Tuning Ag Loading and Particle Size in Ag@g-C3N4 Photocatalysts for Selective CO2 Conversion to CO and CH4
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Structural Characterization of Catalysts
2.3. Photocatalytic Performance Test
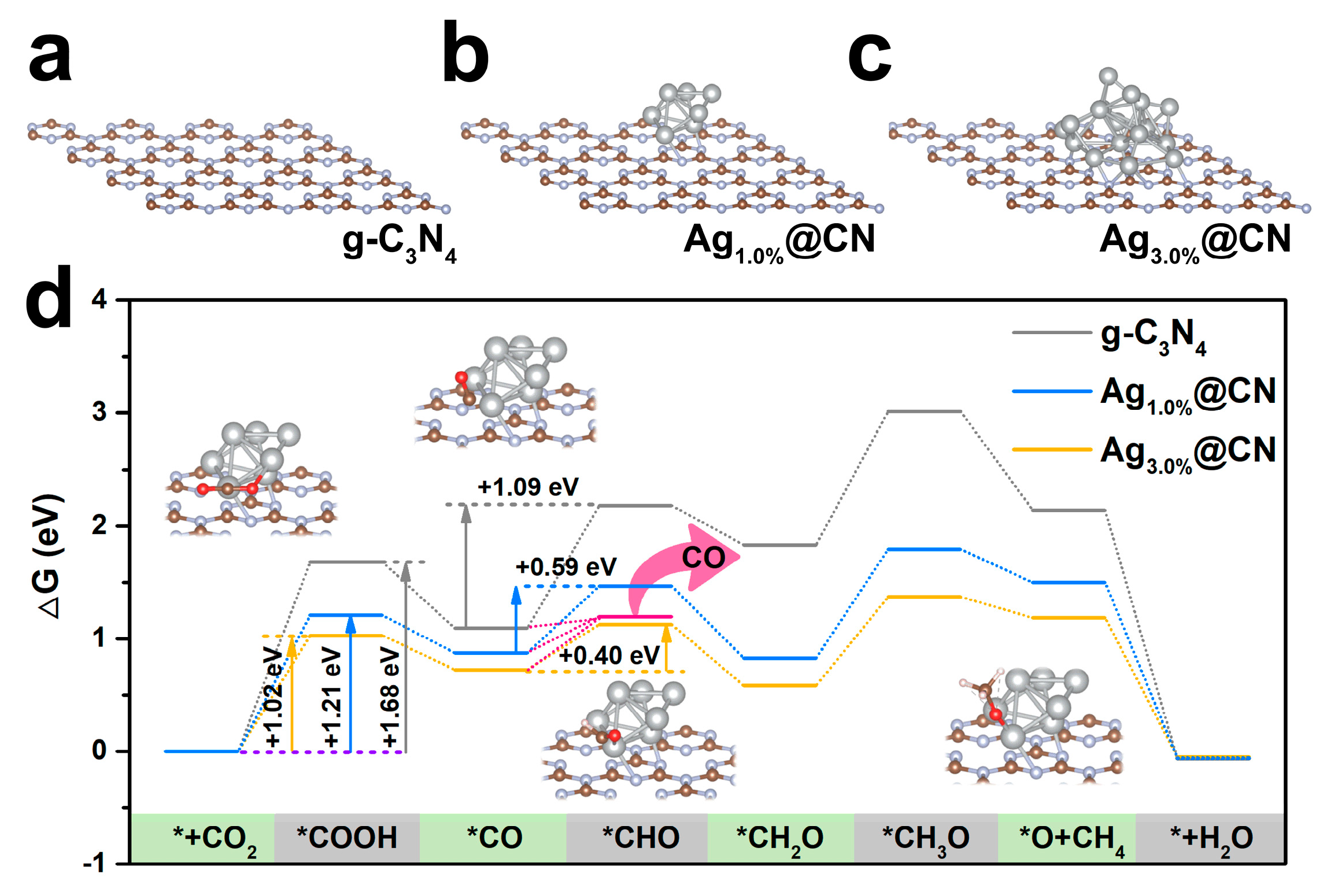
2.4. Density Functional Theory Calculations
3. Results and Discussion
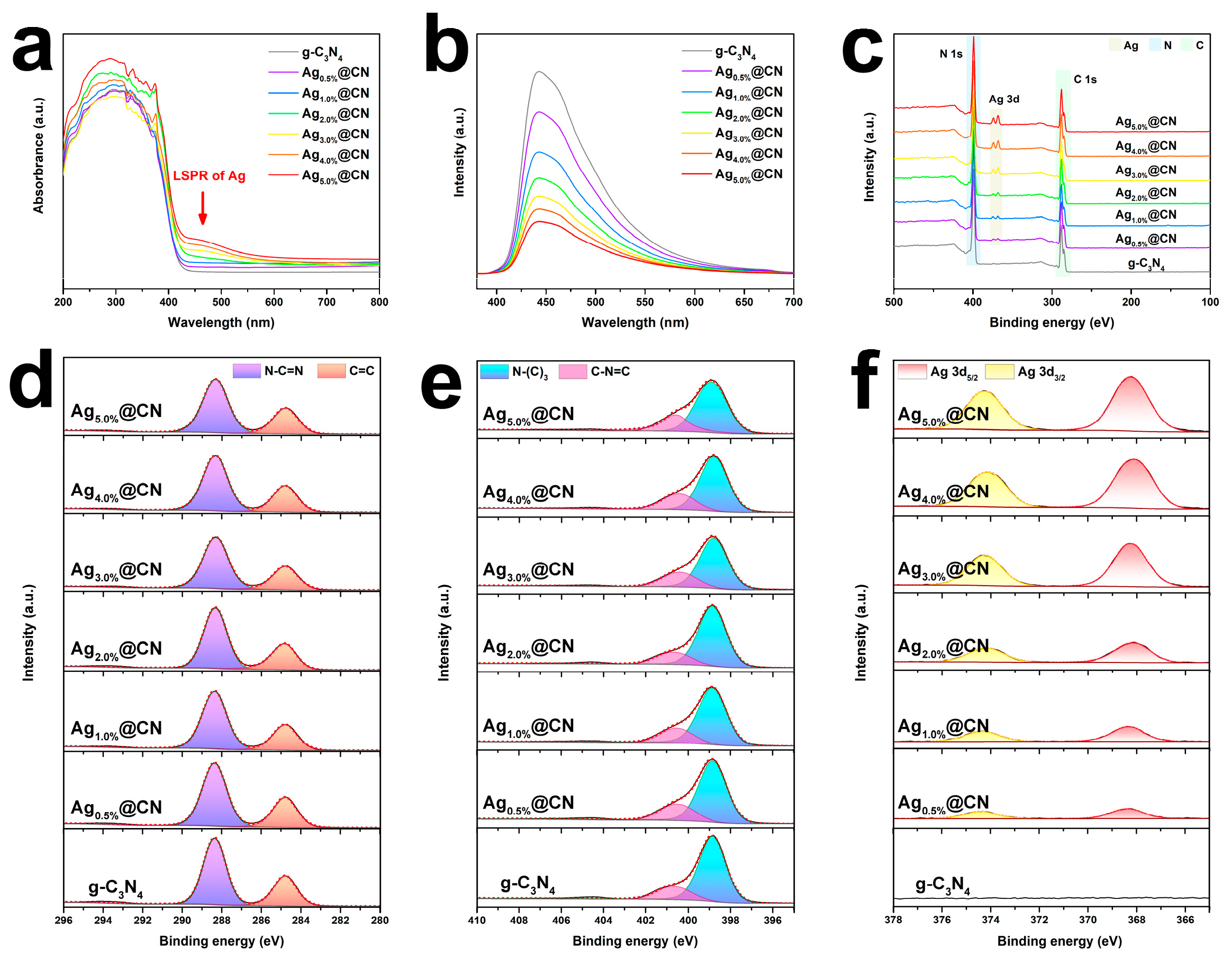
3.1. Structural Characteristics
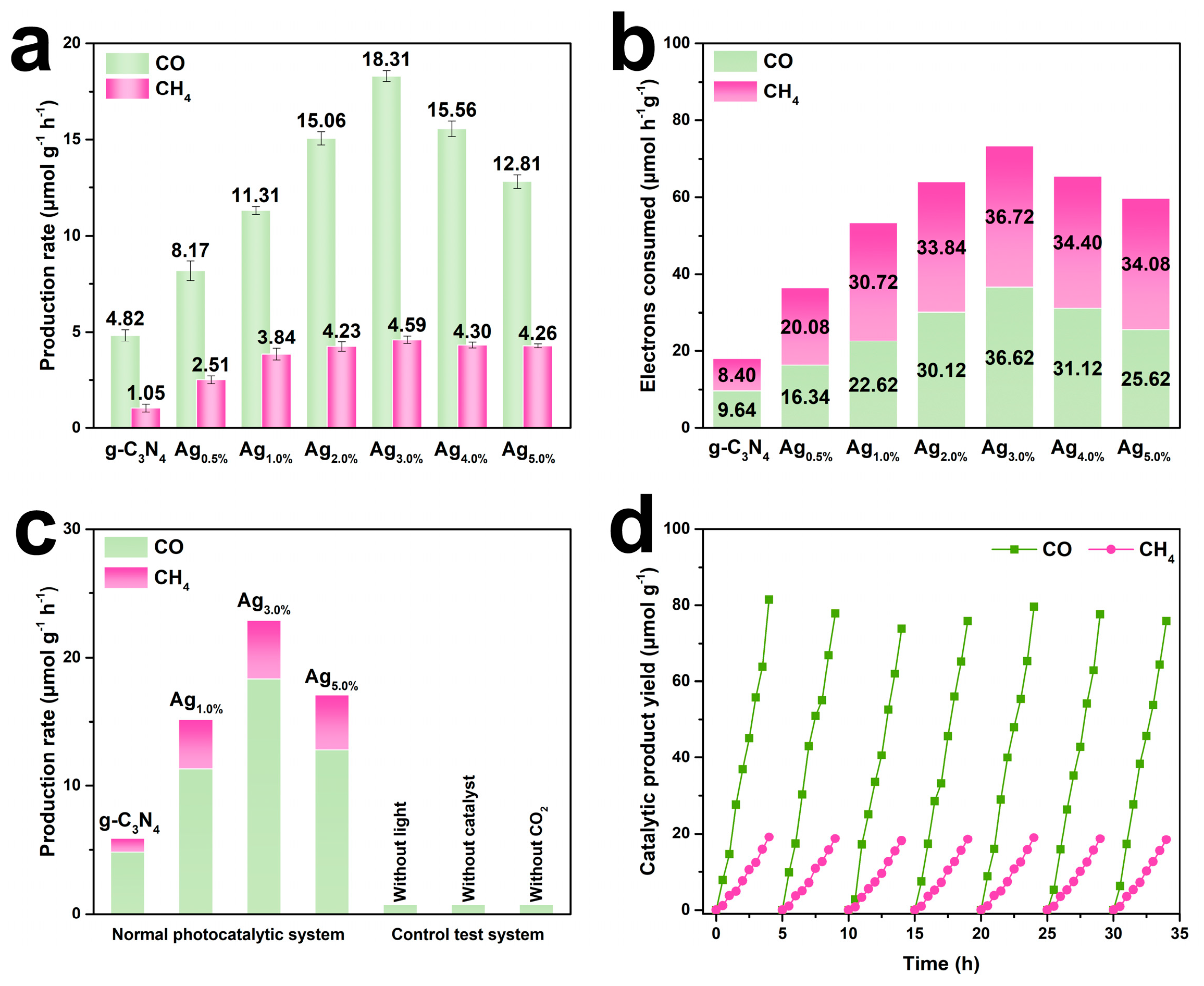
3.2. Photocatalytic Performance Evaluation
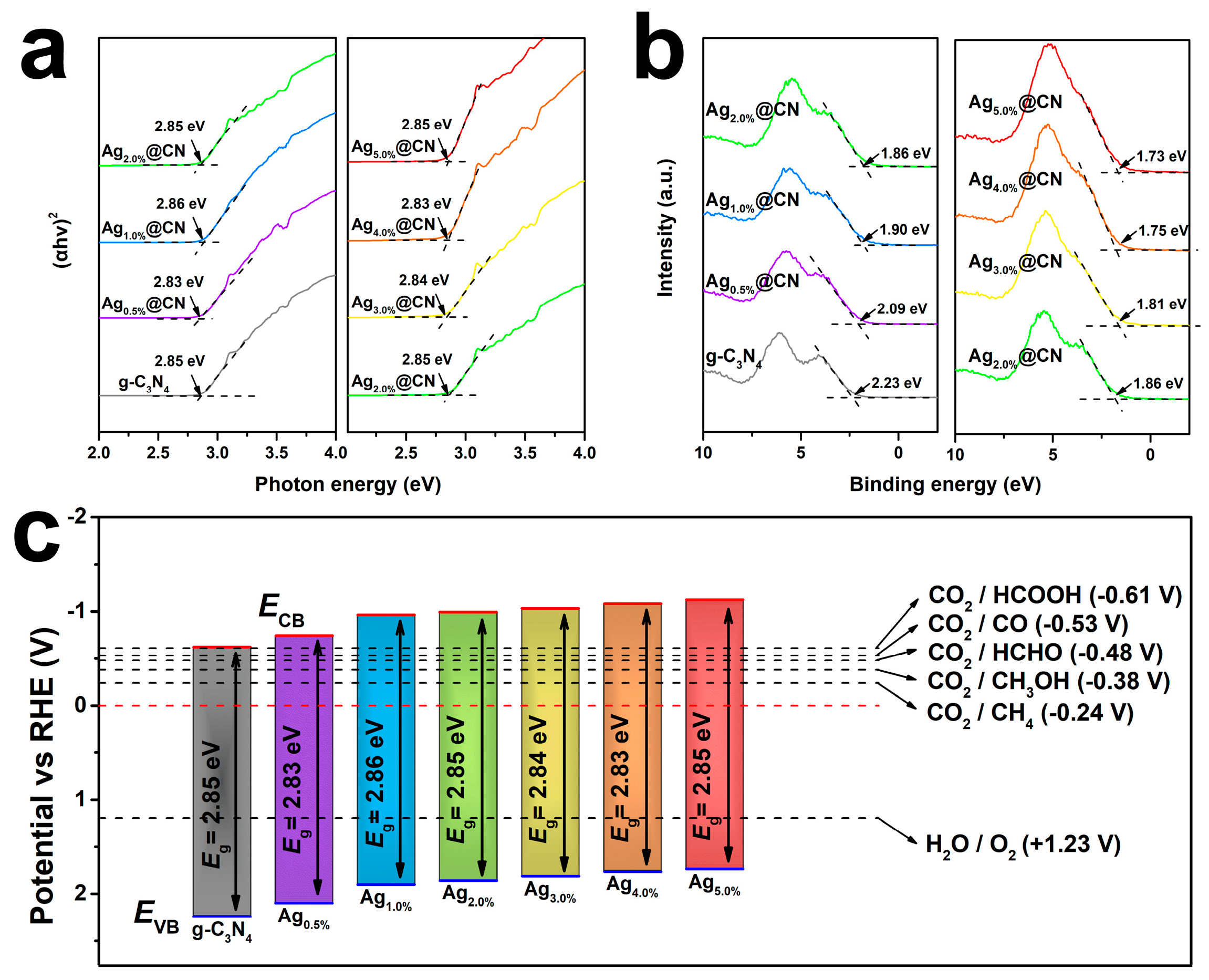
3.3. Electronic Structure and Band Modulation
3.4. DFT Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Pal, U. Interface engineered metal oxide heterojunction nanostructures in photocatalytic CO2 reduction: Progress and prospects. Coord. Chem. Rev. 2024, 516, 215967. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Liu, X.; Inagaki, S.; Gong, J. Heterogeneous Molecular Systems for Photocatalytic CO2 Reduction with Water Oxidation. Angew. Chem. Int. Ed. 2016, 55, 14924–14950. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, G.; Cometto, C.; Ma, B.; Zhao, H.; Groizard, T.; Chen, L.; Fan, H.; Man, W.-L.; Yiu, S.-M.; et al. Selectivity control of CO versus HCOO− production in the visible-light-driven catalytic reduction of CO2 with two cooperative metal sites. Nat. Catal. 2019, 2, 801–808. [Google Scholar] [CrossRef]
- Rao, H.; Schmidt, L.C.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Li, S.; Rao, H.; Qin, J.-s.; She, P.; Cheong, W.-C.; Jing, L. Recent Advances of Photocatalytic CO2 Reduction Based on Hybrid Molecular Catalyst/Semiconductor Photocatalysts: A Review. Small 2025, 21, 2408075. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Han, Y.; Ye, H.; Liu, R.; Lan, Y.; Xue, M.; Xie, X.; Yu, S.; Zhang, L.; et al. g-C3N4-based photocatalysts for organic pollutant removal: A critical review. Carbon Res. 2023, 2, 14. [Google Scholar] [CrossRef]
