Abstract
YVO4 based phosphors have aroused extensive interest in the field of optoelectronics due to their good chemical stability and unique luminescence properties. However, commercialization of YVO4 phosphors requires high luminescence intensity, enhanced conversion efficiency, and a wide excitation spectrum. In this work, Eu3+, Ba2+, Bi3+ co-doped YVO4 was prepared by the sol–gel method. The XRD of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ phosphor analysis confirms the pure tetragonal phase, with a fairly large size of approximately 100 nm for the optimal composition. And the SEM and TEM revealed well-dispersed spherical nanoparticles with sizes of 100–120 nm. The introduction of Ba2+ ions enhanced the luminescence intensity, while the incorporation of Bi3+ ions improved the excitation width of the phosphor. The resulting YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ phosphor exhibited a 1.39-times broader excitation bandwidth and a 2.72-times greater luminescence intensity at 618 nm compared to the benchmark YVO4: 5% Eu3+ sample. Additionally, the transmittance of the films in the 350 nm to 800 nm region exceeded 85%. The YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ film effectively absorbed ultraviolet light and converted it to red emission, enabling potential applications in solar cell window layers, dye-sensitized cell luminescence layers, and solar cell packaging glass.
1. Introduction
YVO4 phosphor with a tetragonal phase shows excellent chemical stability, such as a high decomposition temperature (>1800 °C) and high resistance to moisture and common solvents, and unique luminescence properties. In particular, the Eu3+-doped YVO4 red emission phosphor has received extensive interest for many applications [1,2,3,4,5,6,7,8]. Compared with traditional Y2O3: Eu3+ phosphors, YVO4: Eu3+ phosphor exhibits significantly higher luminescence intensity (reportedly about 2–3 times higher under UV excitation), superior color purity, and a much wider operating temperature range, maintaining high efficiency up to temperatures exceeding 200 °C [1,2,3,4].
Zuo Y Y et al. [9,10,11,12,13,14,15] found that Bi3+-doped YVO4: Eu3+ can broaden the band edges of the absorption and excitation spectrum and red-shift the absorption edge and excitation edge. However, uneven doping and concentration quenching of Bi3+ led to a reduction in photoluminescence intensity. Wangkhem R et al. [16,17,18,19,20] found that the doping of M2+ alkaline ions into YVO4: Eu3+ introduced radiation defect centers and enhanced the luminescence intensity. L. Jiang et al. [21] improved the luminescence intensity by coating YVO4: Eu3+ with SiO2. Enrico C et al. [22] prepared YVO4: Ln3+ (Ln3+ = Eu3+, Sm3+, and Dy3+) phosphors, and studied the effect of co-doped ions on their luminescence properties. Tang L et al. [23] prepared YVO4: Eu3+-trifluoro acetone by inorganic and organic hybridization, which showed significantly enhanced luminescence intensity. Wang GF et al. [24] synthesized water-soluble YVO4: Ln3+ and YVO4: Ln3+, Ba2+ (Ln = Ce, Dy, Eu, Sm) nanocrystals by the polyethylpyrrolidone-assisted hydrothermal method, and found that Ba2+ doping enhanced the luminescence intensity. Park K C et al. [25] found that in Ba3(1-x)Eu2xV2O8, the luminous intensity of VO43− decreased with the increase in Eu3+ content due to the charge transfer transition of VO43−. The excitation spectra of Ba2.1Eu0.6V2O8 only showed charge transfer transition and f-f transition of Eu3+ ions. In the Ba3(1−x)Y2xV2O8 (0 ≤ x ≤ 0.33) system, the luminescence intensity decreases with the increase in Y3+ content. YVO4: Eu3+ phosphors synthesized by the high-temperature solid phase method usually exhibit severe grain agglomeration. The crystal destruction and incomplete shape obtained upon ball milling result in decreased luminescence intensity and increased light decay. Moreover, uneven particles of YVO4: Eu3+ luminescent materials also result in uneven coating, and lead to a series of problems such as poor color temperature consistency, light color consistency, and color rendering index of the display. Traditional synthesis methods also cause low luminous intensity and light conversion efficiency of luminous materials. Therefore, single rare earth and alkaline earth-doped YVO4: Eu3+ luminous materials have narrow excitation spectra, which hinders their commercial applications [26]. Several recent studies have reported on novel hybrid luminescent materials based on YVO4 hollow mesoporous microspheres [27], dynamic color gradient anti-counterfeiting technology with multi-wavelength excitation [28], and nanoporous YVO4 as a luminescent system for molecular encapsulation detection [29]. These findings present broad application prospects of YVO4-based phosphors in the fields of optoelectronic integration, anti-counterfeiting technology, and optical sensing. Developing improved YVO4: Eu3+ phosphors can significantly improve luminescence efficiency and anti-counterfeiting capabilities and also open up new research pathways for developing novel rare-earth complexes.
Although single-doped or double-doped Ba2+ or Bi3+ have been reported, how to simultaneously solve the two key problems of a “narrow excitation spectrum” and “luminescence intensity needs to be improved” faced by YVO4: Eu3+ materials through the synergistic effect of ternary co-doping (Synergistic Effect) has yet to be deeply explored. In this work, YVO4: Eu3+, Ba2+, Bi3+ crystals and films were synthesized by the sol–gel method. In this material, Eu3+ ion serves as the activator, dominating the red emission of phosphor. Ba2+ ion enhances luminescence, while the introduction of Bi3+ ion broadens the excitation spectrum of the samples. The effects of annealing temperature, doping concentration, and coating parameters on the phase structure, microstructural characteristics, and photoluminescence properties of crystals and films were systematically investigated. Additionally, the interaction mechanisms of Eu3+-, Ba2+-, and Bi3+-doping ions were explored, including their roles in morphology control, wide-spectrum excitation, and luminescence enhancement.
2. Experimental
2.1. Materials
Yttrium nitrate (Y(NO3)3·6H2O, 99.99%), europium nitrate (Eu(NO3)3·6H2O, 99.99%), barium nitrate (Ba(NO3)2, 99.99%), bismuth nitrate (Bi(NO3)3, 99.99%), ammonium vanadate (NH4VO3, 99.99%), and citric acid (C6H8O7) (analytical grade, A.R.) were used as the raw materials. Dilute nitric acid, acetone (CH3COCH3) (analytical-grade, A.R.), ammonia water (NH3·H2O) (analytical-grade, A.R.), polyethylene glycol (PEG 4000) (analytical grade, A.R.), and ethanol (C2H5OH) (analytical-grade, A.R.) were used as the solvents. All chemicals were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). All chemicals were used as received, without further purification. Deionized water was used throughout this work.
