3.1. Antibacterial Activity of SeNPs
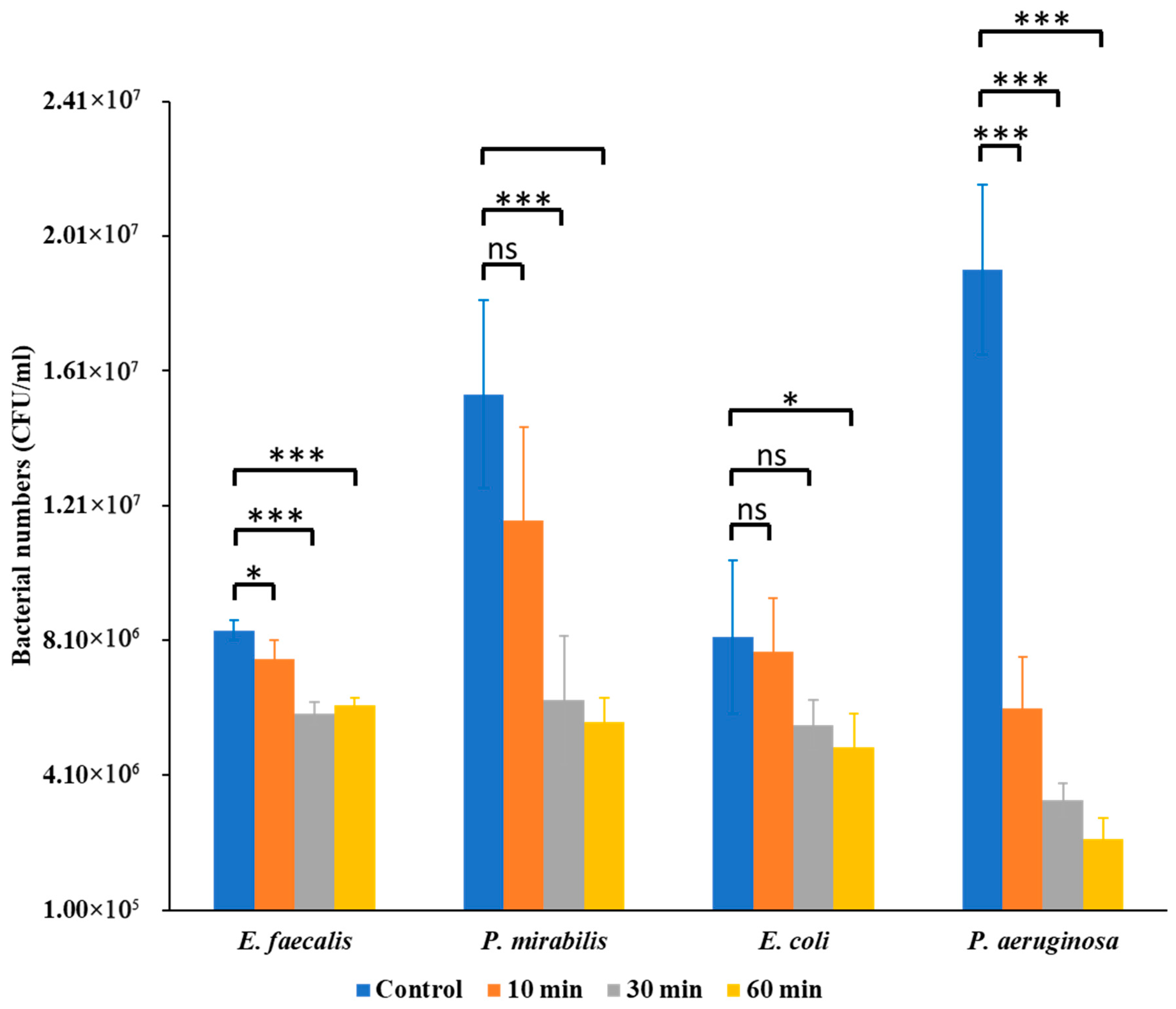
The antibacterial effects of Se NPs varied significantly among the tested uropathogens, revealing species-specific differences in susceptibility. Bacterial enumeration assays revealed that Se NPs exerted a notable inhibitory effect on bacterial CFU counts, but the magnitude of inhibition differed among species (
Figure 1).
E. faecalis showed moderate reductions at 30 min (
p < 0.05) and stronger inhibition at 60 min (
p < 0.01).
P. mirabilis was more resilient, with no significant effect at 10 min but significant decreases at 30 min (
p < 0.01) and 60 min (
p < 0.001).
E. coli exhibited progressive inhibition, significant at 30 min (
p < 0.05) and 60 min (
p < 0.01).
P. aeruginosa was the most susceptible, showing highly significant reductions at 10, 30, and 60 min (
p < 0.001 for all). In all cases, positive control samples showed significantly higher CFU counts than Se NP-treated samples.
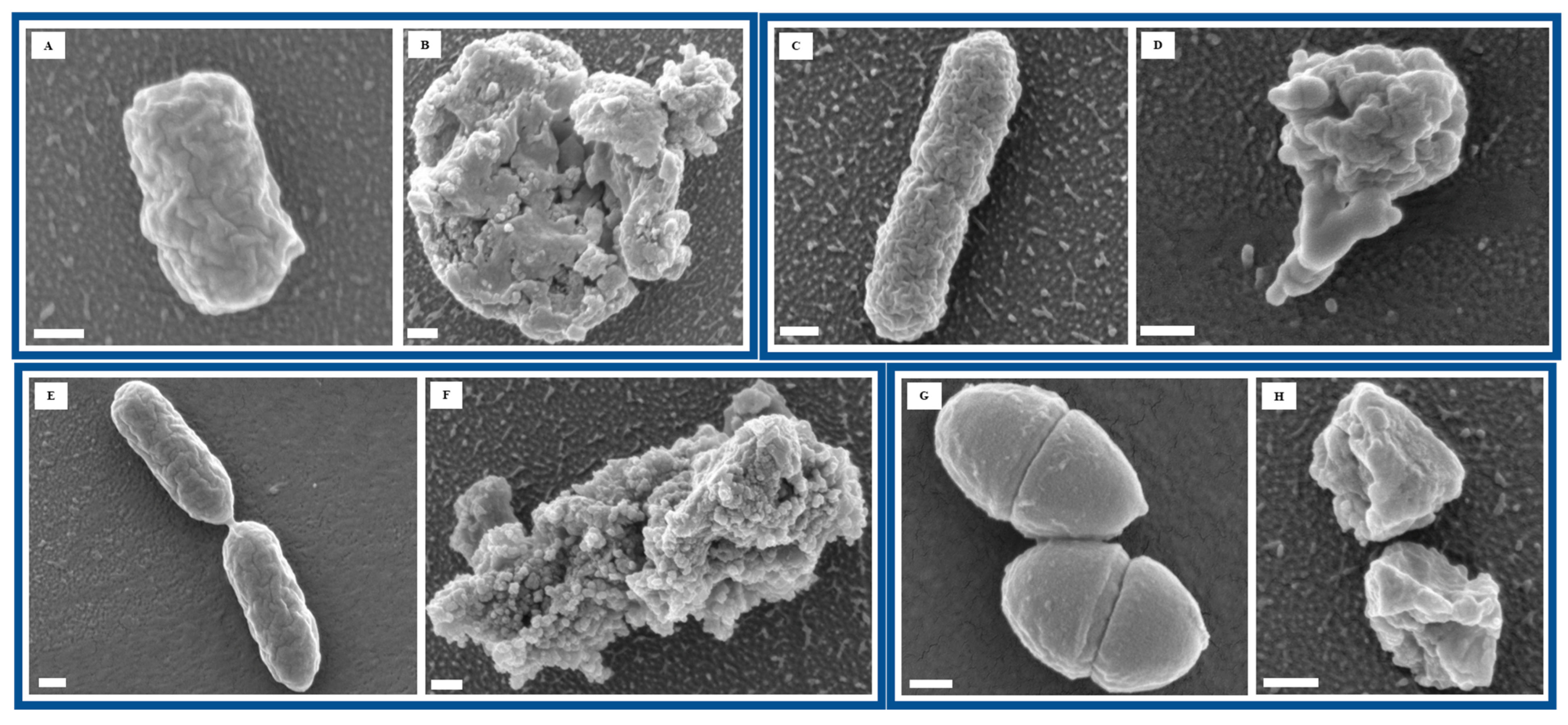
To further assess the impact of Se NP exposure on bacterial morphology, we performed FESEM to visualize structural changes at the cellular level. Imaging of untreated bacterial cells revealed smooth surfaces and intact cellular structures, indicative of healthy bacterial morphology (
Figure 2A,C,E,G). However, after 60 min of Se NP exposure, all four bacterial species exhibited varying degrees of structural damage, suggesting significant morphological alterations in response to NP-induced stress (
Figure 2B,D,F,H). Such morphological disruption points to bacterial membranes as a key target of Se NP action, leading to loss of integrity, leakage of intracellular contents, and eventual cell death.