- Mao, L.; Zhai, B.; Wen, L.; Xiao, W.; Shi, J.; Kang, X.; Liu, Y.; Cheng, C.; Jin, H.; Guo, L. Simultaneous bulk and surface modifications of g-C3N4 via supercritical CO2 -assisted post-treatment towards enhanced photocatalytic activity. Appl. Catal. B Environ. Energy 2025, 362, 124712. [Google Scholar] [CrossRef]
- Yu, W.; You, X.; Wu, Y.; Li, C.; Wang, Q.; Yang, X.; Bian, X.; Lu, Y. Achieving highly efficient and selective CO2 photoreduction to CO over g-C3N4 via micro-environment optimization mediated by HZSM-5 zeolite integration. J. Environ. Chem. Eng. 2025, 13, 116271. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yu, Z.; Jiang, X.; Chen, J.; Tao, L.; Wang, M.; Shen, Y. Artificial photosynthesis of ethanol using type-II g-C3N4/ZnTe heterojunction in photoelectrochemical CO2 reduction system. Nano Energy 2019, 60, 827–835. [Google Scholar] [CrossRef]
- Ye, L.; Wu, D.; Chu, K.H.; Wang, B.; Xie, H.; Yip, H.Y.; Wong, P.K. Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2016, 304, 376–383. [Google Scholar] [CrossRef]
- Tang, J.; Guo, C.; Wang, T.; Cheng, X.; Huo, L.; Zhang, X.; Huang, C.; Major, Z.; Xu, Y. A review of g-C3N4-based photocatalytic materials for photocatalytic CO2 reduction. Carbon Neutralization 2024, 3, 557–583. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Zeng, X.; Xiao, F.; Fang, W.; Du, X.; He, X.; Wang, D.; Li, W.; Chen, H. Efficient photocatalytic reduction of CO2 by improving adsorption activation and carrier utilization rate through N-vacancy g-C3N4 hollow microtubule. Mater. Today Energy 2023, 31, 101211. [Google Scholar] [CrossRef]
