Pd/SiC-Catalyzed Visible-Light-Driven N-Methylation of Nitroaranes Using Formaldehyde
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Catalysts
2.2. Catalyst Characterization
2.3. Catalytic Reaction
3. Results and Discussion
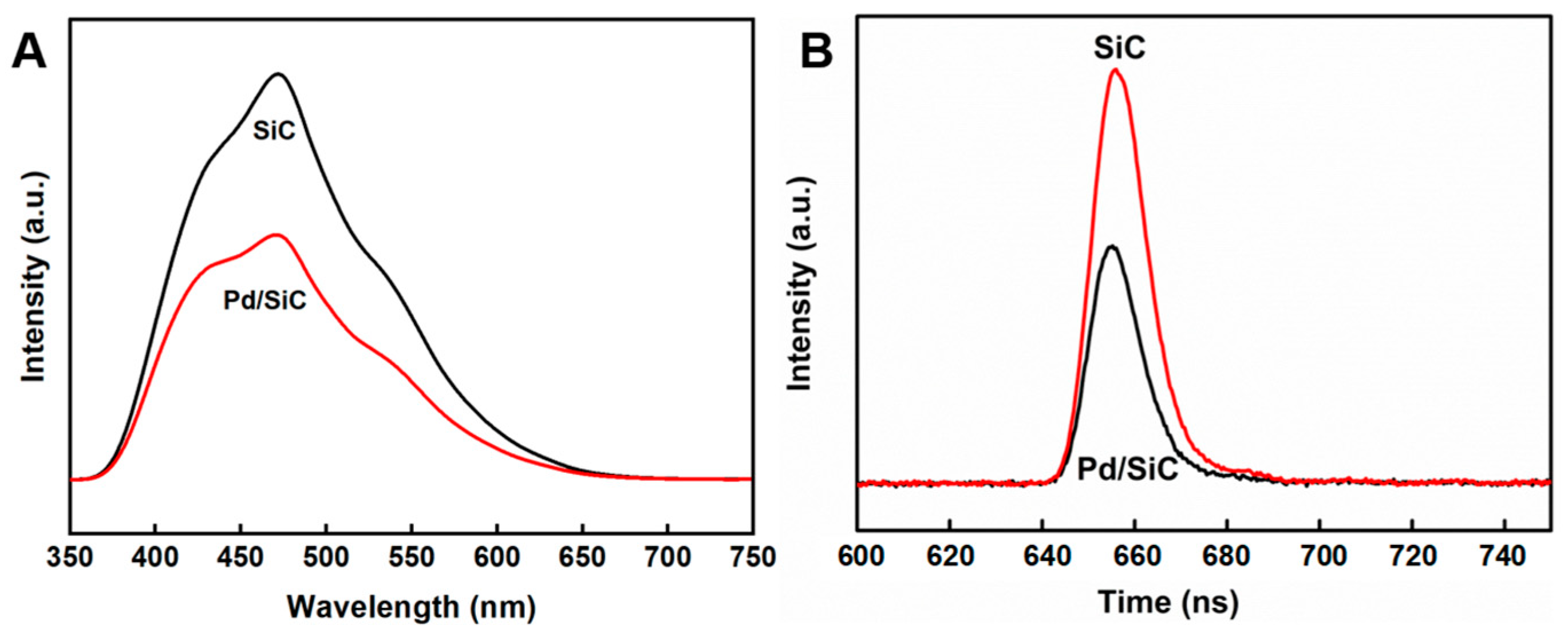
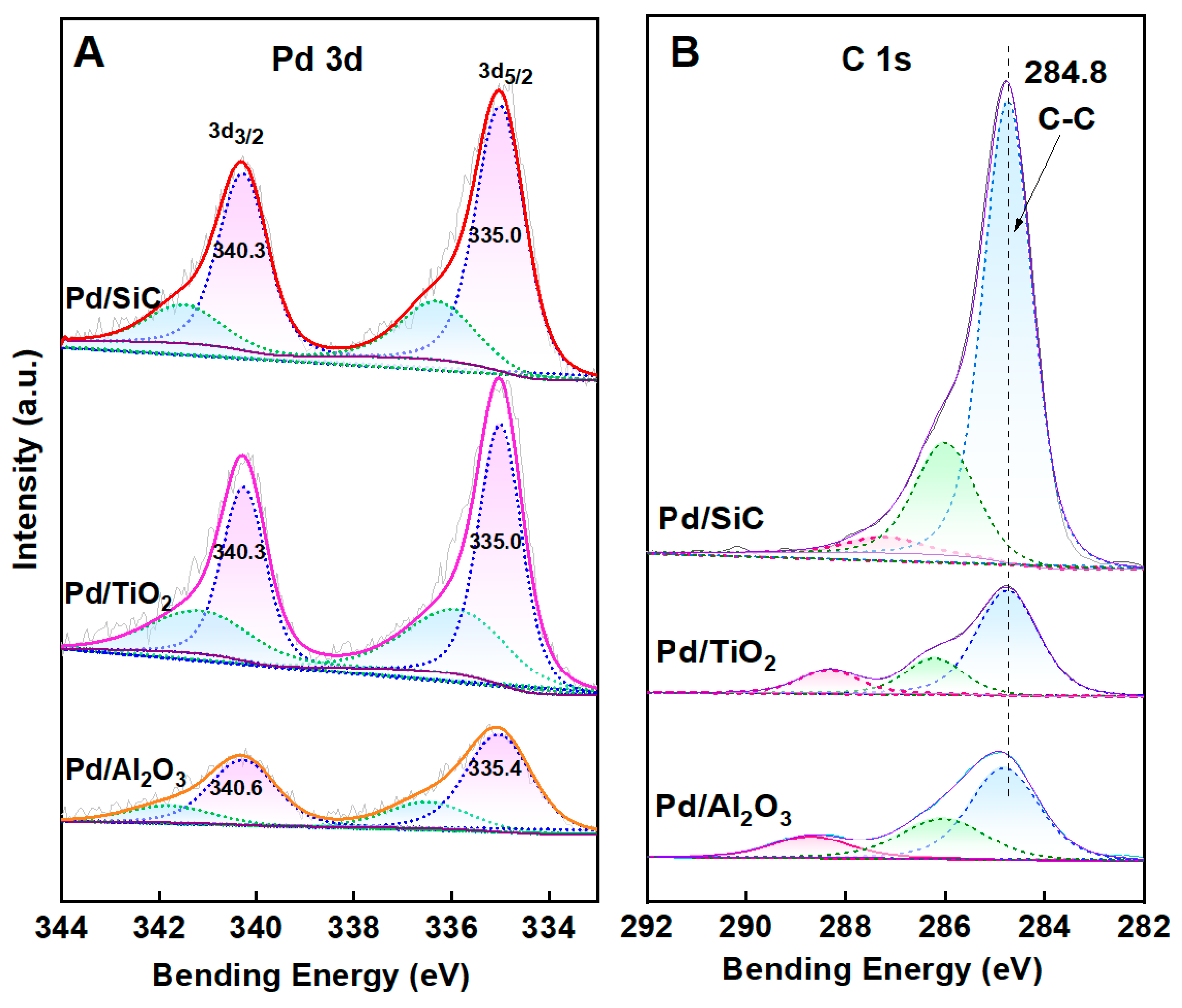
3.1. Synthesis and Characterization
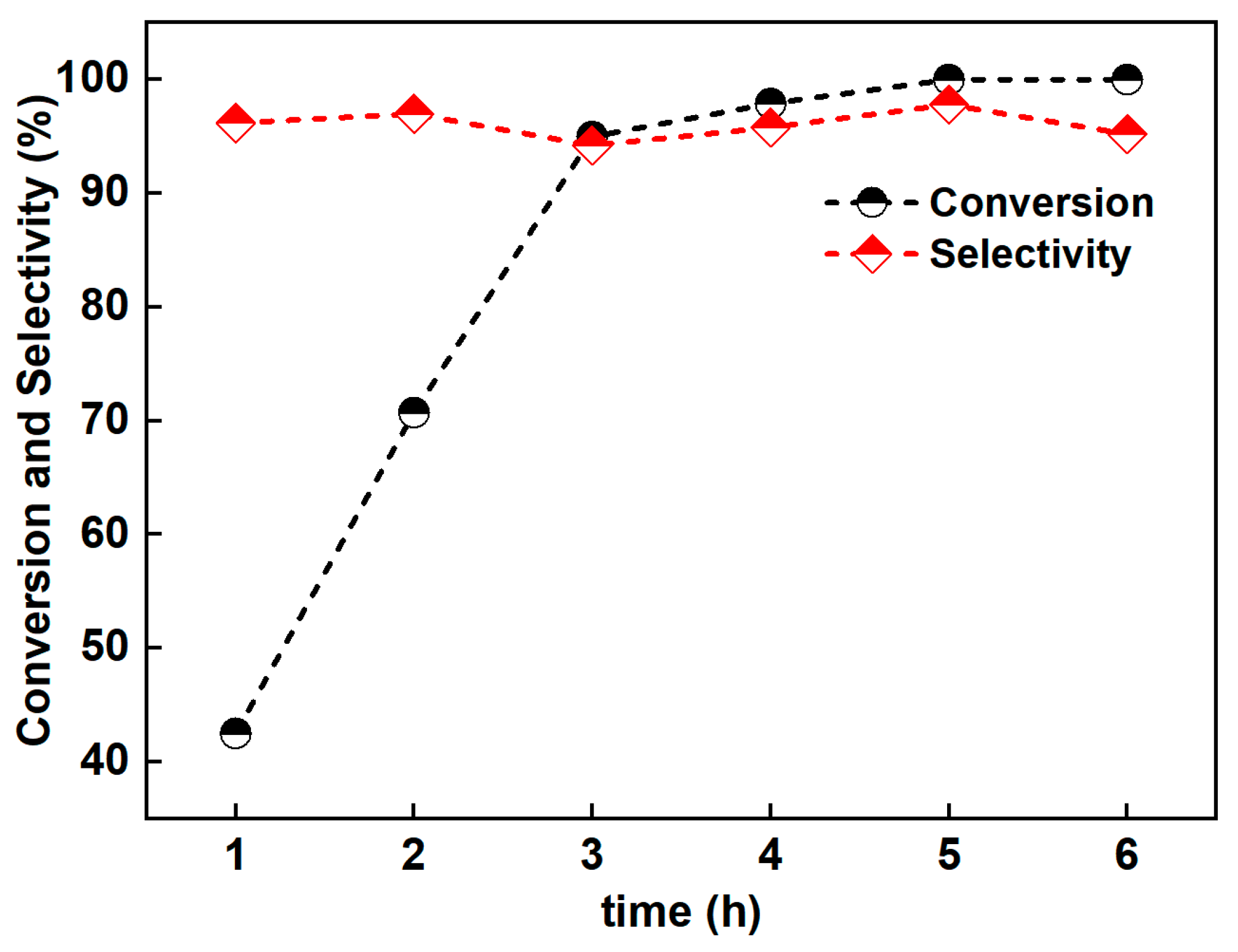
3.2. Photocatalytic Performance of the Catalyst
3.3. Versatility of Catalysts
3.4. Stability of the Catalystsen
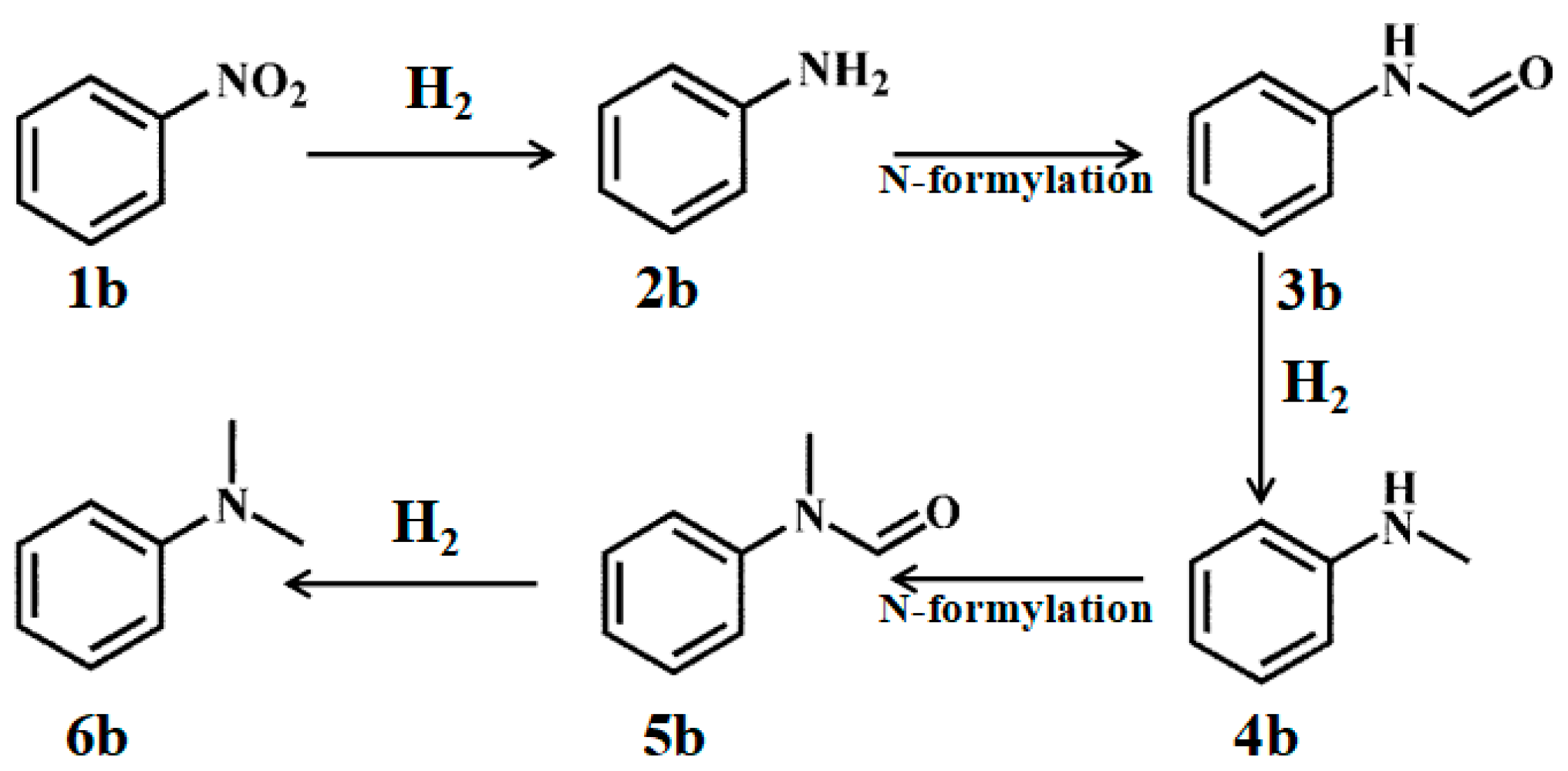
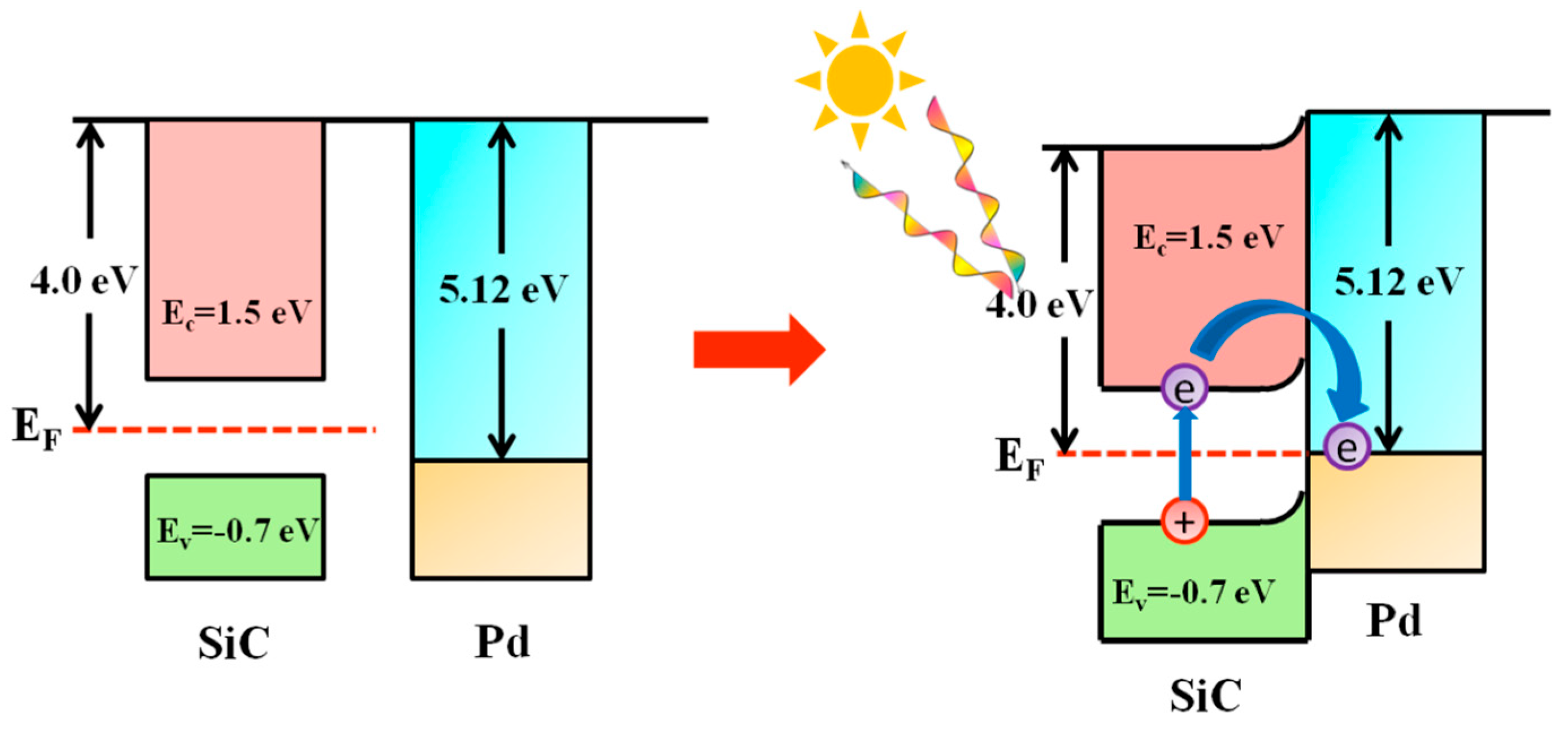
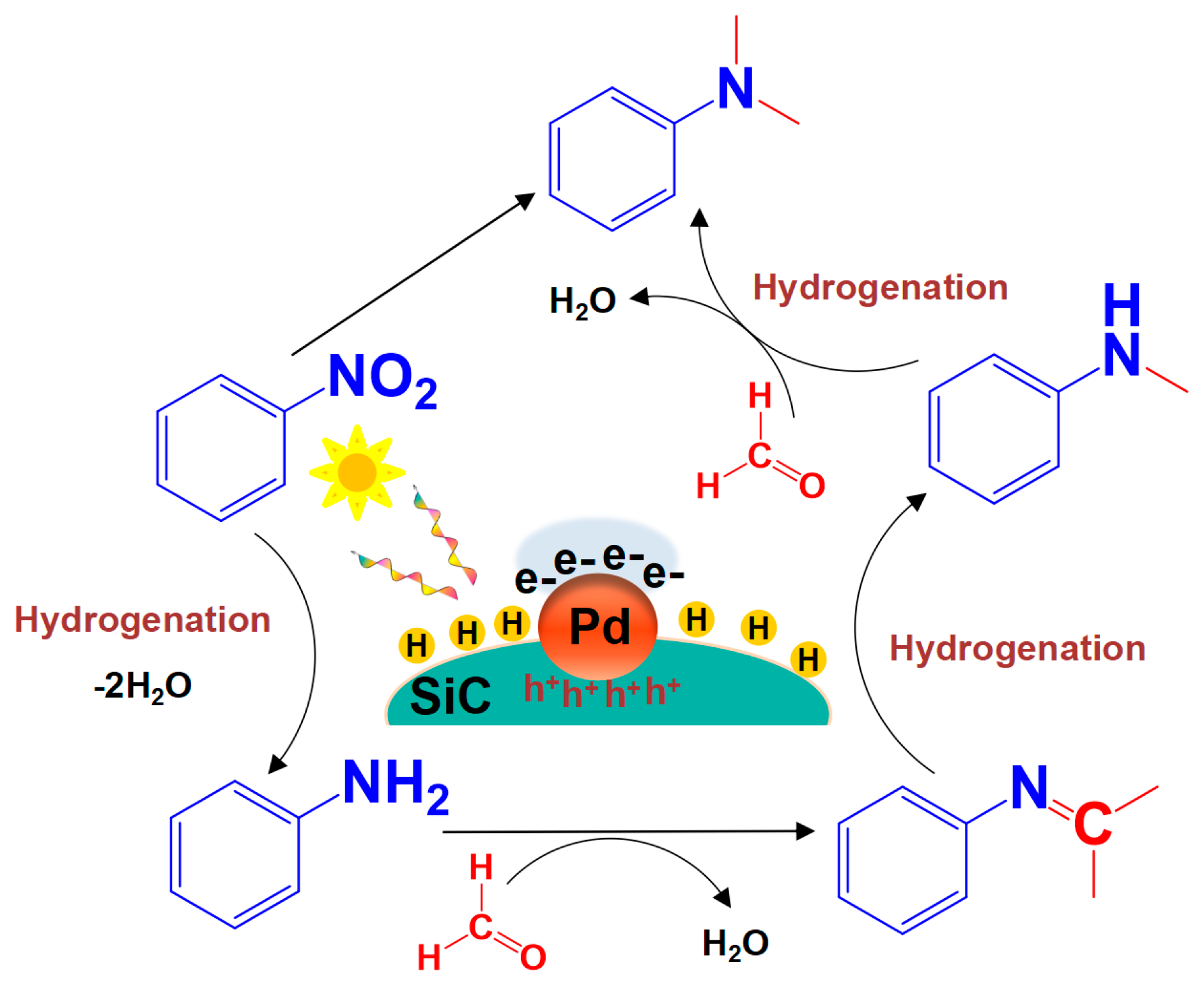
3.5. Investigation of the Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, B.; Shee, S.; Chakrabarti, K.; Kundu, S. Tandem Transformation of Nitro Compounds into N-Methylated Amines: Greener Strategy for the Utilization of Methanol as a Methylating Agent. ChemSusChem 2017, 10, 2370–2374. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076. [Google Scholar]
- Wienhöfer, G.; Sorribes, I.; Boddien, A.; Westerhaus, F.; Junge, K.; Junge, H.; Llusar, R.; Beller, M. General and Selective Iron-Catalyzed Transfer Hydrogenation of Nitroarenes without Base. J. Am. Chem. Soc. 2011, 133, 12875–12879. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Deng, Y.; Shi, F. Reductive N-Alkylation of Nitro Compounds to N-Alkyl and N,N-Dialkyl Amines with Glycerol as the Hydrogen Source. ACS Catal. 2013, 3, 808–811. [Google Scholar]