2.2. Synthesis of YVO4: xEu3+, yBa2+, and zBi3+ Crystals
The preparation process of YVO4: 1%Eu3+ crystal is as follows. First, 4.95 mmol of Y(NO3)3·6H2O and 0.05 mmol of Eu(NO3)3·6H2O were dissolved in H2O (10 mL), and the mixture was stirred evenly. Then, citric acid (metal ion–citric acid = 1:3) was added at a constant temperature of 80 °C with stirring to form solution A. NH4VO3 (5.0 mmol) and NH3·H2O (0.5 mL) were added into 20 mL of deionized water. After heating and stirring at a constant temperature of 90 °C, a transparent yellow solution was formed, which was named solution B. Solution B was slowly added into solution A to form mixed solution C. At this time, the color of mixed solution C changed from brick red to dark red (A and B were mixed after cooling) or dark green (A and B were mixed before cooling). After complete addition of solution B, the mixed solution C was ultrasonicated for 10 min. The pH value of the mixed solution C was accurately adjusted to 7.0 using concentrated ammonia (25–28%) (measured using a pH meter), and a blue, uniform, and transparent solution D was obtained. Solution D was continuously stirred at a constant temperature of 80 °C to slowly form a sol. As the reaction time continued, it gradually formed a thick, blue gel. The obtained gel was dried at 120 °C for 6 h. After drying, a honeycomb-shaped dark blue dry gel was obtained. The dry gel was calcined in a muffle furnace at 500 °C for 3 h, and the organic matter in the dry gel was removed to obtain a yellowish YVO4: 1%Eu3+ powder sample. The calcined YVO4: 1%Eu3+ samples were annealed for 2 h at 800 °C, 900 °C, 1000 °C, 1100 °C, and 1200 °C, respectively, to obtain white powder samples.
According to the preparation scheme of YVO4: 1%Eu3+ samples, a series of YVO4: xEu3+ (x = 3%, 5%, 7%), YVO4: 5%Eu3+, yBa2+ (y = 1%, 3%, 5%, 7%) and YVO4: 5%Eu3+, 5%Ba2+, zBi3+ (z = 0.5%, 1%, 1.5%, 2%) samples were prepared by adjusting the concentration of solute in solution A according to the doping ratio. The samples were finally annealed at 1100 °C for 2 h.
2.3. Synthesis of YVO4: Eu3+, Ba2+, and Bi3+ Films
The preparation process of YVO4: Eu3+, Ba2+, and Bi3+ films was similar to that of YVO4: 5%Eu3+, 5%Ba2+, and 0.5%Bi3+ crystals. After obtaining the uniform blue solution D, 0.06 g/mL of polyethylene glycol (PEG 4000) was added to the blue solution. After stirring for several hours under heated conditions, the gel was obtained. Quartz glass was chosen as the substrate for the film. The substrate was soaked in HNO3 in advance. The mixed solution was prepared according to the ratio of C2H5OH:H2O:CH3COCH3 = 1:1:1. The quartz substrate was soaked in the mixed solution for 30 min and dried before use. The film was coated on the quartz substrate by the rotating coating method (rotating at 4000 rpm for 30 s with a spinner). The gel was dropped on the quartz substrate using a 1 mL dropper, and then the film was formed by rotating the coating at a rotation speed of 4000 r/min for 30 s. After each layer of coating rotation, the wet film was placed into the oven at 80 °C for 10 min. After the film was formed, rotating coating was continued and the steps were repeated to prepare the film with 1, 3, 5, 7, and 9 layers. Finally, the prepared film was calcined in a muffle furnace, and the film sample was obtained by pre-sintering at 500 °C for 3 h and then at 1100 °C for 2 h.
2.4. Characterization
Unless otherwise specified, all measurements were repeated on three independently synthesized batches of samples to ensure reproducibility. The data presented are the average values. X-ray diffraction (XRD, Bruker D8 Advance, Karlsruhe, Germany), scanning electron microscopy (SEM, S-4800, Tokyo, Japan), and transmission electron microscopy (TEM, Tecnai G2 F20 S-TWIN, USA) were used to investigate the phase structures and microstructural characteristics of YVO4: Eu3+, Ba2+, and Bi3+ crystals and films. ICP-OES: Thermo Fisher iCAP 7400 (Thermo Fisher Scientific, Waltham, MA, USA). Photoluminescence excitation and emission spectra were recorded with a Hitachi F-4600 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) equipped with a xenon lamp source. UV–Vis absorption spectra were monitored with a UV3150 spectrophotometer (Shimadzu, Japan). All measurements were conducted in air at room temperature.
3. Results and Discussion
3.1. Photoluminescence Properties
The photoluminescence spectra of YVO4: Eu3+ crystals with different doping concentrations annealed at 1100 °C and the crystals with 5% Eu3+ concentration annealed at different temperatures are shown in Figure 1 and Figure 2. The peak at 260 nm (Figure 1a and Figure 2a) is generated by the Eu–O charge transfer band, and the peak at 320 nm is generated by the charge transfer from the oxygen ligand to the vanadium ion center [30,31]. The 7F0.1 → 5GJ, 5L7 transition at 384 nm, 7F0.1 → 5L6 transition at 395 nm, 7F0.1 → 5D3 transition at 420 nm, and 7F0.1 → 5D2 transition at 470 nm are all characteristic excitation peaks of Eu3+. The 5D1 → 7F1 transition at 538 nm, 5D0 → 7F1 transition at 600 nm, 5D0 → 7F2 transition at 618 nm, and 5D0 → 7F3 transition at 657 nm [32,33] are all characteristic emission peaks of Eu3+ (Figure 1b and Figure 2b). The above results showed that Eu3+ was successfully doped into the YVO4 matrix by the sol–gel method.