- Song, Q.; Hu, J.; Zhou, Y.; Ye, Q.; Shi, X.; Li, D.; Jiang, D. Carbon vacancy-mediated exciton dissociation in Ti3C2Tx/g-C3N4 Schottky junctions for efficient photoreduction of CO2. J. Colloid Interface Sci. 2022, 623, 487–499. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Sun, Z.; Tang, Q.; Liu, Y.; Wang, L.; Wang, H.; Wu, Z. Enhanced CH4 selectivity in CO2 photocatalytic reduction over carbon quantum dots decorated and oxygen doping g-C3N4. Nano Res. 2019, 12, 2749–2759. [Google Scholar] [CrossRef]
- Park, J.; Liu, H.; Piao, G.; Kang, U.; Jeong, H.W.; Janáky, C.; Park, H. Synergistic conversion of CO2 into C1 and C2 gases using hybrid in-doped TiO2 and g-C3N4 photocatalysts. Chem. Eng. J. 2022, 437, 135388. [Google Scholar] [CrossRef]
- Que, M.; Cai, W.; Chen, J.; Zhu, L.; Yang, Y. Recent advances in g-C3N4 composites within four types of heterojunctions for photocatalytic CO2 reduction. Nanoscale 2021, 13, 6692–6712. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/covalent organic framework van der Waals heterojunction toward highly efficient S-scheme CO2 photoreduction. Appl. Catal. B Environ. 2022, 301, 120814. [Google Scholar] [CrossRef]
- Sun, J.; Bian, J.; Li, J.; Zhang, Z.; Li, Z.; Qu, Y.; Bai, L.; Yang, Z.-D.; Jing, L. Efficiently photocatalytic conversion of CO2 on ultrathin metal phthalocyanine/g-C3N4 heterojunctions by promoting charge transfer and CO2 activation. Appl. Catal. B Environ. 2020, 277, 119199. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Zhou, Y.; Cao, J.; Yuan, M.; Lv, H. Phosphorous doped g-C3N4 supported cobalt phthalocyanine: An efficient photocatalyst for reduction of CO2 under visible-light irradiation. J. Colloid Interface Sci. 2021, 594, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, S.; Wu, S.-Y.; Chen, H.