- Wang, X.; Zhao, K.; Wang, H.; Shi, F. Selective Synthesis of N-Monomethyl Amines with Primary Amines and Nitro Compounds. Catal. Sci. Technol. 2021, 11, 7239–7254. [Google Scholar] [CrossRef]
- Campos, J.; Sharninghausen, L.S.; Manas, M.G.; Crabtree, R.H. Methanol Dehydrogenation by Iridium N-Heterocyclic Carbene Complexes. Inorg. Chem. 2015, 54, 5079–5084. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hong, X.; Cui, H.-Z.; Zhan, B.; Li, Z.-M.; Hou, X.-F. Bimetallic Bis-Nhc-Ir(Iii) Complex Bearing 2-Arylbenzo[D]Oxazolyl Ligand: Synthesis, Catalysis, and Bimetallic Effects. Organometallics 2020, 39, 3514–3523. [Google Scholar] [CrossRef]
- Li, F.; Xie, J.; Shan, H.; Sun, C.; Chen, L. General and Efficient Method for Direct N-Monomethylation of Aromatic Primary Amines with Methanol. RSC Adv. 2012, 2, 8645–8652. [Google Scholar] [CrossRef]
- Ogata, O.; Nara, H.; Fujiwhara, M.; Matsumura, K.; Kayaki, Y. N-Monomethylation of Aromatic Amines with Methanol Via Pnhp-Pincer Ru Catalysts. Org. Lett. 2018, 20, 3866–3870. [Google Scholar] [CrossRef]
- Choi, G.; Hong, S.H. Selective Monomethylation of Amines with Methanol as the C1 Source. Angew. Chem. Int. Ed. 2018, 57, 6166–6170. [Google Scholar] [CrossRef]
- Dang, T.T.; Ramalingam, B.; Seayad, A.M. Efficient Ruthenium-Catalyzed N-Methylation of Amines Using Methanol. ACS Catal. 2015, 5, 4082–4088. [Google Scholar] [CrossRef]
- Maji, M.; Chakrabarti, K.; Paul, B.; Roy, B.C.; Kundu, S. Ruthenium(Ii)-Nnn-Pincer-Complex-Catalyzed Reactions between Various Alcohols and Amines for Sustainable C−N and C−C Bond Formation. Adv. Synth. Catal. 2017, 360, 722–729. [Google Scholar] [CrossRef]