These findings are consistent with prior reports that have demonstrated the broad-spectrum antibacterial potential of Se NPs. Shakibaie et al. showed that biogenic Se NPs inhibited
P. aeruginosa and
S. aureus growth, attributing their effects to oxidative stress and membrane damage [
37]. Xu et al. reported that Se NPs penetrated bacterial membranes, triggering ROS-mediated toxicity [
38]. More recently, Lin et al. confirmed Se NP-induced ROS generation and membrane disruption in
S. aureus [
39].
Taken together, these results reinforce the antibacterial potential of SeNPs while emphasizing that their efficacy is pathogen-dependent. The morphological and quantitative evidence provided here adds to the growing body of literature supporting SeNPs as promising antimicrobial candidates.
3.3. COG Functional Analysis
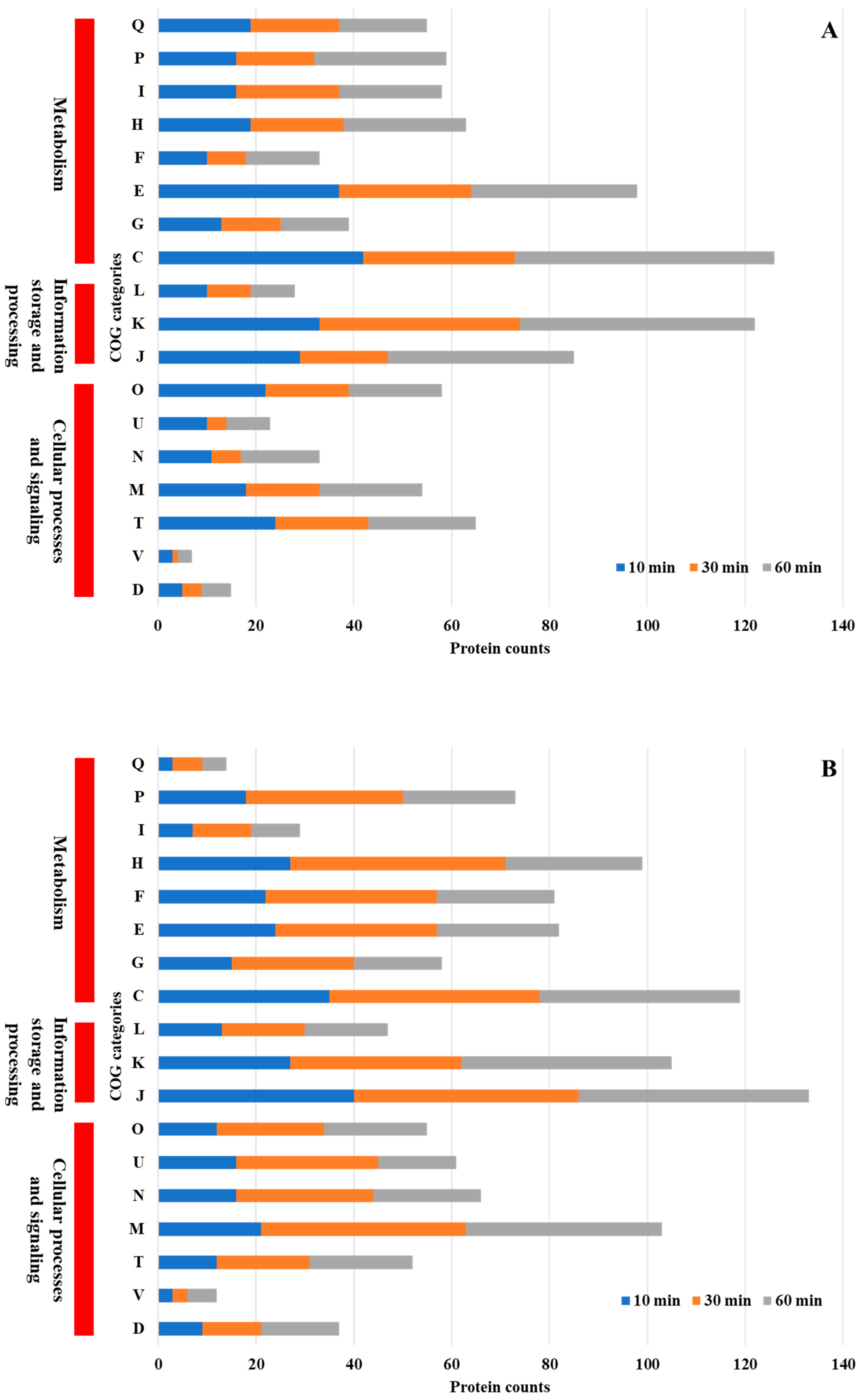
COG functional analysis of differentially expressed proteins revealed both shared and unique adaptive responses in
P. aeruginosa and
P. mirabilis following Se NP treatment (
Figure 4A,B). Both
P. aeruginosa and
P. mirabilis exhibited significant alterations in protein expression related to core cellular functions, including energy production and conversion, transcriptional regulation, amino acid metabolism, and protein synthesis. These shared metabolic responses suggest that both species employ conserved strategies to maintain cellular homeostasis under NP-induced stress. Enhanced expression of proteins involved in energy metabolism and transcriptional regulation indicates that both bacteria activate stress response pathways that promote adenosine triphosphate (ATP) production and transcriptional reprogramming to sustain viability under adverse conditions.