-T.; Zada, A.; Linlin, L.; Ismail, A.; Ali, S.; Raziq, F.; Haider, M.; et al. Synergistic Functionality of Dopants and Defects in Co-Phthalocyanine/B-CN Z-Scheme Photocatalysts for Promoting Photocatalytic CO2 Reduction Reactions. Small 2023, 19, 2208179. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, C.; Xiao, L.; Chen, W.; Lu, W. Artificial light-harvesting 2D photosynthetic systems with iron phthalocyanine/graphitic carbon nitride composites for highly efficient CO2 reduction. Catal. Sci. Technol. 2021, 11, 5952–5961. [Google Scholar] [CrossRef]
- Ji, Y.C.; Yang, R.Q.; Wang, L.W.; Song, G.X.; Wang, A.Z.; Lv, Y.W.; Gao, M.M.; Zhang, J.; Yu, X. Visible light active and noble metal free Nb4N5/TiO2 nanobelt surface heterostructure for plasmonic enhanced solar water splitting. Chem. Eng. J. 2020, 402, 126226. [Google Scholar] [CrossRef]
- Deng, A.; Zhao, E.; Li, Q.; Sun, Y.; Liu, Y.; Yang, S.; He, H.; Xu, Y.; Zhao, W.; Song, H.; et al. Atomic Cobalt–Silver Dual-Metal Sites Confined on Carbon Nitride with Synergistic Ag Nanoparticles for Enhanced CO2 Photoreduction. ACS Nano 2023, 17, 11869–11881. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Li, H.; Li, H.; Qi, W.; Zhang, Q.; Zhu, J.; Zhao, P.; Yang, S. Accelerating photogenerated charge kinetics via the g-C3N4 Schottky junction for enhanced visible-light-driven CO2 reduction. Appl. Catal. B Environ. 2022, 318, 121863. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Wang, D.; Jin, C.; Fang, L.; Li, X.; Jiang, Y.; Wang, X.; Tian, C. Coordination-induced in situ confinement of small-sized Ag nanoparticles on ultrathin C3N4 with strong metal–support interaction for enhanced selective CO2 photoreduction. Inorg. Chem. Front. 2025. [Google Scholar] [CrossRef]