- Grigg, R.; Mitchell, T.R.B.; Sutthivaiyakit, S.; Tongpenya, N. Transition Metal-Catalysed N -Alkylation of Amines by Alcohols. Chem. Commun. 1981, 12, 611–612. [Google Scholar] [CrossRef]
- Elangovan, S.; Neumann, J.; Sortais, J.-B.; Junge, K.; Darcel, C.; Beller, M. Efficient and Selective N-Alkylation of Amines with Alcohols Catalysed by Manganese Pincer Complexes. Nat. Commun. 2016, 7, 12641. [Google Scholar] [CrossRef]
- Bruneau-Voisine, A.; Wang, D.; Dorcet, V.; Roisnel, T.; Darcel, C.; Sortais, J.-B. Mono-N-Methylation of Anilines with Methanol Catalyzed by a Manganese Pincer-Complex. J. Catal. 2017, 347, 57–62. [Google Scholar] [CrossRef]
- Polidano, K.; Allen, B.D.W.; Williams, J.M.J.; Morrill, L.C. Iron-Catalyzed Methylation Using the Borrowing Hydrogen Approach. ACS Catal. 2018, 8, 6440–6445. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Zhu, Y.; Xiang, Y. One-Pot Synthesis of N,N-Dimethylaniline from Nitrobenzene and Methanol. New J. Chem. 2009, 33, 2051–2054. [Google Scholar] [CrossRef]
- Uozumi, Y.; Sakurai, F. Reductive N-Methylation with CO2 and H2 Using CualOx. Synfacts 2014, 10, 0444. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Deng, Y.; Shi, F. N-Methylation of Amine and Nitro Compounds with Co2/H2catalyzed by Pd/Cuzroxunder Mild Reaction Conditions. Chem. Commun. 2014, 50, 13521–13524. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Deng, Y.; Shi, F. Light-Promoted N,N-Dimethylation of Amine and Nitro Compound with Methanol Catalyzed by Pd/Tio2 at Room Temperature. RSC Adv. 2015, 5, 14514–14521. [Google Scholar] [CrossRef]
