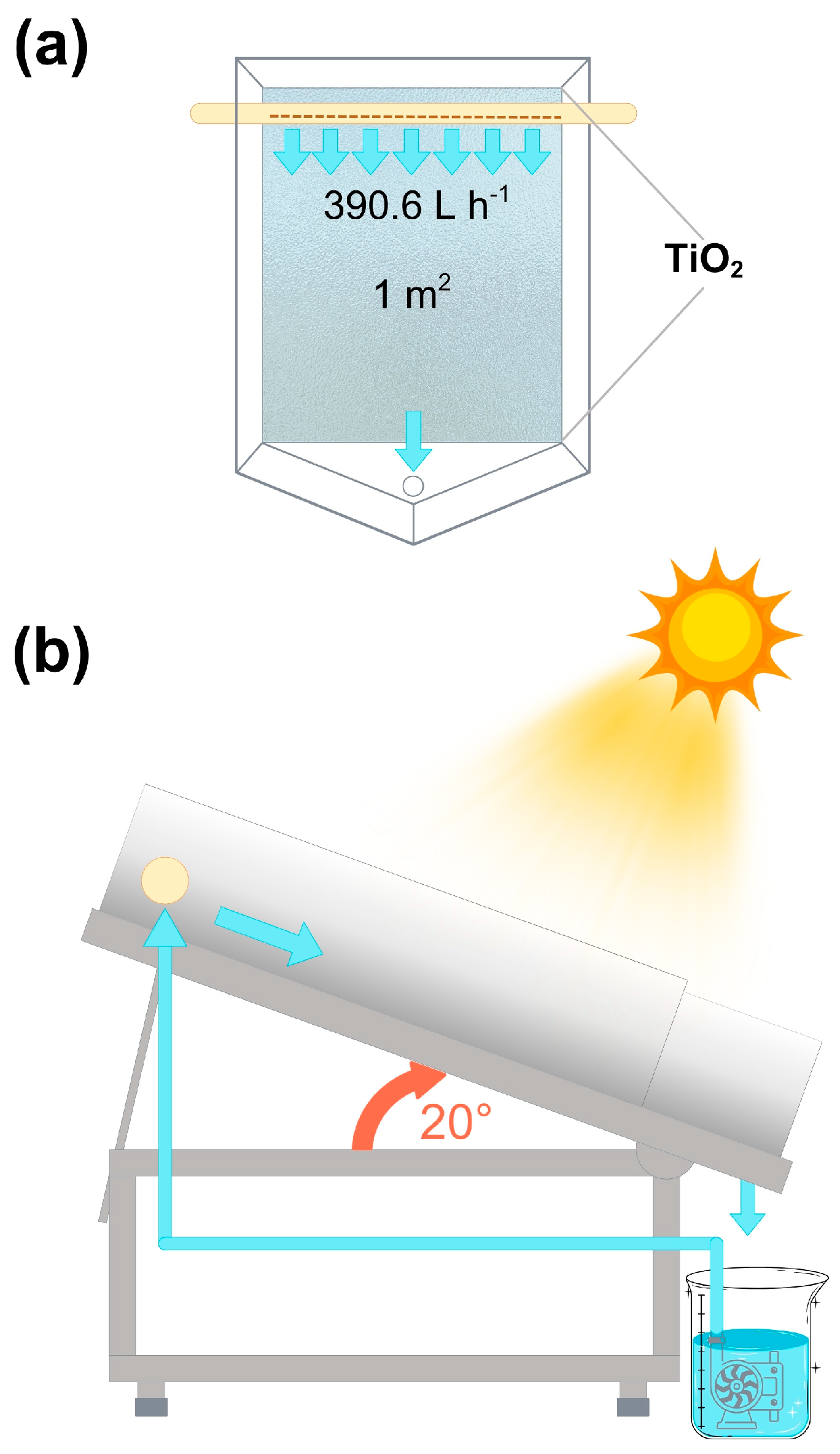
2.3. Photolysis and Photocatalysis Degradation Experiments
2.3.1. Effect of Different TiO2 Structures on COD Removal
2.3.2. Effect of H2O2 Addition
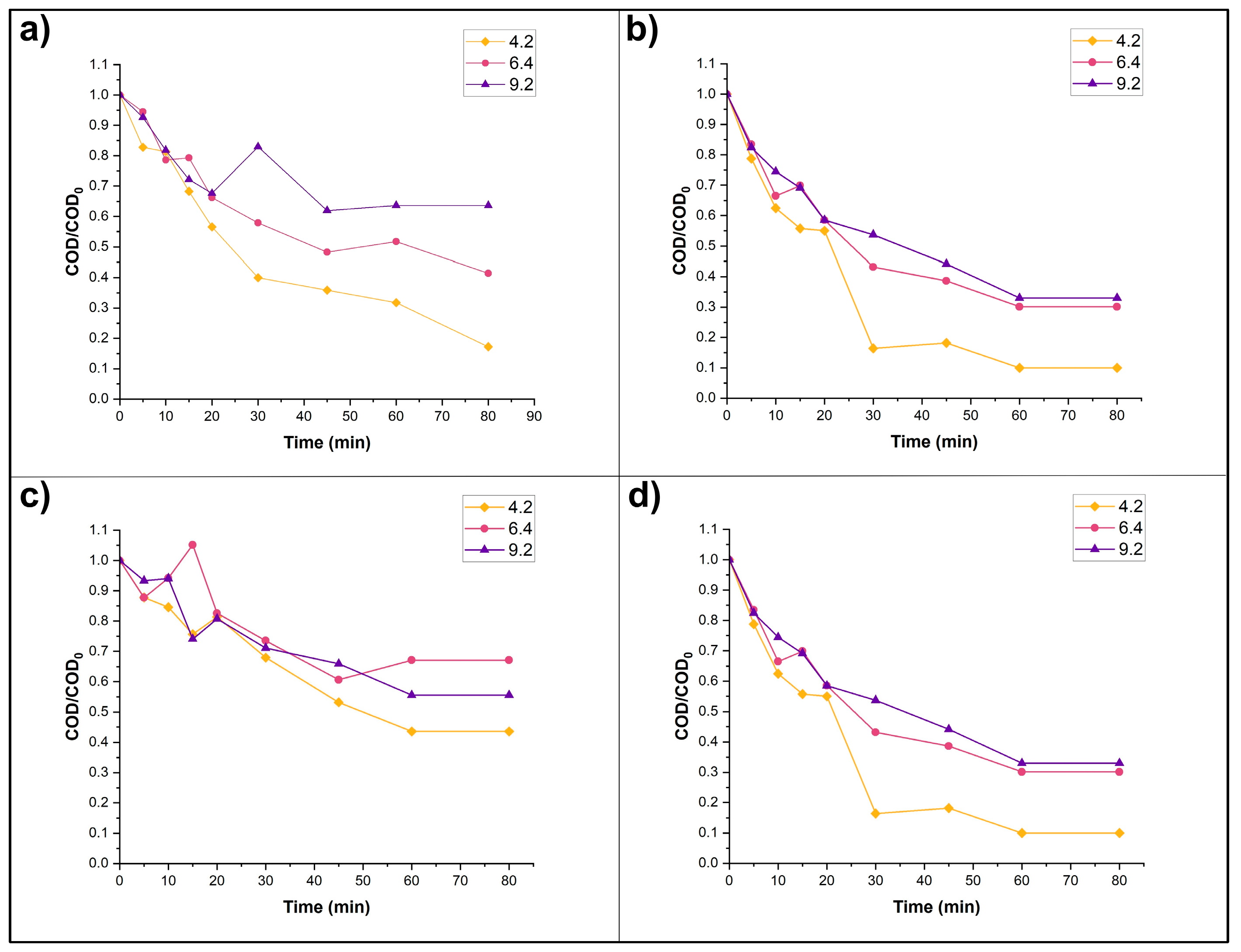
2.3.3. Effect of Initial pH
2.4. Kinetic Parameters Calculation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Photolysis Experiments
3.3. Photocatalysis Experiments: Effects of Catalyst, pH, and H2O2
3.4. Kinetic Analysis
3.5. Statistical Analysis Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MET | Metoprolol |
| WWTP | Wastewater treatment plant |
| AOP | Advanced oxidation process |
| XRD | X-ray diffraction |
| NIR | Near infrared |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| DAD | Diode array detector |
| FPR | Flat-plate reactor |
| COD | Chemical oxygen demand |
| PZC | Point of zero charge |
| EDS | Energy dispersive spectroscopy |
| TOC | Total organic carbon |
References
- Lubes, G. Análisis por modelaje molecular del MET y sus metabolitos. Av. Química 2009, 4, 101–106. [Google Scholar]
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha Técnica de Metoprolol Tartrato; Aurovitas: Madrid, Spain, 2021. [Google Scholar]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Nachweis von Betablockern und Bronchospasmolytika in der aquatischen Umwelt. Determination of beta-blocker and b2-sympatomimetics in the aquatic environment. Vom Wasser 1996, 87, 263–274. [Google Scholar]
- Ternes, T. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3261. [Google Scholar] [CrossRef]
- Jiménez, C. Contaminantes orgánicos emergentes presentes en el ambiente: Productos farmacéuticos. Rev. Lasallista Investig. 2011, 8, 143–153. [Google Scholar]
- Gueye, C.; Diaw, P.A.; Mbaye, M.; Mbaye, O.M.A.; Cissé, L.; Gaye Seye, D.; Aaron, J.J.; Oturan, N.; Oturan, M. Spectrofluorimetric determination of beta-blockers atenolol and bisoprolol fumarate residues in Senegal natural waters. Maced. J. Chem. Chem. Eng. 2023, 42, 79–92. [Google Scholar] [CrossRef]
- Barceló, D.; López, M.J. Contaminación y calidad química del agua: El problema de los contaminantes emergentes. In Jornadas de Presentación de Resultados: El Estado Ecológico de las Masas de Agua; Panel Científico-Técnico de Seguimiento de la Política de Aguas: Sevilla, Spain, 2008; pp. 1–27. [Google Scholar]