Despite these similarities, species-specific adaptations were evident.
P. mirabilis uniquely upregulated proteins associated with cell wall and membrane biogenesis. The enhanced expression of proteins involved in cell envelope synthesis suggests that
P. mirabilis employs a protective strategy that strengthens its outer membrane and reduces NP permeability, thereby minimizing cellular disruption [
40].
A comparison with prior research revealed both conserved and species-specific responses to NP-induced stress. For example,
P. aeruginosa exposed to copper stress exhibited significant changes in inorganic ion transport and metabolism (P), energy production and conversion (C), amino acid transport and metabolism (E), cell wall/membrane/envelope biogenesis (M), and translation, ribosomal structure, and biogenesis (J) [
41]. Similarly, when
Streptococcus suis was exposed to silver NPs, key adaptive responses included translation, ribosomal structure, and biogenesis (J), cell wall/membrane/envelope biogenesis (M), amino acid transport and metabolism (E), transcription (K), and carbohydrate transport and metabolism (G) [
42]. Likewise,
Xanthomonas campestris pv. campestris under copper stress exhibited significant differential regulation of inorganic ion transport and metabolism (P), carbohydrate transport and metabolism (G), amino acid transport and metabolism (E), transcription (K), and replication, recombination, and repair (L) [
43]. The observed similarities with bacterial responses to other NPs and heavy metals suggest that certain stress-adaptive mechanisms are conserved across species, while others are tailored to individual bacterial physiology.
3.4. KEGG Pathway Analysis
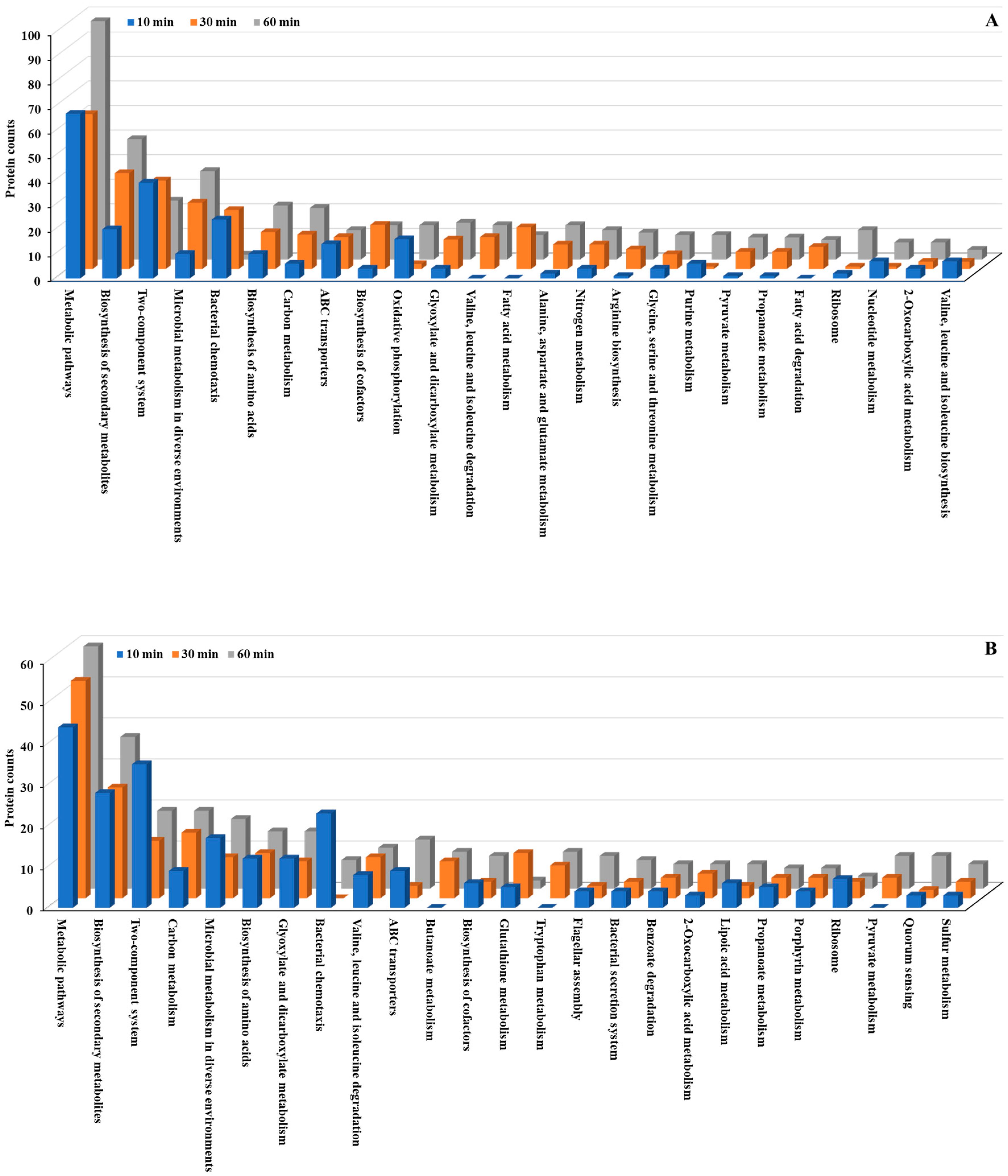
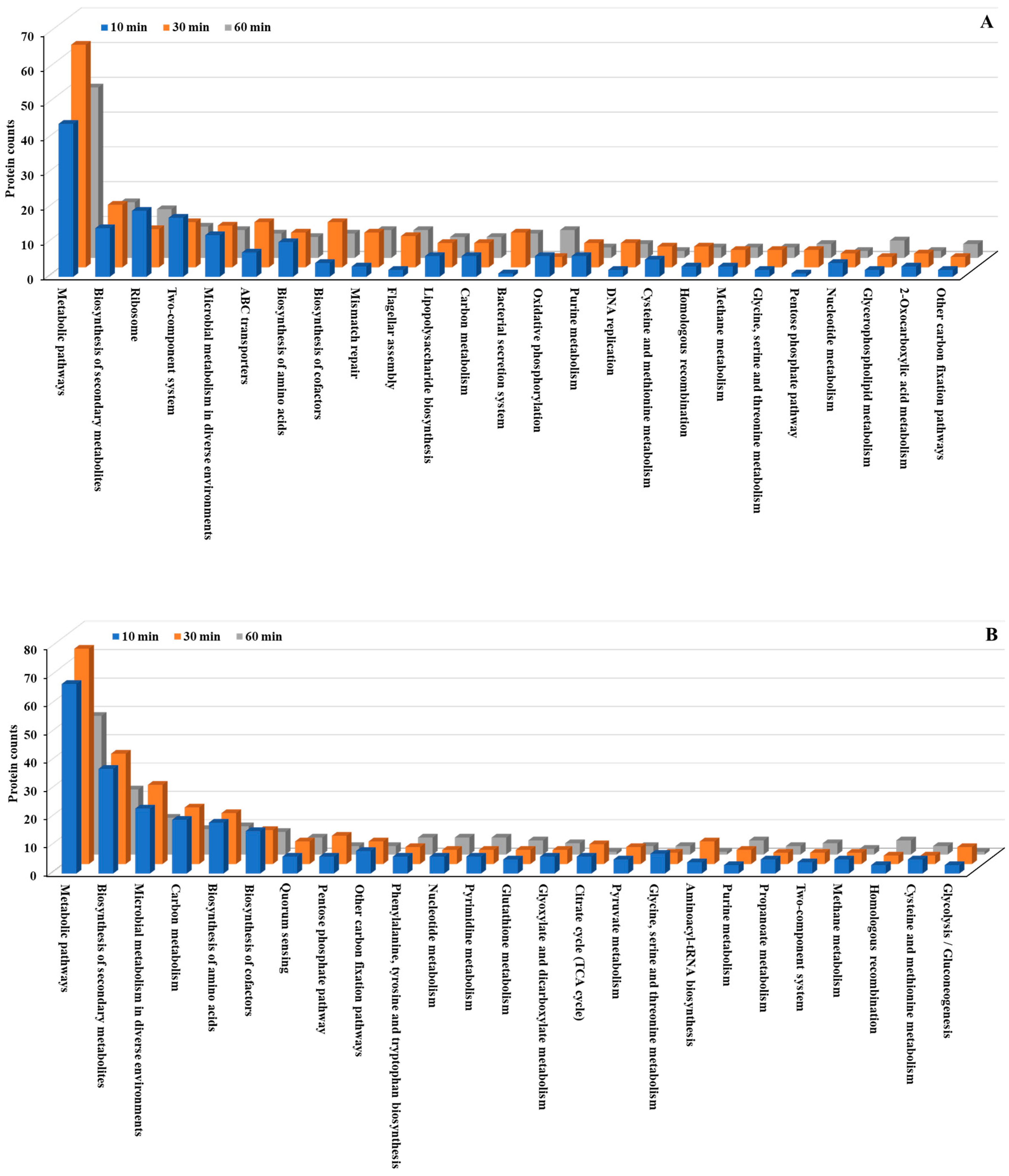
We conducted KEGG pathway analysis to further investigate the molecular mechanisms underlying the bacterial stress response to Se NP exposure. This analysis revealed significant alterations in metabolic pathways across both
P. aeruginosa and
P. mirabilis (
Figure 5 and
Figure 6;
Tables S2, S3, S8 and S9). Metabolic pathways, including biosynthesis of secondary metabolites, microbial metabolism in diverse environments, and biosynthesis of amino acids, exhibited the most significant changes, with both upregulated and downregulated proteins in both bacteria. The extensive involvement of these pathways suggests that Se NP exposure induces a global metabolic shift in both species, requiring the reallocation of cellular resources to support survival under oxidative and structural stresses.
Beyond these fundamental cellular processes, several key pathways were markedly overexpressed in both species. Specifically, multiple two-component regulatory systems, along with pathways related to bacterial chemotaxis, flagellar assembly, and ATP-binding cassette (ABC) transporters, were significantly upregulated. These pathways are essential for bacterial sensing, mobility, and active transport [
44], suggesting that Se NP exposure prompts both species to enhance environmental sensing and resource allocation mechanisms. This pattern underscores the bacteria’s ability to detect and respond to stress stimuli, regulate cellular motility, and manage transmembrane transport processes, all of which are crucial for mitigating NP-induced toxicity.
The two-component system is a highly conserved bacterial signaling mechanism that enables cells to sense environmental changes and rapidly trigger appropriate regulatory responses [
45]. In both
P. aeruginosa and