- Hu, S.; Qiao, P.; Yi, X.; Lei, Y.; Hu, H.; Ye, J.; Wang, D. Selective Photocatalytic Reduction of CO2 to CO Mediated by Silver Single Atoms Anchored on Tubular Carbon Nitride. Angew. Chem. Int. Ed. 2023, 62, e202304585. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, M.; Qu, Z.H.; Cui, X.; Liu, Z.Y.; Piao, C.C.; Li, S.G.; Wang, J.; Song, Y.T. Fabrication of highly active Z-scheme Ag/g-C3N4-Ag-Ag3PO4 (110) photocatalyst photocatalyst for visible light photocatalytic degradation of levofloxacin with simultaneous hydrogen production. Chem. Eng. J. 2020, 382, 122394. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.M.; Cao, X.R.; Wei, X.; Cao, J.Y.; Lin, K.X.; Zhou, Y.Y.; Ai, Y.J. Hydrogenated Si-doped g-C3N4: Promising electrocatalyst for CO2 capture and conversion. Appl. Surf. Sci. 2023, 614, 156195. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, F.X.; Zheng, L.R.; Wang, H.F.; Yang, X.H.; Yang, H.G. Tuning Metal Catalyst with Metal-C3N4 Interaction for Efficient CO2 Electroreduction. ACS Catal. 2018, 8, 11035–11041. [Google Scholar] [CrossRef]
- Omr, H.A.E.; Putikam, R.; Hussien, M.K.; Sabbah, A.; Lin, T.Y.; Chen, K.H.; Wu, H.L.; Feng, S.P.; Lin, M.C.; Lee, H. Design of sculptured SnS/g-C3N4 photocatalytic nanostructure for highly efficient and selective CO2 conversion to methane. Appl. Catal. B-Environ. Energy 2023, 324, 122231. [Google Scholar] [CrossRef]
- Dai, Y.X.; Wang, Y.T.; Zuo, G.C.; Kong, J.J.; Guo, Y.; Sun, C.; Xian, Q.M. Photocatalytic degradation mechanism of phenanthrene over visible light driven plasmonic Ag/Ag3PO4/g-C3N4 heterojunction nanocomposite. Chemosphere 2022, 293, 133575. [Google Scholar] [CrossRef]
- Zhuang, C.Q.; Chang, Y.; Li, W.M.; Li, S.J.; Xu, P.; Zhang, H.; Zhang, Y.H.; Zhang, C.; Gao, J.F.; Chen, G.; et al. Light-Induced Variation of Lithium Coordination Environment in g-C3N4 Nanosheet for Highly Efficient Oxygen Reduction Reactions. ACS Nano 2024, 18, 5206–5217. [Google Scholar] [CrossRef]