- Dzialowski, E.M.; Turner, P.K.; Brooks, B.W. Physiological and reproductive effects of beta adrenergic receptor antagonists in Daphnia magna. Arch. Environ. Contam. Toxicol. 2006, 50, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Lahti, M.; Oikari, A. Microbial transformation of pharmaceuticals naproxen, bisoprolol, and diclofenac in aerobic and anaerobic environments. Arch. Environ. Contam. Toxicol. 2011, 61, 202–210. [Google Scholar] [CrossRef]
- Feiner, M.; Laforsch, C.; Letzel, T.; Geist, J. Sublethal effects of the beta-blocker sotalol at environmentally relevant concentrations on the New Zealand mudsnail Potamopyrgus antipodarum. Environ. Toxicol. Chem. 2014, 33, 2510–2515. [Google Scholar] [CrossRef]
- Iancu, V.-I.; Puiu, D.; Radu, G.-L. Determination of some beta-blockers in surface water samples. Sci. Bull. 2020, 8, 122–128. [Google Scholar]
- Huerta-Fontela, M.; Galceran, M.-T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Abramovic, B.; Kler, S.; Sojic, D.; Lausevic, M.; Radovic, T.; Vione, D. Photocatalytic degradation of MET tartrate in suspensions of two TiO2-based photocatalysts with different surface area. Identification of intermediates and proposal of degradation pathways. J. Hazard. Mater. 2011, 198, 123–132. [Google Scholar] [CrossRef]
- Moctezuma, E.; Leyva, E.; López, M.; Pinedo, A.; Zermeño, B.; Serrano, B. Photocatalytic degradation of MET tartrate. Top. Catal. 2013, 56, 1875–1882. [Google Scholar] [CrossRef]
- Wilde, M.L.; Montipó, S.; Martins, A.F. Degradation of β-blockers in hospital wastewater by means of ozonation and Fe2+/ozonation. Water Res. 2014, 48, 280–295. [Google Scholar] [CrossRef]
- Kovács, K.; Tóth, T.; Wojnárovits, L. Evaluation of advanced oxidation processes for β-blockers degradation: A review. Water Sci. Technol. 2021, 85, 685–705. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry. Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Matijevic, E. Monodispersed Colloids: Art and Science. Langmuir 1986, 2, 12–20. [Google Scholar] [CrossRef]
- Morales, B.A.; Novaro, O.; López, T.; Sánchez, E.; Gómez, R. Effect of Hydrolysis Catalyst on the Ti Deficiency and Crystallite Size of Sol-Gel-TiO2 Crystalline Phases. J. Mater. Res. 1995, 10, 2788–2796. [Google Scholar] [CrossRef]
- Barbé, C.J.; Arendse, F.; Comte, P.; Jirousek, M.; Lenzmann, F.; Shklover, V.; Grätzel, M. Nanocrystalline Titanium Oxide Electrodes for Photovoltaic Applications. J. Am. Ceram. Soc. 1997, 80, 3157–3171. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.; Li, W.; Ni, C.; Shah, S.; Tseng, Y. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Shokri, M.; Elham, H.; Zeininezhad, A. The effect of particle size and crystal structure of titanium dioxide nanoparticles on the photocatalytic properties. J. Environ. Sci. Health Part A 2008, 43, 460–467. [Google Scholar] [CrossRef] [PubMed]