P. mirabilis, Se NP treatment resulted in the upregulation of two-component system-associated proteins, including AlgB, AlgR, CcoN, CydA, CpxA, DctA, FlrC, GlnL, GlnR, NarG, PhoB, PhoR, and RegB. These proteins play essential roles in bacterial stress adaptation by modulating gene expression in response to such external stimuli as oxidative stress, metal ion toxicity, and membrane damage [
46].
These findings align with previous findings. For example, Filipović et al. found that SeNPs with different surface chemistries induce distinct bacterial stress responses, including membrane-targeted effects, in
E. coli and
P. aeruginosa [
12]. Moreover, Vahdati and Moghadam reported synergistic antibacterial effects when SeNPs were combined with lysozyme, among mechanisms that include disruption of bacterial signaling and cell envelope integrity [
47].
The observed upregulation of these components suggests that Se NP exposure activates a broad stress-responsive signaling network, enabling bacteria to dynamically regulate gene expression and cellular functions to enhance survival. This response is consistent with findings from previous studies on bacterial adaptation to environmental stressors. For example,
Salmonella Typhimurium exposed to high-intensity ultrasound exhibited significant upregulation of two-component system proteins [
48]. Similarly,
P. aeruginosa treated with antibiotics showed a comparable upregulation of two-component system components, suggesting that both antibiotic- and NP-induced stresses may elicit overlapping bacterial response mechanisms [
49]. Additional evidence from studies on
Staphylococcus epidermidis under tigecycline treatment and
S. mutans under bacitracin stress further supports the critical role of this pathway in bacterial survival under diverse environmental challenges [
50,
51].
Chemotaxis and motility are crucial bacterial behaviors that facilitate movement toward favorable environments and away from harmful stressors [
52]. In response to Se NP exposure, proteins associated with bacterial chemotaxis, including CheW, CheZ, FliG, FliM, Mcp, MotA, and RbsB, exhibited significant upregulation. Similarly, flagellar assembly proteins such as FlgB, FlgD, FlgF-H, FlgJ, FlgM, FliD, FliF-H, FliK, FliM, and FlrC were also overexpressed. This widespread upregulation suggests that enhanced bacterial motility is a key survival strategy for evading toxic NP interactions.
Previous studies have demonstrated that bacterial motility is a crucial adaptive mechanism under stress conditions. For instance,
P. aeruginosa exposed to antibiotics exhibited a significant increase in bacterial chemotaxis gene expression, reinforcing the role of motility in bacterial adaptation [
49]. Additionally,
Natranaerobius thermophilus relied on bacterial chemotaxis to survive high-salt conditions, suggesting that motility plays a pivotal role in microbial stress responses across diverse bacterial species [
53].
Bacterial cells also upregulated membrane transport mechanisms in response to Se NP-induced stress. ABC transporters, a superfamily of integral membrane proteins, play a vital role in nutrient uptake, efflux of toxic compounds, and overall bacterial homeostasis by utilizing ATP hydrolysis to transport a wide array of substrates [
54]. After Se NP treatment, ABC transporter-associated proteins, including FtsX, LptB, MlaB, PotD, and ZnuA, were significantly upregulated, suggesting that bacteria prioritize transmembrane transport processes to mitigate NP-induced stress. Similarly, bacterial secretion system proteins, such as SecB, ShlA, TatA, TatB, VgrG, and YajC, were overexpressed, further supporting the hypothesis that Se NP exposure triggers an increase in transmembrane transport activity.