- Van Dorp, D.H.; Hijnen, N.; Di Vece, M.; Kelly, J.J. SiC: A Photocathode for Water Splitting and Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-Y.; Wang, Y.-Y.; Tong, X.-L.; Jin, G.-Q.; Guo, X.-Y. Photocatalytic Hydrogen Production over Modified Sic Nanowires under Visible Light Irradiation. Int. J. Hydrogen Energy 2012, 37, 15038–15044. [Google Scholar] [CrossRef]
- Tuci, G.; Liu, Y.; Rossin, A.; Guo, X.; Pham, C.; Giambastiani, G.; Pham-Huu, C. Porous Silicon Carbide (SiC): A Chance for Improving Catalysts or Just Another Active-Phase Carrier? Chem. Rev. 2021, 121, 10559–10665. [Google Scholar] [CrossRef]
- Jiao, Z.-F.; Guo, X.-N.; Zhai, Z.-Y.; Jin, G.-Q.; Wang, X.-M.; Guo, X.-Y. The Enhanced Catalytic Performance of Pd/SiC for the Hydrogenation of Furan Derivatives at Ambient Temperature under Visible Light Irradiation. Catal. Sci. Technol. 2014, 4, 2494–2498. [Google Scholar] [CrossRef]
- Hao, C.-H.; Guo, X.-N.; Sankar, M.; Yang, H.; Ma, B.; Zhang, Y.-F.; Tong, X.-L.; Jin, G.-Q.; Guo, X.-Y. Synergistic Effect of Segregated Pd and Au Nanoparticles on Semiconducting Sic for Efficient Photocatalytic Hydrogenation of Nitroarenes. ACS Appl. Mater. Interfaces 2018, 10, 23029–23036. [Google Scholar] [CrossRef]