- González-Burciaga, L.A.; Núñez-Núñez, C.M.; Morones-Esquivel, M.M.; Avila-Santos, M.; Lemus-Santana, A.; Proal-Nájera, J.B. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts 2020, 10, 118. [Google Scholar] [CrossRef]
- Sánchez-Pérez, J.F.; Comendador-Jiménez, B.; Castro, E.; Cánovas, M.; Conesa, M. Characterization of the effects of vitamin D synthesis and sunburn in the population due to solar radiation exposure using PROBIT methodology. Heliyon 2024, 10, e30864. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef]
- Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y. Difference in TiO2 photocatalytic mechanism between rutile and anatase studied by the detection of active oxygen and surface species in water. Phys. Chem. Chem. Phys. 2015, 17, 18691–18698. [Google Scholar] [CrossRef]
- Draoui, A.; Hebboul, Z.; Boudabia, S.; Lefkaier, I.K.; Naidjate, M.E.; Belbel, A.; Aroudji, H.; Mokhtari, A.; Goumri-Said, S. Cost-effective transformation of rutile to anatase and synthesis of Zn2Ti3O8. Chem. Pap. 2025, 79, 2177–2189. [Google Scholar] [CrossRef]
- Zeng, M. Influence of TiO2 Surface Properties on Water Pollution Treatment and Photocatalytic Activity. Bull. Korean Chem. Soc. 2013, 34, 953–956. [Google Scholar] [CrossRef]
- Oancea, P.; Oncescu, T. The photocatalytic degradation of dichlorvos under solar irradiation. J. Photochem. Photobiol. A 2008, 199, 8–13. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Ramgolam, Y.K.; Soyjaudah, K.M.S. Modelling the impact of spectral irradiance and average photon energy on photocurrent of solar modules. Sol. Energy 2018, 173, 1058–1064. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Daxini, R.; Wu, Y. Review of methods to account for the solar spectral influence on photovoltaic device performance. Energy 2024, 286, 129461. [Google Scholar] [CrossRef]
- Kim, D.S.; Han, S.J.; Kwak, S.Y. Synthesis and photocatalytic activity of mesoporous TiO2 with the surface area, crystallite size, and pore size. J. Colloid Interface Sci. 2007, 316, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Fan, Q.; Chen, C.; Chen, Z.; Alsaedi, A.; Hayat, T. Insights into the crystal size and morphology of photocatalysts. J. Colloid Interface Sci. 2019, 538, 638–647. [Google Scholar] [CrossRef]