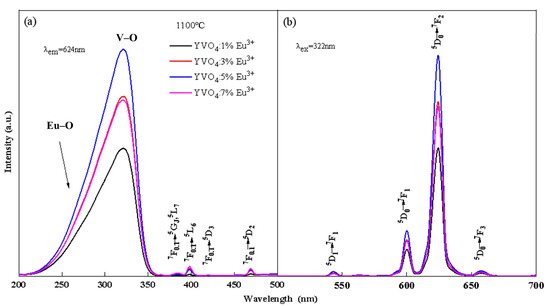
Figure 1.
Excitation (a) and emission (b) spectra of YVO4: xEu3+ (x = 1%, 3%, 5%, 7%) after annealing at 1100 °C.
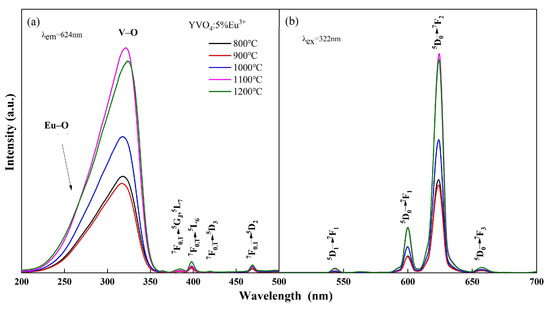
Figure 2.
Excitation (a) and emission (b) spectra of YVO4: 5%Eu3+ annealed at different temperatures.
The luminescence intensity of YVO4: Eu3+ crystals varied with the annealing temperature and doping concentration, as seen from Figure 1 and Figure 2. When the annealing temperature reached 1100 °C, the excitation intensity was 2.28 times that of 800 °C, and the emission intensity at 618 nm was 2.25 times that of 800 °C. When the doping concentration was 5%, the excitation intensity and emission intensity reached the maximum. In the band range of 200–350 nm, the excitation spectral intensity at 5% doping was 1.73 times that of 1%, 1.24 times that of 3%, and 1.26 times that of 7%. The luminescence intensity at 618 nm at 5% doping was 1.72 times that of 1%, 1.27 times that of 3%, and 1.31 times that of 7%.
The change in luminescence properties of YVO4: 5%Eu3+ caused by the variation in annealing temperature can be attributed to several factors. As the annealing temperature increases, the covalent bond interaction strengthens, which facilitates the electron migration from O2− to V5−. This results in a slight red-shift in the V–O electron migration band [34] and enhanced luminescence intensity. Additionally, the improvement in crystallinity with the increase in temperature reduces crystal defects and non-radiative traps, leading to enhanced luminescence intensity [35]. Furthermore, the rapid growth of crystals at higher temperatures results in the formation of submicron- to micron-sized crystals with varying sizes and reduced uniformity, creating more pronounced inter-crystal voids. Consequently, the photoluminescence performance of these micron-sized luminescent materials was inferior to that of nanomaterials.
When the doping concentration of Eu3+ reached 5% and then continued to increase, the luminescence intensity of YVO4: Eu3+ crystals decreased. The reason is that the spacing between the particles gradually decreased as the Eu3+ concentration continued to increase to a certain critical point. When the spacing was less than 1–2 nm, the concentration quenching phenomenon occurred, resulting in a reduction in luminescence intensity [36].
The photoluminescence intensity of YVO4: 5%Eu3+, yBa2+ crystals varied with the doping concentration, as seen in Figure 3a,b. When the concentration of Ba2+ was 5%, the excitation intensity of YVO4: 5%Eu3+, 5%Ba2+ crystals at 200–350 nm was 2.89 times that of undoped Ba2+, and the luminescence intensity of 5D0 → 7F2 at 618 nm was 2.72 times that of undoped Ba2+. As the concentration of Ba2+ continued to increase, the excitation intensity and luminescence intensity of YVO4: Eu3+, Ba2+ crystals decreased. This decrease occurred because the radius of Ba2+ is larger than that of Eu3+, and there is an energy transfer between Ba2+ and Eu3+. In the non-radiative transition process, Ba2+ absorbs more energy than Eu3+. When there is competition between VO43−-Eu3+ energy transfer and Eu3+-Ba2+ non-radiative transition, the increase in Ba2+ doping concentration leads to concentration quenching and a decrease in luminescence intensity [37]. The above results confirmed that Ba2+ was successfully doped into the YVO4: Eu3+ matrix by the sol–gel method.
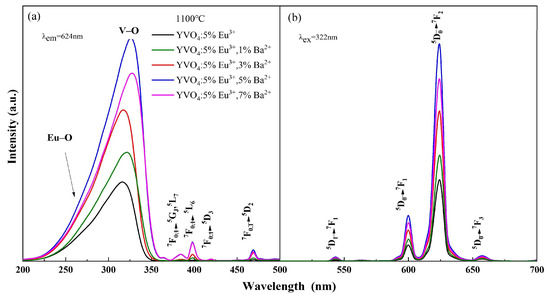
Figure 3.
Excitation (a) and emission (b) spectra of YVO4: 5%Eu3+, yBa2+ (y = 1%, 3%, 5%, 7%).
Ba2+ doping promoted a slight red-shift in the V–O electron migration band of the excitation spectrum (Figure 3a). This is because the charge radius ratio of Ba2+ is smaller than that of Y3+, and the binding of Ba2+ with the oxygen atom is less tight than that of Y3+ with the oxygen atom. When Ba2+ was doped into the lattice, lattice parameters changed, resulting in a looser binding of Y3+/Ba2+ with the oxygen atom in the lattice. Consequently, Ba2+ doping caused a slight lattice distortion. Thus, the charge transfer state (CTS) absorption band [38] was enhanced and the luminescence intensity of the CTS excitation band increased [39].
With the incorporation of Bi3+, the wide-spectrum excitation performance was considerably improved (Figure 4a). The excitation widths of YVO4: 5%Eu3+, 5%Ba2+, zBi3+ (z = 0.5%, 1%, 1.5%, 2%) crystals at 200–350 nm were 1.34 times, 1.29 times, 1.25 times, and 1.32 times that of YVO4: 5%Eu3+, 5%Ba2+, respectively. However, with the increase in Bi3+ doping concentration, the excitation intensity and luminescence intensity of YVO4: 5%Eu3+, 5%Ba2+, zBi3+ (z = 0.5%, 1%, 1.5%, 2%) crystals gradually decreased (Figure 4a,b) [40]. The excitation intensity of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ crystals at 200–350 nm was 0.73 times that of YVO4: 5%Eu3+, 5%Ba2+, and their luminescence intensity at 618 nm was 0.725 times that of YVO4: 5%Eu3+, 5%Ba2+. The excitation intensity of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ crystals at 200–350 nm was 2.11 times that of YVO4: 5%Eu3+, and the excitation width was 1.39 times that of YVO4: 5%Eu3+. Their luminescence intensity at 623 nm was 1.97 times that of YVO4: 5%Eu3+.