- Sun, L.L.; Feng, Y.B.; Ma, K.; Jiang, X.H.; Gao, Z.Y.; Wang, J.G.; Jiang, N.; Liu, X.S. Synergistic effect of single-atom Ag and hierarchical tremella-like g-C3N4: Electronic structure regulation and multi-channel carriers transport for boosting photocatalytic performance. Appl. Catal. B-Environ. Energy 2022, 306, 121106. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Q.; Wen, D.; Wu, C.; Pan, Y.; Liu, X.; Huang, Z.; Li, N. Particle Size-Dependent Charge Transfer Dynamics for Boosting CO2 Photoreduction over Ag/TiO2 Heterojunction. ACS Catal. 2024, 14, 8105–8115. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, Z.Z.; Wang, J.; Liu, H.L.; Liu, P.; Hu, D.; Tan, T.X.; Wang, C. Ultra-stable oxygen species in Ag nanoparticles anchored on g-C3N4 for enhanced electrochemical reduction of CO2. Electrochim. Acta 2021, 390, 138831. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, L.Y.; Cheng, B.; Fan, J.J.; Yu, J.G. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhang, P.; Jiang, S.J.; Wu, X.; Song, S.Q.; Zhu, M.S.; Lou, Z.Z.; Li, Z.; Liu, F.; Liu, Y.H.; et al. Photocatalytic reduction elimination of UO22+ pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst. Appl. Catal. B-Environ. 2017, 200, 378–385. [Google Scholar] [CrossRef]
- Ghafoor, S.; Inayat, A.; Aftab, F.; Duran, H.; Kirchhoff, K.; Waseem, S.; Arshad, S.N. TiO2 nanofibers embedded with g-C3N4 nanosheets and decorated with Ag nanoparticles as Z-scheme photocatalysts for environmental remediation. J. Environ. Chem. Eng. 2019, 7, 103452. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Li, M.; Liu, Y. Annealing temperature effects on monolayer WS(2)-veiled Ag nanoparticle array for surface catalytic reaction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 303, 123137. [Google Scholar] [CrossRef]