- Goldstein, J.-I.; Newbury, D.-E.; Michael, J.-R.; Ritchie, N.-W.; Scott, J.-H.-J.; Joy, D.-C. Scanning Electron Microscopy and X-Ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Su, X.-J.; Hou, G.-L. Effects of Silicon Content on Microstructure and Photocatalytic Activity of TiO2/SiO2 Composite Aerogels. J. Inorg. Mater.-Beijing 2010, 25, 911–915. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Wang, Y.; Ji, H.; Ma, W.; Zang, L.; Zhao, J. Surface modification of TiO2 by phosphate: Effect on photocatalytic activity and mechanism implication. J. Phys. Chem. 2008, 112, 5993–6001. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Yang, S.; Lee, H. Surface analysis of N-doped TiO2 nanorods and their enhanced photocatalytic oxidation activity. Appl. Catal. B 2017, 204, 209–215. [Google Scholar] [CrossRef]
- Kutzner, S.; Schaffer, M.; Börnick, H.; Licha, T.; Worch, E. Sorption of the organic cation MET on silica gel from its aqueous solution considering the competition of inorganic cations. Water Res. 2014, 54, 273–283. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Phototransformations of selected pharmaceuticals under low-energy UVA–vis and powerful UVB–UVA irradiations in aqueous solutions—The role of natural dissolved organic chromophoric material. Anal. Bioanal. Chem. 2009, 394, 1621–1636. [Google Scholar] [CrossRef]
- Romero, V.; Marco, P.; Giménez, J.; Esplugas, S. Adsorption and Photocatalytic Decomposition of the β-Blocker MET in Aqueous Titanium Dioxide Suspensions: Kinetics, Intermediates, and Degradation Pathways. Int. J. Photoenergy 2013, 2013, 138918. [Google Scholar] [CrossRef]
- Rivas, F.J.; Gimeno, O.; Borralho, T.; Carbajo, M. UV-C radiation based methods for aqueous MET elimination. J. Hazard. Mater. 2010, 179, 357–362. [Google Scholar] [CrossRef]
- Filipe, O.M.S.; Santos, E.B.H.; Otero, M.; Gonçalves, E.A.C.; Neves, M.G.P.M.S. Photodegradation of metoprolol in the presence of aquatic fulvic acids. Kinetic studies, degradation pathways and role of singlet oxygen, OH radicals and fulvic acids triplet states. J. Hazard. Mater. 2020, 385, 121523. [Google Scholar] [CrossRef]
- Avilés-García, O.; Espino-Valencia, J.; Mendoza-Zepeda, A.; Donkor, K.; Brewer, S.; Romero, R.; Natividad, R. Removal of MET by means of photo-oxidation processes. Catal. Today 2022, 397, 562–573. [Google Scholar] [CrossRef]