- Jiao, Z.-F.; Zhao, J.-X.; Guo, X.-N.; Tong, X.-L.; Zhang, B.; Jin, G.-Q.; Qin, Y.; Guo, X.-Y. Turning the Product Selectivity of Nitrile Hydrogenation from Primary to Secondary Amines by Precise Modification of Pd/Sic Catalysts Using Nio Nanodots. Catal. Sci. Technol. 2019, 9, 2266–2272. [Google Scholar] [CrossRef]
- De Marchi, S.; Núñez-Sánchez, S.; Bodelón, G.; Pérez-Juste, J.; Pastoriza-Santos, I. Pd Nanoparticles as a Plasmonic Material: Synthesis, Optical Properties and Applications. Nanoscale 2020, 12, 23424–23443. [Google Scholar] [CrossRef] [PubMed]
- Pakizeh, T. Optical Absorption of Nanoparticles Described by an Electronic Local Interband Transition. J. Opt. 2013, 15, 025001. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Li, X.H.; Zhang, Y.N.; Wei, X.; Wang, K.X.; Chen, J.S. Highly Efficient Dehydrogenation of Formic Acid over a Palladium-Nanoparticle-Based Mott–Schottky Photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 11822–11825. [Google Scholar] [CrossRef]
- Cheong, S.; Watt, J.D.; Tilley, R.D. Shape Control of Platinum and Palladium Nanoparticles for Catalysis. Nanoscale 2010, 2, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, X.; Wang, K.; Lv, J.; Chen, S.; Yao, G.; Lang, J.; Lv, F.; Pu, Y.; Yang, R.; et al. Palladium Catalyzed Radical Relay for the Oxidative Cross-Coupling of Quinolines. Nat. Commun. 2022, 13, 4180. [Google Scholar] [CrossRef]