- Tafreshi, S.S.; Moshfegh, A.Z.; de Leeuw, N.H. Mechanism of Photocatalytic Reduction of CO2 by Ag3PO4(111)/g-C3N4 Nanocomposite: A First-Principles Study. J. Phys. Chem. C 2019, 123, 22191–22201. [Google Scholar] [CrossRef]
- Shen, R.C.; Zhang, L.P.; Chen, X.Z.; Jaroniec, M.; Li, N.; Li, X. Integrating 2D/2D CdS/α-Fe2O3 ultrathin bilayer Z-scheme heterojunction with metallic β-NiS nanosheet-based ohmic junction for efficient photocatalytic H2 evolution. Appl. Catal. B-Environ. 2020, 226, 118619. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, C.P.; Wu, S.H.; Li, X.; Chen, Y.J.; Yang, W.L. Construction of Built-In Electric Field within Silver Phosphate Photocatalyst for Enhanced Removal of Recalcitrant Organic Pollutants. Adv. Funct. Mater. 2020, 30, 2002918. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, S.; Wu, L.Q.; Li, M.J.; Chong, Y.A.; Qiu, Y.C.; Chen, G.X.; Zhao, Y.; Feng, C.H.; Ye, D.Q.; et al. Strain-Engineering of Mesoporous Cs3Bi2Br9/BiVO4 S-Scheme Heterojunction for Efficient CO2 Photoreduction. Small 2023, 19, e2302058. [Google Scholar] [CrossRef]
- Zheng, Z.; Du, T.; Chen, P.; Yue, Q.; Wang, H.; Zhou, L.; Wang, Y. 2D/1D nested hollow porous ZnIn2S4/g-C3N4 heterojunction based on morphology modulation for photocatalytic CO2 reduction. J. Environ. Chem. Eng. 2024, 12, 112971. [Google Scholar] [CrossRef]
- Zhang, S.W.; Feng, H.N.; Li, C.Y.; Cao, X.D.; Li, H.; Wu, Y. The reduction mechanism of C1 product from carbon dioxide catalyzed by Ni-doped g-C3N4. Mol. Catal. 2024, 559, 114064. [Google Scholar] [CrossRef]