ABC transporters have been widely implicated in bacterial stress responses. In
Brucella melitensis, ABC transporters were upregulated under salt stress to maintain osmotic balance, and in
S. epidermidis, ABC transporter proteins were overexpressed in response to antibiotics, highlighting their importance in antimicrobial resistance mechanisms [
50,
55]. Furthermore,
S. mutans exposed to theaflavins showed significant upregulation of membrane transport pathways, supporting the importance of transport mechanisms in bacterial adaptation to environmental challenges [
56]. Interestingly, some studies have reported the downregulation of ABC transporters under acid stress, as observed in
Lactiplantibacillus plantarum, where ABC transporter pathways were suppressed in response to pH changes [
57], suggesting that bacterial responses to environmental stressors are context-dependent.
3.5. Expression Pattern (EPN) Analysis
Our EPN analysis revealed distinct yet overlapping adaptive responses. EPN clustering was used to categorize proteins based on their differential expression across time points, providing insights into metabolic and regulatory shifts triggered by NP exposure.
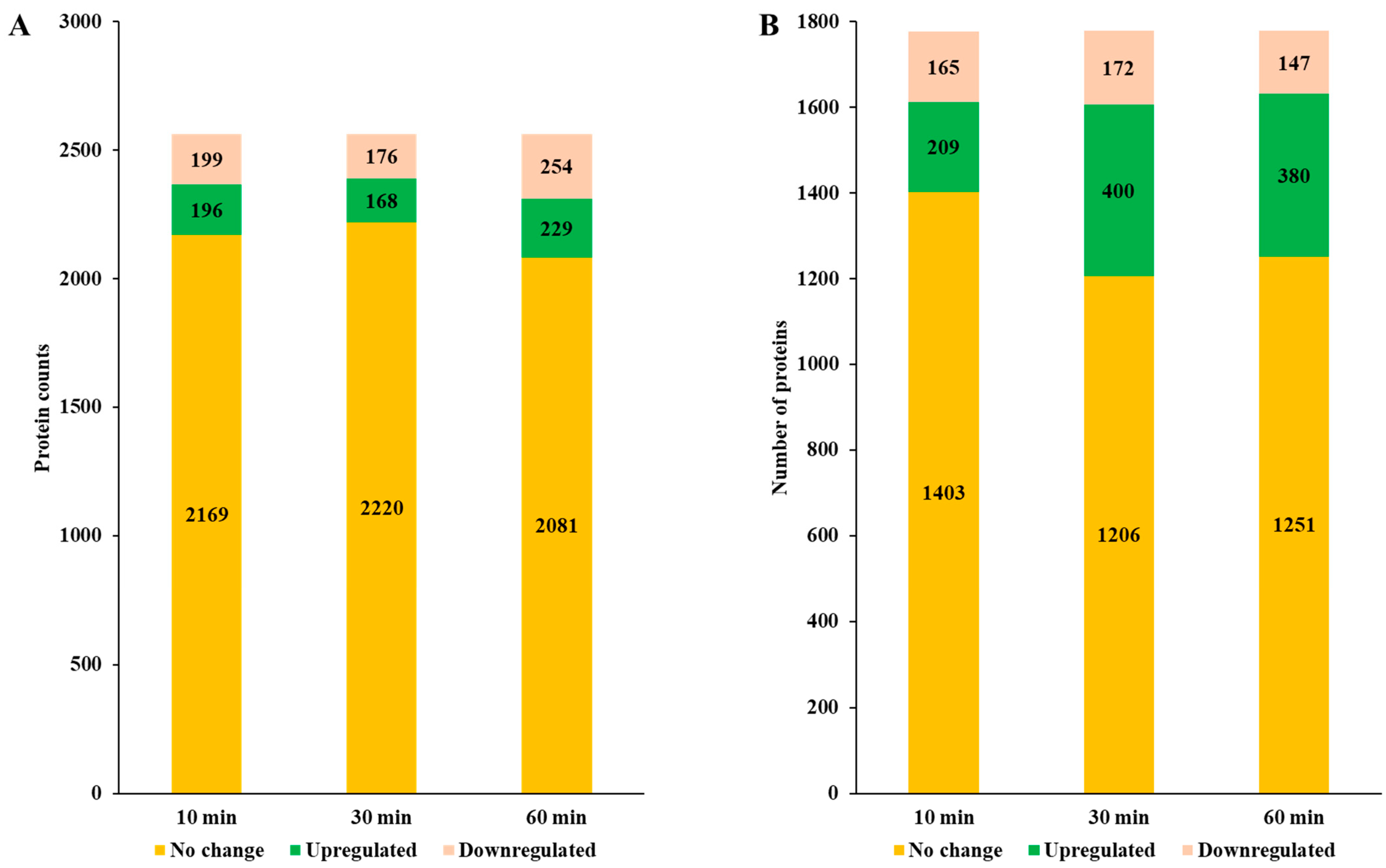
P. aeruginosa exhibited 26 significant EPN clusters, whereas
P. mirabilis had 23 clusters, indicating a comparable but distinct response to Se NP-induced stress (
Tables S1 and S7).
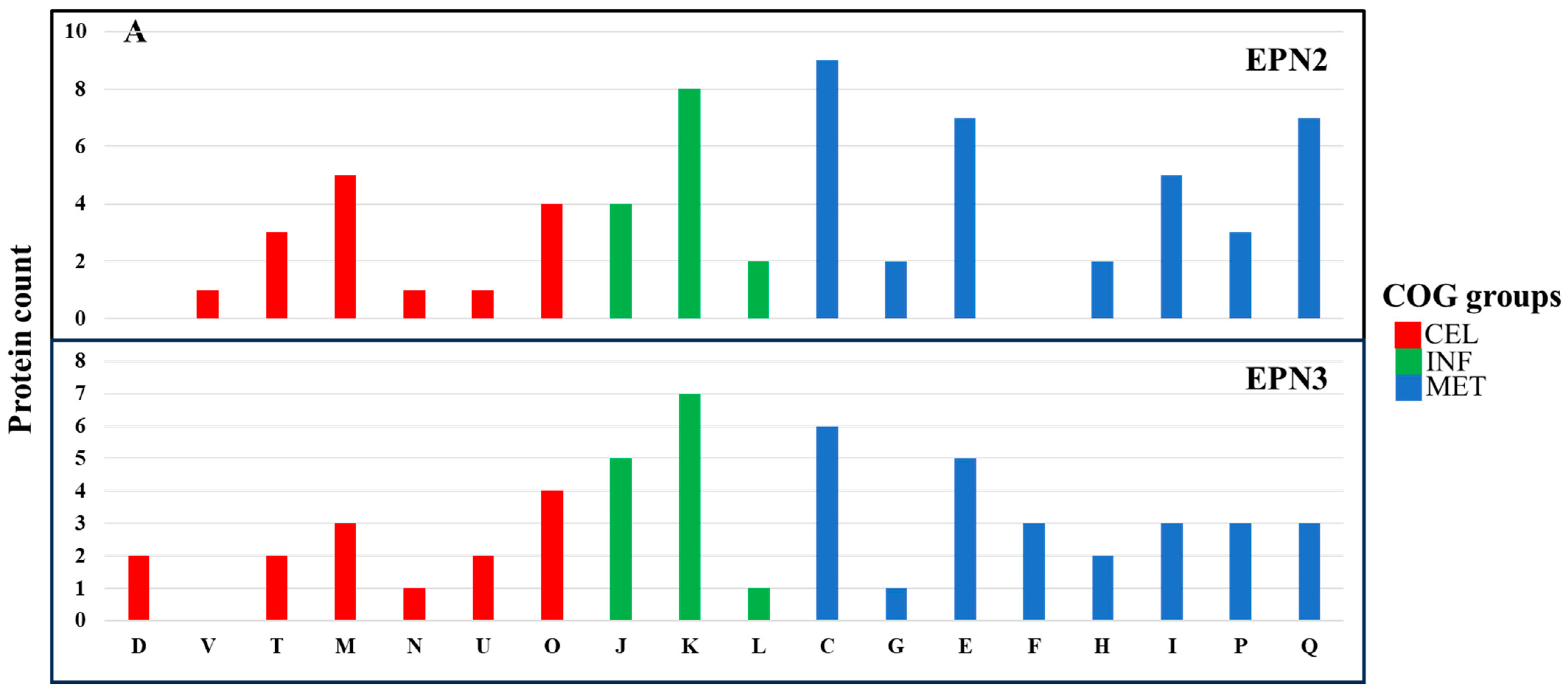
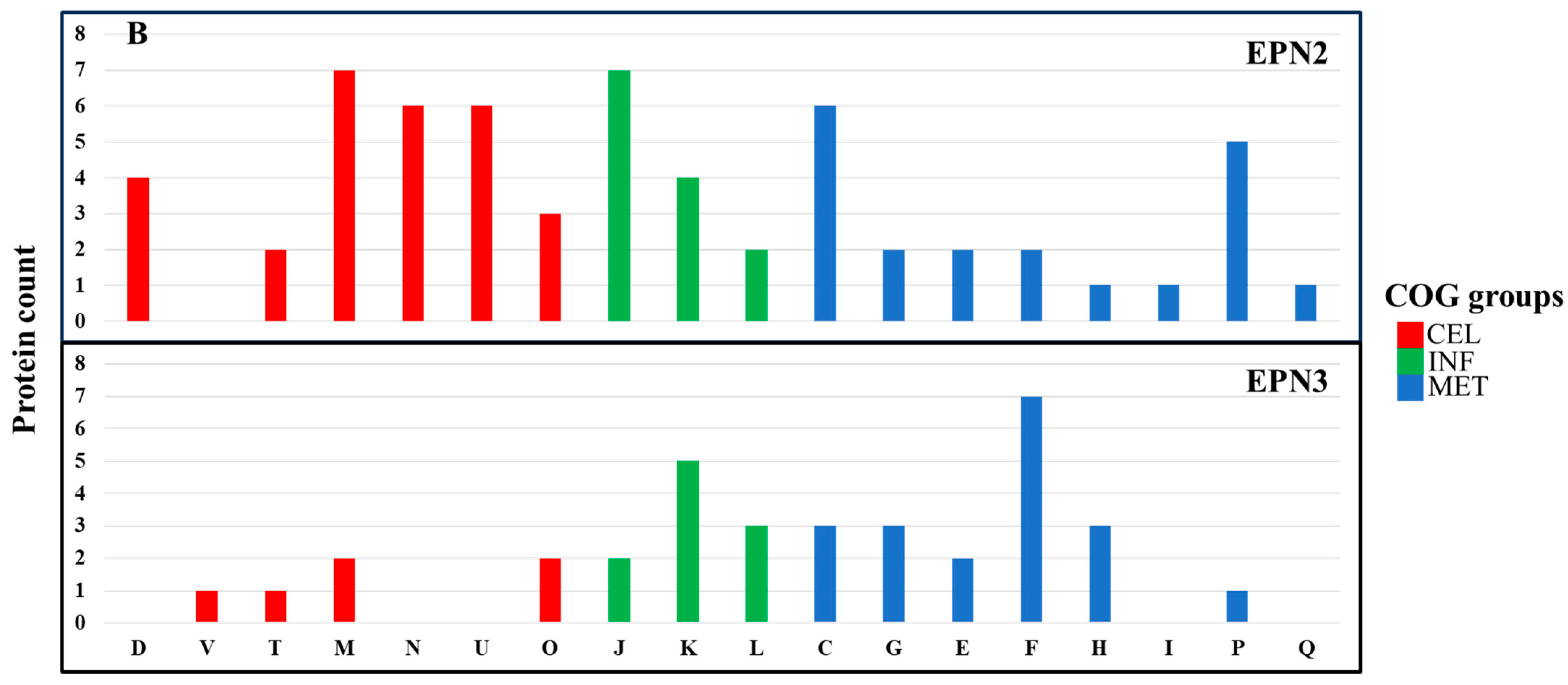
In the EPN2 dataset, which included proteins consistently overexpressed throughout the treatment,
P. aeruginosa showed upregulation of 73 proteins involved primarily in energy production and conversion (C) (
Figure 7A,
Table S5). Among these, key components of the electron transport chain and ATP synthesis machinery, including AtpA, Fpr, GlcE, NapA, NuoM, Qor, RnfC, and RnfG, were markedly overexpressed [
58]. The upregulation of these proteins suggests that