- Canton, P.; Meneghini, C.; Riello, P.; Balerna, A.; Benedetti, A. Thermal Evolution of Carbon-Supported Pd Nanoparticles Studied by Time-Resolved X-Ray Diffraction. J. Phys. Chem. B 2001, 105, 8088–8091. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Zhuang, C.; Peng, T.; Li, R.; Li, X. Highly Asymmetric Phthalocyanine as a Sensitizer of Graphitic Carbon Nitride for Extremely Efficient Photocatalytic H2 Production under near-Infrared Light. ACS Catal. 2013, 4, 162–170. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, T.-C.; Hsu, Y.-J. Znse·0.5N2H4 Hybrid Nanostructures: A Promising Alternative Photocatalyst for Solar Conversion. ACS Appl. Mater. Interfaces 2015, 7, 1616–1623. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin Hexagonal SnS2 Nanosheets Coupled with g-C3N4 Nanosheets as 2D/2D Heterojunction Photocatalysts toward High Photocatalytic Activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Li, X.-H.; Baar, M.; Blechert, S.; Antonietti, M. Facilitating Room-Temperature Suzuki Coupling Reaction with Light: Mott-Schottky Photocatalyst for C-C-Coupling. Sci. Rep. 2013, 3, 1743. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef]
- Chen, S.; Ying, P.; Wang, L.; Wei, G.; Zheng, J.; Gao, F.; Su, S.; Yang, W. Growth of Flexible N-Doped SiC Quasialigned Nanoarrays and Their Field Emission Properties. J. Mater. Chem. C 2013, 1, 4779–4784. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.; Arnold, D.P.; Liu, H.; Zhu, H. Efficient Photocatalytic Suzuki Cross-Coupling Reactions on Au–Pd Alloy Nanoparticles under Visible Light Irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Chen, X.; Tang, G.; Deng, G.-J.; Ji, X.; Huang, H. Carbon Nitride Nanosheet-Supported Bismuth Nanoparticle Catalyst for Reductive Amination of Carbonyl Compounds with Nitroaromatics. ACS Appl. Nano Mater. 2023, 6, 15367–15373. [Google Scholar] [CrossRef]