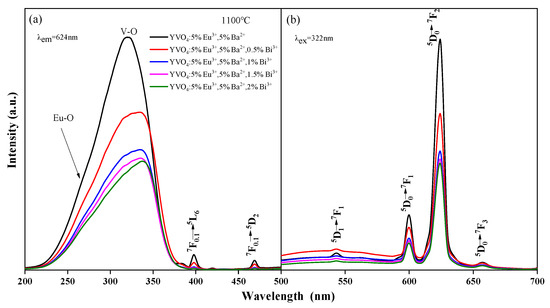
Figure 4.
Excitation (a) and emission (b) spectra of YVO4: 5%Eu3+, 5%Ba2+, zBi3+ (z = 0.5%, 1%, 1.5%, 2%).
After the addition of Bi3+, a slight red-shift was observed in both the main peak and edge of the excitation band (Figure 4a). The main peak moved from 320 to 335 nm. This was due to the absorption of Bi3+ in the ultraviolet region of 200–400 nm, and the presence of an electron transfer between Bi3+-V5+ ions and energy transfer to Eu3+. Bi3+ plays a sensitization role here. The Eu–O and V–O absorption peaks of YVO4: Eu3+, Ba2+, Bi3+ crystals both decreased, mainly because the energy between O2−-Eu3+ and O2−-V5+ was transferred to the O2−-Bi3+ electron transition. Consequently, the absorption peaks of O2−-Eu3+ and O2−-V5+ decreased [41,42,43]. Another reason for the reduced excitation peak strength was the difficulty doping YVO4 at high concentrations. The incorporation of Bi3+ caused lattice distortion, reducing the actual doping amounts of Eu3+ and Ba2+, thus lowering the excitation strength. Furthermore, ICP-OES ion occupancy analysis enables the determination of occupancy characteristics for Eu3+, Ba2+, and Bi3+ ions within the YVO4 host. ICP-OES analysis revealed the following ion incorporation efficiencies: In YVO4: 5% Eu3+, the actual Eu3+ content was determined to be 4.82%; for YVO4: 5% Eu3+, 5% Ba2+, measured values showed 4.79% Eu3+ and 4.44% Ba2+; while in YVO4: 5% Eu3+, 5% Ba2+, 0.5% Bi3+, the incorporated concentrations were quantified as 4.35% Eu3+, 4.84% Ba2+, and 0.28% Bi3+. ICP-OES analysis shows that the actual doping amount of Bi3+ (0.28%) is much lower than its nominal doping concentration (0.5%), and its doping efficiency (56%) is also significantly lower than that of Eu3+ (87%) and Ba2+ (97%). This phenomenon is mainly attributed to the large ion radius mismatch between Bi3+ and Y3+.
The incorporation of both Ba2+ and Bi3+ ions into YVO4: Eu3+ phosphors significantly enhanced their luminescence properties. Ba2+ doping induced lattice expansion and local distortion, which reduced the crystal field symmetry around Eu3+ and strengthened its electric dipole transition (5D0 → 7F2) [10,39], thereby improving red emission intensity. Concurrently, Ba2+ doping introduced positive charge defects, while Bi3+ doping partially compensated for charge imbalance, suppressing the formation of oxygen vacancies and other non-radiative recombination centers to minimize luminescence quenching. Additionally, the strong ultraviolet absorption (300–350 nm) of Bi3+ enabled efficient excitation energy harvesting and subsequent non-radiative energy transfer to Eu3+, thereby boosting excitation width and luminescence efficiency. Thus, the luminescence behavior of YVO4: Eu3+ under Bi3+ doping is a result of the competition between effective sensitization at lower concentrations and concentration quenching effects at higher doping levels.
All emission peaks in the spectra (Figure 3b and Figure 4b) were characteristic peaks of Eu3+, with no emission peaks attributable to Ba2+ or Bi3+. This indicated that Ba2+ and Bi3+ themselves did not emit light, and that the energy transfer between Eu3+ and VO43− groups was efficient. The doping of Ba2+ only improved the luminescence intensity of YVO4: Eu3+ [44,45,46]. This enhancement occurred because doped Ba2+ replaced the position of Y3+ in the crystal lattice, creating a negative potential BaY′ and altering the lattice parameters, which increased the luminescence intensity [44]. Additionally, the sensitizer Bi3+ transferred energy to the active ion Eu3+ after the electron transfer between Bi3+-V5+ ions.
With the increase in the number of film layers, both the excitation intensity and luminescence intensity increased rapidly, reaching the maximum at the third layer (Figure 5a,b). However, as the number of film layers continued to increase, both the excitation intensity and luminescence intensity exhibited a decreasing trend. Specifically, the excitation strength of the film with 3 layers at 304 nm was 2.52, 2.94, 1.95, and 32.38 times that of the 1-layer, 5-layer, 7-layer and 9-layer films, respectively. Similarly, the luminescence intensity of 3-layer film at 618 nm was 2.62 times, 3.02 times, 2.00 times, and 48.52 times that of 1-layer, 5-layer, 7-layer, and 9-layer films, respectively.
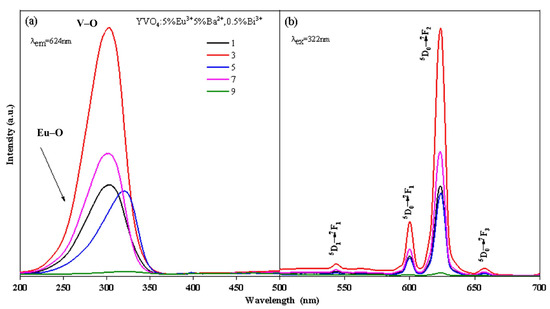
Figure 5.
Excitation (a) and emission (b) spectra of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ films.