P. aeruginosa responded to Se NP stress by increasing ATP production and optimizing oxidative phosphorylation. Additionally, the heightened expression of electron transport chain components, including NuoM and Qor, suggests a greater reliance on oxidative phosphorylation as a primary strategy to mitigate Se NP exposure-induced oxidative stress [
59].
In contrast,
P. mirabilis exhibited overexpression of 84 proteins in the EPN2 dataset, but with a different functional emphasis (
Figure 7B,
Table S11). The most significantly upregulated proteins were associated with cell wall/membrane/envelope biogenesis (M) and translation, ribosomal structure, and biogenesis (J). Proteins such as LptD, MipA, RfaB, RfaQ, Slp, TtgC, and YtfM, which are involved in cell envelope biogenesis [
60], were significantly induced. This suggests that
P. mirabilis reinforced its structural integrity in response to Se NP exposure [
61]. Additionally, protein expression involved in translation, such as RpmF, RpmH, RpsS, RpsU, and TrmA, also had greatly increased, indicating that
P. mirabilis prioritized sustaining ribosomal function to maintain protein synthesis under stress.
The EPN3 dataset, which included proteins consistently downregulated throughout the treatment, revealed further distinctions between the two species. In
P. aeruginosa, 69 proteins were consistently downregulated, with transcription-related proteins (K) exhibiting the most pronounced decreases (
Figure 7A,
Table S6). Key transcriptional regulators such as Anr, ColR, Rnk, SpoT, and YebC showed significant downregulation, suggesting that Se NP exposure suppressed specific regulatory pathways governing stress responses. The suppression of transcriptional regulators such as Anr and ColR indicates a shift away from certain metabolic pathways, likely as an energy conservation strategy in response to stress [
62].
In
P. mirabilis, 40 proteins were consistently downregulated; the most affected category was nucleotide transport and metabolism (F) (
Figure 7B,
Table S12). Proteins such as Add, ChbG, Cmk, GpmB, KdsA, PneB, and Udk, which are involved in nucleotide biosynthesis and metabolism [
63], exhibited significant decreases in expression.