- Mahy, J.; Wolfs, C.; Mertes, A.; Vreuls, C.; Drot, S.; Smeets, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Lambert, S.D. Advanced photocatalytic oxidation processes for micropollutant elimination from municipal and industrial water. J. Environ. Manag. 2019, 50, 109561. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, T.; Koga, C.; Negishi, N.; Takeuchi, K.; Matsuzawa, S. An approach to elucidating photocatalytic reaction mechanisms by monitoring dissolved oxygen: Effect of H2O2 on photocatalysis. Appl. Catal. B 2009, 87, 46–55. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2− and OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Affam, A.C.; Chaudhuri, M. Degradation of pesticides chlorpyrifos, cypermethrin and chlorothalonil in aqueous solution by TiO2 photocatalysis. J. Environ. Manag. 2013, 130, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Qiu, F.; Rong, J.; Zhu, X.; Yan, J.; Yang, D. Enhanced visible light photocatalytic activity of W-doped porous g-C3N4 and effect of H2O2. Mater. Lett. 2016, 164, 127–131. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntema, D.; Rijnaarts, H.-H.-M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B Environ. 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Czech, B.; Rubinowska, K. TiO2-assisted photocatalytic degradation of diclofenac, metoprolol, estrone and chloramphenicol as endocrine disruptors in water. Adsorption 2013, 19, 619–630. [Google Scholar] [CrossRef]
- Azouani, R.; Tieng, S.; Michau, A.; Hassouni, K.; Chhor, K.; Bocquet, J.F.; Vignes, J.L.; Kanaev, A. Elaboration of Doped and Composite Nano-TiO2. Chem. Eng. Trans. 2009, 17, 981–986. [Google Scholar] [CrossRef]
- Do Rosário, L.O.; Castro, M.A.M.; Tranquilin, R.L.; Teodoro, M.D.; Correa, M.A.; Motta, F.V.; Bomio, M.R.D. Direct Z-scheme SrMoO4/g-C3N4 heterostructure with enhanced photocatalytic activity for decontamination in wastewater: UV and solar irradiation approach. J. Photochem. Photobiol. A 2024, 449, 115402. [Google Scholar] [CrossRef]
- Yang, H.; An, T.; Li, G.; Song, W.; Cooper, W.J.; Luo, H.; Guo, X. Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: A case of β-blockers. J. Hazard. Mater. 2010, 179, 834–839. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Núñez-Núñez, C.M.; Osorio-Revilla, G.I.; Villanueva-Fierro, I.; Antileo, C.; Proal-Nájera, J.B. Solar Fecal Coliform Disinfection in a Wastewater Treatment Plant by Oxidation Processes: Kinetic Analysis as a Function of Solar Radiation. Water 2020, 12, 639. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Kowalska, E.; Abdeldayem, H.M. The synergistic effect of anatase and brookite for photocatalytic generation of hydrogen and diclofenac degradation. J. Environ. Chem. Eng. 2021, 9, 106566. [Google Scholar] [CrossRef]