3.2. Phase Structures and Microstructural Characteristics
XRD patterns of YVO4: Eu3+ crystals prepared by the sol–gel method with different doping concentrations and the same doping concentration at different annealing temperatures are shown in Figure 6a,b. The positions and relative intensities of the diffraction peaks of the samples matched well with the standard card (JCPD 17-0341), and corresponded to the YVO4 crystal group. The crystals showed tetragonal zircon structure without any heterogenous phase. With the increase in annealing temperature, doping concentration, and number of film layers, the diffraction peaks became sharper, and the crystallinity of the samples improved. A synthesis temperature of 1100 °C and a heating rate of 20 °C/min were selected in this study through comprehensive optimization of crystallinity, doping efficiency, and process feasibility. The reaction temperature of 1100 °C ensures pure-phase crystallization of YVO4, eliminates interference from impurity phases on luminescent properties, and facilitates effective Eu3+ incorporation into Y3+ lattice sites to enhance doping efficiency. Simultaneously, this temperature enables complete decomposition of organic precursors, and reduces carbon residues and non-radiative recombination centers, thereby improving luminous efficiency. The heating rate of 20 °C/min accelerates nucleation kinetics, yielding uniform and fine crystallites (approximately 100–300 nm) with enhanced luminescence uniformity. The synergistic effects of an appropriate temperature and heating rate optimized crystallization and defect suppression, ultimately achieving excellent luminescent performance.
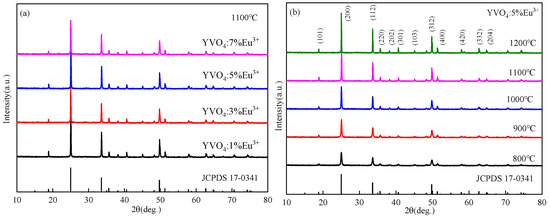
Figure 6.
(a) XRD patterns of YVO4: 5%Eu3+ after annealing at different temperatures (JCPDS card No. 17-0341); (b) XRD patterns of YVO4: xEu3+ (x = 1%, 3%, 5%, 7%) after annealing at 1100 °C (JCPDS card No. 17-0341).
XRD patterns of YVO4: 5%Eu3+, yBa2+ and YVO4: 5%Eu3+, 5%Ba2+, zBi3+ at different Ba2+ doping concentrations are presented in Figure 7a,b, indicating the good crystallization properties of the samples. With the increase in Ba2+ doping concentration, Ba3(VO4)2 heterophase gradually appeared, and the diffraction peak shifted to a small angle (Figure 7a). This shift was due to the larger radius of Ba2+ (0.135 nm) compared to Y3+ (0.088 nm). At a low concentration, Ba2+ can effectively enter the lattice of YVO4 and replace the position of Y3+. However, at a high concentration, Ba2+ cannot mix well into the lattice of YVO4, which is consistent with some reports [47,48,49].
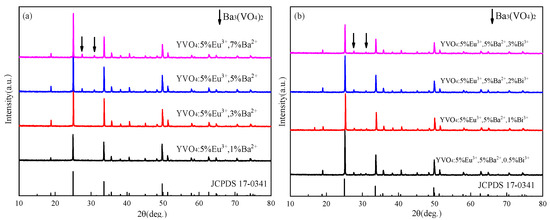
Figure 7.
(a) XRD patterns of YVO4: 5%Eu3+, yBa2+ (y = 1%, 3%, 5%, 7%) after annealing at 1100 °C (JCPDS card No. 17-0341); (b) XRD patterns of YVO4: 5%Eu3+, 5%Ba2+, zBi3+ (z = 0.5%, 1%, 1.5%, 2%) after annealing at 1100 °C (JCPDS card No. 17-0341).
The diffraction peaks shifted towards smaller angles with the incorporation of Bi3+, and the heterodox peaks at 27.6° and 31.2° gradually increased in intensity with a higher Bi3+ doping concentration (Figure 7b). This indicates that at high doping concentrations, Bi3+ cannot be successfully incorporated into the lattice of YVO4.
With the increase in annealing temperature, the crystal size gradually increased (Figure 8a–e). When the annealing temperature reached 1100 °C, the crystals transformed rapidly from a thin scaly shape to a spheroid shape, with a uniform size of about 100 nm and excellent dispersion. When annealed at 1200 °C, the crystals exhibited various sizes and shapes, growing rapidly into ingot-like and rod-like forms with micrometer-scale dimensions ranging from approximately 300 nm to 600 nm.
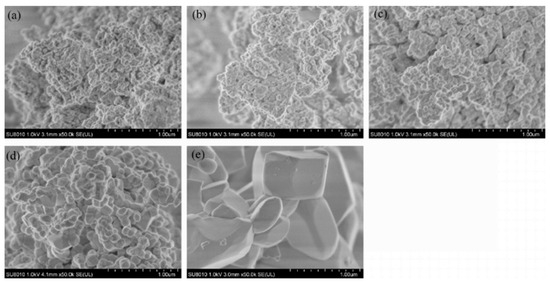
Figure 8.
SEM images of YVO4: 5%Eu3+ at different annealing temperatures: (a) 800 °C, (b) 900 °C, (c) 1000 °C, (d) 1100 °C, and (e) 1200 °C.
The crystal sizes of YVO4: 5%Eu3+ were distributed in the range of 100 nm to 120 nm (Figure 9a), while the crystal sizes of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ were distributed in the range of 150 nm to 310 nm (Figure 9c). All the crystals showed good dispersion. Lattice stripes in the crystals can be clearly observed in Figure 9b,d, indicating that all the crystals possessed good crystallization properties.

Figure 9.
(a,b) TEM images of YVO4: 5%Eu3+ annealed at 1100 °C; (c,d) TEM images of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ annealed at 1100 °C.
3.3. Transmittance Spectra
The transmittance of the film initially increased with increase in the number of film layers, and reached the maximum for the three-layer film (Figure 10). Then, the transmittance showed a downward trend as the number of film layers continued to increase. The transmittance of the uniformly coated film fluctuated greatly between 250 and 350 nm. The transmittance in some regions showed an obvious decrease, especially for the small peaks at 260 nm, 280 nm and 320 nm. These reductions were attributed to the absorption of ultraviolet light by the YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ films in this region. For wavelengths between 350 nm and 1000 nm, the transmittance of all films remained above 75%, with the transmittance of three-layer, five-layer and seven-layer films exceeding 85%. The YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ phosphor film developed in this study exhibited high transmittance (>85%) and UV-to-visible light conversion capability in solar cell window layers, demonstrating potential for future exploration in optical temperature sensing applications. Other systems such as Ca9Mg1.5(PO4)7: Eu2+/Eu3+ [50] and Ca9Zn1.5(PO4)7: Eu, Tb [51] have also demonstrated the advantages of multi-ion co-doping in temperature sensing and white LEDs. Therefore, the tailored design of multifunctional optical materials can be achieved by modulating doped ion interactions.