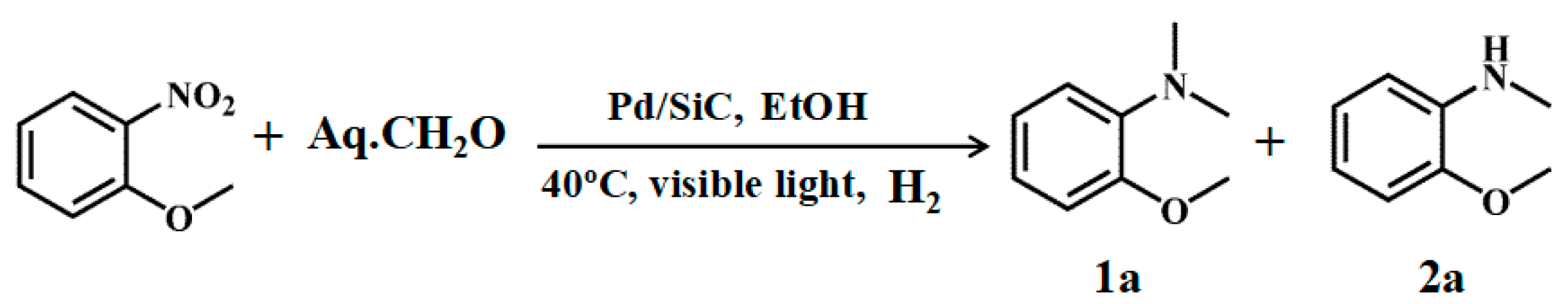


| Entry | Catalyst | Conv. (%) | Select. (%) | |
|---|---|---|---|---|
| 2-methoxy-N,N-dimethylaniline (1a) | 2-methoxy-N-methylaniline (2a) | |||
| 1 | SiC | 0 | — | — |
| 2 | Pd/SiC-light | 95.0 | 94.2 | 5.8 |
| 3 | Pd/SiC-dark | 46.1 | 96.4 | 3.6 |
| 4 | Pd/SiC-Ar-light | 0 | — | — |
| 5 | Pt/SiC-light | 96.6 | 57.3 | 42.7 |
| 6 | Ru/SiC-light | 9.9 | 81.3 | 18.7 |
| 7 | Pd/Al2O3 | 14.5 | 90.8 | 9.2 |
| 8 | Pd/TiO2 | 30.3 | 93.7 | 6.3 |
| 9 | Pd/ZrO2 | 21.6 | 97.1 | 2.9 |
| 10 | Pd/SiC (1 wt%) | 72.3 | 85.0 | 15 |
| 11 | Pd/SiC (5 wt%) | 69.8 | 96.8 | 3.2 |
| Entry | Substrate | Product | Conv. (%) | Select. (%) |
|---|---|---|---|---|
| 1 | 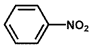 |  | 100 | 100 |
| 2 |  |  | 100 | 100 |
| 3 |  |  | 100 | 100 |
| 4 | 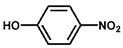 | 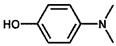 | 79.1 | 100 |
| 5 | 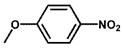 | 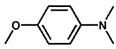 | 98 | 96.7 |
| 6 | 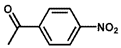 |  | 91.5 | 100 |
| 7 |  | 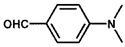 | 100 | 100 |
| 8 a | 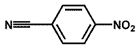 | 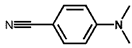 | 100 | 38.7 |
| 9 |  | 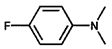 | 67.2 | 68.2 |