3.6. Differentially Expressed Proteins Associated with Virulence Factors
Se NP treatment resulted in both shared and species-specific regulation of virulence-associated proteins.
P. aeruginosa exhibited 47 differentially expressed virulence-related proteins, while
P. mirabilis displayed a broader response, with 59 differentially expressed virulence-associated proteins (
Table 2 and
Table 3). Despite differences in the number of virulence factors affected, both species exhibited overlapping responses in key functional categories, including adherence, biofilm formation, secretion systems, immune modulation, motility, and nutrient acquisition. However,
P. mirabilis displayed additional regulation of stress survival- and exotoxin-related proteins. Proteins are involved in capsular polysaccharide synthesis, lipid A modifications, and efflux-mediated immune resistance [
64]. The broader immune modulation response in
P. mirabilis suggests that this species may rely more extensively on modifying its cell surface structures to resist NP-induced stress. Previous research has shown that antimicrobial agents and NPs can induce modifications in outer membrane components, influencing bacterial immune evasion strategies [
65,
66].
In terms of adherence,
P. aeruginosa regulated such proteins as ChpB, CheW, FimL, FimX, RpoN, RpoS, PilB, PilH, PilI, and PilU, all of which contribute to bacterial attachment and colonization [
67]. Similarly,
P. mirabilis exhibited differential expression of adherence-associated proteins, including Crp, MatB, MetQ, MrfD, Tuf, and Uca. The presence of multiple adherence-related proteins in both bacteria suggests that Se NP exposure may influence bacterial attachment properties, possibly altering surface interactions that impact colonization and biofilm formation. Biofilm-associated proteins were also significantly affected in both species.
P. aeruginosa altered the expression of AlgB, AlgP, AlgR, LasR, and RhlR, which are key regulators of alginate biosynthesis and quorum sensing, crucial for biofilm maintenance [
67]. In contrast,
P. mirabilis differentially expressed such biofilm-related proteins as KGA92257.1 and RpoE. These findings suggest that Se NP exposure may disrupt bacterial aggregation and biofilm maturation, potentially weakening bacterial persistence mechanisms. Previous studies have demonstrated that silver NPs can interfere with bacterial adhesion and biofilm formation by disrupting cell surface structures and altering quorum-sensing pathways [
68].
Secretion system proteins were altered in both species, indicating an increased emphasis on protein translocation and virulence factor secretion under stress. In
P. aeruginosa, several type II and type III secretion system components, including GspE, XcpR-S, and YebC [
67], were differentially expressed. Similarly,
P. mirabilis exhibited differential expression of secretion system-related proteins, including Hcp, ImpB, Impl, PpiA, and TssQ_1, components associated with the type VI secretion system [
64]. These findings suggest that both bacteria respond to Se NP stress by modulating secretion system components, possibly to facilitate the export of virulence factors or stress response proteins. Similar trends have been observed in
Vibrio parahaemolyticus and
S. aureus, where exposure to antimicrobial agents led to the modulation of secretion systems, aiding in bacterial persistence [
69,
70].
Immune modulation-related proteins exhibited distinct patterns between the two species.
P. aeruginosa showed regulation of Gmd, LpxK, RfaG, RfbA, and RhlA, proteins involved in immune evasion and bacterial defense [
67]. In contrast,
P. mirabilis displayed a broader immune modulation response, with differentially expressed proteins including AcpP, GalE, GlmU, KdsA, KdtA, LpxB-C, LpxL, MsbA, RfaC, and WecC. Many of these motility-associated proteins were widely affected in both bacteria, indicating a potential impact on bacterial movement and environmental navigation under stress. In
P. aeruginosa, proteins such as CheW, DctD, FlaG, FleR, FlgD-G, FlgL, FlgN, FliC, FliF, FliH, MotA, MotD, and NtrC were differentially expressed, while
P. mirabilis exhibited regulation of motility-related proteins, including CheZ, FlgB, FlgF-H, FlgJ, FlgM, FliG-H, FliM, GlnG, Tap, and YfhA. These findings indicate that both species may be modulating their flagellar and chemotactic systems in response to Se NP exposure.
A key distinction between the two species was the differential regulation of stress survival proteins.
P. mirabilis, but not
P. aeruginosa, exhibited differential regulation of ClpB, KatA, SodC, and UreC, all of which are associated with bacterial stress survival and oxidative stress resistance [
64]. This suggests that
P. mirabilis may rely on oxidative stress resistance mechanisms to counteract Se NP toxicity, likely through increased antioxidant enzyme activity and proteolysis of damaged proteins. In contrast, the absence of similar stress survival proteins in
P. aeruginosa may indicate that this species mitigates oxidative stress through alternative pathways, such as enhanced energy metabolism or efflux system activation.
Selenium nanoparticles are well known to catalyze the generation of reactive oxygen species, which impose oxidative stress and damage key biomolecules including DNA, proteins, and lipids. Han et al. demonstrated that Se NPs induce intracellular reactive oxygen species accumulation in methicillin-resistant
S. aureus and vancomycin-resistant
Enterococcus, ultimately triggering apoptosis-like bacterial death [
13]. Consistent with this, our proteomic analysis revealed upregulation of stress response proteins, further supporting reactive oxygen species-mediated oxidative stress as a central mechanism of Se NP antibacterial activity.
3.7. Differentially Expressed Proteins Associated with Antimicrobial Resistance
The analysis of differentially expressed proteins associated with antimicrobial resistance in
P. aeruginosa and
P. mirabilis following Se NP treatment revealed distinct regulatory patterns, indicating species-specific strategies for mitigating NP-induced stress and potential antimicrobial effects (
Table 4 and
Table 5). In
P. aeruginosa, 10 antimicrobial resistance-related proteins were differentially expressed, including efflux system components (AdeR, MexG, MexH, MexL, MexV, OpmD, RsmA) and antibiotic target replacement proteins (DfrA3, MyrA, RpoB). In
P. mirabilis, 10 antimicrobial resistance-associated proteins were also differentially expressed, including efflux-related transporters (CpxA, CpxR, Crp, Hns, LptD, MsbA, RsmA, TolC), an antibiotic target alteration regulator (PhoB), and an antibiotic-modifying enzyme (CatA4). The expression patterns suggest that both bacteria employ efflux-mediated resistance and structural modifications to counteract Se NP-induced stress.
Efflux pump-associated proteins were regulated in both species, suggesting that active transport mechanisms play a central role in bacterial adaptation to Se NP exposure. In
P. aeruginosa, MexG, MexH, MexV, and OpmD—components of the MexGHI-OpmD efflux system—were differentially expressed, indicating increased activity of the resistance-nodulation-cell division (RND) efflux system [
71]. These efflux pumps are known to contribute to multidrug resistance by expelling toxic compounds, including antimicrobials and heavy metals, from Gram-negative bacteria. Additionally, the MexGHI-OpmD efflux system has been reported to be involved in stress responses in
P. aeruginosa, further supporting its role in counteracting NP-induced toxicity [
71].