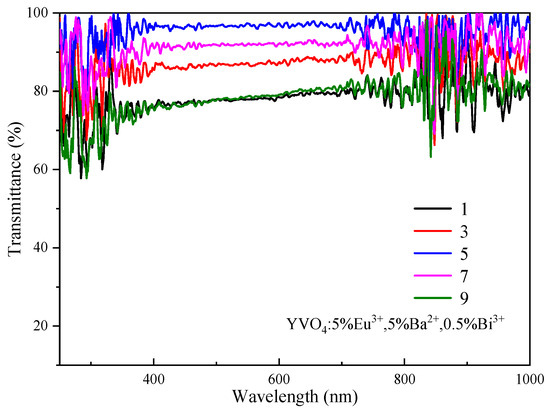
Figure 10.
Transmittance spectra of YVO4: 5%Eu3+, 5%Ba2+, 0.5%Bi3+ films.
4. Conclusions
In conclusion, Eu3+, Ba2+, Bi3+ tri-doped YVO4 phosphors and thin films were successfully synthesized via a sol–gel approach. The incorporation of Ba2+ ions enhanced the luminescence intensity by 2.72 times at 618 nm through lattice expansion and suppression of non-radiative decay, while Bi3+ co-doping broadened the excitation bandwidth by 1.39 times via efficient energy transfer. The obtained films exhibit high transparency (>85%) and effective UV-to-red conversion, demonstrating great potential for solar cell applications. This study provides a strategic ternary-doping strategy that simultaneously overcomes the narrow excitation and moderate emission limitations of traditional YVO4: Eu3+ phosphors. Further studies should focus on thermal stability assessment and device integration to advance practical implementations.
Author Contributions
Y.L. and Y.H.: Validation, Investigation, Data curation; W.Z. and J.L.: Visualization, Methodology, Data curation, Software; J.H., W.Q. and Y.X.: Methodology, Formal analysis, Writing—original draft, Supervision, Funding acquisition, Conceptualization; C.D.: Writing—review and editing, Investigation, Formal analysis, Visualization, Conceptualization; P.H.: Funding acquisition, Resources, Project administration, Validation; W.D. and L.Q.: Resources, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Hunan Province (2022JJ60057) and the Natural Science Foundation of Jiangxi Province (20224BAB214023, 20232BAB204010).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Peng, L.X.; Li, L.P.; Qin, F.; Wang, C.W.; Zhang, Z.G. A multi-mode self-referenced optical thermometer based on low-doped YVO4: Eu3+ phosphor. J. Lumin. 2023, 263, 120168. [Google Scholar] [CrossRef]
- Mentasti, L.; Zucchi, I.A.; Cammarata, A.; Glisoni, R.; Santiago, M.; Barreto, G. Facile functionalization of YVO4: Eu3+: From nanoparticles to luminescent PMMA nanocomposites for radiation detectors. Opt. Mater. 2022, 129, 112566. [Google Scholar] [CrossRef]
- Babu, A.; Réveret, F.; Barros, A.; Cisnetti, F.; Lemoine, K.; Jamon, D.; Suta, M.; Chadeyron, G.; Boyer, D. Efficient Luminescent Coatings Constituted of Hybrid Sol-Gel Matrixes Embedding YVO4: Eu3+ Nanocrystals. Acs Appl. Mater. Interfaces 2025, 17, 43413–43423. [Google Scholar] [CrossRef] [PubMed]
- Mentasti, L.; Martínez, N.; Zucchi, I.A.; Marcazzó, J.; Orellana, G.; Santiago, M.; Barreto, G. Novel materials for radiation sensing in radiotherapy treatments: Development of luminescent YVO4: Eu3+ polymer-based nanocomposites. Opt. Mater. 2024, 150, 115285. [Google Scholar] [CrossRef]
- Pankratov, V.; Popov, A.I.; Shirmane, L.; Kotlov, A.; Feldmann, C. LaPO4:Ce, Tb and YVO4: Eu nanophosphors: Luminescence studies in the vacuum ultraviolet spectral range. J. Appl. Phys. 2011, 110, 053522. [Google Scholar] [CrossRef]
- Kang, J.H.; Nazarov, M.; Bin Im, W.; Kim, J.Y.; Jeon, D.Y. Characterization of nano-size YVO4: Eu and (Y, Gd)VO4: Eu phosphom by low voltage cathodo- and photoluminescence. J. Vac. Sci. Technol. B 2005, 23, 843–848. [Google Scholar] [CrossRef]
- Lim, J.; Na, Y.E.; Lee, Y.S.; Bu, S.D. Conversion of the valence states of Eu ions in YVO4 with the gamma-ray irradiation. Curr. Appl. Phys. 2018, 18, 864–868. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Mao, J.; Zhu, P.F.; Wang, G.F. Tunable multicolor luminescence in vanadates from yttrium to indium with enhanced luminous efficiency and stability for its application in WLEDs and indoor photovoltaics. Nano Res. 2023, 16, 11486–11494. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, Y.; Gao, H. Synthesis and Photoluminescence Properties of (Y, Bi, Sc)VO4: Eu3+ Phosphor. Rare Met. Mater. Eng. 2007, 36, 386–389. [Google Scholar]
- Tomina, E.V.; Sladkopevtsev, B.V.; Novikova, L.A.; Boykov, N.I.; Maltsev, S.A. Microwave and ultrasonic radiation-activated synthesis and luminescent properties of nanopowder YVO4: Bi3+, Eu3+. Russ. Chem. Bull. 2023, 72, 1113–1121. [Google Scholar] [CrossRef]
- Ohno, T.; Iso, Y.; Isobe, T. Low-Temperature Synthesis of YVO4: Bi3+, Eu3+ Nanoparticle Phosphors Using a Methanol Solution of Trivalent Cations. Ecs J. Solid State Sci. Technol. 2016, 5, R142–R145. [Google Scholar] [CrossRef]
- Wang, D.M.; Tie, S.L.; Wan, X. White light emitting from YVO4/Y2O3: Eu3+, Bi3+ composite phosphors for UV light-emitting diodes. Ceram. Int. 2015, 41, 7766–7772. [Google Scholar] [CrossRef]