- Eddy, D.R.; Nur Sheha, G.A.; Permana, M.D.; Saito, N.; Takei, T.; Kumada, N.; Irkham; Rahayu, I.; Abe, I.; Sekine, Y.; et al. Study on triphase of polymorphs TiO2 (anatase/rutile/brookite) for boosting photocatalytic activity of metformin degradation. Chemosphere 2024, 351, 141206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]


| Phase | Spatial Group ** | Phase% | ICCD Card | Cristal Size (nm) | Band Gap (eV) | |
|---|---|---|---|---|---|---|
| TiO2 P25 * | Anatase | I 41/a m d [141] | 85.27 | 00-021-1272 | 20.97 | 3.3 |
| Rutile | P42/m n m [136] | 14.73 | 01-070-7347 | 33.96 | ||
| TiO2 Sigma-Aldrich | Anatase | I 41/a m d [141] | 96.81 | 00-021-1272 | 58.6 | 3.33 |
| Rutile | P42/m n m [136] | 3.19 | 01-070-7347 | |||
| TiO2 Fermont * | Anatase | I 41/a m d [141] | 100 | 00-021-1272 | 80.71 | 3.23 |
| Catalyst | H2O2 Addition (mMol/L) | K (min−1) | Half-Life Time (min) | COD Removal (%) |
|---|---|---|---|---|
| P25 | 0 | 0.0249 | 27.8 | 83.0 |
| 4 | 0.0486 | 14.3 | 90.0 | |
| Sigma-Aldrich | 0 | 0.0185 | 37.5 | 56.4 |
| 4 | 0.0283 | 24.5 | 63.0 | |
| Fermont | 0 | * | * | 16.0 |
| 4 | * | * | 21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrecillas-Rodríguez, I.C.; Rodríguez-González, F.; Tapia-Maruri, D.; Dorantes-Rosales, H.J.; Molina-González, J.L.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. Comparative Efficiencies of TiO2 Photocatalysts on β-Blocker Metoprolol Degradation by Solar Heterogeneous Photocatalysis. Nanomaterials 2025, 15, 1445. https://doi.org/10.3390/nano15181445
Torrecillas-Rodríguez IC, Rodríguez-González F, Tapia-Maruri D, Dorantes-Rosales HJ, Molina-González JL, Núñez-Núñez CM, Proal-Nájera JB. Comparative Efficiencies of TiO2 Photocatalysts on β-Blocker Metoprolol Degradation by Solar Heterogeneous Photocatalysis. Nanomaterials. 2025; 15(18):1445. https://doi.org/10.3390/nano15181445
Chicago/Turabian StyleTorrecillas-Rodríguez, Irma C., Francisco Rodríguez-González, Daniel Tapia-Maruri, Héctor J. Dorantes-Rosales, José L. Molina-González, Cynthia M. Núñez-Núñez, and José B. Proal-Nájera. 2025. "Comparative Efficiencies of TiO2 Photocatalysts on β-Blocker Metoprolol Degradation by Solar Heterogeneous Photocatalysis" Nanomaterials 15, no. 18: 1445. https://doi.org/10.3390/nano15181445
APA StyleTorrecillas-Rodríguez, I. C., Rodríguez-González, F., Tapia-Maruri, D., Dorantes-Rosales, H. J., Molina-González, J. L., Núñez-Núñez, C. M., & Proal-Nájera, J. B. (2025). Comparative Efficiencies of TiO2 Photocatalysts on β-Blocker Metoprolol Degradation by Solar Heterogeneous Photocatalysis. Nanomaterials, 15(18), 1445. https://doi.org/10.3390/nano15181445