Similarly,
P. mirabilis displayed overexpression of TolC, MsbA, and LptD, components of tripartite efflux pumps and lipopolysaccharide-assembly pathways [
72]. These proteins facilitate resistance against antimicrobial agents, metal ions, and environmental stressors. The upregulation of these efflux-related proteins suggests that
P. mirabilis employs efflux-mediated detoxification to reduce intracellular accumulation of Se NPs, a common strategy observed in bacteria exposed to heavy metals and antibiotics [
73,
74].
Regulatory proteins governing antimicrobial resistance and stress responses were also differentially expressed in both species, indicating complex transcriptional and post-transcriptional regulation in response to Se NPs. In
P. aeruginosa, RsmA, AdeR, and MexL were differentially expressed. RsmA plays a crucial role in secondary metabolism and antimicrobial resistance by modulating RNA stability and translation [
75]. AdeR, a well-known regulator of efflux pump expression, facilitates adaptive resistance by modulating bacterial responses to environmental stress [
76]. The observed downregulation of MexL, a negative regulator of the MexXY efflux system, suggests that Se NP exposure may trigger the derepression of certain efflux pumps, possibly leading to increased antimicrobial resistance [
72].
3.8. Differentially Expressed Proteins Associated with Heavy Metal Resistance
The analysis of differentially expressed proteins associated with heavy metal resistance in
P. aeruginosa and
P. mirabilis following Se NP treatment revealed distinct regulatory patterns, reflecting species-specific strategies for coping with metal-induced stress (
Table 6 and
Table 7).
P. aeruginosa exhibited differential expression of 17 heavy metal resistance-related proteins, including metal transporters (CorA, CorD, CueA), mercury detoxification proteins (MerA, MerP, MerR), regulatory transcription factors (CopR, MexT, ComR, CpxR, RpoS), and stress tolerance proteins (NirD/YgiW/YdeI family). In contrast,
P. mirabilis displayed a broader response, with 25 differentially expressed heavy metal resistance proteins, including tellurite resistance proteins (TerB, TerD, TehB), phosphate transporters (PitA, PstB, PstS), copper homeostasis proteins (CutC, CuiD), metal-binding proteins (ZinT, ZntA), and regulatory proteins (ModE, PcoR, CpxR, IclR). These findings suggest that while both species employ metal transport, detoxification, and regulatory mechanisms,
P. mirabilis activates a more extensive set of resistance pathways to counteract Se NP-induced toxicity.
Differential expression of metal transporters in both
P. aeruginosa and
P. mirabilis highlights the crucial role of metal ion homeostasis in their adaptive responses to Se NP stress. In
P. aeruginosa, the expression of CorA (ion transporter) and CorD (a Co
2+/Mg
2+ efflux protein) was modulated, indicating a tightly controlled mechanism for metal ion homeostasis. Additionally, CueA, a copper-translocating P-type ATPase, was differentially expressed, suggesting that
P. aeruginosa employs active copper transport mechanisms to mitigate metal toxicity. These findings are consistent with previous reports showing that CueA contributes to metal homeostasis and resistance in
P. aeruginosa exposed to metal stress [
77].
In
P. mirabilis, multiple transport proteins were modulated, including CutC (copper homeostasis protein), CuiD (blue copper oxidase), ZinT (metal-binding protein), and ZntA (cadmium-translocating P-type ATPase). CutC and CuiD are known to contribute to bacterial copper tolerance, potentially by modulating copper oxidation and transport [
78]. Meanwhile, ZinT and ZntA contribute to resistance against multiple heavy metals, including zinc, lead, cobalt, mercury, and cadmium, suggesting that
P. mirabilis employs a wider range of transporters to maintain intracellular metal ion balance [
79].
A notable difference between the two species was the activation of mercury resistance proteins in
P. aeruginosa. Mer operon components MerA (Mercury(II) reductase), MerP (periplasmic binding protein), and MerR (mercury resistance transcriptional regulator) were differentially expressed. The MerA enzyme plays a crucial role in reducing toxic mercury (Hg
2+) to volatile elemental mercury (Hg
0), which is then expelled from the cell [
80]. Presence of these detoxification proteins suggests that Se NP exposure may regulate heavy metal detoxification pathways in
P. aeruginosa.
Conversely,
P. mirabilis did not exhibit mercury resistance protein expression but instead displayed upregulation of tellurite resistance proteins (TerB, TerD, TehB). Tellurite resistance has been linked to oxidative stress protection, suggesting that
P. mirabilis may rely on tellurite resistance mechanisms rather than mercury detoxification to mitigate NP-induced stress [
81]. The upregulation of tellurite resistance proteins supports previous findings that tellurite resistance pathways enhance bacterial survival under oxidative and metal stress conditions [
82].
Differential expression of several membrane-associated proteins suggests that both species undergo structural remodeling of their outer membranes to adapt to Se NP-induced stress. In
P. aeruginosa, ModC (a heme ABC transporter ATP-binding protein) and VcaM (a lipid A export permease/ATP-binding protein) were differentially expressed, potentially indicating modifications in membrane permeability and lipid transport. Lipid A modifications have been shown to contribute to bacterial resistance against metal toxicity by reducing membrane permeability to toxic ions [
83].
In
P. mirabilis, LptD (lipopolysaccharide-assembly protein) and PitA (a low-affinity phosphate transporter) were differentially expressed, further supporting the hypothesis that membrane integrity and phosphate metabolism play crucial roles in stress adaptation. Increased expression of LptD suggests that
P. mirabilis may reinforce its lipopolysaccharide layer to mitigate Se NP-induced damage, a strategy previously observed in bacteria exposed to metal stress [
84].
A limitation of this study is that we conducted proteomic analysis only on P. aeruginosa and P. mirabilis, which exhibited the highest and lowest susceptibility to Se NPs, respectively. While this approach provided valuable insights into species-specific adaptive responses, it did not capture the full spectrum of proteomic changes across all tested uropathogens, including E. coli and E. faecalis. As a result, potential differences in stress adaptation mechanisms among other bacterial species remain unexplored. Future studies should expand proteomic analysis over a broader range of pathogens to provide a more comprehensive understanding of bacterial responses to Se NP-induced stress.
Another consideration is the potential loss of proteins during sample processing. Since Se NPs induce cell membrane damage, proteins—particularly secreted proteins or intracellular proteins released due to cell rupture—may leak into the surrounding medium and be lost during the centrifugation step post-treatment. This means that the detected proteomic profile primarily reflects proteins retained within partially intact or damaged cells and may not represent the full proteome. Furthermore, differences in proteomic profiles between P. aeruginosa and P. mirabilis could partly be attributed to differences in cellular integrity, as P. aeruginosa is more susceptible and prone to lysis, whereas P. mirabilis is more resistant. Despite this limitation, the detection of differentially regulated proteins provides meaningful insight into bacterial stress responses and adaptation strategies following Se NP exposure.
Finally, due to budgetary constraints, proteome analyses were not conducted in triplicates. Performing proteomic profiling for two bacterial species across three time points would have required substantial additional resources beyond the scope of the current funding. While this represents a limitation in statistical replication, we minimized variability by applying stringent data processing, normalization, and filtering criteria. The observed pathway-level trends were consistent with established bacterial stress responses, lending confidence to the robustness of the findings. Future studies, contingent on available funding, should aim to include triplicate analyses to further enhance the statistical power and reproducibility of the proteomic data.