- Kawai, K.; Narushima, T.; Kaneko, K.; Kawakami, H.; Matsumoto, M.; Hyono, A.; Nishihara, H.; Yonezawa, T. Synthesis and antibacterial properties of water-dispersible silver nanoparticles stabilized by metal-carbon σ-bonds. Appl. Surf. Sci. 2012, 262, 76–80. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Vidal-López, A.; Solá, M.; Poater, A. 2D carbon nitride as a support with single Cu, Ag, and Au atoms for carbon dioxide reduction reaction. Phys. Chem. Chem. Phys. 2023, 25, 8574–8582. [Google Scholar] [CrossRef] [PubMed]

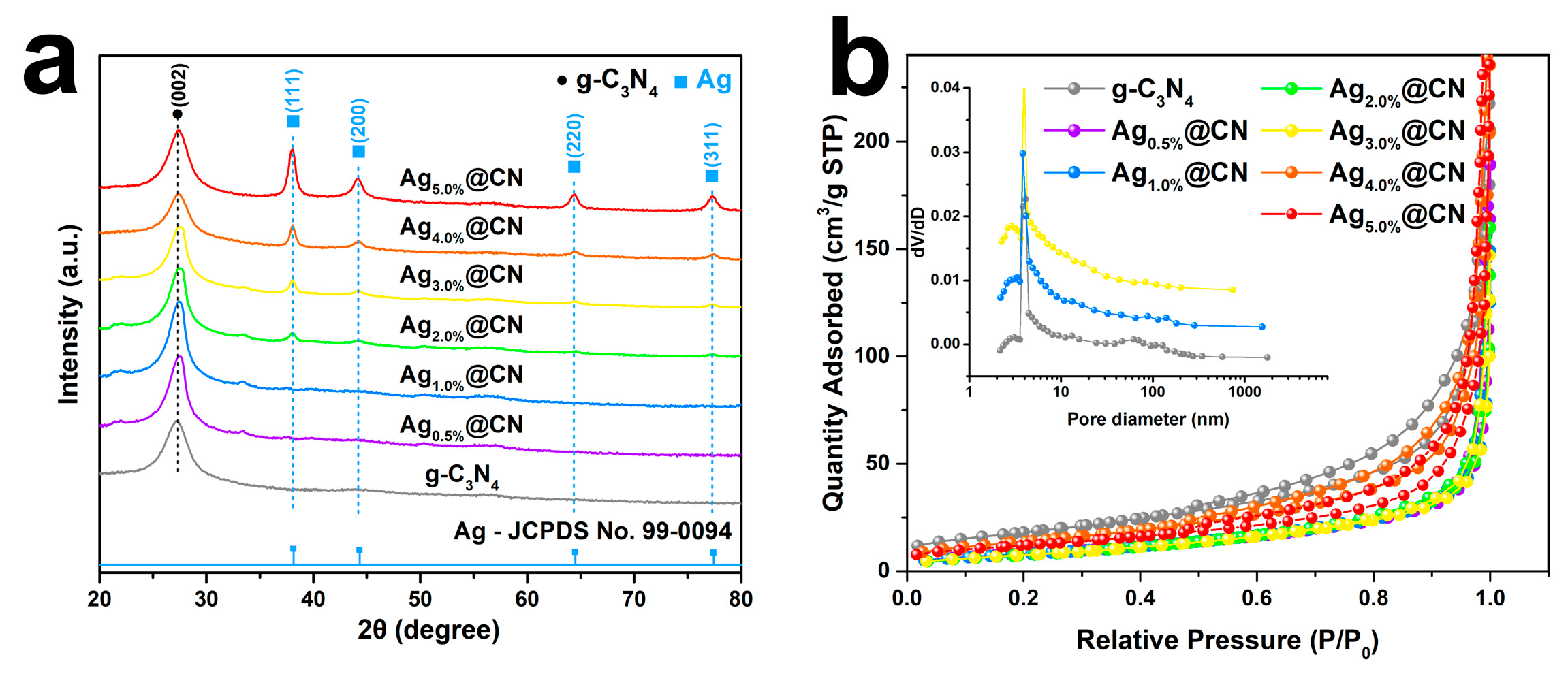
| Catalyst | SBET (m2 g−1) | Vpore (cm3 g−1) | Dpore (nm) |
|---|---|---|---|
| g-C3N4 | 65.629 | 0.222 | 13.532 |
| Ag0.5%@CN | 53.394 | 0.177 | 12.450 |
| Ag1.0%@CN | 43.476 | 0.139 | 11.596 |
| Ag2.0%@CN | 47.153 | 0.156 | 12.359 |
| Ag3.0%@CN | 49.746 | 0.185 | 12.526 |
| Ag4.0%@CN | 50.751 | 0.191 | 13.311 |
| Ag5.0%@CN | 52.744 | 0.204 | 13.543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, N.; Zhou, Q. Tuning Ag Loading and Particle Size in Ag@g-C3N4 Photocatalysts for Selective CO2 Conversion to CO and CH4. Nanomaterials 2025, 15, 1443. https://doi.org/10.3390/nano15181443
Liu S, Li N, Zhou Q. Tuning Ag Loading and Particle Size in Ag@g-C3N4 Photocatalysts for Selective CO2 Conversion to CO and CH4. Nanomaterials. 2025; 15(18):1443. https://doi.org/10.3390/nano15181443
Chicago/Turabian StyleLiu, Shicheng, Na Li, and Qulan Zhou. 2025. "Tuning Ag Loading and Particle Size in Ag@g-C3N4 Photocatalysts for Selective CO2 Conversion to CO and CH4" Nanomaterials 15, no. 18: 1443. https://doi.org/10.3390/nano15181443
APA StyleLiu, S., Li, N., & Zhou, Q. (2025). Tuning Ag Loading and Particle Size in Ag@g-C3N4 Photocatalysts for Selective CO2 Conversion to CO and CH4. Nanomaterials, 15(18), 1443. https://doi.org/10.3390/nano15181443