| 10 b | 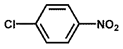 | 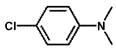 | 100 | 31.9 |
| Entry | Substrate | Conv. (%) | Select. (%) | ||||
|---|---|---|---|---|---|---|---|
| 2b | 3b | 4b | 5b | 6b | |||
| 1 | 1b | 100 | <1 | nd. | nd. | nd. | >99 |
| 2 a | 1b | 61.7 | <1 | nd | 9.3 | nd | 90.6 |
| 3 | 2b | 100 | — | nd. | nd. | nd. | >99 |
| 4 | 3b | <1 | — | — | nd. | nd. | 100 |
| 5 | 4b | 100 | — | — | — | nd. | 100 |
| 6 | 5b | 0.5 | — | — | — | — | 100 |
| 7 b | 5b | 0 | — | — | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, D.; Guo, R.; Dong, X.; Fan, Y.; Wang, J.; Tong, X. Pd/SiC-Catalyzed Visible-Light-Driven N-Methylation of Nitroaranes Using Formaldehyde. Nanomaterials 2025, 15, 1394. https://doi.org/10.3390/nano15181394
Hou D, Guo R, Dong X, Fan Y, Wang J, Tong X. Pd/SiC-Catalyzed Visible-Light-Driven N-Methylation of Nitroaranes Using Formaldehyde. Nanomaterials. 2025; 15(18):1394. https://doi.org/10.3390/nano15181394
Chicago/Turabian StyleHou, Dongfang, Ruifeng Guo, Xianshu Dong, Yuping Fan, Jingru Wang, and Xili Tong. 2025. "Pd/SiC-Catalyzed Visible-Light-Driven N-Methylation of Nitroaranes Using Formaldehyde" Nanomaterials 15, no. 18: 1394. https://doi.org/10.3390/nano15181394
APA StyleHou, D., Guo, R., Dong, X., Fan, Y., Wang, J., & Tong, X. (2025). Pd/SiC-Catalyzed Visible-Light-Driven N-Methylation of Nitroaranes Using Formaldehyde. Nanomaterials, 15(18), 1394. https://doi.org/10.3390/nano15181394