- Takeshita, S.; Isobe, T.; Sawayama, T.; Niikura, S. Effects of the homogeneous Bi3+ doping process on photoluminescence properties of YVO4: Bi3+, Eu3+ nanophosphor. J. Lumin. 2009, 129, 1067–1072. [Google Scholar] [CrossRef]
- Chen, D.Q.; Yu, Y.L.; Huang, P.; Lin, H.; Shan, Z.F.; Zeng, L.W.; Yang, A.P.; Wang, Y.S. Color-tunable luminescence for Bi3+/Ln3+: YVO4 (Ln = Eu, Sm, Dy, Ho) nanophosphors excitable by near-ultraviolet light. Phys. Chem. Chem. Phys. 2010, 12, 7775–7778. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.; Nanda, S.S.; Dash, S. Simultaneous Influence of Dual Sensitizers on Photo-Physical Properties of Dy3+ Activated YVO4 Phosphors. J. Mater. Eng. Perform. 2024, 33, 5268–5278. [Google Scholar] [CrossRef]
- Wangkhem, R.; Singh, N.P.; Singh, N.S. On the mechanism of luminescent YVO4: Eu3+ as turn-off luminescent probe for selective detection of Cu2+ ions. Ceram. Int. 2024, 50, 11588–11596. [Google Scholar] [CrossRef]
- Nirwan, F.M.; Rao, T.K.G.; Gupta, P.K.; Pode, R.B. Studies of defects in YVO4: Pb2+, Eu3+ red phosphor material. Phys. Status Solidi A-Appl. Mater. Sci. 2003, 198, 447–456. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Fang, Y.-C.; Chu, S.-Y. Energy Transfer Sm3+→Eu3+ in Potential Red Phosphor (Ca, Ba)3(VO4)2: Sm3+, Eu3+ for Use in Organic Solar Cells and White Light-Emitting Diodes. J. Am. Ceram. Soc. 2010, 93, 3850–3856. [Google Scholar] [CrossRef]
- Jiu, H.F.; Jiao, H.Q.; Zhang, L.X.; Jia, W.B.; Huang, C.S.; Chang, J.X. Improved luminescence behavior of YVO4: Eu3+ hollow microspheres by Ca2+ doping. Superlattices Microstruct. 2015, 83, 627–634. [Google Scholar] [CrossRef]
- Wangkhem, R.; Singh, N.S.; Singh, N.P.; Singh, S.D.; Singh, L.R. Facile synthesis of re-dispersible YVO4: Ln3+ (Ln3+ = Dy3+, Eu3+, Sm3+) nanocrystals: Luminescence studies and sensing of Cu2+ ions. J. Lumin. 2018, 203, 341–348. [Google Scholar] [CrossRef]
- Jiang, L.S.; Zhang, Z.Y.; Xiao, Y.C.; Wang, Q.M. Novel templates directed synthesis of YVO4: Eu3+ (red) and Y2O3-SiO2: Tb3+ (green) phosphors. J. Lumin. 2012, 132, 2822–2825. [Google Scholar] [CrossRef]
- Cavalli, E.; Angiuli, F.; Belletti, A.; Boutinaud, P. Luminescence spectroscopy of YVO4: Ln3+, Bi3+ (Ln3+ = Eu3+, Sm3+, Dy3+) phosphors. Opt. Mater. 2014, 36, 1642–1648. [Google Scholar] [CrossRef]
- Tang, L.; Gui, W.J.; Ding, K.J.J.; Chen, N.; Du, G.P. Ion exchanged YVO4: Eu3+ nanocrystals and their strong luminescence enhanced by energy transfer of thenoyltrifluoroacetone ligands. J. Alloys Compd. 2014, 590, 277–282. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Zhang, D.; Wang, L.; Wei, G.; Zhu, P.; Kim, R. Enhanced Photoluminescence of Water Soluble YVO4: Ln3+ (Ln = Eu, Dy, Sm, and Ce) Nanocrystals by Ba2+ Doping. J. Phys. Chem. C 2008, 112, 17042–17045. [Google Scholar] [CrossRef]
- Park, K.C.; Mho, S. Photoluminescence properties of Ba3V2O8, Ba3(1−x) Eu2xV2O8 and Ba2Y2/3V2O8: Eu3+. J. Lumin. 2007, 122, 95–98. [Google Scholar] [CrossRef]
- de Sá, G.F.; Malta, O.L.; Donegá, C.D.; Simas, A.M.; Longo, R.L.; Santa-Cruz, P.A.; da Silva, E.F., Jr. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 2000, 196, 165–195. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Zhang, H.P.; Zu, G.N.; Wang, Z.T.; Fu, Y.H. Luminescence properties of rare earth complexes bonded to novel mesoporous spherical hybrid materials. J. Rare Earths 2023, 41, 60–66. [Google Scholar] [CrossRef]
- Wu, Y.F.S.; Chen, X.; Gong, Z.Y.; Tian, B.; Xue, E.B.; Zheng, K.; Liang, J.; Wu, W. Multi-wavelength excitation-dependent fluorescence with dynamic color gradients for information encryption and anti-counterfeiting. J. Mater. Chem. C 2024, 12, 11497–11505. [Google Scholar] [CrossRef]
- Brito, M.L.; Huband, S.; Walker, M.; Walton, R.I.; Filho, P.C.D. Nanoporous YVO4 as a luminescent host for probing molecular encapsulation. Chem. Commun. 2023, 59, 11393–11396. [Google Scholar] [CrossRef]
- Jia, G.; Zhang, C.; Ding, S.; Wang, L.; Li, L.; You, H. Synthesis and enhanced luminescence of uniform and well-dispersed quasispherical YVO4: Ln3+ (Ln = Eu, Dy) nanoparticles by a solvothermal method. CrystEngComm 2012, 14, 573–578. [Google Scholar] [CrossRef]
- Hsu, C.; Powell, R.C. Energy transfer in europium doped yttrium vanadate crystals. J. Lumin. 1975, 10, 273–293. [Google Scholar] [CrossRef]
- Meyssamy, H.; Riwotzki, K.; Kornowski, A.; Naused, S.; Haase, M. Wet-chemical synthesis of doped colloidal nanomaterials: Particles and fibers of LaPO4: Eu, LaPO4: Ce, and LaPO4: Ce, Tb. Adv. Mater. 1999, 11, 840–844. [Google Scholar] [CrossRef]
- Jia, G.; Song, Y.H.; Yang, M.; Liu, K.; Zheng, Y.H.; You, H.P. Facile synthesis and luminescence properties of octahedral YVO4: Eu3+ microcrystals. J. Cryst. Growth 2009, 311, 4213–4218. [Google Scholar] [CrossRef]
- Singh, N.S.; Ningthoujam, R.S.; Luwang, M.N.; Singh, S.D.; Vatsa, R.K. Luminescence, lifetime and quantum yield studies of YVO4: Ln3+ (Ln3+ = Dy3+, Eu3+) nanoparticles: Concentration and annealing effects. Chem. Phys. Lett. 2009, 480, 237–242. [Google Scholar] [CrossRef]
- Tang, L.; Chen, N. White light emitting YVO4: Eu3+, Tm3+, Dy3+ nanometer- and submicrometer-sized particles prepared by an ion exchange method. Ceram. Int. 2016, 42, 302–309. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, P.; Duan, C.; Yan, K.; Yin, M.; Lou, L.; Xia, S.; Krupa, J.-C. Preparation and size effect on concentration quenching of nanocrystalline Y2SiO5: Eu. Chem. Phys. Lett. 1998, 292, 133–136. [Google Scholar] [CrossRef]
- Wang, S.F.; Gu, F.; Lü, M.K.; Zhou, G.J.; Ai, Z.P.; Xu, D.; Yuan, D.R. Preparation and luminescent characteristics of Mn2+, Er3+ co-doped ZrO2 nanocrystals. J. Cryst. Growth 2003, 257, 84–88. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, N.; Du, G.; Jiang, C. Effect of Ba2+ doping on the photoluminescence of YVO4: Eu3+ phosphor and first principles calculations. J. Lumin. 2020, 222, 117117. [Google Scholar] [CrossRef]
- Zhan, Y.C.; Du, G.P.; Chen, N.; Li, Y.Y.; Liu, B.F.; Liu, G.H. Photoluminescence properties of YVO4: Eu3+, Ba2+ nanoparticles prepared by an ion exchange method. Mater. Sci. Semicond. Process. 2016, 41, 233–239. [Google Scholar] [CrossRef]
- Park, W.J.; Jung, M.K.; Yoon, D.H. Influence of Eu3+, Bi3+ co-doping content on photoluminescence Of YVO4 red phosphors induced by ultraviolet excitation. Sens. Actuators B-Chem. 2007, 126, 324–327. [Google Scholar] [CrossRef]
- Riwotzki, K.; Haase, M. Colloidal YVO4: Eu and YP0.95V0.05O4: Eu nanoparticles: Luminescence and energy transfer processes. J. Phys. Chem. B 2001, 105, 12709–12713. [Google Scholar] [CrossRef]
- Iso, Y.; Takeshita, S.; Isobe, T. Electrophoretic Deposition and Characterization of Transparent Nanocomposite Films of YVO4: Bi3+, Eu3+ Nanophosphor and Silicone-Modified Acrylic Resin. Langmuir 2014, 30, 1465–1471. [Google Scholar] [CrossRef]
- Su, J.G.; Mi, X.Y.; Sun, J.C.; Yang, L.X.; Hui, C.L.; Lu, L.P.; Bai, Z.H.; Zhang, X.Y. Tunable luminescence and energy transfer properties in YVO4: Bi3+, Eu3+ phosphors. J. Mater. Sci. 2017, 52, 782–792. [Google Scholar] [CrossRef]
- Tian, L.H.; Mho, S. Enhanced photoluminescence of YVO4: Eu3+ by codoping the Sr2+, Ba2+ or Pb2+ ion. J. Lumin. 2007, 122, 99–103. [Google Scholar] [CrossRef]
- Liao, Y.B.; Chen, N.; Du, G.P. Strong luminescence enhancement of YVO4: Eu3+, Ba2+ phosphors prepared by a solvothermal method. J. Alloys Compd. 2013, 561, 214–219. [Google Scholar] [CrossRef]
- Liao, Y.B.; Zhan, Y.C.; Chen, N.; Du, G.P. Effect of Sr2+ doping on the luminescence properties of YVO4: Eu3+, Sr2+ particles prepared by a solvothermal method. J. Sol-Gel Sci. Technol. 2013, 65, 353–358. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ling, W.J.; Lu, F.P. Luminescence properties of red phosphor YVO4: Eu3+, Ba2+. Optik 2023, 287, 171082. [Google Scholar] [CrossRef]
- Kumari, P.; Manam, J. Enhanced red emission on co-doping of divalent ions (M2+ = Ca2+, Sr2+, Ba2+) in YVO4: Eu3+ phosphor and spectroscopic analysis for its application in display devices. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2016, 152, 109–118. [Google Scholar] [CrossRef]
- Mho, S.-I.; Tian, L.; Park, K.-C.; Yeo, I.-H. 42.4: Enhanced Photoluminescence of (Y, A)V(O, S)4: Eu3+ (A = Sr, Ba) Compared with YVO4: Eu3+. SID Symp. Dig. Tech. Pap. 2005, 36, 1425–1427. [Google Scholar] [CrossRef]
- Ruan, F.P.; Deng, D.G.; Wu, M.; Chen, B.W.; Lei, R.S.; Xu, S.Q. Multichannel luminescence of Eu2+/Eu3+ Co-activated Ca9Mg1.5(PO4)7 phosphors for self-referencing optical thermometry. J. Lumin. 2019, 213, 117–126. [Google Scholar] [CrossRef]
- Ruan, F.P.; Deng, D.G.; Wu, M.; Wu, C.X.; Xu, S.Q. Tunable single-host full-color-emitting Ca9Zn1.5(PO4)7: Eu, Tb phosphor via Eu2+/Eu3+ dual-emitting. J. Lumin. 2018, 198, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).